Abstract
Background
Canine pituitary dwarfism or combined pituitary hormone deficiency (CPHD) in shepherd dogs is associated with an LHX3 mutation and can lead to a wide range of clinical manifestations. Some dogs with CPHD have neurological signs that are localized to the cervical spine. In human CPHD, caused by an LHX3 mutation, anatomical abnormalities in the atlanto‐axial (C1‐C2) joint have been described.
Objectives
To evaluate the presence of atlanto‐axial malformations in dogs with pituitary dwarfism associated with an LHX3 mutation and to investigate the degree of similarity between the atlanto‐axial anomalies found in canine and human CPHD patients with an LHX3 mutation.
Animals
Three client‐owned Czechoslovakian wolfdogs and 1 client‐owned German shepherd dog, previously diagnosed with pituitary dwarfism caused by an LHX3 mutation, with neurological signs indicating a cervical spinal disorder.
Methods
Radiography, computed tomography, and magnetic resonance imaging of the cranial neck and skull, necropsy, and histology.
Results
Diagnostic imaging identified abnormal positioning of the dens axis and incomplete ossification of the suture lines between the ossification centers of the atlas with concurrent atlanto‐axial instability and dynamic compression of the spinal cord by the dens axis. The malformations and aberrant motion at C1–C2 were confirmed at necropsy and histology.
Conclusions and Clinical Importance
The atlanto‐axial abnormalities of the dwarf dogs resemble those encountered in human CPHD patients with an LHX3 mutation. These findings suggest an association between the LHX3 mutation in dogs with CPHD and atlanto‐axial malformations. Consequently, pituitary dwarfs should be monitored closely for neurological signs.
Keywords: Ataxia, Canine, Growth hormone, Hypermetria, Opisthotonus
Abbreviations
- 3D
3‐dimensional
- CPHD
combined pituitary hormone deficiency
- CT
computed tomography
- FSH
follicle‐stimulating hormone
- GH
growth hormone
- GHRH
growth hormone releasing hormone
- H&E
hematoxylin and eosin
- LH
luteinizing hormone
- MPR
multiplanar reconstruction
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- TE
time of echo
- TR
time of repetition
- TSH
thyroid stimulating hormone
- WI
weighted images
The transcription factor LHX3 is an important regulator of early pituitary development. Molecular defects in this gene can result in underdevelopment of the pituitary gland, resulting in combined pituitary hormone deficiency (CPHD) in both humans and dogs.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Individuals with CPHD caused by an LHX3 mutation are deficient in growth hormone (GH), thyroid stimulating hormone (TSH), prolactin, luteinizing hormone (LH) and follicle‐stimulating hormone (FSH), and although some human patients also may have impaired corticotrope function, ACTH secretion is unaffected in dogs with CPHD.6, 10, 11, 12
Canine CPHD caused by an LHX3 mutation is encountered most often in German shepherd dogs and in related breeds such as Saarloos and Czechoslovakian wolfdogs.6, 10 CPHD in these breeds is inherited in an autosomal recessive fashion13 and linked to a contraction of a DNA repeat in intron 5 of canine LHX3, leading to deficient splicing of the LHX3 transcript.6, 10 Common clinical manifestations of canine CPHD are marked growth retardation, retention of secondary hairs (puppy hair coat) with concurrent lack of primary or guard hairs, and bilaterally symmetrical alopecia.11, 12, 14 The disorder also can lead to a wide range of other clinical manifestations, but not all dwarfs share the same clinical signs.14
In humans with CPHD caused by an LHX3 mutation, a short neck, rigid cervical spine, and anatomical abnormalities in the occipito‐atlantoaxial joints in combination with a basilar impression, defined as an upward displacement of vertebral elements into the normal foramen magnum caused by softening of bones at the base of the skull, of the dens axis have been reported.5, 9 So far, there have been no reports of similar abnormalities in canine dwarfs.
Recently, 3 Czechoslovakian wolfdogs and 1 German shepherd dwarf with CPHD were presented to our clinic for neurological signs suggestive of a cervical spinal problem. The investigations demonstrated anatomical abnormalities of the atlanto‐axial joint with concurrent atlanto‐axial instability and dynamic compression of the spinal cord by the dens axis. This article is the first report of cervical abnormalities associated with CPHD caused by an LHX3 mutation in dogs.
Materials and Methods
Dogs
Three Czechoslovakian wolfdogs and 1 German shepherd dog with confirmed CPHD were presented to the Utrecht University Clinic for Companion Animals because of neurological signs. Two of the Czechoslovakian wolfdogs originated from the same litter and were intact females. The other Czechoslovakian wolfdog dwarf was an unrelated intact male. At presentation, the ages of the Czechoslovakian wolfdogs dogs were 7, 8, and 10 months and the body weights of the dwarfs were 11.2, 14.2, and 20.0 kg, respectively. The German shepherd dwarf was a 4‐year‐old intact female with a body weight of 19.8 kg.
The dogs were diagnosed with CPHD at 3–4 months of age using a growth hormone releasing hormone (GHRH) stimulation test as described previously.12 Basal plasma growth hormone (GH) concentrations were low and none of the dogs responded to provocative testing with GHRH with an increase in the plasma GH concentration. The LHX3 mutation was demonstrated by genetic testing, as described previously.6, 10 Briefly, genomic DNA, derived from EDTA‐anticoagulated whole blood, was amplified using polymerase chain reaction (PCR), and DNA fragment length analysis was performed on the PCR products. All dogs were homozygous for the 7‐bp deletion in LHX3 intron 5 that has been reported in shepherd dogs with CPHD.6, 10 The animals were treated with porcine GH1 (0.1–0.3 IU per kg body weight 3 times per week) and synthetic levo‐thyroxine2 (with a starting oral dosage of 0.02 mg/kg body weight, q12h) as described previously.14
Diagnostic Imaging
Magnetic resonance imaging (MRI) was performed with a 0.2T open magnet using a 16S multipurpose coil.3 Sagittal T2‐weighted images (WI) (time of repetition [TR]: 4455 ms, time of echo [TE]: 117 ms), and transverse T1‐WI (TR: 560 ms, TE: 15 ms) of the caudal skull and the cranial cervical vertebral column were obtained in all dogs (both sequences consisted of 3‐mm‐thick slices). Flash 3‐dimensional (3D)‐WI (TR: 34 ms, TE: 12 ms) before and after contrast administration were obtained in 2 Czechoslovakian dogs. In 1 Czechoslovakian dog, transverse T1‐WI were obtained before and after contrast administration and a flash 3D sequence was performed only after contrast administration. All flash 3D series consisted of 1‐mm‐thick T1‐weighted slices that were used for multiplanar reconstruction (MPR) purposes. Patients were imaged in sternal recumbency.
Computed tomography (CT) images were obtained with a single detector helical CT unit4 using 2‐mm‐thick transverse slices with a pitch of 1 (120 kV, 180–260 mA). Patients were imaged in sternal recumbency.
Radiographic imaging was performed with a digital radiography system.5 Two lateral views were obtained in each dog with the cranial neck in a neutral and flexed position to check for potential atlanto‐axial subluxation.
Histopathology
The atlas and axis, including the associated part of the cervical spinal cord, were removed from each animal for gross and histopathological evaluation. Tissues were fixed in 4% neutral buffered formaldehyde. After fixation, a transverse section from the spinal cord in the region of the atlas was routinely processed into 5‐μm paraffin tissue sections. The atlas was decalcified in a neutral buffered 10% EDTA solution and areas that showed gross lesions were trimmed after decalcification and also were routinely processed into 5‐μm paraffin tissue sections. Both tissue sections from the spinal cord and the atlas were stained with hematoxylin and eosin (H&E).
Results
The 3 Czechoslovakian wolfdogs were presented with progressive weakness and proprioceptive ataxia of all limbs. The owners reported that the dogs were falling on their side while remaining fully conscious. They also noticed that the dogs were more quiet than before and that they overextended their neck for abnormally long periods of time as if they were star gazing. The German shepherd dwarf was presented with mild paraparesis that was first noticed when the dog was 7 months of age and had become slightly more prominent as the dog aged.
Physical examination disclosed proportionate dwarfism and retention of lanugo hair on the trunk and proximal extremities with guard hairs on the head and feet and isolated patches of guard hair on the trunk, in combination with bilateral alopecia at points of wear, such as the trunk, neck, and proximal extremities.
On neurological examination, the mental status of the animals was alert to mildly decreased. The Czechoslovakian wolfdog dwarfs had tetraparesis and proprioceptive ataxia affecting the trunk and all limbs. One of the Czechoslovakian wolfdogs had a hypermetric gait in all 4 limbs, whereas the other 2 had hypermetric gait in the thoracic limbs only. The German shepherd dwarf had mild paraparesis and mild proprioceptive ataxia of the pelvic limbs. The dog had no hypermetric gait. Passive flexion of the neck appeared to be painful in all dogs. The Czechoslovakian wolfdog dwarfs had impaired proprioception in all limbs. Cranial nerve assessment and spinal reflexes were unremarkable in all dogs. These findings localized the problem to the cervical spine in the Czechoslovakian wolfdogs. The caudal proprioceptive ataxia and paresis in the German shepherd dwarf was suggestive of a T3‐L3 spinal lesion. However, the dog felt painful on manipulation of the neck, and superficial compression of the cervical spinal cord can cause neurological deficits of the pelvic limbs only. Therefore, a cervical spinal localization was suspected in this dwarf as well. Differential diagnoses for cervical spinal cord lesions resulting in upper motor neuron signs in young dogs include anomalies (eg, atlantoaxial instability, other vertebral, and spinal cord anomalies, dermoid sinus, calcinosis circumscripta, osteochondromatosis), arachnoid cysts, degenerative diseases (eg, leucoencephalomyelopathy, leucodystrophy) and inflammatory diseases such as myelitis (both infectious and sterile) and extensive discospondylitis. Neoplasia affecting the spinal cord is more common in older animals, but can occur at any age. Trauma and vascular disorders were considered less likely in these dogs because of the progressive clinical signs.
Complete blood count disclosed mild leukocytosis with lymphocytosis in 1 dog. Routine biochemical blood testing indicated no abnormalities in any of the animals. The dogs were already being treated for secondary hypothyroidism with synthetic levo‐thyroxine and the plasma total thyroxine concentrations of all dogs were within reference range.
Diagnostic Imaging
CT and MRI images showed complete or partial open sutures between both neural arches of C1 in the dorsal midline (Fig 1) in all dogs and between the body of C1 and the right neural arch, left neural arch or both in the Czechoslovakian wolfdogs. The ends of the ossifying centers of C1 adjacent to the open sutures had a flared or thickened appearance or both. Postcontrast MRI imaging showed enhancement of a minimal to moderate amount of tissue located in the open sutures in C1. Sagittal T2‐WI showed intramedullary hyperintensity and widening of the central canal at the level of C1‐C2 in the Czechoslovakian wolfdog dwarfs. Intramedullary changes at this level were iso‐ to hypo‐intense to the surrounding spinal cord parenchyma on the flash 3D‐WI. At the same level, dorsal displacement of the dens with mild compression of the spinal cord was visible in the Czechoslovakian wolfdog dwarfs. The intramedullary changes were compatible with myelopathy. The dens were attached normally to C2 in all dogs.
Figure 1.
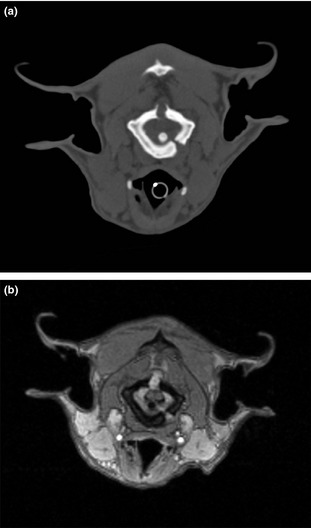
Transverse CT images of one of the Czechoslovakian wolfdog dwarfs at the level of C1 (a). Note the open suture lines of C1. Transverse postcontrast flash 3D‐WI of the same dog at the level of C1 (b). Note the enhancement of the tissue in the dorsal and left ventral suture. Note the dorsal displacement of the spinal cord, which is mildly compressed between the dens and the suture tissue.
Radiographic imaging of the dogs showed no or only minimal changes in the distance between the dorsal aspect of C1 and the cranial aspect of the spinous process of C2 when comparing neutral and flexed lateral views of the cervical spine. However, the deviant anatomy of the atlas compromised these measurements.
Follow‐up
Because the condition of 1 of the Czechoslovakian wolfdogs severely deteriorated, the owner requested euthanasia. One of the other 2 Czechoslovakian wolfdogs initially stabilized, but at the age of 18 months, the dog suddenly died after making an abrupt movement with its head while playing with its litter‐mate. The third Czechoslovakian dwarf was treated conservatively with analgesics and rest, after which the dog gradually improved. After 4 months, opisthotonus, hypermetria, and ataxia of the thoracic limbs had resolved. The function of the pelvic limbs improved, but mild proprioceptive ataxia and paraparesis remained. The dog was still stable after 3 years. The neurological signs of the German shepherd dog stabilized and the dog was euthanized when the dog was 6 years old for reasons unrelated to the neurological problems.
Necropsy and Histology
Necropsy and histology were performed on 2 of the Czechoslovakian wolfdogs and on the German shepherd dog. In addition to the findings described at physical examination, macroscopic evaluation of the pituitary gland showed that all 3 dogs had cystic enlargement of the adenohypophysis.
The atlas and axis were conjointly dissected in all 3 dogs. To evaluate the mobility of these vertebrae, the ligaments connecting these elements were left intact. The incomplete ossification of the atlas resulted in severe instability with free motion between the bony elements of this vertebra in the 2 Czechoslovakian wolfdogs. Flexion of the C1‐C2 junction clearly resulted in compression of the spinal cord by the dens axis (Fig 2). Also, the shape of the dens was abnormal in 1 of the Czechoslovakian wolfdogs, which resulted in an excessively dorsal projection of this structure.
Figure 2.
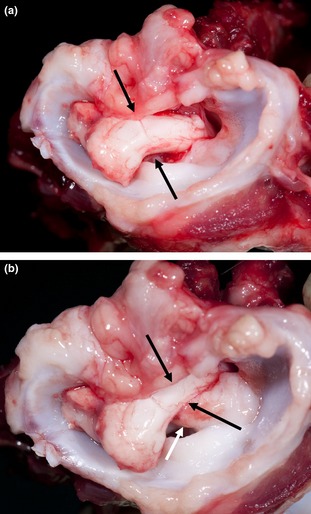
Transverse cranial macroscopic view of the cervical vertebral column including C1, at necropsy of a Czechoslovakian wolfdog with dwarfism. (a) Extension of C1‐C2. There is no compression of the spinal cord (black arrows). (b) Flexion of C1‐C2. Note the compression of the cervical spinal cord (black arrows) caused by dorsal displacement of the dens axis (white arrow).
Histopathological evaluation of the atlas identified discontinuity of the bony structures at the sites that were identified by CT imaging. The gap between the bony parts contained fibrous and cartilaginous tissue. In 1 of the Czechoslovakian wolfdogs, a slit‐like space lined with elongated flattened cells was noticeable within the fibrous tissue, suggestive of a joint‐like structure (Fig 3). The spinal cord at C1 showed moderate to marked degenerative changes that were most prominent in the ventral and lateral funiculi (Fig 4a). These changes consisted of swelling of axons (spheroids), loss of axons, and influx of macrophages consistent with Wallerian degeneration (Fig 4b).
Figure 3.
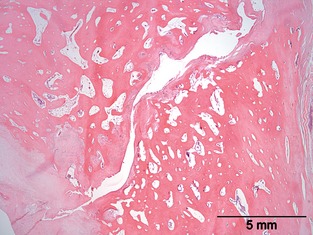
Histopathological picture of an area in the atlas revealing a discontinuity in the bony part of the vertebral arch. H&E.
Figure 4.
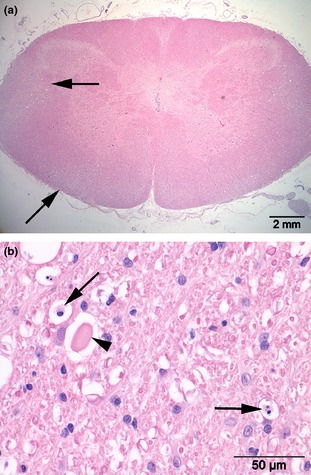
Transverse section of the cervical spinal cord at C1 (a) revealing vacuolar changes in ventral and lateral funiculi (arrows). Marked axonal swelling (arrowhead) and influx of macrophages (arrows) consistent with Wallerian degeneration (b). H&E.
Discussion
This study is the first report of anatomical malformations of the atlanto‐axial joint, leading to instability and dynamic compression of the cervical spinal cord, in shepherd dogs with CPHD. We suggest that these atlanto‐axial malformations are associated with the mutation of LHX3 in these dogs with CPHD. Therefore, shepherd dogs with pituitary dwarfism should be monitored closely for neurological signs.
All dogs were diagnosed with CPHD at an earlier age and displayed typical signs of pituitary dwarfism such as proportionate dwarfism and retention of their puppy hair coat. In addition, the dogs displayed progressive neurological signs. In the Czechoslovakian wolfdogs, the combination of tetraparesis, ataxia of the trunk and limbs and impaired proprioception located the problem to the cervical spine or brain stem. Although hypermetria is a classic sign of cerebellar disease, patients with damage to the superficial spinocerebellar tracts in the cervical spinal cord also will exhibit a similar hypermetric gait.15 The dogs were alert or had mildly decreased mentation, and the owners reported that the animals over‐extended their neck as if they were gazing at the stars. Consciousness is regulated in the cerebrum and brainstem, and dysfunction of the brainstem also may result in opisthotonus. However, a problem of the brainstem was considered less likely because cranial nerve assessment was unremarkable, the dogs showed no abnormalities in respiratory and cardiovascular function, and mentation was only slightly affected. Passive flexion of the neck appeared to be painful, and it therefore was expected that the animals were quieter and held their neck in an abnormal position because of neck pain. The German shepherd dwarf had paraparesis. Although paraparesis most often is caused by lesions involving the T3‐L3 spinal cord segments, lesions of the cervical spinal cord also may result in paraparesis. Mild compressive lesions of the cervical spinal cord affect the outer layers of the cord parenchyma first and these contain tracts that connect the pelvic limbs with the brain and cerebellum. Later in the cervical myelopathy disease process, the inner layers that contain tracts that connect the thoracic limbs with the central nervous system are affected resulting in tetraparesis and tetraplegia. Taken together, the history and clinical signs were consistent with a cervical spinal problem in all dogs.
Incomplete ossification of the atlas in dogs with cervical pain and neurological deficits compatible with a C1‐C5 myelopathy also has been reported in previous studies. One report described the absence of the neural arch of the atlas in a dog with neurological signs and a slight dorsal angulation of the dens, but without abnormal movement on flexion or extension of the cervical spine.16 A second report describes 5 dogs with incomplete ossification of the atlas.17 Of these animals, 4 dogs were diagnosed with concurrent atlantoaxial subluxation with dorsal displacement of the axis relative to the atlas. In the third report, 12 dogs with incomplete ossification of the atlas were described, of which 5 were diagnosed with atlantoaxial subluxation based on the width of the space between the dens and the intercentrum of the atlas.18 Incomplete ossification of the atlas was thought to be associated with deficiencies of the various atlantoaxial ligaments or their attachments, allowing laxity of the atlantoaxial joint. However, due to lack of necropsy in any affected dog, this hypothesis has not been tested so far. In this study, MRI and CT images identified incomplete ossification of the atlas with ≥1 of the 3 suture lines between the 3 ossification centers of C1 still open in all dogs. In 3 dogs, a variable degree of compression of the spinal cord was noted at the level of the dens between C1 and C2. The dens were attached to C2 in a normal fashion in all dogs. It was not possible to determine the integrity of the atlantoaxial ligaments or their attachments, even at post‐mortem examination. However, on necropsy, the incomplete ossification of the atlas clearly resulted in free motion between the bony elements of this vertebra in the 2 Czechoslovakian wolfdogs. Therefore, the incomplete ossification of the bony elements of C1 is expected to have resulted in instability of C1. This instability and resulting movements between the bony elements of C1 probably caused the excessive soft tissue formation located in the open sutures in C1. Additionally, flexion of the C1‐C2 junction resulted in increased dorsal displacement of the dens axis, causing compression of the cervical spinal cord. These findings could indicate weakness of the atlantoaxial ligaments, and add support to the hypothesis that incomplete ossification of the atlas is associated with deficiencies of the various atlantoaxial ligaments, allowing laxity of the atlantoaxial joint. The dorsal displacement of the dens axis could explain the ataxia, hypermetria and para‐ and tetra‐paresis displayed by these dogs. By hyper‐extending their necks, the dogs decreased compression of the spinal cord. In the German shepherd dwarf, the neurological abnormalities were milder than in the other dwarfs, and at necropsy no instability of the ring structure of C1 could be identified. However, trauma of the cervical spinal cord was confirmed by the histological findings in this dwarf as well. Wallerian degeneration is a process resulting from acute focal damage of a myelinated axon in which the part of the axon separated from the neuron's cell body degenerates distal to the injury. Therefore, also in the German shepherd dwarf, the incomplete closure of the ossification center is expected to have resulted in instability of C1.
Mutations in the LHX3 gene are associated with pituitary dwarfism in both human and canine patients. LHX3 is a DNA‐binding transcription factor expressed in early fetal life and an essential regulator of pituitary gland development.19 In human CPHD patients, anatomical malformations of the atlas and axis have been reported in addition to pituitary abnormalities.5, 9 In humans, CPHD can be caused by a number of different LHX3 mutations, which may explain why in some CPHD patients anatomical defects of the atlanto‐axial joint occur whereas in other patients they are absent. In contrast, shepherd dogs with CPHD are expected to be affected by the same mutations.6, 10 This raises the question of whether these vertebral malformations are not just coincidental findings that are unrelated to the 7‐bp deletion in LHX3. However, the atlanto‐axial malformations occured in dwarfs of 2 different breeds, and similar findings are described in human CPHD. Additionally, the phenotype of pituitary dwarfism is highly variable in other aspects as well, which could be caused by possible variations in the extent of residual activity of the LHX3 protein among dwarfs.6 Therefore, we suggest that anatomical abnormalities of the atlanto‐axial joint are associated with canine CPHD caused by an LHX3 mutation and that these dwarfs should be monitored closely for neurological signs.
The anatomical malformations of the atlanto‐axial joint reported here do not completely resemble those described in human CPHD patients. Of the 6 human CPHD patients with atlanto‐axial malformations, 5 had a cleft in the caudal arch of the atlas, 1 of these patients also had a defect in the anterior arch of the atlas combined with os odontoideum5 and a different patient only had a cleft in the atlas body and anterior arch.9 In the dogs described here, the dens was still attached to the atlas. Human patients also showed a disturbance of the normal convex‐convex relationship in the sagittal plane between the surfaces of the lateral intervertebral joints in the atlanto‐axial articulation. In addition, the inclination of the atlanto‐axial joint surface was increased in the coronal plane.5, 9 In our dogs, we did not detect these abnormalities. In the physiological situation, these structures already are shaped differently in dogs. This is probably because of the different position of the head in relation to the spine and therefore different exertion of muscles and the gravitational force on these structures in dogs.
Because LHX3 expression is not detected in the sclerotome,20 it is not clear why mutations in this gene would result in bony malformations. Perhaps LHX3 is needed for the activation of a as yet unidentified gene involved in regulation of the development of the atlas, and loss of activation of this gene might lead to the bony malformations seen in these dogs. Additional research would be needed to explore this theory.
Acknowledgments
The authors are grateful to the owners of the dogs that were part of this study.
The study was not supported by a grant or otherwise.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was performed at Utrecht University.
Part of the paper was presented at the Voorjaarsdagen Congress 2012.
Footnotes
Reporcin, Zamira Life Sciences Pty Ltd, Knoxfield, Australia
Forthyron, Eurovet Animal Health, Bladel, The Netherlands
Magneton Open Viva, Siemens AG, Germany
Philips Secura, Philips NV, Eindhoven, the Netherlands
Philips Bucky Diagnost, Philips NV, Eindhoven, the Netherlands
References
- 1. Netchine I, Sobrier ML, Krude H, et al. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet 2000;25:182–186. [DOI] [PubMed] [Google Scholar]
- 2. Bhangoo AP, Hunter CS, Savage JJ, et al. Clinical case seminar: A novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab 2006;91:747–753. [DOI] [PubMed] [Google Scholar]
- 3. Pfaeffle RW, Savage JJ, Hunter CS, et al. Four novel mutations of the LHX3 gene cause combined pituitary hormone deficiencies with or without limited neck rotation. J Clin Endocrinol Metab 2007;92:1909–1919. [DOI] [PubMed] [Google Scholar]
- 4. Rajab A, Kelberman D, de Castro SC, et al. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum Mol Genet 2008;17:2150–2159. [DOI] [PubMed] [Google Scholar]
- 5. Kriström B, Zdunek AM, Rydh A, et al. A novel mutation in the LIM homeobox 3 gene is responsible for combined pituitary hormone deficiency, hearing impairment, and vertebral malformations. J Clin Endocrinol Metab 2009;94:1154–1161. [DOI] [PubMed] [Google Scholar]
- 6. Voorbij AMWY, van Steenbeek FG, Vos‐Loohuis M, et al. A contracted DNA repeat in LHX3 intron 5 is associated with aberrant splicing and pituitary dwarfism in German shepherd dogs. PLoS ONE 2011;6:e27940. doi:10.1371/journal.pone.0027940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonfig W, Krude H, Schmidt H. A novel mutation of LHX3 is associated with combined pituitary hormone deficiency including ACTH deficiency, sensorineural hearing loss, and short neck: A case report and review of the literature. Eur J Pediatr 2011;170:1017–1021. [DOI] [PubMed] [Google Scholar]
- 8. Bechtold‐Dalla Pozza S, Hiedl S, Roeb J, et al. A recessive mutation resulting in a disabling amino acid substitution (T194R) in the LHX3 homeodomain causes combined pituitary hormone deficiency. Horm Res Paediatr 2012;77:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sobrier ML, Brachet C, Vié‐Luton MP, et al. Symptomatic heterozygotes and prenatal diagnoses in a nonconsanguineous family with syndromic combined pituitary hormone deficiency resulting from two novel LHX3 mutations. J Clin Endocrinol Metab 2012;97:E503–509. [DOI] [PubMed] [Google Scholar]
- 10. Voorbij AMWY, Leegwater PA, Kooistra HS. Pituitary dwarfism in Saarloos and Czechoslovakian wolf dogs is associated with a mutation in LHX3. J Vet Intern Med 2014;28:1770–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamann F, Kooistra HS, Mol JA, et al. Pituitary function and morphology in two German shepherd dogs with congenital dwarfism. Vet Rec 1999;144:644–646. [DOI] [PubMed] [Google Scholar]
- 12. Kooistra HS, Voorhout G, Mol JA, Rijnberk A. Combined pituitary hormone deficiency in German shepherd dogs with dwarfism. Domest Anim Endocrinol 2000;19:177–190. [DOI] [PubMed] [Google Scholar]
- 13. Andresen E, Willeberg P. Pituitary dwarfism in German shepherd dogs: Additional evidence of simple autosomal recessive inheritance. Nord Vet Med 1976;28:481–486. [PubMed] [Google Scholar]
- 14. Voorbij AMWY, Kooistra HS. Pituitary dwarfism in German shepherd dogs. J Vet Clin Sci 2009;2:4–11. [Google Scholar]
- 15. Taylor SM. Intracranial disorders In: Nelson RW, Couto CG, eds. Small Animal Internal Medicine, 4th ed St. Louis, MO: Mosby Elsevier; 2009:1019–1026. [Google Scholar]
- 16. Owen MC, Davis SH, Worth AJ. Imaging diagnosis—Traumatic myelopathy in a dog with incomplete ossification of the dorsal lamina of the atlas. Vet Radiol Ultrasound 2008;49:570–572. [DOI] [PubMed] [Google Scholar]
- 17. Warren‐Smith CMR, Kneissl S, Benigni L, et al. Incomplete ossification of the atlas in dogs with cervical signs. Vet Radiol Ultrasound 2009;50:635–638. [DOI] [PubMed] [Google Scholar]
- 18. Parry AT, Upjohn MM, Schlegl K, et al. Computed tomography variations in morphology of the canine atlas in dogs with and without atlantoaxial subluxation. Vet Radiol Ultrasound 2010;51:596–600. [DOI] [PubMed] [Google Scholar]
- 19. Sheng HZ, Zhadanov AB, Mosinger B, et al. Specification of pituitary cell lineages by the LIM homeobox gene LHX3. Science 1996;272:1004–1007. [DOI] [PubMed] [Google Scholar]
- 20. Kelberman D, Rizzoti K, Lovell‐Badge R, et al. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 2009;30:790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]


