Abstract
Background
Atypical hyperadrenocorticism (AHAC) is considered when dogs have clinical signs of hypercortisolemia with normal hyperadrenocorticism screening tests.
Hypothesis/Objectives
To compare cortisol concentrations and adrenal gland size among dogs with pituitary‐dependent hyperadrenocorticism (PDH), atypical hyperadrenocorticism (AHAC), and healthy controls.
Animals
Ten healthy dogs, 7 dogs with PDH, and 8 dogs with AHAC.
Method
Dogs were prospectively enrolled between November 2011 and January 2013. Dogs were diagnosed with PDH or AHAC based on clinical signs and positive screening test results (PDH) or abnormal extended adrenal hormone panel results (AHAC). Transverse adrenal gland measurements were obtained by abdominal ultrasound. Hourly mean cortisol (9 samplings), sum of hourly cortisol measurements and adrenal gland sizes were compared among the 3 groups.
Results
Hourly (control, 1.4 ± 0.6 μg/dL; AHAC, 2.9 ± 1.3; PDH, 4.3 ± 1.5) (mean, SD) and sum (control, 11.3 ± 3.3; AHAC, 23.2 ± 7.7; PDH, 34.7 ± 9.9) cortisol concentrations differed significantly between the controls and AHAC (P < .01) and PDH (P < .01) groups. Hourly (P < .01) but not sum (P = .27) cortisol concentrations differed between AHAC and PDH dogs. Average transverse adrenal gland diameter of control dogs (5.3 ± 1.2 mm) was significantly less than dogs with PDH (6.4 ± 1.4; P = .02) and AHAC (7.2 ± 1.5; P < .01); adrenal gland diameter did not differ (P = .18) between dogs with AHAC and PDH.
Conclusions and Clinical Importance
Serum cortisol concentrations in dogs with AHAC were increased compared to controls but less than dogs with PDH, while adrenal gland diameter was similar between dogs with AHAC and PDH. These findings suggest cortisol excess could contribute to the pathophysiology of AHAC.
Keywords: Adrenal, Atypical Cushing's Syndrome, Endocrine
Abbreviations
- 17 OHP
17‐hydroxyprogesterone
- AHAC
atypical hyperadrenocorticism
- ALP
alkaline phosphatase
- CBC
complete blood count
- HAC
hyperadrenocorticism
- LDDS
low dose dexamethasone suppression
- PDH
pituitary‐dependent hyperadrenocorticism
- SD
standard deviation
- UCCR
urine cortisol to creatinine ratio
Hyperadrenocorticism (HAC), or Cushing's syndrome, is one of the most common endocrinopathies of older dogs. Common tests for screening dogs for HAC include the ACTH stimulation test, the low‐dose dexamethasone suppression (LDDS) test, and the urine cortisol:creatinine ratio (UCCR). Differences in cortisol concentrations among dogs with pituitary‐dependent HAC (PDH), adrenal‐dependent HAC, and healthy animals have been detected when blood was collected at 30 minutes intervals for 8 hours.1 Further evaluation of that data suggests equivalent results for sampling every 60 minutes versus 30 minutes. Therefore, regular sampling can reveal trends in basal cortisol concentration.
Atypical hyperadrenocorticism (AHAC) is considered when dogs have clinical signs of hypercortisolemia, no evidence of a sex hormone‐secreting adrenal tumor, and HAC screening test results within the normal reference interval.2, 3, 4 Alterations in concentrations of adrenocortical precursor hormones before and after ACTH stimulation, historically, have been used to diagnose AHAC in dogs.2, 3, 4 Because these hormones precede cortisol production in the cortisol pathway, dogs with HAC also have elevations in adrenocortical precursor hormones.5 Dogs with nonadrenal neoplasia and no evidence of HAC can also have increased concentrations of 17‐hydroxyprogesterone (17 OHP) after ACTH stimulation,6 and serum 17 OHP concentrations in healthy intact female dogs during estrus, diestrus, and pregnancy are greater than those of dogs with HAC.7 In addition, dogs with alopecia and no other clinical signs can have increased concentrations of adrenal precursor hormones.8 Based on these studies it is unlikely that precursor hormones are responsible for the clinical signs noted in dogs with AHAC.
The etiopathogenesis of AHAC remains unknown. Some dogs diagnosed with AHAC progress to typical HAC.5 We propose that AHAC not associated with an adrenal tumor is due to increased 24‐hour cortisol secretion which may not be detected using routine screening tests. The purpose of this study was to assess differences in basal cortisol concentration in dogs with PDH, AHAC, and healthy dogs by collecting hourly blood samples over an 8 hours period. A secondary objective was to compare adrenal gland size among the 3 groups of dogs.
Materials and Methods
Experimental Subjects
Client‐owned dogs were eligible for prospective enrollment in this study, approved by the University of Tennessee Institutional Animal Care and Use Committee, between November 2011 and January 2013. Written consent was obtained from all participants. Dogs weighing a minimum of 6 kg were included in the study to allow for adequate blood sampling. Dogs with unilateral adrenal gland enlargement, adrenal glands ≥2.0 cm in transverse (dorsal–ventral) diameter, or enlarged irregular margins on ultrasonography evaluation were excluded from the study. Case evaluation and enrollment, including dynamic adrenal function testing and adrenal ultrasound, were performed 1 week to 1 month before blood sampling for basal cortisol concentrations.
Dogs were eligible as controls if they were 7 years of age or older, had no health issues in the past 3 months, were on no medications other than routine ecto‐ and endoparasite prophylaxis, had no clinical signs of disease, and had no abnormalities on physical examination. In addition, baseline complete blood count, chemistry panel, urinalysis and adrenal ultrasonographic evaluation were within normal limits.
Dogs were eligible for inclusion in the PDH group if they had 2 or more clinical signs of HAC,2, 3 increased alkaline phosphatase (ALP) activity, and hypercortisolemia based on ACTH stimulation (cortisol >22.0 μg/dL) or LDDS (cortisol >1.4 μg/dL at 8 hours) testing.
Dogs were eligible for inclusion in the AHAC group if they had 2 or more clinical signs of HAC,2, 3 increased ALP activity, did not have hypercortisolemia based on ACTH stimulation and LDDS testing, and had increases in post‐ACTH stimulation concentrations of at least 2 of the adrenal sex hormones or steroid hormone intermediates.9 Hormones measured included progesterone, 17 OHP, estradiol, and androstenedione.
Adrenal Ultrasound
Adrenal ultrasound was performed with a real‐time ultrasound machine1 using 14 MHz linear, 10 MHz phased array or 6 MHz curved array transducers by a board‐certified veterinary radiologist (GAH). Dogs were positioned in dorsal recumbency. At least 3 images in longitudinal planes were obtained and the largest transverse (dorsal–ventral) diameter measurement perpendicular to the long axis was recorded. Adrenal gland enlargement was defined as having at least 1 adrenal gland larger than 6 mm for dogs weighing less than or equal to 10 kg and larger than 7.5 mm for dogs weighing greater than 10 kg.10
Basal Cortisol Concentration Measurement
On the day of blood sampling, each dog had a 20 gauge cephalic vein catheter placed at approximately 8:00 am. Blood was collected hourly starting at 9:00 am for a total of 9 sampling times. Dogs had 2–3 mL blood drawn through the catheter at each sampling for a maximum of 27 mL. The first 0.5 mL of blood was collected in a separate syringe and discarded before each sample collection. Catheters were flushed with 2 mL saline and then 0.5 mL heparinized saline at the completion of each collection. To minimize stress, dogs were confined between samplings and were walked just after a blood collection depending on their need. Catheters were removed after the last sample collection, and dogs were returned to their owners. Blood was allowed to clot at room temperature before centrifugation. Serum was separated and stored frozen at −70°C until completion of case enrollment and sample collection. Samples were mailed together overnight on ice for cortisol analysis.2
Cortisol was measured in serum/plasma samples with a commercially available radioimmunoassay kit.3 Antibody‐coated polystyrene tubes, 125I‐ labeled cortisol, and standards containing human serum were supplied in the kit. This assay has been commonly used in veterinary endocrine laboratories and validation data for this assay in dogs have appeared in several publications.11, 12, 13
Statistical Analysis
A mixed model ANOVA [PROC MIXED]4 was used to distinguish cortisol measurements over the 9 hours sampling period. Group (control, AHAC, or PDH), hour and dog were included as class variables. Cortisol measurement was included as the dependent variable; group, hour and the group X hour interaction were included as independent variables. Hour was included as a repeated measure with subject as dog. Dog was also included as a random variable. A similar model was used to assess cortisol values over time after eliminating the baseline measurement at the first sampling. A second mixed model ANOVA was used to compare the sum of cortisol measurements for each group over the 8 hours sampling period. The model included group as a class variable with sum of cortisol measurements as the dependent variable and group as the independent variable. Dog was included as a random variable in the model. A third mixed model ANOVA was used to distinguish measurements of the adrenal glands among the 3 groups. Group, side, and dog were included as class variables in the model. Measurement was included as the dependent variable and dog weight, group, side, and the group X side interaction were included as independent variables. Dog was included as a random variable in the model. Continuous variables (age and weight) were log transformed and compared among groups using a fourth ANOVA model with dog and group entered as class variables, age or weight as the independent variable, group as the dependent variable, and dog within group as random variable. The assumption that the residuals from each model approximate a normal distribution was examined using the test statistic of Shapiro‐Wilk. In all models, means separation for status within group (PDH, AHAC or control) was done using the method of Tukey. Data are presented as unadjusted mean ± one standard deviation (SD). A value of ≤0.05 was used to determine statistical significance in all tests.
Results
Control dogs (n = 10) consisted of 4 mixed breed dogs, 2 Labrador Retrievers and 1 each of Border Collie, Dachshund, Jack Russell Terrier, and Rat Terrier. There were 6 neutered male and 4 spayed female dogs. Ages ranged from 7 to 12 years (median 8 years), with 1 considered geriatric with no age assigned. Body weights for this group ranged from 6 to 30 kg (median 10.9 kg). The PDH group (n = 7) included 2 Miniature Schnauzers and 1 each of Beagle, Cocker Spaniel, Rhodesian Ridgeback, Shih Tzu, and mixed breed dog. There were 6 spayed female dogs and 1 neutered male dog. Ages ranged from 7 to 9 years (median 8 years) and body weights from 9.9 to 49 kg (median 32 kg). The AHAC group (n = 8) included 4 mixed breed dogs and 1 each of Australian Cattle Dog, Basenji, Cairn Terrier, and Miniature Schnauzer. There were 3 neutered male and 5 spayed female dogs. Ages ranged from 10 to 13 years (median 11.5 years) and body weights from 6 to 30 kg (median 11.6 kg). Although there was no difference in weights among the 3 groups (P = .09), dogs with AHAC were significantly older than the other 2 groups (P < .01).
Dogs with PDH had 1 to 5 common clinical signs of HAC (median 4) and 2 to 5 total clinical signs of HAC as defined in the ACVIM Consensus Statement.2 The most frequently reported signs were polyuria and polydipsia (5) and pendulous abdomen (5). Clinical findings reported less frequently included polyphagia (3), hepatomegaly (3), panting (2), calcium oxalate urolithiasis (2), and 1 each of endocrine alopecia, hypertension, and weight gain. Laboratory abnormalities consistent with HAC included hypercholesterolemia (4), proteinuria (2), hypertriglyceridemia (1), and thrombocytosis (1). The median ALP concentration was 636.5 μ/L (range 291–1065 μ/L). ACTH stimulation test results were diagnostic for Cushing's syndrome in 3 of 7 dogs (42.9%). LDDS was diagnostic for Cushing's syndrome in all 4 dogs in which it was performed. Three dogs had LDDS results consistent with partial pituitary suppression and escape, eg, the 4 hours cortisol concentration was <1.4 μg/dL or was decreased by greater than 50% compared with baseline in addition to having cortisol concentrations consistent with HAC at 8 hours.1 Three dogs had ACTH stimulation test results within the reference interval but had LDDS test results diagnostic for HAC.
Dogs with AHAC had 2 to 5 common clinical signs of HAC (median 4) and 3 to 7 total clinical signs of HAC as defined in the ACVIM Consensus Statement.2 The most frequently reported signs were polyuria and polydipsia (7), polyphagia (6), and pendulous abdomen (5). Other clinical findings included recurrent skin infections (4), panting (2), weight gain (2), endocrine alopecia (2) and 1 each with hypertension, muscle weakness, and hyperpigmentation of skin. Laboratory abnormalities consistent with HAC included proteinuria (2) and hypercholesterolemia (1). The median ALP concentration was 841 μ/L (range 181–2278 μ/L). Cortisol and cortisol precursor concentrations before and after ACTH stimulation test are summarized in Table 1. The cortisol concentration at the 8 hours sampling of the LDDS was less than 0.5 μg/dL in 5 dogs, equal to 0.75 μg/dL in 1 dog and between 1.0 and 1.4 μg/dL in 2 dogs. No dogs showed an inverse pattern of cortisol concentration in the LDDS in which the 4 hours sample was elevated but the 8 hours sample was within reference range.14
Table 1.
Adrenal hormone concentrations before and after ACTH stimulation in 8 dogs with atypical hyperadrenocorticism (AHAC)
| Reference Intervala | Median (range) | |||
|---|---|---|---|---|
| Baseline | After Stimulation | Baseline | After Stimulation | |
| Cortisol (μg/dL) | 0.2–5.9 | ≤22.0b | 2.3 (1.6–9.0) | 15.8 (12.7–17.7) |
| Androstenedione (ng/mL) | 0.05–0.57 | 0.27–3.97 | 0.42 (0.21–3.94) | 5.89 (1.32–8.42) |
| Estradiol (pg/mL) | 30.8–69.9 | 27.9–69.2 | 78 (58.7–165.1) | 73.8 (55.8–168.3) |
| Progesterone (ng/mL) | 0.03–0.49 | 0.10–1.50 | 0.13 (<0.03–1.11) | 1.78 (1.22–3.83) |
| 17‐OH Progesterone (ng/mL) | 0.08–0.77 | 0.40–1.62 | 0.15 (<0.08–0.72) | 1.78 (1.25–5.89) |
Reference intervals from University of Tennessee Veterinary Medical Center Clinical Endocrinology Service.
Cortisol concentrations greater than 22.0 μg/dL considered diagnostic for HAC.
When all groups were combined, cortisol concentrations were significantly higher at the first sampling time (T0) than any of the other time points (P < .01) (Fig 1). When looking at individual groups, T0 was significantly different from all other times for the AHAC group (P < .05) only. T0 was significantly different from T1 only for the PDH group (P < .01). The first sampling time for all groups was, therefore, excluded from further analysis, even though it did not change the statistical significance of the results. The mean cortisol concentration over all times, excluding the first sampling, was significantly different between the control (1.4 ± 0.6 μg/dL [mean, SD]) and AHAC (2.9 ± 1.3) groups (P < .01), between the control and PDH (4.3 ± 1.5) groups (P < .01), and between the AHAC and PDH groups (P < .01). The sum of cortisol measurements (T1 to T8) was also statistically different between the control (11.3 ± 3.3) and AHAC (23.2 ± 7.7) groups (P = .01), and between the control and PDH (34.7 ± 9.9) groups (P < .01). The sum of cortisol measurements between AHAC and PDH groups was not statistically different (P = .27), although the AHAC group had lower sum cortisol measurements than PDH.
Figure 1.
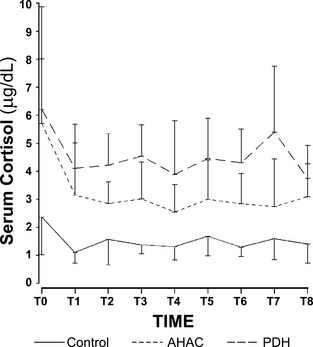
Hourly cortisol concentrations (mean, SD) of control dogs (n = 10), dogs with AHAC (n = 8), and dogs with PDH (n = 7). T0 mean cortisol for dogs with AHAC was significantly greater (P < .01) than other sampling times.
The average transverse diameter of the adrenal glands was compared among the 3 groups (Table 2). When adjusted for weight and side (left and right), the average diameter of the adrenal glands of the control dogs were significantly less than those of dogs with PDH (P = .02) and AHAC (P < .01). There was no difference in diameter of the adrenal gland among dogs with AHAC and PDH (P = .18). Adrenomegaly was present in 4 of 7 dogs (57%) with PDH, 5 of 8 dogs (62.5%) with AHAC, and 0 of 10 control dogs.
Table 2.
Average transverse diameter (mm ± SD) of adrenal glands in control dogs (n = 10), dogs with AHAC (n = 8), and dogs with PDH (n = 7). Different letters indicate significant difference (P < .01)
| Left Adrenal | Right Adrenal | Average of Both Glands | |
|---|---|---|---|
| Control | 5.6 ± 1.1 | 5.0 ± 1.3 | 5.3 ± 1.2a |
| AHAC | 7.1 ± 1.5 | 7.2 ± 1.7 | 7.2 ± 1.5b |
| PDH | 6.3 ± 1.3 | 6.5 ± 1.6 | 6.4 ± 1.4b |
Discussion
Serum cortisol concentrations over a 9 hours period in dogs with AHAC were significantly increased when compared to control dogs but less than those of dogs diagnosed with PDH. These results support the hypothesis that dogs with AHAC have increased cortisol concentrations. Dogs with AHAC also had significantly larger adrenal gland measurements than control dogs, but adrenal gland size did not differ between dogs with AHAC and PDH. These findings along with similar clinical signs between the 2 groups suggest that cortisol excess could contribute to the pathophysiology of AHAC.
The term AHAC has been used to describe dogs with clinical signs of hypercortisolemia in which standard screening tests for HAC are within normal limits or low as in the case of adrenal tumors.3, 4, 9 Because of limitations in the diagnostic accuracy of currently available screening tests for PDH, results of both the ACTH stimulation test and the LDDS test should be confirmed to be within reference intervals before evaluation for AHAC.2, 3 Three dogs with PDH in this study had abnormal LDDS and normal ACTH stimulation test results.
The established cortisol reference ranges for the LDDS test to differentiate dogs with HAC from normal dogs were developed many years ago.15, 16 There is interest in revisiting these ranges to determine if the same cut‐off values still apply.2 It is thought that some of the dogs considered to have AHAC would actually be diagnosed with typical HAC once this occurs. In this study, 5 dogs with AHAC had cortisol 8 hours postdexamethasone less than 0.5 μg/dL (reference range <1.4 μg/dL). It is unlikely that different cortisol reference ranges would have provided a more definitive diagnosis of HAC for these dogs. The 4 hours cortisol assessment in the LDDS test has been used to differentiate dogs with PDH from those with adrenal tumors.16 Recently, an inverse pattern of cortisol concentration has been described in dogs with HAC in which the 4 hours cortisol concentration of the LDDS test is increased but the 8 hours cortisol concentration is within the reference range.14 Based on this study, reliance on only the 8 hours cortisol results could lead to false negative test interpretation. No dogs with AHAC in this study had an inverse pattern of their LDDS test results.
There is not a consensus on cortisol concentration post‐ACTH stimulation that is compatible with a diagnosis of HAC, with ranges from greater than 20 to 25 μg/dL.1, 15, 17 The authors used 22 μg/dL, which is the standard for their hospital; however, all dogs with AHAC in this study had cortisol concentration post‐ACTH stimulation less than 18 μg/dL.
The urinary cortisol to creatinine ratio (UCCR) was developed to avoid the need for 24‐hour urine collection and to screen the patient for HAC.18, 19 Because dogs under the stress of an examination, with renal disease, and with other disorders can have values that overlap those found in HAC, the test is not specific for HAC (specificity as low as 21%).18 While some feel that it can be used to exclude the diagnosis in dogs with polyuria and polydipsia, sensitivities as low as 50% have been described.20 For this reason, UCCR were not performed as part of enrollment criteria in this study. One dog, which met all inclusion criteria for this study but was withdrawn before the 9 hours blood cortisol collection, had normal UCCR but abnormal LDDS results. The diagnostic value of the UCCR in dogs with AHAC is unknown.
Diagnosing AHAC is controversial.2, 4, 21 Currently, it is made based on presence of increased concentrations of one or more of adrenal sex hormones (17 OHP, progesterone, estradiol, and androstenedione) pre‐ and post‐ACTH stimulation.9 Because these hormones are precursors to cortisol, they can also be increased in dogs with HAC3, 4, 5, 6, 22, 23 and up to 31% of dogs with nonadrenal illness.6 They are also increased in dogs with alopecia and no other clinical abnormalities.8 Additionally, increased adrenal sex hormones and steroid hormone intermediates post‐ACTH stimulation have been documented before development of hypercortisolemia in at least one dog with HAC.5
Clinical signs of HAC and vacuolar hepatopathy have been shown to be associated with excess cortisol administration.24 The roles that adrenal sex hormones and steroid hormone intermediates contribute to clinical signs in dogs with AHAC is unknown. Progestins are known to cross‐react with the cortisol receptor, potentially resulting in its activation.25 Dogs and cats with progesterone‐secreting adrenal tumors can present with clinical signs of hypercortisolemia including polydipsia, polyuria, polyphagia, thin skin, alopecia, and diabetes mellitus.26, 27, 28, 29 However, 73% of healthy dogs with alopecia in which both HAC and hypothyroidism had been ruled out had at least one baseline or post‐ACTH stimulation adrenal sex hormone or steroid hormone intermediate greater than the reference interval.8 With the exception of alopecia, these dogs did not have clinical signs of HAC. Additionally, healthy intact female dogs have increased concentrations of progesterone and 17 OHP for prolonged periods of time during estrus, diestrus, and pregnancy without development of clinical signs of HAC.7 Finally, corticosteroid‐induced ALP expression is upregulated in hepatocytes by cortisol, but not 17 OHP, progesterone, estradiol, or androstenedione administration.5 Results of these studies suggest that excess adrenal sex hormone concentrations are unlikely to be the cause of the clinical and clinicopathologic findings in dogs with AHAC.
The sum and mean cortisol concentrations of dogs with AHAC were intermediate between control and PDH dogs. The reason why serum cortisol concentrations are lower in dogs with AHAC than dogs with PDH is unknown. It is possible that these dogs are presenting earlier after onset of PDH; however, this explanation would not explain the significantly older age of dogs with AHAC in this study. Baseline cortisol concentration with regard to age and body size has been evaluated in previous studies.30, 31 While age had no effect, smaller breeds had significantly greater baseline cortisol concentration. In this study, there was no significant difference of body weight among the 3 groups, although dogs with AHAC weighed less than dogs in the other groups. If weight influenced results of this study, it should have resulted in falsely higher, not lower, cortisol concentrations for the AHAC group. Another explanation could be that pituitary tumors in dogs with AHAC produce less ACTH or secrete it in a manner different from dogs with PDH, resulting in less production of cortisol than in PDH. Unfortunately, endogenous ACTH concentrations were not determined as part of this study. More research is needed to investigate the presence and function of a pituitary tumor in dogs with AHAC.
The first blood sample collected was excluded from analysis even though it did not change the final statistical outcome because the cortisol concentrations from the first time‐point were greater than at other sampling times. Catheters were placed at approximately 8 AM for all dogs. The placement of catheters can be stressful and likely resulted in an increase in cortisol production. The first sample period occurred less than 1 hour after catheter placement, which apparently was not sufficient time for normalization of cortisol secretion. Previous studies have shown that diagnostic procedures, such as skin scrapings and mock ultrasound, result in temporary increases in cortisol secretion.32, 33 In the latter study, cortisol concentrations returned to baseline 2 hours after mock ultrasound.33
Ultrasonographic determination of adrenal gland size was performed on all dogs in this study to rule out adrenal neoplasia and to compare adrenal size among the 3 groups. Ultrasound measurement of the transverse diameter of the adrenal gland is considered more accurate than the length to determine whether or not adrenal size is increased.34 Because small breed dogs have smaller adrenal glands,10 adjustment for weight of dog was used when statistically comparing adrenal gland diameters among the 3 groups because dogs with AHAC weighed less even though this was not statistically significant. We showed the adrenal glands of dogs with AHAC are similar in size to those of dogs with PDH but statistically larger than those of healthy dogs, supporting an adrenal hormone source. Lack of ultrasonographic adrenomegaly does not rule out PDH.1, 10 Consistent with previous reports, 43% of dogs with PDH had adrenal glands within normal limits based on ultrasound in this study.
Dogs with chronic illness can have increased adrenocortical precursor hormones and cortisol concentrations.6 Because a control group comprised of dogs with nonadrenal illness was not included, the impact of concurrent disease on assessment of 8 hours basal cortisol concentrations remains unknown. Dogs with clinical signs suggestive of nonadrenal illness were excluded from this study. Because comprehensive clinicopathologic analysis and diagnostic imaging, including thoracic radiographs and complete abdominal ultrasound, were not performed in all dogs, possible enrollment of some dogs with concurrent nonadrenal illness cannot be definitively ruled out.
One additional limitation of this study was the small sample size which may have resulted in a false separation of AHAC and PDH dogs. Interestingly, dogs with AHAC were significantly older than dogs with PDH, supporting the statistical differentiation of these groups. The significance of this finding is unknown but may point toward a different underlying etiopathologic basis of AHAC.
Results of this study suggest cortisol excess could contribute to the pathophysiology of AHAC. The terminology of this condition remains confusing. The condition is neither occult nor atypical if clinical signs are attributed to hypercortisolemia. Perhaps this is a low grade form of hypercortisolemia; however, until specific pathophysiologic mechanisms have been examined it may be best to leave the name as atypical Cushing's syndrome.
Acknowledgments
Supported by the AKC Canine Health Foundation ACORN grant. The authors thank Jacqueline Davis for her technical assistance.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Siemens Sequoia 512, Siemens medical Solutions USA, Inc., Mountain View, CA
Diagnostic Center for Population and Animal Health – Endocrine Diagnostic Section, Lansing, MI
Coat‐A‐Count Cortisol, Siemens Medical Solutions Diagnostics, Los Angeles, CA
SAS version 9.4, SAS Institute, Cary, NC
Behrend EN, Kemppainen RJ, Kennis RA, et al. Assessment by quantitative PCR of ability of sex hormones to induce expression of classic glucocorticoid‐induced genes in canine hepatocytes (abstr). J Vet Intern Med 2011;25:EN‐10
References
- 1. Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushing's syndrome) In: Feldman EC, Nelson RW, eds. Canine and Feline Endocrinology and Reproduction, 3rd ed St Louis, MO: Saunders Co; 2004:253–357. [Google Scholar]
- 2. Behrend EN, Kooistra HS, Nelson R, et al. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J Vet Intern Med 2013;27:1292–1304. [DOI] [PubMed] [Google Scholar]
- 3. Ristic JME, Ramsey IK, Heath FM, et al. The use of 17‐hydroxyprogesterone in the diagnosis of canine hyperadrenocorticism. J Vet Intern Med 2001;16:433–439. [DOI] [PubMed] [Google Scholar]
- 4. Benitah N, Feldman EC, Kass PH, Nelson RW. Evaluation of serum 17‐hydroxyprogesterone concentration after administration of ACTH in dogs with hyperadrenocorticism. J Am Vet Med Assoc 2005;227:1095–1101. [DOI] [PubMed] [Google Scholar]
- 5. Frank LA, Schmeitzel LP, Oliver JO. Steroidogenic response of adrenal tissues after administration of ACTH to dogs with hypercortisolemia. J Am Vet Med Assoc 2001;218:214–216. [DOI] [PubMed] [Google Scholar]
- 6. Behrend EN, Kemppainen RJ, Boozer AL, et al. Serum 17‐α‐hydroxyprogesterone and corticosterone concentrations in dogs with nonadrenal neoplasia and dogs with suspected hyperadrenocorticism. J Am Vet Med Assoc 2005;227:1762–1767. [DOI] [PubMed] [Google Scholar]
- 7. Brömel C, Feldman EC, Davidson AP, et al. Serum 17α‐hydroxyprogesterone concentrations during the reproductive cycle in healthy dogs and dogs with hyperadrenocorticism. J Am Vet Med Assoc 2010;236:1208–1214. [DOI] [PubMed] [Google Scholar]
- 8. Frank LA, Hnilica KA, Rohrbach BW, Oliver JW. Retrospective evaluation of sex hormones and steroid hormone intermediates in dogs with alopecia. Vet Dermatol 2003;14:91–97. [DOI] [PubMed] [Google Scholar]
- 9. Scott‐Moncrieff JCR. Atypical and subclinical hyperadrenocorticism In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XIV. St Louis, MO: Saunders Co; 2009:219–224. [Google Scholar]
- 10. Choi J, Kim H, Yoon J. Ultrasonographic adrenal gland measurements in clinically normal small breed dogs and comparison with pituitary‐dependent hyperadrenocorticism. J Vet Med Sci 2011;73:985–989. [DOI] [PubMed] [Google Scholar]
- 11. Kemppainen RJ, Thompson FN, Lorenz MD. Use of a low dose synthetic ACTH challenge test in normal and prednisone‐treated dogs. Res Vet Sci 1983;35:240–242. [PubMed] [Google Scholar]
- 12. Watson AD, Church DB, Emslie DR. Plasma cortisol concentrations in dogs given cortisone or placebo by mouth. Res Vet Sci 1993;55:379–381. [DOI] [PubMed] [Google Scholar]
- 13. Russell NJ, Foster S, Clark P, et al. Comparison of radioimmunoassay and chemiluminescent assay methods to estimate canine blood cortisol concentrations. Aust Vet J 2007;85:487–494. [DOI] [PubMed] [Google Scholar]
- 14. Mueller C, Sieber‐Ruckstuhl N, Wenger M, et al. Low‐dose dexamethasone test with “inverse” results: a possible new pattern of cortisol response. Vet Rec 2006;159:489–491. [DOI] [PubMed] [Google Scholar]
- 15. Feldman EC. Comparison of ACTH response and dexamethasone suppression as screening tests in canine hyperadrenocorticism. J Am Vet Med Assoc 1983;182:506–510. [PubMed] [Google Scholar]
- 16. Mack RE, Feldman EC. Comparison of two low‐dose dexamethasone suppression protocols as screening and discrimination tests in dogs with hyperadrenocorticism. J Am Vet Med Assoc 1990;197:1603–1606. [PubMed] [Google Scholar]
- 17. Hill K, Scott‐Moncrieff JC, Moore G. ACTH stimulation testing: A review and a study comparing synthetic and compounded ACTH products. Vet Med 2004;February:134–147. [Google Scholar]
- 18. Smiley LE, Peterson ME. Evaluation of a urine cortisol:creatinine ratio as a screening test for hyperadrenocorticism in dogs. J Vet Intern Med 1993;7:163–168. [DOI] [PubMed] [Google Scholar]
- 19. Jensen AL, Iversen L, Koch J, et al. Evaluation of the urinary cortisol:creatinine ratio in the diagnosis of hyperadrenocorticism in dogs. J Small Anim Pract 1997;38:99. [DOI] [PubMed] [Google Scholar]
- 20. Zerbe CA. Screening tests to diagnose hyperadrenocorticism in cats and dogs. Compend Cont Educ Pract Vet 2000;22:17–31. [Google Scholar]
- 21. Behrend EN, Kennis R. Atypical Cushing's syndrome in dogs: Arguments for and against. Vet Clin Small Animal 2010;40:285–296. [DOI] [PubMed] [Google Scholar]
- 22. Monroe WE, Panciera DL, Zimmerman KL. Concentrations of noncortisol adrenal steroids in response to ACTH in dogs with adrenal‐dependent hperadrenocorticism, pituitary‐dependent hyperadrenocorticism, and nonadrenal illness. J Vet Intern Med 2012;26:945–952. [DOI] [PubMed] [Google Scholar]
- 23. Chapman PS, Mooney CT, Ede J, et al. Evaluation of the basal and post‐adrenocorticotrophic hormone serum concentations of 17‐hydroxyprogesterone for the diagnosis of hyperadrenocorticism in dogs. Vet Rec 2003;153:771–775. [PubMed] [Google Scholar]
- 24. Huang HP, Yang HL, Liang SL, et al. Iatrogenic hyperadrenocorticism in 28 dogs. J Am Anim Hosp Assoc 1999;35:200–207. [DOI] [PubMed] [Google Scholar]
- 25. Selman PJ, Mol JA, Rutteman GR, et al. Effects of progestin administration on the hypothalamic‐pituitary‐adrenal axis and glucose homeostasis in dogs. J Reprod Fertil Suppl 1997;51:345–354. [PubMed] [Google Scholar]
- 26. Syme HM, Scott‐Moncrieff JC, Treadwell NG, et al. Hyperadrenocorticism associated with excessive sex hormone production by an adrenocortical tumor in two dogs. J Am Vet Med Assoc 2001;219:1725–1728. [DOI] [PubMed] [Google Scholar]
- 27. Boord M, Griffin C. Progesterone secreting adrenal mass in a cat with clinical signs of hyperadrenocorticism. J Am Vet Med Assoc 1999;214:666–669. [PubMed] [Google Scholar]
- 28. Rossmeisl JH, Scott‐Moncrieff JC, Siems J, et al. Hyperadrenocorticism and hyperprogesteronemia in a cat with an adrenocortical adenocarcinoma. J Am Anim Hosp Assoc 2000;36:512–517. [DOI] [PubMed] [Google Scholar]
- 29. DeClue AE, Breshears LA, Pardo ID, et al. Hyperaldosteronism and hyperprogesteronism in a cat with an adrenal cortical carcinoma. J Vet Intern Med 2005;19:355–358. [DOI] [PubMed] [Google Scholar]
- 30. Reimers TJ, Lawler DF, Sutaria PM, et al. Effects of age, sex, and body size on serum concentrations of thyroid and adrenocortical hormones in dogs. Am J Vet Res 1990;51:454–457. [PubMed] [Google Scholar]
- 31. Mongillo P, Prana E, Babai G, et al. Effect of age and sex on plasma cortisol and dehydroepiandrosterone concentrations in the dog (Canis familiaris). Res Vet Sci 2014;96:33–38. [DOI] [PubMed] [Google Scholar]
- 32. Frank LA, Kunkle GA, Beale KM. Comparison of serum cortisol concentration before and after intradermal testing in sedated and nonsedated dogs. J Am Vet Med Assoc 1992;200:507–510. [PubMed] [Google Scholar]
- 33. May ER, Frank LA, Hnilica KA, Lane IF. Effects of a mock ultrasonographic procedure on cortisol concentrations during low‐dose dexamethasone suppression testing in clinically normal adult dogs. J Am Vet Res 2004;65:267–270. [DOI] [PubMed] [Google Scholar]
- 34. Barberet V, Pey P, Duchateau L, et al. Intra‐ and interobserver variability of ultrasonographic measurements of the adrenal glands in healthy beagles. Vet Rad Ultrasound 2010;51:656–660. [DOI] [PubMed] [Google Scholar]


