Abstract
Background
Transvenous pacemaker implantation in dogs is associated with a relatively high complication rate. At our institution, pacemaker implantation in dogs with high‐grade atrioventricular block (HG‐AVB) frequently is performed as an after‐hours emergency.
Hypothesis
Among dogs with HG‐AVB, the rate of major complications is higher when pacemakers are implanted after hours (AH) compared to during business hours (BH).
Animals
Client‐owned dogs with HG‐AVB that underwent transvenous pacemaker implantation between January 2002 and December 2012 at the North Carolina State University Veterinary Teaching Hospital.
Methods
Retrospective medical record review. Two‐year follow‐up was required for complications analysis.
Results
Major complications occurred in 14/79 dogs (18%) and included lead dislodgement, lead or generator infection, lead or generator migration, and pacing failure. Incidence of major complications was significantly higher AH (10/36, 28%) compared to BH (4/43, 9%; P = .041), and all infectious complications occurred AH. Median survival time for all dogs was 27 months and did not differ between AH and BH groups for either all‐cause (P = .70) or cardiac (P = .40) mortality. AH dogs were younger than BH dogs (P = .010), but there were no other clinically relevant differences between BH and AH groups in terms of demographic, clinical, or procedural variables.
Conclusions and Clinical Importance
At our institution, AH transvenous pacemaker placement is associated with a higher rate of major complications (especially infections) compared to BH placement. This difference may be because of a variety of human factor differences AH versus BH.
Keywords: Canine, Heart block, Transvenous
Abbreviations
- AH
after hours
- BH
business hours
- CANPACERS
Companion Animal Pacemaker Registry and Repository
- CHF
congestive heart failure
- ECG
electrocardiogram
- HG‐AVB
high‐grade atrioventricular block
- MST
median survival time
- NCSU
North Carolina State University
- PAP
permanent artificial pacemaker
Since the first reported pacemaker implantation in a dog in 1968,1 artificial pacing has become the standard of care for treatment of certain bradyarrhythmias in dogs, with many referral institutions performing dozens of these procedures annually.2 Transvenous pacemaker implantation, performed using a jugular venotomy with the pacing lead placed into the right ventricle under fluoroscopic guidance, generally is considered the preferred method of pacemaker placement in dogs.3, 4, 5
The most common indication for permanent artificial pacemaker (PAP) implantation in dogs is high‐grade atrioventricular block (HG‐AVB).2, 3, 4 This diagnosis encompasses both high‐grade second degree (3 : 1 conduction or less) and third degree (complete) AVB. Schrope et al6 demonstrated that without PAP placement, dogs with HG‐AVB have a high incidence of sudden death (up to 40% within 6 months of diagnosis). Because of this, many institutions consider HG‐AVB to be a surgical emergency requiring urgent PAP implantation. Based on the timing of presentation and perceived patient stability, some affected dogs undergo emergency PAP placement after hours (AH), whereas others are hospitalized and monitored until the next business day. Procedures performed AH inevitably involve fewer personnel and decreased technical support.
Several large‐scale retrospective studies2, 3, 4, 5, 7 have reviewed the outcomes and complication rates associated with PAP placement in dogs. Rates of major complications ranged from 55 to 71% in the 1980s8, 9 but have decreased to 13–33% in the 2000s.2, 3, 4, 7 Higher complication rates have been reported in dogs with pre‐existing structural heart disease,4 postoperative infections,10 previously used pulse generators,4 and inexperienced operators.2 In comparison, large‐scale retrospective studies in humans report major complication rates of 2.3–11.2%,11, 12, 13, 14 with higher rates associated with dual‐chamber pacemakers,12, 13 untined versus tined leads,14 pre‐existing structural heart disease,13, 15 and inexperienced operators.13
The primary aim of this study was to compare complication rates associated with transvenous pacemaker placement in dogs with HG‐AVB performed during normal business hours (BH) versus AH. We hypothesized that the major complication rate would be higher for AH procedures. A secondary aim of this study was to compare long‐term survival between dogs with pacemakers placed BH versus AH.
Materials and Methods
Record Review
A retrospective medical record review of all dogs diagnosed with HG‐AVB that underwent permanent transvenous pacemaker implantation at the North Carolina State University (NCSU) Veterinary Teaching Hospital between January 1, 2002 and December 31, 2012 was performed. The study was limited to dogs with HG‐AVB because other symptomatic bradyarrhythmias in dogs (eg, sick sinus syndrome, atrial standstill) rarely require emergency pacing. Limiting our study population to a single electrocardiographic diagnosis eliminated a variable that may have affected complication rate or outcome.
The following information was obtained for each patient: signalment, weight, clinical signs (type and duration), time between referral to NCSU and PAP placement, electrocardiogram (ECG) diagnosis, escape heart rate, presence of ventricular ectopy, QRS duration, response to atropine, systolic blood pressure, presence and severity of structural heart disease apart from expected bradycardia‐induced changes (classified as mild, moderate or severe), CHF (classified as left‐sided, right‐sided or both), azotemia (BUN concentration >26 mg/dL, serum creatinine concentration >1.7 mg/dL, or both; classified based on International Renal Interest Society Acute Kidney Injury grading criteria as mild for serum creatinine concentration <2.5 mg/dL and moderate for serum creatinine concentration 2.6–5.0 mg/dL), lead fixation type (tined or active), use of temporary pacing during PAP implantation (transthoracic or transvenous), starting time and duration of PAP implantation, incidence of hypotension (mean arterial blood pressure <60 mmHg) under anesthesia, expertise level of primary operator (faculty, 1‐year resident, 2‐year resident, or 3‐year resident), total hours of hospitalization, total cost of visit, presence or absence of complications related to PAP placement (defined below), and long‐term outcome and survival (defined below).
Procedures were defined as occurring AH if the procedure began outside of the time frame of 8:00 am–5:00 pm Monday through Friday. Based on personnel policies at our institution, a cardiology technician was not present during AH procedures. Complications were identified and categorized as major and minor as previously described.2, 3, 4 Major complications of pacemaker implantation were those considered to be life threatening, capable of causing sudden loss of pacing, or those requiring replacement of the pacemaker system (eg, lead dislodgement, infection, right ventricular perforation). Only those patients with a minimum of 2 years of follow‐up data were considered in the analysis of major complications; this time frame was chosen because of the known occurrence of late infectious complications in our study population. Minor complications were those considered not life threatening (eg, small wound seromas, muscle twitching at generator site, minor hemorrhage, minor arrhythmias, sensing issues) or complications associated with general anesthesia.
Survival was assessed by identifying the date and cause of death, either by medical record review or by contacting owners and referring veterinarians by phone or mail. Cause of death was categorized as cardiac (including directly pacemaker‐related, CHF, or sudden death), or noncardiac.
Pacemaker Implantation Technique
With few exceptions, patients received a standardized preoperative diagnostic evaluation including systolic blood pressure measurement, ECG, atropine response test, echocardiogram, thoracic radiographs, CBC, and serum biochemistry panel. Some dogs also had abdominal ultrasound examination and urinalysis performed based on clinical indication. Between the time of presentation and the time of PAP placement, all patients had transthoracic pacing pads placed, received a parenteral dose of an anticholinergic medication (either atropine or glycopyrrolate) every 4–6 hours, and were monitored by continuous ECG. Anticholinergic drugs were given regardless of response to atropine with the goal of preventing surges in vagal tone that could further decrease the ventricular response rate.
A consistent technique was used for transvenous pacemaker placement. Patients were premedicated with a combination of hydromorphone and an anticholinergic drug. General anesthesia was induced using either propofol or a combination of midazolam and etomidate, and maintained using isoflurane gas. If temporary transthoracic pacing was performed during anesthesia, atracurium typically was administered to decrease muscle contractions.
A right jugular cut‐down and venotomy were performed, and a bipolar permanent pacing lead was placed in the right ventricular apex under fluoroscopic guidance. Proper lead placement was confirmed by both fluoroscopy and threshold testing. A pulse generator,1 obtained through the Companion Animal Pacemaker Registry and Repository (CANPACERS) program, was connected to the lead and secured within a muscular pocket in the right dorsolateral neck region. Incisions were closed routinely in 3 layers with absorbable suture and skin staples. Dogs were hospitalized and monitored for approximately 12–24 hours after the procedure before discharge. Analgesia and sedation (opioids, acepromazine) were administered postoperatively to minimize pain, movement and agitation. Anantibiotic (cefazolin) was administered IV peri‐operatively, and an antibiotic (usually cephalexin) was administered PO for 7–10 days after discharge. In most cases, a nonsteroidal anti‐inflammatory drug (usually carprofen) also was administered for 3–7 days to alleviate discomfort. The neck was bandaged for 1–3 days after the procedure. Skin staples were removed 10–14 days after pacemaker implantation, usually by the referring veterinarian. Pacemaker interrogation and adjustment generally was performed 3–6 months after the procedure and was recommended every 6–12 months thereafter, but client adherence to this schedule was variable.
Statistical Analysis
Comparison of variables between AH and BH groups was performed with proprietary software2 using Student's t‐test for continuous variables and Fisher's Exact Test for categorical variables. Wilcoxon rank‐sum tests were used for data that were not normally distributed. Survival times were modeled and differences in outcomes were tested using nonparametric log‐rank tests. Survival times were analyzed separately for all‐cause mortality and cardiac mortality. Dogs that were still alive at the time of writing or were lost to follow‐up were included in the survival analysis as censored observations. Additional variables were tested for independent associations with either presence of complications or final outcome using analysis of variance for continuous variables and Fisher's Exact test for categorical variables. Wilcoxon 2‐sample tests were used to determine associations between survival time and presence of complications. Kruskal–Wallis tests were used to determine associations between survival time and other variables. Bonferroni's correction was used to control the Type I error rate. Significance was set at P < .05.
Results
Demographic and Clinical Findings
Ninety‐seven dogs met the inclusion criteria (Table 1). Average age at pacemaker implantation was 10 ± 3.2 years (range, 2–16 years). Commonly represented breeds included Labrador retrievers (18), Chow Chows and crosses (10), Cocker Spaniels (9), Beagles and crosses (7), German Shepherds(6), mixed breed dogs (6), Boxers and crosses (5), and Boston Terriers (5).
Table 1.
Demographic, clinical, procedural, and survival data for dogs with HG‐AVB undergoing transvenous PAP implantation at NCSU between 2002 and 2012. See text for definitions of individual variables. BH and AH groups are compared with Student's t‐tests (continuous variables) or Fisher's Exact test (categorical variables). All data are given as mean ± standard deviation unless otherwise noted
| Variable | All dogs | BH | AH | P value |
|---|---|---|---|---|
| Number of dogs | 97 | 55 | 42 | – |
| Age (years) | 10 ± 3.2 | 10.7 ± 3.2 | 9.0 ± 3.1 | .025a |
| Weight (kg) | 23 ± 10 | 22 ± 9.8 | 25 ± 11 | .43 |
| ECG diagnosis (3rd degree AVB) | 87/97 (90%) | 48/55 (87%) | 39/42 (93%) | .68 |
| Ventricular ectopy | 17/97 (18%) | 7/55 (13%) | 10/42 (24%) | .24 |
| Escape heart rate (bpm) | 42 ± 11 | 43.6 ± 9.6 | 40.8 ± 12.0 | .58 |
| QRS duration (ms) | 61 ± 18 | 58.5 ± 18.2 | 65.1 ± 18.6 | .26 |
| Atropine responsive | 4/77 (5%) | 3/47 (6%) | 1/34 (3%) | .64 |
| Structural heart disease | 55/97 (57%) | 32/55 (58%) | 23/42 (55%) | .38 |
| Mild: 46 | Mild: 29 | Mild: 17 | ||
| Moderate: 5 | Moderate: 2 | Moderate: 3 | ||
| Severe: 4 | Severe: 1 | Severe: 3 | ||
| Congestive heart failure | 9/97 (9%) | 3/55 (5%) | 6/42 (14%) | .40 |
| Left: 4 | Left: 2 | Left: 2 | ||
| Right: 4 | Biventricular: 1 | Right: 4 | ||
| Biventricular: 1 | ||||
| Azotemia | 25/97 (26%) | 15/51 (29%) | 10/41 (24%) | .40 |
| Mild: 17 | Mild: 11 | Mild: 6 | ||
| Moderate: 8 | Moderate: 4 | Moderate: 4 | ||
| Time between referral/pacemaker (days) | 0.52 ± 0.60 | 0.81 ± 0.58 | 0.12 ± 0.32 | <.0001a |
| Lead fixation (% passive) | 88/94 (94%) | 49/54 (91%) | 39/40 (98%) | .31 |
| Operator experience | F: 16/97 | F: 9/55 | F: 7/42 | .57 |
| R3: 22/97 | R3: 13/55 | R3: 9/42 | ||
| R2: 34/97 | R2: 18/55 | R2: 16/42 | ||
| R1: 25/97 | R1: 15/55 | R1: 10/42 | ||
| Faculty present? | 79/97 (81%) | 43/55 (78%) | 36/42 (86%) | .46 |
| Temporary pacing used | 44/97 (45%) | 24/55 (44%) | 20/42 (48%) | .82 |
| Hypotension under anesthesia | 37/97 (38%) | 23/50 (46%) | 14/41 (34%) | .49 |
| Procedure duration (minutes) | 90 ± 27 | 91 ± 27 | 88 ± 26 | .26 |
| Hospitalization (hours) | 39 ± 16 | 45 ± 14 | 32 ± 16 | <.0001a |
| Cost of procedure (US$) | 2414 ± 608 | 2411 ± 644 | 2416 ± 564 | .47 |
| Major complications | 14/79 (17.7%) | 4/43 (9.3%) | 10/36 (27.8%) | .041a |
| Minor complications | 14/79 (17.7%) | 6/43 (14%) | 8/36 (22.2%) | .39 |
| MST (all‐cause) (months) | 27 | 23.5 | 29 | .70 |
| MST (cardiac) (months) | 16 | 18 | 13.5 | .40 |
Indicate statistically significant differences between BH and AH groups.
Clinical signs included syncope in 39 dogs (40%), lethargy in 18 dogs (19%), both syncope and lethargy in 14 dogs (14%), and signs of CHF (eg, dyspnea, abdominal distension) in 6 dogs (6%). Twenty dogs (21%) were presented for an incidental bradyarrhythmia noted on routine examination and were reported to be asymptomatic. Duration of clinical signs generally was short (median, 5 days) with substantial variation (range, 1–365 days). A summary of other demographic, clinical, diagnostic, and procedural variables is presented in Table 1.
Fifty‐five dogs (57%) had pacemakers implanted during BH, whereas 42 dogs (43%) had pacemakers implanted AH. Compared to BH dogs, AH dogs were younger, had shorter time between referral and pacemaker placement, and had shorter hospitalization duration. There were no significant differences between BH and AH dogs for any other variables analyzed (Table 1).
Complication Rates
Seventy‐nine dogs had sufficient follow‐up data to analyze complication rates. Major complications occurred in 14 dogs (18%). Major complications included lead dislodgement (5 dogs), lead infection or vegetation (3 dogs), lead thrombus (1 dog), infection at the pulse generator site (2 dogs), SC migration of the pulse generator (2 dogs), ventricular perforation by the pacing lead (1 dog), and pacing failure with suspected exit block (1 dog). The incidence of major complications was significantly higher AH (10/36 dogs, 28%) compared to BH (4/43 dogs, 9%; P = .041). All infectious complications (3 lead infections and 2 generator infections) occurred in the AH group. There were no significant trends in major complication rate over time; major complication rate did not differ by year during the study period (P = .85). Median time from pacemaker implantation to major complication was 60 days (range, 1 hours–978 days). The majority (64%) of complications occurred within 3 months of pacemaker implantation.
Minor complications were observed in 14 dogs (14%); all minor complications occurred either peri‐operatively or within days of PAP implantation. Some dogs had >1 minor complication. Rates of minor complications did not differ between BH (6/55 dogs, 11%) and AH (8/42 dogs, 19%; P = .39). Minor complications included minor ventricular arrhythmias (9 dogs), small seromas over pulse generator sites (6 dogs), muscle twitching after PAP implantation (2 dogs), esophageal stricture postanesthesia requiring subsequent bougienage (1 dog), and transient acute kidney injury postanesthesia (1 dog). All minor complications resolved with treatment.
Survival Analysis
Long‐term follow‐up data were available for 82 dogs (85%). Nineteen dogs (23%) were alive at the time of writing (average follow‐up time, 24 months), and 63 dogs were dead. No dogs died intra‐ or peri‐operatively; all dogs survived to hospital discharge. Median survival time (MST) after PAP implantation for all dogs was 27 months (range, 2 weeks–114 months; mean, 31.5 ± 24.7 months). Of the 63 deaths, 44 (70%) died or were euthanized for noncardiac causes, and 19 (30%) died or were euthanized for cardiac causes. MST for dogs that experienced cardiac death was 16 months, which was significantly shorter than MST for noncardiac death (P = .017).
Survival (all‐cause mortality) did not differ between BH dogs (MST, 23.5 months) and AH dogs (MST, 29 months; P = .70; Fig 1). When considering cardiac mortality only, survival did not differ between the 9 BH dogs (MST, 18 months) and 10 AH dogs (MST, 13.5 months) that experienced cardiac death (P = .40; Fig 2).
Figure 1.
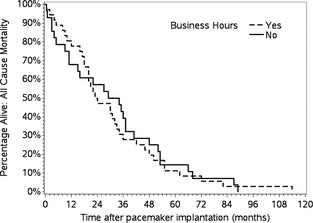
Kaplan–Meier survival curve (all‐cause mortality) for dogs with HG‐AVB undergoing pacemaker implantation during business hours versus after hours. Survival did not differ between groups (P = .70).
Figure 2.
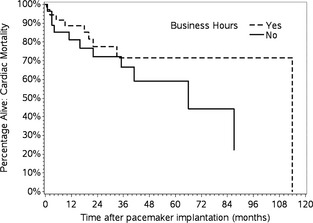
Kaplan–Meier survival curve (cardiac mortality only) for dogs with HG‐AVB undergoing pacemaker implantation during business hours versus after hours. Survival did not differ between groups (P = .40).
Of the 19 dogs that experienced a cardiac cause of death, 6 deaths (32%, 9.5% of total) appeared to be directly attributable to pacemaker complications, 11 (58%, 17% of total) from CHF, and 2 dogs (11%, 3.2% of total) died suddenly. Pacemaker‐related death occurred 0.5–22 months after PAP implantation, whereas death because of CHF occurred 2–114 months after PAP implantation. Dogs with major complications related to pacemaker placement were significantly more likely to die of cardiac causes (7/8, 88%) than dogs without major complications (12/55, 22%; P < .001), and presence of a major complication also was associated with shorter survival times (P = .026). Presence of a minor complication was not associated with a survival difference (P = .35).
Azotemia on presentation (either mild or moderate) was associated with decreased survival time for all‐cause mortality (P = .0029), but not cardiac mortality. Analysis of other patient variables identified no other independent associations with presence of complications or with survival (all‐cause or cardiac).
Discussion
Results of this study supported our hypothesis that major complications associated with PAP implantation occurred more frequently AH compared to BH. In particular, all infectious complications occurred in dogs with pacemakers placed AH. There were no clinical, demographic, or procedural variables that differed between BH and AH groups to explain the difference in complication rate, suggesting that personnel differences BH versus AH may have been a relevant factor.
Patient characteristics in this study were similar to those previously reported2, 3, 4, 7 including clinical signs, concurrent heart diseases, and incidence of CHF on presentation. Limiting this study to dogs with HG‐AVB may explain the slightly higher mean age of patients in this study compared to some previous studies (10.5 years versus 8–8.5 years). Pacemaker implantation technique in this study was similar to that previously described, with some differences: we used tined leads (versus active fixation) in the vast majority of cases; we used transthoracic (versus transvenous) pacing in cases requiring temporary pacing; and, all pulse generators were implanted in the dorsolateral neck region rather than in the intrascapular or lateral thoracic regions.
The overall rate of major complications in our study was 18%, which is within the range reported in other large‐scale studies of pacemaker placement in dogs over the past 15 years (13–33%).2, 3, 4, 7 As with previous studies, our most common complications were lead dislodgement and infection. Interestingly, we had more dogs develop infection of the lead (rather than generator), which in all cases eventually led to death or euthanasia. Although venous thrombosis and cranial vena cava syndrome have been reported in dogs with transvenous pacemakers,16, 17, 18, 19 lead vegetation was not a common complication noted in the previous large‐scale retrospective studies of pacemakers in dogs. The occurrence of lead infection or thrombosis in our patients could be related to lead hardware, poor sterile technique, or underlying prothrombotic conditions not apparent at the time of PAP implantation. Generator failure was a rare major complication in our study (1% of dogs, compared to the previously reported 2–6%2, 4, 7), possibly because CANPACERS no longer distributes previously used pulse generators. The overall rate of minor complications in this study (14.4%) was similar to that of previous studies (11–31%).2, 3, 4, 7
Despite our somewhat older patient population, median survival time after pacemaker placement (27 months) was slightly higher than in previous studies (22–26 months).3, 7 Incidence of cardiac death in our study (30%) was similar to that previously reported (22–44%).3, 4 Not surprisingly, dogs with major complications were more likely to experience cardiac death than dogs without major complications. Indeed, 6/14 dogs with major complications experienced death or euthanasia that was directly attributable to the pacemaker complication (3 dogs with lead infection, 1 dog with lead thrombosis, 1 dog with lead migration and perforation, and 1 dog with pacing failure). None of the dogs with lead dislodgement died from this complication; all had the pacing lead replaced with a subsequent procedure.
Despite the increased complication rate AH, there was no survival difference BH versus AH. This may be because one of the most common complications (lead dislodgment) was uniformly nonfatal after replacement of the lead. Alternatively, the lack of survival difference between groups may reflect the relatively low incidence of cardiac death (30%) and specifically pacemaker‐related death (9.5%) in this population.
The only factors found to have an independent association with survival (all‐cause or cardiac) were presence of a major complication and azotemia on presentation. These findings are in contrast to some previous studies that found that the presence pre‐existing heart disease4 and operator inexperience2 were correlated with decreased survival. Primary operator experience was not associated with the presence of complications or survival in our study. This may be because of our institutional policy that a cardiology faculty member or final‐year resident must be present for and assist with every PAP placement, regardless of timing. This policy also explains the lack of difference in operator level BH versus AH (P = .57). In addition, there was no difference in complication rates or survival when comparing cases in which the clinician supervising a junior resident as primary operator was a faculty member versus a third‐year resident.
Although several previous studies have reported complication rates in canine PAP implantation, this study was the first to investigate complication rates in reference to the timing of the procedure. One possible explanation for higher complication rates AH would be selection bias. Clinicians may select the most severely affected and most unstable dogs for AH placement, and these dogs may be independently predisposed to higher complication rates. However, our data suggest that BH and AH groups did not differ in clinical variables reflecting disease severity. Indeed, the groups differed only in age (AH dogs were younger than BH dogs), time between referral and pacemaker placement (shorter for AH dogs), and duration of hospitalization (shorter for AH dogs). We could identify no specific reason for the younger age of AH dogs.
We therefore suspect that the disparity in complication rate was related to procedure timing and associated personnel differences. At our institution during BH, the following personnel are directly involved in a pacemaker implantation: a board‐certified cardiologist, 1–2 cardiology residents, 1–2 registered technicians with Veterinary Technician Specialist (Cardiology) certification, an anesthesia resident with or without an anesthesia technician, and 2 senior veterinary students. Furthermore, additional board‐certified personnel are available in the building to troubleshoot technical or anesthetic problems (eg, cardiologists, anesthesiologists, radiologists, criticalists). In contrast, the personnel involved in an AH pacemaker implantation generally include the following: a board‐certified cardiologist (or 3‐year cardiology resident), a cardiology resident, 1 anesthesia resident or technician, and 1–2 senior veterinary students.
These personnel differences may have affected complication rates in a number of ways. Decreased numbers and expertise of personnel may diminish the AH team's ability to troubleshoot problems encountered during pacemaker implantation. The absence of a cardiology technician may have compromised routine aseptic patient preparation. Many of the personnel on emergency call for PAP implantation have completed a normal daytime shift, and fatigue may be a contributing factor. Ancillary team members AH may be less well‐trained in cardiology procedures and may be less familiar with the cardiology personnel performing the procedure, possibly contributing to procedural errors or communication difficulties AH. Our study did not allow us to differentiate among the many personnel‐related variables that may have contributed to the higher complication rate AH. However, the fact that all infectious complications occurred in patients paced AH raises specific concern for the quality of aseptic patient preparation and adherence to sterile technique AH.
We could find no veterinary studies investigating differences in complication rates of any surgical procedure (cardiac or otherwise) as a function of procedure timing. Human literature regarding timing‐ or personnel‐based differences in complication rates also is limited. One study in human medicine found no difference in complication rates between PAP implantation performed in an operating room versus a catheterization laboratory,11 but the timing of the procedure and personnel involved were the same in each group. Another recent study in humans3 found that patients undergoing laparoscopic cholecystectomy had higher complication rates if the procedure was performed as an AH emergency (after 7:00 pm), as opposed to a planned BH procedure. In addition, a large‐scale study of nonelective hospital admissions in humans suggested that patients admitted on weekends had higher mortality than patients admitted during the week.20
Our study had several limitations. The retrospective descriptive study design allowed no way to assess the various personnel factors that may have contributed to the difference in complication rates BH versus AH. In addition, because cases were not randomly assigned to BH or AH PAP implantation, we do not know whether additional morbidity or mortality might have occurred if AH cases had been delayed until the next business day. Furthermore, all data were collected at a single institution. Our AH environment may not accurately reflect the experience of other institutions with different emergency management protocols and personnel policies. Finally, long‐term follow‐up data were not available for all dogs. Some dogs were lost to follow‐up such that survival data was unavailable, and some dogs had insufficient follow‐up to be included in the analysis of complication rates.
In conclusion, the results of this study support the hypothesis that major complications (especially infections) occurred more frequently in dogs with HG‐AVB in which pacemakers were implanted AH. For some patients, the increased risk of AH complications may balance or outweigh the risk of clinical deterioration associated with delaying PAP implantation until the next business day. However, these data do not imply that all pacemaker procedures should be delayed, because some unstable patients always will require emergency pacing. We hope this study will be used to support reasonable policies to lower the still excessive complication rate associated with PAP implantation in any setting (eg, rigorous task analysis, checklist implementation, augmentation and training of personnel for AH procedures). Given institutional variation in the number and type of personnel available for AH procedures, our findings should be interpreted within the context of an individual institution before being generalized into new policy initiatives.
Acknowledgments
This study was not supported by any grant or other funding source.
Conflict of Interest: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work was performed at the College of Veterinary Medicine, North Carolina State University, Raleigh, NC.
This study was presented in abstract form at the North Carolina State University College of Veterinary Medicine Research Forum in April 2014.
Footnotes
Medronic, Minneapolis, MN
SAS version 9.3, Cary, NC
Wray CJ, Harvin JA, Phatak UR, Kao LS, Ko TC, Riall TS. Predictors of bile duct injury during laparoscopic cholecystectomy: Experience from two hospitals. Paper presented at: Society for Surgery of the Alimentary Tract 54thAnnual Meeting; 2013 May 17–21; Orlando, FL
References
- 1. Buchanan J, Dear M, Pyle R, Berg P. Medical and pacemaker therapy of complete heart block and congestive heart failure in a dog. J Am Vet Med Assoc 1968;152:1099–1109. [PubMed] [Google Scholar]
- 2. Oyama MA, Sisson DD, Lehmkuhl LB. Practices and outcome of artificial cardiac pacing in 154 dogs. J Vet Intern Med 2001;15:229–239. [DOI] [PubMed] [Google Scholar]
- 3. Wess G, Thomas WP, Berger DM, Kittleson MD. Applications, complications, and outcomes of transvenous pacemaker implantation in 105 dogs (1997‐2002). J Vet Intern Med 2006;20:877–884. [DOI] [PubMed] [Google Scholar]
- 4. Johnson MS, Martin MWS, Henley W. Results of pacemaker implantation in 104 dogs. J Small Anim Pract 2007;48:4–11. [DOI] [PubMed] [Google Scholar]
- 5. Sisson D, Thomas WP, Woodfield J, et al. Permanent transvenous pacemaker implantation in forty dogs. J Vet Intern Med 1991;5:322–331. [DOI] [PubMed] [Google Scholar]
- 6. Schrope DP, Kelch WJ. Signalment, clinical signs, and prognostic indicators associated with high‐grade second‐or third‐degree atrioventricular block in dogs: 124 cases (January 1, 1997–December 31, 1997). J Am Vet Med Assoc 2006;228:1710–1717. [DOI] [PubMed] [Google Scholar]
- 7. Domenech O, Santilli R, Pradelli D, Bussadori C. The implantation of a permanent transvenous endocardial pacemaker in 42 dogs: A retrospective study. Med Sci Monit 2005;6:168–175. [PubMed] [Google Scholar]
- 8. Lombard CW, Tilley LP, Yoshioka M. Pacemaker implantation in the dog: Survey and literature review. J Am Anim Hosp Assoc 1981;17:751–758. [Google Scholar]
- 9. Bonagura J, Helphrey M, Muir W. Complications associated with permanent pacemaker implantation in the dog. J Am Vet Med Assoc 1983;182:149–155. [PubMed] [Google Scholar]
- 10. Fine DM, Tobias AH. Cardiovascular device infection in dogs: Report of 8 cases and review of the literature. J Vet Intern Med 2007;21:1265–1271. [DOI] [PubMed] [Google Scholar]
- 11. Miller G, Leman R, Gratz J. Comparison of lead dislodgement and pocket infection rates after pacemaker implantation in the operating room versus the catheterization laboratory. Am Heart J 1988;115:1048–1051. [DOI] [PubMed] [Google Scholar]
- 12. Kiviniemi MS, Pirnes MA, Eränen HJ, et al. Complications related to permanent pacemaker therapy. Pacing Clin Electrophysiol 1999;22:711–720. [DOI] [PubMed] [Google Scholar]
- 13. Eberhardt F, Bode F, Bonnemeier H, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart 2005;91:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazama S, Nishiyama K, Machii M, et al. Long‐term follow‐up of ventricular endocardial pacing leads: Complications, electrical performance, and longevity of 561 right ventricular leads. Jpn Heart J 1993;34:193–200. [DOI] [PubMed] [Google Scholar]
- 15. Mayosi BM, Little F, Millar RN. Long‐term survival after permanent pacemaker implantation in young adults: 30 year experience. Pacing Clin Electrophysiol 1999;22:407–412. [DOI] [PubMed] [Google Scholar]
- 16. Van De Wiele CM, Hogan DF, Green HW, Parnell NK. Cranial vena caval syndrome secondary to transvenous pacemaker implantation in two dogs. J Vet Cardiol 2008;10:155–161. [DOI] [PubMed] [Google Scholar]
- 17. Murray JD, O'Sullivan ML, Hawkes KC. Cranial vena caval thrombosis associated with endocardial pacing leads in three dogs. J Am Anim Hosp Assoc 2010;46:186–192. [DOI] [PubMed] [Google Scholar]
- 18. Mulz JM, Kraus MS, Thompson M, Flanders JA. Cranial vena caval syndrome secondary to central venous obstruction associated with a pacemaker lead in a dog. J Vet Cardiol 2010;12:217–223. [DOI] [PubMed] [Google Scholar]
- 19. Cunningham SM, Ames MK, Rush JE, Rozanski EA. Successful treatment of pacemaker‐induced stricture and thrombosis of the cranial vena cava in two dogs by use of anticoagulants and balloon venoplasty. J Am Vet Med Assoc 2009;235:1467–1473. [DOI] [PubMed] [Google Scholar]
- 20. Ricciardi R, Roberts PL, Read TE, et al. Mortality rate after nonelective hospital admission. Arch Surg 2001;146:545–551. [DOI] [PubMed] [Google Scholar]


