Abstract
Background
Acute kidney injury (AKI) is common in dogs. Few studies have assessed sequential changes in indices of kidney function in dogs with naturally occurring AKI.
Objective
To document sequential changes of conventional indices of renal function, to better define the course of AKI, and to identify a candidate marker for recovery.
Animals
Ten dogs with AKI.
Methods
Dogs were prospectively enrolled and divided into surviving and nonsurviving dogs. Urine production was measured with a closed system for 7 days. One and 24‐hour urinary clearances were performed daily to estimate solute excretion and glomerular filtration rate (GFR). Solute excretion was calculated as an excretion ratio (ER) and fractional clearance (FC) based on both the 1‐ and 24‐hour urine collections.
Results
Four dogs survived and 6 died. At presentation, GFR was not significantly different between the outcome groups, but significantly (P = .03) increased over time in the surviving, but not in the nonsurviving dogs. Fractional clearance of Na decreased significantly over time (20.2–9.4%, P < .0001) in the surviving, but not in the nonsurviving dogs. The ER and FC of solutes were highly correlated (r, 0.70–0.95).
Conclusion and Clinical Impact
Excretion ratio might be used in the clinical setting as a surrogate marker to follow trends in solute excretion. Increased GFR, urine production, and decreased FC of Na were markers of renal recovery. The FC of Na is a simple, noninvasive, and cost‐effective method that can be used to evaluate recovery of renal function.
Keywords: Acute renal failure, Kidney, Renal/Urinary tract, Excretion ratio, Urinary clearance, Prognostic indicators
Abbreviations
- AKI
acute kidney injury
- GFR
glomerular filtration rate
- ER
excretion ratio
- FC
fractional clearance
- Na
sodium
- K
potassium
- NGAL
neutrophil gelatinase‐associated lipocalin
- VMTH
veterinary medical teaching hospital
- CKD
chronic kidney disease
- C
renal clearance
- Cx
clearance solute x
- Ccr
clearance creatinine
- Ucr
urine creatinine concentration
- Pcr
plasma creatinine concentration
- V
urine flow rate
- MAT
microscopic agglutination test
Acute kidney injury (AKI) is associated with a sudden decrease in the glomerular filtration rate (GFR) and changes in urine volume and renal solute excretion. It is associated with high morbidity and mortality, influenced mainly by the underlying etiology and options available for management.1, 2, 3, 4, 5, 6, 7, 8, 9 The major causes for AKI in dogs include infectious diseases, toxic insults, hemodynamic instability, immune‐mediated diseases, and a variety of miscellaneous and idiopathic etiologies.1, 2, 3, 4, 5, 6, 7, 8, 9 The therapeutic approach for AKI generally is supportive and directed at elimination of the underlying cause and correction of the hemodynamic and biochemical consequences of the attendant uremia. When medical management fails to control clinical signs and clinicopathologic abnormalities, more advanced treatment such as hemodialysis can be applied, if available.
On the basis of limited studies, some risk factors for mortality have been identified in dogs. In 1 study, the severity of azotemia (serum creatinine concentration >10 mg/dL), hypocalcemia (<8.6 mg/dL), and qualitatively assessed proteinuria were found to be risk factors for mortality.2 In that study, dogs that survived >5 days were more likely to recover.2 In a study of hospital‐acquired AKI in dogs, anion gap and serum phosphorus concentration at presentation were found to be risk factors for mortality, but there was no association between the magnitude of azotemia and survival.1 Older dogs were shown to be at increased risk for AKI and less likely to recover.1
In human medicine, there has been active investigation to identify and characterize biomarkers of renal injury in the hope they may allow early identification of kidney damage before functional decreases in renal function (eg, urine output, serum creatinine concentration). Serum and urinary markers used in human medicine include neutrophil gelatinase‐associated lipocalin, kidney injury molecule‐1, cysteine‐rich protein, cystatin C, and urine interleukin‐18.10, 11, 12 Neutrophil gelatinase‐associated lipocalin also has been recently evaluated in dogs with AKI.13, 14 Nevertheless, there are no current indices used routinely in the acute stages of AKI to predict recovery of renal function or survival.
Because renal injury may promote changes in glomerular filtration, urine production or renal solute excretion, these parameters potentially could be used as markers for renal recovery in patients with AKI. No recent studies have documented daily or sequential changes in these renal function parameters to characterize the clinical course of AKI or predict repair of renal injury and recovery of function. Sequential changes in glomerular or tubular function could serve as surrogates to predict outcome in AKI.
The traditional and most accurate assessments of renal function include GFR and fractional clearance (FC) of solutes, which are based on timed collections of urine, but these methods are time‐consuming, require placement of a urinary catheter, and are cumbersome to perform. The solute excretion ratio (ER) is the mathematical equivalent of the FC for a solute and can be calculated using spot urine and plasma samples as a predictor of renal excretory function. The ER is more clinically applicable because it eliminates the need for urinary catheter placement and timed urine collection. The comparative accuracy of the solute ER, however, has been questioned in a previous study.15
The objective of this study was to characterize the course of the disturbances in kidney function in dogs with naturally acquired AKI. Specifically, we sought (1) to assess sequential changes in GFR, urine production, and FC of solutes as indices of glomerular and tubular injury; (2) to assess the correlation between the renal ER and FC of solutes; and (3) to identify among these kidney function parameters potential markers for renal recovery and predictors of survival in dogs with AKI.
Materials and Methods
This study was approved by the Institutional Animal Care and Use Committee at the University of California‐Davis. It was a prospective, observational clinical trial of dogs with naturally occurring AKI. Dogs presented to the University of California Veterinary Medical Teaching Hospital (VMTH) between March 2005 and March 2006 with AKI were considered for study. Inclusion criteria were acute onset of clinical signs (<7 days), serum creatinine concentration >5 mg/dL, urine production <0.5 mL/kg/h, urine specific gravity <1.025, absence of ultrasonographic evidence of chronic kidney disease, and body weight >15 kg. Ten dogs fulfilled the inclusion criteria and were enrolled for further study after their owners had signed an informed consent form. Hemodialysis was initiated when medical management failed, based on an assessment that was made for each individual dog by the attending clinicians and the nephrologist based on severity of azotemia, clinical signs, presence of hyperkalemia, fluid overload, and urine production.
The study period included 7 consecutive days after presentation to the VMTH. All dogs had urinary catheters placed upon presentation, and urine was collected continuously throughout the study period by using a closed system. The urine collection bag was kept on ice to prevent degradation of solutes. Urine volume was estimated gravimetrically q12h using an analytic scale, and collected specimens were stored at −70°C pending analysis, which was performed up to 8 weeks from the time of collection. Daily measurements included bodyweight, urine production (q12h), serum and urine chemistries, GFR (estimated by endogenous creatinine clearance), urinary solute clearance, solute FC (determined on 60‐minute and 24‐hour urine collections), and solute ER (determined on spot urine and serum chemistry results). Solute clearance and excretion were determined for urea, sodium, potassium, calcium, phosphorus, magnesium, and glucose. Assessment of hydration status was done subjectively based on skin turgor, moisture of mucous membranes, presence of chemosis, and sequential changes in body weight.
Urinary Clearances
Urine and blood samples to calculate the ER were collected each day just before performing the clearance. The clearance of creatinine was used to estimate GFR as has been described previously.16, 17, 18 Briefly, 2 sequential 30‐minute quantitative urine collections were performed daily between 8:00 am and 10:00 am for assessment of GFR and renal solute excretion. Blood was collected at the beginning of the first urine collection and at the end of the second urine collection. The bladder was emptied and rinsed 3 times with 10 mL of sterile water at the beginning and end of each collection, and the bladder rinses at the end of each collection period were added to the urine collection. Urine volume for each collection was measured gravimetrically using an analytical scale. An aliquot was stored at −70°C pending analysis. Sodium, potassium, urea, creatinine, calcium, magnesium, phosphorus, and glucose concentrations were measured on all serum and urine samples at the hospital's laboratory.1
The results for the 2 individual 30‐minute clearances were averaged to provide an estimate for the renal clearance of each solute.
The renal clearance (C), FC, and ER for each solute (x) were calculated as previously described:19
where, Cx = clearance of solute x, Ccr = clearance of creatinine, V = urine flow rate (mL/min), Ux = urine concentration of solute x, Px = plasma concentration of solute x, Pcr = plasma creatinine concentration, and Ucr = urine creatinine concentration.
Outcome
Dogs were deemed to have survived if they had complete clinical recovery or partial recovery of renal function, which was amenable to medical management for at least 30 days after discharge. Dogs were classified as not having survived if they remained dialysis dependent or were euthanized because of failure to improve. All dogs were managed for at least 14 days before a decision to euthanize was made.
Statistical analysis
Descriptive statistics were used to document sequential changes in kidney function and solute excretion over the study period. Continuous parameters (eg, urine production, GFR) were compared between outcome groups using the Mann‐Whitney U‐test when they were not normally distributed and by the Student's t‐test when they were normally distributed. Correlations between ER and FC at 1 and 24 hours were performed using multiple linear regression with ER as the dependent variable, FC at 1 and 24 hours as predictor variables, and indicator variables for individual dog included in each model, allowing for intercepts to vary, but the slope to be constant for all dogs. Mixed effects analysis of variance was performed to assess differences in solute excretion between the outcome groups, with survival group and day as fixed effects, and individual dog as the random effect. Models initially were fit with interactions between the fixed effects, and if interactions were not significant, the main effects models were fit. Posthoc comparisons were adjusted using a Bonferroni correction. Statistical analysis was performed by statistical software.2 P < .05 was considered statistically significant.
Results
The study population consisted of 6 castrated male and 4 spayed female dogs of the following breeds: Queensland Heeler (1), Labrador Retriever (3), Belgian Tervuren (1), Australian Shepherd (1), and mixed breed dogs (4). The mean age was 9 ± 1.9 years (range, 7–12 years). Mean body weight was 26.1 ± 5.9 kg (range, 17.9–35.2 kg).
The etiology of AKI included: leptospirosis (5), acute glomerulonephritis based on renal biopsy (2), and unknown etiology (3). A diagnosis of leptospirosis was confirmed if the titer of a single microscopic agglutination test was >1 : 800 for nonvaccinal serovars or if there was an evidence of a 4‐fold increase in titers on paired serum samples.
At presentation, median serum creatinine concentration was 11.3 mg/dL (range, 6–18.2 mg/dL), median serum urea nitrogen concentration was 112 mg/dL (range, 69–160 mg/dL), and median urine specific gravity was 1.012 (range, 1.007–1.016). Fluids were administered at presentation to only 3/10 dogs because of decreased urine production. Fluids were discontinued in 2 of the 3 dogs after 2 days because of low urine production and risk of overhydration. Only 1 dog received IV fluids throughout the study period to match urine losses.
Four dogs survived and 6 dogs died or were euthanized. Of the nonsurviving dogs, 5 were euthanized because of lack of renal improvement between 18 and 55 days (median, 35 days) after presentation, and 1 dog died from complications of pancreatitis and aspiration pneumonia 42 days after presentation. Eight dogs were managed using hemodialysis, and of these, 3 survived. Medical management only was used in 2 dogs, of which 1 survived.
Most surviving dogs had only partial recovery of renal function at the end of the 7‐day study period, and maintained an increased serum creatinine concentration at day 7 (median, 2.5 mg/dL; range, 1.3–5.4 mg/dL; reference range [RR], 0.3–1.2 mg/dL). Median serum urea nitrogen concentration of surviving dogs was 25.5 mg/dL (range, 9.0–61.0 mg/dL; RR, 5–21 mg/dL). One dog was still dialysis dependent at the end of the study period, but became nondialysis dependent by day 9 after presentation. For the nonsurviving dogs, the median serum creatinine concentration at day 7 was 5.8 mg/dL (range, 3.7–7.3 mg/dL; RR, 0.3–1.2 mg/dL) and the median serum urea nitrogen concentration was 52 mg/dL (range, 33–135 mg/dL; RR, 5–21 mg/dL).
Urine Production
There was no significant interaction between surviving and nonsurviving groups and time, but across all times there was a significant difference between the groups (P = .004, Fig 1). The predicted mean urine production across all times in survivors was 4.67 (1.10) mL/kg/h, and the predicted mean across all time in nonsurvivors was 0.91 (0.93) mL/kg/h.
Figure 1.
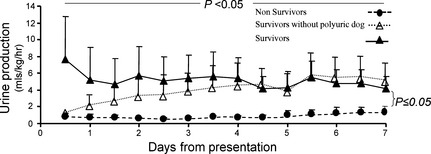
Changes in urine production in survivors (n = 4) and nonsurvivors (n = 6) during the 7‐day study period. Presented are the mean and the standard deviation (which is presented only as a positive or a negative deviation to prevent overlap).
Glomerular Filtration Rate
There was a significant interaction between group and time (P < .05), with significant group differences on day 6 (P = .016) and day 7 (P = .001, Fig 2). Means and standard deviations in survivors on days 6 and 7 results were 0.503 (0.466) and 0.622 (0.441) mL/min/kg, respectively; whereas in nonsurvivors on days 6 and 7 results were 0.053 (0.051) and 0.074 (0.097) mL/min/kg, respectively.
Figure 2.
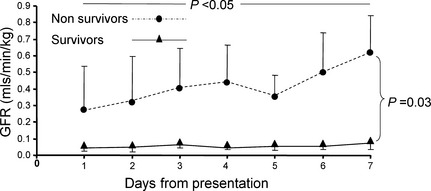
Changes in glomerular filtration rate in survivors (n = 4) and nonsurvivors (n = 6) over the 7‐day study period. Presented are the mean and the standard deviation (which is presented only as a positive or a negative deviation to prevent overlap).
Correlation between Excretion Ratio and Fractional clearance
The ER for sodium and potassium were compared to the FC determined from the 1‐ and 24‐hour urine collections for these solutes to assess the utility of ER as a surrogate for FC. Overall, there was significant (P < .001) and high correlation (r = 0.835 and r = 0.921) between ER and FC (based on 1‐ and 24‐hour urine collections), respectively. Correlations between sodium ER and 1‐ and 24‐hour sodium FC were 0.835 and 0.823, respectively. Correlations between potassium ER and 1‐ and 24‐hour potassium FC were 0.921 and 0.814, respectively.
Sequential Changes in Solute Excretion as Potential Markers for Survival
The mean ER for sodium decreased over the study period in the surviving (0.21 ± 0.08 to 0.09 ± 0.07), but not in the nonsurviving dogs (0.25 ± 0.15 to 0.22 ± 0.19, Fig 3). There was a significant group difference between the outcome groups on day 7 (P = 2.006), with mean ER of sodium (ERNa) decreased in the surviving dogs, but unchanged in the nonsurviving dogs (Fig 3).
Figure 3.
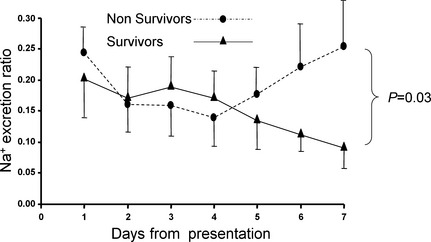
Changes in excretion ratio of sodium in survivors (n = 4) and nonsurvivors (n = 6) over the 7‐day study period. Presented are the mean and the standard deviation (which is presented only as a positive or a negative deviation to prevent overlap).
The mean ER for potassium (ERK) for the surviving dogs decreased from 2.3 ± 0.8 on day 1 to 0.86 ± 0.4 on day 7, and for the nonsurviving dogs, the ERK decreased from 1.59 ± 0.64 to 0.83 ± 0.3. When the surviving dogs were compared to the nonsurviving dogs over the study period, there was no significant difference between the groups (Fig 4).
Figure 4.
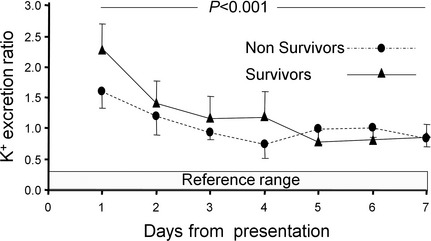
Changes in excretion ratio of potassium in survivors (n = 4) and nonsurvivors (n = 6) over the 7‐day study period. Presented are the mean and the standard deviation (which is presented only as a positive or a negative deviation to prevent overlap).
The mean ER for glucose, urea, phosphorus, calcium, and magnesium also were compared between the surviving and nonsurviving groups. The median daily blood glucose concentration for all dogs was lower than the renal threshold (200 mg/dL).20 There were no significant differences between outcome groups, nor was there a significant change over time (Table 1).
Table 1.
Fractional clearance (%) of solutes based on 1‐hour clearance
| Solute | Survivors | Nonsurvivors | ||
|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | |
| Median (range) | Median (range) | Median (range) | Median (range) | |
| Magnesium | 37.2 (22.8–66.6) | 24.9 (4.1–34.1) | 55.7 (4.9–68.3) | 27.1 (0.0–76.1) |
| SUNa | 71.8 (34.2–93.5) | 93.6 (36.3–109.9) | 76.9 (24.5–100) | 54.8 (0.0–92.2) |
| Glucose | 26.3 (1.1–100.5) | 4.5 (2.3–29.9) | 20.0 (0.0–41.0) | 3.0 (0.0–57.5) |
| Phosphorus | 88.1 (34.6–90.2) | 23.9 (6.7–50.3) | 57.7 (19.8–93.7) | 32.9 (0.0–63.2) |
| Calcium | 20.4 (15.1–39.8) | 14.9 (4.3–27.5) | 26.6 (1.2–31.7) | 14.6 (0.0–45.4) |
SUN, serum urea nitrogen, There were no statistical differences between day 1 and day 7 in both outcome groups in any of the solutes measured.
Discussion
In this study, we have documented sequential changes in GFR, urine production, and renal solute excretion in dogs with naturally occurring AKI. The results of the present study suggest that ER can be used in the clinical setting as a surrogate for FC and that the ER of specific electrolytes (eg, Na) can be used to predict renal recovery.
The mortality rate in this study was 6/10 (60%). Leptospirosis was diagnosed in 5/10 dogs and, of those, 4 survived. This finding is comparable to the survival rate reported recently for dogs undergoing hemodialysis for AKI.3, 21 Traditionally, recovery from AKI has been associated with a transition from an initial oliguric to a polyuric phase.2, 9 However, many animal patients with AKI are nonoliguric at initial presentation.2 Nonoliguric AKI appears to afford a better prognosis than oliguric AKI and is more common with nephrotoxic rather than ischemic renal injury.9, 22 Approximately, 33% of dogs presenting to a referral center with AKI are reported to be nonoliguric, another 33% are reported as anuric and the remaining are oliguric.2, 9 In a retrospective study of AKI in dogs, normal or increased urine output was reported in 39% of the dogs.2 In the current study, there was no significant difference in urine production between surviving and nonsurviving dogs at presentation, but the surviving group produced significantly more urine over the study period compared to the nonsurviving group (Fig 1). Based on these results, anuria or oliguria that persists beyond 7 days should be considered a more negative prognostic indicator than the presence of anuria or oliguria at initial presentation. Decreased urine production also has been found to be a risk factor for mortality in various studies of AKI in dogs and cats.1, 2, 4, 7, 23.
Glomerular filtration rate and urine production were not statistically different between the 2 outcome groups at presentation, but significant differences were found between the outcome groups. Over time, the GFR of survivors increased, whereas there was no significant change in the GFR of the nonsurviving dogs. Most surviving dogs were nondialysis dependent at the end of the study period, but their GFR was still markedly decreased. Even dogs with complete clinical recovery and resolution of their azotemia on day 7 had a persisting decrease in GFR (<1 mL/min/kg). These observations emphasize that dogs that have seemingly recovered from AKI still have a marked decrease in their kidney function and are likely to be at risk for additional renal injury.
Conventional measurement of the FC of solutes by the kidneys is based on clearance techniques using timed urine collections. These techniques are considered a more accurate method of measuring solute excretion, but require placement of a urinary catheter and are time‐consuming and cumbersome to perform in clinical practice. The simultaneous collection of nontimed urine and blood (spot) samples, and calculation of ER is easier to perform and less invasive, but the physiologic validity and accuracy of this method has been questioned. A study comparing the ER and the FC of electrolytes in 5 healthy cats suggested that this spot method is unreliable.15 The results of the current study demonstrated that the ER for Na and K correlated with the simultaneously measured FC of these solutes when measured using either a 1‐ or 24‐hour timed urinary collection. For K, higher correlations were found between ER and FC determined with the 1‐hour versus FC determined with a 24‐hour urine collection. These differences may have resulted from changes in the FC of solutes throughout the 24‐hour period that were not temporally associated with a spot sample collection used for the ER determination. These observations indicate that the ER for Na and K suitably predicted the trends in renal excretion of these solutes in the early maintenance stage of AKI in these dogs with minimal invasiveness, inconvenience, and low cost. Although less precise, these results suggest further that ER might be useful in clinical settings as a surrogate for FC and predictor of outcome. It is yet to be determined if these changes occur in different causes of AKI other than leptospirosis.
The FC of K in dogs with normal renal function is <20%, and the FC for Na is <1%.24 At presentation, all dogs had markedly increased FC (>150% for K and >20% for Na) and ER for these solutes indicating extensive dysregulation of solute excretion associated with AKI in dogs at this early stage of disease. Potassium excretion decreased progressively over the entire study period in both surviving and nonsurviving dogs, but there were no significant differences in the pattern or magnitude of K excretion between these groups. This observation is in contrast to Na excretion in which there was a significant difference between the outcome groups. These observations might reflect differences in the respective renal regulation and tubular handling of K and Na. The marked increase in K excretion (>100%) suggests secretion of this solute, likely by the distal tubule, which is less susceptible to damage in AKI compared to the proximal tubule. The decrease in FCK over the study period may reflect improved tubular regulation of this solute as seen with Na, improvement in the external balance for K because of the imposed medical or dialytic treatment, decreased K intake, decreased K load because of progressive elimination throughout the study period or some combination of these factors. This marked increase in FCK may be a mechanism by which animals with AKI maintain potassium homeostasis despite substantial decreases in the GFR.
The marked increase in the FC and ER for Na at the beginning of the study documents the extensive disruption of tubular reabsorptive function by the injured kidneys in both outcome groups. Differences in FC and ER for Na between surviving and nonsurviving dogs became apparent at day 4 with the onset of marked disassociation in the excretion pattern for Na between groups. The progressive decrease in FC Na in surviving dogs to reference values suggests that proximal tubular function was being restored. This is in marked contrast to the sudden and progressive increase in FC Na observed in the nonsurviving dogs suggesting ongoing disruption of tubular function. To the extent that improvements in FC Na forecast recovery from renal injury, ER becomes a simple, noninvasive, and cost‐effective tool to monitor these events and may prove useful as a prognostic indicator and a marker for renal recovery. When a gradual decrease in ER for Na is observed, it is likely that epithelial repair is taking place. It is yet to be determined if improvement in the FC Na is a more sensitive indicator for recovery compared to serum creatinine concentration or urine production, but this parameter of tubular function may be especially useful for dogs managed with renal replacement treatments in which serum creatinine concentration is maintained artificially at a concentration below steady state making improvement in renal function more difficult to assess.
Fractional clearance of Na can be influenced by administration of Na‐containing fluids. Such fluids can increase the FC Na, and might negate differences between the outcome groups. Once hydrated, fluids were administered to match ongoing losses (eg, urinary losses). Consequently, dogs with increasing urine production received larger amounts of fluids compared to anuric dogs. Nevertheless, FC Na was high in nonsurvivors and decreased gradually in survivors, even in the polyuric dog that received relatively large amounts of fluids.
The FCNa has been evaluated in human medicine both as a prognostic indicator and as a marker for differentiating volume‐responsive AKI (ie, prerenal azotemia) from more severe forms of AKI and acute azotemia. A high FCNa profile was more common with severe morphological tubular injury, whereas a lower FCNa more commonly was documented in transient, nonoliguric AKI. It was, however, found to be of limited prognostic value.25, 26, 27, 28
The FC of other solutes such as urea nitrogen, magnesium, calcium, phosphorus, and glucose often were abnormal. Fractional clearance of these solutes has not been reported extensively in dogs with AKI, but has been reported for normal dogs.29 The current study suggests that FC of these solutes also is deranged in AKI (Table 1). The high FC of glucose likely indicated the presence of proximal renal tubular damage because none of the dogs had blood glucose concentrations above the renal threshold. Nonetheless, none of the other solutes provided significant differentiation between groups or response over time and provided no utility as markers for survival in dogs with AKI during the first week of hospitalization.
This study had a few limitations. First, the small sample size might have rendered this study underpowered to document potential differences between the outcome groups relative to a specific solute (type II error). Therefore, it is a preliminary study and additional studies involving a larger sample size are required to confirm and expand on the findings. Second, 50% of the dogs studied had leptospirosis as the etiology of their AKI. As has been documented, leptospirosis has a shorter time course and better outcome for survival than other etiologies of AKI, and the association between the observed improvements between FCNa and ERNa and survival may not be characteristic for AKI of other etiologies. Although this may be considered a limitation, it is a reflection of the prevalence of leptospirosis patients at the VMTH, as was previously reported.21 Third, the presence of oliguria or anuria in some study dogs hampered the quantification of renal solute excretion, especially Na. The bladder rinses used to assure complete recovery of urine at each collection interval resulted in dilution of Na to the point of nondetection in the 1‐hour clearances in dogs with severe oliguria. To avoid this problem, an air rinse could be used as an alternative.
In conclusion, an increase in urine production, GFR, and a decrease in the FCNa were associated with survival in dogs with AKI and may serve as early prognostic markers of renal repair. In this setting, ER proved to be an accurate and convenient estimate for the FC for a variety of solutes, and the FCNa may be similarly useful as an early prognostic marker of improvement in renal function and survival. The changes in FCNa and ERNa occurred in surviving dogs before any evidence of resolution of their azotemia or dialysis dependency. These markers should be further investigated in a larger cohort to further substantiate these preliminary findings.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The patients were treated at the William R. Pritchard Veterinary Medical Teaching Hospital, University of California, Davis, One Shields Avenue, Davis, California
Footnotes
Hitachi 917: Roche Laboratories, Indianapolis, IN
NCSS 6.0.22 software, NCSS, Kaysville, UT
References
- 1. Behrend EN, Grauer GF, Mani I, et al. Hospital‐acquired acute renal failure in dogs: 29 cases (1983‐1992). J Am Vet Med Assoc 1996;208:537–541. [PubMed] [Google Scholar]
- 2. Vaden SL, Levine J, Breitschwerdt EB. A retrospective case‐control of acute renal failure in 99 dogs. J Vet Intern Med 1997;11:58–64. [DOI] [PubMed] [Google Scholar]
- 3. Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990‐1998). J Am Vet Med Assoc 2000;216:371–375. [DOI] [PubMed] [Google Scholar]
- 4. Brown SA, Barsanti JA, Crowell WA. Gentamicin‐associated acute renal failure in the dog. J Am Vet Med Assoc 1985;186:686–690. [PubMed] [Google Scholar]
- 5. Bruchim Y, Klement E, Saragusty J, et al. Heat stroke in dogs: A retrospective study of 54 cases (1999‐2004) and analysis of risk factors for death. J Vet Intern Med 2006;20:38–46. [DOI] [PubMed] [Google Scholar]
- 6. Eubig PA, Brady MS, Gwaltney‐Brant SM, et al. Acute renal failure in dogs after the ingestion of grapes or raisins: A retrospective evaluation of 43 dogs (1992‐2002). J Vet Intern Med 2005;19:663–674. [DOI] [PubMed] [Google Scholar]
- 7. Langston CE. Acute renal failure caused by lily ingestion in six cats. J Am Vet Med Assoc 2002;220:49–52, 36. [DOI] [PubMed] [Google Scholar]
- 8. Stokes JE, Forrester SD. New and unusual causes of acute renal failure in dogs and cats. Vet Clin North Am Small Anim Pract 2004;34:909–922, vi. [DOI] [PubMed] [Google Scholar]
- 9. Segev G, Kass PH, Francey T, et al. A novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med 2008;22:301–308. [DOI] [PubMed] [Google Scholar]
- 10. Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule‐1 (KIM‐1): A novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62:237–244. [DOI] [PubMed] [Google Scholar]
- 11. Herget‐Rosenthal S, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int 2004;66:1115–1122. [DOI] [PubMed] [Google Scholar]
- 12. Parikh CR, Jani A, Melnikov VY, et al. Urinary interleukin‐18 is a marker of human acute tubular necrosis. Am J Kidney Dis 2004;43:405–414. [DOI] [PubMed] [Google Scholar]
- 13. Segev G, Palm C, Leroy B, et al. Evaluation of neutrophil gelatinase‐associated lipocalin as a marker of kidney injury in dogs. J Vet Intern Med 2013;27:1362–1367. [DOI] [PubMed] [Google Scholar]
- 14. Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. BMC Vet Res 2012;8:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finco DR, Brown SA, Barsanti JA, et al. Reliability of using random urine samples for “spot” determination of fractional excretion of electrolytes in cats. Am J Vet Res 1997;58:1184–1187. [PubMed] [Google Scholar]
- 16. Finco DR, Tabaru H, Brown SA, et al. Endogenous creatinine clearance measurement of glomerular filtration rate in dogs. Am J Vet Res 1993;54:1575–1578. [PubMed] [Google Scholar]
- 17. Bovee KC, Joyce T. Clinical evaluation of glomerular function: 24‐hour creatinine clearance in dogs. J Am Vet Med Assoc 1979;174:488–491. [PubMed] [Google Scholar]
- 18. Finco DR, Coulter DB, Barsanti JA. Simple, accurate method for clinical estimation of glomerular filtration rate in the dog. Am J Vet Res 1981;42:1874–1877. [PubMed] [Google Scholar]
- 19. Cowgill LD, Goldfarb S, Lau K, et al. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest 1979;63:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shnnon JA, Fisher S. The renal tubular reabsorption of glucose in the normal dog. Am J Physiol 1938;122:765–774. [Google Scholar]
- 21. Segev G, Kass PH, Francey T, et al. Novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med 2007;21:599. [DOI] [PubMed] [Google Scholar]
- 22. Smithies MN, Cameron JS. Can we predict outcome in acute renal failure? Nephron 1989;51:297–300. [DOI] [PubMed] [Google Scholar]
- 23. Worwag S, Langston CE. Acute intrinsic renal failure in cats: 32 cases (1997‐2004). J Am Vet Med Assoc 2008;232:728–732. [DOI] [PubMed] [Google Scholar]
- 24. Laroute V, Chetboul V, Roche L, et al. Quantitative evaluation of renal function in healthy Beagle puppies and mature dogs. Res Vet Sci 2005;79:161–167. [DOI] [PubMed] [Google Scholar]
- 25. Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976;236:579–581. [DOI] [PubMed] [Google Scholar]
- 26. Pepin MN, Bouchard J, Legault L, et al. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007;50:566–573. [DOI] [PubMed] [Google Scholar]
- 27. Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984;36:20–23. [DOI] [PubMed] [Google Scholar]
- 28. Saha H, Mustonen J, Helin H, et al. Limited value of the fractional excretion of sodium test in the diagnosis of acute renal failure. Nephrol Dial Transplant 1987;2:79–82. [PubMed] [Google Scholar]
- 29. Lefebvre HP, Dossin O, Trumel C, et al. Fractional excretion tests: A critical review of methods and applications in domestic animals. Vet Clin Pathol 2008;37:4–20. [DOI] [PubMed] [Google Scholar]


