Abstract
Background
Ghrelin is a growth hormone secretagogue. It is a potent regulator of energy homeostasis. Ghrelin concentration is down‐regulated in humans with hypersomatotropism (HS) and increases after successful treatment. Additionally, ghrelin secretion seems impaired in human diabetes mellitus (DM).
Hypothesis
Serum ghrelin concentration is down‐regulated in cats with HS‐induced DM (HSDM) compared to healthy control cats or cats with DM unrelated to HS and increases after radiotherapy.
Animals
Cats with DM (n = 20) and with HSDM (n = 32), 13 of which underwent radiotherapy (RT‐group); age‐matched controls (n = 20).
Methods
Retrospective cross‐sectional study. Analytical performance of a serum total ghrelin ELISA was assessed and validated for use in cats. Differences in serum ghrelin, fructosamine, IGF‐1 and insulin were evaluated.
Results
Ghrelin was significantly higher (P < .001) in control cats (mean ± SD: 12.9 ± 6.8 ng/mL) compared to HSDM‐ (7.9 ± 3.3 ng/mL) and DM‐cats (6.7 ± 2.3 ng/mL), although not different between the HSDM‐ and DM‐cats. After RT ghrelin increased significantly (P = .003) in HSDM‐cats undergoing RT (from 6.6 ± 1.9 ng/mL to 9.0 ± 2.2 ng/mL) and the after RT ghrelin concentrations of HSDM cats were no longer significantly different from the serum ghrelin concentration of control cats. Serum IGF‐1 did not significantly change in HSDM‐cats after RT, despite significant decreases in fructosamine and insulin dose.
Conclusion and Clinical Importance
Ghrelin appears suppressed in cats with DM and HSDM, although increases after RT in HSDM, suggesting possible presence of a direct or indirect negative feedback system between growth hormone and ghrelin. Serum ghrelin might therefore represent a marker of treatment effect.
Keywords: Acromegaly, Cat, Insulin growth factor 1, Pituitary adenomas, Secondary diabetes
Abbreviations
- BSH
British shorthair
- CT
computed tomography
- DLH
domestic longhair
- DM
diabetes mellitus
- DSH
domestic shorthair
- ELISA
enzyme‐linked immunosorbent assay
- GH
growth hormone
- Gy
gray
- HS
hypersomatotropism
- IGF‐1
insulin growth factor 1
- RIA
radioimmunoassay
- RT
radiotherapy
- rt
room temperature
Feline hypersomatotropism (HS) is caused by a functional somatotropic adenoma or hyperplasia of the pars distalis of the anterior pituitary gland resulting in excessive growth hormone (GH) secretion.1 The diabetogenic effect of excess GH leads to diabetes mellitus (DM), which is often the initial clinical presentation. In the long run, the anabolic effects of GH‐induced insulin‐like growth factor 1 (IGF‐1) can lead to characteristic physical changes, including soft tissue growth resulting in the syndrome of acromegaly.1, 2 The diagnosis of HS remains difficult, since no single test can provide a satisfactory definitive diagnosis. Additionally, feline GH assays are not widely available commercially and therefore have currently limited value in a clinical setting despite acceptable sensitivity and specificity.5 Although the usefulness of serum IGF‐1 as a diagnostic marker for HS has been documented, false negative and false positive results do occur and positive results therefore require confirmation of a pituitary lesion with advanced imaging; this is expensive and requires heavy sedation or general anesthesia.4, 6, 7 Refinement of existing endocrine testing or development of alternative tests for HS is therefore desirable. Easier, more reliable and accessible diagnostics would facilitate earlier diagnosis of HS as the inciting cause of DM in cats and facilitate more rapid and appropriate treatment, increasing the chance for diabetic remission. Alternative endocrine tests might help establish the true efficacy of HS treatment, which is especially relevant after radiotherapy (after RT), since IGF‐1 seems an unreliable marker for this purpose, thus rendering many cats vulnerable to potentially serious iatrogenic hypoglycemia.7, 8
Although ghrelin was first identified as an endogenous ligand for the GH secretagogue receptor, it is now mostly known as an orexigenic peptide.9 Little is known about the role of ghrelin in endogenous GH‐secretion during normal physiological conditions. Nevertheless, studies in humans have shown that serum ghrelin concentrations are lower when HS is present, indicating existence of a possible direct or indirect negative feedback system between GH and ghrelin.10, 11 Additionally, serum ghrelin rises after successful surgical removal of a somatotrophinoma.10, 12 Ghrelin has also been shown to suppress insulin release and modify insulin sensitivity, processes at the heart of the etiopathogenesis of type 2 DM, which currently remains incompletely understood.13, 14 Screening for dysregulation of ghrelin secretion and correlations between ghrelin and the level of glycemic control in uncomplicated or primary feline DM (non‐HS‐induced feline DM) is therefore of additional interest, alongside its use as a control group for HS‐induced diabetic cats.
The aim of this study was to validate a serum ghrelin ELISA for use in cats and to evaluate serum ghrelin in feline HS and DM, specifically comparing serum ghrelin in cats with HS‐induced DM (HSDM), cats with uncomplicated or primary DM (DM) and healthy cats, as well as assessing the effect of radiotherapy (RT) on circulating ghrelin concentration in HSDM‐cats, compared to the traditionally used serum IGF‐1.
Material and Methods
Sample Recruitment
Residual serum was collected from samples from three groups of cats: healthy cats (control‐cats, N = 20), insulin treated cats with uncomplicated or primary DM (DM‐cats, N = 20) and cats with HSDM (HSDM‐cats, N = 32). All groups were age‐matched; DM‐cats and control‐cats were weight‐matched. From a subgroup (N = 13) of the HSDM‐cats samples were also collected before and after RT. All cats were starved for 8–10 hours before sampling and all samples were taken before insulin administration. Serum was separated through centrifugation, separated into aliquots and stored at −80°C within 1 hour of collection. Fructosamine, IGF‐1 and ghrelin were measured in samples obtained at the same time. Cats were categorized into the DM‐group on the basis of clinical signs (polyuria, polydipsia, and polyphagia), clinicopathological findings consistent with DM (persistent hyperglycemia and concurrent glycosuria, serum fructosamine concentration >350 μmol/L, serum total IGF‐1 concentration <700 ng/mL) and modest insulin requirements with adequate diabetic control at time of sampling and during follow‐up (<1.5 IU/kg/injection). All cats had been diagnosed, and administered exogenous insulin, a minimum of 1 month before sample collection and follow‐up data (minimum of 6 months) were available for all cats. Cats were categorized into the HSDM‐group on the basis of a diagnosis of DM (criteria above excluding insulin requirements condition), a serum total IGF‐1 concentration >900 ng/mL (all determined by the same RIA, Cambridge Specialist Laboratories Services, Cambridge, UK) and demonstration of a pituitary lesion on brain imaging studies (contrast‐enhanced computed tomography [CT] or magnetic resonance imaging [MRI]). In 13 HSDM cats additional serum samples were obtained after completion of hypo‐or hyper‐fractionated RT (12 cats: total dose of 37 gyay (Gy) in five once‐weekly fractions and 1 cat: total dose of 37 (Gy) in ten escalating fractions delivered three times weekly; median collection time after RT completion: 12 months; range 3–32 months, 25–75% percentile 5–14 months). The upper limit for the IGF‐1 RIA assay used was 2000 ng/mL and values above this limit were therefore reported as 2,000 ng/mL. Residual sample from 20 age‐matched cats undergoing a routine health screen were used as healthy controls; all had normal serum biochemistry (including glucose), urinalysis, and serum total thyroxine results during this health screen. Ethical approval for sample collection had been obtained previously from the Royal Veterinary College local ethical committee.
Ghrelin Assay Protocol
A competitive enzyme‐linked immunosorbent assay (ELISA) kit for Rat/Mouse Ghrelin1 , 2 was validated for this study. The use of this particular assay for the measurement of ghrelin in cats has previously been reported and is based on the similarities between rat and cat ghrelin.15, 16 The assay was conducted in accordance with the manufacturer's instructions. In brief, 15 μL of serum was added to 135 μL of assay buffer, vortexed and 50 μL transferred into wells primed with secondary antibody, followed by addition of primary antibody and biotinylated peptide. The plate was incubated for 2 hours at room temperature (rt) during orbital shaking (300–400 rpm) and then thoroughly washed using assay buffer. Streptavidin‐peroxidase enzyme was added and left for 1 hours at rt during orbital shaking followed by washing and addition of 3,3′,5,5′‐tetramethylbenzidine (TMB) substrate; the reaction was stopped after 1 hour of incubation by adding hydrochloric acid (0.5N). The color intensity was measured immediately at 450 nm.3 A standard curve was generated through dilution of provided standard recombinant rat/mouse ghrelin (concentrations: 0.01, 0.1, 1.0, 10, and 100 ng/mL). Based on duplicate measurements, concentrations were determined by multiplying the concentrations obtained from intra‐polation of the standard curve data points with the dilution factor (1 : 10).
Ghrelin Assay—Analytical Performance
The precision of the ELISA was evaluated by calculating intra‐ and inter‐assay coefficients of variation (CV). For determination of the intra‐assay CV, three serum samples from cats in the control‐ and HSDM‐group with a range of ghrelin concentrations were each determined ten times during one ELISA run. For determination of the interassay CV, three serum samples from cats from the same groups with a range of ghrelin concentrations were determined in duplicate in three ELISA runs. Separately frozen aliquots from the same residual sample were used to exclude interference from freeze–thaw cycles. CVs were expressed as a percentage and calculated by dividing the standard deviation (SD) by the mean of the measurements.
In the absence of purified feline ghrelin, the accuracy of the assay was evaluated indirectly by linearity under dilution and recovery studies. For linearity under dilution, serum from two of the control cats was serially diluted (2‐fold) using the provided assay buffer. Additionally, to exclude matrix effects, two samples with high and low concentrations of ghrelin were mixed at different ratios to create percentage dilutions of 100%, 75%, 50%, 25%, and 0%. Feline ghrelin recovery was expressed as a percentage ([measured/expected concentration] × 100) for each dilution.17 The limit of detection (LoD) was determined on the basis of 18 repeat measurements of the zero standard and defined as the mean plus three standard deviations.
Statistical Analysis
In order to evaluate sample matrix effects, linearity under dilution was assessed by ordinary linear regression analysis comparing measured and expected ghrelin concentrations.
Data were evaluated for normality using the Kolmogorov‐Smirnov test and normally distributed data were described using mean and SD, whereas non‐normally distributed data were described using median and range. Comparison of variables between DM, HSDM and control was performed using one‐way ANOVA followed by posthoc Tukey's multiple comparison test. Comparison between two groups were performed using unpaired or paired t‐test for unpaired or paired normally distributed data and the Mann‐Whitney U‐test or paired‐sample Wilcoxon signed rank test for unpaired or paired non‐normally distributed data. Additionally, Bonferroni posthoc correction was performed for multiple t‐test comparisons between paired observations (before and after RT) and controls.
Pearson (parametric) or Spearman (nonparametric) correlation analyses were used to assess for a correlation between changes in ghrelin and insulin dose, IGF‐1 and fructosamine before and after RT and for a correlation between ghrelin and fructosamine concentrations in DM and HSDM cats. P‐values of <.05 were considered statistically significant. GraphPad Software was used.4
Results
Ghrelin Assay—Analytical Performance
Linearity under serial dilution of feline samples was demonstrated with linear regression equations showing a correlation coefficient approximating 1.0 (Fig 1A). The mean recovery when mixing feline samples with high and low ghrelin concentration was 96.4 ± 6.9% (range 86–103%) (Fig 1B). The assay LoD was 0.64 ng/mL (mean 0.22 ± 0.14 ng/mL) and values below this were considered to be zero.
Figure 1.
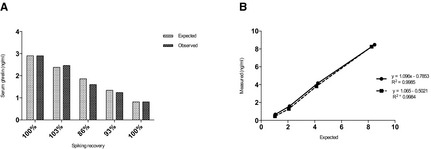
(A) Linearity under dilution of two control samples with ghrelin concentrations of 8.5 ng/mL (solid line) and 8.3 ng/mL (dashed line), respectively, diluted 100 : 0%, 50 : 50%, 25 : 75%, and 12.5 : 87.5%. (B) Spiking recovery expressed as percentage when mixing samples of high and low ghrelin concentration by single measurements of different ratios (100 : 0%; 75 : 25%; 50 : 50%; 25 : 75%; 0 : 100%). Recovery % = (ghrelinmeasured/ghrelinexpected) × 100%.
Mean intra‐CV was 8.7%, 5.1%, and 11.2% for samples with low (4.3 ng/mL), medium (5.4 ng/mL), and high (14.1 ng/mL) ghrelin concentrations, respectively, and inter‐CV was 7.8%, 8.3%, and 9.1% for a sample with low (3.2 ng/mL), medium (6.7 ng/mL) and high (10.2 ng/mL) ghrelin concentration, respectively.
Assessed Population
Mean body weight was significantly different between the three groups (P < .001). Mean body weight was not significantly different between the DM‐cats and control cats, though the bodyweight of HSDM‐cats was significantly higher than both other groups (P < .05; posthoc Tukey's multiple comparison test) (Table 1). The median insulin dose/kg in the HSDM‐group was significantly higher when compared to the DM group (P < .001, Mann‐Whitney U‐test). For the subgroup of HSDM‐cats that underwent RT (HSDM+RT), median insulin dose and mean serum fructosamine concentrations were both significantly lower after RT when compared to pretreatment values (insulin: paired‐sample Wilcoxon signed rank test, P = .01; fructosamine: paired t‐test, P = .02). The mean serum IGF‐1 concentration for the HSDM+RT‐cats was not significantly lower after RT (paired t‐test, P = .24). Insulin dose, IGF‐1, fructosamine and ghrelin concentrations for the group of HSDM‐cats that underwent RT are illustrated in Fig 2.
Table 1.
Physical characteristics, median (range) insulin dose and mean ± SD serum fructosamine and IGF‐1 concentrations of cats with diabetes mellitus (DM), cats with hypersomatotropism and diabetes mellitus (HSDM) including the subgroup of cats that underwent RT (RT) and healthy age‐matched control cats
| DM | HSDM | RT (Pre) | RT (Post) | Controls | |
|---|---|---|---|---|---|
| Number (n) | 20 | 32 | 13 | 13 | 20 |
| Age (years) | 12.1 (7–17) | 10 (6–14) | 10 (5–13) | 11 (7–13) | 11 (9–17) |
| Breed | 19 DSH/1 Abysinian | 29 DSH/2 DLH/1 BSH | 11 DSH/1 DLH/1 BSH | 18 DSH/1 DLH/1 BSH | |
| Sex | 12 MN/6 FN/2 FE | 21 MN/9 FN/2 FE | 9 MN/4 FN | 12MN/8 FN | |
| BW (kg) | 3.9 (2.7–6) | 5.5 (3.9–9.1)a | 5.8 (3.9–9.1) | 5.4 (3.9–10.1) | 4.6 (2.1–5.8) |
| Insulin dose (IU/kg/inj) | 0.8 (0.2–1.) | 2.7 (1–9.6)b | 3.2 (1.4–9.6) | 1.1 (0–4)c | NA |
| Fructosamine (μmol/L) | 495 ± 95 | 613 ± 179b | 623 ± 157 | 475 ± 174c | NA |
| IGF‐1 (ng/mL) | 257 ± 72 | 1562 ± 381 | 1597 ± 389 | 1412 ± 387 | NA |
Significant difference compared to the DM‐ and control group (ANOVA; post‐hoc Tukeys).
Significant difference compared to the DM‐group (insulin: Mann‐Whitney U‐test/fructosamine unpaired t‐test).
Significant difference compared to the before RT group (insulin: paired‐sample Wilcoxon signed rank test; fructosamine: paired t‐test).
Figure 2.
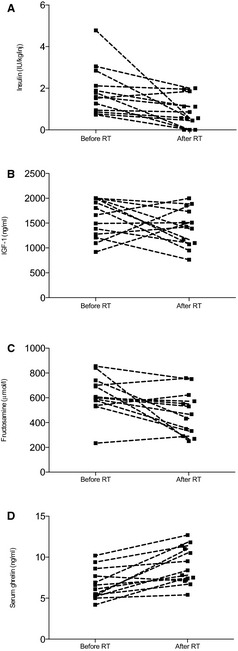
Insulin (A), IGF‐1 (B), fructosamine (C) and ghrelin (D) concentrations before and after RT for the 13 cats with HSDM that underwent RT (HSDM+RT). Dashed lines connect pairs of observations before and after RT.
Feline Ghrelin Concentrations in DM‐, HSDM‐, and Control‐Cats
The mean ± SD serum concentration of total ghrelin in the HSDM‐cats was 7.9 ± 3.3 ng/mL (range 1.7–18.3 ng/mL). The mean ± SD total ghrelin concentration in the DM‐cats was 6.7 ± 2.3 ng/mL (range 3.6–12.7 ng/mL), whereas the mean total ghrelin in the control‐cats was 12.9 ± 6.8 ng/mL (range 2.4–32.3 ng/mL). Total ghrelin concentration was significantly different between groups (ANOVA, P < .001) and posthoc Tukey's analysis identified that control cats had a significantly higher total ghrelin concentration compared to HSDM and DM cats (Fig 3).
Figure 3.
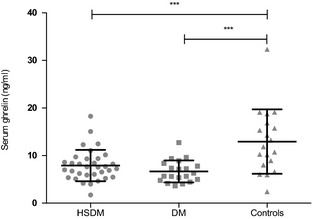
Serum ghrelin concentrations in cats with hypersomatotropism and diabetes mellitus (HSDM) (circles), cats with diabetes mellitus (DM) (squares) and healthy control cats (triangles). Mean values are indicated by long horizontal lines and standard deviations (SD) are indicated by short horizontal lines. ***Indicates a statistically significant difference in means between groups (P < .05) (ANOVA and posthoc Tukey's).
The subpopulation of HSDM‐cats that underwent RT had a mean ± SD pretreatment serum ghrelin concentration of 6.6 ± 1.8 ng/mL (range 4.2–10.2 ng/mL). The after RT total ghrelin was 9.0 ± 2.3 ng/mL (range 5.4–12.7 ng/mL), which was significantly higher (paired t‐test, P < .0008) (Fig 4). Total serum ghrelin in HSDM pre‐RT cats (P = .0028), though not in HSDM after RT cats (P = .055), was significantly lower compared to control cats (multiple t‐tests with Bonferroni posthoc significance level α = 0.025).
Figure 4.
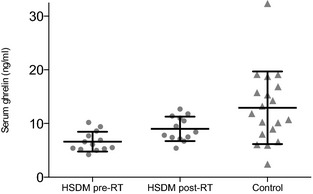
Serum ghrelin concentration in cats with hypersomatotropism and diabetes mellitus (HSDM) before (circles) and after (squares) RT versus control cats (triangles). Mean values are indicated by long horizontal lines and standard deviation (SD) are indicated by short horizontal lines. *Indicates significant difference, P = .0028 (multiple t‐tests; Bonferroni posthoc significance level α = 0.025), no significant difference was observed between post‐RT and controls (P = .055).
Changes in serum ghrelin before and after RT did not significantly correlate with changes in insulin dose pre‐ and after RT (Spearman: r = 0.36; P = .22), changes in serum IGF‐1 (Pearson: r = 0.16; P = .59) or fructosamine (Pearson: r = 0.02; P = .94). Additionally, there was no significant correlation between serum fructosamine and ghrelin in the DM‐cats (Pearson: r = −0.08; P = .074) or HSDM‐cats (Pearson: r = −0.12; P = .51).
Discussion
Serum ghrelin in cats with HSDM and cats with uncomplicated or primary DM has not previously been studied. This study suggests that, in parallel to the situation in man,10, 11, 12, 18, 19 serum ghrelin is suppressed in cats with HS‐induced DM and in cats with DM unrelated to HS. Serum ghrelin is therefore not useful as a diagnostic for determining the presence or absence of HS in the diabetic cat. However, it might prove useful as a marker for treatment effect, since serum ghrelin increased to normal following RT in cats with HSDM.
The overlapping ranges of the ghrelin concentrations in the before and after RT group might indicate a limitation to the use of ghrelin as an indicator for treatment effect and is also encountered in human acromegaly.20 Nevertheless, of all 13 assessed cats undergoing RT, 12 (92%) showed an increase in serum ghrelin, and in 11/12 this was of a magnitude greater than the detection limit of the assay. The rise in ghrelin concentrations was accompanied by improved glycemic control reflected by significant lowering of serum fructosamine and insulin dose, indicating treatment effect. Interestingly, serum IGF‐1 concentrations were not significantly changed after treatment, supporting current belief that this represents a poor indicator of treatment effect when it concerns RT for feline HS.8 Whether serum ghrelin can actually predict a fall in insulin requirements has yet to be determined and would require a future prospective study with several sample time points after RT before a decrease in insulin requirement. The latter will, however, be a difficult study to perform, since the RT effect might be seen 6 months to a year after treatment, if it will take effect at all. In humans suffering from acromegaly, IGF‐1 concentrations attenuate very slowly after RT and maximal control of the release of GH may require more than 15 years. Radiotherapy is therefore generally reserved for tumors that have recurred or persisted after surgery.21 On the other hand, successful hypophysectomy leads to an immediate reduction in GH and IGF‐1 and both parameters are therefore used to assess biochemical control of disease, as well as predict the risk of recurrence.22 This also highlights the superiority of hypophysectomy over RT in terms of HS‐treatment efficacy in humans, rather than a mere lack of sensitivity of IGF‐1 after RT. Feline case reports suggest this is also true for the hypersomatotropic cat.3, 8, 23, 24
The diurnal variation in circulating ghrelin is a topic of ongoing research in humans, with some conflicting results in terms of influences on its secretion pattern.25 Whether there is diurnal variation in feline circulating ghrelin concentrations is currently unknown. The relatively small differences between before and after radiation ghrelin concentrations could therefore also have been confounded by such unknown natural ghrelin dynamics. For instance, ghrelin is known to be influenced by meals in man.26 Nevertheless, on 24‐hour serial assessment of circulating ghrelin concentrations in humans, it was shown that samples taken before breakfast correlated strongly with 24‐hour integrated area under the curve values (r = 0.873), suggesting that a single starved morning sample could be used as a surrogate for 24‐hour profiles to estimate overall ghrelin concentrations.27 Therefore, to minimize the effects of daily ghrelin dynamics in this study, all cat samples were taken in the early morning and before food. Additionally, the reported amplitudes of ghrelin secretion pulses in humans are usually in the range of 0.5 ± 0.1 ng/mL,26 which therefore could only pose a modest confounding factor, given that the difference between the mean concentration of serum ghrelin before and after radiotherapy in our cats was almost 5‐fold greater (2.4 ng/mL). Specific feline ghrelin physiology research is nevertheless indicated to further evaluate these issues, as well as an assessment of ghrelin stability and ideal storage conditions.
The ELISA used in this study evaluated total ghrelin concentration, despite the existence of two major forms of ghrelin in the body. The octanoylated or “active” form of ghrelin known as acyl ghrelin can activate the GH secretagogue receptor.9 The major form in blood is the nonoctanoylated or “inactive” form known as desacyl ghrelin. Nevertheless, increasing numbers of studies report also biological effects of desacyl ghrelin and studies in humans have shown that both forms are suppressed in acromegaly, justifying the use of a total ghrelin ELISA.11, 28, 29 The study employed, after approriate validation, a Rat/Mouse Ghrelin ELISA for measuring feline ghrelin. The absence of commercially available (recombinant) feline ghrelin posed a limitation in the assessment of accuracy. Nevertheless, the choice of the used ELISA system was based on its previous use for this purpose16 and the fact that ghrelin has a highly conserved amino acid sequence across species. Only one amino acid difference exists between rat and feline ghrelin and synthetic rat ghrelin is able to stimulate GH secretion in cats.15 This, in combination with the serial dilution and spiking recovery data, obtained using mixtures of feline serum, suggests an adequate level of accuracy of the chosen assay.
In humans, dogs and cats, the relationship between endogenous ghrelin secretion and pituitary GH secretion is not fully elucidated.15, 30, 31, 32 Humans with acromegaly have lower ghrelin concentrations and these tend to increase after surgery in correlation with normalization of GH and IGF‐I and improved insulin sensitivity, suggesting negative feedback to occur exerted by excess GH on ghrelin secretion.10, 12, 33 Based on the before and after RT results of this study, this hypothesized negative feedback effect could also be present in the cat. The effect may be independent or could be confounded by changes in insulin secretion and sensitivity, as well as glycemic control, induced by HS and its treatment. Indeed, in humans, low ghrelin concentrations have been associated with insulin resistance and development of type 2 DM.14, 18, 28, 34 In addition, hyperinsulinemia, acute hyperglycemia and poor long‐term glycemic control in cats with DM might impair ghrelin secretion in humans.35, 36 However, although in this study the mean insulin dose and fructosamine concentration for the HSDM+RT group were significantly lower following RT, there was no significant correlation between the changes in serum ghrelin concentration and the changes in fructosamine or insulin dose. Additionally, no correlations could be documented between fructosamine and ghrelin in the entire DM and HSDM cat populations.
In order to prevent inclusion of hypersomatotropic cats in the DM group, only cats with serum total IGF‐1 concentration <700 ng/mL and modest insulin requirements with adequate diabetic control at time of sampling were included. Additionally, these cats showed no evidence of insulin resistance during a minimum of 6 months follow‐up. At time of IGF‐1 determination, all cats had been diagnosed with DM and had been started on exogenous insulin for a minimum of 1 month, making a false negative IGF‐1 result, because of the inhibitory effect of portal insulin deficiency on hepatic IGF‐1 production unlikely.37
Low fasting ghrelin concentrations have been consistently associated with obesity independent of the presence of type 2 DM in people and negative feedback from excess leptin produced by the numerous adipocytes has been implicated in this process.38, 39, 40, 41, 42 In contrast, a previous study in obese and lean cats found that fasting serum acylated ghrelin concentrations were in fact slightly higher in an obese state (though the mean difference was only 9 pg/mL).43 Nevertheless, because of such possible impact of obesity or body condition on ghrelin, the DM‐group and control cats in this study were weight‐matched; additionally, none were classified as obese by the attending clinician; furthermore, average body weight in these two groups was not strongly suggestive of underdiagnosed obesity. Body condition scores or DEXA scan results were unfortunately not available for these two groups. The HSDM‐group cats were not weight‐matched since weight gain and a greater stature formed part of the pathogenesis of the disease in many of this group, as evidenced by the significantly higher body weights compared to the DM‐ and control‐cats. Nevertheless, the relationship between ghrelin concentration and weight seen in the human nonacromegaly population has been documented to be absent in human patients with acromegaly.10 Additionally, body condition scores were available for the feline HSDM group and did not indicate presence of obesity in any of the 32 HSDM cats. Body condition scores and weight did not significantly change after RT in the assessed subpopulation of HSDM cats undergoing this treatment, rendering it unlikely that the detected significant increase in serum ghrelin after RT was caused by a change in body condition or body fat.
In conclusion, the analytical performance of a rat and mouse ghrelin ELISA was assessed to be appropriate in the cat with satisfactory inter‐ and intra‐assay precision, linearity under dilution and recovery studies. This study also suggests that in cats with HSDM and cats with DM serum ghrelin is suppressed compared to healthy cats. Serum ghrelin increased significantly following successful RT in cats with HSDM to a level that was comparable to control cats, highlighting the potential existence of a direct or indirect negative feedback system between ghrelin and GH. Similar to the situation in human DM, the role of ghrelin in feline DM remains to be elucidated. Finally, further studies are necessary to investigate the exact utility of serum ghrelin as a diagnostic tool to assess RT effect in HSDM, whereas IGF‐1 has been confirmed to be insensitive for this purpose.
Acknowledgments
The authors thank the RVC Clinical Investigations Centre team for their help in sample collection and the radiotherapy department at VRCC, London, UK for providing radiotherapy on some of the animals. This study was supported by a grant kindly awarded by PetPlan Charitable Trust.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
This research was performed at the Department of Clinical Sciences and Services of The Royal Veterinary College.
Previously presented as part of an abstract at the 22nd European College of Veterinary Internal Medicine‐Companion Animals (ECVIM‐CA) Congress in Maastricht, September 6–8, 2012, The Netherlands and published as an abstract in Journal of Veterinary Internal Medicine 2012; 26(1).
Footnotes
The Peoples Dispensary for Sick Animals, Bow and Beaumont Sainsbury Animal's Hospital, Camden
Ghrelin (Rat Mouse) EIA Kit (range 0–100 μg/mL), Phoenix Pharmaceuticals Inc, Belmont, CA
Bio‐Tek EL808 8 channel absorbance reader, Potton, UK
GraphPad Prism version 5.0a for Windows, GraphPad Software, San Diego, CA
References
- 1. Feldman EC, Nelson RW. Feline acromegaly In: Feldman EC, Nelson RW, ed. Disorders of Growth Hormone. Canine and Feline Endocrinology and Reproduction, 3rd ed Philadelphia, PA: WB Saunders; 2004:69–84. [Google Scholar]
- 2. Niessen SJM, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Animal Pract 2013;43:319–350. [DOI] [PubMed] [Google Scholar]
- 3. Niessen SJM, Petrie G, Gaudiano M, et al. Feline acromegaly: An underdiagnosed endocrinopathy? J Vet Intern Med 2007;21:899–905. [DOI] [PubMed] [Google Scholar]
- 4. Berg RIM, Nelson RW, Feldman EC, et al. Serum insulin‐like growth factor‐I concentration in cats with diabetes mellitus and acromegaly. J Vet Int Med 2007;21:892–898. [DOI] [PubMed] [Google Scholar]
- 5. Niessen SJM, Khalid M, Petrie G, Church DB. Validation and application of a radioimmunoassay for ovine growth in the diagnosis of acromegaly in cats. Vet Rec 2007;160:902–907. [DOI] [PubMed] [Google Scholar]
- 6. Tschuor F, Zini E, Schellenberg S, et al. Evaluation of four methods used to measure plasma insulin‐like growth factor‐I concentrations in healthy cats and cats with diabetes mellitus or other diseases. Am J Vet Res 2012;73:1925–1931. [DOI] [PubMed] [Google Scholar]
- 7. Niessen S. Feline acromegaly: An essential differential diagnosis for the difficult diabetic. J Feline Med Surg 2010;2:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunning MD, Lowrie CS, Bexfield NH, et al. Exogenous insulin treatment after hypofractionated radiotherapy in cats with diabetes mellitus and acromegaly. J Vet Int Med 2009;23:243–249. [DOI] [PubMed] [Google Scholar]
- 9. Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 10. Freda PU, Reyes CM, Conwell IM, et al. Serum ghrelin levels in acromegaly: Effects of surgical and long‐acting octreotide therapy. J Clin Endocrinol Metabol 2003;88:2037–2044. [DOI] [PubMed] [Google Scholar]
- 11. Kawamata T, Inui A, Hosoda H, et al. Perioperative plasma active and total ghrelin levels are reduced in acromegaly when compared with in nonfunctioning pituitary tumours even after normalization of serum GH. Clin Endocrinol 2007;67:140–144. [DOI] [PubMed] [Google Scholar]
- 12. Cappiello V, Ronchi C, Morpurgo PS, et al. Circulating ghrelin levels in basal conditions and during glucose tolerance test in acromegalic patients. Eur J Endocrinol 2002;147:189–194. [DOI] [PubMed] [Google Scholar]
- 13. Gelling RW, Overduin J, Morrison CD, et al. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin‐induced feeding. Endocrinol 2004;145:4575–4582. [DOI] [PubMed] [Google Scholar]
- 14. Yada T, Dezaki K, Sone H, et al. Ghrelin regulates insulin release and glycemia: Physiological role and therapeutic potential. Curr Diabetes Rev 2008;4:18–23. [DOI] [PubMed] [Google Scholar]
- 15. Ida T, Miyazato M, Naganobu K, et al. Purification and characterization of feline ghrelin and its possible role. Domest Anim Endocrinol 2007;32:93–105. [DOI] [PubMed] [Google Scholar]
- 16. Belsito KR, Vester BM, Keel T, et al. Impact of ovariohysterectomy and food intake on body composition, physical activity, and adipose gene expression in cats. J Anim Sci 2009;87:594–602. [DOI] [PubMed] [Google Scholar]
- 17. Jensen A, Kjeldgaard‐Hansen M. Diagnostic Test Validation In: Weiss D, ed. Schalm's Veterinary Hematology, 6th ed Oxford, UK, IA: Blackwell Publishing; 2010:1027–1033. [Google Scholar]
- 18. Soriano‐Guillén L, Barrios V, Lechuga‐Sancho A, et al. Response of circulating ghrelin levels to insulin therapy in children with newly diagnosed type I diabetes mellitus. Pediatr Res 2004;55:830–835. [DOI] [PubMed] [Google Scholar]
- 19. Pöykkö SM, Kellokoski E, Hörkkö S, et al. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 2003;52:2546–2553. [DOI] [PubMed] [Google Scholar]
- 20. Roemmler J, Otto B, Steffen B, et al. Serum leptin and ghrelin levels in active and inactive acromegalic patients during an oral glucose test. Exp Clin Endocrinol Diabetes 2009;117:135–141. [DOI] [PubMed] [Google Scholar]
- 21. Jenkins PJ, Bates P, Carson MN, et al. Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin‐like growth factor‐I in patients with acromegaly. J Clin Endocrinol Metab 2006;91:1239–1245. [DOI] [PubMed] [Google Scholar]
- 22. Barkan AL. Biochemical markers of acromegaly: GH vs IGF‐I. Growth Horm IGF Res 2004;14:S97–S100. [DOI] [PubMed] [Google Scholar]
- 23. Meij BP, Auriemma E, Grinwis G, et al. Successful treatment of acromegaly in a diabetic cat with transsphenoidal hypophysectomy. J Feline Med Surg 2010;12:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brearley MJ, Polton GA, Littler RM, Niessen SJM. Coarse fractionated radiation therapy for pituitary tumours in cats: A retrospective study of 12 cases. Vet Comp Oncol 2006;4:209–217. [DOI] [PubMed] [Google Scholar]
- 25. Sofer S, Eliraz A, Kaplan S, et al. Changes in daily leptin, ghrelin and adiponectin profiles following a diet with carbohydrates eaten at dinner in obese subjects. Nutr Metab Cardiovasc Dis 2013;23:744–750. [DOI] [PubMed] [Google Scholar]
- 26. Natalucci G, Riedl S, Gleiss A, et al. Spontaneous 24‐h ghrelin secretion pattern in fasting subjects: Maintenance of a meal‐related pattern. Eur J Endocrinol 2005;152:845–850. [DOI] [PubMed] [Google Scholar]
- 27. Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714–1719. [DOI] [PubMed] [Google Scholar]
- 28. Kojima M, Kangava K. Ghrelin: Structure and function. Physiol Rev 2005;85:495–522. [DOI] [PubMed] [Google Scholar]
- 29. Bedendi I, Alloatti G, Marcantoni A, et al. Cardiac effects of ghrelin and its endogenous derivatives des‐octanoyl ghrelin and des‐Gln14‐ghrelin. Eur J Pharmacol 2003;476:87–95. [DOI] [PubMed] [Google Scholar]
- 30. Peino R, Baldelli R, Rodriguez‐Garcia J, et al. Ghrelin‐induced growth hormone secretion in humans. Eur J Endocrinol 2000;143:R11–R14. [DOI] [PubMed] [Google Scholar]
- 31. Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin stongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 2000;85:4908–4911. [DOI] [PubMed] [Google Scholar]
- 32. Bhatti SFM, De Vliegher SP, Mol JA, et al. Ghrelin‐stimulation test in the diagnosis of canine pituitary dwarfism. Res Vet Sci 2006;81:24–30. [DOI] [PubMed] [Google Scholar]
- 33. Kozakowski J, Rabijewski M, Zgliczyński W. Decrease in serum ghrelin levels in patients with acromegaly normalize after successful surgical treatment. Endokrynol Polska 2005;56:862–870. [PubMed] [Google Scholar]
- 34. Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 2008;118:239–249. [DOI] [PubMed] [Google Scholar]
- 35. Saad MF. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab 2002;87:3997–4000. [DOI] [PubMed] [Google Scholar]
- 36. Briatore L, Andraghetti G, Cordera R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur J Endocrinol 2003;149:403–406. [DOI] [PubMed] [Google Scholar]
- 37. Reusch CE, Kley S, Casella M, et al. Measurements of growth hormone and insulin‐like growth factor 1 in cats with diabetes mellitus. Vet Rec 2006;158:195–200. [DOI] [PubMed] [Google Scholar]
- 38. Ueno H, Shiiya T, Mizuta M, et al. Plasma ghrelin concentrations in different clinical stages of diabetic complications and glycemic control in Japanese diabetics. Endocrin J 2007;54:895–902. [DOI] [PubMed] [Google Scholar]
- 39. McLaughlin T. Plasma ghrelin concentrations are decreased in insulin‐resistant obese adults relative to equally obese insulin‐sensitive controls. J Clin Endocrinol Metab 2004;89:1630–1635. [DOI] [PubMed] [Google Scholar]
- 40. Appleton DJ, Rand JS, Sunvold GD. Plasma leptin concentrations are independently associated with insulin sensitivity in lean and overweight cats. J Feline Med Surg 2002;4:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erdmann J, Lippl F, Wagenpfeil S, et al. Differential association of basal and postprandial plasma ghrelin with leptin, insulin and type II diabetes. Diabetes 2005;54:1371–1378. [DOI] [PubMed] [Google Scholar]
- 42. Krzyzanowska‐Świniarska B , Kempa A , Miazgowski T, et al. Serum acylated ghrelin adiponectin and leptin levels in normal‐weight and obese menopausal women. Horm Metab Res 2007;39:835–839. [DOI] [PubMed] [Google Scholar]
- 43. Martin LJ, Siliart B, Lutz TA, et al. Postprandial response of plasma insulin, amylin and acylated ghrelin to various test meals in lean and obese cats. Br J Nutr 2010;103:1610–1619. [DOI] [PubMed] [Google Scholar]


