Abstract
Background
Currently, functional assessment to monitor therapeutic response in feline lower airway disease (FLAD) has limited application.
Objectives
To evaluate if expiratory indices derived from pseudo‐tidal breathing flow‐volume loop (pTBFVL) representing lower airway obstruction would decrease after clinical improvement and to investigate the correlation between functional phenotype and inflammatory cell type in bronchoalveolar lavage (BAL) fluid.
Animals
Nineteen client‐owned cats with FLAD.
Methods
Prospective observational study. Functional assessment with pTBFVL indices (eg, peak to mid‐expiratory flow; PEF/EF50) and conventional barometric whole body plethysmography (BWBP) parameters (eg, enhanced pause) was carried out before receiving treatment. BAL was performed to analyze inflammatory cell types. Signs were assessed by scoring. The cats were treated with glucocorticoids daily and functional testing was repeated.
Results
Loop indices PEF/EF50 and PEF/EF25 were significantly decreased after treatment (P < .001). Conventional BWBP parameters were not significantly different before and after treatment. Cats with PEF/EF50 > 1.51 before treatment had a significantly higher granulocyte (eosinophil plus neutrophil) percentage in BAL fluid (P = .014). Granulocyte percentage in BAL fluid was strongly correlated with PEF/EF25 (P = .001, r s = 0.74) and moderately correlated with PEF/EF50 (P = .022, r s = 0.57), whereas eosinophil or neutrophil percentage alone had no significant correlation with functional parameters.
Conclusions and Clinical Importance
Functional parameters including PEF/EF50 and PEF/EF25 can be used for monitoring therapeutic response. The presence of airflow limitation during mid‐ to late expiration is affected by the overall extent of granulocyte infiltration.
Keywords: Asthma, Barometric whole body plethysmography, Chronic bronchitis, Pulmonary function test
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BWBP
barometric whole body plethysmography
- CB
chronic bronchitis
- COPD
chronic obstructive pulmonary disease
- EF25, 50, or 75
expiratory flow at end tidal volume plus 25, 50, and 75% tidal volume
- FLAD
feline lower airway disease
- IF25, 50 or 75
inspiratory flow at end tidal volume plus 25, 50 and 75% tidal volume
- PEF
peak expiratory flow
- Penh
enhanced pause
- PFT
pulmonary function test
- PIF
peak inspiratory flow
- pTBFVL
pseudo‐tidal breathing flow‐volume loop
- TNCC
total nucleated cell count
Feline lower airway disease (FLAD) is a common chronic respiratory disorder in small animal clinical medicine. The disease was estimated to affect 1–5% of the cat population,1 but no reliable prevalence data have been reported in recent years. In humans, the prevalence of chronic airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) is increasing worldwide compared with the last decade, probably in association with air pollution or for unknown reasons.2, 3, 4 The same trend also may be observed in companion animals because of shared environmental factors.5 Pulmonary function testing (PFT) could identify airflow obstruction, provide quantitative assessment, and help predict prognosis in chronic airway diseases.2, 6
Airway sampling such as bronchoalveolar lavage (BAL) often is considered the gold standard for identifying the presence of inflammation and indicating glucocorticoid treatment in FLAD.5 Therapeutic response is mostly determined by subjective observation of clinical signs because of the lack of a noninvasive monitoring tool. Nevertheless, subclinical inflammation despite high‐dose glucocorticoid therapy was reported in 1 study using serial BAL, which raised the question of whether or not tapering glucocorticoid therapy based on the resolution of clinical signs is sufficient.7
Feline lower airway disease currently is believed to consist of various noninfectious airway inflammatory disorders, including asthma and chronic bronchitis (CB).1, 5 In recent years, discriminating asthma from CB has drawn much attention because the 2 diseases may have different diagnostic criteria, therapeutic approaches, and prognoses.1, 5, 8 Eosinophilic inflammation in BAL fluid usually is thought to indicate asthma, whereas nonseptic neutrophilic inflammation is associated with CB.1, 7, 8, 9, 10, 11, 12 This classification is somewhat questionable, however, because the role of mixed inflammation or nonuniform inflammation in different lung segments is not clear at present.5, 10 Additional information on pulmonary function could enhance understanding of these airway diseases and guide future classification.
Noninvasive PFT with a barometric whole body plethysmography (BWBP) system is well‐tolerated in conscious cats and suitable for repeated functional measurements. Although decreased airway responsiveness after treatment with steroids or bronchodilators could be evaluated by bronchoprovocation testing in experimental cats,13, 14 bronchospasm at presentation prohibits further provocative stimulation15 and limits the ability to compare the provocative index before and after treatment in client‐owned cats. Aside from conventionally measured BWBP parameters and airway responsiveness testing, breathing pattern depicted by pseudoflow and pseudovolume may indirectly estimate respiratory mechanics during tidal breathing.16, 17 The occurrence of small airway obstruction in cats with FLAD can result in substantial airflow limitation in the mid‐ to late‐expiratory phase, and a peak to midexpiratory flow ratio >1.51 identifies this obstructive pattern during tidal breathing.17 We believe that with clinical improvement after treatment, respiratory function should also show improvement. In this study, we hypothesized that expiratory indices representing lower airway obstruction would decrease after clinical improvement. In addition, the correlation between inflammatory cell type in BAL fluid and functional parameters was investigated.
Materials and Methods
Animals and Study Design
Cats with respiratory signs were screened for inclusion in the study. Diagnostic and therapeutic plans were determined according to individual cat's condition and the owner's willingness to participate. Cats were considered eligible for the study if FLAD was diagnosed. Diagnosis of FLAD was based on both clinical signs and BAL analysis indicating eosinophilic, neutrophilic or mixed inflammatory lower airway disease. If the owners refused BAL, a clinical diagnosis of FLAD was assigned according to the following findings: (1) a history of chronic (>2 months) coughing, (2) episodes of repeated, but spontaneous recovery of, coughing or respiratory distress (ie, disease evidence of reversibility), (3) radiographic evidence of bronchial or bronchointerstitial disease pattern, (4) history or subsequent finding of a response to glucocorticoid treatment, (5) no clinical signs suggestive of upper airway infection, (6) lack of pleural space disease or congestive heart failure (eg, pneumothorax, pleural effusion, pulmonary edema, enlarged left atrium) based on radiographic or ultrasonographical findings or both, and (7) no suspicion of pneumonia or infectious etiology (eg, increased body temperature, leukocytosis, alveolar infiltration on thoracic radiographs, or other systemic signs). A questionnaire about historical information was filled out and a consent form was signed by the owners and described the purpose, need for long‐term follow‐up, and noninvasive nature of the subsequent functional assessment study. Clinical data were recorded, including breed, age, body weight, body condition score (BCS), physical examination findings and other clinical findings. Historical information obtained from the owners included specific signs observed in the cat, the time when the signs were first noted, frequency and extent of signs such as cough, the timing of signs (ie, at rest or after exercise), presence of increased respiratory effort, exercise tolerance, lifestyle, possible risk factors such as environmental hazard (eg, passive tobacco smoke exposure), previous diagnosis or treatment, treatment response, and other related history. All cats had a CBC, serum biochemistry profile, and thoracic radiograph performed. Cats then were prospectively evaluated, including changes of clinical signs, general condition and other associated information. PFT was assessed and recorded before any treatment. BAL then was performed on the study cats if no glucocorticoids or antibiotics had been prescribed recently. The cats were scheduled to be treated daily with glucocorticoids for a minimum of 3 weeks and have PFT reevaluated while still receiving their initial dose of glucocorticoids at the time of re‐assessment.7, 18 Prednisolone (1.2–2.0 mg/kg/daily PO) was the preferred choice of glucocorticoid therapy. If there was a contraindication or owner concern about oral prednisolone use, inhaled fluticasone (150–250 μg, q12h) was prescribed and delivered with a commercial spacing chamber.18, 19, 20 If concern about possible slow‐onset of the inhaled form of glucocorticoid existed, cats receiving inhaled fluticasone were scheduled to be treated for a minimum of 6 weeks before PFT re‐assessment. Bronchodilators were not routinely prescribed, but owners were allowed to give terbutaline (0.42–0.625 mg PO) or inhaled salbutamol (100 μg) for several doses if the cat had severe respiratory distress or exacerbation of clinical signs. Whenever pretreatment PFT and BAL results were available or the scheduled treatment could be consistently administered with a post‐treatment PFT result available at the scheduled time, cats were included in the final analysis. Cats were excluded if glucocorticoids, immunosuppressive drugs, or bronchodilators were given within 1 month before functional assessment, if clinically relevant upper airway obstruction with excessive inspiratory effort was present, or if other life‐threatening conditions (eg, persistent respiratory distress, fever, hypothermia, other severe systemic signs) were present.
Functional Assessment
Respiratory function was assessed by a noninvasive BWBP system (Barometric whole‐body plethysmograph).1 The pressure signal of the system was calibrated before each measurement by dynamic injection of 50 mL air into the chamber. Under quiet tidal breathing, the pseudotidal breathing flow‐volume loop (pTBFVL) and conventionally‐used BWBP parameters were acquired at the same time as described elsewhere.17, 21, 22, 23 Artifactual waveforms caused by sniffing, vocalization, and body movements were automatically excluded and manually eliminated by the rejection setting of the commercial software (BioSystem XA 2.11.0 software1) and simultaneous visual inspection, respectively. Indices of pTBFVL were obtained as previously described.17 Briefly, the points that represented peak inspiratory and expiratory flow (PIF and PEF) and expiratory or inspiratory flow at end tidal volume plus 75%, 50% and 25% tidal volume (EF75, IF75, EF50, IF50, EF25, IF25) on a pTBFVL loop were identified, and ratio forms of the indices were used in the study, including PEF/PIF, PEF/EF50, PEF/EF25, EF50/EF25, PIF/IF50, PIF/IF25, IF50/IF25, EF75/IF75, EF50/IF50 and EF25/IF25. Conventional BWBP parameters were recorded from the commercial software, including respiratory rate (RR; breaths/min), tidal volume (TV; mL), minute volume (MV; mL), inspiratory and expiratory time (T i and T e; s), peak inspiratory and expiratory flow (PIF and PEF; mL/s), relaxation time (RT; s; time point when 65% of tidal volume is expired), end‐inspiratory and expiratory pause (EIP and EEP; ms), pause (PAU; unitless; [T e − RT]/RT), and enhanced pause (Penh; unitless; [PEF/PIF] × PAU).
Bronchoalveolar Lavage
Bronchoalveolar lavage was performed using a blind technique as previously described.7, 12, 24, 25 Aseptic technique was applied during the procedure to minimize oropharyngeal and other possible contamination. Cats were anesthetized (propofol at an induction dosage of 2–6 mg/kg IV followed by 0.1–0.4 mg/kg/min as a continuous rate infusion) and carefully intubated with a sterile endotracheal tube. Lateral recumbency was used to aid in retrieving samples from the dependent side, and both left and right sides were sampled unless the cat was not stable. A sterile 6 or 8 French gauge polyvinylchloride pediatric feeding tube then was passed through the endotracheal tube and wedged in a distal airway. One to 2 aliquots of 6–12 mL (approximately 2 mL/kg) warm sterile saline was instilled into the lung lobe and then aspirated immediately to obtain representative samples with surfactant. BAL fluid (BALF) was analyzed immediately after collection. Four aliquots of 0.5–1.0 mL BALF were submitted for microbiological examination, including semiquantitative aerobic and anaerobic bacterial culture, Mycoplasma culture, and fungal culture. Total nucleated cell count (TNCC/μL) was determined on undiluted and unfiltered BALF with a hemocytometer. Differential cell counts (%) were performed on BALF cytospin preparations with Liu's and Giemsa stain. At least 200 cells per sample were counted. Eosinophilic inflammation was defined as ≥17% eosinophils, neutrophilic inflammation as ≥7% neutrophils, and mixed inflammation as ≥17% eosinophils and ≥7% neutrophils.12
Statistical Analysis
Data were examined for Gaussian distribution by the Shapiro‐Wilk test and presented as mean ± SD for normally distributed data or median with interquartile range (IQR) for nonparametric data. Statistical analysis was processed by commercial software.2 A scoring system described in previous studies was used to semiquantify the observed clinical signs and radiographic abnormalities,15, 17 and clinical scores were given based on historical and physical examination findings before statistical analysis (Table 1). Pre‐ and post‐treatment clinical scores and functional parameters were compared with the Wilcoxon signed rank test. To investigate the clinical outcomes among different inflammatory types and functional phenotypes, BALF eosinophil percentage and functional parameter pTBFVL PEF/EF50 were used for assignment of subgroups. To be comparable with a previous study,15 inflammatory cell types were further assigned based on the presence of ≥17% BALF eosinophils or not (eosinophilic or noneosinophilic bronchitis). Functional phenotypes were categorized into pTBFVL PEF/EF50 > 1.51 or not based on a previously described indicator for the concave expiratory pattern in FLAD.17 Mann–Whitney rank sum test was used for comparison of body weight, BCS, age, clinical data and functional parameters among subgroups. Correlation between BALF and functional parameters was analyzed by Pearson's and Spearman's correlation. In the correlation analysis, the logarithmic value of Penh was calculated and used. The level of significance was defined as P < .05.
Table 1.
Details of the clinical and radiographic score system used in this study
| 12‐point clinical score | |
| Cough frequencya | 0 = absent (no coughing noted at all) |
| 1 = occasional (interval longer than a month) | |
| 2 = infrequent (longer than a week, but occurred at least once a month) | |
| 3 = frequent (longer than every other day, but occurred at least once a week) | |
| 4 = intensive (once daily or every other day) | |
| 5 = very intensive (several times daily) | |
| Respiratory distressb | 0 = absent |
| 1 = present only after exertion or stress | |
| 2 = moderate when breathing difficulties had been observed more than once at home | |
| 3 = severe if present permanently at rest | |
| Thoracic auscultationb | 0 = no abnormal sounds |
| 1 = increased respiratory sounds | |
| 2 = abnormal sounds at thoracic auscultation such as crackles/wheezes | |
| General conditionb | 0 = normal attitude/appetite |
| 1 = lethargic/reduced appetite | |
| 2 = unresponsive/anorexic | |
| 10‐point radiographic scoreb | |
| Bronchial pattern | 0 = absent, 1 = mild, 2 = moderate, 3 = severe |
| Interstitial pattern | 0 = absent, 1 = mild, 2 = moderate, 3 = severe |
| Alveolar infiltration | 0 = absent, 1 = focal, 2 = generalized |
| Lung hyperinflation/hyperlucency | 0 = absent, 1 = presence |
| Lobar atelectasis | 0 = absent, 1 = presence |
Results
Nineteen client‐owned cats were enrolled for the final analysis. Breeds included domestic shorthair (12), Persian (3), Russian Blue (2), domestic longhair (1), and Ragdoll (1). Mean age was 5.3 ± 3.2 years (range, 7.5 months to 11 years) and mean body weight was 4.9 ± 1.0 kg (range, 3.1–6.8 kg). Median 9‐point BCS was 6 (interquartile range [IQR], 5–6). The duration of FLAD clinical signs ranged from 2 to 60 months with a median of 15 months (IQR, 6–31.5 months). The most common clinical sign observed by the owners was cough and median cough frequency score was 4 (IQR, 3–5). Only 4 cats showed respiratory distress after exertion or stress (2/19) or had breathing difficulties observed more than once at home (2/19; median respiratory distress score, 0; IQR, 0–0). Median thoracic auscultation score was 2 (IQR, 0.5–2). All of the cats had normal activity and appetite and were assigned a general condition score of 0. Mean value of the 12‐point cumulative clinical score was 5.3 ± 2.3 (range, 2–9). The most common thoracic radiographic abnormality was a bronchointerstitial pattern, and mean of the 10‐point radiographic score was 2.9 ± 1.1 (range, 1–5). Baseline functional assessment data are shown in Table 2. BAL was performed in 16 cats. Inflammatory cell types in BALF were mixed in 9/16, eosinophilic in 4/16, and neutrophilic in 3/16 of the study cats. On further classification by eosinophil percentage in BALF,15 there were 13/16 eosinophilic and 3/16 noneosinophilic bronchitis cats. Follow‐up period ranged from 6 months to 3 years.
Table 2.
The baseline functional assessment data in 19 FLAD cats
| Variable | Median (IQR) |
|---|---|
| pTBFVL indices | |
| PEF/PIF | 0.88 (0.77–1.12) |
| PEF/EF50 | 1.55 (1.28–1.79) |
| PEF/EF25 | 2.25 (1.75–2.48) |
| EF50/EF25 | 1.28 (1.18–1.40) |
| PIF/IF50 | 1.12 (1.09–1.17) |
| PIF/IF25 | 1.24 (1.17–1.40) |
| IF50/IF25 | 1.16 (1.05–1.20) |
| EF75/IF75 | 0.90 (0.74–1.08) |
| EF50/IF50 | 0.68 (0.60–0.77) |
| EF25/IF25 | 0.61 (0.56–0.67) |
| Conventional BWBP parameters | |
| RR (cycles/min) | 50 (45–63) |
| TV/BW (mL/kg) | 5.3 (4.5–6.3) |
| MV/BW (mL/kg) | 272.5 (224.3–322.0) |
| T i (s) | 0.49 (0.38–0.55) |
| T e (s) | 0.76 (0.64–0.82) |
| PIF/BW (mL/s/kg) | 16.5 (13.5–19.0) |
| PEF/BW (mL/s/kg) | 13.9 (11.4–17.7) |
| RT (s) | 0.35 (0.29–0.39) |
| EIP (ms) | 24.1 (22.9–26.1) |
| EEP (ms) | 25.7 (11.6–61.5) |
| PAU (unitless) | 1.11 (0.89–1.23) |
| Penh (unitless) | 1.00 (0.69–1.18) |
FLAD, feline lower airway disease; and IQR, interquartile ranges.
Data are presented as median with IQR.
The prescheduled treatment plan with post‐treatment PFT was completed in 15 cats. All cats responded to either oral prednisolone (14) or inhaled fluticasone (1) treatment, with significantly decreased cough frequency score (P < .001) and thoracic auscultation score (P = .001) at the time of functional assessment re‐evaluation. Median pre‐ and post‐treatment 12‐point cumulative clinical scores were 5 (IQR, 3–7) and 0 (IQR, 0–1), respectively. Indices of pTBFVL were significantly different before and after treatment, including PEF/EF50 (P = .001), PEF/EF25 (P = .001) and PIF/IF50 (P = .022; Table 3). None of the conventionally‐used BWBP parameters were found to be significantly different before and after treatment.
Table 3.
Clinical scores and functional parameters before and after treatment in 15 cats
| Variable | Pretreatment | Post‐treatment | P‐Value |
|---|---|---|---|
| 12‐point cumulative clinical score | 5 (3–7) | 0 (0–1) | |
| Cough frequency score | 4 (3–5) | 0 (0–0) | <.001 |
| Respiratory distress score | 0 (0–1) | 0 (0–0) | .75 |
| Thoracic auscultation score | 2 (0–2) | 0 (0–0) | .002 |
| General condition score | 0 (0–0) | 0 (0–0) | 1.0 |
| pTBFVL indices | |||
| PEF/PIF | 0.93 (0.76–1.18) | 0.83 (0.70–0.91) | .055 |
| PEF/EF50 | 1.63 (1.44–1.83) | 1.27 (1.15–1.38) | .001 |
| PEF/EF25 | 2.28 (1.76–2.57) | 1.60 (1.42–2.00) | .001 |
| EF50/EF25 | 1.28 (1.17–1.52) | 1.26 (1.21–1.49) | .60 |
| PIF/IF50 | 1.14 (1.09–1.20) | 1.08 (1.06–1.11) | .022 |
| PIF/IF25 | 1.28 (1.16–1.40) | 1.26 (1.14–1.41) | .39 |
| IF50/IF25 | 1.17 (1.05–1.21) | 1.16 (1.08–1.23) | .89 |
| EF75/IF75 | 0.91 (0.73–1.12) | 0.85 (0.69–0.96) | .095 |
| EF50/IF50 | 0.68 (0.58–0.77) | 0.72 (0.61–0.78) | .68 |
| EF25/IF25 | 0.60 (0.53–0.71) | 0.62 (0.53–0.72) | .14 |
| Conventional BWBP parameters | |||
| RR (cycles/min) | 49 (44–58) | 51 (35–62) | .76 |
| TV/BW (mL/kg) | 5.3 (4.4–6.2) | 5.8 (4.3–7.0) | .76 |
| MV/BW (mL/kg) | 272.5 (204.2–339.1) | 293.2 (191.6–330.1) | .85 |
| T i (s) | 0.49 (0.40–0.55) | 0.48 (0.41–0.64) | .72 |
| T e (s) | 0.76 (0.65–0.83) | 0.77 (0.67–0.98) | .56 |
| PIF/BW (mL/s/kg) | 16.1 (13.1–19.2) | 16.4 (13.6–18.6) | 1.0 |
| PEF/BW (mL/s/kg) | 14.4 (11.6–17.8) | 12.5 (10.3–15.4) | .14 |
| RT (s) | 0.35 (0.31–0.39) | 0.38 (0.30–0.45) | .33 |
| PAU (unitless) | 1.12 (0.88–1.28) | 1.01 (0.78–1.32) | .39 |
| Penh (unitless) | 1.10 (0.64–1.21) | 0.77 (0.60–1.46) | .39 |
PEF, peak expiratory flow; PIF, peak inspiratory flow; RR, respiratory rate; TV, tidal volume; MV, minute volume; BW, barometric whole; PAU, pause; Penh, enhanced pause; and IQR, interquartile ranges.
Data are presented as median with IQR.
Bolded values indicating two‐tailed P < .05.
Clinical outcomes were compared among subgroups of inflammatory cell types and functional phenotypes, respectively. Signalment, duration of clinical signs, pretreatment clinical and radiographic scores, Penh, log Penh, pTBFVL PEF/EF50 and PEF/EF25 were not different between eosinophilic and noneosinophilic bronchitis cats (Table 4). Post‐treatment clinical scores between cats with eosinophilic and noneosinophilic bronchitis also were not significantly different.
Table 4.
Signalment, clinical scores, and functional parameters between eosinophilic and noneosinophilic bronchitis in 16 cats
| Variable | Eosinophilic Bronchitis (n = 13) | Noneosinophilic Bronchitis (n = 3) | P‐Value |
|---|---|---|---|
| Age (years) | 5.0 (1.7–6.5) | 5.0 (4.0–8.0) | .54 |
| Body weight (kg) | 4.6 (3.6–5.6) | 5.2 (5.0–5.2) | .69 |
| 9‐point body condition score | 5 (5–6) | 5 (5–5.5) | .82 |
| Duration of clinical signs (months) | 10 (6–24) | 42 (24–51) | .23 |
| 12‐point cumulative clinical score | 5 (3–7) | 3 (2.5–5) | |
| Cough frequency score | 4 (3–5) | 1 (1–3) | .25 |
| Respiratory distress score | 0 (0–0) | 0 (0–0) | 1.0 |
| Thoracic auscultation score | 1 (0–2) | 2 (1.5–2) | .30 |
| General condition score | 0 (0–0) | 0 (0–0) | 1.0 |
| 10‐point radiographic score | 3 (3–3) | 2 (2–3) | .56 |
| pTBFVL PEF/PIF | 0.88 (0.77–1.04) | 0.76 (0.76–0.81) | .36 |
| pTBFVL PEF/EF50 | 1.55 (1.29–1.63) | 1.25 (1.17–1.46) | .36 |
| pTBFVL PEF/EF25 | 2.25 (1.76–2.46) | 1.56 (1.42–1.92) | .24 |
| Penh | 0.93 (0.74–1.20) | 0.64 (0.62–0.87) | .30 |
IQR, interquartile ranges.
Data are presented as median with IQR.
Feline lower airway disease cats with PEF/EF50 > 1.51 before treatment had higher but not statistically different cough frequency score (P = .055), significantly lower macrophage percentage in BALF (P = .007), and significantly higher granulocyte (eosinophil plus neutrophil) percentage (P = .014) than cats with pretreatment PEF/EF50 ≤ 1.51 (Table 5). There were no significant differences between 2 functional subgroups in terms of signalment, duration of clinical signs and radiographic scores. After glucocorticoid treatment, 3 cats still had PEF/EF50 > 1.51. These 3 cats had significantly longer duration of clinical signs before treatment (median, 48 months versus 6.5 months; P = .004) and significantly higher pre‐ and post‐treatment respiratory distress score (P = .004 and .029, respectively) than cats with post‐treatment PEF/EF50 ≤ 1.51.
Table 5.
Signalment, clinical scores, and BALF results between cats with and without PEF/EF50 > 1.51 before treatment
| Variable | N | Cats with PEF/EF50 > 1.51 (n = 11) | Cats with PEF/EF50 ≤ 1.51 (n = 8) | P‐Value |
|---|---|---|---|---|
| Age (years) | 19 | 5.0 (3.0–8.0) | 4.5 (2.8–6.5) | .35 |
| Body weight (kg) | 19 | 5.2 (3.6–5.7) | 5.1 (4.7–5.6) | .27 |
| BCS (9 points) | 19 | 6 (5–6.5) | 5.5 (5–6) | .41 |
| Duration of clinical signs (months) | 19 | 10 (6–32) | 17 (6–33) | .48 |
| 12‐point cumulative clinical score | 19 | 6 (5–7) | 3 (2.5–6) | |
| Cough frequency score | 19 | 5 (3.5–5) | 3 (1.5–4.5) | .055 |
| Respiratory distress score | 19 | 0 (0–0.5) | 0 (0–0) | .26 |
| Thoracic auscultation score | 19 | 2 (1–2) | 1 (0–2) | .18 |
| General condition score | 19 | 0 (0–0) | 0 (0–0) | 1.0 |
| 10‐point radiographic score | 19 | 3 (3–3.5) | 2.5 (2–3) | .14 |
| Variable | N | Cats with PEF/EF50 > 1.51 (n = 8) | Cats with PEF/EF50 ≤ 1.51 (n = 8) | P‐Value |
|---|---|---|---|---|
| BALF TNCC (cells/μL) | 16 | 1,459 (841–1,964) | 1,236 (731–1,577) | .32 |
| BALF Eosinophil (%) | 16 | 44 (23–70) | 23 (12–43) | .065 |
| BALF Neutrophil (%) | 16 | 29 (5–47) | 22 (12–36) | .40 |
| BALF Macrophage (%) | 16 | 21 (13–31) | 46 (32–65) | .007 |
| BALF Lymphocyte (%) | 16 | 4 (3–6) | 2 (2–5) | .17 |
| BALF Granulocyte (%) | 16 | 74 (64–83) | 52 (33–66) | .014 |
BALF, bronchoalveolar lavage fluid; BCS, body condition score; and IQR, interquartile ranges.
Data are presented as median with IQR.
Bolded values indicating one‐tailed P < .05.
Bronchoalveolar lavage fluid macrophage percentage was significantly and inversely correlated with PEF/EF50 (P = .012, r = −0.61, R 2 = 0.37 from Pearson; P = .015, r s = −0.60 from Spearman) and PEF/EF25 (P = .003, r = −0.69, R 2 = 0.47 from Pearson; P = .001, r s = −0.74 from Spearman). BALF granulocyte percentage also was significantly correlated with PEF/EF50 (P = .015, r = 0.59, R 2 = 0.35 from Pearson; P = .022, r s = 0.57 from Spearman) and PEF/EF25 (P = .003, r = 0.69, R 2 = 0.48 from Pearson; P = .001, r s = 0.74 from Spearman; Fig 1). Correlation between log Penh and BALF eosinophil (P = .78, r = 0.076, R 2 = 0.006 from Pearson; P = .36, r s = 0.24 from Spearman), neutrophil (P = 0.12, r = 0.40, R 2 = 0.16 from Pearson; P = .61, r s = 0.14 from Spearman), macrophage (P = .060, r = −0.48, R 2 = 0.23 from Pearson; P = .072, r s = −0.46 from Spearman), and granulocyte (P = .066, r = 0.47, R 2 = 0.22 from Pearson; P = .085, r s = 0.44 from Spearman) percentage was not statistically significant. There were no statistically significant correlations between BALF TNCC and PEF/EF50, PEF/EF25, and log Penh.
Figure 1.
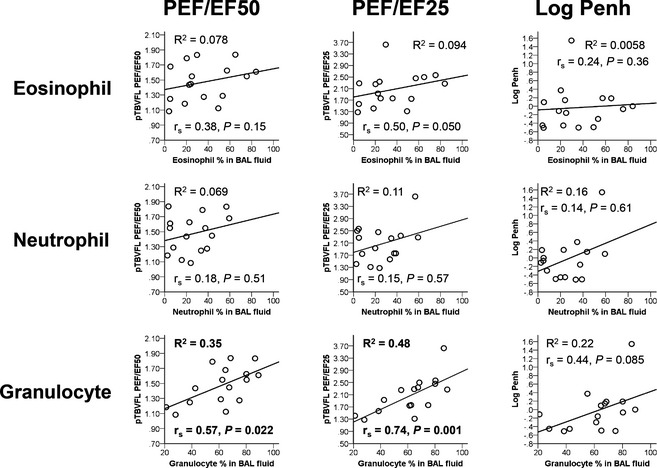
Scatter plots showing the correlations between specific cell (eosinophil, neutrophil, and granulocyte) percentage in bronchoalveolar lavage fluid (BALF) and functional parameters representing airflow obstruction (PEF/EF50, PEF/EF25 and Log Penh). R 2 from Pearson correlation, Spearman's rho correlation (r s), and P values were included for each plot.
Discussion
This prospective observational study demonstrates that changes in pulmonary function after treatment could be identified by noninvasive assessment. Selected pTBFVL indices may serve as indicators for monitoring therapeutic response. Functional phenotype could imply clinical severity and change in proportion of cells in BALF. Correlation between functional changes and BALF cytology represented mainly an overall increase in the granulocyte population.
In this study, PEF/EF50 and PEF/EF25 were shown to decrease significantly after glucocorticoid treatment. Airflow limitation caused by bronchoconstriction or accumulation of mucus was mainly identified in the midexpiratory phase during tidal breathing.4, 26 The most significant difference between cats with and without FLAD also was indicated by the ratio of peak to midexpiratory pseudoflow.17 In humans, the ratio of EF25/PEF previously has been documented to increase significantly after bronchodilator inhalation in young asthmatic children.27 Decreased flow during late expiration also was observed in horses with COPD and inflammatory airway disease under an induced maneuver of forced expiration, and the severity of small airway obstruction in COPD crisis was worse than during remission.28 As a consequence, it was expected that indices describing mid‐ or late expiratory flow changes could identify the improvement of airflow limitation after treatment for the underlying etiology. The results of this study support the use of PEF/EF50 or PEF/EF25 as a noninvasive method for monitoring treatment response in cats with lower airway diseases.
A slight but statistically significant change in PIF/IF50 after treatment was an unexpected finding in this study because no differences in the shape of the pTBFVL and TBFVL inspiratory curves were found previously between cats with and without FLAD.17, 29 TBFVL PIF/IF50 was relatively higher in dogs with laryngeal diseases and in brachycephalic dogs than in healthy dogs.30, 31, 32 Laryngeal edema was noted in 1 of our cats during BAL without correlated upper airway signs, and it was judged not to be clinically relevant. Subclinical upper airway problems of either primary or secondary cause may have been present in several FLAD cats in this study, and functional assessment was sensitive enough to detect it.
Loop indices PEF/EF50 and PEF/EF25 were better than Penh in detecting lower airway functional change in this study. Baseline measurements of conventionally‐used BWBP parameters did not change in 5 experimental cats with mild CB after glucocorticoid treatment although a bronchoprovocative index C‐Penh 300% was significantly increased.20 Repeated BWBP assessment was performed on clinical cats with chronic bronchial disease in 2 recent studies, and significantly lower Penh (P = .048, n = 19) and higher C‐Penh 300% (P = .049, n = 10) than before treatment were showed in 1 report,33 whereas no statistically significant difference between any conventional parameters or C‐Penh 300% before and after treatment (n = 7) were found in the other study.15 Our results suggest that evaluating pTBFVL indices simultaneously should be helpful for monitoring patients with airflow limitation.
The lack of significant difference for Penh before and after treatment in this study was due to many factors, including lung mechanic changes and the sensitivity of the test. Using Penh as a surrogate index for bronchoconstriction is controversial.34 It is difficult to determine exactly why Penh failed to show significant changes in this study. Penh is a calculated index involving expiratory time, relaxation time, and peak inspiratory and expiratory flow, which could be affected by several factors and should be interpreted with caution. Furthermore, the phenomenon of airflow obstruction is complicated in lower airway disease. The decrease in small airway diameter can be caused by bronchospasm, smooth muscle hypertrophy, or mucus obstruction,5, 35, 36 which may not always be distinguishable separately. The above components responsible for airflow limitation could exist to a variable extent in cats with naturally occurring FLAD. Under the premise that Penh could properly indicate the existence of bronchoconstriction, bronchospasm might wax and wane among study cats and not necessarily appear during a short period of PFT recording without provocative stimulation, whereas airflow limitation associated with mucus accumulation or remodeling changes may be more consistently recognized before steroid therapy. Another possible explanation is that most cats in this study were not in critical condition, which might be more common when bronchospasm is present.
There was no statistically significant correlation between BALF eosinophil percentage and functional parameters representing airflow obstruction in our study, but this correlation became significant with eosinophil plus neutrophil (granulocyte) percentage. The significantly decreased macrophage percentage is a compensatory reduction that reflects the significant increase in overall extent of inflammatory cell infiltration. In an earlier study, BALF granulocyte percentage correlated better with log Penh than did eosinophil percentage.15 Earlier study findings and our findings imply that obstructive functional pattern is the outcome of excessive infiltration of inflammatory cells, rather than only eosinophils. TNCC was not correlated with functional parameters in both studies, perhaps because this variable was unreliable without estimation of epithelial lining fluid in BALF or processing of BALF may have differed in different studies.10, 37 In addition, PEF/EF25 may be used to predict the extent of infiltration.
In contrast to a previous study,15 there was no significant difference in log Penh or Penh in our study between cats with eosinophilic and noneosinophilic bronchitis. Eosinophilic inflammation in BALF is an important feature of feline asthma,9 and there was evidence that cats with eosinophilic inflammation were more likely to show airway hyperresponsiveness than cats with neutrophilic inflammation.8 A recent study found that log Penh was significantly higher (P = .031) in cats with eosinophilic bronchitis compared to those with noneosinophilic bronchitis, suggesting a correlation between eosinophilic inflammation and bronchoconstriction.15 All 3 obstruction‐associated parameters (Penh, PEF/EF50 and PEF/EF25) in this study were not statistically different between cats with and without eosinophilic bronchitis. This finding suggests that bronchoconstriction is not consistently present or easily recognized in cats with eosinophilic bronchitis. Considering that bronchospasm is a feature of asthma, it may call into question the role of eosinophils in bronchoconstriction of the asthmatic phenotype. In human airway disease, there is substantial overlap in clinical manifestations and pulmonary function results between asthma and COPD.35, 38 COPD in human medicine is defined as a group of diseases (eg, CB) of heterogeneous etiology.39 Some COPD patients exhibit airway hyperresponsiveness or have eosinophilic airway inflammation,38, 39 whereas asthma patients can develop incompletely reversible airflow obstruction or have neutrophilic inflammation.35, 38 As a result, phenotype‐specific management has been proposed to be a more proper approach than using traditional categorization‐based treatment guidelines.40 Although FLAD may or may not share the same features with human airway diseases, identifying functional aspects could expand understanding of these diseases and facilitate future classifications.
Aside from inflammatory cell type, functional phenotype may provide more information to explain clinical outcome in cats with FLAD. Functional phenotype of PEF/EF50 > 1.51 represents an expiratory pattern characterized by a concave expiratory curve or dramatically decreased flow in the midexpiratory phase.17 FLAD cats with PEF/EF50 > 1.51 before treatment showed significantly higher granulocyte percentage, indicating more severe inflammatory cell infiltration in small airways. Of interest, 3 cats with postglucocorticoid treatment PEF/EF50 > 1.51 had significantly longer duration of clinical signs and higher respiratory distress score, suggesting the possibility of more severe or persistent lower airway obstruction that had not fully responded to the present glucocorticoid treatment protocol. Unfortunately, BALF was not available in 2 of these cats. Whether much longer glucocorticoid treatment, higher glucocorticoid dosage or a combination of drugs including bronchodilators is beneficial for these cats should be investigated in additional clinical trials. Functional phenotype recognized by noninvasive procedures might be used to predict the severity of airway obstruction associated with inflammation before treatment and to evaluate the reversibility of airflow limitation after treatment.
This study had several limitations, such as the low number of cats in each BALF inflammatory type. There were only 3/16 cats with BALF eosinophil percentage <17% assigned to the noneosinophilic subgroup, which might contribute to underpowered results. However, the same pitfall also existed in another comparable study using clinical cats with eosinophilic (8/12) and noneosinophilic (4/12) bronchitis.15 The main reason is the requirement of relatively invasive diagnostic testing, which limits the number of cats with suspected lower airway disorders that can be evaluated. Furthermore, post‐treatment BAL was not performed in these FLAD cats, and persistent subclinical inflammation in individual cats cannot be ruled out. However, it is difficult for cat owners to consent to repeated BAL after improvement of clinical signs because of the concerns of cost and safety. In addition, clinical outcomes according to clinical scores are relatively subjective, which may have influenced the results. Another study limitation is that placebo effect might be considered for some cats with clinical improvement because FLAD clinical signs could wax and wane. Spontaneous waning of disease could be problematic in studying clinical cases of FLAD, and a true therapeutic response may not be definitely confirmed. Finally, cats with persistent respiratory distress were excluded from our study, and this may eliminate some cats with the most severe and acute form of FLAD.
In conclusion, changes in respiratory function after treatment could be detected with a noninvasive assessment in clinical cats with respiratory disease. Measuring functional parameters such as pTBFVL PEF/EF50 or PEF/EF25 could allow monitoring for therapeutic response. An increase in PEF/EF25 is well correlated with an increase in granulocyte percentage, implying more severe extent of inflammatory cell infiltration. Functional assessment could be repeatedly and noninvasively performed in cats with lower airway disease for monitoring purposes on a functional basis. The potential role of using functional phenotype in guiding therapeutic strategies for chronic airway diseases deserves further study.
Acknowledgments
We thank Dr Jiunn‐Horng Lin for Mycoplasma culture; Dr Pei‐Ying Lo and Dr. Chung‐Ling Liu for airway sample collection and management; biostatistician We‐Ting Hung for statistical consultation. The study was not supported by any grant.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
C.‐H. Liu and J.‐J. Lee contributed equally to this work.
The work was done at Graduate Institute of Veterinary Medicine, School of Veterinary Medicine, National Taiwan University, Taiwan; Section of Small Animal Internal Medicine, National Taiwan University Veterinary Hospital, Taiwan.
Footnotes
Buxco Electronics, Wilmington, NC
SPSS 19.0.0; IBM Corporation, Armonk, NY
References
- 1. Padrid P. Chronic bronchitis and asthma in cats In: Bonagura JD, Twedt DC, ed. Current Veterinary Therapy XV. St. Louis, MO: Elsevier; 2014:673–680. [Google Scholar]
- 2. Schikowski T, Adam M, Marcon A, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J 2014;44:614–626. [DOI] [PubMed] [Google Scholar]
- 3. Cosio BG, Rosado JR, Rossi FF. Asthma: Epidemiology, pathophysiology, and risk factors In: Spiro SG, Silvestri GA, Agustí A, eds. Clinical Respiratory Medicine, 4th ed Philadelphia, PA: W.B. Saunders; 2012:487–500. [Google Scholar]
- 4. Diaz EA, Chung Y, Lamoureux DP, et al. Effects of fresh and aged traffic‐related particles on breathing pattern, cellular responses, and oxidative stress. Air Qual Atmos Health 2012;6:431–444. [Google Scholar]
- 5. Reinero CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet J 2011;190:28–33. [DOI] [PubMed] [Google Scholar]
- 6. Culver BH. Pulmonary function testing In: Spiro SG, Silvestri GA, Agustí A, eds. Clinical Respiratory Medicine, 4th ed Philadelphia, PA: W.B. Saunders; 2012:133–142. [Google Scholar]
- 7. Cocayne CG, Reinero CR, DeClue AE. Subclinical airway inflammation despite high‐dose oral corticosteroid therapy in cats with lower airway disease. J Feline Med Surg 2011;13:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirt RA, Galler A, Shibly S, et al. Airway hyperresponsiveness to adenosine 5′‐monophosphate in feline chronic inflammatory lower airway disease. Vet J 2011;187:54–59. [DOI] [PubMed] [Google Scholar]
- 9. Trzil JE, Reinero CR. Update on feline asthma. Vet Clin North Am Small Anim Pract 2014;44:91–105. [DOI] [PubMed] [Google Scholar]
- 10. Ybarra WL, Johnson LR, Drazenovich TL, et al. Interpretation of multisegment bronchoalveolar lavage in cats (1/2001–1/2011). J Vet Intern Med 2012;26:1281–1287. [DOI] [PubMed] [Google Scholar]
- 11. Johnson LR, Vernau W. Bronchoscopic findings in 48 cats with spontaneous lower respiratory tract disease (2002–2009). J Vet Intern Med 2011;25:236–243. [DOI] [PubMed] [Google Scholar]
- 12. Nafe LA, DeClue AE, Reinero CR. Storage alters feline bronchoalveolar lavage fluid cytological analysis. J Feline Med Surg 2011;13:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leemans J, Kirschvink N, Clercx C, et al. Effect of short‐term oral and inhaled corticosteroids on airway inflammation and responsiveness in a feline acute asthma model. Vet J 2012;192:41–48. [DOI] [PubMed] [Google Scholar]
- 14. Leemans J, Kirschvink N, Bernaerts F, et al. A pilot study comparing the antispasmodic effects of inhaled salmeterol, salbutamol and ipratropium bromide using different aerosol devices on muscarinic bronchoconstriction in healthy cats. Vet J 2009;180:236–245. [DOI] [PubMed] [Google Scholar]
- 15. Allerton FJ, Leemans J, Tual C, et al. Correlation of bronchoalveolar eosinophilic percentage with airway responsiveness in cats with chronic bronchial disease. J Small Anim Pract 2013;54:258–264. [DOI] [PubMed] [Google Scholar]
- 16. Kirschvink N. Barometric whole body plethysmography and enhanced pause (PENH): How relevant are they? Vet J 2008;176:125–126. [DOI] [PubMed] [Google Scholar]
- 17. Lin CH, Lee JJ, Liu CH. Functional assessment of expiratory flow pattern in feline lower airway disease. J Feline Med Surg 2014;16:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reinero CR, Decile KC, Byerly JR, et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma. Am J Vet Res 2005;66:1121–1127. [DOI] [PubMed] [Google Scholar]
- 19. Cohn LA, DeClue AE, Cohen RL, et al. Effects of fluticasone propionate dosage in an experimental model of feline asthma. J Feline Med Surg 2010;12:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirschvink N, Leemans J, Delvaux F, et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis. J Feline Med Surg 2006;8:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirschvink N, Leemans J, Delvaux F, et al. Non‐invasive assessment of growth, gender and time of day related changes of respiratory pattern in healthy cats by use of barometric whole body plethysmography. Vet J 2006;172:446–454. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman AM, Dhupa N, Cimetti L. Airway reactivity measured by barometric whole‐body plethysmography in healthy cats. Am J Vet Res 1999;60:1487–1492. [PubMed] [Google Scholar]
- 23. Hirt RA, Dederichs D, Boehler A, et al. Relationship of age, sex, body weight, and hematologic and respiratory variables with airway reactivity in adult cats. Am J Vet Res 2003;64:26–31. [DOI] [PubMed] [Google Scholar]
- 24. Foster SF, Martin P, Braddock JA, et al. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995–2000). J Feline Med Surg 2004;6:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foster SF, Martin P. Lower respiratory tract infections in cats: Reaching beyond empirical therapy. J Feline Med Surg 2011;13:313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoymann HG. Lung function measurements in rodents in safety pharmacology studies. Front Pharmacol 2012;3:156. doi: 10.3389/fphar.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlsen KH, Lodrup Carlsen KC. Tidal breathing analysis and response to salbutamol in awake young children with and without asthma. Eur Respir J 1994;7:2154–2159. [DOI] [PubMed] [Google Scholar]
- 28. Couetil LL, Rosenthal FS, DeNicola DB, et al. Clinical signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary function in horses with inflammatory respiratory disease. Am J Vet Res 2001;62:538–546. [DOI] [PubMed] [Google Scholar]
- 29. McKiernan BC, Dye JA, Rozanski EA. Tidal breathing flow‐volume loops in healthy and bronchitic cats. J Vet Intern Med 1993;7:388–393. [DOI] [PubMed] [Google Scholar]
- 30. Amis TC, Kurpershoek C. Tidal breathing flow‐volume loop analysis for clinical assessment of airway obstruction in conscious dogs. Am J Vet Res 1986;47:1002–1006. [PubMed] [Google Scholar]
- 31. Amis TC, Kurpershoek C. Pattern of breathing in brachycephalic dogs. Am J Vet Res 1986;47:2200–2204. [PubMed] [Google Scholar]
- 32. Amis TC, Smith MM, Gaber CE, et al. Upper airway obstruction in canine laryngeal paralysis. Am J Vet Res 1986;47:1007–1010. [PubMed] [Google Scholar]
- 33. Galler A, Shibly S, Bilek A, et al. Inhaled budesonide therapy in cats with naturally occurring chronic bronchial disease (feline asthma and chronic bronchitis). J Small Anim Pract 2013;54:531–536. [DOI] [PubMed] [Google Scholar]
- 34. van den Hoven R. A jack‐in‐the‐box of respiratory research: Is the technique of barometric whole body plethysmography a disappointing surprise? Vet J 2007;173:250–251. [DOI] [PubMed] [Google Scholar]
- 35. Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest 2004;126:117S–124S. [DOI] [PubMed] [Google Scholar]
- 36. Bay JD, Johnson LR. Feline bronchial disease/asthma In: King LG, ed. Textbook of Respiratory Disease in Dogs and Cats. St. Louis, MO: Saunders; 2004:388–396. [Google Scholar]
- 37. Mills PC, Litster A. Using urea dilution to standardise cellular and non‐cellular components of pleural and bronchoalveolar lavage (BAL) fluids in the cat. J Feline Med Surg 2006;8:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: What are its features and how important is it? Thorax 2009;64:728–735. [DOI] [PubMed] [Google Scholar]
- 39. MacNee W. Chronic obstructive pulmonary disease: Epidemiology, pathophysiology, and clinical evaluation In: Spiro SG, Silvestri GA, Agustí A, eds. Clinical Respiratory Medicine, 4th ed Philadelphia, PA: W.B. Saunders; 2012:531–552. [Google Scholar]
- 40. Pavord ID. Complex airway disease: An approach to assessment and management. The Lancet Respiratory Medicine 2013;1:84–90. [DOI] [PubMed] [Google Scholar]


