Abstract
Background
Only few pharmacologic compounds have been validated for treatment of atrial fibrillation (AF) in horses. Studies investigating the utility and safety of flecainide to treat AF in horses have produced conflicting results, and the antiarrhythmic mechanisms of flecainide are not fully understood.
Objectives
To study the potential of flecainide to terminate acutely induced AF of short duration (≥15 minutes), to examine flecainide‐induced changes in AF duration and AF vulnerability, and to investigate the in vivo effects of flecainide on right atrial effective refractory period, AF cycle length, and ventricular depolarization and repolarization.
Animals
Nine Standardbred horses. Eight received flecainide, 3 were used as time‐matched controls, 2 of which also received flecainide.
Methods
Prospective study. The antiarrhythmic and electrophysiologic effects of flecainide were based on 5 parameters: ability to terminate acute pacing‐induced AF (≥15 minutes), and drug‐induced changes in atrial effective refractory period, AF duration, AF vulnerability, and ventricular depolarization and repolarization times. Parameters were assessed at baseline and after flecainide by programmed electrical stimulation methods.
Results
Flecainide terminated all acutely induced AF episodes (n = 7); (AF duration, 21 ± 5 minutes) and significantly decreased the AF duration, but neither altered atrial effective refractory period nor AF vulnerability significantly. Ventricular repolarization time was prolonged between 8 and 20 minutes after initiation of flecainide infusion, but no ventricular arrhythmias were detected.
Conclusions and Clinical Importance
Flecainide had clear antiarrhythmic properties in terminating acute pacing‐induced AF, but showed no protective properties against immediate reinduction of AF. Flecainide caused temporary prolongation in the ventricular repolarization, which may be a proarrhythmic effect.
Keywords: Atrial electrophysiology, Equine, Pacing, Programmed electrical stimulation
Abbreviations
- aEGM
atrial electrogram
- aERP
atrial effective refractory period
- AF
atrial fibrillation
- AFCL
atrial fibrillation cycle length
- bpm
beats per minute
- MAP
mean arterial blood pressure
- S‐ECG
surface ECG
- SR
sinus rhythm
- TVEC
transvenous electrical cardioversion
The utility and safety of flecainide to treat atrial fibrillation (AF) in horses has been evaluated in a few studies and with conflicting results.1, 2, 3, 4 In acute experimentally induced AF studies, flecainide was reported to be a safe antiarrhythmic compound with high efficacy in restoring sinus rhythm (SR) in horses with AF of 15 minutes′ duration,1 but caused sudden death in 1 of 2 treated horses with 7 days of AF.4 Disappointing efficacy and questionable safety were found in horses with longer lasting AF, with evidence of electrical and mechanical remodeling.2, 3, 4
Atrial fibrillation is the most common clinically relevant arrhythmia affecting performance in athlete horses.5, 6, 7, 8 Orally administered quinidine sulfate is the pharmacologic treatment of choice for restoring SR in horses regardless of the duration of AF,9 and has a fairly high efficacy (65–90%) in horses with no underlying cardiac disease.8 However, treatment frequently is terminated because of cardiac or noncardiac adverse effects or both.9, 10 Transvenous electrical cardioversion (TVEC) is effective in restoring SR in horses without underlying cardiac disease with reported success rates of approximately 90%.11, 12 However, this procedure requires specialized hospital facilities and equipment. Hence, alternative treatment options are desirable.
Atrial fibrillation is a self‐sustaining arrhythmia characterized by rapid uncoordinated atrial activation and irregular ventricular contractions.13 The fundamental mechanisms underlying AF are still debated, but regardless of the initiating event, there is broad consensus that AF is maintained by rapid focal activation or by re‐entrant wavelets of depolarization moving randomly across the atria.13, 14, 15 Flecainide is, because of its sodium channel blocking properties, classified as a class IC antiarrhythmic drug. One of the prominent features of flecainide is slowing of the conduction velocity throughout the heart. However, the exact pharmacologic mechanisms are not fully understood. In vivo studies have produced conflicting results with regard to flecainide‐induced changes in atrial effective refractory period (aERP).16, 17, 18, 19, 20 Flecainide may slow the atrial activation rate and eventually terminate AF by decreasing excitability, increasing the size and decreasing the number of re‐entrant circuits,18 primarily by widening the temporal excitable gap (the difference between AF cycle length [AFCL] and aERP during AF),16, 17 by changing the appearance of the rotating wavefront or some combination of these effects.21, 22, 23 In animal tachypacing models and in human AF patients, flecainide caused increased AFCL, decreased conduction velocity and caused a minor tachycardia‐dependent increase in aERP.17, 24, 25, 26, 27, 28, 29 In horses, a marked flecainide‐induced increase in AFCL has been reported,2 but the effects of flecainide on aERP, AF duration, and AF vulnerability have not yet been investigated.
The purpose of this study was to evaluate the potential of flecainide to terminate acutely induced AF of short duration and to assess the properties of flecainide on AF duration and AF vulnerability in healthy horses. In addition, the acute electrophysiologic effects of flecainide on aERP, AFCL, and ventricular depolarization and repolarization time (QRS, QTc, and JTc) were investigated by standardized programmed stimulation methods.30
Materials and Methods
Nine Standardbred horses were included: 3 geldings, 6 mares; age, 7.9 ± 3.9 years; and, body weight, 475 ± 47 kg (Table S2 in Data S1). Eight horses were included in a flecainide group and 3 horses in a control group. Two horses participated in both groups. The horses showed no signs of cardiovascular disease based on history, clinical examination, thorough cardiac auscultation, 24‐hour ECG, and routine echocardiographic examination. The studies were approved by The Danish Animal Experiments Inspectorate (license number 2012‐15‐2934‐00198) and performed in accordance with the Danish guidelines for animal experiments according to the European Commission Directive 86/609/EEC.
All experiments were performed in standing nonsedated horses restrained in a stock. Before catheterization, injection sites were aseptically prepared and locally anesthetized1 by using SC infiltration. In total, 3 12‐gauge IV catheters2 were placed: 2 in the right and 1 in the left jugular vein. The left‐sided catheter was used for drug administration, blood sampling, and routine access. The right‐sided catheters were placed in the lower half of the vein approximately 10 cm apart and replaced by introducer sheaths.3 A multipolar steerable nonfixative electrode4 was introduced through each introducer sheath and advanced into the right atrium: 1 for atrial pacing, and the second for recording intra‐atrial electrograms (aEGM). The electrodes were positioned in the atrium so that deflections on the aEGM appeared in close association with the beginning of the P wave on the 2 simultaneously recorded surface ECGs (S‐ECG1 + S‐ECG2), and pacing at 60 beats per minute (bpm) resulted in consistent atrial capture. Self‐adhesive ECG electrodes5 were positioned as shown in Figure 1. S‐ECG1 was optimized to show atrial activity (P wave lead), whereas S‐ECG2 was a modified base‐apex lead focusing on ventricular activity. In S‐ECG1, the positive electrode was adjusted dorso‐ventrally until the recorded P wave had the highest amplitude possible. Both intra‐atrial and surface ECGs were monitored during the experiments and stored6 for later analysis. Furthermore, an additional surface ECG (S‐ECG3) identical to S‐ECG2 was recorded with the Televet system5 and continued for 12–24 hours after the experiment. The experimental protocol of the study is demonstrated in Figure 2.
Figure 1.
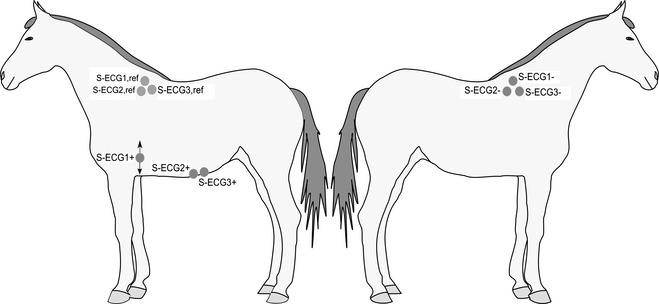
ECG leads. S‐ECG1 and S‐ECG2: surface ECGs recorded simultaneously during the experiments. S‐ECG3: modified base‐apex surface ECG recorded during and 12–24 hours after the experiment. +/−/ref indicate positive, negative, and reference electrodes, respectively. Arrows at S‐ECG1+ indicate that this electrode was adjusted dorso‐ventrally until the recorded P wave had the highest amplitude possible.
Figure 2.
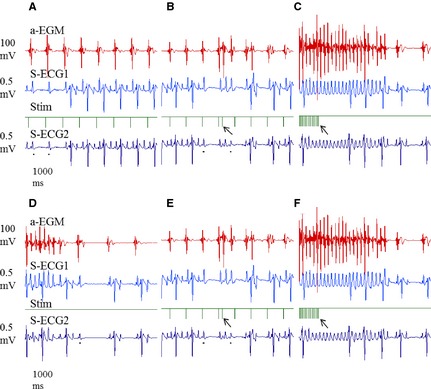
The experimental protocol of the study based on ECG recordings. To emphasize that the same protocol was used before and after flecainide, identical ECG sequences are presented in B and E and in C and F. (A) Positioning of electrodes and atrial pacing threshold determination. (B) atrial effective refractory period (aERP) measurements at multiple atrial pacing rates (60, 75, 120, and 182 bpm). (C) Atrial fibrillation (AF) duration and AF vulnerability measurements. AF was induced and the time to cardioversion at different induction settings (see Fig 3) was recorded. (D) If AF ≥ 15 minutes occurred horses were treated with flecainide (2 mg/kg IV) and cardioversion times recorded. (E) Repeated threshold determination and measurements of aERP at identical atrial pacing rates as before flecainide treatment. (F) Remeasuring of AF duration and AF vulnerability as described under (C).  : A black mark under a P wave indicates a second degree AV block. Arrow in (B) and (E): S2 stimuli associated with atrial capture. Arrow in (C) and (F): Tachypacing period leading to AF and subsequent spontaneous cardioversion.
: A black mark under a P wave indicates a second degree AV block. Arrow in (B) and (E): S2 stimuli associated with atrial capture. Arrow in (C) and (F): Tachypacing period leading to AF and subsequent spontaneous cardioversion.
Atrial Effective Refractory Period
A constant pulse width of 2 milliseconds was used throughout the experiment. Measurements of aERP were conducted using twice baseline atrial pacing threshold. The aERP was determined once before and once after flecainide treatment at atrial pacing rates of 60, 75, 120, and 182 bpm, respectively. aERP was measured with 10 basic stimuli (S1) followed by an extra stimulus (S2) applied with 10 milliseconds increments, and was defined as the longest S1–S2 failing to elicit a new action potential. Every S1–S2 interval was applied multiple times (5–10 repetitions) at each pacing rate, and if intermittent atrial capture occurred, the S2 value below the one with ≥50% captures was defined as the aERP.
Atrial Fibrillation Cycle Length
Atrial fibrillation cycle length was measured as a surrogate for tissue refractoriness, and represents the time interval between consecutive atrial depolarizations. In the present study, AFCL was determined by manually analyzing 15‐second sequences on the aEGM the last 15 seconds before the initiation of flecainide treatment and repeated in the last 15 seconds before AF termination.
Induction of AF and Flecainide Treatment
Atrial fibrillation was repeatedly induced using programmed electrical stimulation. A comprehensive induction protocol was used because the sensitivity of horses to induced AF has not been described previously, and because detailed information was needed to evaluate possible flecainide‐induced changes in AF duration and AF vulnerability. A schematic outline of the induction protocol is illustrated in Figure 3. The duration of each tachypacing period was 2–6 seconds (Table S3 in Data S1). If the duration of an induced AF episode exceeded 5 minutes at any given induction setting, tachypacing was repeated 5 times. If episodes were shorter than 5 minutes, 10–15 inductions were made and mean AF duration was calculated. Comparisons (baseline versus post‐flecainide) were based on results obtained at the baseline induction setting capable of inducing a mean AF duration >1 minute. In addition, the first single AF episode >1 minute in duration defined AF vulnerability (for vulnerability scoring, see Table S1 in Data S1). If only a few or short‐lasting AF episodes were induced, the stimulation frequency was stepwise increased and at the final step (burst pacing at 3,000 bpm; ie, 50 Hz), the current was subsequently increased (Fig 3). Whenever an AF episode exceeded 15 minutes in duration, AF termination was attempted by IV infusion of flecainide7 (2 mg/kg) over 10 minutes according to previous recommendations.1 If no AF episodes ≥15 minutes occurred and spontaneous termination occurred repeatedly, horses were treated with flecainide while in SR. Seven horses were treated while in AF; 1 while in SR. After flecainide infusion, aERP measurements and AF induction protocols were repeated. Subsequently, the effects of flecainide on AF duration and AF vulnerability were assessed. Blood pressure8 (noninvasive) and blood samples9 were collected at baseline and at specific time points after flecainide treatment to evaluate flecainide‐induced changes (for details see Data S1).
Figure 3.
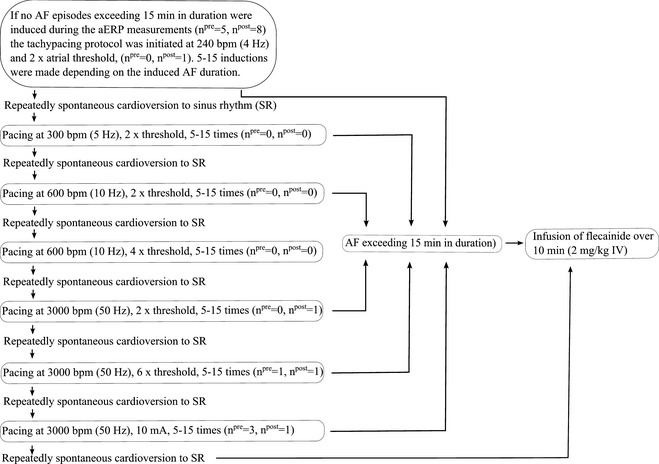
Flow diagram illustrating the atrial fibrillation (AF) induction protocol. For each horse, AF duration and AF vulnerability were measured at the induction setting capable of inducing AF episodes exceeding 1 minute in duration (see text). npre and npost represent the number of horses entering AF ≥ 15 minutes at that particular induction setting before and after flecainide treatment, respectively.
Time‐Matched Control Studies
Three horses were assigned to participate in time‐matched control studies. Two horses completed the control study after a flecainide wash‐out period, whereas the last horse was included only in the control group. The control studies followed a protocol as described for the flecainide studies except that only isotonic saline was infused instead of flecainide. After infusion, the horses were monitored for spontaneous cardioversion for 30 minutes. If a horse was still in AF after 30 minutes, the experiment was ended and the horse was released from all equipment including intracardiac electrodes. However, S‐ECG3 was left in position to capture the exact cardioversion time.
Analysis of ECG Intervals
QT intervals and QRS durations were manually analyzed on S‐ECG2; JTc intervals were calculated as (QTc minus QRS). Representative ECG tracings illustrating QT and QRS measurements in SR and AF, as well as information regarding QRS/QTc‐methods are provided in Data S1. Heart rate was calculated in the same sections as QRS and QT were measured.
Data Analysis
Results are presented as mean ± SD. Statistical analyses were performed by specialized software.10 All datasets were normally distributed allowing the use of parametric tests. However, logarithmic transformation of AF duration data was necessary to accomplish normal distribution. The combined effects of flecainide and atrial stimulation rates on aERP were examined by 2‐way repeated‐measures ANOVA followed by Bonferroni′s posthoc test. A paired t‐test was used to analyze flecainide‐induced changes in AF duration, whereas a Wilcoxon matched pairs t‐test was used to compare changes in AF vulnerability. Flecainide‐induced changes in blood pressure (MAP), heart rate, QRS, QTc, and JTc interval were analyzed by 1‐way repeated ANOVA followed by Dunnett′s posthoc test using the time point just before initiated infusion of flecainide as reference (Time − 1). P<.05 was considered statistically significant.
Results
To investigate the electrophysiologic effects of flecainide, we examined the atrial pacing threshold, AFCL, and aERP before and after infusion of flecainide. Flecainide did not alter the atrial pacing threshold compared to baseline (1.0 ± 0.9 versus 0.8 ± 0.5; Table S2 in Data S1). AFCL increased significantly before flecainide‐induced AF termination compared to baseline with a mean increase of 35 ± 19% (P < .001; Table S2 in Data S1). However, treatment with flecainide did not result in statistically significant changes in aERP at any stimulation rate (Fig 4). Faster atrial pacing rate resulted in a decrease in aERP (P = .014; Table S3 in Data S1).
Figure 4.
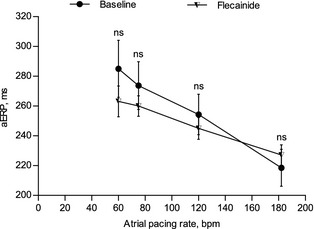
Baseline and flecainide‐induced changes in right atrial effective refractory period (aERP) at 60, 75, 120, and 182 bpm, respectively, in healthy Standardbred horses (n = 8).
The antiarrhythmic potential of flecainide was studied by evaluating drug‐induced termination time, changes in AF duration and AF vulnerability. The outcome of the induction protocol varied markedly among horses. However, AF episodes of variable duration were induced in all horses. Three of 8 horses were highly sensitive and developed AF episodes ≥15 minutes during baseline aERP measurements at an atrial pacing rate of 182 bpm (Table S3 in Data S1). In total, 7 of 8 horses developed AF ≥ 15 minutes during the baseline protocol and were treated with flecainide while in AF. Duration of the last AF episode before initiation of treatment was 21 ± 5 minutes (Table S3 in Data S1). In 1 horse, spontaneous AF termination occurred repeatedly, and only several short episodes of AF could be induced, and thus, this horse received flecainide while in SR. The 7 horses treated with flecainide while in AF, all cardioverted to SR during flecainide infusion with a termination time of 4.6 ± 2.5 minutes (Table S3 in Data S1).
Post‐flecainide AF inductions were initiated 42 ± 13 minutes and ended 72 ± 24 minutes after completed infusion. Plasma concentrations of flecainide (ng/mL) 1 minute to 24 hours after completed infusion are illustrated in Figure 5 and plasma half‐life (T ½) values are presented in Table S4 in Data S1. The flecainide‐induced changes in AF duration and AF vulnerability are shown in Figure 6A,B. All horses completed the post‐treatment aERP protocol without developing any AF episodes ≥15 minutes. Flecainide significantly shortened AF duration (P = .003; Fig 6A, Table S3 in Data S1), but had no statistically significant effect on AF vulnerability (P = .136; Fig 6B, Table S3 in Data S1).
Figure 5.
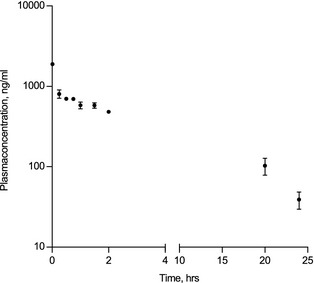
Flecainide plasma concentrations 1 minute to 24 hours after completed infusion of flecainide (2 mg/kg IV over 10 minutes) in healthy Standardbred horses (n = 7). Note that the Y‐axis is log‐transformed. For details, please see Table S3 in Data S1.
Figure 6.
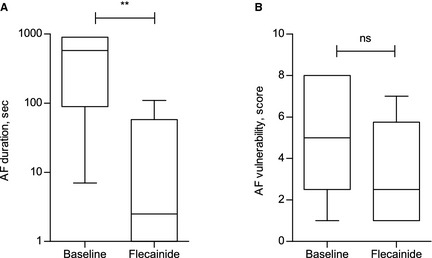
Antiarrhythmic and potential protective effects of flecainide. Results presented as box plot, whiskers represent minimum to maximum values. (A) Atrial fibrillation (AF) duration at baseline and after flecainide treatment, **P = .003. Note that the Y‐axis is log‐transformed. (B) AF vulnerability at baseline and after flecainide treatment, ns: nonsignificant, P = .136. For each horse, AF duration was measured and compared using the AF induction setting shown at baseline to be effective at inducing AF episodes with a mean duration >1 minute (see text). For each horse, AF vulnerability was measured and compared using the AF induction setting shown to be effective at inducing a single AF episode >1 minute in duration before and after flecainide, respectively.
Baseline MAP was 78 ± 12 mmHg. Flecainide did not significantly change MAP during a 60‐minute period after completion of infusion compared to baseline (Fig 7).
Figure 7.
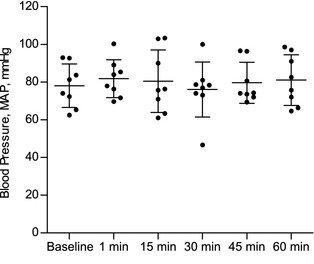
Flecainide‐induced (2 mg/kg IV) changes in blood pressure in healthy Standardbred horses (n = 8).
To evaluate flecainide‐induced changes in the ventricular conduction pattern, we examined changes in HR, and QRS, QTc, and JTc intervals. QRS, QTc, and JTc were prolonged between 8 and 30 minutes, 6 and 25 minutes, and 8 and 20 minutes after initiation of flecainide infusion, respectively (Fig 8A,C,D). HR was calculated over 20 ± 1.8 beats and was stable throughout the experiments (Fig 8B). No ventricular arrhythmias were detected during infusion and in a 90‐minute period after flecainide treatment.
Figure 8.
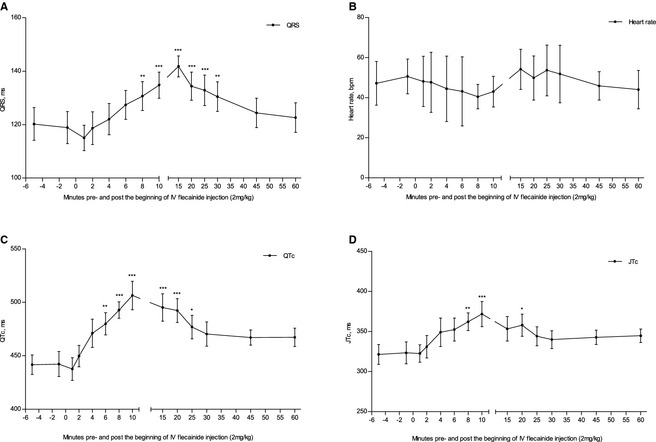
Flecainide‐induced (2 mg/kg IV) changes in QRS (A), heart rate (B), QTc (C), and JTc (D) in healthy Standardbred horses (n = 8). Time (minute): T0 and T10 represent the beginning and end of flecainide infusion, respectively. Asterisks indicate the significance level compared to baseline (T‐1); *P < .05, **P < .01, ***P < .001.
Control Studies
To measure AFCL at comparable time points in the flecainide and in control groups, AFCL after saline infusion was measured at the previously recorded time of cardioversion from AF to SR after flecainide infusion (4.6 minute after starting infusion = during infusion). Baseline atrial pacing threshold, pre‐saline AF duration, pre‐saline AFCL, AFCL at 4.6 minutes after saline infusion was started, and baseline aERP at 60 and 182 bpm in the control experiments are presented in Table S5 in Data S1. All 3 horses developed AF episodes ≥15 minute during the baseline protocol with a mean AF duration of 22 ± 5 minutes before saline infusion was started. AF was not terminated by saline infusion, and spontaneous termination time was 5.8 ± 3.2 hours (Table S5 in Data S1).
Discussion
This study was designed to investigate flecainide‐induced effects on atrial electrophysiology in horses with acutely induced AF of short duration. We assessed the antiarrhythmic potential of flecainide by its cardioversion potential and effects on AF duration and AF vulnerability. The in vivo electrophysiologic profile of flecainide was evaluated by changes in aERP, AFCL, QRS, QTc, and JTc.
Flecainide is a clinically important compound in human patients with paroxysmal AF, but for safety reasons, candidates for treatment are carefully selected and the use of flecainide is restricted to patients without structural heart disease and with preserved left ventricular function.31, 32 When these criteria are fulfilled, treatment with flecainide demonstrates cardioversion rates of 57–92% in human patients with AF of short duration.32 Flecainide terminated all AF episodes in the present study with a termination time comparable to that observed in previous studies,1, 27 and at the evaluated stimulation rates, the presented baseline values of aERP in horses were consistent with those reported by others.4, 33 Our study identified no flecainide‐induced changes in aERP, indicating that flecainide did not have an influence on atrial repolarization at the chosen stimulation rates. Depending on the model, flecainide has been reported to have variable effects on aERP in vivo.16, 17, 18, 19, 20 In humans, some reported a slight but not significant flecainide‐induced increase in aERP, whereas others reported significant and use‐dependent increases.20, 34 A flecainide‐induced reduction in aERP accommodation to atrial activation rates, which may be important in suppressing AF, has been demonstrated by others.18, 34, 35, 36 However, this mechanism of rate‐dependency and increased drug action at rapid atrial activation rates could not be proven by the present study. Still, despite the lack of aERP prolongation at the atrial rates measured in the present study, we cannot rule out possible increases in aERP during the rapid rates typical of AF. Our observations are in agreement with results from studies conducted in goats where aERP was unaffected, AFCL increased, and AF vulnerability unchanged by flecainide.17, 24 The variable flecainide‐induced changes in aERP may be related to species differences and species‐specific atrial ion channel composition.
Atrial fibrillation cycle length shortens when AF is maintained,4, 27, 37, 38 prolongs immediately before spontaneous or drug‐induced termination18 and might provide a risk assessment for AF recurrence.38 The observed flecainide‐induced increase in AFCL in the present study correlated well with the individual termination time (Tables S2, S3 in Data S1). However, in 2 horses the termination times were strikingly short and correlated with very limited increases in AFCL, indicating a questionable drug‐related termination of AF. However, in the majority of horses, the flecainide‐induced increase in AFCL corresponded well with results reported by others.2, 17, 24, 27 A single study investigating flecainide‐induced changes in AFCL in horses with spontaneous AF reported comparable increases, nevertheless none of the treated horses cardioverted in response to treatment.2 These conflicting results can most likely be explained by the difference in AF duration and hence differences in the degree of electrical remodeling. Van Loon et al. reported baseline AFCL values approximately 30 milliseconds shorter than ours, which supports the assumption of extensive electrical remodeling in the previous study.2 These findings emphasize the notion that AF should be terminated as quickly as possible to prevent electrophysiologic remodeling which will potentially drive the tissue into the cycle of “AF begets AF.”
In the present study, we found a flecainide‐induced decrease in induced AF duration, but no significant influence on AF vulnerability. No previous studies that were methodically comparable with regard to flecainide‐induced changes in AF duration and AF vulnerability were found in the literature. However, a study in dogs, investigating flecainide′s properties against AF‐promoted electrical remodeling identified no benefit of flecainide on either of the 2 parameters, and a goat model showed 100% reinducibility of AF after cardioversion with flecainide.24, 39 The results from the present study suggest that flecainide is effective in terminating acutely induced AF of short duration, has a positive diminishing effect on AF duration but does not protect against immediate reinduction of induced AF in healthy horses.
The safety profile of flecainide was evaluated by flecainide‐induced changes in QRS and QTc. QRS durations were measured as a surrogate for the effect of flecainide on ventricular conduction velocity, and our results were in agreement with results reported by others.1, 2 Because of sodium channel blockade, and hence depressant effects on cardiac conduction, a flecainide‐induced widening of QRS was expected.20, 40 In humans, recommendations are that flecainide treatment should be discontinued if QRS prolongation exceeds 25% of baseline.36, 41 The same limit of 25% is used in equine medicine to suggest quinidine toxicity,42 but such recommendations are not available for flecainide. However, because both quinidine and flecainide are sodium channel blockers, it might be reasonable to expect a similar limit to indicate flecainide toxicity. Toxicity was not indicated in any of the horses included in the present study because the maximum widening of QRS did not exceed 25% at any time during the experiments. However, the effect of flecainide is use‐dependent, and therefore flecainide‐induced changes in QRS may be increased at higher heart rates than reported in the present study. To investigate whether flecainide affected ventricular repolarization, we evaluated changes in QTc and JTc intervals. In a previous study of horses, no flecainide‐induced changes in QT intervals were reported.1 In our study, QTc was significantly prolonged between 6 and 25 minutes after flecainide infusion was started. To obtain a more specific ventricular repolarization time, JTc intervals were calculated, and in contrast to what has been documented in humans,20 flecainide temporarily prolonged JTc in horses. The present study however did not identify any potentially dangerous ventricular arrhythmias, which is in agreement with other studies.1, 3 However, 3 cases of flecainide‐induced wide QRS‐tachycardia have been reported in horses2, 4 of which 1 episode led to ventricular fibrillation and sudden cardiac death. Combined with the altered ventricular repolarization observed in the present study, it is important to recognize the potential proarrhythmic properties of flecainide.
Acutely induced AF in horses is a unique technique to mimic AF. The present study reports results on pacing‐induced short episodes of AF in healthy horses and the applicability to longer lasting spontaneous AF is therefore uncertain. Two studies investigated the antiarrhythmic potential and safety of flecainide on longer lasting naturally occuring AF in horses and reported disappointing results.2, 3 Only 1 of 14 attempts to terminate naturally occuring AF restored SR. In addition, the horse that responded positively to treatment had recent‐onset AF of only 12 days′ duration. Another study used flecainide to cardiovert persistent burst pacing‐induced AF of 7 days′ duration. Only 1 of 2 horses regained SR.4 Such observations support the theory of AF duration being an extremely important parameter when estimating the potential efficacy of flecainide, and that treatment with antiarrhythmic drugs always carries a risk of inducing devastating ventricular arrhythmias.
In conclusion, flecainide had clear antiarrhythmic effects terminating acute pacing‐induced AF of short duration (21 minutes) and resulted in decreased AF duration in healthy horses, but flecainide had no protective properties against immediate reinduction of AF and caused temporary prolongation in ventricular repolarization, which may be a potentially dangerous proarrhythmic effect.
Supporting information
Data S1. Materials and Methods.
Acknowledgments
We acknowledge all staff members at The Large Animal Teaching Hospital involved in the project and especially Peter Urban for help and assistance. The work was supported by The Danish Horse Levy Foundation.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
All practical work was carried out at The Large Animal Teaching Hospital, Department of Large Animal Sciences, University of Copenhagen, Denmark.
Footnotes
Carbocain 20 mg/kg, AstraZeneca, Copenhagen, DK
Intraflon 2 (PTFE), 12G, Vycon, Wiltshire, UK
One Piece/Tuohy‐Borst Catheter Introducer with Integral Hemostasis Valve, 8F, Argon Medical Devices, Holte, DK
Inquiry Steerable Diagnostic Catheter, 6F/110 cm, St. Jude Medical, Inc, Glostrup, DK
Kruuse, Langeskov, DK
LabChart 7 Pro, ADInstruments, Oxford, UK
Tambocor, 10 mg/mL, Meda AS, Allerød, DK
PM‐9000Vet, Portable Veterinary Monitor, Mindray, tail‐cuff system
Venosafe Lithium Heparin tubes, 9 mL, Terumo, SE
GraphPad Prism 5 Software, San Diego, CA
References
- 1. Ohmura H, Nukada T, Mizuno Y, et al. Safe and efficacious dosage of flecainide acetat for treating equine atrial fibrillation. J Vet Med Sci 2000;62:711–715. [DOI] [PubMed] [Google Scholar]
- 2. van Loon G, Blissitt KJ, Keen JA, et al. Use of intravenous flecainide in horses with naturally‐occurring atrial fibrillation. Equine Vet J 2004;36:609–614. [DOI] [PubMed] [Google Scholar]
- 3. Birettoni F, Porciello F, Rishniw M, et al. Treatment of chronic atrial fibrillation in the horse with flecainide: Personal observation. Vet Res Commun 2007;31:273–275. [DOI] [PubMed] [Google Scholar]
- 4. De Clercq D, van Loon G, Tavernier R, et al. Atrial and ventricular electrical and contractile remodeling and reverse remodeling owing to short‐term pacing‐induced atrial fibrillation in horses. J Vet Intern Med 2008;22:1353–1359. [DOI] [PubMed] [Google Scholar]
- 5. Leroux AA, Detilleux J, Sandersen CF, et al. Prevalence and risk factors for cardiac diseases in a hospital‐based population of 3,434 horses (1994–2011). J Vet Intern Med 2013;27:1563–1570. [DOI] [PubMed] [Google Scholar]
- 6. Deem DA, Fregin GF. Atrial fibrillation in horses: A review of 106 clinical cases, with consideration of prevalence, clinical signs and prognosis. J Am Vet Med Assoc 1982;180:261–265. [PubMed] [Google Scholar]
- 7. Reef VB, Reimer JM, Spencer PA. Treatment of atrial fibrillation in horses: New perspectives. J Vet Intern Med 1995;9:57–67. [DOI] [PubMed] [Google Scholar]
- 8. Reef VB, Bonagura J, Buhl R, et al. Recommendations for management of equine athletes with cardiovascular abnormalities. J Vet Intern Med 2014;28:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reef VB, Levitan CW, Spencer PA. Factors affecting prognosis and conversion in equine atrial fibrillation. J Vet Intern Med 1988;2:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Morris DD, Fregin GF. Atrial fibrillation in horses: Factors associated with response to quinidine sulfate in 77 clinical cases. Cornell Vet 1982;72:339–349. [PubMed] [Google Scholar]
- 11. McGurrin MK, Physick‐Sheard PW, Kenney DG. Transvenous electrical cardioversion of equine atrial fibrillation: Patient factors and clinical results in 72 treatment episodes. J Vet Intern Med 2008;22:609–615. [DOI] [PubMed] [Google Scholar]
- 12. McGurrin MKJ, Physick‐Sheard PW, Kenney DG, et al. Transvenous electrical cardioversion in equine atrial fibrillation: Technique and successful treatment of 3 horses. J Vet Intern Med 2003;17:715–718. [PubMed] [Google Scholar]
- 13. Khairy P, Nattel S. New insights into the mechanisms and management of atrial fibrillation. CMAJ 2002;167:1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 14. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–220. [DOI] [PubMed] [Google Scholar]
- 15. Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Circ Res 1977;41:9–18. [DOI] [PubMed] [Google Scholar]
- 16. Allessie MA, Wijffels MCEF, Dorland R. Mechanisms of pharmacologic cardioversion of atrial fibrillation by Class I drugs. J Cardiovasc Electrophysiol 1998;9:S69–S77. [PubMed] [Google Scholar]
- 17. Wijffels MC, Dorland R, Mast F, et al. Widening of the excitable gap during pharmacological cardioversion of atrial fibrillation in the goat: Effects of cibenzoline, hydroquinidine, flecainide, and d‐sotalol. Circulation 2000;102:260–267. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Pagé P, Nattel S. Mechanism of flecainide's antiarrhythmic action in experimental atrial fibrillation. Circ Res 1992;71:271–287. [DOI] [PubMed] [Google Scholar]
- 19. Rivard L, Sinno H, Shiroshita‐Takeshita A, et al. The pharmacological response of ischemia‐related atrial fibrillation in dogs: Evidence for substrate‐specific efficacy. Cardiovasc Res 2007;74:104–113. [DOI] [PubMed] [Google Scholar]
- 20. Hellestrand KJ, Bexton RS, Nathan AW, et al. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J 1982;48:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fast VG, Kleber AG. Role of wavefront curvature in propagation of cardiac impulse. Cardiovasc Res 1997;33:258–271. [DOI] [PubMed] [Google Scholar]
- 22. Kneller J, Leon J, Nattel S. How do class 1 antiarrhythmic drugs terminate atrial fibrillation?—A quantitative analysis based on a realistic ionic model. Circulation 2001;104:5–5. [Google Scholar]
- 23. Kneller J, Kalifa J, Zou R, et al. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically‐realistic mathematical model. Circ Res 2005;96:e35–e47. [DOI] [PubMed] [Google Scholar]
- 24. Wijffels MC, Dorland R, Allessie MA. Pharmacologic cardioversion of chronic atrial fibrillation in the goat by class IA, IC, and III drugs: A comparison between hydroquinidine, cibenzoline, flecainide, and d‐sotalol. J Cardiovasc Electrophysiol 1999;10:178–193. [DOI] [PubMed] [Google Scholar]
- 25. Kirchhof P, Engelen M, Franz MR, et al. Electrophysiological effects of flecainide and sotalol in the human atrium during persistent atrial fibrillation. Basic Res Cardiol 2005;100:112–121. [DOI] [PubMed] [Google Scholar]
- 26. Tuan J, Osman F, Jeilan M, et al. Increase in organization index predicts atrial fibrillation termination with flecainide post‐ablation: Spectral analysis of intracardiac electrograms. Europace 2010;12:488–493. [DOI] [PubMed] [Google Scholar]
- 27. Biffi M, Boriani G, Bronzetti G, et al. Electrophysiological effects of flecainide and propafenone on atrial fibrillation cycle and relation with arrhythmia termination. Heart 1999;82:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duytschaever M, Blaauw Y, Allessie M. Consequences of atrial electrical remodeling for the anti‐arrhythmic action of class IC and class III drugs. Cardiovasc Res 2005;67:69–76. [DOI] [PubMed] [Google Scholar]
- 29. Eijsbouts S, Ausma J, Blaauw Y, et al. Serial cardioversion by class IC Drugs during 4 months of persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 2006;17:648–654. [DOI] [PubMed] [Google Scholar]
- 30. Schwarzwald CC, Hamlin RL, Bonagura JD, et al. Atrial, SA Nodal, and AV nodal electrophysiology in standing horses: normal findings and electrophysiologic effects of quinidine and diltiazem. J Vet Intern Med 2007;21:166–175. [DOI] [PubMed] [Google Scholar]
- 31. Cardiac Arrhythmia Suppression Trial (CAST) Investigators . Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989;321:406–412. [DOI] [PubMed] [Google Scholar]
- 32. Camm AJ, Kirchhof P, Lip GYH, et al. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 33. Van Loon G, Duytschaever M, Tavernier R, et al. An equine model of chronic atrial fibrillation: Methodology. Vet J 2002;164:142–150. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe H, Watanabe I, Nakai T, et al. Frequency‐dependent electrophysiological effects of flecainide, nifekalant and d, l‐sotalol on the human atrium. Jpn Circ J 2001;65:1–6. [DOI] [PubMed] [Google Scholar]
- 35. O'Hara G, Villemaire C, Talajic M, et al. Effects of flecainide on the rate dependence of atrial refractoriness, atrial repolarization and atrioventricular node conduction in anesthetized dogs. J Am Coll Cardiol 1992;19:1335–1342. [DOI] [PubMed] [Google Scholar]
- 36. Aliot E, Capucci A, Crijns HJ, et al. Twenty‐five years in the making: Flecainide is safe and effective for the management of atrial fibrillation. Europace 2011;13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wijffels MCEF, Kirchhof CJHJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 38. De Clercq D, Decloedt A, Sys SU, et al. Atrial fibrillation cycle length and atrial size in horses with and without recurrence of atrial fibrillation after electrical cardioversion. J Vet Intern Med 2014;28:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shinagawa K, Shiroshita‐Takeshita A, Schram G, et al. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: Insights into the mechanism of the superior efficacy of amiodarone. Circulation 2003;107:1440–1446. [DOI] [PubMed] [Google Scholar]
- 40. Cros C, Skinner M, Moors J, et al. Detecting drug‐induced prolongation of the QRS complex: New insights for cardiac safety assessment. Toxicol Appl Pharmacol 2012;265:200–208. [DOI] [PubMed] [Google Scholar]
- 41. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014. doi:10.1016/j.jacc.2014.03.022 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Marr CM, Bowen IM, eds. Cardiology of the Horse, 2nd ed Edinburgh, UK: Saunders, Elsevier; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and Methods.


