Abstract
Giving strong prognostic information, T‐cell infiltration is on the verge of becoming an additional component in the routine clinical setting for classification of colorectal cancer (CRC). With a view to further improving the tools for prognostic evaluation, we have studied how Th1 lymphocyte infiltration correlates with prognosis not only by quantity, but also by subsite, within CRCs with different molecular characteristics (microsatellite instability, CpG island methylator phenotype status, and BRAF and KRAS mutational status). We evaluated the Th1 marker T‐bet by immunohistochemistry in 418 archival tumour tissue samples from patients who underwent surgical resection for CRC. We found that a high number of infiltrating Th1 lymphocytes is strongly associated with an improved prognosis in patients with CRC, irrespective of intratumoural subsite, and that both extent of infiltration and patient outcome differ according to molecular subgroup. In brief, microsatellite instability, CpG island methylator phenotype‐high and BRAF mutated tumours showed increased infiltration of Th1 lymphocytes, and the most pronounced prognostic effect of Th1 infiltration was found in these tumours. Interestingly, BRAF mutated tumours were found to be more highly infiltrated by Th1 lymphocytes than BRAF wild‐type tumours whereas the opposite was seen for KRAS mutated tumours. These differences could be explained at least partly by our finding that BRAF mutated, in contrast to KRAS mutated, CRC cell lines and tumour specimens expressed higher levels of the Th1‐attracting chemokine CXCL10, and reduced levels of CCL22 and TGFB1, stimulating Th2/Treg recruitment and polarisation. In conclusion, the strong prognostic importance of Th1 lymphocyte infiltration in CRC was found at all subsites evaluated, and it remained significant in multivariable analyses, indicating that T‐bet may be a valuable marker in the clinical setting. Our results also indicate that T‐bet is of value when analysed in molecular subgroups of CRC, allowing identification of patients with especially poor prognosis who are in need of extended treatment.
Keywords: colorectal cancer, Th1 lymphocytes, intratumoural subsites, molecular subgroups, BRAF, KRAS, prognosis
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer‐related death in the Western world 1. Curative therapy is based on surgical resection, but approximately 40% of patients die from metastatic disease. The success scores for accurate prediction of patient prognosis remain discouraging. In addition to the TNM staging system, the state of the tumour immune response has been shown to be critical for prognosis in CRC 2, 3, and implementation of an immunoscore in clinical practice is in progress, work initiated by J Galon et al 4.
The prognostic importance of immune cell infiltration is multifaceted and depends on the microenvironment of the tumour, immune cell composition and the dominant expression profiles of cytokines and chemokines 5. Inflammation can act in favour of cancer development and tumour progression but, in the local tumour immune response, different subsets of lymphocytes have been shown to act as potent suppressors of tumour growth 6, 7, 8. We and others have shown that infiltration of cytotoxic T‐lymphocytes (CTLs) and regulatory T‐lymphocytes (Tregs) results in a better prognosis in CRC patients 2, 9, 10. We also found that the intratumoural subsite of these two T‐cell subsets is important. The most significant prognostic effect of CTLs was found when they infiltrated the tumour epithelium, whereas Tregs proved a stronger prognosticator when analysed in the stroma surrounding the tumour.
CRC is a heterogeneous disease that develops through different molecular pathways, three of which have been well described 11: the classical microsatellite stable (MSS) adenoma to carcinoma pathway 12, which is responsible for approximately 85% of CRCs, the microsatellite instability (MSI) pathway first described by Ionov et al and Thibodeau et al 13, 14, and the CpG island methylator phenotype (CIMP) 15. An association has been shown between MSI and increased infiltration of immune cells, which might explain the better outcome in this patient group 16, 17. Another association has been seen between MSI tumours and BRAF mutation, where more than 50% of BRAF mutated tumours were found to be MSI 18, 19. Although the oncogenic protein KRAS is part of the same MAPK signalling pathway as BRAF, KRAS mutation is more often seen in MSS tumours. KRAS and BRAF mutations are mostly considered to be poor prognostic factors, mainly in MSS tumours 20, 21. The results on BRAF mutation in MSI tumours are inconclusive, with some studies showing that BRAF mutation worsens the prognosis 22 whereas other studies have found no difference in patient outcome between MSI/BRAF wild‐type and MSI/BRAF mutated groups 21, 23. Few studies have evaluated T‐cell subtypes in relation to molecular subgroups of CRC but it has been shown that MSI tumours seem to recruit a higher number of CTLs, T helper 1 (Th1) lymphocytes and CD45RO effector memory T lymphocytes compared to MSS tumours 24. As this may be important in the development of new prognostic tools and in therapeutic decisions, our aim is to study this further.
In the present study, we investigated the prognostic impact of Th1 lymphocytes, which have important functions in supporting the activity of CTLs 25. This was performed by immunohistochemical staining of the Th1 marker T‐bet. The degree of infiltrating T‐bet+ cells was assessed in 418 formalin fixed paraffin‐embedded (FFPE) archival tumour tissue sections from patients who underwent surgical resection for CRC. The average extent of infiltration along the tumour‐invasive front, in the centre of the tumour, and within the tumour epithelium was evaluated semiquantitatively using a four‐grade scale. The prognostic value of infiltrating T‐bet+ lymphocytes was also evaluated in molecular subgroups of CRC defined by MSI status, CIMP status and BRAF and KRAS mutational status.
Materials and methods
Study population
The tumour specimens used in this study were from the Colorectal Cancer in Umeå Study (CRUMS) 26. They were collected from patients who had undergone surgical resection for CRC between 1995 and 2003 at the Department of Surgery, Umeå University Hospital, Sweden. The handling of tissue samples and patient data in the present study was approved by the Regional Ethical Review Board of Umeå, Sweden, and in accordance with the Declaration of Helsinki. FFPE tissue was sampled from all patients and clinicopathological and molecular variables were defined according to procedures described by Dahlin et al 26, except for an updated follow‐up of survival, which was performed during the autumn of 2012. Using routine haematoxylin and eosin stained sections, lymphovascular invasion was recorded as present or absent (yes or no), and peritumoural lymphocytic infiltration was semiquantitatively recorded using a four‐graded scale, modified from Jass et al 27. Tumours were further divided into groups of low (1–2) or high (3–4) peritumoural lymphocytic infiltration. Altogether, 418 patients were included in the study. Exclusion criteria included an unavailable or insufficient tumour sample, and/or lack of clinical information. Preoperative radiation therapy was administered to 63 (45%) of the rectal cancer patients. Twenty‐eight patients were excluded from survival analysis, due to incomplete follow‐up data or to death from perioperative complications (death within 30 days of operation).
MSI screening status, CIMP status and BRAF and KRAS mutational status have previously been analysed in this patient cohort 23, 26. Briefly, MSI screening status was determined by immunohistochemistry with a positive MSI screening status (MSI) – in contrast to a negative screening status (MSS) – describing tissue samples with tumour cells lacking nuclear staining for one or more of the proteins MLH1, MSH2, MSH6 and PMS2. CIMP status was determined by evaluation of hypermethylation of an eight‐gene panel (CDKN2A, MLH1, CACNA1G, NEUROG1, RUNX3, SOCS1, IGF2 and CRABP1) by the MethyLight method (quantitative real‐time PCR) with previously described primer and probe sequences; CIMP‐negative tumours, 0 genes; CIMP‐low tumours, 1–5 genes; and CIMP‐high tumours, 6–8 genes. BRAFV600E mutation was determined by the Taqman allelic discrimination assay, described in 28 (with reagents from Applied Biosystems, Life Technologies, Stockholm, Sweden). Mutational analysis of KRAS was performed by sequencing of codon 12 and 13 using Big Dye v.3.1 (Applied Biosystems), which has been described previously 23.
Immunohistochemistry
For immunohistochemical staining, 4‐µm FFPE sections were cut, dried, de‐waxed, and rehydrated. T‐bet antibody (H‐210; Santa Cruz Biotechnology, Heidelberg, Germany) was used at a dilution of 1:50 on an automated Ventana Benchmark Ultra staining machine with the iVIEW DAB Detection Kit for visualisation (Ventana, Illkirch CEDEX, France). Immunohistochemical staining was evaluated with light microscopy as the most representative area at different intratumoural subsites: the invasive tumour front, the centre of the tumour, and within the tumour epithelium (intraepithelial expression) as previously described 9. T‐bet+ cells were semiquantitatively scored as 1–4: 1 (no/sporadic), 2 (moderate), 3 (abundant), or 4 (highly abundant), according to a previously published scale 26. The specimens were evaluated twice by the same observer under supervision of an experienced pathologist, and discordant cases were reviewed a third time, followed by a conclusive judgment. A total score was also obtained for each tumour according to Ogino et al and Dahlin et al 26, 29, adding together the information from T‐bet expression at the different subsites: T‐bet total score 3–4, low expression; 5–6, moderate expression; and 7–12, abundant expression. In our previously published work with this cohort, the expression of CD3, CD8, FOXP3, NOS2 and CD163 9, 26, 30 was analysed immunohistochemically.
Cell culture
The colon cancer cell line Caco2 (ATCC, Manassas, VA, USA) and its derivatives were grown in Dulbecco's modified Eagle's medium with glutaMAX supplemented with 10% fetal bovine serum (Gibco, Life Technologies, Stockholm, Sweden) and maintained at 37°C in an atmosphere of 5% CO2. The stable transfectants expressing mutant BRAF (Caco2‐BRAFV600E) or mutant KRAS (Caco2‐KRASG12V) have been described 31.
In situ and in vitro evaluation of cytokine and chemokine expression by semi‐quantitative reverse transcriptase PCR (RT‐PCR)
For analysis of expression of cytokine or chemokine genes in tumour tissues from 12 CRC patients, RNA was isolated from four FFPE‐embedded tumour sections (4 µM) per tumour using the High Pure RNA Paraffin Kit (Roche Diagnostics, Bromma, Sweden) and then converted to cDNA using the Superscript VILO cDNA Synthesis Kit (Invitrogen, Life Technologies, Stockholm, Sweden) according to the manufacturer's protocols. For cultured CRC cells, the NucleoSpin RNA Kit (Macherey‐Nagel, Duren, Germany) was used for isolation of total RNA, and cDNA was synthesised using Superscript II Reverse Transcriptase (Invitrogen). The primers used for normalisation were designed for FFPE sections, RPL13A forward: 5′‐GTACGCTGTGAAGGC‐3′ and reverse: 5′‐GTTGGT GTTCATCCG‐3′; and for cultured cells, GAPDH forward: 5′‐TGCACCACCAACTGCTTAGC‐3′ and reverse: 5′‐GGCATGGACTGTGGTCATGAG‐3′ (DNA Technology, Risskov, Denmark). The primers used for TGF‐β1 were, TGF‐β1 forward: 5′‐CCCAGCATCTGCAAAGCTC‐3′ and reverse: 5′‐GTCAATGTACAGCTGCCGCA‐3′ (DNA Technology). For the remaining genes, Quantitect Primer Assays (Quiagen, Sollentuna, Sweden) were used. The semi‐quantitative RT‐PCR‐reactions were run on a Taqman 7900HT (Applied Biosystems) and the following cycling parameters were used: 50°C for 2 min and then an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s.
Statistical analysis
PASW Statistics 22 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Cross‐tabulations were analysed with χ 2 tests and linear relationships were tested with the exact linear‐by‐linear association test. Correlations between categorical variables were analysed using the Spearman rank correlation test. The nonparametric Mann–Whitney U‐test was used for differences in continuous variables between groups. Kaplan–Meier survival analysis was used to estimate cancer‐specific survival, and the log‐rank test was used for comparisons of differences in outcome between groups. The definition of cancer‐specific survival was death with known disseminated or recurrent disease. Multivariable survival analyses were performed using Cox proportional hazard models. p < 0.05 was considered statistically significant.
Results
Distribution of T‐bet+ lymphocytes in CRC
The presence of Th1 lymphocytes was determined in specimens from 418 CRC patients by immunohistochemical evaluation of T‐bet expression (Figure 1). T‐bet expression was assessed at three different subsites: the invasive tumour front, the centre of the tumour and within the tumour epithelium (intraepithelial expression), as previously described 9. A total score was created by combining the scores for the different subsites. A modest to massive infiltration (total score 5–12) of T‐bet+ cells was found in 65% of tumours, while the remaining tumours showed weak or no infiltration (total score 3–4). The invasive front showed the highest numbers of infiltrating T‐bet+ cells, followed by the tumour centre. T‐bet+ cells within the tumour epithelium were rare.
Figure 1.
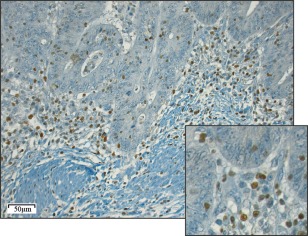
T‐bet immunoreactivity in CRC tumour specimens. Representative light microscopic image of immunohistochemical staining for T‐bet in a CRC specimen (20 objective magnification). The smaller square represents a close‐up using 40 objective magnification.
Infiltration of T‐bet+ Th1 lymphocytes was highly and positively correlated to infiltration of the previously analysed pan T‐lymphocytes (CD3+) 26, CTLs (CD8+) and regulatory T‐lymphocytes (FOXP3+) 9 (Table 1). T‐bet infiltration was also correlated to the previously analysed infiltration of macrophage subsets, where NOS2 and CD163, respectively, were used as markers to discriminate between macrophage subsets of a predominant M1 or M2 phenotype 30 (Table 1).
Table 1.
The correlation of expression of T‐bet and various immune cell markers
| Immune markers | Tbet r s | Tbet p‐value |
|---|---|---|
| CD3 | 0.617 | <0.001 |
| CD8 | 0.570 | <0.001 |
| FOXP3 | 0.572 | <0.001 |
| NOS2 | 0.298 | <0.001 |
| CD163 | 0.435 | <0.001 |
r s, Spearman's rank correlation coefficient. Total score 3–4, 5–6, 7–12, was used for correlations of Tbet, CD3, CD8 and FOXP3. For NOS2 and CD163, score 1–4 at the tumour front was used.
Infiltrating T‐bet+ lymphocytes in relation to clinicopathological parameters
The total score of infiltrating T‐bet+ cells in CRC specimens was correlated to clinicopathological variables (Table 2). No additional information was gained by relating these parameters to T‐bet infiltration at the different intratumoural subsites. Numbers of infiltrating T‐bet+ cells were increased in the right colon (p = 0.005) and reduced in preoperatively irradiated rectal tumours (p = 0.005). T‐bet expression also showed a strong inverse association with tumour stage (p = 0.005), lymphovascular invasion (p = 0.009) and peritumoural lymphocytic infiltration (p < 0.001).
Table 2.
Total score for T‐bet expression in relation to clinicopathological characteristics in CRC
| T‐bet Total score | ||||
|---|---|---|---|---|
| 3–4 | 5–6 | 7–12 | p‐value | |
| Frequency (%) | 107 (27.4) | 147 (37.7) | 136 (34.9) | |
| Gender, n (%) | 0.644 | |||
| Male | 58 (26.5) | 87 (39.7) | 74 (33.8) | |
| Female | 49 (28.7) | 60 (35.1) | 62 (36.3) | |
| Age, n (%) | 0.047/0.394a | |||
| ≤59 | 20 (27.4) | 24 (32.9) | 29 (39.7) | |
| 60–69 | 34 (35.4) | 29 (30.2) | 33 (34.4) | |
| 70–79 | 35 (25.9) | 63 (46.7) | 37 (27.4) | |
| ≥80 | 18 (20.9) | 31 (36.0) | 37 (43.0) | |
| Localization, n (%) | 0.005/0.001a | |||
| Right colon | 21 (17.4) | 47 (38.8) | 53 (43.8) | |
| Left colon | 40 (31.7) | 40 (31.7) | 46 (36.5) | |
| Rectum | 46 (32.9) | 58 (41.4) | 36 (25.7) | |
| Stage, n (%) | 0.005/<0.001a | |||
| I | 10 (18.2) | 20 (36.4) | 25 (45.5) | |
| II | 32 (21.1) | 59 (38.8) | 61 (40.1) | |
| III | 25 (30.5) | 33 (40.2) | 24 (29.3) | |
| IV | 39 (41.9) | 31 (33.3) | 23 (24.7) | |
| Lymphovascular invasion, n (%) | 0.009 | |||
| No | 62 (22.1) | 108 (38.6) | 110 (39.3) | |
| Yes | 26 (40.6) | 19 (29.7) | 19 (29.7) | |
| Grade, n (%) | 0.837 | |||
| Low | 52 (26.5) | 77 (39.3) | 67 (34.2) | |
| High | 54 (28.6) | 69 (36.5) | 66 (34.9) | |
| Growth pattern, n (%) | 0.175 | |||
| Pushing | 28 (22.2) | 47 (37.3) | 51 (40.5) | |
| Infiltrating | 78 (30.1) | 97 (37.5) | 84 (32.4) | |
| Histology type, n (%) | 0.496 | |||
| Mucinous | 10 (20.4) | 20 (40.8) | 19 (38.8) | |
| Nonmucinous | 96 (28.5) | 124 (36.8) | 117 (34.7) | |
| Peritumoural lymphocytic infiltration, n (%) | <0.001 | |||
| Low | 87 (41.0) | 83 (39.2) | 42 (19.8) | |
| High | 20 (11.6) | 61 (35.3) | 92 (53.2) | |
| Preoperative radiation therapy b , n (%) | 0.005/0.001a | |||
| No | 80 (24.7) | 122 (37.7) | 122 (37.7) | |
| Yes | 27 (42.9) | 23 (36.5) | 13 (20.6) | |
χ 2 tests were used for categorical variables.
Exact linear‐by‐linear association test was used to test for linear relationship between variables.
Preoperative radiation therapy in rectal cancers only.
Prognostic importance of infiltrating T‐bet+ lymphocytes in CRC
Cancer‐specific survival was addressed in CRC patients with different levels of infiltrating T‐bet+ cells. Figure 2 shows Kaplan–Meier plots of cancer‐specific survival in patients according to T‐bet infiltration at the different subsites: the invasive tumour front, the centre of the tumour or within the tumour epithelium (intraepithelial expression) – or as a total score. Increased infiltration of T‐bet+ cells (presented as a total score) was significantly associated with an improved prognosis (log‐rank p = 0.003) (Figure 2). A higher number of infiltrating T‐bet+ cells were also beneficial for prognosis at the different subsites (Figure 2). The prognostic importance of total score remained significant in a multivariable Cox regression analysis adjusting for stage, age, sex, localisation and preoperative radiation (HR 0.64, 95% CI 0.41–0.98, p = 0.041). T‐bet total score 3–4 (low) was used as the reference category.
Figure 2.
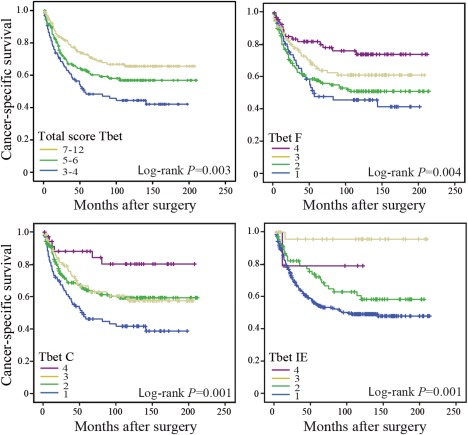
Cancer‐specific survival in CRC patients. Kaplan–Meier plots of cases scored for T‐bet total score: 3–4, low expression; 5–6, moderate expression; or 7–12, abundant expression; or T‐bet expression at intratumoural subsites: T‐bet F (front), T‐bet C (centre), and T‐bet IE (intraepithelium); score 1–4: 1 (no/sporadic), 2 (moderate), 3 (abundant), and 4 (highly abundant). Log‐rank test was used to calculate p‐values.
Infiltration of T‐bet+ lymphocytes in relation to molecular parameters of CRC and prognosis
The infiltration of T‐bet+ cells was investigated in molecular subgroups of CRC defined by MSI screening status, CIMP status and oncogenic mutations in BRAF (BRAFV600E) or KRAS (codon 12 and 13). T‐bet infiltration was significantly associated with tumours classified as MSI, CIMP‐high or BRAF mutated (p < 0.001, p = 0.027 and p < 0.001, respectively) (Table 3). In contrast, KRAS mutant tumours were less infiltrated by T‐bet+ cells (p = 0.005) (Table 3). Furthermore, highly infiltrated MSI tumours were more often found to be BRAF mutated (p = 0.016) (Table 3).
Table 3.
Total score for T‐bet expression in relation to molecular characteristics in CRC
| T‐bet | ||||
|---|---|---|---|---|
| Total score | 3–4 | 5–6 | 7–12 | p‐value |
| MSI screening statusa, n (%) | <0.001 | |||
| MSS | 93 (29.2) | 127 (39.9) | 98 (30.8) | |
| MSI | 12 (19.7) | 15 (24.6) | 34 (55.7) | |
| CIMP statusb, n (%) | 0.027 | |||
| CIMP‐negative | 58 (31.4) | 68 (36.8) | 59 (31.9) | |
| CIMP‐low | 41 (26.3) | 64 (41.0) | 51 (32.7) | |
| CIMP‐high | 8 (17.0) | 13 (27.7) | 26 (55.3) | |
| BRAF status, n (%) | <0.001 | |||
| Wild type | 98 (29.6) | 130 (39.6) | 103 (31.1) | |
| Mutated | 7 (13.2) | 14 (26.4) | 32 (60.4) | |
| KRAS status, n (%) | 0.005 | |||
| Wild type | 80 (25.9) | 108 (35.0) | 121 (39.2) | |
| Mutated | 26 (33.8) | 36 (46.8) | 15 (19.5) | |
| Combined MSI screening and BRAF status, n (%) | 0.016 | |||
| MSI wild type | 9 (30.0) | 10 (33.3) | 11 (36.7) | |
| MSI mutated | 3 (10.0) | 5 (16.7) | 22 (73.3) | |
| 0.433 | ||||
| MSS wild type | 87 (29.8) | 116 (39.7) | 89 (30.5) | |
| MSS mutated | 4 (18.2) | 9 (40.9) | 9 (40.9) | |
χ 2 tests were used for categorical variables.
Cases lacking nuclear staining of tumour cells for at least one of MLH1, MSH2, MSH6 or PMS2 were considered to have a positive MSI screening status.
Phenotype determined according to hypermethylation of an eight‐gene panel with the following number of hypermethylated genes found for CIMP‐negative, 0 genes; CIMP‐ low, 1–5 genes, and CIMP‐high, 6–8 genes. MSI, microsatellite instability; MSS, microsatellite stable; CIMP, CpG island methylator phenotype. BRAF mutated (V600E); KRAS mutated (codon 12 and 13).
The prognostic importance of infiltrating T‐bet+ cells was also assessed in the different molecular subgroups of CRC. In general, the effect of T‐bet infiltration on patient outcome varied slightly according to the different subgroups of CRC. A significantly improved prognosis was found for patients with MSI tumours that were more highly infiltrated by T‐bet+ cells (log‐rank p = 0.015) (Figure 3A). In MSS tumours, T‐bet infiltration was of borderline prognostic importance (log‐rank p = 0.074) (Figure 3A). For CIMP tumours, a stepwise increased prognostic relationship was found between T‐bet infiltration and CIMP‐negative tumours, CIMP‐low tumours, and CIMP‐high tumours (Figure 3B). Furthermore, infiltration of T‐bet+ cells had prognostic importance in subgroups of CRC, depending on their BRAF and KRAS mutations. Patients with BRAF mutated tumours with low T‐bet infiltration had an especially poor prognosis compared to the other two groups combined (Figure 3C) (log‐rank p = 0.022; p = 0.070 when comparing all three groups). T‐bet infiltration was also found to have prognostic importance in patients with BRAF wild‐type tumours (log‐rank p = 0.014) (Figure 3C) and KRAS wild‐type tumours (log‐rank p = 0.014) (Figure 3D). However, after adding MSI screening status, CIMP status and BRAF and KRAS mutation status to the multivariable Cox regression model presented above, the prognostic importance of T‐bet infiltration was found also to be independent of these molecular attributes (HR 0.58, 95% CI 0.37–0.92, p = 0.022).
Figure 3.
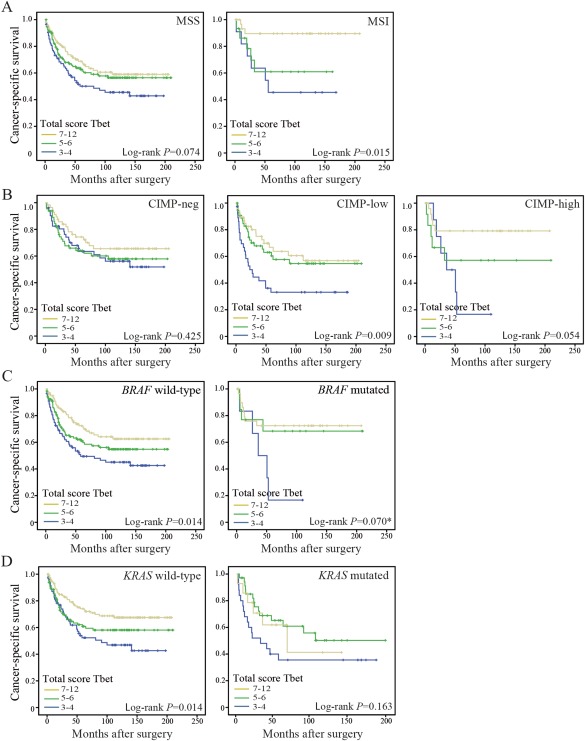
Cancer‐specific survival in molecular subgroups of CRC. Kaplan–Meier plots of patients with (A) MSS and MSI tumours; (B) CIMP‐neg, CIMP‐low and CIMP‐high tumours; (C) BRAF wild‐type and mutated (V600E) tumours; and (D) KRAS wild‐type and mutated (codon 12 and 13) tumours. Cases were scored for T‐bet total score: 3–4, low expression; 5–6, moderate expression; or 7–12, abundant expression. Log‐rank test was used to calculate p‐values. *Log‐rank p = 0.022 when comparing BRAF mutated tumours with low T‐bet infiltration to the other two groups combined.
Cytokine and chemokine expression in BRAF and KRAS mutated CRC cell lines and tumour specimens
It was an interesting finding that tumours with BRAF mutation were more highly infiltrated with T‐bet+ cells than BRAF wild‐type tumours, while tumours with KRAS mutations, a signalling protein in the same signalling pathway, were less infiltrated with T‐bet+ cells than KRAS wild‐type tumours. We, therefore, wanted to investigate whether these mutations differentially affected the expression of cytokines (IL6, IL10 and TGF‐β1) and chemokines (CCL5, CXCL10, CCL22 and CCL24) that regulate the recruitment and polarisation of T‐lymphocytes 5, 32, 33. Using semi‐quantitative RT‐PCR, we analysed expression of cytokine and chemokine genes in the CRC cell line Caco2 (wild‐type in BRAF and KRAS) and stable transfectants expressing either mutant BRAF (Caco2‐BRAFV600E) or mutant KRAS (Caco2‐KRASG12V). While some of the cytokines/chemokines were not expressed at all, or at very low levels, we found that Caco2‐BRAFV600E cells expressed significantly higher levels of the Th1‐attracting chemokine CXCL10 than Caco2 cells and Caco2‐KRASG12V cells (Figure 4A). In addition, Caco2‐BRAFV600E cells expressed significantly lower levels of CCL22 and TGFB1, which stimulate the Th2/Treg axis, than Caco2 and Caco2‐KRASG12V cells. To confirm our in vitro findings, we also analysed the expression of CXCL10, CCL22 and TGFB1 by semi‐quantitative RT‐PCR in tumour specimens from CRC patients carrying oncogenic mutations in BRAFV600E or KRAS (codon 12 and 13). We found that expression of CXCL10 was significantly higher in tumours with the BRAF mutation than in tumours with KRAS mutation (p = 0.010) (Figure 4B), while the expression of both CCL22 and TGFB1 tended to be lower in BRAF mutated tumours (p = 0.150 and p = 0.262, respectively).
Figure 4.
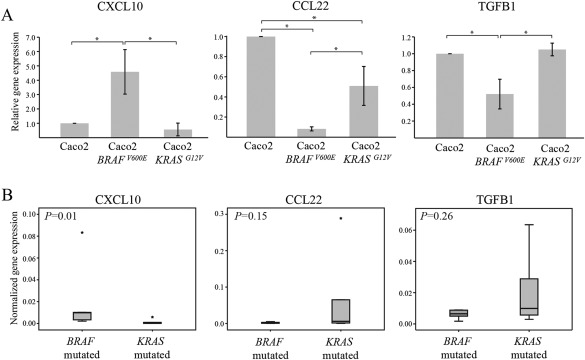
Expression of cytokine and chemokine genes in CRC. (A) Expression of CXCL10, CCL22 and TGFB1 was analysed by semi‐quantitative RT‐PCR in (A) human colon cancer cells Caco2, Caco2 cells stably expressing BRAFV600E (Caco2‐BRAFV600E), and Caco2 cells stably expressing KRASG12V (Caco2‐KRASG12V). Shown is the fold gene expression from four independent experiments ±standard deviation, with Caco2 control cells set as 1. Significant differences are indicated by * (p < 0.05). (B) Expression of CXCL10, CCL22 and TGFB1 was analysed by semi‐quantitative RT‐PCR in tumour specimens carrying oncogenic mutations in either BRAF (V600E) (n = 6) or KRAS (codon 12 and 13) (n = 6), and illustrated with box plots. Normalised gene expression is represented as the mean of 2‐ΔCt for each sample normalised against RPL13A. Outlier values (o) and far‐out values (*) are indicated.
Discussion
Since the establishment of tumour immunity as a key player in tumour control, the prognostic importance of different types of immune cells has been investigated. When designing an immunoscore as a component of cancer classification 4, it is important to find the T‐cell subtypes that give the strongest prognostic information. As pointed out by Galon et al the evaluation of a novel marker, among other traits, should be feasible, reproducible, standardized, pathology‐based and powerful. The markers used at present are CD3 and CD8, analysed in two regions; the tumour invasive margin and tumour centre 34.
Previous studies have shown that Th1 immunity has beneficial effects on prognosis in CRC 2, 35. Here, we have studied the extent of Th1 lymphocyte infiltration and its prognostic value in different molecular subgroups of CRC. In our analysis, we assessed Th1 infiltration not only according to quantity but also according to subsite within the tumour. We found that the prognostic importance of Th1 lymphocyte infiltration seems not to be dependent on the intratumoural subsite and is significant even within molecular subgroups of CRC. The prognostic importance of Th1 lymphocyte infiltration remained significant in multivariable analysis, which indicates that T‐bet is a suitable general prognostic marker in the scoring of immune cell infiltration in the clinical setting and may be a good complement to the Immunoscore. In our hands, T‐bet appears to be an even more valuable marker than CD8, as the prognostic importance of CD8‐positive lymphocytes was lost in multivariable analyses when evaluated at the tumour front and centre 9. The strongest prognostic importance was found for CD8‐positive intraepithelial lymphocytes, underscoring the need to identify the most important tumour subsite of the different infiltrating T cells. We found that the prognostic importance of Th1 cells, even though they are rarely present within the tumour epithelium, does not depend significantly on the intratumoural subsite. This could perhaps be explained by the fact that Th1 cells do not engage in close contact with tumour cells themselves. Instead, cytokine excretion by Th1 cells stimulates the recruitment and activation of the CTLs that have direct anti‐tumour activities 25. Previous studies have shown an association between high Th1 cell infiltration and improved prognosis in several types of cancer 5 – not only in CRC 2, 35, 36, but also, for example, in breast carcinoma 37, gastric cancer 38 and renal cell carcinoma 39.
CRC is a disease of vast heterogeneity. Researchers such as Tejpar et al have taken on the daunting task of subclassifying CRC further according to differences in clinical, molecular and pathological features 20, 40. When evaluating Th1 infiltration in molecular subgroups of CRC defined by CIMP status, MSI status and BRAF and KRAS mutation status, we found significant differences in the extent of infiltration and prognostic value. In our previous study on CTLs and Tregs 9, we could see that Th1 infiltration varies significantly more than that of both CTLs and Tregs when analysed in subgroups of MSI and CIMP. Furthermore, BRAF mutated tumours were more highly infiltrated by Th1 lymphocytes than BRAF wild‐type tumours, while the opposite was true of tumours mutated in KRAS, a signalling protein in the same signalling pathway. When stratifying for MSI screening status, we further found that MSI tumours highly infiltrated by Th1 lymphocytes were frequently BRAF mutated, suggesting that BRAF mutation may contribute to the prognostic importance of MSI in CRC.
We found a significantly better prognosis in patients with MSI tumours highly infiltrated with Th1 cells and a particularly poor prognosis in CIMP‐high and BRAF mutated tumours with a low extent of Th1 cell infiltration. In general, however, even though numbers of infiltrating Th1 cells varied according to molecular attributes, the prognostic importance of Th1 infiltration was found in multivariable analysis to be independent of MSI and CIMP status and of BRAF and KRAS mutation status.
The contrasting Th1 infiltration seen in BRAF and KRAS mutated tumours caused us to search for possible differences in the expression of chemokines and cytokines that regulate the recruitment and polarisation of T‐lymphocytes in BRAF and KRAS mutated CRC cells. We found that BRAF mutated CRC cells expressed significantly higher levels of the Th1‐attracting chemokine CXCL10 compared to KRAS mutated and KRAS wild‐type CRC cells. Furthermore, BRAF mutated CRC cells expressed significantly lower levels of CCL22 and TGFB1, which stimulate Th2/Treg recruitment and polarisation, compared to KRAS mutated and KRAS wild‐type CRC cells. A strength of our in vitro data is that the role of BRAF and KRAS mutation in the regulation of cytokine/chemokine secretion was studied in the same genetic and epigenetic background in Caco2 cells (MSS, CIMP negative, BRAF and KRAS wild type 41). When cytokine/chemokine secretion was compared in BRAF and KRAS mutated CRC cell lines with different genetic backgrounds, the results were contradictory 42. However, our in vitro findings could also reflect the results of later analysis of the expression of CXCL10, CCL22 and TGFB1 in tumour specimens from CRC patients carrying oncogenic mutations in either BRAF or KRAS. Boissière‐Michot et al have found MSI CRC to express higher levels of CXCL10 24 and, considering the close association between BRAF mutational status and MSI, one could suspect the differences found here, in our study, to be MSI status related. The CRC cell line used was, however, MMR proficient 41 and the differences in cytokine and chemokine expression in the tumour specimens were not entirely dependent on MSI status. Furthermore, in a previous study on this patient cohort we found a tendency that BRAF mutation was of prognostic importance even within the subgroup of MSI 23 (p = 0.090 for the present study). Together, these results suggest that MSI is not the only contributor to the prognostic importance of BRAF mutation and our findings would explain – at least partially – the higher and lower infiltration of Th1 lymphocytes seen in BRAF and KRAS mutated tumours, respectively, and why BRAF mutated tumours are generally more highly infiltrated. The cytokine‐ and chemokine‐expression analysis was, however, performed in only a small proportion of these tumours. Studies on a larger scale are needed to verify these findings. An association between KRAS mutation and decreased expression of genes related to Th1 immunity has been previously suggested 43.
In immunohistochemical analyses, there is often the question of marker exclusivity. T‐box transcription factor T‐bet is essential for effector Th1 lymphocyte development 44 and thus strongly linked to this T‐cell subset. There have, however, been studies showing that other cell types also express T‐bet. For example, Natural killer cells have been shown to require the expression of T‐bet for their development and maintenance 45, and activated Tregs are believed to express T‐bet (at least transiently) in order to maintain homeostasis 46, 47. So there are findings that complicate the matter of exclusivity, but T‐bet is currently the most specific marker for Th1 lymphocytes 24. In this study, the extent of infiltrating T‐bet+ lymphocytes was evaluated semiquantitatively. Compared to a quantitative automated method, the method advocated in the Immunoscore, this has the disadvantage of interobserver variability but also the advantage – considering the heterogeneous T‐cell dispersion within a single tumour section – of identifying the different tumour compartments and avoiding necrotic areas. A strength of this study was that T‐bet expression was analysed in a large number of CRC patients with a long follow‐up time (>200 months), whose tumours had undergone both clinico‐pathological and molecular characterisation.
In conclusion, this study has shown that high Th1 lymphocyte infiltration is strongly associated with a better prognosis in patients with CRC, independently of intratumoural subsite, indicating that T‐bet is a potentially valuable marker in the clinical setting. Our findings that the extent of Th1 infiltration, and patient outcome, differ in different molecular subgroups of CRC also suggest that T‐bet may be of additional value if analysed in subgroups of CRC as a way of selecting patients with an especially poor prognosis who are in need of extended treatment.
Statement of Author Contributions
AL, IVL, SE and RP conceived the study. AL, IVL, VE, MLW, ÅÖ, SE and RP collected and analysed data. AL, IVL, SE and RP interpreted data. AL, IVL, SE and RP were involved in writing the paper. All authors critically reviewed and gave final approval of the submitted version.
Acknowledgements
We are grateful to Kerstin Näslund for expert technical assistance and Dr. Anna Dahlin for previous evaluations in CRUMS.
Disclosure of potential conflicts of interest: The authors disclose no potential conflicts of interest.
Contract/grant details: This study was supported by grants from the Swedish Cancer Society (CAN2011/839 RP), Swedish Research Council (B03488901 RP), Cancer Research Foundation in Northern Sweden (LP 12‐1959 SE) and the Syskonen Svenssons Foundation for Medical Research (2014 SE).
References
- 1. Siegel R, Ma J, Zou Z, et al Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Galon J, Costes A, Sanchez‐Cabo F, et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 3. Mlecnik B, Tosolini M, Kirilovsky A, et al Histopathologic‐based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 4. Galon J, Pages F, Marincola FM, et al The immune score as a new possible approach for the classification of cancer. J Transl Med 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fridman WH, Pages F, Sautes‐Fridman C, et al The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 7. Svennevig JL, Lunde OC, Holter J, et al Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer 1984;49:375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ropponen KM, Eskelinen MJ, Lipponen PK, et al Prognostic value of tumour‐infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997;182:318–324. [DOI] [PubMed] [Google Scholar]
- 9. Ling A, Edin S, Wikberg ML, et al The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer 2014;110:2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salama P, Phillips M, Grieu F, et al Tumor‐infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009;27:186–192. [DOI] [PubMed] [Google Scholar]
- 11. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007;50:113–130. [DOI] [PubMed] [Google Scholar]
- 12. Vogelstein B, Fearon ER, Hamilton SR, et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988;319:525–532. [DOI] [PubMed] [Google Scholar]
- 13. Ionov Y, Peinado MA, Malkhosyan S, et al Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558–561. [DOI] [PubMed] [Google Scholar]
- 14. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–819. [DOI] [PubMed] [Google Scholar]
- 15. Toyota M, Ahuja N, Ohe‐Toyota M, et al CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999;96:8681–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–618. [DOI] [PubMed] [Google Scholar]
- 17. Deschoolmeester V, Baay M, Lardon F, et al Immune Cells in Colorectal Cancer: prognostic Relevance and Role of MSI. Cancer Microenviron 2011;4:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weisenberger DJ, Siegmund KD, Campan M, et al CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787–793. [DOI] [PubMed] [Google Scholar]
- 19. French AJ, Sargent DJ, Burgart LJ, et al Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 2008;14:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sinicrope FA, Shi Q, Smyrk TC, et al Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015;148:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samowitz WS, Sweeney C, Herrick J, et al Poor survival associated with the BRAF V600E mutation in microsatellite‐stable colon cancers. Cancer Res 2005;65:6063–6069. [DOI] [PubMed] [Google Scholar]
- 22. Ogino S, Shima K, Meyerhardt JA, et al Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res 2012;18:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eklof V, Wikberg ML, Edin S, et al The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer 2013;108:2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boissiere‐Michot F, Lazennec G, Frugier H, et al Characterization of an adaptive immune response in microsatellite‐instable colorectal cancer. Oncoimmunology 2014;3:e29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szabo SJ, Kim ST, Costa GL, et al A novel transcription factor, T‐bet, directs Th1 lineage commitment. Cell 2000;100:655–669. [DOI] [PubMed] [Google Scholar]
- 26. Dahlin AM, Henriksson ML, Van Guelpen B, et al Colorectal cancer prognosis depends on T‐cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011;24:671–682. [DOI] [PubMed] [Google Scholar]
- 27. Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet 1987;1:1303–1306. [DOI] [PubMed] [Google Scholar]
- 28. Benlloch S, Paya A, Alenda C, et al Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real‐time chemistry methodology. J Mol Diagn 2006;8:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogino S, Nosho K, Irahara N, et al Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009;15:6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edin S, Wikberg ML, Dahlin AM, et al The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PloS One 2012;7:e47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lundberg IV, Lofgren Burstrom A, Edin S, et al SOX2 expression is regulated by BRAF and contributes to poor patient prognosis in colorectal cancer. PLoS One 2014;9:e101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mantovani A, Sica A, Sozzani S, et al The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 33. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889–896. [DOI] [PubMed] [Google Scholar]
- 34. Galon J, Mlecnik B, Bindea G, et al Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014;232:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tosolini M, Kirilovsky A, Mlecnik B, et al Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011;71:1263–1271. [DOI] [PubMed] [Google Scholar]
- 36. Camus M, Tosolini M, Mlecnik B, et al Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009;69:2685–2693. [DOI] [PubMed] [Google Scholar]
- 37. Oldford SA, Robb JD, Codner D, et al Tumor cell expression of HLA‐DM associates with a Th1 profile and predicts improved survival in breast carcinoma patients. Int Immunol 2006;18:1591–1602. [DOI] [PubMed] [Google Scholar]
- 38. Ubukata H, Motohashi G, Tabuchi T, et al Evaluations of interferon‐gamma/interleukin‐4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 2010;102:742–747. [DOI] [PubMed] [Google Scholar]
- 39. Kondo T, Nakazawa H, Ito F, et al Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1‐type immune response. Cancer Sci 2006;97:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tejpar S, Bertagnolli M, Bosman F, et al Prognostic and predictive biomarkers in resected colon cancer: current status and future perspectives for integrating genomics into biomarker discovery. Oncologist 2010;15:390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mouradov D, Sloggett C, Jorissen RN, et al Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res 2014;74:3238–3247. [DOI] [PubMed] [Google Scholar]
- 42. Khan S, Cameron S, Blaschke M, et al Differential gene expression of chemokines in KRAS and BRAF mutated colorectal cell lines: role of cytokines. World J Gastroenterol 2014;20:2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lal N, Beggs AD, Willcox BE, et al An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. Oncoimmunology 2015;4:e976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullen AC, High FA, Hutchins AS, et al Role of T‐bet in commitment of TH1 cells before IL‐12‐dependent selection. Science 2001;292:1907–1910. [DOI] [PubMed] [Google Scholar]
- 45. Gordon SM, Chaix J, Rupp LJ, et al The transcription factors T‐bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012;36:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu F, Sharma S, Edwards J, et al Dynamic expression of transcription factors T‐bet and GATA‐3 by regulatory T cells maintains immunotolerance. Nat Immunol 2015;16:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koch MA, Tucker‐Heard G, Perdue NR, et al The transcription factor T‐bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009;10:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]


