Abstract
The reported incidence of local recurrence of peripheral atypical lipomatous tumours is highly variable and is likely to reflect the different inclusion criteria of cases, and the design of previous studies. We aimed to study the incidence of local recurrence of 90 cases of atypical lipomatous tumours and an additional 18 cases of de novo dedifferentiated liposarcoma. All tumours were diagnosed on the basis of MDM2 amplification: all patients had their first treatment in the same specialist sarcoma unit and were followed for a minimum of 60 months. The tumours were diagnosed between 1997 and 2009 and followed until the end of 2014. Seventy cases (78%) of atypical lipomatous tumours were located in the thigh (mean size 195 mm on presentation). Eight atypical lipomatous tumours (8.9%) recurred locally, of which 50% recurred after 60 months. The only two tumours with intralesional excisions recurred. Seven of the eight recurrent tumours were detected by the patient by self‐examination. One case recurred a second time as a dedifferentiated liposarcoma. Seventeen per cent of the de novo dedifferentiated liposarcomas recurred within 60 months of presentation. Extending the study period revealed that atypical lipomatous tumour could recur up to 40 years after the first surgery. Furthermore, of 26 tumours that recurred in the extended study, 27% recurred more than once, and three of the seven that recurred more than once transformed into a dedifferentiated liposarcoma. We recommend that, following post‐operative wound care, patients with atypical lipomatous tumour are referred back to their general practitioner for follow up, but that in the event of a suspected recurrence they have rapid access back to the specialist unit using a ‘supported discharge’ scheme. In the event of an intralesional excision and if a lesion recurs, patients are followed in a specialist unit at regular intervals: whether MRI scanning is a valuable means of monitoring such patients is unclear and requires an evidence base
Keywords: atypical lipomatous tumour, well differentiated liposarcoma, dedifferentiated liposarcoma, fluorescence in situ hybridisation, MDM2
Introduction
Benign and malignant lipomatous neoplasms account for approximately 50% of all soft tissue tumours. Liposarcoma, including the atypical lipomatous tumours (ALT) and dedifferentiated (DDLS), myxoid and pleomorphic variants, is the single most common soft tissue sarcoma of adulthood, accounting for approximately 20% of all soft tissue sarcomas. ALT account for approximately 45% of all liposarcomas: these well‐differentiated low grade lipomatous tumours are slow‐growing and locally aggressive, and only show potential for metastasis following dedifferentiation. The clinical outcome of centrally sited (retroperitoneal and chest cavity) tumours is well recognised as being poor, with recurrence and mortality being as high as 80% over a period of 10–20 years 1. In contrast, the documented incidence of local recurrence for peripheral ALT appears to be considerably lower but highly variable [2–8] ranging from 8% 2, 9 to 27% 1, 5 to 52% 4. The variation in these figures requires explanation.
In view of the widely variable reported incidence of local recurrence of peripheral ALT, which is likely to reflect the different criteria for inclusion into the studies, our primary aim was to provide an evidence base on which to plan clinical management and follow‐up of patients presenting with a peripheral ALT. This was achieved by generating robust information on the clinical outcome of 90 patients who presented with peripherally‐sited ALT: the tumours were diagnosed using the strict criterion of the presence of tumour MDM2 amplification, and all patients had their first surgical treatment in our specialist unit and were followed for a minimum of 60 months. The secondary aim of the project was to identify the percentage of ALT that presented with a dedifferentiated component and to determine the risk of recurrence of these tumours.
Materials and methods
The study was undertaken as part of a departmental audit and was also covered by ethical approval EC17.14 under Human Tissue Authority license 12055. An electronic search of the pathology archive was undertaken for all peripherally‐sited ALT, presenting between January 1997 and June 2009, including those with and without a de novo dedifferentiated component. This provided a patient cohort with minimum of a 60 months follow‐up. The first search included only those patients who had their first surgery at a specialist sarcoma centre and only those tumours with MDM2 amplification were included.
The search was then extended to include all patients diagnosed with ALT (all with MDM2 amplification) and comprised patients who had their first surgical treatment outside the specialist sarcoma centre. The purpose was to determine the range of time from primary resection of ALT to local recurrence and to increase the numbers to identify how often recurrent tumours recurred more than once and how often these transformed into dedifferentiated liposarcoma (secondary DDLS).
All cases were reviewed by experienced histopathologists (AMF, RT, MFA). The diagnoses of ALT and DDLS were classified according to criteria reported in 2013 10. ALT were subclassified as ‘lipoma‐like’, sclerosing, inflammatory, and DDLS with heterologous differentiation 10. Fluorescence in situ hybridisation (FISH) for MDM2 amplification was performed on all samples.
Surgical excision was considered ‘marginal’ if tumour was covered with less than 10 mm of normal or reactive tissue and ‘wide’ if excised with 10 mm or more of normal or reactive surrounding tissue. Surgical excision was classified as intralesional if tumour was exposed during the surgical procedure.
Fluorescence in situ hybridisation
FISH for MDM2 amplification was performed on all samples. In brief, and as previously described, a full tissue block section from each lipomatous neoplasm was examined by FISH using the ZytoLight SPEC MDM2/CEN12 Dual Colour Probe kit (ZytoVision GmbH, Bremerhaven, Germany). This probe cocktail decorates the human chromosomal region MDM2 with a green signal and the alpha satellite centromeric region of chromosome 12 (D12Z3 sequences) with a red signal. The centromere of chromosome 12 (CEN12) is detected as a strong, intense red signal, whereas integrated alpha satellite 12 sequences (alphoid 12 signals) located at ring or marker chromosome are detected as faint signals compared with that of CEN12. FISH was performed according to the manufacturer's protocol 11.
Evaluation of the sampled tissue sections was carried out using fluorescence microscopy on an Olympus BX‐51 (Olympus, Southall, Middlesex, UK). The number of each signal for MDM2 (green) and CEN12 (centromere 12; red) was scored by counting a minimum of 50 nonoverlapping nuclei per case, and the average number of MDM2 and CEN12 signals was then calculated. A ratio of greater than 2.0 was considered to represent MDM2 amplification, a ratio of less than or equal to 2 was considered to be nonamplified. Regardless of the MDM2: CEN12 ratio, an average of 3 or more signals for CEN12 was considered to represent copy number gain of both CEN12 and MDM2 (referred to by others previously as aneusomy or polysomy). All FISH was reviewed by at least two experienced individuals.
Results
Atypical lipomatous tumours: 1997–2009 (n = 90)
Ninety‐six patients, 53 men and 43 women, presented with ALT between 1997 and 2009 but six patients died of another disease before reaching 60 months follow‐up and these were excluded from the study. The mean age of the remaining 90 patients at presentation was 57 years (median: 61; range: 22–92). The most common location was the thigh (n = 70); other less common locations were: upper arm/shoulder (n = 5), buttock (n = 3), groin (n = 4), lower leg/calf (n = 4), axilla (n = 3) and back (n = 1). The mean size of these tumours at diagnosis was 195 mm (range: 35–340 mm): only two cases measured less than 50 mm. Eighty‐eight were classified as ‘lipoma‐like’ variant, one as sclerosing variant and one as inflammatory variant.
All 90 patients who presented with ALT were followed for a minimum of 60 months. The median and mean follow‐up period of 90 individuals was 96 and 112 months, respectively. Eight of the 90 (8.9%) tumours recurred locally: seven recurred once and one recurred twice. The earliest recurrence was at 12 months, the latest was at 156 months and four of the eight recurrences occurred after 60 months (Table 1). In the one patient whose tumour recurred twice, the second recurrence presented as a DDLS (110 mm). All patients with recurrences are alive and none of the tumours has metastasised. Seven of the eight recurrent tumours were detected by self‐examination by the patient. The other was detected by follow‐up magnetic resonance scanning.
Table 1.
Clinical data on 8 of 90 patients presenting with recurrent ALT between 1997 and 2009
| Site | Margin status | Year of presentation and tumour size at each presentation | Follow‐up period from first presentation (months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary tumour | Tumour size (mm) | First recurrence | Tumour size (mm) | Second recurrence | Tumour size (mm) | ||||
| Case 1 | Thigh | Marginal | 1997 | 245 | 2010 | 210 | 2012 | 110 | 192 |
| Case 2 | Leg | Intralesional | 1999 | 265 | 2013 | 110 | 168 | ||
| Case 3 | Axilla | Marginal | 2006 | 240 | 2013 | 110 | 84 | ||
| Case 4 | Thigh | Marginal | 2002 | 230 | 2008 | 180 | 132 | ||
| Case 5 | Thigh | Marginal | 1999 | 235 | 2004 | 75 | 156 | ||
| Case 6 | Arm | Marginal | 2008 | 100 | 2012 | 60 | 60 | ||
| Case 7 | Thigh | Marginal | 2008 | 340 | 2012 | 90 | 60 | ||
| Case 8 | Thigh | Intralesional | 2005 | 170 | 2006 | 130 | 96 | ||
The majority of tumours (88/90) were resected with a marginal/complete excision margin (Figure 1). Two cases had documented evidence of intralesional excisions, and both of these recurred. The mean size of the eight recurrent tumours (first recurrent events) was 133 mm (range 75–280 mm). The size of the first recurrence in each case was smaller than the primary tumour (one‐tailed t‐test, p = 0.0043). The size of the case which recurred a second time (secondary DDLS) was 110 mm and was smaller than the presenting tumour (245 mm) and the first recurrence (280 mm).
Figure 1.
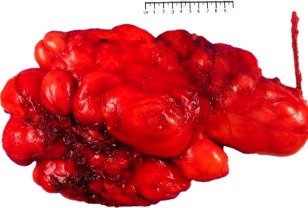
Large bosselated ALT which appears to be completely excised and covered by a thin film of loose fibrous tissue (marginal excision). Maximum dimension of tumour = 240 mm.
Extended study
In addition to the 90 cases of ALT included in the primary study (vide supra), an extended search of our archive revealed an additional 175 cases giving a total of 265 cases that presented to the RNOH between 1997 and 2014. Of these, 155 cases presented after 2009 and, therefore, had limited follow‐up. Furthermore, 18 of this extended group presented at RNOH with recurrent disease. One of these recurrent cases (thigh) presented at RNOH 40 years after the first excision. Twenty‐six (including eight in the primary study) of the 265 (10%) cases presented with recurrent ALT: 19 (73%) recurred once as ALT and 7 (27%) recurred more than once as ALT: 3 of the latter (n = 7) presented as secondary DDLS on the final recurrence. Details of these seven tumours are shown in Table 2. The mean size of the recurrent tumours was 143 mm (range 40–250 mm). Only two cases measured less than 100 mm. The majority (89%) of these tumours were resected with a complete/marginal excision margin.
Table 2.
Clinical data on 7 of 26 patients who had more than one tumour recurrence
| Site | Year of presentation and tumour size at each presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary tumour | Tumour size (mm)a | First recurrence | Tumour size (mm) | Second recurrence | Tumour size (mm) | Third recurrence | Tumour size (mm) | Fourth recurrence | Tumour size (mm) | ||
| Case 1 | Thigh | 1984 | Unknown | 1994 | 270 | 2004 | 230 | 2009 | 220 | 2013 | 190 |
| Case 2 | Thigh | 1981 | Unknown | 1987 | 240 | 2007 | 210 | 2011 | 110 | ||
| Case 3 | Forearm | 1995 | Unknown | 2004 | 140 | 2005 | 90 | ||||
| Case 4 | Arm | 1999 | Unknown | 2003 | 120 | 2010 | 90 | ||||
| Case 5 | Thigh | 2002 | Unknown | 2004 | 100 | 2006 | 170 | ||||
| Case 6 | Thigh | 1995 | Unknown | 1999 | 160 | 2005 | 50 | ||||
| Case 7 | Thigh | 1997 | 245 | 2010 | 210 | 2012 | 110 | ||||
All tumours with an unknown size did not have surgery at RNOH.
These patients had their primary surgery anytime from 1980 onwards.
De novo dedifferentiated liposarcoma: 1997–2009 (n = 18)
Eighteen peripherally‐sited de novo DDLS were identified during 1997–2009, representing 16% of all ALT (those with and without a de novo dedifferentiated component). This patient group comprised nine men and nine women with a mean age of 60 (range: 30–86 years). The anatomical locations of the de novo DDLS were similar to that of the fully mature ALT: thigh (n = 8), upper arm/shoulder (n = 4), buttock (n = 2) and lower leg/calf (n = 2) and chest wall (n = 2). &&The mean size of theses tumours at first presentation was 161 mm (range 40–360 mm). Two of 18 cases had a heterologous component, one with an osteosarcomatous component and the other with a rhabdomyosarcomatous component (Figure 2).
Figure 2.
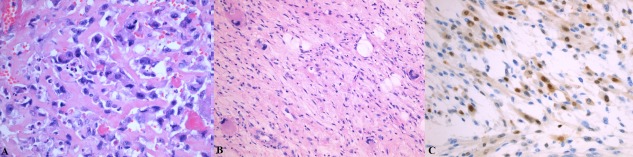
Light photomicrographs of de novo dedifferentiated ALT showing evidence of heterologous differentiation. (a) Haematoxylin and eosin‐stained section showing osteosarcomatous differentiation with osteoid deposition [HE, × 200]. (b) Rhabdomyosarcomatous differentiation [HE, × 100] is confirmed by (c) myogenin immunoreactivity [×200].
Of the 18 patients who presented with a de novo DDLS, 11 were followed for a minimum of 60 months; the other seven patients died before this time, but only two died of their disease. The median and mean follow‐up period was 72 months, and 75 months, respectively. Three (17%) of the de novo DDLS recurred, two recurring locally once and a third case recurring locally once and subsequently developing metastatic disease at two sites. All three tumours recurred within 60 months of presentation. The mean size of the recurrent tumours was 220 mm (range 160–290 mm).
Discussion
We studied 90 patients with ALT, presenting between 1997 and 2009, all of whom underwent their first resection at a single specialist sarcoma unit, and showed that with a minimum follow‐up of 60 months the recurrence rate was 8.9%. Fifty percent of the tumours recurred within the first 60 months, with the remainder recurring up to 12 years later. One patient represented 40 years following their first surgical excision indicating that relapse can occur several decades after the first treatment.
There have been numerous reports of the incidence of recurrence of peripheral ALT, based on datasets that are often not comparable 12. In some studies, the follow up period is short, a factor highlighted by Kooby et al 8 who showed that in their cohort (mean follow‐up 47 months) recurrences only occurred beyond 60 months. Whereas this is likely to under‐represent the number of recurrences, recurrences may be over‐represented in studies that include a significant number of patients referred with relapsed disease to a specialist unit, whose initial surgery was not undertaken in a specialist unit. This is supported by the observation that the publications with the highest incidence of recurrent disease are reported from large highly specialist centres and, therefore, likely to reflect selection bias 1, 4, 7. This is further highlighted in a study by Mavrogenis et al 12 in which the local recurrence rate in patients diagnosed and treated at their institution at the time of first presentation was 10.6%, which is not dissimilar to our figure of 8.9%. However, when patients referred for treatment of recurrent disease were included in their calculations, the recurrence rate increased to 25% 12. Our incidence of recurrence increased to 10% when we included patients referred with recurrent disease. However, this figure is not a true reflection of the recurrence rate for two reasons: firstly patients referred with a recurrent tumour introduces a selection bias into the calculation, and secondly this group includes 155 patients with ALT who have had limited follow up (less than 60 months).
Distinguishing peripheral ALT from large lipomas using microscopy alone is challenging 7, 11, 13. The presence of either ring chromosomes and/or giant marker chromosomes detected by cytogenetics or MDM2 amplification by FISH or PCR has been shown by several groups to be valuable for distinguishing these tumours 5, 7, 9, 11, 13, 14. Zhang et al 7 and Neuville et al 13 showed that this molecular test resulted in the diagnosis of ALT being changed to lipoma in 21% (11/52) and 18% (6/33) of cases respectively, but that the reverse was much lower (∼2%) 7. In the past, this molecular biomarker was not always used 1, 2, 8, 12, 15 and, therefore, is also likely to have contributed to the different reported incidence of recurrence of ALT.
Since ALT are frequently large at presentation (mean of 195 mm, range of 35–340 mm in this study), and often have a complex, bosselated contour, they are surgically challenging. In the UK, it is recommended, that such tumours are excised at specialist sarcoma units and this is likely to account for the low incidence of ALT (2/96) that were found to have demonstrable intralesional excisions. In the two cases with obvious intralesional excision, a clinical decision was made that this was the appropriate surgical management to preserve the adjacent neurovascular structure. However, as both tumours recurred, it suggests that intralesional excision is a significant risk factor for recurrence and highlights the need for detailed examination of the gross specimen by the pathologist.
Local recurrence is associated with an increased risk of secondary dedifferentiation which has metastatic potential 4, 12, 16: this was confirmed in our extended study, which included ALT presenting outside the 1997–2009 period and patients referred from other hospitals because of recurrent disease. These data showed that three of seven patients with multiple recurrent ALT developed a dedifferentiated component either at or subsequent to the second recurrence. Taken together, the findings underscore the need for appropriate initial surgical management.
ALT are low grade neoplasms and the majority presents as large tumours (>10 cm): by definition they are slow‐growing and therefore are likely to have been present for many years before presentation. In view of the evidence that dedifferentiation is time‐dependent 1, it is therefore not surprising that we identified that 17% (18 of 108) of all ALT at presentation had a dedifferentiated component. This figure is consistent with the reports by others 1, 3, 4. De novo DDLS behave more aggressively than conventional ALT: of our 18 cases, 17% recurred, behaviour comparable to that of other high grade sarcomas of the limb 17. As with other intermediate and high grade sarcomas in our centre, DDLS is frequently treated with post‐operative radiotherapy and patients are monitored according to the ESMO/ European Sarcoma Network Working Group, 2012 guidelines 17.
Tumours are conventionally classified as benign and malignant, although these categories often do not reflect the wide spectrum of behaviour of tumours. ALT is an example of the challenge of conveying to patients the degree of seriousness of a disease which some doctors refer to as cancer, whereas others may refer to it is as a tumour with a low risk of recurrence and may even label it as benign. The robust outcome data for patients with ALT in this study will help doctors clarify for their patients what they want to know about their disease. However, acquiring such data can be challenging if a disease is rare, and this is compounded when the tumour is slow‐growing and recurs late but highlights the needs for specialist hospitals.
There is considerable difference in opinion as to how best follow up patients treated for excision of peripherally sited ALT. Should such patients be seen regularly and indefinitely on the basis that a tumour can recur as late as 40 years, or discharged on the basis that only 8% recur within 5 years (if treated in a specialist unit)? The recent publication by Cassier et al 18 further complicates the issue because in their multicentre study including 283 ALT of the extremities, trunk and girdle (median follow up of 61.7 months) they found that adjuvant radiotherapy reduces the risk of local relapse. However, they comment that the use of radiotherapy for a ‘benign’ tumour should be considered with caution, because of the potential side effects of radiotherapy (in particular, risk of second malignancy). On the evidence accrued from our study, we consider that there is justification for the current practice used in our institution: our guidelines require a post‐operative assessment at approximately 6 weeks following surgery and the patients are educated about self‐examination for detection of tumour recurrence. There is good evidence that this is effective as seven of the eight patients with local recurrence in our study detected their recurrent disease on self‐examination. Provided there is good wound healing and no other complications, the patient is given a ‘supported discharge’ note for their general practitioners: this provides them with rapid and direct access to our specialist care unit if they have concerns about possible recurrent disease. However, if an intralesional excision is performed the patient is assessed at regular intervals by clinical examination and magnetic resonance imaging, although we acknowledge that the benefit of monitoring patients using the latter is not evidence‐based and requires prospective data collection.
Contract/grant details
The research was funded by Skeletal Cancer Action Trust (SCAT), UK. Support was provided by the National Institute for Health Research, NIHR UCLH Biomedical Research Centre (AMF, BMS) and the CRUK UCL Experimental Medicine Cancer Centre (AMF). We are grateful to Siobhan Roche, RNOH, for her technical support.
Author contributions
The study was designed by AMF: data collection was made by SNK, CT, GF, KL, BS, POD, RP, RT and MFA; data analysis, and interpretation was made by AMF, SNK, BS, HTY, NP, RT and FA; the literature search, generation of figures was performed by SNK and RT; the manuscript was written by AMF and SNK.
Acknowledgements
We are grateful to the patients for participating in the research and to the clinicians and support staff in the London Sarcoma Service involved in their care. The research was undertaken as part of the RNOH Musculoskeletal Research Programme and Biobank.
Disclosure/Conflict of Interest: We would like to declare that there are no known conflicts of interest.
References
- 1. Weiss SW, Rao VK. Well‐differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow‐up study of 92 cases with analysis of the incidence of “dedifferentiation”. Am J Surg Pathol 1992; 16: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 2. Sommerville SM, Patton JT, Luscombe JC, et al. Clinical outcomes of deep atypical lipomas (well‐differentiated lipoma‐like liposarcomas) of the extremities. ANZ J Surg 2005; 75: 803–806. [DOI] [PubMed] [Google Scholar]
- 3. Lucas DR, Nascimento AG, Sanjay BK, et al. Well‐differentiated liposarcoma. The mayo clinic experience with 58 cases. Am J Clin Pathol 1994 1994; 102: 677–683. [DOI] [PubMed] [Google Scholar]
- 4. Rozental TD, Khoury LD, Donthineni‐Rao R, et al. Atypical lipomatous masses of the extremities: Outcome of surgical treatment. Clin Orthop Relat Res 2002; 398: 203–211. [DOI] [PubMed] [Google Scholar]
- 5. Bassett MD, Schuetze SM, Disteche C, et al. Deep‐seated, well differentiated lipomatous tumors of the chest wall and extremities: The role of cytogenetics in classification and prognostication. Cancer 2005; 103: 409–416. [DOI] [PubMed] [Google Scholar]
- 6. Serpell JW, Chen RY. Review of large deep lipomatous tumours. ANZ J Surg 2007; 77: 524–529. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Erickson‐Johnson M, Wang X, et al. Molecular testing for lipomatous tumors: Critical analysis and test recommendations based on the analysis of 405 extremity‐based tumors. Am J Surg Pathol 2010; 34: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 8. Kooby DA, Antonescu CR, Brennan MF, et al. Atypical lipomatous tumor/well‐differentiated liposarcoma of the extremity and trunk wall: Importance of histological subtype with treatmentrecommendations. Ann Surg Oncol 2004; 11: 78–84. [DOI] [PubMed] [Google Scholar]
- 9. Billing V, Mertens F, Domanski HA, et al. Deep‐seated ordinary and atypical lipomas: Histopathology, cytogenetics, clinical features, and outcome in 215 tumours of the extremity and trunk wall. J Bone Joint Surg Br Vol. 2008; 90: 929–933. [DOI] [PubMed] [Google Scholar]
- 10. Chistopher DM, Fletcher JAB, Pancras CW, et al. WHO classification of tumours of soft tissue and bone. IARC Press, Lyon, 2013; 33–38. [Google Scholar]
- 11. Kashima T, Halai D, Ye H, et al. Sensitivity of MDM2 amplification and unexpected multiple faint alphoid 12 (alpha 12 satellite sequences) signals in atypical lipomatous tumor. Mod Pathol 2012; 25: 1384–1396. [DOI] [PubMed] [Google Scholar]
- 12. Mavrogenis AF, Lesensky J, Romagnoli C, Alberghini M, Letson GD, Ruggieri P. Atypical lipomatous tumors/well‐differentiated liposarcomas: Clinical outcome of 67 patients. Orthopedics 2011; 34: e893–898. [DOI] [PubMed] [Google Scholar]
- 13. Neuville A, Ranchere‐Vince D, Dei Tos AP, et al. Impact of molecular analysis on the final sarcoma diagnosis: A study on 763 cases collected during a european epidemiological study. Am J Surg Pathol 2013; 37: 1259–1268. [DOI] [PubMed] [Google Scholar]
- 14. Sirvent N, Coindre JM, Maire G, et al. Detection of mdm2‐cdk4 amplification by fluorescence in situ hybridization in 200 paraffin‐embedded tumor samples: Utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real‐time PCR. Am J Surg Pathol 2007; 31: 1476–1489. [DOI] [PubMed] [Google Scholar]
- 15. Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: A study of 61 cases, with a minimum follow‐up of 10 years. Am J Surg Pathol 2007; 31: 1–14. [DOI] [PubMed] [Google Scholar]
- 16. Folpe AL, Weiss SW. Lipoleiomyosarcoma (well‐differentiated liposarcoma with leiomyosarcomatous differentiation): A clinicopathologic study of nine cases including one with dedifferentiation. Am J Surg Pathol 2002; 26: 742–749. [DOI] [PubMed] [Google Scholar]
- 17. Rothermundt C, Whelan JS, Dileo P, et al. What is the role of routine follow‐up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 2014; 110: 2420–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassier PA, Kantor G, Bonvalot S, et al. Adjuvant radiotherapy for extremity and trunk wall atypical lipomatous tumor/well‐differentiated lps (alt/wd‐lps): A French sarcoma group (gsf‐geto) study. Ann Oncol 2014; 25: 1854–1860. [DOI] [PubMed] [Google Scholar]


