Abstract
Brain tumour stem cells and microglia both promote the growth of astrocytomas, the commonest form of primary brain tumour, with recent emerging evidence that these cell types may interact in glioma models. It is unclear whether microglia and stem cells are associated in human gliomas. To investigate this question, we used the technique of tissue microarrays to perform a correlative study of a large number of tumour samples. We quantified immunostaining of human astrocytic tumour tissue microarrays (86 patients; World Health Organisation grade II–IV) for microglia Ionized calcium binding adaptor molecule 1 (Iba1) and CD68, and stem cell nestin, SOX2 and CD133. Ki67 was used to assess proliferation and GFAP for astrocytic differentiation. Immunoreactivity for both microglial markers and stem cell markers nestin and SOX2 significantly increased with increasing tumour grade. GFAP was higher in low grade astrocytomas. There was a positive correlation between: (i) both microglial markers and nestin and CD133, (ii) nestin and tumour cell proliferation Ki67 and (iii) both microglial markers and Ki67. SOX2 was not associated with microglia or tumour proliferation. To test the clinical relevance, we investigated the putative association of these markers with clinical outcomes. High expression for nestin and Iba1 correlated with significantly shorter survival times, and high expression for nestin, Iba1, CD68 and Ki67 was associated with faster tumour progression on univariate analysis. On multivariate analysis, nestin, CD133 and Ki67 remained significant predictors of poorer survival, after adjustment for other markers. These results confirm previous in vitro findings, demonstrating their functional relevance as a therapeutic target in humans. This is the first report of a novel correlation between microglia and stem cells that may drive human astrocytic tumour development.
Keywords: glioma, microglia, stem cells, brain tumour
Introduction
The commonest primary brain tumours are gliomas, graded I–IV according to the World Health Organisation (WHO) system based on histological features 1. Glioblastoma (GBM) is the most aggressive form (grade IV) which despite advances in surgery, radiotherapy and chemotherapy has a median survival of around 14 months 2. The ‘cancer stem cell hypothesis’ proposes that tumour initiation and propagation is driven by tumour stem cells (brain‐tumour initiating cells). These cells typically comprise a small percentage of the total tumour cell population and have the characteristics of normal stem cells, such as the ability to self‐replicate, differentiate and create a cellular hierarchy with heterogeneous cells 3. A variety of cell markers have been investigated to identify stem cells, perhaps the most well‐known ones being CD133, nestin and SOX2. CD133 (prominin‐1) was first identified in membrane protrusions of mouse neuroepithelial stem cells 4. CD133 has also been proposed as a marker for cancer stem cells as it is highly expressed in tumour‐initiating cells of brain tumours 5 and high levels of CD133 correlate with poor outcomes in paediatric medulloblastoma 6.
Animal models provided additional support for this idea with the injection of 100 CD133+ cells into a mouse brain can generate a tumour 7. Studies with human GBMs have demonstrated that CD133 identifies a sub‐population of glioma stem cells although not all glioma stem cells are positive for this marker 8, 9, 10. Moreover, CD133+ cells promote resistance of GBM to radiotherapy by activating the DNA damage checkpoint following radiation 11. Another marker of stem cells is nestin, which was originally used to identify adult neural stem cells in the subventricular zone, yet also found to mark a subpopulation of endogenous glioma stem cells that can propagate glioma growth after chemotherapy 12. Both nestin and CD133 predict a poorer prognosis in glioma patients 13, 14, 15. SOX2 is important for self‐renewal of neural stem cells and is found in GBM. Silencing of this gene abrogates proliferation and tumourigenicity in immunodeficient mice 16.
During development, microglia the macrophage of the brain, interact with normal neural stem cells, promoting their growth 17 and evidence is mounting that microglia may support glioma growth. Indeed, microglia form a substantial population of glioma cells, up to a third in some cases 18, 19, 20. In the presence of glioma cells, microglia lack the ability to produce pro‐inflammatory cytokines (eg tumour necrosis factor (TNF) alpha, IL1 and IL6), to up‐regulate co‐stimulatory molecules and to be stimulated by Toll‐like receptors and, thus, appear unable to mount an effective immune response against gliomas 21, 22, 23. Indeed, the microglial cytokine profile in gliomas is consistent with an anti‐inflammatory (M2) rather than a pro‐inflammatory (M1) phenotype 24, 25 which may favour tumour propagation. In gliomas, microglia are associated with high grade and poor prognosis 24 and recently in animal models, inhibition of CSF‐1R was found to alter the phenotype of glioma‐infiltrating microglia such that their tumour‐promoting functions were impaired, and this was associated with a reduced tumour growth 26.
Recent work suggests that microglia interact with brain‐tumour initiating cells. Glioma stem cells induced an immunosuppressive phenotype in microglia in vitro 27, and microglia enhanced the invasiveness of these tumour‐initiating cells 28. Moreover, microglia cultured from glioma patients were unable to reduce sphere‐forming capacity of brain‐tumour initiating cells but microglia from non‐glioma patients could do so. We aim to investigate whether there is any association between microglia and stem cells in human tumour samples that would demonstrate the relevance of this recent experimental work in vivo. Tissue microarrays (TMAs) are a relatively novel tool for exploring protein expression from a large number of tissue samples, ideal for exploring correlations between molecular and clinic‐pathological findings, onto a single slide for analysis at one time 29, 30. The TMA allows the high‐throughput assessment of biomolecules in tissue samples in a more standardized manner, reducing the variability that may be seen when performing assays on individual samples 31. Here, use of TMAs allowed analysis of many tumour samples by immunohistochemistry with a panel of markers. Using human glioma TMAs, we sought to determine if microglial markers (CD68 and Ionized calcium binding adaptor molecule 1 [Iba1]) are associated with key stem cell markers (CD133, nestin and SOX2), and whether such markers correlate with proliferation (Ki67).
Methods
Ethical approval
The study was performed under ethical approval from Southampton and South West Hampshire Local Research Ethics Committees (Reference No: REC 10/H0504/32).
Cases
Biopsies of astrocytic tumours (WHO grades II–IV) from 83 consecutive patients who had given consent for research were obtained from the archives of the Department of Cellular Pathology, Southampton General Hospital, UK (27 grade II, 27 grade III, and 29 grade IV). Samples of solid tumour without necrosis were used to construct TMA paraffin blocks. Clinical data were collected retrospectively from the hospital records. The age range of all the patients was 3–77 years (median 51 years; 51 male, 35 female – Table 1). All the samples used for grade IV tumours were from primary GBMs, in that they were not from tumours which were previously diagnosed as low grade and then subsequently transformed to a higher grade. The median age of grade IV tumours was significantly higher than of grade II and III tumours [(p < 0.001), one‐way analysis of variance (ANOVA)], but there was no significant difference in ages of patients with grade II and III tumours (p = 0.051). Tumour locations included occipital, frontal, parietal and temporal in both hemispheres. The majority of patients had surgical resection and postoperative chemotherapy and/or radiotherapy. Median follow‐up duration was 28 months, ranging from two weeks (the shortest survival time) to 131 months. Two patients were lost to follow‐up, but clinical data was obtained for 81 of the 83 patients. With the exception of one patient who died from multiple cerebral infarcts (which themselves were likely to be related to the tumour), all deaths in the follow‐up were due to the underlying glioma.
Table 1.
Characteristics of the patients
| Male | Female | Mean age in years (± standard deviation) | Number of tumours with diameter >6cm | |
|---|---|---|---|---|
| Grade II | 15 | 12 | 34.5 (±18.3) | 7 |
| Grade III | 18 | 9 | 44.3 (±15.2) | 9 |
| Grade IV | 18 | 11 | 61.8 (±7.81) | 7 |
Immunohistochemistry
Ten micrometre sections of formalin‐fixed paraffin‐embedded tissue from the tumour TMA blocks were immunostained in a single batch. Immunohistochemistry was performed using the appropriate antigen retrieval methods for each primary antibody. Biotinylated secondary antibodies (rabbit anti‐mouse, swine anti‐rabbit and rabbit anti‐goat) were from Dako, normal serum and avidin‐biotin complex were from Vector Laboratories. Bound antibody was visualized using the avidin‐biotin‐peroxidase complex method (Vectastain Elite ABC) with 3′3 diaminobenzidine as chromogen and 0.05% hydrogen peroxide as substrate to obtain a brown precipitate. All sections were dehydrated before to be mounted in DePeX (BDH Laboratory Supplies).
The primary antibodies used were: microglia: Iba1 (all microglia‐Wako Laboratories), CD68 (phagocytic activity – clone PG‐M1, Dako); stem cell: CD133 (orb18124, Biorbyt), nestin (ab22035, Abcam), SOX2 (clone Y‐17, Santa Cruz Biotechnology); GFAP (glial acid fibrillary protein – Z0334, Dako), and Ki67 (cell proliferation – clone MIB1, Dako).
Quantification of the immunostaining in human gliomas
The TMAs were scanned using the Olympus dotSlide system (×20 objective) and TMA software (Olympus, UK). Image analysis of the immunostaining was performed with ImageJ (Wayne Rasband, National Institutes of Health (NIH), Bethesda, MD, version 1.47g) to obtain protein load defined as the percentage area of the image stained. The use of protein load as the standard quantification measure for all immunostaining in human glioma samples provided consistency within the measurement allowing fair comparisons between sample groups.
Statistical analysis
Data were assessed for normality using the Shapiro–Wilk test and through the examination of quantile–quantile plots. For comparison between tumour grades, one‐way ANOVA or a Kruskal–Wallis test were used for normally and non‐normally distributed data, respectively. To analyse relationships between markers, Spearman's rank correlation coefficient was used as the data were non‐parametric. Linear regression analysis was further used to test whether combinations of immunohistochemical markers improved prediction of tumour proliferation (Ki67). To compare baseline demographic characteristics of the patient cohorts, student's tests were used. Kaplan–Meier plots have been used when looking at survival outcomes. The log‐rank test has been used to test for significant differences in survival between patients with tumours containing different protein loads of specified markers. Two clinical outcomes were assessed: overall survival being the time from clinical diagnosis to death, and time for tumour progression being the time from diagnosis to either progression of the tumour on magnetic resonance imaging (MRI) scan in the absence of surgical resection (eg enhancement, larger size) or to recurrence on imaging of the tumour after surgery. For overall survival, cases were censored if they were alive at the most recent follow‐up, and for tumour progression cases were censored if there was no progression to higher grade or recurrence at the last follow‐up or if they had died before such events occurred. Multivariate analysis combining all stem cell, microglial and proliferative markers was performed by Cox regression to identify significant predictors of overall and progression‐free survival. In addition, hazard ratios (HR) were obtained from the Cox‐regression analysis. p values less than 0.05 have been considered statistically significant throughout. Data analysis was performed using IBM SPSS Statistics version 20.
Results
GFAP and Ki67
As expected, all tumours contained GFAP positive cells, reflecting their glial differentiation (Figure 1A). GFAP load was higher in grade II than grade III tumours (median 74.7% vs 59.9%; p = 0.034) reflecting the greater degree of differentiation of low grade tumours, but was not significantly different in grade IV tumours. Quantification of Ki67, which labels cells in the cell cycle and, therefore, assesses cell proliferation, showed an increase from grade II to III (mean 0.20% vs 1.12%; p < 0.001) and grade III to IV (mean 1.12% vs 3.10%; p = 0.003), consistent with increased cell proliferation with increasing grade of tumour (Figure 1B).
Figure 1.

Quantification and illustration of the different markers in astrocytic tumours of grade II to IV. (A) GFAP is significantly decreased between tumours grade II and III (p = 0.034). (B) The proliferation marker Ki67 shows a significant increase in grade IV compared to grades II (p < 0.001) and III (p = 0.003). Microglial association with tumour growth is demonstrated by (C) the microglial marker Iba1 which is significantly increased between grades (II–III: p = 0.003; II–IV: p = 0.020) and (D) the increased microglial protein CD68 between grades II and IV (p < 0.001). For the stem cell markers, (E) nestin is significantly increased in tumour grade IV compared to grade II (p = 0.005) and grade III (p < 0.001), as also observed for (F) SOX2 (II–IV: p = 0.025; III–IV: p = 0.005). (G) CD133 shows a trend to increase in tumour between grades II to IV. Scale bar = 50μm.
Microglial markers
Iba1 immunostaining revealed substantial numbers of microglial cells within the tumours. Iba1 load was higher in grade IV tumours than grade II (mean 12.02% vs 3.43%; p = 0.003) and III (mean 12.02% vs 6.08%; p = 0.020) tumours (Figure 1C). Similarly, there was a higher CD68 load in grade IV tumours than in grade II (median 8.59% vs 2.42%; p < 0.001) tumours, but there was no significant difference when comparing CD68 staining in grade III astrocytomas with the other grades. Overall, the data showed that both microglial markers increased significantly with tumour grade.
Stem cell markers
The nestin load was significantly higher in grade IV tumours than grade II or III (median 13.05% vs 0.82; p = 0.005 – median 13.05% vs 0.79%; p < 0.001; Figure 1E). The SOX2 load was also higher in grade IV tumours than grade II (median 1.40% vs 0.43%; p = 0.025) and grade III (median 1.40% vs 0.56%; p = 0.005; Figure 1F). The CD133 load showed a non‐significant trend to increase in grade IV compared to grades II and III (Figure 1G). Overall, the data showed that the stem cell markers nestin and SOX2 increased significantly with tumour grade.
Correlations between markers
Correlations between markers were performed across all tumour grades. GFAP correlated with CD133 (ρ = 0.325; p = 0.008; Figure 2A), Ki67 with both microglial markers Iba1 (ρ = 0.352; p = 0.006; Figure 2B), CD68 (ρ = 0.369; p = 0.002; Figure 2C), and also with nestin: (ρ = 0.447; p < 0.001; Figure 2D). There was a correlation between both microglial markers Iba1 and CD68 (ρ = 0.634; p < 0.001; Figure 2E) and between the stem cell markers nestin and CD133 (ρ = 0.312; p = 0.0015; Figure 2F). The analysis of microglial and stem cell markers demonstrated highly significant correlations between Iba1 and nestin (ρ = 0.613; p < 0.001; Figure 2G), Iba1 and CD133 (ρ = 0.355; p = 0·.006; Figure 2H), CD68 and nestin (ρ = 0.519; p < 0.001; Figure 2I) and CD68 and CD133 (ρ = 0·471; p < 0.001; Figure 2J).
Figure 2.
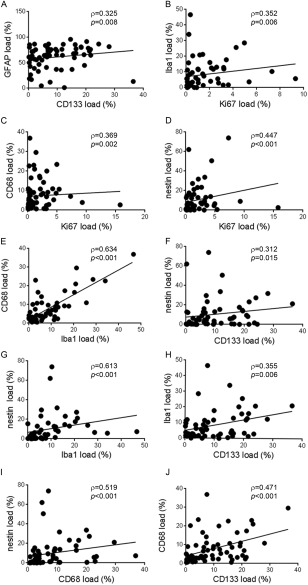
Relationship between glial differentiation, proliferation, stem cells and microglia. (A) GFAP shows a positive correlation with cancer stem cell CD133 (p = 0.008). Ki67 is positively correlated with microglial markers (B) Iba1 (p = 0.006) and (C) CD68 (p = 0.002), and with (D) stem cell marker nestin (p < 0.001) indicating that both tumour stem cells and microglia correlate with proliferation. Statistical analysis shows (E) both microglial markers are associated (p < 0.001) as well as (F) the stem cell markers nestin and CD133 (p = 0.015); but also a positive correlation between microglia and stem cells with Iba1 correlated with (G) nestin (p < 0.001) and (H) CD133 (p = 0.006) and CD68 related to (I) nestin (p < 0.001) and (J) CD133 (p < 0.001).
Predictors of tumour development and patient survival
We tested whether any of the markers predicted overall survival of patients and/or clinical development of the tumours. Statistical modelling demonstrated significant differences when the top 20% of the data were used for Ki67, Iba1, CD68 and CD133 and the top 50% for nestin. Log‐rank tests demonstrated, illustrated by Kaplan–Meier plots, that a high Ki67 load (top 20%) gave both poorer overall survival (p = 0.033; median survival of high Ki67 = 11 months, low Ki67 = 30 months; HR = 2.2; Figure 3A) and faster tumour progression (p = 0.006; median survival of high Ki67 = 13 months, low Ki67 = 30 months; HR = 3.28; Figure 3D). High nestin load (top 50%) was also associated with worse overall survival (p = 0.002, median survival of high nestin = 11 months, low nestin = 50 months; HR = 2.65; Figure 3B) and shorter progression‐free survival (p = 0.004; median survival of high nestin = 14 months, low nestin = 34 months; HR = 2.62; Figure 3E). A high Iba1 load (top 20%) was associated with worse overall survival (p = 0.010; median survival of high Iba1 = 9 months, low Iba1 = 39 months; HR = 2.56; Figure 3C) and worse progression‐free survival (p = 0.006, median survival of high Iba1 = 11 months, low Iba1 = 30 months; HR = 3.37; Figure 3F). High CD133 (top 20%) predicted worse progression‐free survival (p = 0.021; median survival of high CD133 = 10 months, low CD133 = 23 months; HR = 2.70; Figure 3G) as did CD68 (top 20%; p = 0.039; median survival of high CD68 = 20 months, low CD68 = 23 months; HR = 2.29; Figure 3H). SOX2 and GFAP were not associated with either of the outcome measures. Multivariate Cox‐regression analysis for the overall survival demonstrates that Ki67 (p = 0.005, HR 1.332), nestin (p = 0.001, HR 1.047) and CD133 (p = 0.046, HR 1.049) independently predicted the outcome. For progression‐free survival, multivariate Cox‐regression analysis revealed a trend for worse outcome with nestin (p = 0.056, HR 1.062), but none of the other markers were significant predictors.
Figure 3.

Analysis with clinical information shows the probability of survival to be significantly associated with (A) the proliferation (high vs low Ki67; p = 0.033), (B) stem cell (high vs low nestin; p = 0.002) and (C) microglia (high vs low Iba1; p = 0.010). The probability of time to progression is also associated with the (D) proliferation (high vs low Ki67; p = 0.002), (E) stem cell (high vs low nestin; p = 0.007) and (F) microglia (high vs low Iba1; p = 0.005); but also with (G) cancer stem cell CD133 (high vs low p = 0.010) and phagocytic activity of microglia (high vs low CD68; p = 0.024).
Discussion
Our study used TMAs to explore associations between a panel of markers, and it reveals novel correlations between microglia and stem cells in human gliomas, which also correlate with tumour cell proliferation. This confirms the functional relevance in humans of emerging in vitro work that demonstrates microglia facilitate the invasiveness brain‐tumour initiating cells: the novel association between microglia and stem cells in human tumours suggests this cooperation may indeed be occurring in humans. The putative findings that microglia and stem cells may be associated with worse survival and faster tumour progression further support this notion.
Recent work has demonstrated that microglia in gliomas have an anti‐inflammatory or M2 profile providing an immunosuppressive environment supportive of tumour cell proliferation, invasion and angiogenesis 21. Specifically, microglia in gliomas secrete metalloproteinase, which promote tumour invasion, and transforming growth factor‐beta (TFG) and epidermal growth factor (EGF) that promote tumour proliferation and angiogenesis 32. Ablation of microglia in vivo reduces glioma growth and pharmacological activation of microglia increases glioma size by increasing proliferation and reducing apoptosis 33. However, previous studies have not explored the relationship between microglia and stem cells in human gliomas as investigated by us here. Our findings provide further insight into the role of microglia in gliomas by suggesting that the likely M2 microglial anti‐inflammatory profile may promote the tumour development at least partly through actions on the tumour stem cells. Likewise, nestin was the only stem cell marker that correlated with Ki67, survival and progression‐free survival in the human tumours.
Microglia are the most abundant immune cells in gliomas 18, 19 and our data show an increase of microglia with increasing tumour grade. Specifically, the correlation between CD68 immunoreactivity and tumour grade suggests that microglial phagocytic activity is an important factor. Both Iba1 and CD68 correlate with proliferation rate of the glioma, suggesting that microglia and their phagocytic activity may be important for driving proliferation of the tumour. In vitro studies have demonstrated that gliomas actively recruit microglia 34 and that their phagocytic activity may be facilitating invasion and, therefore, growth of the tumour 35. Moreover, the anti‐immune profile of microglia may be promoting proliferation of glioma cells themselves 23. Our observation of a putative association between microglia, proliferation and tumour progression on univariate analysis lends further support to this idea. More studies are needed to define exactly what microglia phagocytose to facilitate tumour invasion, for example degenerating tumour cells or brain tissue components, especially as many of the studies suggest abnormal microglial function in gliomas. Experimental models will be particularly helpful in determining whether microglial phagocytic activity can promote glioma growth and by what mechanisms. Indeed, recent work demonstrates CD74 to be a marker of glioma‐associated microglia of an M1 polarisation that is also associated with improved prognosis 36, so further work is needed to define how glioma stem cells may associate and interact with different microglial populations.
CD133 was the only stem cell marker in this study that correlated with GFAP, whose expression decreased with glioma grade, likely because glioma cells are less differentiated in high grade versus low grade tumours. It may point towards these cells having an important role early on in tumour development at the time of tumour initiation, particularly as some low grade gliomas have a high level of CD133 expression. The potential association between CD133 expression and faster tumour progression is in keeping with this interpretation, although this was not significant on multivariate analysis. Moreover, CD133 was significantly associated with poor overall survival on multivariate analysis. This is also supported by another study showing that CD133 cells in vivo can create a glial tumour that is phenotypically similar to a patient's original tumour 6. In association with our findings, this implies that CD133 cells might be more important for initiation of the tumour rather than its growth. Previous studies showed that CD133‐positive cells can differentiate into endothelial cells in high grade gliomas, and may, therefore, be important for angiogenesis, a hallmark of such tumours 37. The association between the microglia and CD133 merits further experimental exploration into whether there is any cooperation between microglia and CD133‐positive cells that facilitates angiogenesis via differentiation of CD133‐cells into endothelial cells, which would be a potential mechanism by which co‐operation between microglia and stem cells would support tumour growth. The stem cell marker nestin identified cells with different properties. In contrast to CD133, nestin level was 15‐fold higher in grade IV compared to low grade gliomas and correlated with tumour proliferation. These pieces of evidence point towards an important role of nestin‐expressing stem cells in driving glioma proliferation and aggressiveness, and the putative association between nestin and patient survival as well as progression of tumours is consistent with this hypothesis. A recent study in a mouse model showed that nestin‐expressing tumour stem cells can propagate tumour regrowth after chemotherapy and that this growth was suppressed by ablation of these specific cells 12. The evidence we present here supports the concept that nestin stem cells are indeed associated with glioma progression, which microglia may well be augmenting given the association we have observed with nestin.
Interestingly, SOX2 was the only stem cell marker which did not associate with tumour proliferation, development, survival or microglia. As the different stem cell markers label different subpopulations of cells, these findings imply that SOX2‐positive cells do not collaborate with microglia to drive tumour growth. Rather, any potential co‐operation between microglia and stem cells are likely to be with CD133 and nestin‐positive cells, for which significant associations were observed.
In our study, we used TMAs to allow many tumour samples to be analysed for a panel of immunohistochemical markers onto a single slide in a standardized manner. We used markers to predict clinical outcomes by Kaplan–Meier analysis without taking into account other potential confounding factors because this would require a larger number of tumours to be analysed and our primary aim was to demonstrate any associations between microglial, stem cell and proliferative markers. Further specific studies looking into each of these markers separately will help in determining if other patient characteristics are confounding factors, such as isocitrate dehydrogenase (IDH) expression, extent of resection, nature of treatment, tumour size and patient age and sex. Several studies have already analysed how stem cell associate with prognosis in gliomas, the results of which have been conflicting 13, 14, 38, 39.
Our work has demonstrated a novel association between microglia and cancer stem cells in human gliomas, and further shown that microglial phagocytic activity as well as increased stem cells was associated with proliferative rate and potentially poorer clinical outcomes. A recent study demonstrated that manipulation of microglia influences brain‐tumour initiating cells and tumour proliferation, confirming a pivotal role of microglia in brain tumour development 40, and tumour stem cells were able to induce immunosuppressive microglia in vitro 27. This novel association found in human tissue may, therefore, be important in the context of the development of microglial ligands for positron emission tomography (PET) imaging and the potential for manipulation of microglia for therapeutic purposes to slow glioma growth.
Author contribution
IN and GP help performed the experiments and collected the data. PLG and GS provided the clinical information of the patients. SWM provided stem cell expertise and help with the interpretation of the data. IN and SH provided the data analysis. GJT provided the ethics and expertise in tissue micro‐arrays helping with the interpretation of the data. JARN and DB conceived and designed the study and interpreted the data. All authors were involved in the writing of the paper and had final approval of the submitted version.
Acknowledgements
We thank the patients for their consent to participation in research. We also thank the staff of the Department of Cellular Pathology, Southampton General Hospital, and of the Histochemistry Research Unit, University of Southampton in helping to facilitate tissue preparation and staining. This study was supported by donations from patients and relatives to the Wessex Brain Tumour Research Fund and the Faculty of Medicine, University of Southampton.
No conflicts of interest were declared.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stopschinski BE, Beier CP, Beier D. Glioblastoma cancer stem cells–from concept to clinical application. Cancer Lett 2013; 338: 32–40. [DOI] [PubMed] [Google Scholar]
- 4. Yin AH, Miraglia S, Zanjani ED, et al AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90: 5002–5012. [PubMed] [Google Scholar]
- 5. Singh SK, Clarke ID, Terasaki M, et al Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–5828. [PubMed] [Google Scholar]
- 6. Singh SK, Hawkins C, Clarke ID, et al Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401. [DOI] [PubMed] [Google Scholar]
- 7. Beier D, Hau P, Proescholdt M, et al CD133(+) and CD133(−) glioblastoma‐derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007; 67: 4010–4015. [DOI] [PubMed] [Google Scholar]
- 8. Liu Q, Nguyen DH, Dong Q, et al Molecular properties of CD133+ glioblastoma stem cells derived from treatment‐refractory recurrent brain tumors. J Neurooncol 2009; 94: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christensen K, Schroder HD, Kristensen BW. CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol 2011; 104: 129–143. [DOI] [PubMed] [Google Scholar]
- 10. Kahlert UD, Bender NO, Maciaczyk D, et al CD133/CD15 defines distinct cell subpopulations with differential in vitro clonogenic activity and stem cell‐related gene expression profile in in vitro propagated glioblastoma multiforme‐derived cell line with a PNET‐like component. Folia Neuropathologica/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences 2012; 50: 357–368. [DOI] [PubMed] [Google Scholar]
- 11. Bao S, Wu Q, McLendon RE, et al Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760. [DOI] [PubMed] [Google Scholar]
- 12. Chen J, Li Y, Yu TS, et al A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012; 488: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang M, Song T, Yang L, et al Nestin and CD133: valuable stem cell‐specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res 2008; 27: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeppernick F, Ahmadi R, Campos B, et al Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 2008; 14: 123–129. [DOI] [PubMed] [Google Scholar]
- 15. Arai H, Ikota H, Sugawara K, et al Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high‐grade gliomas. Brain Tumor Pathol 2012; 29: 160–167. [DOI] [PubMed] [Google Scholar]
- 16. Gangemi RM, Griffero F, Marubbi D, et al SOX2 silencing in glioblastoma tumor‐initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 2009; 27: 40–48. [DOI] [PubMed] [Google Scholar]
- 17. Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 2009; 158: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 18. Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech 2001; 54: 106–113. [DOI] [PubMed] [Google Scholar]
- 19. Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia 2002; 40: 252–259. [DOI] [PubMed] [Google Scholar]
- 20. Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res 2005; 81: 447–455. [DOI] [PubMed] [Google Scholar]
- 21. Hussain SF, Yang D, Suki D, et al The role of human glioma‐infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol 2006; 8: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain SF, Yang D, Suki D, et al Innate immune functions of microglia isolated from human glioma patients. J Transl Med 2006; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Billingham C, Powell MR, Jenner KA, et al Rat astrocytic tumour cells are associated with an anti‐inflammatory microglial phenotype in an organotypic model. Neuropathol Appl Neurobiol 2013; 39: 243–255. [DOI] [PubMed] [Google Scholar]
- 24. Komohara Y, Ohnishi K, Kuratsu J, et al Possible involvement of the M2 anti‐inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216: 15–24. [DOI] [PubMed] [Google Scholar]
- 25. Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 2013; 39: 3–18. [DOI] [PubMed] [Google Scholar]
- 26. Pyonteck SM, Akkari L, Schuhmacher AJ, et al CSF‐1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 2013; 19: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu A, Wei J, Kong LY, et al Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol 2010; 12: 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye XZ, Xu SL, Xin YH, et al Tumor‐associated microglia/macrophages enhance the invasion of glioma stem‐like cells via TGF‐beta1 signaling pathway. J Immunol 2012; 189: 444–453. [DOI] [PubMed] [Google Scholar]
- 29. Bubendorf L, Nocito A, Moch H, et al Tissue microarray (TMA) technology: miniaturized pathology archives for high‐throughput in situ studies. J Pathol 2001; 195: 72–79. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Wang H, Zhang W, et al Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol 2002; 12: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takikita M, Chung JY, Hewitt SM. Tissue microarrays enabling high‐throughput molecular pathology. Curr Opin Biotechnol 2007; 18: 318–325. [DOI] [PubMed] [Google Scholar]
- 32. Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol 2012; 14: 958–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia 2011; 59: 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okada M, Saio M, Kito Y, et al Tumor‐associated macrophage/microglia infiltration in human gliomas is correlated with MCP‐3, but not MCP‐1. Int J Oncol 2009; 34: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 35. Ghosh A, Chaudhuri S. Microglial action in glioma: a boon turns bane. Immunol Lett 2010; 131: 3–9. [DOI] [PubMed] [Google Scholar]
- 36. Zeiner PS, Preusse C, Blank AE, et al MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1‐polarized immune milieu, and prolonged patient survival in gliomas. Brain Pathol 2014. Aug 31. doi: 10.1111/bpa.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang R, Chadalavada K, Wilshire J, et al Glioblastoma stem‐like cells give rise to tumour endothelium. Nature 2010; 468: 829–833. [DOI] [PubMed] [Google Scholar]
- 38. Kim KJ, Lee KH, Kim HS, et al The presence of stem cell marker‐expressing cells is not prognostically significant in glioblastomas. Neuropathology 2011; 31: 494–502. [DOI] [PubMed] [Google Scholar]
- 39. Pallini R, Ricci‐Vitiani L, Montano N, et al Expression of the stem cell marker CD133 in recurrent glioblastoma and its value for prognosis. Cancer 2011; 117: 162–174. [DOI] [PubMed] [Google Scholar]
- 40. Sarkar S, Doring A, Zemp FJ, et al Therapeutic activation of macrophages and microglia to suppress brain tumor‐initiating cells. Nat Neurosci 2014; 17: 46–55. [DOI] [PubMed] [Google Scholar]


