Abstract
Background
Germline mutations in the BRCA1 and BRCA2 genes are the most frequent known hereditary causes of familial breast cancer. Little is known about the interaction of age at diagnosis, estrogen receptor (ER) and progesterone receptor (PgR) expression and outcomes in patients with BRCA1 or BRCA2 mutations.
Methods
A PubMed search identified publications exploring the association between BRCA mutations and clinical outcome. Hazard ratios (HR) for overall survival were extracted from multivariable analyses. Hazard ratios were weighted and pooled using generic inverse-variance and random-effect modeling. Meta-regression weighted by total study sample size was conducted to explore the influence of age, ER and PgR expression on the association between BRCA mutations and overall survival.
Results
A total of 16 studies comprising 10,180 patients were included in the analyses. BRCA mutations were not associated with worse overall survival (HR 1.06, 95% CI 0.84–1.34, p = 0.61). A similar finding was observed when evaluating the influence of BRCA1 and BRCA2 mutations on overall survival independently (BRCA1: HR 1.20, 95% CI 0.89–1.61, p = 0.24; BRCA2: HR 1.01, 95% CI 0.80–1.27, p = 0.95). Meta-regression identified an inverse association between ER expression and overall survival (β = -0.75, p = 0.02) in BRCA1 mutation carriers but no association with age or PgR expression (β = -0.45, p = 0.23 and β = 0.02, p = 0.97, respectively). No association was found for BRCA2 mutation status and age, ER, or PgR expression.
Conclusion
ER-expression appears to be an effect modifier in patients with BRCA1 mutations, but not among those with BRCA2 mutations.
Introduction
Mutations in BRCA1 and BRCA2 genes explain approximately 15% of familial breast cancers and are the most common hereditary lesions in breast cancer. Compared with women without mutations, those with somatic mutations in BRCA1 and BRCA2 genes are at increased risk for the development of breast cancer (life time breast cancer risk of 50–80%). These mutations are inherited in an autosomal dominant fashion and lead to the synthesis of an inefficient protein that impairs DNA repair mechanisms, specifically the homologous recombination pathway [1, 2].
The association between BRCA genes and cancer predisposition is well described. There are substantial data showing that after adjustment for stage, breast cancers associated with BRCA mutations are associated with similar outcomes to sporadic breast cancers [3]. Less is known regarding whether survival outcomes are influenced by age at diagnosis of breast cancer, or degree of estrogen receptor (ER) or progesterone receptor (PgR) expression. Here we aimed to identify studies evaluating mutations in BRCA1 or BRCA2 genes and clinical outcome in breast cancer to assess the influence of age and hormonal receptor expression on survival. We hypothesized that BRCA mutation carriers with ER-negative tumors have better outcomes than women with BRCA wildtype and ER-negative tumors.
Methods
This analysis was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (S1 Table) [4].
Data sources and searches
Medline (Host: PubMed) was searched for studies published between January 1998 and February 2016), which evaluated DNA repair pathways in breast cancer. We used the MeSH terms ((((BRCA1) AND BRCA2) AND Breast cancer) AND survival) and added the limitation of human studies. The search was restricted to publications in English. Additional studies were identified through reviews of citation lists.
Study selection and data extraction
Two reviewers (LD, AO) independently evaluated all the titles identified by the search strategy. The results were then pooled and all potentially relevant publications retrieved in full and assessed for eligibility. Disagreement was resolved by consensus. For the primary analysis the following inclusion criteria for selection of studies were used: (i) studies in breast cancer reporting outcomes for overall survival (OS) in patients with BRCA1 or BRCA2 germline mutations compared to no such mutations (ii) availability of an adjusted hazard ratio (HR) for the mutational status with its 95% confidence interval (CI) or the associated p-value.
The following information was captured using predesigned data abstraction forms: First author, year of publication, BRCA type (if available), age of patients at diagnosis, duration of follow-up, number of patients with and without BRCA mutation, proportion of tumors with ER and PgR expression, proportion of patients with metastatic disease. Furthermore, HRs for mutational status from multivariable models with 95% CI and/or p-value and the variables used for adjustments were captured.
Data synthesis and statistical analyses
The primary outcome of interest was OS. The overall effect of BRCA mutational status on overall survival was assessed in a meta-analysis. Estimates of HRs were weighted and pooled using the generic inverse variance and random-effect model. All meta-analyses were conducted using RevMan 5.2 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Statistical heterogeneity was assessed using Cochran’s Q and I2statistics and considered present if the P-value for Cochran’s Q was <0.10 or I2> 50%. Initial analysis was conducted for all studies with available adjusted HRs of overall survival for germline BRCA mutational status (BRCA1 and BRCA2 combined). Subsequent analyses explored the individual effect of BRCA1 or BRCA2 or unspecified BRCA. Differences between subgroups were assessed using methods described by Deeks et al. [5]. Sensitivity analyses were done with exclusion of studies that reported HRs which were not adjusted for age or hormonal receptor status. Meta-regression was conducted to explore the influence of age and ER/PgR expression on the association between BRCA mutation status and survival. Specifically, a linear regression weighted by total study sample size (weighted least square regression) was carried out to evaluate the impact of median/mean age at diagnosis or proportion of patients who were ER/PgR-positive on the HR for survival for BRCA mutational status. Once again, the primary analysis of a pooled HR for BRCA1 and BRCA2 was used, if both were reported in the same study. Subsequent analyses were done separately for BRCA1 and BRCA2 mutations. Meta-regression analyses were done using SPSS version 20 (IBM Corp, Armonk, NY). All statistical tests were two-sided, and statistical significance was defined as p<0.05. No corrections were made for multiple testing.
Results
Characteristics of studies
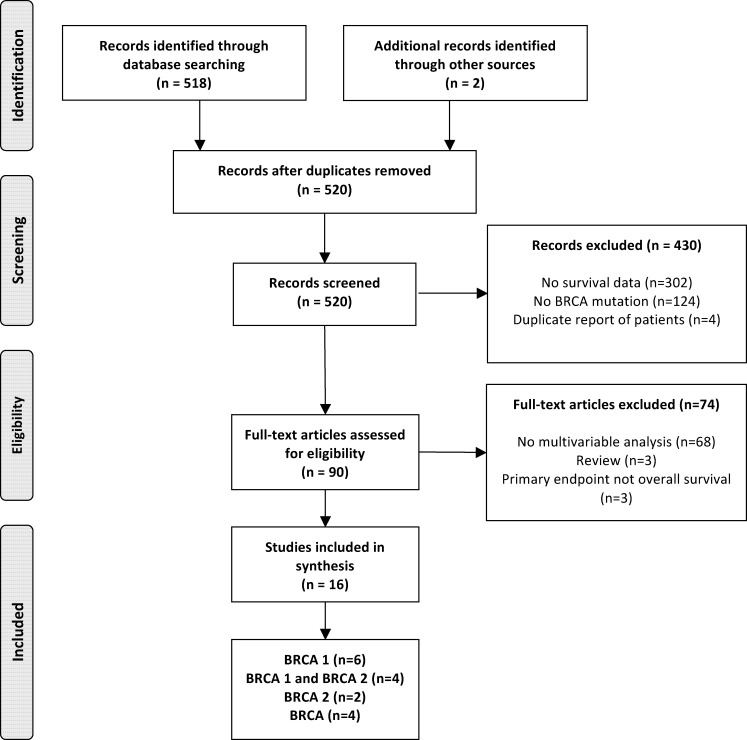
A total of 16 studies were identified (Fig 1) and characteristics are presented in Table 1. These studies comprised a total of 10,180 patients (1,325 patients [13%] had BRCA mutations) with a mean or median follow-up of 69 months (range of medians/means 34 to 228 months). Six studies [6–11] reported outcomes for BRCA1 alone, four [3, 12–14] for both BRCA1 and BRCA2, two [15, 16] for BRCA2 alone, and four studies [17–20] reported data for unspecified BRCA mutations.
Fig 1. Selection of included studies.
Table 1. Characteristics of Included Studies.
| Reference | Mutation | BRCA mut | BRCA- | Total patients | Study population | Age (mean or median) | Follow-up (mean or median) | Proportion ER+ | Proportion PR+ | Proportion metastatic | HR adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayraktar 2011 | BRCA | 114 | 113 | 227 | High-risk women with TNBC referred for genetic testing | 41 | 41 | 0% | 0% | 0% | Age, clinical stage, [*] |
| Bayraktar 2013 | BRCA1/BRCA2 | 41 | 154 | 195 | Women referred for BRCA testing | 39 | 34 | 59% | 43% | 100% | Nodal status, Grade, bisphosphonates, tripple negativity |
| Brekelmans 2007 | BRCA1/BRCA2 | 260 | 238 | 498 | Cases and controls (comparable BC patients) | 43 | 57 | 61% | 59% | 0% | T- and N-stage, grade, ER status, chemotherapy, endocrine therapy |
| Budroni 2009 | BRCA2 | 44 | 464 | 508 | Consecutive patients consenting for testing | 50 | nr | 74% | 56% | 6% | Pathologic T stage, pathologic N stage, M, ER, PR |
| Cortesi 2010 | BRCA1 | 80 | 931 | 1011 | High and intermediate risk patients undergoing testing; sporadic BC as control | nr | 72 | 67% | 62% | 0% | Stage, ER, PR, grade, age, chemotherapy |
| Goffin 2003 | BRCA1 | 30 | 248 | 278 | Ashkenazi Jewish women | 53 | 96 | 63% | nr | 41% | Tumor size, LN status, grade, p53 status |
| Gonzalez-Angulo 2011 | BRCA | 15 | 62 | 77 | Patients with TNBC | 51 | 43 | 0% | 0% | 0% | Pathological stage, grade, [*] |
| Goodwin 2012 | BRCA1/BRCA2 | 166 | 1550 | 1716 | Population-based cohort study | 45 | 95 | 71% | 70% | 2% | Age, tumor and LN stage, grade, ER, PR status, year of diagnosis |
| Hamann 2000 | BRCA1 | 36 | 49 | 85 | Patients with hereditary BC | 42 | 68 | nr | nr | 0% | Age, bilaterality |
| Huzarski 2013 | BRCA1 | 233 | 3112 | 3345 | Unselected women with newly diagnosed BC | 42 | 89 | 59% | 66% | 49% | Year of birth, age, ER, PR, Her2, size, nodes, oophorectomy, tamoxifen, chemotherapy |
| Nilsson 2014 | BRCA1/BRCA2 | 20 | 201 | 221 | Unselected women offered BRCA1/2 germline testing | 36 | 228 | 51% | 58% | 0% | Age, TNM stage, (neo)adjuvant chemotherapy, tumor grade, ER status |
| Rennert 2007 | BRCA1/BRCA2 | 128 | 1189 | 1317 | Incident cases of invasive breast cancer | 56 | nr | 62% | nr | 42% | Age, tumor size, LN status, metatases |
| Stoppa-Lyonnet 2000 | BRCA1 | 42 | 150 | 192 | Patients with BC and a family history of breast and/or ovarian cancer | 42 | 58 | 56% | 60% | 26% | LN status |
| Verhoog 1998 | BRCA1 | 49 | 196 | 245 | BRCA1 carriers matched with controls with sporadic BC | 40 | nr | 61% | 61% | 4% | Tumor stage, [**] |
| Verhoog 1999 | BRCA2 | 28 | 112 | 140 | BRCA2 carriers matched with controls with sporadic BC | 46 | nr | 86% | 81% | 1% | Tumor stage, [**] |
| Veronesi 2005 | BRCA | 39 | 86 | 125 | Patients with breast cancer and a family history of breast or ovarian cancer | 40 | 69 | 68% | 68% | 43% | Age, grade |
BC, breast cancer; ER, estrogen receptor; Her2, human epithelial growth factor receptor 2; LN, lymphnode; nr, not reported; OS, overall survival; PR, progesterone receptor.
* studies involved only patients with triple negative breast cancer;
** groups were matched according to age
Influence of age at diagnosis and ER/PgR expression
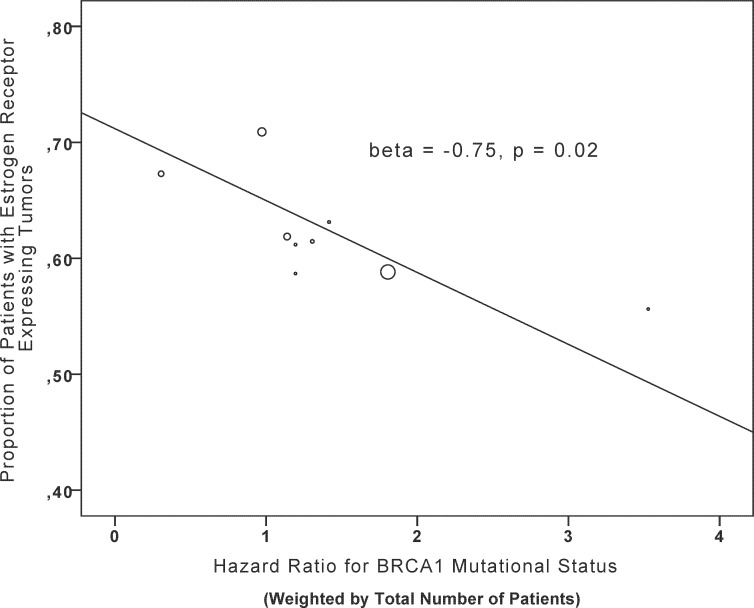
In meta-regression analysis, there was an inverse association between BRCA1 mutation status and ER expression and overall survival (β = -0.75, p = 0.02, Table 2, Fig 2). No association was seen with age or PgR expression (Table 2). Also, no association was found for unspecified BRCA mutations or for BRCA2 mutation status with age, ER, or PgR expression mutations (Table 2). Sensitivity analyses including only studies with HRs adjusted for age and hormone receptor expression yielded similar results (Table 3). Specifically, the magnitude of the association of ER and overall survival in BRCA1 mutation carriers remained similar (β = -0.80 and β = -0.79 for studies with HRs adjusted for hormonal receptors and age, respectively).
Table 2. Meta-regression (weighted by total sample size of studies).
| BRCA | BRCA1 | BRCA2 | ||||
|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | |
| Age | -0.39 | 0.15 | -0.45 | 0.23 | 0.45 | 0.38 |
| ER expression | -0.13 | 0.65 | -0.75 | 0.02 | -0.32 | 0.54 |
| PgR expression | 0.25 | 0.40 | 0.02 | 0.97 | 0.58 | 0.31 |
ER, estrogen receptor; PgR, progesterone receptor
Fig 2. Meta-regression.
Association of BRCA1 germline mutational status and proportion of patients with estrogen receptor expressing tumors.
Table 3. Meta-regression (weighted by total sample size of studies); studies with HRs with and without adjustment for age (upper part) and hormonal receptors excluded (lower part) only.
| BRCA | BRCA1 | BRCA2 | |||||
|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | ||
| Studies with adjustment for age | |||||||
| Age | -0.45 | 0.22 | -0.59 | 0.30 | 0.45 | 0.70 | |
| ER expression | -0.15 | 0.70 | -0.79 | 0.12 | -0.87 | 0.33 | |
| PgR expression | 0.24 | 0.58 | 0.19 | 0.81 | -1.00 | nd | |
| Studies without adjustment for age | |||||||
| Age | -0.31 | 0.54 | -0.16 | 0.84 | 0.12 | 0.93 | |
| ER expression | -0.06 | 0.91 | -0.80 | 0.20 | -0.08 | 0.95 | |
| PgR expression | 0.36 | 0.55 | 0.40 | 0.74 | 0.94 | 0.22 | |
| Studies with adjustment for hormonal receptors | |||||||
| Age | -0.62 | 0.10 | -0.79 | 0.22 | 0.05 | 0.95 | |
| ER expression | -0.07 | 0.86 | -0.80 | 0.104 | 0.29 | 0.72 | |
| PgR expression | 0.36 | 0.34 | 0.13 | 0.84 | 0.91 | 0.092 | |
| Studies without adjustment for hormonal receptors | |||||||
| Age | -0.35 | 0.45 | -0.56 | 0.32 | 1.00 | nd | |
| ER expression | -0.56 | 0.24 | -0.93 | 0.072 | -1.00 | nd | |
| PgR expression | -0.64 | 0.36 | -1.00 | nd | nd | nd | |
ER, estrogen receptor; PgR, progesterone receptor; nd, not determined
Association of BRCA1/2 mutations with overall survival (OS)
Pooled analysis of all 16 studies reporting data for BRCA1, BRCA2 or unspecified BRCA mutations showed no association between the presence of mutations and overall survival (HR 1.06, 95% CI 0.84–1.34, p = 0.61) (S1 Fig). There was evidence of inter-study heterogeneity (Cochran’s Q p = 0.001, I2 = 60%), which was mainly introduced by the study of Cortesi et al. [6]. Excluding this study did not significantly change the overall findings (HR = 1.16, p = 0.14), but reduced heterogeneity (Cochran’s Q p = 0.04, I2 = 43%).
When studying specific mutations, for the 10 studies evaluating BRCA1, no association with overall survival was observed (HR 1.21, 95% CI 0.91–1.61, p = 0.20, see S2B Fig). There was significant heterogeneity (Cochran’s Q p = 0.005, I2 = 62%) introduced by one outlier study, Cortesi et al. [6], which reported better outcomes for women with BRCA1 mutations. After exclusion of this study BRCA1 mutations were associated with a similar effect on overall survival (HR 1.34, 95% CI 1.12–1.60).
Similarly, no association with prognosis was observed for the studies evaluating BRCA2 (HR 1.01, 95% CI 0.80–1.27, p = 0.95), without evidence of heterogeneity (Cochran’s Q p = 0.053, I2 = 0%, see S2B Fig).
No association between BRCA mutations and overall survival was found for the pooled analyses of four studies evaluating populations with unspecified BRCA mutations (HR 0.84, 95% CI 0.38–1.84, p = 0.66, see S2C Fig).
The overall results were unchanged when excluding studies for which reported HRs were not adjusted for age and hormonal receptors, respectively (S2 Table).
Publication bias
Visual inspection of the Funnel plot did not indicate evidence of publication bias (S3 Fig).
Discussion
The association between the presence of mutations in BRCA1 and BRCA2 genes and an increased risk of developing breast cancer is well known. Additionally, it has been established that after diagnosis of breast cancer, presence of BRCA mutation does not appear to influence cancer outcomes after adjustment for tumor stage. However it is less clear how age at diagnosis and ER or PgR expression contributes to the oncogenic phenotype of tumors wearing these mutations therefore affecting prognosis. In the present article we explored the hypothesis that BRCA mutation carriers with ER-negative tumors do better than women with BRCA wildtype and ER-negative tumors and found that ER-expression appears to be an effect modifier in patients with BRCA1 mutations, but not among those with BRCA2 or unspecified BRCA mutations. Furthermore, we confirm that BRCA mutation status does not affect survival.
These novel findings have relevant clinical implications. It may be reassuring for patients and their families that their long-term prognosis is not negatively influenced purely by the presence of a BRCA mutation. These data reinforce the concept that presence of germline mutations facilitate tumor initiation but do not influence tumor behavior. However, in the subgroup of women with BRCA1 germline mutations and low or absent expression of hormone receptors prognosis may be less favorable. Yet, these data do not provide data to inform of treatment choice. In the recently reported Treating to New Targets (TNT) trial, patients with BRCA mutations and triple negative breast cancer had similar outcomes as those with sporadic triple negative breast cancer [21]. However, despite this finding, response to taxane and platinum-based chemotherapy was discordant in those with mutant and wildtype BRCA status. As such, the role of individualized therapy in BRCA mutation carriers remains an area where further research is warranted.
A mutation in a gene is relevant when it is necessary and sufficient to initiate and promote an oncogenic process. However, even in this situation, oncogenic mutations are not always linked with worse outcome [22]. During tumor evolution, acquired molecular alterations differentiate tumoral clones with a more aggressive phenotype. In this context, it is unclear which molecular alteration or combinations of molecular alterations facilitate this state; however, as our data show, germline mutations of these genes do not contribute to this phenotype. Our observations suggest that mutations in BRCA2 but not BRCA1 genes are linked with the initiation of the oncogenic process rather than with a clear role in the progression of the tumor or sensitivity to anti-cancer treatment.
An earlier meta-analysis of 11 studies comparing overall and disease-free survival rates between BRCA1/2 mutation carriers and non-carriers found significantly lower short-term and long-term survival rates for BRCA1 mutation carriers (HR = 1.92 and 1.33, respectively) while both short-term and long-term survival rates of BRCA2 mutation carriers did not differ from non-carriers [23]. A more recent review of the literature and meta-analysis by Zhong and colleagues [24] identified 13 studies that examined the effects of BRCA1/2 on breast cancer survival and found that BRCA1 mutation carriers had worse overall survival than non-carriers (HR = 1.50, p = 0.009) whereas progression-free survival was not different. BRCA2 mutation was not associated with breast cancer prognosis. Reasons for the difference in findings compared to our results may be due to the fact that both prior meta-analyses also included HRs from univariable analyses. It is known that breast cancer associated with BRCA mutations are more likely to be associated with young age and in the case of BRCA1 mutations with triple negative phenotype. The unadjusted enrichment for breast cancer with these characteristics may lead to erroneous associations with worse outcomes among BRCA mutation carriers. In the work by Zhong et al. a subgroup analysis of studies using multivariable analyses, BRCA1 mutation carriers only had borderline worse overall survival (HR = 1.40, p = 0.05) and also another recent review of the literature and meta-analysis did not find worse breast cancer survival in the adjuvant setting for BRCA1/2 mutation carriers [25].
Our study has limitations. Included studies used predominantly case-control methodology; thus, control for confounders is difficult with this design. To account for this problem, at least in part, we only included studies reporting HRs from multivariable analyses. However, the variables included in the various multivariable models were heterogeneous and adjustment is only able to control for measured confounders and not all studies reported HRs with adjustment for age at diagnosis or expression of hormonal receptors. To account for this we weighted all analyses by total sample size as studies reporting adjusted HRs for age and hormonal receptors comprised 83% and 77% of all patients. In addition, we performed sensitivity analyses excluding studies with HR not adjusted for the respective variables, what did not change the overall findings. However, the potential for residual confounding remains. Furthermore, our study is a meta-analysis of the literature rather of patient level data and there is a potential for selection bias by studies reporting positive results (although visual inspection of the Funnel plot did not indicate that this was a major issue). A further concern is the inter-study variability in a number of our analyses.
In conclusion, there is no apparent difference in overall survival in BRCA mutation carriers and non-carriers. However, there appears to be a strong and statistically significant association between ER expression and overall survival in patients with BRCA1 germline mutations but not with age or PgR expression.
Supporting Information
(PDF)
Pooled estimates for BRCA1 (A), BRCA2 (B), and unspecified BRCA mutations (C).
(PDF)
(PDF)
(DOC)
(DOCX)
Data Availability
All data underlying the findings of our study are provided in the manuscript, Tables, Figures, or Supporting Information.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nature genetics. 2008;40(1):17–22. 10.1038/ng.2007.53 [DOI] [PubMed] [Google Scholar]
- 2.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nature reviews Cancer. 2004;4(9):665–76. 10.1038/nrc1431 [DOI] [PubMed] [Google Scholar]
- 3.Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(1):19–26. 10.1200/JCO.2010.33.0068 [DOI] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine. 2009;6(7):e1000100 Epub 2009/07/22. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7305):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC cancer. 2010;10:90 10.1186/1471-2407-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goffin JR, Chappuis PO, Begin LR, Wong N, Brunet JS, Hamel N, et al. Impact of germline BRCA1 mutations and overexpression of p53 on prognosis and response to treatment following breast carcinoma: 10-year follow up data. Cancer. 2003;97(3):527–36. 10.1002/cncr.11080 [DOI] [PubMed] [Google Scholar]
- 8.Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(26):3191–6. 10.1200/JCO.2012.45.3571 [DOI] [PubMed] [Google Scholar]
- 9.Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, Gautier C, Gauthier-Villars M, Bourstyn E, et al. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(24):4053–9. [DOI] [PubMed] [Google Scholar]
- 10.Verhoog LC, Brekelmans CT, Seynaeve C, van den Bosch LM, Dahmen G, van Geel AN, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351(9099):316–21. [DOI] [PubMed] [Google Scholar]
- 11.Hamann U, Sinn HP. Survival and tumor characteristics of German hereditary breast cancer patients. Breast cancer research and treatment. 2000;59(2):185–92. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar S, Gutierrez-Barrera AM, Lin H, Elsayegh N, Tasbas T, Litton JK, et al. Outcome of metastatic breast cancer in selected women with or without deleterious BRCA mutations. Clin Exp Metastasis. 2013;30(5):631–42. 10.1007/s10585-013-9567-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43(5):867–76. 10.1016/j.ejca.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 14.Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. The New England journal of medicine. 2007;357(2):115–23. 10.1056/NEJMoa070608 [DOI] [PubMed] [Google Scholar]
- 15.Budroni M, Cesaraccio R, Coviello V, Sechi O, Pirino D, Cossu A, et al. Role of BRCA2 mutation status on overall survival among breast cancer patients from Sardinia. BMC cancer. 2009;9:62 10.1186/1471-2407-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhoog LC, Brekelmans CT, Seynaeve C, Dahmen G, van Geel AN, Bartels CC, et al. Survival in hereditary breast cancer associated with germline mutations of BRCA2. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(11):3396–402. [DOI] [PubMed] [Google Scholar]
- 17.Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast cancer research and treatment. 2011;130(1):145–53. 10.1007/s10549-011-1711-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(5):1082–9. 10.1158/1078-0432.CCR-10-2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronesi A, de Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, et al. Familial breast cancer: characteristics and outcome of BRCA 1–2 positive and negative cases. BMC cancer. 2005;5:70 10.1186/1471-2407-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson MP, Hartman L, Idvall I, Kristoffersson U, Johannsson OT, Loman N. Long-term prognosis of early-onset breast cancer in a population-based cohort with a known BRCA1/2 mutation status. Breast cancer research and treatment. 2014;144(1):133–42. 10.1007/s10549-014-2842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tutt A, Ellis P, Kilburn L, Gilett C, Pinder S, Abraham J, et al. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). San Antonio Breast Cancer Sysmposium. 2014:abstract S3-01. [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. Epub 2011/03/08. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 23.Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast cancer research and treatment. 2010;122(1):11–25. 10.1007/s10549-010-0859-2 [DOI] [PubMed] [Google Scholar]
- 24.Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(1):211–20. 10.1158/1078-0432.CCR-14-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Broek AJ, Schmidt MK, van 't Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PloS one. 2015;10(3):e0120189 10.1371/journal.pone.0120189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Pooled estimates for BRCA1 (A), BRCA2 (B), and unspecified BRCA mutations (C).
(PDF)
(PDF)
(DOC)
(DOCX)
Data Availability Statement
All data underlying the findings of our study are provided in the manuscript, Tables, Figures, or Supporting Information.