Abstract
Objective
Anhedonia, disrupted reward processing, is a core symptom of major depressive disorder. Recent findings demonstrate altered reward-related ventral striatal reactivity in depressed individuals, but the extent to which this is specific to anhedonia remains poorly understood. The authors examined the effect of anhedonia on reward expectancy (expected outcome value) and prediction error-(discrepancy between expected and actual outcome) related ventral striatal reactivity, as well as the relationship between these measures.
Method
A total of 148 unmedicated individuals with major depressive disorder and 31 healthy comparison individuals recruited for the multisite EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care) study underwent functional MRI during a well-validated reward task. Region of interest and whole-brain data were examined in the first- (N=78) and second- (N=70) recruited cohorts, as well as the total sample, of depressed individuals, and in healthy individuals.
Results
Healthy, but not depressed, individuals showed a significant inverse relationship between reward expectancy and prediction error-related right ventral striatal reactivity. Across all participants, and in depressed individuals only, greater anhedonia severity was associated with a reduced reward expectancy-prediction error inverse relationship, even after controlling for other symptoms.
Conclusions
The normal reward expectancy and prediction error-related ventral striatal reactivity inverse relationship concords with conditioning models, predicting a shift in ventral striatal responding from reward outcomes to reward cues. This study shows, for the first time, an absence of this relationship in two cohorts of unmedicated depressed individuals and a moderation of this relationship by anhedonia, suggesting reduced reward-contingency learning with greater anhedonia. These findings help elucidate neural mechanisms of anhedonia, as a step toward identifying potential biosignatures of treatment response.
Anhedonia, the disruption of reward processing, is a core symptom of depressive illness (1, 2). Numerous demonstrations of the influence of anhedonia on reward-guided behavior are reported (3, 4), for example, absence of a reward-related response bias in a signal detection task (5). However, examination of the neural underpinnings of this effect has yielded inconsistent findings. While several studies report reduced reward-related reactivity in striatal and medial frontal regions in individuals with major depressive disorder (6) or with high levels of anhedonia (7), other studies show robust anticipatory striatal activation in depressed individuals (8), as well as different affected loci within the striatum (9).
Some of these findings may be reconciled using reinforcement learning models to capture the variation of neural reactivity and to conceptualize abnormalities observed in individuals with major depressive disorder (10). One such model is the temporal difference model, which proposes that during learning, the prediction of future reward is updated based on the difference between the expected reward magnitude (from previous experience) and the actual reward outcome (11, 12). Prediction error signals are tracked in the ventral striatum (13, 14) and, as learning progresses, ventral striatal responding shifts from reward outcome (i.e., prediction error) to reward cues (i.e., reward expectancy), reflecting the process of conditioning (11). Preliminary evidence implicates reduced prediction error encoding in major depressive disorder, which is associated with severity of anhedonia symptoms (15).
We recently reported an inverse relationship between reward expectancy and prediction error ventral striatal reactivity in healthy individuals, consistent with a transfer of ventral striatal responding from prediction error to reward expectancy predicted by the temporal difference model (16). Importantly, this association was absent in medicated depressed individuals with bipolar disorder or major depressive disorder, which provides further evidence of disrupted temporal difference encoding in depressed individuals (16). Given that this finding was reported in medicated individuals with major depressive disorder and that psychotropic medications, including antidepressants, can modulate prefrontalstriatal dopamine function (17), it is important to determine whether a similar pattern of altered ventral striatal functioning is present in unmedicated depressed individuals with major depressive disorder. Furthermore, the extent to which this pattern of altered reward expectancy and prediction error-related ventral striatal reactivity is specifically associated with anhedonia, rather than with other symptom dimensions in major depressive disorder, remains unknown.
We first sought to adopt a conventional diagnostic categorical approach and compare reward expectancy and prediction error-related ventral striatal reactivity in a large group of unmedicated depressed individuals with major depressive disorder and a group of healthy individuals using a well-validated reward task. In a novel step forward, we then adopted a dimensional approach, paralleling the approach advocated by the National Institute of Mental Health Research Domain Criteria (18), and determined, across both groups, the extent to which alteration in the expected inverse relationship between reward expectancy and prediction error-related ventral striatal reactivity was moderated specifically by the severity of anhedonia rather than other symptoms. Participants were recruited for the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care) study, a large multisite randomized controlled trial aiming to identify biomarkers of treatment response in major depressive disorder (data available upon request from MH Trivedi, PJ McGrath, M Weissman, R Parsey, and M Fava, principal investigators; ClinicalTrials.gov identifier: NCT01407094 [also see reference 19]). The design of EMBARC allowed us to test the following hypotheses separately in the first and second recruited cohorts of 100 depressed individuals and in the total sample of 200 depressed individuals.
In accordance with the temporal difference model and a previous report (16), we hypothesized that healthy individuals would demonstrate an inverse relationship between reward expectancy and prediction error-related ventral striatal reactivity and that this association would be absent in depressed individuals with major depressive disorder. Based on the disruptive effect of anhedonia on reward-related reactivity (7, 20, 21) and functional connectivity (20), as well as specifically on prediction error encoding in the ventral striatum (15), we further hypothesized that the relationship between reward expectancy and prediction error-related ventral striatal reactivity in depressed individuals with lower, compared with higher, anhedonia severity would more closely follow the pattern observed in healthy individuals.
METHOD
Participants
Participants were 200 unmedicated depressed individuals with major depressive disorder and 40 healthy individuals recruited for EMBARC. The study was conducted at four clinical sites: Columbia University, Massachusetts General Hospital, the University of Michigan, and the University of Texas Southwestern Medical Center. All individuals were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P [22]) to confirm diagnoses of major depressive disorder in patients and absence of diagnoses of lifetime axis I mood, anxiety, and psychotic disorders and current substance abuse in healthy individuals. To be eligible for the study, individuals had to be 18–65 years old, had to report an age at onset of depression occurring before 30 years old, and had to be fluent in English. Additional inclusion/exclusion criteria are presented in the data supplement accompanying the online edition of this article.
Fifty depressed patients and nine healthy individuals were excluded because of excessive motion (>4 mm), low slice signal-to-noise ratio (<80), and severe artifacts in MRI data. This exclusion rate is in line with other neuroimaging data sets (23). Two depressed patients were excluded because of a large number of omission errors (≥11) for the reward task. This yielded a final sample of 148 depressed patients (97 women; mean age=37.11 years [SD=12.93]) and 31 healthy individuals (19 women; mean age=38.42 years [SD=15.74]). A total of 158 participants were right-handed, 20 were left-handed, and one was ambidextrous. The two groups did not differ in age, sex ratio, handedness, and education level. The study was approved by the institutional review boards at each of the four clinical sites. All participants provided written, informed consent.
Clinical Measures
All participants were rated on the Hamilton Depression Rating Scale (HAM-D [24]) for severity of depression. Participants also completed the anhedonic depression subscale from the Mood and Anxiety Symptom Questionnaire (25), as well as the Snaith-Hamilton Pleasure Scale (26), to assess anhedonia severity, the anxious arousal subscale from the Mood and Anxiety Symptom Questionnaire (25) to assess somatic arousal severity, the Spielberger State-Trait Anxiety Inventory (27) to assess state anxiety, and the Altman Self-Rating Mania Scale (28) to determine hypomania severity. All self-report questionnaires were completed on the scanning day except the Altman Self-Rating Mania Scale, which was completed during an initial evaluation visit.
Reward Task
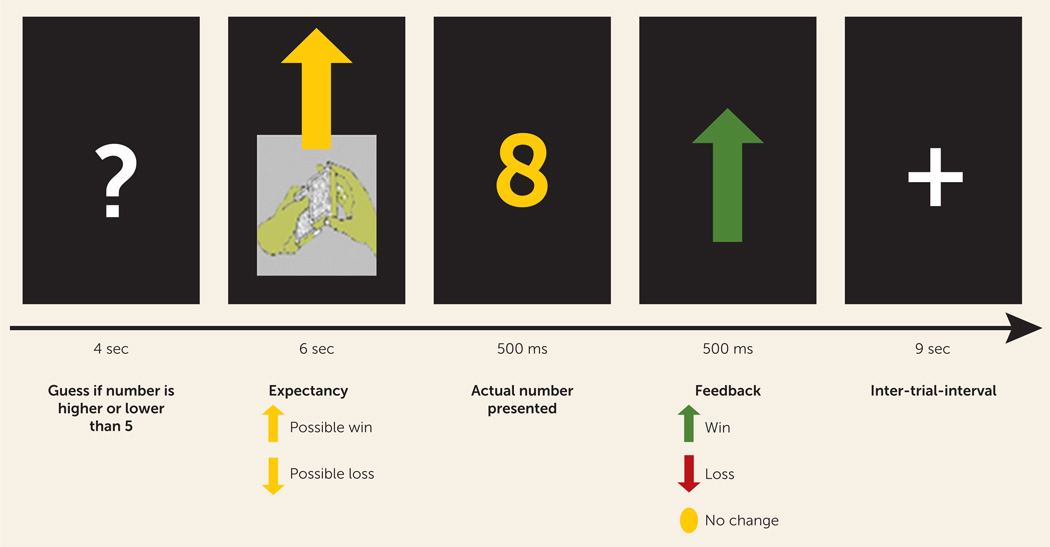
A well-validated monetary reward task comprised 24 trials presented in pseudorandom order with predetermined outcomes (29–31). There were four possible trial types (N=6 each): the expectation of a possible win, followed by a win or no change outcome, and the expectation of a possible loss, followed by a loss or no change outcome (Figure 1). During each trial, individuals guessed using button press whether the value of an upcoming card would be higher or lower than the number 5 (presentation of a question mark; 4 seconds). An upward or downward arrow was then presented for 6 seconds, representing a possible win or possible loss, respectively, while the participant anticipated the outcome. The outcome then appeared for 1 second (a number for 500 ms and then an upward or downward arrow for win and loss outcomes, respectively, or a yellow circle for no change outcomes, for 500 ms), followed by a 9-second intertrial interval. Participants were informed that their performance would determine a monetary reward after the scan, with $1.00 for each win and 50 cents deducted for each loss. The total possible earnings were, in fact, fixed at $3.00 to equalize rewards between participants. Previous data indicate that participants are unaware of the latter and believe that outcome is determined by chance (29). Participants completed a practice run of the task prior to the scan.
FIGURE 1.
Diagram of Given Trial From the Reward Taska
a The paradigm consists of 24 trials: 12 are reward-expectation trials, in which an arrow points upward and the possible outcomes are a win (six trials) or no change (six trials), and 12 are loss-expectation trials, in which the arrow points downward and the possible outcomes are a loss (six trials) or no change (six trials).
Image Acquisition
Neuroimaging data were collected using 3-T MRI scanners at all sites (for imaging parameters at each site, see the online data supplement).
Image Analysis
Preprocessing procedures were performed with Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Functional images for each participant were realigned to the first volume in the time series, coregistered with the corresponding anatomical image and smoothed with an 8-mm Gaussian kernel. The first-level model included the following four regressors: response (4-second presentations of a question mark), anticipation (6-second presentations of an arrow), outcome (1-second presentations of the number and feedback arrow), and baseline (3-second presentation of an orienting cross). In addition, we included two regressors representing reward expectancy and prediction error. The reward expectancy regressor, coupled with the anticipation phase, reflected the expected value of the arrow, set to +0.5 for the up arrow condition (given the 50% chance of winning $1.00) and to −0.25 for the down arrow condition (given the 50% chance of losing 50 cents). The prediction error regressor, coupled with the outcome, was determined by the difference between the outcome and the expected value (i.e., +0.5 for a win following an up arrow, −0.5 for no win following an up arrow, +0.25 for a no loss following a down arrow, −0.25 for a loss following a down arrow).
To model omission errors, we included another regressor, which lasted 17 seconds from the onset of the question mark and replaced other trial events during this period. Finally, we included the six motion parameters from the realignment phase as covariates of no interest. Serial autocorrelations were modeled using a first-order autoregressive process.
The main conditions of interest were reward expectancy and prediction error. We conducted a region of interest analysis focused on the ventral striatum. Mean parameter estimates reflecting reward expectancy and prediction error reactivity were extracted using two separate functional masks for the right and left ventral striatum, based on our previous findings (16). We conducted bivariate and partial correlational analyses (covarying for age, sex, site, and slice signal-to-noise ratio) to examine the relationship between reward expectancy and prediction error-related ventral striatal reactivity in the two groups (major depressive disorder group and healthy group).
We conducted hierarchical regression analyses to examine the effect of anhedonia, anxiety, and depression severity on the relationship between reward expectancy and prediction error-related ventral striatal reactivity, as well as on reward expectancy and prediction error-related ventral striatal reactivity per se, using the covariates described above.
We also conducted whole-brain analyses that paralleled the region-of-interest analyses using a family-wise error cluster threshold of p<0.05 (see the online data supplement).
The above analyses were conducted in the first-recruited cohort of 100 depressed patients (referred to as cohort MDD100a; N=78 with usable data) and healthy individuals (N=31 with usable data), in the second-recruited cohort of 100 depressed patients (referred to as cohort MDD100b; N=70 with usable data) and healthy individuals, and in all 200 depressed patients (referred to as cohort MDD200; N=148) and healthy individuals.
RESULTS
Demographic Variables
There were no group differences in age, sex ratio, and level of education for the MDD100a cohort (all p values >0.28), the MDD100b cohort (all p values >0.1), or the MDD200 cohort (all p values >0.11) and healthy individuals (Table 1).
TABLE 1.
Demographic, Clinical, and Behavioral Data for the First-Recruited Cohort of Individuals With Major Depressive Disorder (MDD100a), the Second-Recruited Cohort (MDD100b), the Total Sample of Depressed Participants (MDD200), and Healthy Comparison Subjectsa
| MDD100a Cohort (N=78) |
MDD100b Cohort (N=70) |
MDD200 Cohort (N=148) |
Healthy Comparison Subjects (N = 31) |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 38.47 | 13.21 | 35.60 | 12.54 | 37.11 | 12.9 | 38.42 | 15.74 |
| Education (years) | 15.13 | 2.46 | 14.60 | 2.90 | 14.88 | 2.68 | 15.88 | 4.77 |
| Clinical measures | ||||||||
| Hamilton Depression Rating Scale (24-item) scoreb–d |
25.91 | 5.70 | 27.20 | 5.49 | 26.52 | 5.62 | 0.79 | 1.08 |
| Mood and Anxiety Symptom Questionnaire anhedonic depression subscale scoreb–d |
43.12 | 6.62 | 44.14 | 4.68 | 43.61 | 5.78 | 25.35 | 6.91 |
| Snaith-Hamilton Pleasure Scale (ordinal) scoreb–d |
33.04 | 5.50 | 33.93 | 5.44 | 33.46 | 5.48 | 20.52 | 5.45 |
| Mood and Anxiety Symptom Questionnaire anxious arousal subscale scoreb–d |
17.56 | 5.48 | 18.49 | 6.43 | 18.00 | 5.95 | 10.81 | 0.98 |
| Spielberger State- Trait Anxiety Inventory scoreb–d |
46.90 | 10.80 | 48.56 | 10.61 | 47.68 | 10.7 | 23.84 | 4.43 |
| Altman Self-Rating Mania Scale score |
1.84 | 2.15 | 1.28 | 1.48 | 1.58 | 1.89 | 1.39 | 1.8 |
| Reward task (reaction time) (ms) |
732.00 | 203.73 | 686.14 | 246.45 | 710.3 | 225 | 672.2 | 241.7 |
| Reward task (omission errors) |
0.410 | 0.653 | 0.500 | 0.897 | 0.45 | 0.78 | 0.355 | 0.608 |
The female/male ratio for the MDD100a cohort, MDD100b cohort, MDD200 cohort, and healthy comparison subjects is as follows: 52/26, 45/25, 98/50, and 19/12, respectively.
Significant difference between cohort MDD100a and comparison groups (p<0.001, t test).
Significant difference between cohort MDD100b and comparison groups (p<0.001, t test).
Significant difference between MDD200 and comparison groups (p<0.001, t test).
Clinical Measures
Depressed individuals had higher scores on the HAM-D, Mood and Anxiety Symptom Questionnaire anhedonic depression subscale, Snaith-Hamilton Pleasure Scale, Mood and Anxiety Symptom Questionnaire anxious arousal subscale, and Spielberger State-Trait Anxiety Inventory than healthy individuals (all p values <0.001 for cohorts MDD100a, MDD100b, and MDD200 [see Table 1]). There were no group differences in Altman Self-Rating Mania Scale scores between cohorts MDD100a (p=0.3), MDD100b (p=0.75), or MDD200 (p=0.6) and healthy individuals. For one depressed participant, scores on the Mood and Anxiety Symptom Questionnaire anhedonic depression and anxious arousal subscales were not available, and for six depressed participants, scores on the Altman Self-Rating Mania Scale were not available. For two healthy participants, HAM-D scores were not available.
Reward Task
Behavioral measures
There were no group differences in reaction time and number of omission errors between cohorts MDD100a (p=0.19 and p=0.69, respectively), MDD100b (p=0.79 and p=0.41, respectively), or MDD200 (p=0.4 and p=0.51, respectively) and healthy individuals (Table 1).
Neural reactivity
Region-of-interest analysis: reward expectancy and prediction error
There were no differences in reward expectancy and prediction error-related reactivity in the right or left ventral striatum between cohorts MDD100a (all p values >0.38), MDD100b (all p values >0.31), or MDD200 (all p values >0.47) and healthy individuals.
Relationship between reward expectancy and prediction error-related ventral striatal reactivity
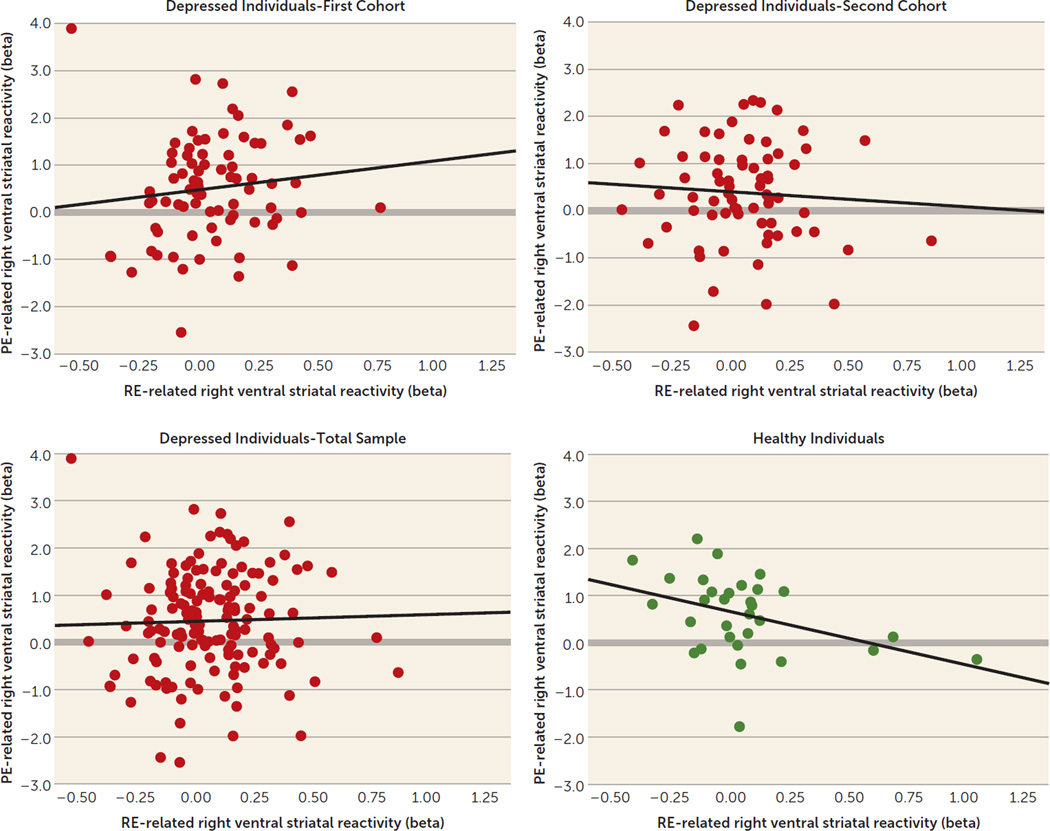
There was a significant inverse correlation between reward expectancy and prediction error-related right ventral striatal reactivity in healthy individuals (r=−0.39, df=29, p=0.03) but not in depressed individuals (cohort MDD100a: r=0.12, df=76, p=0.31; cohort MDD100b: r=−0.07, df=68, p=0.58; cohort MDD200: r=0.03, df=146, p=0.72 [Figure 2]).These correlation coefficients differed significantly between the healthy group and cohort MDD100a (z=−2.4, p=0.02) and cohort MDD200 (z=−2.15, p=0.03) but not cohort MDD100b (z=−1.55, p=0.12). The latter comparison was nearly significant for a one-tailed t test (p=0.06).
FIGURE 2.
Association Between Reward Expectancy and Prediction Error-Related Reactivity in the Right Ventral Striatum in the First-Recruited Cohort (N=78), Second-Recruited Cohort (N=70), and the Total Sample (N=148) of Depressed Individuals and Healthy Comparison Subjects (N=31)a
a RE=reward expectancy; PE=prediction error.
There was a similar pattern of relationships between the left ventral striatal reward expectancy and prediction error-related reactivity in healthy and depressed individuals (healthy participants: r=−0.22, df=29, p=0.25; cohort MDD100a: r=0.12, df=76, p=0.31; cohort MDD100b; r=−0.06, df=68, p=0.62; cohort MDD200: r=0.04, df=146, p=0.67), but the major depressive disorder group compared with healthy comparison group differences in correlation coefficients were not significant (z=−1.63, p=0.1, z=−0.7, p=0.48 and z=−1.23, p=0.22, respectively). Similar between-group differences in correlational patterns between the right ventral striatal reward expectancy and prediction error-related reactivity were observed when controlling for age, sex, and site (healthy participants: r=−0.41, df=24, p=0.04; cohort MDD100a: r=0.07, df=71, p=0.59; cohort MDD100b: r=−0.144, df=62, p=0.26; cohort MDD200: r=−0.03, df=140, p=0.74).
Effects of anhedonia, anxiety, and depression severity on the relationship between reward expectancy and prediction error-related ventral striatal reactivity
MDD100a and healthy individuals
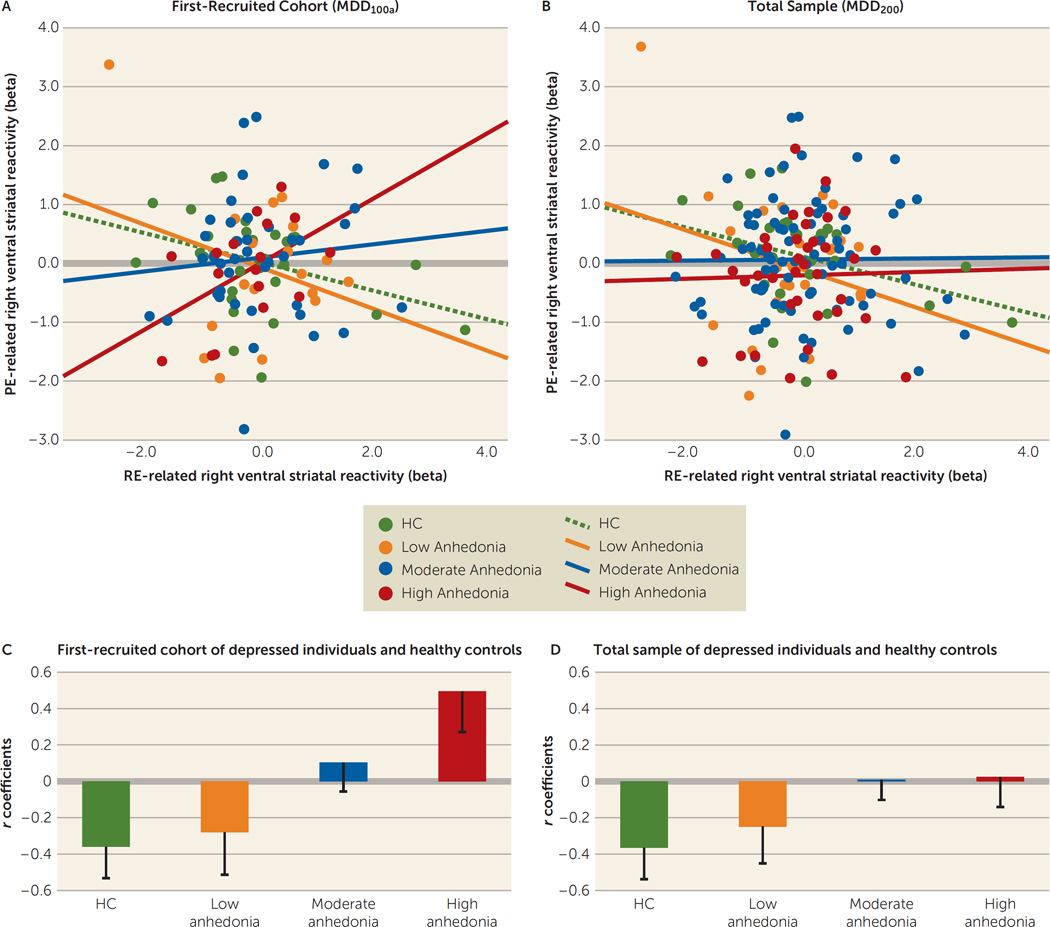
We conducted a hierarchical multiple regression with right ventral striatal prediction error-related reactivity as the dependent variable. To control for age, sex, site, and slice signal-to-noise ratio, we included these covariates in the first step of the model. We then entered the two independent variables of interest: right ventral striatal reward expectancy-related reactivity and scores on the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale. Finally, we added the interaction term for the two independent variables. The interaction term accounted for significant variance in right ventral striatal prediction error-related reactivity (R2change=0.12, Fchange=14.6, df=1, 98, p<0.001). To examine this finding further, we subdivided individuals with major depressive disorder into three subgroups defined by tertile split of scores on the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale: major depressive disorder-low anhedonia, major depressive disorder-moderate anhedonia, and major depressive disorder-high anhedonia. We then examined the interaction between anhedonia and right ventral striatal reward expectancy-related reactivity using these three subgroups and healthy individuals. This analysis revealed that a reduced inverse regression slope was associated with higher anhedonia severity (F=3.75, df=3, 94, p=0.01 [Figure 3A]).
FIGURE 3.
The Relationship Between Reward Expectancy and Prediction Error-Related Right Ventral Striatal Reactivity in Healthy Comparison (HC) Subjects and in Individuals With Low, Moderate, and High Symptoms of Anhedonia in the First-Recruited Cohort (MDD100a) and Total Sample (MDD200) of Individuals With Major Depressive Disordera
a The scatter plots (A, B) show the relationship between reward expectancy and prediction error-related right ventral striatal reactivity. The bar graphs (C, D) show correlation coefficients and standard errors for HC subjects and anhedonia subgroups (defined by tertile split of anhedonia scores) within the MDD100a cohort and for HC subjects and anhedonia subgroups within the MDD200 cohort. RE=reward expectancy; PE=prediction error.
Similar analyses were conducted to examine the effect of somatic arousal (Mood and Anxiety Symptom Questionnaire anxious arousal subscale), depression severity (HAM-D), and state anxiety (Spielberger State-Trait Anxiety Inventory) on the relationship between reward expectancy and prediction error-related right ventral striatal reactivity. There was a significant moderation effect for the Mood and Anxiety Symptom Questionnaire anxious arousal subscale (R2change=0.05, Fchange=5.4, df=1, 98, p=0.02), an effect that fell short of statistical significance for HAM-D (p=0.07), and no effect for the Spielberger State-Trait Anxiety Inventory (p=0.37).
To determine to what extent the moderation effects observed were specific to anhedonia, we conducted a moderation analysis with scores from all symptom measures (Mood and Anxiety Symptom Questionnaire anhedonic depression and anxious arousal subscales, HAM-D, and Spielberger State-Trait Anxiety Inventory) included in one regression model. First, we entered the covariates to the model, next we entered all the independent variables of interest, and then we entered the three interaction terms for the Mood and Anxiety Symptom Questionnaire anxious arousal subscale, HAM-D, and Spielberger State-Trait Anxiety Inventory and right ventral striatal reward expectancy-related reactivity. In the last step, we added the interaction term for the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale and right ventral striatal reward expectancy-related reactivity. The Mood and Anxiety Symptom Questionnaire anhedonic depression subscale remained a significant moderator of the relationship between right ventral striatal reward expectancy and prediction error-related reactivity, with all other symptom measures included (R2change=0.1, Fchange=12.19, df=1, 90, p=0.001). Furthermore, t tests examining the effect of each predictor (beta) in the model showed that the interaction term for the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale and right ventral striatal reward expectancy-related reactivity was the only significant predictor of right ventral striatal prediction error-related reactivity (t=3.49, p=0.001).
Moderation effect of anhedonia within each depressed cohort and all depressed individuals only
The moderation effects of anhedonia with and without other symptom measures were significant for cohort MDD100a (R2change=0.16, Fchange=14.33, df=1, 67, p<0.001; R2change=0.17, Fchange=15.82, df=1, 61, p<0.001, respectively) and MDD200 (R2change=0.04, Fchange=5.91, df=1, 135, p=0.02 and R2change=0.06, Fchange=9.721, df=1, 129, p=0.002, respectively) but not for MDD100b (both p values >0.1). However, the correlation coefficients for reward expectancy and prediction error-related ventral striatal reactivity did not differ between the two MDD cohorts (p=0.28). A possible factor for the absence of an anhedonia moderation effect on the ventral striatal region of interest in cohort MDD100b was the restricted range of Mood and Anxiety Symptom Questionnaire anhedonic depression subscale scores in this cohort (21 compared with 34 in cohort MDD100a), particularly at the low end of the scale.
Moderation effect of anhedonia within all depressed and healthy individuals
The moderation effect of the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale on the relationship between right ventral striatal reward expectancy and prediction error-related reactivity was significant (R2change=0.03, Fchange=6.53, df=1, 166, p=0.01 [Figure 3B]). This effect of anhedonia remained significant with all other symptom measures added to the model (R2change=0.04, Fchange=8.54, df=1, 158, p=0.004).
Effects of anhedonia, anxiety symptoms, and depression levels on reward expectancy and prediction error-related ventral striatal reactivity
To test whether scores on the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale, Snaith-Hamilton Pleasure Scale, Mood and Anxiety Symptom Questionnaire anxious arousal subscale, HAM-D, or Spielberger State-Trait Anxiety Inventory were significantly associated with reward expectancy or prediction error-related right ventral striatal reactivity in depressed and healthy individuals, we conducted simple regressions with each symptom measure as an independent variable and either reward expectancy or prediction error-related right ventral striatal reactivity as the dependent variable; all covariates were included for these analyses. There was no effect for any of the symptom measures on either reward expectancy or prediction error-related right ventral striatal reactivity in cohort MDD100a (all p values >0.1), cohort MDD200 (all p values >0.12), or healthy individuals (all p values >0.1), except for the Mood and Anxiety Symptom Questionnaire anxious arousal subscale on prediction error-related right ventral striatal reactivity in cohort MDD100b (p=0.02; all other p values >0.12).
Whole-brain moderation analysis (accounting for covariates)
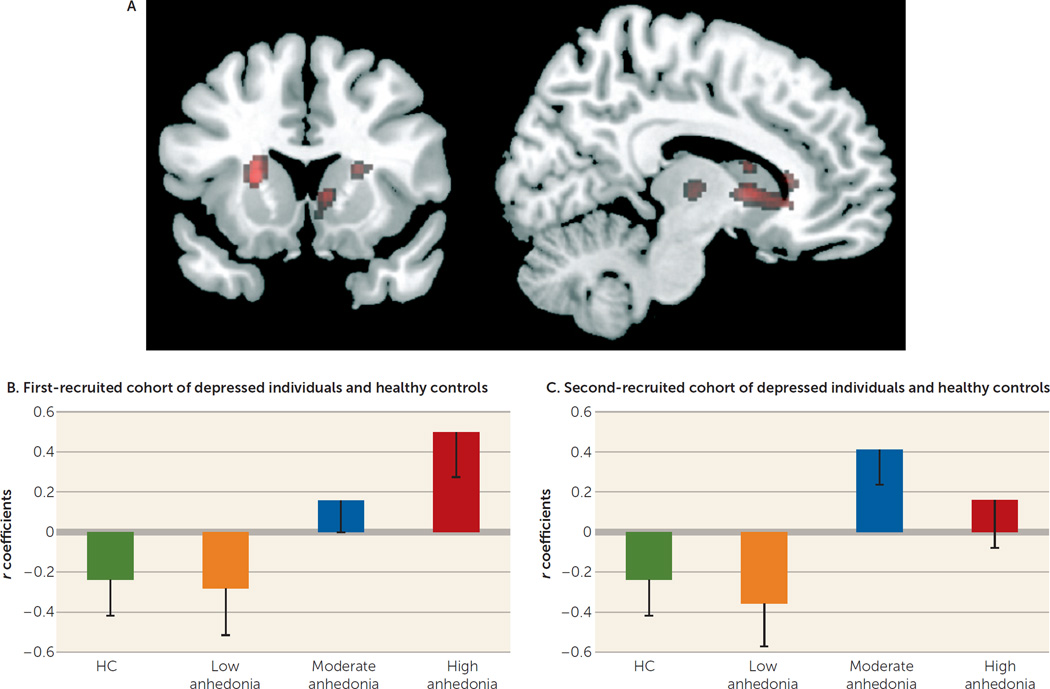
Two separate whole-brain analyses showed, in the first (MDD100a) cohort and healthy individuals and in the second (MDD100b) cohort and healthy individuals, moderation effects of the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale on the relationship between reward expectancy and prediction error-related reactivity in the anterior caudate, just anterior to the ventral striatal region of interest above (see the online data supplement). Across all participants, there was a significant moderation effect of the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale on this relationship in four striatal loci (Figure 4). Furthermore, correlation coefficients between reward expectancy and prediction error-related reactivity, based on parameter estimates extracted from the right anterior caudate, from the latter whole-brain analysis, showed comparable patterns in each of the two MDD cohorts for anhedonia range-equivalent subgroups (Figure 4).
FIGURE 4.
Whole-Brain Moderation Analysis Across All Participantsa
a Whole-brain analysis across all participants was conducted to investigate the significant moderation effect of anhedonia at a voxel-wise level. A regression model was constructed in which prediction error was predicted on the basis of reward expectancy, Mood and Anxiety Symptom Questionnaire anhedonic depression subscale scores, and Mood and Anxiety Symptom Questionnaire anhedonic depression subscale-by-reward expectancy interaction, as well as the other covariates used for the region-of-interest analysis. This regression model (A) was fitted to each voxel. The resulting map was thresholded at a t statistic of 4.6 and a cluster size of 5, corresponding approximately to the peak/cluster family-wise-error corrected threshold (the map was thresholded at t>3.4 for display purposes). For the Mood and Anxiety Symptom Questionnaire anhedonic depression subscale-by-reward expectancy interaction effect, four clusters reflecting an increasingly positive/decreasingly negative correlation between reward expectancy and prediction error with increasing anhedonia were obtained. Two clusters were centered slightly more anteriorly with respect to the ventral striatum regions of interest, in the anterior caudate (left: peak voxel: t=5.36, df=167, p<0.05 [family-wise error] [coordinates: −16, 28, 2; 46 voxels]; right: peak voxel: t=5.60, df=167, p<0.05 [family-wise error] [coordinates: 16, 26, 0; 26 voxels]), while a small cluster was slightly more posterior, on the right (peak voxel: t=4.75, df=167, p<0.05 [family-wise error] [coordinates: 8, 12, 2; 5 voxels]). The fourth cluster was centered on the left dorsal striatum (peak voxel: t=5.63, df=167, p<0.05 [family-wise error] [coordinates: −22, 16, 8; 41 voxels; all reported coordinates are in Montreal Neurological Institute space]). There were no significant voxels for the opposite direction of the interaction. The bar graphs show correlation coefficients (and standard errors) between reward expectancy and prediction error-related reactivity (extracted from a 6-mm sphere centered at coordinates 16, 26, 0) for healthy comparison subjects and anhedonia range equivalent subgroups (defined by tertile split of anhedonia scores) in the (B) first-recruited cohort (MDD100a) and (C) second-recruited cohort (MDD100b) of depressed individuals.
DISCUSSION
The goal of the present study was to determine the extent to which anhedonia disrupts normal patterns of functioning in a key region of reward circuitry, the ventral striatum, during uncertain reward and loss expectancy and outcome. Our findings indicate that while depressed and healthy individuals exhibited similar reward expectancy and prediction error-related ventral striatal reactivity, there were marked group differences in the relationship between the two measures. Healthy, but not depressed, individuals showed an inverse correlation between right ventral striatal reward expectancy and prediction error-related reactivity. Across participants, increased anhedonia severity was associated with a reduced inverse correlation between reward expectancy and prediction error-related right ventral striatal reactivity. These findings were present in the first-recruited cohort of depressed and healthy individuals and the larger sample of all recruited participants. Whole-brain analyses showed a similar moderation effect of anhedonia on the reward expectancy prediction error relationship in the anterior caudate (just anterior to the ventral striatal region of interest), a region key to disrupted reward processing in major depressive disorder (6), in both the first and second cohorts of depressed individuals and healthy individuals and across all participants.
A core feature of conditioning is the transfer in the control of behavior from reinforcement itself to antecedent stimuli that predict reinforcement (32). The temporal difference model provides a unifying account of this transfer, with a single signal that becomes coupled to the earliest reliable predictor of reward (11). Our finding in healthy individuals of an inverse relationship across individuals between reward expectancy and prediction error-related right ventral striatal reactivity is consistent with this model, since right ventral striatal reactivity transferred from the outcome (prediction error) to its antecedent cue (reward expectancy). Here, individuals who show greater reward expectancy than prediction error-related ventral striatal reactivity may show a faster reward cue-outcome contingency learning rate. The absence of this relationship in depressed individuals and the moderating effect of anhedonia upon this relationship suggest that more severely anhedonic individuals may show deficient temporal difference encoding and/or reward cue-outcome contingency learning in rewarding or potentially rewarding contexts. Our findings also highlight the specificity of this deficit to anhedonia, rather than this being a feature of major depressive disorder in general.
Previous findings indicate reduced prediction error-related ventral striatal reactivity in depressed individuals during reward learning (10, 15) and an association between decreased prediction error-related ventral striatal reactivity and greater anhedonia severity (15). Our study is the first, to our knowledge, to examine the relationship among reward expectancy and prediction error-related ventral striatal and whole-brain reactivity and the moderating effect of anhedonia on this relationship in a large sample of unmedicated depressed individuals. Furthermore, while these previous studies reported attenuated prediction error-related ventral striatal reactivity in depressed individuals when using temporal difference (10), or similar (15, 33) modeling approaches, cue-outcome contingency learning was captured by a fixed (10, 15) or individually determined (15, 33) learning rate. The fit of neural reactivity was therefore obtained by matching the observed pattern of dynamically changing ventral striatal reactivity with the parametric model of this reactivity. Thus, a poor fit in depressed individuals could be obtained for two reasons: 1) a general failure to activate the ventral striatum or 2) a failure of the model to reflect the abnormal pattern of fluctuation in ventral striatal reactivity in these individuals. Our findings support the latter, rather than the former account, given that depressed individuals showed normal levels of reward expectancy and prediction error-related ventral striatal reactivity but an aberrant relationship between these measures, especially in more severely anhedonic individuals.
The neurobiological basis of temporal difference learning is thought to involve modulation of ventral striatal activity by the midbrain (ventral tegmental area) dopamine system. The ventral tegmental area, which projects to the ventral striatum, is calibrated to optimize its signal-to-noise ratio by adapting to contextual rates of reinforcement (34). The dysregulation of this contextual adaptation in depressed individuals with greater anhedonia may thus be associated with variability of ventral tegmental area firing and lead to a ventral striatal reactivity pattern that does not tightly correspond to the temporal difference signal. Nevertheless, the ventral striatum may still show robust prediction error-related reactivity, as we show in depressed individuals in the present study, as well as in our previous findings (16).
Our findings are the first to show an absence of the expected inverse relationship between reward expectancy and prediction error-related ventral striatal reactivity in a large group of unmedicated individuals with major depressive disorder and suggest a neural mechanism for deficits in temporal difference learning in the illness. Furthermore, this aberrant pattern of striatal reactivity was associated with greater severity of anhedonia, even after controlling for other symptoms, in both cohorts of depressed individuals and all participants. The identification of a neural measure that may reflect a pathophysiological process underlying a core symptom of major depressive disorder is an important step forward in elucidating biomarkers of different affective symptom dimensions that, in turn, can help identify biomarkers and biosignatures that predict differential treatment response in the illness. These findings may also point to focused target of treatment in the future.
Supplementary Material
Acknowledgments
Supported by NIMH/NIH under awards U01MH092221 (to Dr. Trivedi) and U01MH092250 (to Drs. McGrath, Parsey, and Weissman), as well as by the EMBARC National Coordinating Center at the University of Texas Southwestern Medical Center and the Data Center at Columbia University and Stony Brook University. Valeant Pharmaceuticals donated the bupropion hydrochloride extended-release used in this study.
The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Dr. Almeida has received support from the American Academy of Child and Adolescent Psychiatry Pilot Research Award for General Psychiatry Residents, supported by Pfizer. Dr. Deckersbach has received research funding from the Depressive and Bipolar Disorder Alternative Treatment study, the International OCD Foundation, NARSAD, NIMH, TSA, and Tufts University; he has received honoraria, consultation fees, and/or royalties from Boston University, BrainCells, Catalan Agency for Health Technology Assessment and Research, Clintara, LLC, the Massachusetts General Hospital Psychiatry Academy, the Massachusetts Medical Society, the National Association of Social Workers-Massachusetts, the National Institute on Drug Abuse, NIMH, Oxford University Press, Systems Research and Applications Corporation, and Tufts University; and he has participated in research funded by the Agency for Healthcare Research and Quality, Cyberonics, Forest Research Institute, Janssen Pharmaceuticals, Medtronic, NIA, NIH, Northstar, Shire Development, and Takeda. Dr. Toups has received travel funds from Janssen Research and Development and currently receives compensation for serving on a data safety and monitoring board for Otsuka Pharmaceuticals. Dr. Kurian has received research funding/grants from Evotec, Forest Pharmaceuticals, Johnson and Johnson, Naurex, NIMH, Pfizer, Rexahn, and Targacept. Dr. Phillips has received funding from NIMH and the Emmerling-Pittsburgh Foundation. Dr. Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale and has received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current study; she was the recipient of a grant from Eli Lilly to support a year’s salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D.; she has received unrestricted educational grants and/or lecture fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Otsuka, Pfizer, Sanofi-Aventis, and Shire; and her family owns stock in Bristol-Myers Squibb. Dr. McGrath has received research grant support from Forest, Naurex, and Sunovion. Dr. Fava has received research support from Abbot Laboratories, Alkermes, American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir Pharmaceuticals, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clintara, LLC, Covance, Covidien, Eli Lilly, EnVivo Pharmaceuticals, Euthymics Bioscience, Forest Pharmaceuticals, Ganeden Biotech, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen Research and Development, LLC, Jed Foundation, Johnson and Johnson Pharmaceutical Research and Development, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbec, MedAvante, Methylation Sciences, NARSAD, the National Center for Complementary and Alternative Medicine, the National Institute of Drug Abuse, NIMH, Neuralstem, Novartis AG, Organon Pharmaceuticals, Pamlab, LLC, Pfizer, Pharmacia-Upjohn, Pharmaceutical Research Associates, Pharmavite, LLC, PharmoRx Therapeutics, Photothera, Reckitt Benckiser, Roche Pharmaceuticals, RCT Logic, LLC (formerly Clinical Trials Solutions, LLC), Sanofi-Aventis US LLC, Shire, Solvay Pharmaceuticals Stanley Medical Research Institute, Synthelabo, and Wyeth-Ayerst Laboratories; he has received advisory/consulting fees from Abbott Laboratories, Affectis Pharmaceuticals AG, Alkermes, Amarin Pharma, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, BioMarin Pharmaceuticals, Biovail Corporation, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, CNS Response, Compellis Pharmaceuticals, Cypress Pharmaceutical, DiagnoSearch Life Sciences (P) Ltd., Dinippon Sumitomo Pharma Co., Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, GenOmind, LLC, GlaxoSmithKline, Grunenthal GmbH, i3 Innovus/Ingenis, Janssen Pharmaceutica, Jazz Pharmaceuticals, Johnson and Johnson Pharmaceutical Research and Development, LLC, Knoll Pharmaceuticals Corp., Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MSI Methylation Sciences, Naurex, Neuralstem, Neuronetics, NextWave Pharmaceuticals, Novartis AG, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, LLC, Pfizer, PharmaStar, Pharmavite LLC, PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, LLC (formerly Clinical Trials Solutions, LLC), Rexahn Pharmaceuticals, Ridge Diagnostics, Roche, Sanofi-Aventis US LLC, Sepracor, Servier Laboratories, Schering-Plough Corporation, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical Company Limited, Tal Medical, Tetragenex Pharmaceuticals, TransForm Pharmaceuticals, Transcept Pharmaceuticals, and Vanda Pharmaceuticals; he has received speaking/publishing fees from Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, Imedex, Massachusetts General Hospital Psychiatry Academy/Primedia, Massachusetts General Hospital Psychiatry Academy/Reed Elsevier, Novartis AG, Organon Pharmaceuticals, Pfizer, PharmaStar, United Bio-Source, and Wyeth-Ayerst Laboratories; he is a shareholder with Compellis and PsyBrain; and he has received patents/other income/royalties from the following: patent for Sequential Parallel Comparison Design (licensed by Massachusetts General Hospital Psychiatry to Pharmaceutical Product Development, LLC) and patent application for a combination of ketamineplus scopolamine in major depressive disorder, Copyright for the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire, Sexual Functioning Inventory, Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs and Symptoms, and SAFER, royalties from Lippincott, Williams and Wilkins, Wolters Kluwer, and World Scientific Publishing. Dr. Weissman has received funding from the Interstitial Cystitis Association, NARSAD, the National Institute on Drug Abuse, NIMH, the Sackler Foundation, and the Templeton Foundation; and she receives royalties from American Psychiatric Publishing, MultiHealth Systems, Oxford University Press, and Perseus Press. Dr. Trivedi is or has served as an advisor/consultant and has received fees from Abbott Laboratories, Abdi Ibrahim, Akzo (Organon Pharmaceuticals), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb, Cephalon, Cerecor, CME Institute of Physicians, Concert Pharmaceuticals, Eli Lilly, Evotec, Fabre Kramer Pharmaceuticals, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson and Johnson Pharmaceutical Research and Development, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Pfizer, PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, TalMedical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories; and he has received grant/research support from the Agency for Healthcare Research and Quality, Corcept Therapeutics, Cyberonics, Merck, NARSAD, NIMH, and the National Institute on Drug Abuse.
Footnotes
All other authors report no financial relationships with commercial interests.
REFERENCES
- 1.Argyropoulos SV, Nutt DJ. Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J Psychopharmacol. 2013;27:869–877. doi: 10.1177/0269881113494104. [DOI] [PubMed] [Google Scholar]
- 2.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chase HW, Frank MJ, Michael A, et al. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychol Med. 2010;40:433–440. doi: 10.1017/S0033291709990468. [DOI] [PubMed] [Google Scholar]
- 4.Huys QJ, Pizzagalli DA, Bogdan R, et al. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WN, Chang SH, Guo LY, et al. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 7.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson B, Bhanji JP, Cooney RE, et al. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P, Waiter G, Ahearn T, et al. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 12.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Doherty JP, Dayan P, Friston K, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 14.Rohe T, Weber B, Fliessbach K. Dissociation of BOLD responses to reward prediction errors and reward receipt by a model comparison. Eur J Neurosci. 2012;36:2376–2382. doi: 10.1111/j.1460-9568.2012.08125.x. [DOI] [PubMed] [Google Scholar]
- 15.Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 16.Chase HW, Nusslock R, Almeida JR, et al. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 18.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 19.Phillips ML, Chase HW, Sheline Y, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. 2015;172:124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller J, Young CB, Kelley E, et al. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiatr Res. 2013;47:1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Keedwell PA, Andrew C, Williams SC, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 23.McGrath CL, Kelley ME, Holtzheimer PE, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson D, Clark LA. The Mood and Anxiety Symptom Questionnaire. Iowa City Iowa: University of Iowa; 1991. [Google Scholar]
- 26.Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger CD, Gorsuch RL, Lushene RE, et al. Manualfor the State-Trait Anxiety Inventory (form Y) Palo Alto, Calif: Consulting Psychologists Press; 1983. [Google Scholar]
- 28.Altman EG, Hedeker D, Peterson JL, et al. The Altman Self-Rating Mania Scale. Biol Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- 29.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado MR, Nystrom LE, Fissell C, et al. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 31.Holm SM, Forbes EE, Ryan ND, et al. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- 33.Dombrovski AY, Szanto K, Clark L, et al. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.