Abstract
The liver is a complex organ with a variety of tissue components that require a precise architecture for optimal function of metabolic and detoxification processes. As a result of the delicate orchestration required between the various hepatic tissues, it is not surprising that impairment of hepatic function can be caused by a variety of factors leading to chronic liver disease. Despite the growing rate of chronic liver disease, there are currently few effective treatment options besides orthotopic liver transplantation. Better therapeutic options reside in the potential for genetic and cellular therapies that promote progenitor cell activation aiding de novo epithelial and vascular regeneration, cell replacement, or population of bioartificial hepatic devices. In order to explore this area of new therapeutic potential, it is crucial to understand the factors that promote hepatic function through regulating cell identities and tissue architecture. In this commentary, we review the signals regulating liver cell fates during development and regeneration; and highlight the importance of patterning the hepatic vascular systems to set the groundwork for the macro and micro hepatic architecture of the epithelium.
Keywords: hepatic vasculature, hepatic cell lineage plasticity, oxygen gradients
IMPORTANCE OF UNDERSTANDING THE ESTABLISHMENT AND MAINTENANCE OF LIVER CELL IDENTITIES AND ARCHITECTURE
In the United States, 1 in 2,500 infants have severe liver disease, and an estimated one quarter of Americans will suffer from a liver or biliary disease at some point during their life. Liver diseases have a variety of etiologies, including: genetic, viral, nutritional, toxicity (alcohol or medicinal), and oncogenic. For many patients with severe liver disease and liver failure, the only viable treatment option is liver transplantation. Unfortunately, the need for transplantation organs greatly exceeds the supply of donated organs each year. To further compound this dilemma, a decrease in suitable donors is projected due to the high prevalence of obesity (Ogden et al., 2014). Therefore, recent research efforts have focused on discovering new ways to treat liver disease using cell-based therapies. This work has focused on understanding the potential and molecular regulation of hepatic cellular plasticity and exploiting these cellular characteristics to aid liver regeneration under a chronic injury condition. Ideally, the present research will uncover ways to promote endogenous progenitor cell activation and drive the regeneration of specific liver tissues. For these efforts to succeed and contribute to new regenerative therapies or bioartificial organs, we must be able to reestablish the conditions that promote cell differentiation and function. Hepatic function is highly dependent on having both the correct cell identities as well as having the tissues arranged in a precise lobular architecture. Currently, there is a limited understanding of how the liver vasculature forms even though its formation is a primary and essential step in directing the hepatic architecture. In this commentary, we will describe the crucial three-dimensional hepatic architecture of the epithelial and vascular tissues, the unique mechanism of differentiation and development of each tissue, and the importance of inter-tissue interaction during development and disease; while highlighting the fundamental role the portal vein and hepatic vasculature have in establishment and maintenance of proper liver configuration.
OVERVIEW OF LIVER ARCHITECTURAL ESTABLISHMENT AND SIGNALING PATHWAYS INVOLVED
Importance of liver architecture for function
Proper liver function requires a precise spatial arrangement of liver tissues: the portal vein (PV), central vein (CV), hepatic artery (HA), intrahepatic bile duct (IHBD), and hepatocyte zonation (Figure 1). The importance of the spatial associations of liver tissues is apparent in the structure and function of the hepatic lobule. Hepatic lobules are thought to be the smallest functional unit of the liver. Two dimensionally they appear in an approximate hexagonal shape with a PV at each vertex and a CV branch in the middle. The HA and IHBD branches are arranged at the vertices in close association with PV branches in structures known as portal triads. Hepatocytes arranged in cords fill in the space between the PV and the CV. The organized arrangement of the hepatocyte cords is vital for the exocrine and endocrine functions performed by the hepatocytes. Extending along the basal side of hepatocyte cords are the sinusoids. Sinusoids are specialized capillary structures that connect the PV and HA to the CV. Hepatocytes are allowed to exchange factors with and maintain the composition of nutrients in circulation through the intricate sinusoidal network. On the apical side of hepatocytes are canaliculi, small channels that allow passive transport of bile produced by hepatocytes into the IHBDs. Apart from their relationship in the lobule (micro architecture), each of these epithelial and vascular tissues also has its own architecture and spatial organization within the liver (macro architecture).
Figure 1. Spatial architecture and hepatocyte zonal characteristics in the hepatic lobule.
Hepatocyte cords run along the radius of the lobule between PVs and CVs. Bile is secreted into small canalicular channels and transported to the IHBDs. The sinusoidal capillaries carry blood from the PV and HA past the hepatocytes and ultimately into the CV. Oxygen tensions vary by vascular compartment of the liver with the highest oxygen tension found with the HA (74–104 mm Hg). In the PV, oxygen tensions are lower (34–46 mm Hg) and even lower in the CV (30–35 mm Hg). The sinusoids have a gradient of oxygen tension within periportal sinusoids at 65 mm Hg and the pericentral sinusoids at 30–35 mm Hg. The hepatocytes are arranged into three zones: zone 1 (purple) near the portal vein, zone 2 (medium blue) intermediate hepatocytes, and zone 3 (light blue) near the central vein.
Hepatic vascular development and architecture
The PV and HA architectures are essential to establish and maintain liver function. Oxygenated blood is supplied to the liver by the HA arising from the celiac artery via the descending aorta. The PV collects nutrient-rich effluent blood from the other peritoneal organs and brings it to the liver. In humans, the PV supplies approximately 75% of the afferent hepatic blood flow. The other 25% of hepatic blood flow is supplied by the HA (Tygstrup et al., 1962; Lautt, 2009). The PV and HA both empty into the hepatic sinusoids, and the CV collects the blood that has passed through the sinusoids and returns it to the inferior vena cava (Figure 1).
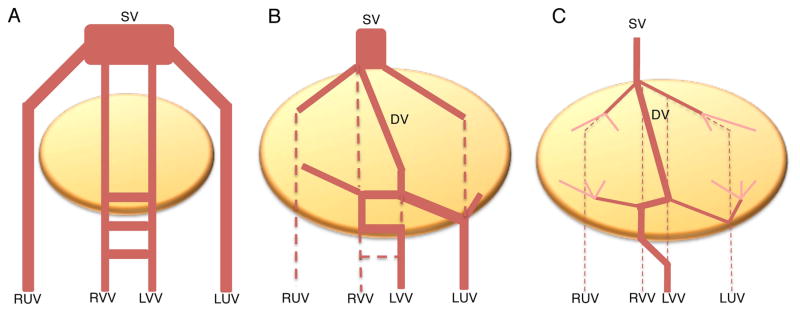
The PV and CV have a unique mechanism of development as extrapolated from the analysis of human fetuses (Lassau and Bastian, 1983; Gouysse et al., 2002; Collardeau-Frachon and Scoazec, 2008) and mouse embryos (Crawford et al., 2010). Both vessels derive from the fetal vitelline and umbilical veins. At the time of liver bud delamination and expansion, circa E9.5 in mouse, the hepatoblasts surround and disrupt the vitelline and umbilical veins. The veins remodel into a modified fetal circulatory network. The pre-hepatic and post-hepatic portions of the veins remain intact and give rise to the bases of the PV and CV (Figure 2) (Gouysse et al., 2002; Collardeau-Frachon and Scoazec, 2008; Crawford et al., 2010).
Figure 2. The vestigial hypothesis of PV and CV development.
According to histological studies of human fetuses, the PV and CV are hypothesized to emerge from the remodeled fetal vitelline and umbilical veins. A. Prior to liver bud formation, the right umbilical vein (RUV), left umbilical vein (LUV), right vitelline vein (RVV), and left vitelline vein (LVV) empty into the sinus venosus (SV). B. As the fetal liver bud expands, it envelops these fetal vessels and causes a remodeling event, generating a new structure, the ductus venosus (DV). C. At the time of birth, the DV collapses. Hepatic circulation after birth goes only through the PV and CV, which are proposed to retain components of the fetal veins.
The PV, CV and IHBD all form hierarchical branched structures that bifurcate approximately 17 to 20 times in human forming smaller and smaller branches to achieve their macro hepatic architecture (Crawford, 2002). Whether the hierarchical branched vascular structures of the PV and CV are formed by angiogenesis and/or vasculogenesis is unknown at this time. Developmentally, the PV is the first to establish its branching architecture, followed by the IHBDs and lastly the HA. The hepatic artery is proposed to develop as an angiogenic sprout originating from the dorsal aorta (Gouysse et al., 2002; Collardeau-Frachon and Scoazec, 2008). Signals derived from the IHBD are suggested to direct the development of the HA intrahepatically. This is supported by a failure to generate mature HAs in a mouse model where IHBD morphogenesis is genetically impaired (Fabris et al., 2008). In humans, congenital hepatic vascular malformations result in redirecting blood in and around the liver. The vascular shunting can occur from the HA to the CV (arteriovenous shunt), from the HA to the PV (arterioportal shunt), or from the PV to the systemic circulation (portosystemic shunt) (DeLeve et al., 2009). Hepatic vascular malformations are rare and can be asymptomatic. Often times, the vascular malformations are diagnosed during unrelated tests or scans. Congenital portosystemic shunts or Abernathy malformations are due to abnormal development of the vitelline venous system (extrahepatic) (Morgan and Superina, 1994; Murray et al., 2003) or failure to close the ductus venosus after birth (intrahepatic) (Yoshimoto et al., 2004). In a case study of a portosystemic shunt from the main PV to the inferior vena cava (Abernathy type I), the portal triads lacked PV branches, but IHBDs and HA branches were present (Emre et al., 2007). In contrast, the portal triads were observed to have large malformed HAs and no visible patent IHBDs in an example of Abernathy type II, a portosystemic shunt from the main PV to the inferior vena cava and a hypoplastic branch connected to and draining into the liver (Schaeffer et al., 2013). Therefore, patients with Abernethy malformations do not clearly support a required sequence of PV, IHBD, and then formation of HAs. An alternative explanation for these various intrahepatic portal triad malformations is that PVs are initially formed providing a pattern for the IHBDs and HAs, but are not maintained due to a shunt resulting in a lack of sustained blood flow.
The sinusoids, lined by liver sinusoidal endothelial cells (LSECs), traverse the lobule, bridging the PV and the CV. The origin of LSECs in the liver is not known. LSECs are presumed to arise from endothelial cells (ECs) resident in the septum transversum mesenchyme (STM), including the ECs that surround that hepatic bud at the time of evagination and delamination (Matsumoto et al., 2001). LSECs provide a lenient selective barrier between the sinusoidal blood and the hepatocytes, allowing for particles to pass through and access the hepatocytes. Sensitivity of LSECs to radiation, ischemia, toxins, chemotherapy and various medication leads to sinusoidal obstructive syndrome. The change in LSECs morphology causes obstructions or gaps in the endothelial barrier hindering sinusoidal blood flow (DeLeve, 211).
Signaling pathways controlling vascular morphogenesis
The main pathway implicated in regulating vascular morphogenesis is vascular endothelial growth factor (VEGF) signaling. VEGF is a crucial signaling pathway governing vascular development and behavior throughout the body. In mammals, there is one principal Vegf ligand and two principal receptors, VEGF receptor 1 (VEGFR1, also known as Flt1) and VEGFR2 (also known as KDR and Flk1) (Ferrara and Davis-Smyth, 1997). The VEGF signaling pathway is essential during development. Mice homozygous for a VEGFR1 or VEGFR2 deletion are embryonic lethal between embryonic day 8.5–9.5 due to impaired or altered vascular development and failed blood-island formation (Fong et al., 1995; Shalaby et al., 1995; Carmeliet et al., 1996; Ferrara et al., 1996). Important to the liver, the timing of lethality associated with a deficiency in either VEGF receptor corresponds to the formation of the hepatic diverticulum, after hepatic specification and prior to hepatic vascularization (Gualdi et al., 1996; Crawford et al., 2010). Therefore, new mouse models need to be generated for investigating the role of VEGF signaling in hepatic vascular development and macro architectural patterning.
In general, VEGF signaling has several known functions in vascular development and homeostasis, including: angiogenic directional growth and branching behavior, vascular permeability, endothelial fenestration, endothelial proliferation, and endothelial cell survival (Connolly et al., 1989; Gerber et al., 2002; Gerhardt et al., 2003; Carpenter et al., 2005; Lee et al., 2007; Krueger et al., 2011; Leung et al., 2013). Angiogenesis is the process of vascular development by which new vessels are generated and grow by sprouting from an existing vessel and extending directionally. The directionality of the angiogenic growth is directed by the graded concentration of Vegf protein (Figure 3). During angiogenesis, ECs in the growing vessel can take on one of two identities: tip or stalk (Phng and Gerhardt, 2009). Tip cells are located at the growing end of blood vessels. They display filopodial extensions and are responsible for pathfinding and directionality (Gerhardt et al., 2003). Stalk cells are all cells of the new vessel that trail behind the tip cell; these form a luminal structure and perform the proliferation required for vessel extension and growth.
Figure 3. Notch and VEGF signaling collaborate to specify tip and stalk cells during angiogenesis.
Angiogenic growth is directed by a gradient of Vegf protein (blue). The tip cell (light red) expresses VEGF receptor (VEGFR), allowing it to respond to Vegf protein. Activation of VEGFR causes the upregulation of Dll4 and VEGFR in the tip cell. Dll4 signals to activate Notch in stalk cells (dark red). The activation of Notch causes the down-regulation of VEGFR, making the stalk cells less responsive to environmental Vegf protein. Together, Notch and VEGF signaling generate positive feedback loops that reinforce the separate tip and stalk cell identities.
Tip and stalk cells engage in lateral inhibition through VEGF and Notch signaling. Both tip and stalk cells express VEGFR2. Upon exposure to Vegf protein, VEGFR2 is activated, leading to the upregulation of the Notch ligand Delta-like4 (Dll4) and VEGFR2, in a positive feedback loop (Phng and Gerhardt, 2009). Dll4 interacts activates Notch receptors expressed on surrounding cells. The activation of Notch inhibits the expression of VEGFR2 and upregulates VEGFR1, making the Notch-activated cell less sensitive to Vegf protein (Phng and Gerhardt, 2009). Through these feedback loops, one cell adopts the tip cell identity while the surrounding cells are designated as stalk cells. The functions of Notch and VEGF signaling in the tip/stalk designation have been defined in detail in several papers, mainly using the cultured mouse retina as a model system (Gerhardt et al., 2003; Hellstrom et al., 2007; Lobov et al., 2007; Suchting et al., 2007; Trindade et al., 2008; Benedito et al., 2009).
Vegf expression and consequent angiogenesis is often promoted by hypoxia. Under hypoxic conditions, cells stabilize the hypoxia inducible factor1α (HIF1α) protein, and the HIF1α/β heterodimer binds enhancer sequences of the Vegfa gene, stimulating the production of Vegf protein (Jones et al., 2001). Secretion of Vegf from a region of hypoxic tissue directs angiogenesis towards that area, ultimately increasing blood flow to alleviate the hypoxic condition.
VEGF signaling has also been shown to be important for the maintenance of LSEC characteristics (DeLeve et al., 2004; Walter et al., 2014a). In experiments with cultured primary LSECs, the loss of fenestrae and upregulation of the platelet-derived endothelial cell adhesion molecule (PECAM) expression occur within days after isolation and culture. Treatment of cultured LSECs with exogenous Vegf or co-cultures of LSECs with a Vegf-producing cell type such as hepatocytes or stellate cells is sufficient to maintain LSEC characteristics (DeLeve et al., 2004). Similar results are observed in vivo. Liver-specific Vegf deficient mice exhibit an expansion of cells expressing PECAM and laminin deposition, demonstrating a loss of LSEC identity (Walter et al., 2014a). Due to the close spatial relationship between LSECs and hepatocytes, it is likely that the hepatocytes are a source of Vegf that maintains LSEC identity in vivo.
Another important signaling pathway in hepatic vascularization is Notch. Notch signaling components are necessary for arteriovenous specification during embryogenesis (Gridley, 2010). Overexpression of Notch4 in the adult hepatic endothelium produces enlarged vessels and arterialization (Carlson et al., 2005). Interestingly, when constitutive activation of Notch4 was ceased, the vessels returned to their normal state. This suggests a role for Notch4 in adult arteriovenous specification and maintenance.
IHBD development and architecture depends on PV establishment
The IHBD has a hierarchical branching architecture that follows the branching pattern of the PV, stemming from a developmental connection (Figure 4). In the first step of IHBD morphogenesis, ductal plate formation, hepatoblasts adjacent to the portal vein mesenchyme activate expression of biliary epithelial cell (BEC) marker genes such as Sry-related HMG box 9 (Sox9). The specified BECs then undergo a remodeling event during which the cells become polarized and generate a lumen adjacent to the PV. The ductal plate cells that are not incorporated into IHBDs regress, turn off BEC markers, and differentiate as periportal hepatocytes (Antoniou et al., 2009; Si-Tayeb et al., 2010; Carpentier et al., 2011). Ductal plate structures are first observed in the proximal hilar regions of the liver and appear in the more distal regions progressively following the PV during embryonic and early postnatal development.
Figure 4. IHBD has a hierarchical branching architecture with a close association with the PV.
Ink injection highlights the hierarchical branching architecture and close proximity of the IHBD (black) and PV (white) systems in the mouse left lobe. This close association stems from a developmental process of ductal plate formation around PVs, culminating in the emergence of lumenal IHBDs spatially associated with PVs.
The smallest components of the biliary system are the canaliculi. The apical surfaces of adjacent hepatocytes are joined to form a narrow canalicular channel. Hepatocytes secrete bile into the canaliculi that form a connected network extending throughout the parenchymal epithelium (Treyer and Musch, 2013). The canaliculi are eventually connected to the small peripheral IHBD branches that merge into fewer, larger branches, until finally one single branch carries the bile out of the liver and transports it into the gallbladder for storage and ultimately into the intestine to aid in digestion. The IHBD system relies on its highly regulated three-dimensional structure to access all of the hepatocytes and effectively clear bile out of the liver. IHBD architectural formation is a highly complex and regulated process wherein ductal plate regression and tube establishment must occur in a coordinated fashion along the three-dimensional PV network to form a connected IHBD network.
Signaling pathways regulating IHBD morphogenesis
The primary pathway that has been implicated in IHBD morphogenesis is Notch signaling. Notch is a conserved signaling pathway that plays a role in reiterative cell fate decisions in several organs during development. Notch signaling has known roles in stem cell maintenance, cell fate decisions, proliferation, and tissue patterning (Andersson et al., 2011). In mammals, there are five canonical Notch ligands across two families (Delta1, 3, and 4, and Jagged1 and 2) and four Notch receptors (Notch1, 2, 3, and 4). Notch ligands and receptors are both membrane bound and participate in juxtacrine signaling between adjacent cells. Therefore, the Notch intercellular signaling pathway disposes the proximal spatial localization of the PV and IHBD.
Ductal plate morphogenesis begins at E13.5 in mouse, at which time the mesenchymal cells surrounding the PV express the Notch ligand Jagged1. Jagged1 interacts with the fundamental hepatic Notch receptor, Notch2, expressed on bipotential hepatoblasts to activate the Notch pathway (Geisler et al., 2008; Hofmann et al., 2010). The expression of a constitutively activated form of Notch2 or Notch1 is sufficient to promote BEC differentiation (Tchorz et al., 2009; Sparks et al., 2010). Additionally, Notch signaling is required for remodeling of the specified BECs into mature, lumenal IHBDs (McCright et al., 2002; Tanimizu and Miyajima, 2004; Geisler et al., 2008; Lozier et al., 2008; Hofmann et al., 2010; Sparks et al., 2010; Jeliazkova et al., 2013). Reduced Notch activity, accomplished through hepatic specific loss of Notch2, results in BEC specification, but inability to remodel into communicating IHBDs (Sparks et al., 2010). Further reduction of Notch activity via hepatic specific loss of Rbpj, the DNA-binding co-factor for all four Notch receptors, results in reduced BEC specification (Zong et al., 2009; Sparks et al., 2010).
In addition to Notch signaling, the transcription factors Sox9, Hnf1β, and Hnf6 are important for biliary morphogenesis; without each of these genes, delays or disruptions in biliary development are observed in mice (Clotman et al., 2002; Coffinier et al., 2002; Antoniou et al., 2009). It is likely that Notch acts upstream of Sox9 during biliary morphogenesis, as Sox9 is a known target of Notch signaling in several organs (Muto et al., 2009; Haller et al., 2012; Chen et al., 2013). The control of Hnf1β and Hnf6 by Notch signaling is less clear, occurring either in parallel or as an epistatic relationship. Hnf1β and Hnf6 expression are both increased with hepatic loss of Rbpj at embryonic day 16.5 (Vanderpool et al., 2012). Therefore, the increase in Hnf6 is likely compensatory and may contribute to the observed increase in its target Hnf1β expression (Clotman et al., 2002). Initial regulation of both Hnf1β and Sox9 by Hnf6 appears to occur during early embryonic time points, with expression of both factors approaching or equaling control mice by E17.5 in an Hnf6 global loss model (Clotman et al., 2002; Antoniou et al., 2009). An alternate possibility would be an epistatic model in which Notch signaling occurs upstream of Hnf6, acting as an attenuator of Hnf6. However, previous experimental models have shown that constitutive Notch activation does not down-regulate expression of Hnf6 (Tanimizu and Miyajima, 2004; Zong et al., 2009). The possibility of Hnf6 occurring upstream of Notch signaling is unlikely, given that Sox9 is a Notch target (Zong et al., 2009) and isolated hepatoblast-specific loss of Hnf6 did not result in any changes in Sox9 at embryonic and early postnatal stages (Vanderpool et al., 2012). Although mechanistic studies remain to be performed, published genetic data suggest that control of factors essential for early IHBD development occurs along parallel mechanisms through Hnf6 and Notch signaling.
Hepatocyte zonal organization is tightly correlated to the architecture of hepatic vascular systems
Within the lobule, there is spatial organization of hepatocytes that is important for function. Approximately 15–25 hepatocytes span the distance between the portal and central veins in the rat liver, forming a series of hepatocyte plates that radiate between the central vein and the portal vein vertices (Colnot and Perret, 2011). The hepatocytes collectively perform a wide variety of tasks, including gluconeogenesis, urea genesis, β-oxidation, and liponeogenesis (Bhatia et al., 1996). These functions are segregated between different subpopulations of hepatocytes. The lobule is organized into three spatial zones: zone 1 is the periportal zone, zone 2 is the intermediate zone, and zone 3 is the pericentral zone (Figure 1). Zone 1 hepatocytes specialize in gluconeogenesis and urea formation, while zone 3 hepatocytes specialize in liponeogenesis, glutamine synthesis, and glycolysis (Colnot and Perret, 2011). Accordingly, the expression of metabolic genes varies between the zones. For example, in rats and mice, one enzyme involved in urea synthesis, glutamine synthetase, is only expressed in the 1–2 cell layers of hepatocytes that immediately juxtapose the central veins. Cell morphologies also change zonally in the rat liver; periportal hepatocytes are smaller, approximately 7–15 μm in diameter, than pericentral hepatocytes, measuring approximately 30–40 μm (Bhatia et al., 1996).
Signaling pathways controlling lobular hepatocyte zonation
The main factor implicated in the formation and maintenance of hepatocyte zonation is Wnt/β-catenin signaling. β-catenin stabilization is both necessary and sufficient for expression of the pericentral enzyme glutamine synthetase (GS) in vivo in the mouse liver (Colnot and Perret, 2011). In a normal liver, stabilized β-catenin is observed only in the zone 3 pericentral hepatocytes. In a liver-specific β-catenin knockout mouse model, the expression of GS is completely absent from the liver. In contrast, the liver-specific knockout of Apc, a negative regulator of β-catenin stabilization, activates the expression of GS in all hepatocytes in the liver (Benhamouche et al., 2006; Colnot and Perret, 2011). The expression of Wnt ligands has been found in several liver cell types, including: hepatocytes, BECs, LSECs, stellate cells (pericytes in the liver sinusoids), and Kupffer cells (resident macrophages in the liver) (Zeng et al., 2007). It has been hypothesized, although without any concrete evidence, that Wnt/β-catenin signaling in the pericentral zone is directed by LSECs (Colnot and Perret, 2011). To investigate which hepatic cell type is responsible for providing Wnt ligands for proper hepatic zonation, a Wntless mouse line was used (Yang et al., 2014). Wntless is required to facilitate the secretion of all Wnt ligands from the cell membrane to signal to other cells. Targeted deletion of Wntless from hepatic epithelial cells, endothelial cells and macrophages were examined by using various Cre lines to eliminate the ability of each cell lineage to secrete Wnt ligands. The results indicate that hepatic epithelial cells and macrophages are not the source of Wnt proteins for β-catenin activation necessary for hepatic zonation. The complete loss of Wntless from hepatic endothelial cells results in embryonic lethality, preventing assessment of proper hepatic zonation. Therefore, further studies will be required to determine whether LSECs provide the source of Wnt ligands in the pericentral zone.
An additional key molecule mediating hepatic zonation is Hnf4α. Hnf4α opposes Wnt signaling to promote the expression of zone 1 periportal hepatocyte genes and inhibit the expression of zone 3 pericentral hepatocyte genes (Colletti et al., 2009). In an Hnf4α deficient liver, the expression of pericentral hepatocyte genes, including GS, is expanded into the periportal zone (Stanulovic et al., 2007). Evidence indicates that Hnf4α and β-catenin, along with its DNA-binding co-factors Tcf/Lef, directly compete for binding on the same consensus enhancer motifs thereby regulating zonal hepatocyte gene expression (Colletti et al., 2009; Colnot and Perret, 2011).
However, what sets up the localized expression pattern of molecular signals may be a more basic physiological principle and intimately regulated by vascular patterning. Oxygen pressure regulates hepatic zonation. The oxygen pressure both in the sinusoids and in the hepatocytes is graded along the axis of the hepatic lobule between the PV and the CV. The blood oxygen tension in the rat sinusoid ranges from approximately 65 mm Hg in the periportal zone to approximately 30–35 mm Hg in the pericentral zone (Figure 1) (Jungermann and Kietzmann, 1996; Lautt, 2009). The oxygen gradient within the sinusoid also generates a gradient in hepatocyte intracellular oxygen tension. The hepatocyte oxygen tensions are considered to range from 45–50 mm Hg in the periportal hepatocytes to 15–20 mm Hg in the pericentral hepatocytes (Jungermann and Kietzmann, 2000). There is evidence that hepatocytes are able to sense oxygen tension through a non-respiratory chain ferro-heme protein, revealing a potential pathway for oxygen to regulate hepatocyte zonal gene expression (Kietzmann et al., 1992; Kietzmann et al., 1993). In cultured primary rat hepatocytes, oxygen tensions influence the gene expression of some zonal genes, including the periportal gene phosphoenolpyruvate carboxykinase (PEPCK, involved in gluconeogenesis) and the pericentral gene glucokinase (GK, involved in glycolysis) (Wolfle and Jungermann, 1985; Jungermann and Kietzmann, 1996). However, it appears that only certain classes of zonal hepatocyte genes are sensitive to oxygen tension. Genes involved in glucose and drug metabolism are more readily influenced by blood flow and oxygen tension, while genes involved in ammonia detoxification and glutamine synthesis have a more stable and defined expression pattern in the face of oxygenation manipulations (Wagenaar et al., 1993; Wagenaar et al., 1994; Bhatia et al., 1996; Jungermann and Kietzmann, 1997; Allen and Bhatia, 2003; Colnot and Perret, 2011). While it is clear that oxygen pressure can influence hepatocyte zonation, an in vivo mechanism through which the regulation occurs and the importance of oxygen pressure during developmental zone establishment and homeostasis remains unknown.
An example demonstrating the importance of proper PV and HA architecture to pattern hepatocyte zonation is observed in a mouse model where Vegf was specifically deleted from the liver epithelial lineages at midgestation (Walter et al., 2014a). Epithelial derived-Vegf is required for the progressive postnatal elaboration of the PV and HA architecture. The changes in hepatic vasculature correlate with observed changes in hypoxic regions across the hepatic epithelium. These changes culminate in perturbation of the hepatocyte zones. Localization of glutamine synthetase, a pericentral hepatocyte marker, is expanded along with carbamoyl phosphate synthestase 1 (CPS1), expressed around the portal vein and intermediate hepatocytes. These results demonstrate the requirement for VEGF signaling in the hepatic epithelium to establish the proper liver vasculature, hepatic oxygenation, and hepatocyte zonal architecture.
KEY ISSUES OF HEPATIC CELL PLASTICITY THAT REMAIN TO BE ADDRESSED FOR REGENERATIVE AND CELL REPLACEMENT THERAPIES
Relationship of hepatocyte zonal identities to cell plasticity and progenitor activity
To further demonstrate the importance of PV establishment, ductular reactions are only observed in periportal regions of chemical-injury and genetic-driven experimental models (Desmet, 2011; Walter et al., 2014b). Additionally, in a variety of experimental injury models and human chronic liver diseases, there is evidence of coincident hepatocyte-to-BEC lineage conversion with the presence of ductular reactions (Michalopoulos et al., 2005; Limaye et al., 2008; Yanger et al., 2013; Sekiya and Suzuki, 2014; Walter et al., 2014b). Together, these data indicate the biological phenomenon of hepatocyte-to-BEC conversion is restricted to zone 1 and zone 2 hepatocytes during liver injury. Even in experimental mouse models where activated Notch is overexpressed in all hepatoblasts or hepatocytes, conversion of hepatoblasts/hepatocytes into BECs only occurs in zone 1 and zone 2 hepatocytes; zone 3 hepatocytes do not fully activate a biliary program expressing cytokeratin 19 despite expressing activated Notch (Sparks et al., 2010; Jeliazkova et al., 2013; Yanger et al., 2013). Potential mechanisms for the spatial restriction in BEC differentiation include 1) a difference in competency of hepatocytes in different zones to receive and/or respond to a pro-BEC differentiation signal or 2) the spatial restriction of a pro-BEC signal to periportal areas. Additionally, differences in the oxygen tension gradient may directly, within the hepatocyte, or indirectly, through vascular macro architecture, influence hepatocyte competency. If an endogenous hepatocyte zonal identity is crucial for cell plasticity, focusing either exclusively on periportal hepatocytes or attempting to identify a zonal signal or oxygen level required to activate hepatocyte-to-BEC conversion may aid current research efforts.
Often times, the signaling pathways and mechanisms that control embryonic cell fates and tissue architecture retain function during adult homeostasis and are re-activated during organ regeneration. In the liver, several signaling pathways with known roles during hepatogenesis, including Notch and Wnt, have roles during injury and regeneration as well. Sox9, a direct target of Notch signaling in the hepatic ductal plate, has been implicated as a marker of hepatocyte-to-BEC conversion during mouse liver injury (Yanger et al., 2013). Thus, the ductal plate differentiation program may be utilized for the generation of new BECs in injured livers of adult mice. During liver injury in adult mice, disrupting Notch signaling is demonstrated to reduce the expression of Sox9 in hepatocytes and to consequently restrict the process of hepatocyte-to-BEC conversion, confirming the link between Notch signaling and Sox9 in cell lineage decisions and plasticity in both the embryonic and adult liver (Yanger et al., 2013; Sekiya and Suzuki, 2014). Studies have demonstrated that Notch signaling plays a role in the generation of the ductular reaction in rats and in mice, as inhibiting Notch signaling can reduce or delay the formation of the ductular reaction (Darwiche et al., 2011; Fiorotto et al., 2013). However, loss of Hnf6 within the background of deficient Notch signaling, via Rbpj, results in initial ductal insufficiency and progressive regeneration of communicating IHBDs (Vanderpool et al., 2012; Walter et al., 2014b). This demonstrates the plasticity of the liver in a ductopenic model and its ability to regenerate bile ducts without the presence Hnf6 or Rbpj.
In addition to Notch, Wnt signaling has also been examined for its role in directing cell fate decisions in the regenerating adult rodent liver. Current thinking suggests that Notch and Wnt may have opposing roles in directing cell fate decisions during liver regeneration, with Notch promoting BEC specification and Wnt promoting hepatocyte fates (Boulter et al., 2012; Strazzabosco and Fabris, 2013). When acute and chronic human liver diseases were analyzed for activation of Notch or Wnt signaling, Wnt signaling was found to be upregulated in hepatic progenitor cells (HPCs) in a parenchymal disease, while Notch was activated in the HPCs of a biliary disease (Spee et al., 2010). These findings suggest different regenerative responses occur in the liver depending on the affected tissue and extent of the injury.
Besides the known capacity of mature hepatocytes to proliferate and produce large numbers of hepatocytes, the process of liver regeneration has long implicated facultative stem cells to rescue hepatic function in conditions where hepatocytes are unable to meet the required demands (Miyajima et al., 2014). Recently there has been much debate on the topic of whether liver stem cells exist in mouse models of hepatic injury associated with ductular reactions (Grompe, 2014). Clonally derived cells isolated from the reactive ductular populations demonstrate bipotentiality in culture and the ability to engraft and generate hepatocytes upon transplantation (Dorrell et al., 2011; Huch et al., 2013). However, in vivo lineage tracing using genetic tools in mouse models of hepatic injury does not support the presence of a facultative liver stem cell (Schaub et al., 2014; Yanger et al., 2014). A caveat to the current mouse injury models of ductular reactions is the fact that some hepatocytes retain the ability to proliferate and therefore may be able to meet the demands of hepatic function without instigating the facultative stem cell compartment. Thus, unveiling a facultative liver stem cell in mice conceivably awaits development of a novel hepatic injury model.
Vascular implications for de novo liver regenerative therapies and generation of bioartificial organs
The PV structure appears to be the first occurrence of an organized three-dimensional architecture during development of the liver. Based on the observed sequence of hepatogenesis in humans (Gouysse et al., 2002; Collardeau-Frachon and Scoazec, 2008; Fabris et al., 2008) and experimental mouse models (Fabris et al., 2008; Hofmann et al., 2010), it can be extrapolated that the establishment of the PV is crucial for directing the development of the IHBD and the HA, and together, the spatial relationship of the macro architecture. The PV, HA, and CV are then involved in generating and maintaining the micro structure of the lobule and the spatial identities of hepatocytes by establishing the oxygen gradient across the lobule. As such, cell replacement therapies aimed at regenerating large portions of the liver after, for example, resection, should carefully consider the vascular architecture. It is likely that the implantation of hepatocytes without an appropriate and regulated vascular architecture may not result in proper hepatocyte survival and function, as well as establishment and maintenance of an IHBD system.
Additionally, studies aimed at generating bioartificial organs from decellularized liver extracellular matrices obtain the most success from protocols utilizing reagents that decellularize the liver while maintaining the native vascular architecture (Uygun et al., 2010). The liver vasculature is a primary step for directing hepatocyte zonal identity as well as providing nutrients and oxygen to nourish the hepatocytes seeded onto the decellularized liver matrix. The extracellular matrix (ECM) of the liver provides structural support, however, it also aids the liver tissue by releasing biochemical cues such as variety of growth factors. Although there have been many advances in bioartificial livers, there are still challenges that remain, one of which is low efficiency of hepatocyte engraftment. To try and overcome this limitation, one research group designed liver scaffolds that encompassed liver-derived ECM and growth factors that were then implanted into the rat liver (Hammond et al., 2011). They found that with this technique they had increased infiltration of the hepatocytes into the scaffold as well as an increase in proliferation around the implanted scaffold (Hammond et al., 2011). Future work in this field will be focused on understanding the composition of native liver ECM for effective synthesis in the lab, as well as, uncovering the complexities of the growth factors, how they promote migration and induce proliferation into the matrix, and what concentrations are optimal for these processes (Bhatia et al., 2014; Handa et al., 2014).
CONCLUSIONS
In order to make advances in future therapeutic options for chronic liver disease, a greater understanding of hepatic vasculature development is crucial. As discussed, the proper establishment of the PV and other hepatic vasculature networks are central for directing subsequent liver tissue architecture, hepatocyte zonation, and ultimately liver function. In addition to hepatic vascular architecture, a more thorough understanding of signaling pathways directing hepatic vasculature formation and endothelial diversity is needed. Endothelial cells from different organs harbor combinations of transcription factors and other factors important for microvascular specification such as angiocrine growth factors and chemokines that are distinguishable by organ type (Nolan et al., 2013). The microvasculature depends on these cues from their environment for proper specification and functionality (Nolan et al., 2013). A greater understanding of these unique factors for each organ will be beneficial in optimizing bioartifical organs of all kinds. All of these areas that direct macro and micro hepatic architecture need to be understood to ultimately drive better therapeutic options and optimal innovation of bioartifical livers to address chronic liver disease.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (NIH) to S.S.H (R01DK078640).
Footnotes
Disclosures: The authors have nothing to disclose.
References
- Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng. 2003;82:253–262. doi: 10.1002/bit.10569. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Toner M, Foy BD, Rotem A, O’Neil KM, Tompkins RG, Yarmush ML. Zonal liver cell heterogeneity: effects of oxygen on metabolic functions of hepatocytes. Cellular Engineering. 1996;1:125–135. [Google Scholar]
- Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr242. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A. 2005;102:9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carpenter B, Lin Y, Stoll S, Raffai RL, McCuskey R, Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development. 2005;132:3293–3303. doi: 10.1242/dev.01902. [DOI] [PubMed] [Google Scholar]
- Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. 1438 e1431–1434. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tao J, Bae Y, Jiang MM, Bertin T, Chen Y, Yang T, Lee B. Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox9. J Bone Miner Res. 2013;28:649–659. doi: 10.1002/jbmr.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Gresh L, Fiette L, Tronche F, Schutz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- Collardeau-Frachon S, Scoazec JY. Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken) 2008;291:614–627. doi: 10.1002/ar.20679. [DOI] [PubMed] [Google Scholar]
- Colletti M, Cicchini C, Conigliaro A, Santangelo L, Alonzi T, Pasquini E, Tripodi M, Amicone L. Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology. 2009;137:660–672. doi: 10.1053/j.gastro.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Colnot S, Perret C. Liver Zonation. In: Monga SPS, editor. Molecular Pathology of Liver Diseases. Molecular Pathology Library; 2011. pp. 7–16. [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JM. Development of the intrahepatic biliary tree. Semin Liver Dis. 2002;22:213–226. doi: 10.1055/s-2002-34508. [DOI] [PubMed] [Google Scholar]
- Crawford LW, Foley JF, Elmore SA. Histology atlas of the developing mouse hepatobiliary system with emphasis on embryonic days 9.5–18.5. Toxicol Pathol. 2010;38:872–906. doi: 10.1177/0192623310374329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwiche H, Oh SH, Steiger-Luther NC, Williams JM, Pintilie DG, Shupe TD, Petersen BE. Inhibition of Notch signaling affects hepatic oval cell response in rat model of 2AAF-PH. Hepat Med. 2011;3:89–98. doi: 10.2147/HMER.S12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeve LD. Vascular Liver Disease and the Liver Sinusoidal Endothelial Cell. In: DeLeve LD, Garcia-Tsao G, editors. Vascular Liver Disease - Mechanisms and Management. Springer; New York: 211. pp. 25–40. [Google Scholar]
- DeLeve LD, Valla DC, Garcia-Tsao G American Association for the Study Liver D. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261–270. doi: 10.1007/s00428-011-1049-2. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH, Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre S, Arnon R, Cohen E, Morotti RA, Vaysman D, Shneider BL. Resolution of hepatopulmonary syndrome after auxiliary partial orthotopic liver transplantation in Abernethy malformation. A case report. Liver Transpl. 2007;13:1662–1668. doi: 10.1002/lt.21349. [DOI] [PubMed] [Google Scholar]
- Fabris L, Cadamuro M, Libbrecht L, Raynaud P, Spirli C, Fiorotto R, Okolicsanyi L, Lemaigre F, Strazzabosco M, Roskams T. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–728. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Fiorotto R, Raizner A, Morell CM, Torsello B, Scirpo R, Fabris L, Spirli C, Strazzabosco M. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–130. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouysse G, Couvelard A, Frachon S, Bouvier R, Nejjari M, Dauge MC, Feldmann G, Henin D, Scoazec JY. Relationship between vascular development and vascular differentiation during liver organogenesis in humans. J Hepatol. 2002;37:730–740. doi: 10.1016/s0168-8278(02)00282-9. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M. Liver stem cells, where art thou? Cell Stem Cell. 2014;15:257–258. doi: 10.1016/j.stem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- Haller R, Schwanbeck R, Martini S, Bernoth K, Kramer J, Just U, Rohwedel J. Notch1 signaling regulates chondrogenic lineage determination through Sox9 activation. Cell Death Differ. 2012;19:461–469. doi: 10.1038/cdd.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JS, Gilbert TW, Howard D, Zaitoun A, Michalopoulos G, Shakesheff KM, Beckingham IJ, Badylak SF. Scaffolds containing growth factors and extracellular matrix induce hepatocyte proliferation and cell migration in normal and regenerating rat liver. J Hepatol. 2011;54:279–287. doi: 10.1016/j.jhep.2010.06.040. [DOI] [PubMed] [Google Scholar]
- Handa K, Matsubara K, Fukumitsu K, Guzman-Lepe J, Watson A, Soto-Gutierrez A. Assembly of human organs from stem cells to study liver disease. Am J Pathol. 2014;184:348–357. doi: 10.1016/j.ajpath.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeliazkova P, Jors S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, Siveke JT, Geisler F. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- Jones A, Fujiyama C, Blanche C, Moore JW, Fuggle S, Cranston D, Bicknell R, Harris AL. Relation of vascular endothelial growth factor production to expression and regulation of hypoxia-inducible factor-1 alpha and hypoxia-inducible factor-2 alpha in human bladder tumors and cell lines. Clin Cancer Res. 2001;7:1263–1272. [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203. doi: 10.1146/annurev.nu.16.070196.001143. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney Int. 1997;51:402–412. doi: 10.1038/ki.1997.53. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Schmidt H, Probst I, Jungermann K. Modulation of the glucagon-dependent activation of the phosphoenolpyruvate carboxykinase gene by oxygen in rat hepatocyte cultures. Evidence for a heme protein as oxygen sensor. FEBS Lett. 1992;311:251–255. doi: 10.1016/0014-5793(92)81113-z. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Schmidt H, Unthan-Fechner K, Probst I, Jungermann K. A ferro-heme protein senses oxygen levels, which modulate the glucagon-dependent activation of the phosphoenolpyruvate carboxykinase gene in rat hepatocyte cultures. Biochem Biophys Res Commun. 1993;195:792–798. doi: 10.1006/bbrc.1993.2115. [DOI] [PubMed] [Google Scholar]
- Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, le Noble F. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassau JP, Bastian D. Organogenesis of the Venous Structures of the Human-Liver - a Hemodynamic Theory. Anatomia Clinica. 1983;5:97–102. [Google Scholar]
- Lautt WW. Hepatic Circulation: Physiology and Pathophysiology. San Rafael (CA): 2009. [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Ciau-Uitz A, Pinheiro P, Monteiro R, Zuo J, Vyas P, Patient R, Porcher C. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013;24:144–158. doi: 10.1016/j.devcel.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye PB, Alarcon G, Walls AL, Nalesnik MA, Michalopoulos GK, Demetris AJ, Ochoa ER. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab Invest. 2008;88:865–872. doi: 10.1038/labinvest.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg. 1994;29:1239–1241. doi: 10.1016/0022-3468(94)90812-5. [DOI] [PubMed] [Google Scholar]
- Murray CP, Yoo SJ, Babyn PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614–620. doi: 10.1007/s00247-003-1002-x. [DOI] [PubMed] [Google Scholar]
- Muto A, Iida A, Satoh S, Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Muller glial cell development in mouse retina. Exp Eye Res. 2009;89:549–558. doi: 10.1016/j.exer.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Schaeffer DF, Laiq S, Jang HJ, John R, Adeyi OA. Abernethy malformation type II with nephrotic syndrome and other multisystemic presentation: an illustrative case for understanding pathogenesis of extrahepatic complication of congenital portosystemic shunt. Hum Pathol. 2013;44:432–437. doi: 10.1016/j.humpath.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya S, Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am J Pathol. 2014;184:1468–1478. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sparks EE, Huppert KA, Brown MA, Washington MK, Huppert SS. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- Stanulovic VS, Kyrmizi I, Kruithof-de Julio M, Hoogenkamp M, Vermeulen JL, Ruijter JM, Talianidis I, Hakvoort TB, Lamers WH. Hepatic HNF4alpha deficiency induces periportal expression of glutamine synthetase and other pericentral enzymes. Hepatology. 2007;45:433–444. doi: 10.1002/hep.21456. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M, Fabris L. The balance between Notch/Wnt signaling regulates progenitor cells’ commitment during liver repair: mystery solved? J Hepatol. 2013;58:181–183. doi: 10.1016/j.jhep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- Tchorz JS, Kinter J, Muller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009;50:871–879. doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- Treyer A, Musch A. Hepatocyte polarity. Compr Physiol. 2013;3:243–287. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS, Duarte A. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112:1720–1729. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tygstrup N, Winkler K, Mellemgaard K, Andreassen M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J Clin Invest. 1962;41:447–454. doi: 10.1172/JCI104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool C, Sparks EE, Huppert KA, Gannon M, Means AL, Huppert SS. Genetic interactions between hepatocyte nuclear factor-6 and Notch signaling regulate mouse intrahepatic bile duct development in vivo. Hepatology. 2012;55:233–243. doi: 10.1002/hep.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar GT, Chamuleau RA, de Haan JG, Maas MA, de Boer PA, Marx F, Moorman AF, Frederiks WM, Lamers WH. Experimental evidence that the physiological position of the liver within the circulation is not a major determinant of zonation of gene expression. Hepatology. 1993;18:1144–1153. [PubMed] [Google Scholar]
- Wagenaar GT, Chamuleau RA, Maas MA, de Bruin K, Korfage HA, Lamers WH. The physiological position of the liver in the circulation is not a major determinant of its functional capacity. Hepatology. 1994;20:1532–1540. doi: 10.1002/hep.1840200624. [DOI] [PubMed] [Google Scholar]
- Walter TJ, Cast AE, Huppert KA, Huppert SS. Epithelial VEGF signaling is required in the mouse liver for proper sinusoid endothelial cell identity and hepatocyte zonation in vivo. Am J Physiol Gastrointest Liver Physiol. 2014a;306:G849–862. doi: 10.1152/ajpgi.00426.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Vanderpool C, Cast AE, Huppert SS. Intrahepatic bile duct regeneration in mice does not require Hnf6 or Notch signaling through Rbpj. Am J Pathol. 2014b;184:1479–1488. doi: 10.1016/j.ajpath.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle D, Jungermann K. Long-term effects of physiological oxygen concentrations on glycolysis and gluconeogenesis in hepatocyte cultures. Eur J Biochem. 1985;151:299–303. doi: 10.1111/j.1432-1033.1985.tb09100.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. Beta-catenin signaling in murine liver zonation and regeneration: A Wnt-Wnt situation! Hepatology. 2014 doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y, Shimizu R, Saeki T, Harada T, Sugio Y, Nomura S, Tanaka H. Patent ductus venosus in children: a case report and review of the literature. J Pediatr Surg. 2004;39:E1–5. doi: 10.1016/j.jpedsurg.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Zeng G, Awan F, Otruba W, Muller P, Apte U, Tan X, Gandhi C, Demetris AJ, Monga SP. Wnt’er in liver: expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]