Abstract
The full role of adult hippocampal neurogenesis (AHN) remains to be determined, yet it is implicated in learning and emotional functions, and is disrupted in negative mood disorders. Recent evidence indicates that AHN is decreased in persistent pain consistent with the idea that chronic pain is a major stressor, associated with negative moods and abnormal memories. Yet, the role of AHN in development of persistent pain has remained unexplored. In this study, we test the influence of AHN in postinjury inflammatory and neuropathic persistent pain-like behaviors by manipulating neurogenesis: pharmacologically through intracerebroventricular infusion of the antimitotic AraC; ablation of AHN by x-irradiation; and using transgenic mice with increased or decreased AHN. Downregulating neurogenesis reversibly diminished or blocked persistent pain; oppositely, upregulating neurogenesis led to prolonged persistent pain. Moreover, we could dissociate negative mood from persistent pain. These results suggest that AHN-mediated hippocampal learning mechanisms are involved in the emergence of persistent pain.
Keywords: Inflammatory, Neuropathic, Learning, Mood, Neurogenesis
1. Introduction
Mechanisms underlying the development of chronic pain remain minimally understood. Recent advances suggest that brain anatomy and physiology, especially components of the limbic brain, play an important role in the process. Yet, detailed cellular and molecular contributions to the transition to chronic pain are not available. As chronic pain is a complex behavior, associated with abnormal mood and memory, it is expected to involve hippocampal processes, and indeed recent evidence from human patients with chronic pain and rodent models demonstrates multiple abnormalities in hippocampal function, changes in associated behavior, and decreases in adult hippocampal neurogenesis (AHN).14,36,37,39,47 Furthermore, depression and anxiety disorders are highly comorbid with chronic pain,22,43 perhaps because of the stress effects of the condition,17 and both are associated with decreases in hippocampal neurogenesis, and antidepressants’ positive effects have been shown to be reliant on increased hippocampal neurogenesis.35,41 Consistent with the latter notion, recent evidence shows that rodents with a reversible neuropathic injury, where mechanical allodynia is relieved weeks after the initial injury, continue to exhibit depression-like and anxiety-like behaviors coupled with persistent decreased AHN,14 implying that even after relief from the physical pain of the neuropathy, its more long-term stressing consequences endure on the nervous system, and that impaired neurogenesis is a reflection of these consequences.
Patients with chronic pain often have poor performance on memory tasks13,29,38 and exhibit difficulty with classical conditioning20; and ample evidence points to the tight interrelationship between pain and learning mechanisms especially within the fear-conditioning literature.2 Even though the exact role of AHN in cognition and behavior remains unclear, there is extensive evidence of its contribution to learning and to memory formation.12,16,19,23,40 Therefore, an additional role of AHN may be its involvement in memory processes that possibly underlie development of chronic pain. Given that chronic pain is associated with reorganization of underlying neuronal circuits, from the periphery to the cortex,3,27,50 where mesocorticolimbic circuits seem to play a critical role,4,33 it is likely that AHN-mediated learning processes are involved in this reorganization. However, the role of AHN in the development of persistent pain remains unexplored. We test the latter hypothesis by enhancing or suppressing AHN using a variety of approaches: (1) intracerebroventricular infusion of AraC, (2) ablation of AHN with x-irradiation, and (3) transgenic bone morphogenetic protein (BMP) and Noggin mice, which are known to express increased/decreased AHN, respectively, to examine their effects on persistent pain as a consequence of inflammatory or neuropathic peripheral injuries. By manipulating levels of AHN before the induction of peripheral injury, we determine the influence of AHN on the development of postinjury persistent or chronic pain.
2. Methods
2.1. Animals
C57Bl/6 male mice obtained from Harlan were used for intracerebroventricular (ICV) infusion experiments and for x-ray ablation experiments. The x-ray ablation followed the methods described previously.41 Mice were anesthetized with ketamine and xylozine, placed in a stereotaxic frame, and radiated using a Siemens Stabilopen x-ray system operated at 300 kVp and 20 mA. Lead shields were used to only expose the subgranular zone (SGZ) of the hippocampus. A cumulative 15 Gy dose was delivered in 3 sessions. The development of NSE-Noggin (neuron specific enolase–noggin) and NSE-BMP4 transgenic mice used was described previously.23 These mice were initially derived on the FVB background strain and were backcrossed 5 to 7 generations onto C57Bl/6. All mice were males and at 8 to 9 weeks of age at the start of experiments. This study was approved by the Animal Care and Use Committee of Northwestern University and Columbia University.
2.2. Inflammatory pain model: carrageenan
In these experiments, 100 μL carrageenan solution (10 mg/mL in physiological saline) was injected into the plantar surface of the left hind paw, and 100 μL physiological saline into the right.
2.3. Neuropathic pain model: spared-nerve injury
Spared-nerve injury (SNI) has been previously described in detail.10 Mice were anesthetized with isoflurane 1.5% to 2% and a mixture of 30% N2O and 70% O2. The left hind-leg sciatic nerve was exposed at the level of the trifurcation into the sural, tibial, and common peroneal nerves. The tibial and common peroneal nerves were tightly ligated and severed, leaving the sural nerve intact. Animals in the Sham surgery group had their sciatic nerve exposed as in the SNI procedure but received no further manipulation.
2.4. Assessing pain behaviors
All pain-related behaviors were assessed using double-blind procedures.
2.4.1. Tactile sensitivity
Paw withdrawal thresholds to Von Frey filament (VF) stimulation were used to assess mechanical sensitivity of the hind paws. Animals were placed in a Plexiglas box with a wire grid floor and allowed to habituate. Filaments of various forces (Stoelting) were applied to the lateral plantar surface of each hind paw, which is innervated by the sural nerve (the only nerve intact after SNI surgery), thus the only surface with sensitivity on the injury side. Of note, 50% thresholds were calculated according to Chaplan et al.6
2.4.2. Cold sensitivity
A blunt needle connected to a syringe was used to drop 50 μL of acetone on the lateral hind paw. Mice were observed for 5 minutes, and their withdrawal behavior and the duration of their withdrawal reaction were recorded according to previous reports.8 Paw withdrawals due to locomotion or weight shifting were not counted.
2.4.3. Heat sensitivity
Animals were placed in an acrylic box with glass pane floor, and the plantar surface of their hind paw was exposed to a beam of infrared radiant heat (Ugo Basile; Stoelting).24 Paw withdrawal latencies were recorded and were measured twice per session, separated by a minimum interval of 5 minutes. Again, paw withdrawals due to locomotion or weight shifting were not counted and the trials repeated.
2.5. Mood-related behavioral testing
2.5.1. Forced swim test
Mice were placed in the center of an upright cylinder filled with lukewarm water (25°C–26°C) to 10 cm below its opening and a depth of 20 cm. Animals were observed in the water for a period of 6 minutes and were monitored for the time spent in a stereotyped immobile posture. Secondary video analysis was used to confirm the consistency and threshold of scoring, as checked by a second masked observer. The cumulative time spent immobile throughout the exposure during each of three 2-minute segments was recorded.
2.5.2. Tail suspension test
Mice were affixed with tape near the middle of the tail to a thin cord that was used to suspend the animals 5 cm above the ground for 5 minutes. Animals were scored for levels of immobility as described for the forced swim test above.
2.5.3. Open field
Mice were placed in the 60- × 60-cm field surrounded by 50-cm high wall under dim light. Activity was measured for 5 minutes to assess total distance traveled and the time spent in the central zone (area >12 cm from walls). Total distance traveled was indicative of general level of activity, whereas the percent time spent in the center was used as an index of the anxiety state of mice.46
2.5.4. Black-box emergence test
A 9 × 9 × 9-cm 3-sided covered box was placed in the center of a 40 × 40 × 40-cm open field illuminated by bright light. Mice were placed in the dark box, and the latency to leave the box was recorded by a video camera and analyzed by Any-Maze (San Diego Instruments, Inc.). This latency was interpreted as anxiety-like behavior.30
2.6. Drug delivery
AraC (Cytosine-β-d-Arabino-Furanoside, 2% in saline, Sigma-Aldrich) or saline was ICV administered with a microosmotic pump (0.25 μL/h, 14 days, Model 1002; Alzet).23 The coordinates of ICV delivery were 0.4 mm posterior to the bregma, 1 mm lateral to the midline, and 2 mm ventral to the skull. Mice show full behavioral and functional recovery soon after surgery.
2.7. Bromodeoxyuridine labeling
For the AraC experiment, all mice received intraperitoneal injections of bromodeoxyuridine (BrdU) (Sigma-Aldrich) 50 mg/kg dissolved in sterile phosphate buffered saline (PBS) (10 mg/mL) and filtered. Injections were given starting day 1 after SNI surgery or day 15 after SNI surgery and continued for 5 days. Mice were perfused 1 week after the last injection.
For the x-ray experiment and the transgenic mice, all mice received a single intraperitoneal injection of BrdU (50 mg/kg) dissolved in sterile PBS (10 mg/mL) and filtered, 3 days before killing the animals.
2.8. Immunohistochemistry
Immunohistochemistry was performed in 3 separate laboratories, using slightly different approaches.
2.8.1. The AraC experiment tissue was processed in RJM laboratory
Mice were transcardially perfused with 4% paraformaldehyde (PFA) in phosphate buffer. Brains were postfixed for 48 hours and sectioned on a vibratome (Leica Microsystems) and collected in cold PBS. To count the BrdU in the hippocampal dentate gyrus SGZ, free-floating sections were then incubated with primary antibody for BrdU (1:500; Fitzgerald) and biotin conjugated secondary (Jackson ImmunoResearch, West Grove, PA) along with Alexa Fluor 633 (Invitrogen) was used. Slices were mounted in Vectashield (Vector) and observed with a fluorescence microscope (Olympus Fluoview FV10i, Confocal Laser Scanning Microscope, Center Valley, PA) for labeling at × 10. To determine the number of BrdU+ cells in the SGZ, we counted every sixth section (6 sections per brain) and multiplied the result by 6.42 Representative slices from each group were separately double stained for doublecortin (DCX) and BrdU to determine whether BrdU+ cells were also DCX+. Free-floating sections were incubated with primary antibodies for Brdu (1:500) and DCX (1:300; Millipore). After primary incubation, the corresponding secondary antibody conjugated with Alexa Fluor 633 (Invitrogen) for DCX and biotin conjugated secondary (Jackson ImmunoResearch) along with Alexa 546 (Jackson ImmunoResearch) was used for Brdu.
2.8.2. The x-ray experiment tissue was processed in RH laboratory
Mice were anesthetized with ketamine and perfused with 4% paraformaldehyde in PBS. Brains were removed, postfixed overnight in 4% PFA, and then cryoprotected in 30% sucrose for at least 72 hours. Tissue was shipped to RH laboratory. A cryostat (Leica, Nussloch, Germany) was used to cut 35 μm coronal sections through the entire dorsoventral axis of the hippocampus. Sections were stored at 4°C to 8°C in PBS with 0.1% NaN3. Free-floating sections were quenched with 0.3% hydrogen peroxide in PBS: methanol for 15 minutes. They were then washed with PBS containing 0.3% Triton X-100, blocked with 10% Normal Donkey Serumin PBS-0.3% Triton X-100 for 2 hours, and incubated overnight at 4°C to 8°C with anti-DCX antibody (goat polyclonal, 1:500; Santa Cruz Biotechnology, Dallas, TX) in PBS-0.3% Triton X-100. After being washed in PBS, tissue was incubated in biotinylated donkey anti-goat secondary antibody (1:500; Jackson ImmunoResearch Laboratories) in PBS for 2 hours, washed in PBS, and incubated with ABC reagent (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) for 1 hour. Tissue was visualized with diaminobenzidine (DAB Substrate kit; Vector Laboratories). An investigator blind to treatment status used a Zeiss (Ober-kochen, Germany) Axioplan-2 upright microscope to count immunoreactive cells bilaterally in the granule cell layer of the dentate gyrus. Ten sections from the entire extent of the dentate gyrus were included in the analysis. Cells were counted bilaterally every sixth section.
2.8.3. The transgenic mice tissue was processed in JAK laboratory
Mice were perfused with ice-cold saline and then 4% PFA. Brains were removed, postfixed in 4% PFA, dehydrated in 30% sucrose in PBS, and rapidly frozen for sectioning on a Leica CM3050S cryostat. Ten-micrometer sections were mounted on slides and were processed for antigen retrieval by incubating in 10 mM sodium citrate pH 7.1 at 95°C for 20 minutes. After cooling for 30 to 45 minutes, sections were washed in PBS, and were incubated in 2N HCl at 37°C for 20 minutes and cooled to room temperature for 10 minutes to expose epitopes in the DNA. After acid treatment, sections were neutralized in 0.1M Borax pH 8.5 for 5 minutes, washed in PBS, blocked in 10% fetal bovine serum for 2 hours, washed again, and exposed to primary antibodies diluted in PBS with 1% bovine serum albumin (BSA) and 0.25% Triton X-100 overnight at 4°C. Primary antibodies included mouse anti-BrdU (1:200; BD) and goat anti-doublecortin C-terminal (1: 500; Santa Cruz Biotechnology). The sections were then washed in PBS and incubated for 2 hours with appropriate fluorescent-labeled secondary antibodies (Alexa-Fluor-405/488/594/647; Molecular Probes) to visualize the primary antibodies. Finally, sections were washed, counterstained with Hoechst 33,342 (Sigma), and mounted with cover slips using ProLong Gold reagent (Invitrogen). Using unbiased stereology, 10 random sections were selected from each animal, and the mean cell count was used as the single data point for statistical analysis.
3. Results
3.1. Pharmacological reduction of AHN reversibly blocks neuropathic tactile and cold allodynia
To determine the impact of AHN levels on development of persistent pain, we pharmacologically, and reversibly, decreased hippocampal neurogenesis rates by ICV infusion of the antimitotic drug Cytosine-β-d-Arabino-Furanoside (AraC) (Fig. 1A, B). AraC is known to decrease Brdu-labeled cells in the hippocampal dentate gyrus SGZ and to subsequently induce learning deficits specific to functions of the dentate gyrus.23 Furthermore, after the termination of AraC infusion, neurogenesis levels are shown to quickly return to normal.15 Therefore, we used this approach to reversibly disrupt AHN and followed neuropathic pain behavior in wild-type mice (WT). Additionally, to ascertain efficacy of the AHN block and also its reversibility, in these same animals, we tracked AHN levels by counting Brdu-labeled proliferating cells and tested for double-labeling with Brdu and DCX to verify that counted cells were developing neurons. We used the SNI model for studying neuropathic pain, as this is a robust model where every animal with SNI exhibits tactile and cold allodynia (and minimal thermal hyperalgesia) for the rest of life.10
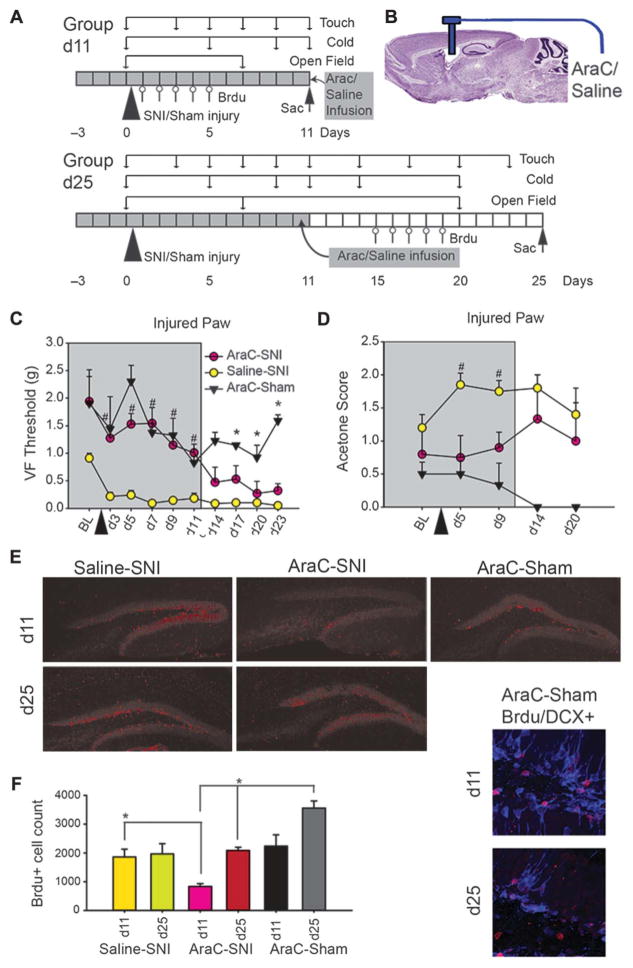
Figure 1.
Reversible decrease in adult hippocampal neurogenesis by intracerebroventricular administration of antimitotic AraC reversibly blocks tactile and cold allodynia in spared-nerve injury (SNI) neuropathic mice. (A) Diagram of experimental timeline. Group d11 and group d25 were tested for touch, cold (on injured and noninjured paws), and open field at indicated times. Group d11 mice were killed at day 11, and group d25 at day 25 after SNI or sham injury (triangle). Bromodeoxyuridine (Brdu) injections were given for 5 days, 1 week before euthanization (arrow). All animals received lateral ventricle infusion of AraC or saline for 14 days, starting 3 days before peripheral injury. (B) AraC or saline was delivered in the lateral ventricle using an osmotic pump. (C, D)Mean ± SEM, touch and cold sensitivity of injured paw in AraC-SNI (n = 10 up to day 11 and n = 4 for days 14–23), Saline-SNI (n = 8 up to day 11 and n = 4 for days 14–23), and AraC-Sham (n=3 up to day 11 and n=2 for days 14–23)WT mice, during brain perfusion (grey, up to day 11) and after cessation of perfusion (days 14–23). (C) Touch sensitivity (Von Frey [VF] 50% threshold in grams) for the injured paw and during AraC infusion (days 3–11, grey) showed a significant effect of group (F2,6=37.72, P<0.001), time (F9,54 = 12.07, P < 0.001), and group-by-time interaction (F18,54 = 4.23, P < 0.001), as determined by 2-way analysis of variance (ANOVA). T-tests determined that the Saline-SNI animals had significantly lower mechanical thresholds than AraC-SNI or AraC-Sham groups at every time point (days 3–11) (#P<0.001), whereas AraC-SNI and AraC-Sham groups did not differ from each other during AraC infusion. Post hoc 2-way ANOVA on AraC groups only indicate a significant decrease in VF threshold (F4,68=3.93, P<0.01). After cessation of AraC infusion (days 14–23), AraC-SNI animals begin exhibiting tactile allodynia and in time nearly match that of Saline-SNI. Two-way ANOVA indicated that there was a significant group (F2,6 = 24.5, P < 0.001), insignificant time (F3,18 = 2.38, P =0.1), and borderline group-by-time interaction (F6,18=2.51, P=0.06). AraC-Sham animals significantly differed from both AraC-SNI and Saline-SNI at every time point (days 14–23) (*P < 0.01). Kruskall–Wallis ANOVA by ranks indicated that baseline (BL) thresholds were not different across groups (P = 0.1). (D) Cold sensitivity (acetone test) for the injured paw and during AraC infusion (grey) was significantly higher in Saline-SNI than AraC-SNI or AraC-Sham group at day 5 (U=0.00, Z=3.80, #P<0.001) and day 9 (U=15.00, Z=2.36, #P=0.02). After cessation of AraC, at days 14 and 20, AraC-SNI and Saline-SNI groups were no longer different in cold sensitivity. (E) Examples of labeling for Brdu-cells in the SGZ for each experimental group at time points day 11 and day 25. These images were white balanced in Photoshop. Right insets are examples of double-labeled DCX-Brdu–positive cells. (F) Mean ± SEM, Brdu-labeled cells in different groups (calculated based on 6 slices per animal), at day 11 (during AraC perfusion) and day 25 (after AraC cessation). On day 11, AraC-Sham (n=2) had significantly more Brdu-labeled cells bilaterally in subgranular zone (mean=2632 cells) than AraC-SNI (n=5) (t=4.82, P=0.04, mean=837), whereas on day 25, AraC-Sham had more Brdu cells (mean = 3555) than AraC-SNI (n = 4) (t = 3.36, P = 0.04, mean = 2084). Moreover, on day 11, Saline-SNI (n = 4) mice had significantly greater Brdu-labeled cells as AraC-SNI (n = 5) (t = 4.55, P = 0.04), which was reversed after AraC cessation as illustrated by increased Brdu cells in AraC-SNI at day 25 (n = 4) as compared to AraC-SNI at day 11 (n = 5) (t = 7.68, P < 0.01) (*P < 0.05).
All saline-infused animals (Saline-SNI), a subgroup of AraC-infused animals (AraC-SNI), underwent SNI surgery 3 days after onset of infusion, and the rest of the AraC animals received sham peripheral surgeries (AraC-Sham). Touch (VF hair thresholds) and cold (acetone test) sensitivities, and also open field behavior, were tracked for up to 23 days after SNI or sham injury. Subgroups were injected with Brdu at different times and killed to quantify AHN disruption (Fig. 1A). AraC or saline was administered ICV through an osmotic pump (Fig. 1B).
During AraC infusion, mechanical thresholds of SNI mice for the injured paw were similar to sham operated mice (days 3–11, Fig. 1C), as there was a significant effect of group, time, and group-by-time interaction. At every time point during infusion of AraC (days 3–11), Saline-SNI animals had significantly lower mechanical thresholds than AraC-SNI or AraC-Sham groups. Moreover, AraC-SNI and AraC-Sham did not differ from each other. Thus, during AraC infusion, the neuropathic injury did not modify these animals’ tactile sensitivity. There was, however, a slight decrease in VF threshold from d3 to d11 for the AraC groups. After cessation of AraC infusion (days 14–23, Fig. 1C), AraC-SNI animals exhibited tactile allodynia that eventually nearly matched that of Saline-SNI, and animals of AraC-SNI and AraC-Sham groups differed significantly from each other. This indicates an emergence of pain behavior dependent on the presence of AraC. There were some small, transient, and borderline differences in healthy paw mechanical thresholds between Saline-SNI and AraC-SNI groups and also between AraC-SNI and AraC-Sham groups, with no evidence for tactile allodynia (Figure S1A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). There were no significant differences between healthy and injured paw baseline thresholds within or across groups, as determined by paired t test.
Sensitivity to cold stimuli for injured paw in SNI mice showed a similar reversible pattern of dependence on AraC availability as observed for mechanical stimuli (Fig. 1D). Saline-SNI animals showed more hypersensitivity than AraC-SNI or AraC-Sham group to cold stimulation during AraC infusion at days 5 and 9 on the injured paw. After cessation of infusion, there was no significant difference between Saline-SNI and AraC-SNI group, demonstrating emergence of cold allodynia. No differences in cold threshold were observed in the healthy paw (Figure S1B, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). Additionally, no differences were seen between groups on either total mobility or time spent in the center zone of the open field test, as measured at baseline, during AraC infusion, and after cessation of infusion (data not shown), implying that AraC infusion has minimal effects on mobility and anxiety-like behavior, replicating earlier results.23
Influence of drug infusion (AraC) and neuropathic injury (SNI) on the rate of AHN was examined both during (day 11) and after cessation of AraC infusion (day 25) by counting Brdu-labeled cell in SGZ, and also verifying that these were developing neurons (Fig. 1E). There was both an impact of drug infusion and neuropathic injury on hippocampal neurogenesis, as measured by Brdu labeling in SGZ (Fig. 1F). The lowest count of Brdu-labeled cells was observed for the AraC-SNI day 11 group, which reverted to levels comparable to those seen in Saline-SNI, day 11 or day 25, groups after cessation of AraC infusion (AraC-SNI day 25 group). Furthermore, AraC decreased the number of Brdu-labeled cells, as predicted by the literature,15,23 and this decrease reversed after cessation of infusion (comparing between AraC-SNI day 11 vs day 25 groups, and between AraC-Sham day 11 vs day 25 groups).
These results demonstrate minimal persistent pain behavior during an acute transient decrease of AHN and resumption of tactile and cold allodynia for the injured paw of SNI mice when neurogenesis levels returned to levels observed in neuropathic mice that did not receive AraC infusions.
3.2. Ablation of AHN by x-ray delays and diminishes expression of neuropathic tactile allodynia
Manipulations of AHN by various methods have resulted in distinct outcomes when assessing its role in memory functions and in negative moods.16 Therefore, we tested an alternative method for diminishing AHN, x-irradiation of SGZ that results in long-term ablation of AHN,41 on neuropathic pain behavior.
To test the effects of x-ray ablation of AHN on SNI pain behavior, sham or x-ray radiation was directed to the SGZ (performed in the laboratory of R.H. in New York City) in WT mice. After 12 days of recovery, these animals were shipped to Chicago, quarantined, and all received an SNI injury. Tactile and cold sensitivities were monitored at baseline and up to 42 days after injury (Fig. 2A, B). X-ray radiation delayed the onset of tactile allodynia for the SN-injured paw by approximately 3 days and diminished magnitude of tactile allodynia for 30 days, after which tactile sensitivity matched to sham-radiated mice (Fig. 2C). There was no group difference in cold sensitivity for the injured paw (Fig. 2D), and tactile and cold sensitivity for the uninjured paw matched that of sham-irradiated mice and did not differ from baseline (Figure S2A, B, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). These animals were also tested for anxiety-like behavior using the black box emergence task, which has previously shown to differentiate between SNI and Sham animals.37 The task did not differentiate between the groups but did show evidence for task repetition-based learning (Figure S2C, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146), suggesting that x-ray radiation decreased SNI-associated anxiety complementing the diminished tactile allodynia observed in the same animals.
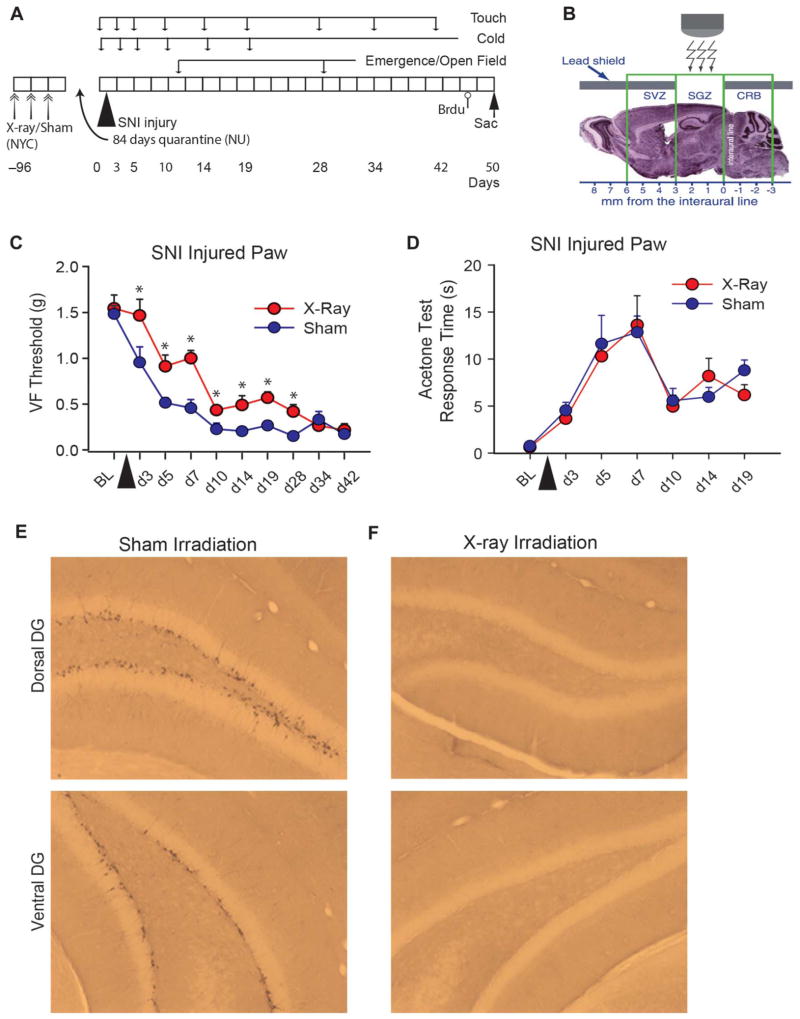
Figure 2.
Long-term ablation of adult hippocampal neurogenesis by x-irradiation delays onset of tactile, but not cold, allodynia in spared-nerve injury (SNI) neuropathic mice. (A) Diagram of experimental timeline. Wild-typemice received either x-ray (n=10) or sham (n=10) brain irradiation over 1 week (double arrows; performed at Columbia U., New York city), 96 days before SNI injury. After 12 days of recovery period, all mice were shipped to Chicago (Northwestern U.), quarantined for 84 days, then underwent unilateral SNI injury, and tested for touch, cold (on injured and noninjured paws), open field, and black box emergence at indicated times. All mice were killed 52 days after peripheral injury. Bromodeoxyuridine (Brdu, open circle) injection was given 2 hours before killing (arrow). (B) X-irradiation was directed towards the subgranular zone (SGZ) portion of the hippocampus. (C)Mean ± SEM, touch sensitivity of the SNI-injured paw in Sham and x-ray–radiated mice, monitored for 42 days. There was a significant effect of group (F1,18=19.71, P<0.0004), time (F9,162=50.46, P<0.00001), and group-by time interaction (F9,162=2.58, P<0.009). At every time point, except at baseline (BL) and days 34 and 42, x-ray mice had higher tactile thresholds than sham mice (*P < 0.05). (D) Mean ± SEM, cold sensitivity (acetone test response duration in seconds) of the SNI-injured paw in Sham and x-ray–radiated mice, monitored for 19 days. There was only a significant effect of time (F6,108 = 10.32, P < 0.00001) but no group or group-by-time interaction effects. (E, F) Doublecortin labeling. Representative slices in Sham-irradiated and SNI-injured mice (E), and x-ray–irradiated and SNI-injured animals (F), in the dorsal (top) and ventral (bottom) dentate gyrus stained for doublecortin. There was almost complete absence of doublecortin-positive cells in the x-ray–irradiated SNI–injured animals. Doublecortin labeling was counted in 2 Sham and 2 x-ray–irradiated mice (10 left and 10 right hemisphere slices per animal). Total doublecortin-labeled cells (left + right) were 1787, 2860 in Sham, and 2, 2 in x-ray–irradiated SNI mice. VF, Von Frey.
Previously published work, using the same x-ray irradiation protocol,34,41 indicates a permanent and near-complete ablation of AHN. To confirm these observations, we examined AHN in 2 x-ray and 2 sham-irradiated SNI animals. Histological examination and counts of doublecortin-labeled cells of hippocampus tissue from these mice show almost complete ablation of AHN (>99%), bilaterally, in dorsal and ventral granular layers for animals with x-ray irradiation, in comparison to Sham radiation (Fig. 2E, F).
3.3. Effects of manipulating AHN in transgenic mice on neuropathic and inflammatory pain
For the next set of experiments, we used 2 well-characterized animal models5,18: one previously shown to have remarkable gains in AHN and cognitive learning behavior (NSE-Noggin, hereon designated as Noggin animals) and the other shown to have diminished AHN and impaired learning behaviors (NSE-BMP4, hereon BMP animals).23 We hypothesized that those with down-regulated neurogenesis would have decreased persistent pain behavior and those with enhanced neurogenesis will exhibit enhanced persistent pain behavior. To generalize the involvement of AHN in postinjury pain, we studied pain behavior in 2 models, neuropathic (SNI) and inflammatory (carrageenan), that underlie relatively distinct afferent nociceptors and spinal cord circuitry.27,50
3.4. Bone morphogenetic protein transgenic mice have decreased AHN and do not exhibit neuropathic tactile allodynia
To determine the impact of genetic manipulation of AHN on neuropathic pain, we examined the influence of BMP4 over-expression (BMP mice) on SNI pain behavior. The SNI pain behavior in WT rodents persists for the life of the animal,10 and resultant tactile allodynia reaches a threshold level close to zero as observed above (Fig. 1C, saline-SNI, and Fig. 2C, sham); thus there is no opportunity to study further enhancement in SNI allodynia or the recovery from SNI allodynia, and therefore Noggin animals were not included in these experiments.
Bone morphogenetic protein and WT littermates underwent either SNI or sham surgery. Mechanical sensitivity was measured at presurgery baseline and for 14 days after surgery (cold sensitivity was not measured in these animals). We observed that BMP SNI animals did not develop tactile allodynia throughout the period of monitoring (Fig. 3A). There were no group differences seen in the healthy paw (Figure S3, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). This result replicates the effects of AraC on SNI pain behavior and is consistent with the effects of x-ray on SNI pain behavior. Thus, all 3 methods of decreasing AHN result in diminished or complete blockade of SNI pain behavior.
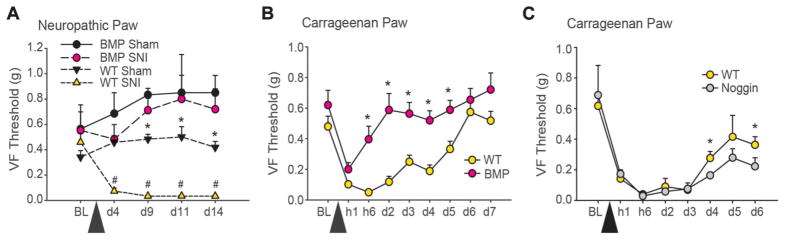
Figure 3.
Neuropathic and inflammatory persistent pain behaviors are diminished in transgenic mice with decreased adult hippocampal neurogenesis (AHN) (Bone morphogenetic protein [BMP]) (A, B), whereas recovery from inflammatory pain is delayed in transgenic mice with increased AHN (Noggin) (C). (A)Mean ± SEM, touch sensitivity of the spared-nerve injury (SNI) paw in BMP and wild-type (WT) littermates who underwent either SNI or sham surgery (BMP SNI n=8, BMP Shamn=6, WT SNI n=8, WT Shamn=7). Tactile sensitivity of the neuropathic paw was monitored at presurgery baseline (BL) and for subsequent 14 days. BMP-SNI animals did not develop tactile allodynia throughout the period of monitoring. After injury, WT-SNI animals had significantly lower mechanical thresholds from all other groups (group, F3,25 = 29.17, P < 0.001; time, F4,100 = 2.43, P = 0.05; group-by-time, F12,100 = 3.00, P < 0.01) (WT-SNI vs all others post hoc, #P < 0.05). BMP-Sham and WT-Sham animals differed significantly from each other on days 9 through 14 (post hoc, *P<0.05). (B)Mean ± SEM, touch sensitivity of the carrageenan-injected paw in BMP (n = 13) and WT (n = 12) littermates. Tactile sensitivity of the inflamed paw was monitored at preinjury BL and for subsequent 7 days. BMP animals showed significantly less mechanical allodynia in the carrageenan-injected paw compared with WT animals (group effect, F1,23=20.20 and P<0.001), and this was observed over time (time effect, F8,176 = 7.85 and P < 0.001, and group-by-time interaction, F8,176 = 6.66 and P < 0.01) with post hoc differences (*P < 0.05) seen at hour 6 through day 5. (C) Mean ± SEM, touch sensitivity of the carrageenan-injected paw in Noggin (n=5) and WT (n=5) littermates. No significant group differences were observed. However, post hoc comparison shows a small difference at days 4 and 6 (*P < 0.05). VF, Von Frey.
3.5. Bone morphogenetic protein transgenic mice exhibit decreased inflammatory pain
Inflammatory tissue injuries give rise to peripheral and spinal cord reorganization specific to such injuries.27,50 Such injuries are commonly associated with increased sensitivity to tactile, heat, and cold stimuli, and importantly the related behaviors and neural reorganization are reversed within a few days after the inflammation subsides.26 Thus inflammatory injury models provide the opportunity to examine the role of AHN on both emergence of pain and on recovery from pain, which we tested when AHN was decreased (BMP mice) or increased (Noggin mice).
We investigated the impact of decreased AHN, in BMP mice, on carrageenan inflammatory pain. Pain behavior was assessed before injury, animals were then injected with carrageenan in 1 paw and saline in the other, and pain behavior followed over the next week. Bone morphogenetic protein animals showed less mechanical allodynia in the carrageenan-injected paw compared with WT animals, starting from 6 hours after injury through day 5 (Fig. 3B), demonstrating that the injury-related tactile allodynia recovered to baseline levels within 6 hours in BMP as compared with WT where the allodynia persisted for 5 days. Similarly, BMP animals exhibited reduced thermal and cold hyperalgesia in the carrageenan-injected paw (Figure S4, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). Importantly, there was little difference in mechanical, heat, and cold responsiveness in the saline-injected paw between the BMP and WT mice (Figure S5, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146), indicating that acute nociception was unaffected in BMP mice.
3.6. Noggin transgenic mice have increased AHN and exhibit delayed recovery from inflammatory tactile allodynia
We investigated the impact of increased AHN, in Noggin mice, on carrageenan inflammatory pain. Pain behavior was assessed before injury, animals were then injected with carrageenan in 1 paw and saline in the other, and pain behavior followed over the next week.
Both Noggin and WT animals showed a similar reduction in VF threshold after injection of carrageenan for the injured paw. However, on days 4 to 6, Noggin animals exhibited more tactile sensitivity than WT animals (Fig. 3C). No significant differences in heat or cold sensitivity were observed in Noggin animals for the injured paw (Figure S6, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). No significant differences were seen between Noggin and WT animals in the saline-injected paw for touch, heat, and cold stimuli (Figure S6A, B, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146), although Noggin animals had significantly longer reaction times to cold at day 5 compared with WT (Figure S6C–E, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A146). Thus, in Noggin animals, increased AHN does not modify development of inflammatory tactile allodynia; instead, it delays recovery from tactile allodynia.
Taken altogether, above results demonstrate that reduced AHN, using 3 different techniques, decreases persistent pain behavior across modalities and conditions. Reciprocally and consistently, when AHN was enhanced recovery from postinflammation, tactile allodynia was prolonged. As observed, effects were specific to persistent and not to acute pain behavior, and we can conclude that AHN is involved in the persistent aspects of postinjury pain in both inflammatory and neuropathic pain conditions.
3.7. Bone morphogenetic protein and Noggin transgenic mice exhibit distinct mood states
The 2 primary consequences most consistently associated with manipulating AHN are changes in learning and memory abilities and changes in depression-like behavior.12,16,35,40,41 Even though above results implicate AHN in postinjury persistent pain, they do not identify which of these 2 processes is the underlying cause. Recent evidence shows that BMP and Noggin mice exhibit profound differences in hippocampus-dependent learning abilities, with BMP showing learning deficits and Noggin mice showing enhanced learning abilities.23 These findings together with above results suggest that persistent pain may be closely related to AHN-dependent learning and memory processes. Alternatively, as persistent pain is a stressor commonly comorbid with depression, influence of AHN on persistent pain may be indirectly through pain first giving rise to depression and the latter in turn influencing rate of AHN. To differentiate between the alternative hypotheses, we examined depression-like behavior in BMP and Noggin mice.
BMP, Noggin, and WT littermates (with no peripheral injuries) were examined on the forced swim test and tail suspension test, to assess depression-like behavior with 2 different methods. On both tests, BMP animals were immobile for longer durations than WT, whereas Noggin animals were less immobile than WT (Fig. 4A, B), indicating that BMP animals have depression-like behavior and Noggin animals do not. The results in BMP mice are most noteworthy because in these mice, we observe a profound decrease in postinjury neuropathic and inflammatory pain behaviors, yet these animals also exhibit depression-like behavior, learning deficits, and decreased AHN. Thus, these data imply that the role of AHN in postinjury pain is not dependent on the depressive effects of pain and more likely involves AHN-dependent memory formation processes.
Figure 4.
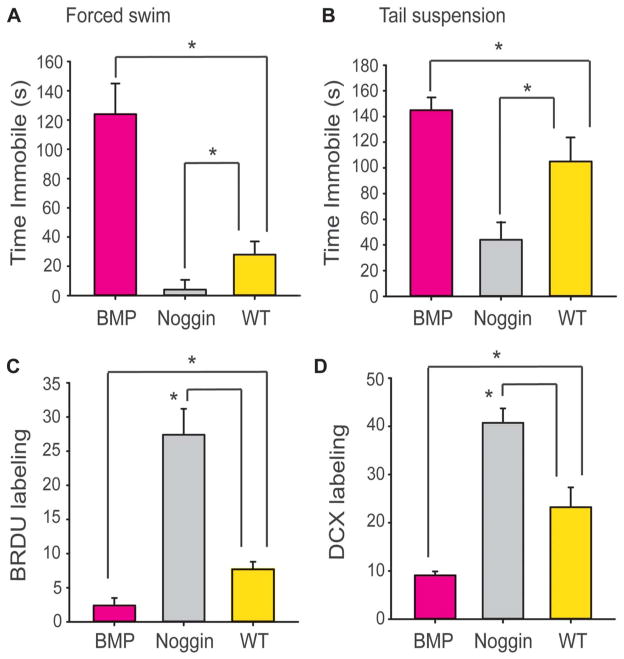
Bone morphogenetic protein (BMP) exhibit heightened depression-like behavior, whereas Noggin shows reduced depression-like behavior, relative to wild-type (WT) littermates. (A) Mean ± SEM, depression-like behavior assessed by time spent immobile during the forced swim test. BMP mice (n = 15) spent significantly more time immobile than WT (n = 15) (t-value28 = 2.73, P < 0.005). In contrast, Noggin mice (n = 15) spent significantly less time immobile than WT (t-value28 = −2.11, P < 0.02). (B) Mean ± SEM, depression-like behavior assessed by the tail suspension test (n = 15 per group, same mice as in A). Neuron-specific enolase (NSE)–BMP animals showed increased tail suspension immobility (t-value28 = 2.10, P = 0.04), and NSE-Noggin animals show reduced tail suspension immobility (t-value28=−2.12, P < 0.02), as compared with WT controls. *P < 0.05. (C) Mean ± SEM, bromodeoxyuridine (Brdu) labeling (n = 5 per group). NSE-BMP animals showed reduced Brdu labeling, and NSE-Noggin animals showed increased Brdu labeling, as compared with WT controls (group F2,12 = 142.1, P < 0.0001). *P < 0.05. (D) Mean ± SEM, DCX labeling (n = 5 per group). NSE-BMP animals showed reduced DCX labeling, and NSE-Noggin animals showed increase DCX labeling, as compared with WT controls (group F2,12 = 154.4, P < 0.0001). *P < 0.05. BMP, bone morphogenetic protein.
Previous work indicates that BMP animals exhibit reduced AHN, whereas Noggin animals exhibit increased AHN.23 To replicate these findings, a subgroup of the BMP, Noggin, and WT littermates (with no peripheral injuries) (n = 5 per group) were used to examine AHN. Histological examination and counts of doublecortin and Brdu-labeled cells (Fig. 4A, D) confirmed that BMP mice show reduced AHN, whereas Noggin mice show increased AHN.
4. Discussion
Collectively, our results show, for the first time, that AHN is involved in the emergence and also to some extent maintenance of postinjury persistent pain. Three different methods were used to manipulate AHN, and in all cases, decreased AHN led to either complete blockade or delayed and decreased postinjury pain behavior of varying amounts, for tactile allodynia, and heat and cold sensitivity, for neuropathic and inflammatory injuries. Moreover, transgenic BMP mice with decreased AHN exhibited heightened depression but dramatically decreased postinjury pain, dissociating the 2 and implying that AHN-dependent memory formation processes are engaged in the emergence of persistent pain. Thus, generally, these results imply that persistent pain is contingent on the interaction between nociceptive signals and hippocampal neurogenesis-dependent learning mechanisms.
Although it is well known that modulating supraspinal neural activity can influence pain behavior, here we show that neurogenesis in a supraspinal structure can have similar effects. All 3 methods used to decrease AHN resulted in diminished persistent pain behavior. Yet, the relationship between extent of AHN disruption and postinjury pain behavior remains unclear. Decreasing AHN by approximately 50% by AraC resulted in reversible full blockade of neuropathic injury behavior. In contrast, x-ray decreased AHN by >85% for the long term but only delayed neuropathic tactile allodynia by 3 days, did not alter cold allodynia, and within 30 days tactile allodynia matched the level observed in sham-radiated SNI mice. In transgenic BMP mice, where the magnitude of decreased AHN matches the levels we see with x-ray treatment,23 we again observe a blockade of neuropathic tactile allodynia and diminished tactile, heat, and cold sensitivities for an inflammatory injury. The most parsimonious explanation for the observed results is that they are a consequence of disruption of adult born granule cells within the dentate gyrus. Still, each of the methods used to manipulate AHN has specific confounds and side effects that may influence our outcomes in unique ways, and future experiments would benefit from confirming the specificity of each treatment. Previous evidence shows that long-term ablation of AHN by radiation or genetically is accompanied with homeostatic pathological changes in the dentate gyrus network that functions to restore its plasticity.44 Moreover, the transgenic models we used are not hippocampus-specific and are known to have peripheral side effects over time,28 yet the lack of changes in baseline or acute pain thresholds rule out peripheral abnormalities and instead suggest a primarily hippocampal-dependent impact of BMP and noggin levels on postinjury pain. Given these caveats, the AraC results are most remarkable as the procedure is an acute and reversible manipulation. Specificity of ICV infusion of AraC on AHN has been previously demonstrated,23 and ICV infusion mitigates systemic side effects and allows for much lower overall dosing for manipulating hippocampal neurogenesis. We saw no differences in motor performance or anxiety (open field) that may influence pain responses; baseline and healthy paw thresholds did not differ between groups in any consistent way, and we thus conclude that observed changes in pain behavior in the AraC experiment, for the most part, do not involve spinal cord or peripheral influences (although nonspecific confounds cannot be fully ruled out). Moreover, within about a week after AraC infusion cessation, pain behavior was back to levels matching SNI animals with ICV saline, suggesting that young and more excitable dividing granule cells are engaged in the postinjury pain behavior.21 Importantly, given that after 2 weeks from the initial peripheral injury, pain sensitivity recovered to levels similarly seen in the ICV saline SNI, implies continuous presence of a nociceptive signal impinging on the hippocampus, which induces re-emergent pain behavior within days of unblocking neurogenesis. It is also remarkable that a nociceptive response believed to be primarily a reflection of spinal reflex circuitry,49 tactile allodynia, shows dependence on hippocampal neurogenesis, implying that hippocampal circuitry modulates descending pathways, and there is good evidence that blocking descending modulation per se can abolish neuropathic pain behavior.48
The hippocampus is emerging as a key area of brain engaged in chronic pain in humans and in rodent models of persistent pain. Along with the effects of neurogenesis seen here, previous studies show altered hippocampal synaptic plasticity,31,37 cytokine expression,1,11,39 and Erk phosphorylation37 in rodent models of chronic pain and decreased volume37,51 and altered functional connectivity in the hippocampus of patients with chronic pain.32,36 The human hippocampus also undergoes AHN at a rate comparable to that reported in the rodent.16,45 Yet, to our knowledge, there are no systematic studies of the impact of damaging the dentate gyrus, or any other portions of the hippocampus, on human pain perception. In 1 subject, patient H.M. (most intensively studied patient in neuroscience), who underwent bilateral resection of the amygdala, hippocampus, and parahippocampal gyrus for intractable epilepsy and have severe retrograde amnesia for the rest of his life, was tested for thermal pain sensitivity 30 years after the brain surgery. H.M. did not feel acute thermal pain applied over diverse body parts.25 Additionally, there are 2 case reports of 3 patients with diverse chronic pain conditions that after an episode of significant memory loss experience dramatic decrease in their pain and in opiate use.7,9 Given our results, the simplest explanation for these cases is that the memory loss is primarily due to hippocampal damage, which leads to disrupting persistent pain-related memory circuitry and diminished pain decrease even after substantial recovery from the amnesia. More systematic studies along these lines are urgently needed.
Our results indicate that neurogenesis-dependent hippocampal-associative learning mechanisms are involved in postinjury persistent pain, which distinguishes corresponding clinical chronic pain conditions from acute pain (a primary reinforcer independent of learning circuitry) and implies that the former are secondary re-enforcers. We emphasize that this study suggests a direct link between AHN and emergence of persistent pain behavior but certainly does not establish a causal relationship between these processes. In fact, to date, there is no convincing evidence for a causal link between AHN and any behavior, and especially in the pathophysiology of depression (the condition most intensely studied relative to AHN).35 Future efforts that may reveal underlying cellular and molecular mechanisms of hippocampal learning mechanisms related to persistent pain should point to novel therapeutic avenues for prevention and amelioration of chronic pain.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grants NINDS NS057704 (A.V.A.), NIDCR DE022746 (A.V.A.), and NINDS NS20778 (J.A.K.). L. Kan was supported by a grant from the University of Pennsylvania and Center for Research in FOP and related disorders. R. J. Miller provided the resources for quantification of hippocampal neurogenesis. X-irradiation and immunohistochemistry were performed in RH laboratory. The authors thank members of Apkarian laboratory for reading earlier versions of this manuscript.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A146.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
Conflict of interest statement
The authors declare no competing financial interests.
References
- 1.Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol. 2011;228:30–40. doi: 10.1016/j.expneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–8. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. PAIN. 2011;152:s49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–19. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72:1068–84. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Choi DS, Choi DY, Whittington RA, Nedeljkovic SS. Sudden amnesia resulting in pain relief: the relationship between memory and pain. PAIN. 2007;132:206–10. doi: 10.1016/j.pain.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. PAIN. 1994;59:369–76. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 9.Chon JY, Hahn YJ, Sung CH, Moon HS. Amnesia and pain relief after cardiopulmonary resuscitation in a cancer pain patient: a case report. J Korean Med Sci. 2012;27:707–10. doi: 10.3346/jkms.2012.27.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 11.del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. PAIN. 2011;152:2827–35. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104:1223–9. doi: 10.1213/01.ane.0000263280.49786.f5. tables of contents. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov EL, Tsuda MC, Cameron HA, Usdin TB. Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J Neurosci. 2014;34:12304–12. doi: 10.1523/JNEUROSCI.0312-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013;20:710–29. doi: 10.1101/lm.026542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat Rev Neurosci. 2015;16:189–200. doi: 10.1038/nrn3855. [DOI] [PubMed] [Google Scholar]
- 18.Fan X, Xu H, Huang Y, Cai W. A combined in situ hybridization and RT-PCR method to detect spatial and temporal patterns of Noggin gene expression in embryonic and postnatal rat hippocampus. Brain Res Brain Res Protoc. 2004;13:99–105. doi: 10.1016/j.brainresprot.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Fan XT, Cai WQ, Yang Z, Xu HW, Zhang JH. Effect of antisense oligonucleotide of noggin on spatial learning and memory of rats. Acta Pharmacologica Sin. 2003;24:394–7. [PubMed] [Google Scholar]
- 20.Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. PAIN. 2002;95:111–18. doi: 10.1016/s0304-3959(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 21.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–65. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerrits MM, van Marwijk HW, van Oppen P, van der Horst H, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosomatic Res. 2015;78:64–70. doi: 10.1016/j.jpsychores.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4:e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 25.Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H. M Behav Neurosci. 1985;99:1031–9. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- 26.Honore P, Catheline G, Le Guen S, Besson JM. Chronic treatment with systemic morphine induced tolerance to the systemic and peripheral antinociceptive effects of morphine on both carrageenin induced mechanical hyperalgesia and spinal c-Fos expression in awake rats. PAIN. 1997;71:99–108. doi: 10.1016/s0304-3959(97)03345-9. [DOI] [PubMed] [Google Scholar]
- 27.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 28.Kan LX, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165:1107–15. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SH, Kim SK, Nam EJ, Han SW, Lee SJ. Spatial versus verbal memory impairments in patients with fibromyalgia. Rheumatol Int. 2012;32:1135–42. doi: 10.1007/s00296-010-1762-1. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–19. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 31.Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol. 2007;574:127–32. doi: 10.1016/j.ejphar.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 32.Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2013;218:903–12. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Brain white matter structural properties predict transition to chronic pain. PAIN. 2013;154:2160–8. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 35.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–8. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutso A, Petre B, Huang L, Baliki M, Torbey S, Herrmann KM, Schnitzer T, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111:1065–76. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–56. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oosterman JM, Derksen LC, van Wijck AJM, Veldhuijzen DS, Kessels RPC. Memory functions in chronic pain examining contributions of attention and age to test performance. Clin J Pain. 2011;27:70–5. doi: 10.1097/AJP.0b013e3181f15cf5. [DOI] [PubMed] [Google Scholar]
- 39.Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology. 2011;36:979–92. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 42.Schultz KM, Banisadr G, Lastra RO, McGuire T, Kessler JA, Miller RJ, McGarry TJ. Geminin-deficient neural stem cells exhibit normal cell division and normal neurogenesis. PLoS One. 2011;6:e17736. doi: 10.1371/journal.pone.0017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc Natl Acad Sci U S A. 2011;108:5437–42. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 47.Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–27. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 48.Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–20. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Vierck CJ, Jr, Kline R, Wiley RG. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats1. Behav Neurosci. 2004;118:627–35. doi: 10.1037/0735-7044.118.3.627. [DOI] [PubMed] [Google Scholar]
- 50.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73:1567–70. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.