Abstract
Background
Currently available plasma cell markers include CD138 and CD38. However, CD38 is not specific to plasma cells. It is also expressed by many epithelial cells and other hematopoietic cells. In addition, rare CD138 negative plasma cell neoplasms may be exceedingly difficult to diagnose. MIST1 is a transcription factor expressed by mouse and human neoplastic and non-neoplastic plasma cells. Our goals were to compare MIST1 expression to CD38/CD138 in neoplasms with plasmacytic differentiation, assess reactivity in normal samples and non-plasmacytic cell lineages, and to determine if MIST1 is expressed in CD138 negative plasma cell neoplasms
Design
85 neoplasms with plasma cell differentiation (marginal zone lymphoma, lymphoplasmacytic lymphoma, plasmablastic lymphoma, plasma cell neoplasm) and 2 non-neoplastic cases (normal marrow) were tested with MIST1 immunohistochemistry. CD138/38 expression for each case was compared to MIST1 reactivity.
Results
Plasma cells were MIST1 positive in all cases interrogated. CD38 and/or CD138 expression was reviewed in all cases and found to be concordant in 46/47 (97.8%) of tested cases, with the exception of one case of plasmablastic lymphoma which showed MIST1 positivity and no CD138 expression. All other cell lineages were negative, with the exception of MZL and LPL, in which MIST1 highlighted a subset of the lymphocytes, with plasmacytic differentiation.
Conclusion
MIST1 is a sensitive and specific marker of plasmacytic differentiation. CD138+ plasma cells expressed MIST1 in all tested cases; however one plasmablastic lymphoma showed MIST1 positivity and no CD138 expression, suggesting MIST1 may be useful in certain CD138-negative cases. In MZL and LPL, MIST1 highlights a subset of the lymphocytes in lymphomas with plasmacytic differentiation.
Introduction
MIST1 is a basic helix-loop-helix transcription factor initially described in 1997 by Lemercier et al(1). They describe its expression in tissues with high secretory function including stomach, liver, lung, salivary gland, pancreas, and spleen(1). MIST1 binds E-boxes within regulatory regions of genomic DNA and regulates several target genes involved in secretory vesicle trafficking and maintenance in addition to apical cytoskeletal rearrangements(2–4).
In vitro studies show expression of MIST1 mRNA to be enriched in plasma cells in response to forced expression of the transcription factor XBP1, in addition Mist1 is expressed at the transcript level in mouse plasma cells (2, 5–8). Capoccia et al showed preservation of plasma cell function in MIST1 knockout mice, suggesting that MIST1 in plasma cells may be functionally redundant; however it can be utilized as a marker of plasmacytic cell differentiation(6). This study showed that MIST1 was reliably expressed by normal plasma cells and by cells with plasmacytic differentiation in a small cohort of 12 plasma cell neoplasms. Preliminary characterization of MIST1 in plasma cells also showed reliable expression of MIST1 in plasma cells in comparison with other B-cells(6).
The goal of this study is to characterize MIST1 expression via immunohistochemistry in various lymphoid neoplasms including those with plasmacytic differentiation. We hypothesize that MIST1 will be a reliable and useful marker of plasmacytic differentiation.
Materials and methods
Immunohistochemistry optimization
The anti-MIST1 antibody used for immunohistochemistry in this study is a rabbit anti-human MIST1 [1:200, provided by Dr. Jason Mills] described previously(3, 9). Antigen retrieval was performed with Ventana Benchmark XT (Ventana medical Systems, INC, Tuscon, AZ) CC1 protocol in 1mM EDTA at pH 8.0 at a primary antibody concentration of 1:400. The antibody was incubated with the tissue for 32 minutes at 37degrees Celsius. The counterstains used were hematoxylin II and blueing reagent.
Patient samples
A search of the archives of the Lauren V Ackerman Laboratory of surgical pathology Barnes-Jewish Hospital (Washington University in St. Louis, MO,USA) was performed for cases from 2005 to 2010. Additional cases of rare entities such as plasmablastic lymphoma involved extending the search to include cases from 2001 to 2005. The cases were reviewed by two hematopathologists (CY, JLF) and those with ample diagnostic tissue in the parrafin blocks were selected to include in the study. Classification of lymphomas was performed utilizing the new 2008 WHO classification on all plasmablastic lymphoma and plasma cell neoplasms (WHO 2008).
CD138 or CD38 expression
All cases of PCN, MZL, LPL, PB were reviewed for expression of CD38 via flow cytometry (FC500, Beckman Coulter) or for expression of CD138 via immunohistochemistry.
Monoclonal CD38 and CD138 antibodies (surface CD38 FITC PNIM0775U and CD38 PC-5 PNIM2651U, Beckman Coulter) were used in the flow cytometry assays. Prediluted commercially available mouse monoclonal CD138 (B-A38, Ventana medical Systems, INC, Tuscon, AZ) was used for immunohistochemistry.
Flow cytometry was performed on the bone marrow aspirates, fresh surgical tissue (including lymph node, spleen, and other soft tissue sites) or peripheral blood. Monoclonal antibodies targeting CD38 and CD138 were used and the samples were interrogated using a Beckman-Coulter FC500 flow cytometer (Beckman Coulter, Brea, CA).
Immunohistochemistry for MIST1 was performed on 86 archived paraffin embedded patient samples representing various tissue types and lymphoid malignancy or non-neoplastic controls. Included in these are cases of plasmablastic lymphoma (10), plasma cell neoplasm (21), follicular lymphoma (8), mantle cell lymphoma (8), marginal zone lymphoma (10), chronic lymphocytic lymphoma/small lymphocytic lymphoma (9), lymphoplasmacytic lymphoma (7), spleen with extramedullary hematopoiesis (1), normocellular marrow without evidence of a hematopoietic malignancy (9), a CD138 negative plasmablastic lymphoma (1), and a posttransplant lymphoproliferative disorder (PTLD) with plasmablastic differentiation (1).
Results
Patient samples
86 samples were interrogated with MIST1 immunohistochemistry. Patient demographics and diagnoses are listed in table 1.
Table 1.
Case demographics by diagnosis subtype
| Diagnosis | # cases | M:F | Patient Age range | Avg Age of Paraffin Blocks (yrs) |
|---|---|---|---|---|
| PCN | 21 | 11:09 | 43–86 | 3.05 |
| PBL | 10 | 7:04 | 40–63 | 6.7 |
| MZL | 10 | 4:05 | 48–87 | 2.66 |
| LPL | 7 | 5:03 | 51–79 | 1.5 |
| FL | 8 | 5:03 | 45–82 | 2 |
| CLL/SLL | 9 | 2:07 | 34–78 | 2 |
| MCL | 8 | 5:03 | 53–82 | 2 |
| Normal | 10 | 7:03 | 42–87 | 2 |
| DLBCL | 1 | 1:00 | 63 | 7 |
| PTLD | 1 | 0:01 | 4 | 1 |
| CD138 neg PB | 1 | 1:00 | 40 | 1 |
plasma cell neoplasm (PCN), plasmablastic lymphoma (PB), marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), follicular lymphoma (FL), chronic lymphocytic lymphoma/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), Diffuse large B cell lymphoma (DLBCL), a CD138 negative plasmablastic lymphoma (CD138 neg PB), and a post-transplant lymphoproliferative disorder with plasmablastic differentiation (PTLD).
Figure 1 shows a composite digital image for the various hematopoietic malignancies reacting with MIST1. In normal controls, CLL/SLL, FL, and MCL only scattered small plasma cells demonstrate dark nuclear reactivity with MIST1 (Q, S, T, U). In plasma cell neoplasms, there is uniform dark nuclear reactivity among plasma cells (K). In plasmablastic lymphoma, there appears to be dimmer but still prominent nuclear reactivity with MIST1 (N). In marginal zone lymphoma and lymphoplasmacytic lymphoma, a subset of the small lymphocytes is noted to show nuclear reactivity with MIST1 in addition to dark nuclear reactivity among the plasma cells (E, H). One advantage to using MIST1 immunohistochemistry in marginal zone lymphomas is that it highlights lymphoepithelial lesions, whereas CD138 will strongly react with both the lymphocytes and the mucosal lining epithelium making the identification of lymphoepithelial lesions difficult (I vs H). MIST 1 is negative in the majority of lymphocytes, adipocytes, endothelial cells and granulocytes.
Figure 1.
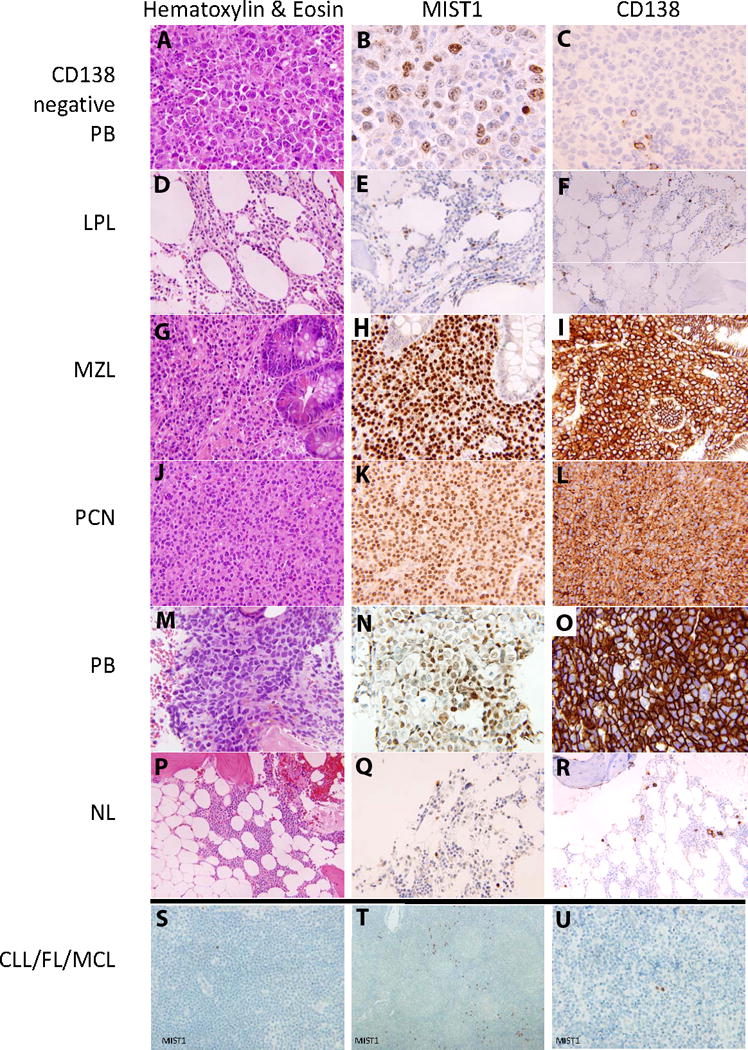
Composite pictures of H&E and immunohistochemistry for MIST1 and CD138.
A. H&E of CD138 negative plasmablastic lymphoma (400× magnification).
B. MIST1 of CD138 negative plasmablastic lymphoma (400× magnification).
C. CD138 of CD138 negative plasmablastic lymphoma (400× magnification).
D. H&E of Lymphoplasmacytic lymphoma (400× magnification).
E. MIST1 of Lymphoplasmacytic lymphoma (400× magnification).
F. CD138 of Lymphoplasmacytic lymphoma (200× magnification).
G. H&E of Marginal Zone Lymphoma (400× magnification).
H. MIST1 of Marginal Zone Lymphoma (400× magnification).
I. CD138 of Marginal Zone Lymphoma (400× magnification).
J. H&E of Plasma cell neoplasm (400× magnification).
K. MIST1 of Plasma cell neoplasm (400× magnification).
L. CD138 of Plasma cell neoplasm (400× magnification).
M. H&E of Plasmablastic lymphoma (400× magnification).
N. MIST1 of Plasmablastic lymphoma (400× magnification).
O. CD138 of Plasmablastic lymphoma (400× magnification).
P. H&E of normal bone marrow (400× magnification).
Q. MIST1 of normal bone marrow (400× magnification).
R. CD138 of normal bone marrow (400× magnification).
S. MIST1 of Chronic Lymphocytic Leukemia/Small Lymphocytic lymphoma (400× magnification).
T. MIST1 of Follicular lymphoma (200× magnification).
U. MIST1 of Mantle cell lymphoma (400× magnification).
Discussion
This is the first study to systematically apply MIST1 immunohistochemistry to a set of B- non-Hodgkin lymphomas and plasma cell neoplasms to investigate the utility of MIST1 as a marker of plasmacytic differentiation. In order to include significant numbers of relatively infrequent malignances such as plasmablastic lymphomas, we included cases which are over 10 years old. Notably, the MIST1 antibody appears to react robustly, with continued preservation even in 10 year-old paraffin blocks.
Currently, only two markers of plasmacytic differentiation (CD38, CD138) are widely used clinically. CD38 is expressed by a various hematopoietic cells including bone marrow hematopoietic precursors, activated T-and B-cells, plasma cells, and some non-Hodgkin lymphoma most notably CLL/SLL(10). In addition, clinical use of CD38 is primarily in flow cytometry and in frozen tissue. CD138 is more specific marker of plasmacytic differentiation(11), however this antigen has also been shown to be expressed by other cells such as acute myeloid leukemia, lymphoblastic leukemia, benign myeloid cells, benign and malignant plasma cells, and metastatic carcinoma(12–13).
There are rare cases of CD38 and CD138 negative plasma cell neoplasms (11,14) in which a definitive diagnosis may be difficult, and which may be misdiagnosed as lymphomas. MIST1 may be of use in these rare cases in highlighting the malignant cells and indicating their plasma cell origin.
Our data indicate that CD138+ plasma cells coexpressed MIST1 in 100% of cases. In addition, in a rare case of CD138 negative plasmablastic lymphoma, MIST1 expression was observed. This indicates that MIST1 may be a more sensitive marker of plasmacytic differentiation as compared to CD138. An interesting observation we have also made with MIST1 is that plasmablastic lymphomas tend to have weaker reactivity compared with plasma cell neoplasms.
MIST1 helps highlight lymphoepithelial lesions in MZL and a subset of lymphocytes in addition to highlighting the plasma cells in LPL and MZL. While some MIST1 reactivity in the adjacent gastric mucosa is present, there is a comparative difference in the intensity of MIST1 reaction allowing easier identification of lymphoepithelial lesions in some MZL which show a greater degree of plasmacytic differentiation. MIST1 reacts with normal gastric chief cells strongly, but in the setting of chronic gastritis, most epithelial MIST1 is lost (9). Unlike CD38/CD138, MIST1 has exclusive nuclear localization; thus the pattern of reactivity is complimentary to that of cell surface markers, providing utility in in-situ analysis of plasmacytic differentiation.
Our data indicate that MIST1 is a robust and reliable marker of plasmacytic differentiation. MIST1 is useful in showing plasmacytic differentiation in CD138 negative plasmablastic lymphoma. MIST1 also has utility in the diagnosis of MZL by highlighting lymphoepithelial lesions. There may also be added utility in the diagnosis of MZL and LPL in that MIST1 seems to highlight not only the plasma cells but also a subset of the lymphocytes which are presumably undergoing plasmacytic differentiation. MIST1 is also a good negative marker in other B non-Hodgkin lymphomas such as MCL and CLL/SLL. In cases where the diagnosis of a plasma cell neoplasm is difficult due to equivocal laboratory or radiologic findings, MIST1 can serve as additional evidence of plasmacytic differentiation. From our observations of MIST1 immunohistochemistry characteristics, it also appears that the intensity of MIST1 reaction inversely correlates with the aggressiveness of the neoplasm. However, this observation is based upon a limited number of cases and additional studies are required to establish this relationship.
In conclusion, MIST1 is a useful immunohistochemical marker of plasmacytic differentiation. MIST1 has clinical utility as a confirmatory marker in the diagnosis of lymphomas with plasmacytic differentiation, as a negative marker in other lymphoma types, and can be useful in the evaluation of difficult plasma cell neoplasms and lymphomas.
Table 2.
MIST 1 Immunohistochemistry characteristics
| # cases | CD38 or CD138 | MIST1 characteristics in non-neoplastic cells | MIST1 characteristics in neoplastic cells | |
|---|---|---|---|---|
| PCN | 21 | Positive in 19 cases (2 not done) | negative in lymphocytes | All plasma cells with strong nuclear reactivity |
| PBL | 10 | Positive in 9 cases, negative in 1 case | negative in lymphocytes | Strong nuclear reactivity (3/10), variable nuclear reactivity (1/10), weak nuclear reactivity (6/10) |
| MZL | 10 | Positive in subset | negative in lymphocytes | Strong nuclear reactivity in plasma cells and lymphocytes (5/9), strong nuclear reactivity in plasma cells (6/9) |
| LPL | 7 | Positive in subset | negative in lymphocytes | Strong nuclear reactivity in plasma cells and lymphocytes |
| FL | 8 | N/A | negative in lymphocytes, positive in scattered single plasma cells | Negative in the majority of cells |
| CLL/SLL | 9 | N/A | Nuclear reactivity in scattered single plasma cells | Negative in the majority of cells |
| MCL | 8 | N/A | Nuclear reactivity in scattered single plasma cells | Negative in the majority of cells, |
| Normal | 10 | N/A | Nuclear reactivity in scattered single plasma cells | Negative in the majority of cells |
| DLBCL | 1 | N/A | Nuclear reactivity in scattered single plasma cells | Negative in the majority of cells |
| PTLD | 1 | Positive in anaplastic portion of PTLD | Negative in lymphocytes | Negative with sparse cells positive in monomorphic B cell portion of PTLD, dim positive in anaplastic portion of PTLD |
| CD138 neg PB | 1 | negative | Negative in lymphocytes | Weak nuclear reactivity in plasma cells |
plasma cell neoplasm (PCN), plasmablastic lymphoma (PB), marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), follicular lymphoma (FL), chronic lymphocytic lymphoma/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), Diffuse large B cell lymphoma (DLBCL), a CD138 negative plasmablastic lymphoma (CD138 neg PB), and a posttransplant lymphoproliferative disorder with plasmablastic differentiation (PTLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemercier C, To RQ, Swanson BJ, Lyons GE, Konieczny SF. Mist1: a novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev Biol. 1997 Feb 1;182(1):101–13. doi: 10.1006/dbio.1996.8454. [DOI] [PubMed] [Google Scholar]
- 2.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Blazewska KM, McKenna CE, et al. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol. 2010 Mar;30(5):1269–84. doi: 10.1128/MCB.01328-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001 Nov 12;155(4):519–30. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007 Jan;134(1):211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 5.Doherty JM, Geske MJ, Stappenbeck TS, Mills JC. Diverse adult stem cells share specific higher-order patterns of gene expression. Stem Cells. 2008 Aug;26(8):2124–30. doi: 10.1634/stemcells.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capoccia BJ, Lennerz JK, Bredemeyer AJ, Klco JM, Frater JL, Mills JC. The transcription factor MIST1 in terminal differentiation of mouse and human plasma cells. Physiol Genomics. 2010 Nov 23; doi: 10.1152/physiolgenomics.00084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007 Jul 6;27(1):53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, et al. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010 Dec;139(6):2038–49. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010 Sep;177(3):1514–33. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessio M, Roggero S, Funaro A, De Monte LB, Peruzzi L, Geuna M, et al. CD38 molecule: structural and biochemical analysis on human T lymphocytes, thymocytes, and plasma cells. J Immunol. 1990 Aug 1;145(3):878–84. [PubMed] [Google Scholar]
- 11.Bayer-Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod Pathol. 2001 Oct;14(10):1052–8. doi: 10.1038/modpathol.3880435. [DOI] [PubMed] [Google Scholar]
- 12.Seftalioglu A, Karakus S. Syndecan-1/CD138 expression in normal myeloid, acute lymphoblastic and myeloblastic leukemia cells. Acta Histochem. 2003;105(3):213–21. doi: 10.1078/0065-1281-00706. [DOI] [PubMed] [Google Scholar]
- 13.Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, et al. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010 Sep 1;127(6):1356–62. doi: 10.1002/ijc.25358. [DOI] [PubMed] [Google Scholar]
- 14.Reid S, Yang S, Brown R, Kabani K, Aklilu E, Ho PJ, et al. Characterisation and relevance of CD138-negative plasma cells in plasma cell myeloma. Int J Lab Hematol. 2010 Dec;32(6 Pt 1):e190–6. doi: 10.1111/j.1751-553X.2010.01222.x. [DOI] [PubMed] [Google Scholar]


