Abstract
It has long been known that differentiated cells can switch fates, especially in vitro, but only recently has there been a critical mass of publications describing the mechanisms adult, post-mitotic cells use in vivo to reverse their differentiation state. We propose that this sort of cellular reprogramming is a fundamental cellular process akin to apoptosis or mitosis. Because reprogramming can invoke regenerative cells from mature cells, it is critical to the longterm maintenance of tissues like the pancreas, which encounter large insults during adulthood but lack constitutively active adult stem cells to repair the damage. However, even in tissues with adult stem cells, like stomach and intestine, reprogramming may allow mature cells to serve as reserve (“quiescent”) stem cells when normal stem cells are compromised. We propose that the potential downside to reprogramming is that it increases risk for cancers that occur late in adulthood. Mature, long-lived cells may have years of exposure to mutagens. Mutations that affect the physiological function of differentiated, post-mitotic cells may lead to apoptosis, but mutations in genes that govern proliferation might not be selected against. Hence, reprogramming with reentry into the cell cycle might unmask those mutations, causing an irreversible progenitor-like, proliferative state. We review recent evidence showing that reprogramming fuels irreversible metaplastic and precancerous proliferations in stomach and pancreas. Finally, we illustrate how we think reprogrammed differentiated cells are likely candidates as cells of origin for cancers of the intestine.
Keywords: reserve stem cells, epithelial homeostasis, tissue metaplasia
Overview
In 1957, Conrad Waddington proposed his highly influential “landscape model” suggesting that cellular differentiation was like a ball rolling downhill (1). Stem cells are higher up on the hill with multiple channels still open to them; mature cells with their specialized, physiological roles, are at the bottom, each in their unique, mature groove. When a cell reaches its resting state at the bottom, it is “terminally differentiated”, implying there is no going back uphill. That irreversible commitment view of differentiation has long been the dogma. On the other hand, even since Waddington’s model was proposed, there have also been experimental observations suggesting that differentiation could be reversed. Even in the year Waddington’s landscape model was published, Elizabeth Hay was using ultrastructural analysis to show how mature cells in the salamander could dedifferentiate, re-enter the cell cycle, expand and then redifferentiate to regenerate a limb following amputation (2), and two years earlier Carol Mosher was observing reprogramming of mature mesenteric stromal cells to regenerate the digestive system of sea cucumbers (3, 4). However, it has been only much more recently that the concept that mammalian differentiated cells could be returned to a stem cell state has found substantial experimental support, with some of the earliest arguments and supporting data for plasticity of differentiated cells coming from Zipori and colleagues(5). Perhaps the watershed was the work from Takahashi, Yamanaka and colleagues showing that differentiated mammalian cells could be directly reprogrammed in vitro to become stem cells (6, 7). It was incontrovertibly shown, thus, that, at least in tissue culture, differentiation does not have to be permanent. In the last few years, it has become increasingly clear that cell dedifferentiation to a regenerative and/or stem cell state is not simply an artifact of tissue culture. A burgeoning literature has shown that cells in vivo in mammalian organs also have the capacity for dedifferentiation, expansion, and regeneration of damaged tissue, just like Hays’ salamander limb cells. In short, it’s now clear that the ball can roll uphill (8).
Organization of review and caveats
Here we will review the bloom of recent literature on reprogramming of differentiated cells in organs of the digestive system, specifically pancreas, stomach, and intestines. We will review both the cell and tissue changes and the signaling networks that appear to govern reprogramming in each organ. Finally, we will state some hypotheses to be tested and theories of our own that we hope will stimulate both experiments and thinking in this budding field.
Terms
The field of study we address in this review is emerging, and the terminology far from standardized. We would like to be as explicit as possible in our use of terms, allowing that often there isn’t sufficient data to know which terms to apply to which observed cellular phenomenon. We will attempt to apply terms as we think are most appropriate and as the majority of investigators in the field are using them. We use the term “plasticity” to mean the capacity of mature cells to reverse their differentiated cell fate while acquiring the potential, even if temporary, of adopting the fate of another lineage. We use “reprogramming” to refer to the molecular events that control “plasticity” of differentiated cells. We use “transdifferentiation” to mean the conversion of a differentiated cell of one lineage to the differentiated state of a different cell lineage. We use the term “dedifferentiation” to mean that a differentiated cell reverses its differentiated fate to acquire properties it had previously during development (i.e., it returns to a state resembling the progenitor, precursor, and/or stem cell state it passed through to become a differentiated cell). The strictest sense of the term “dedifferentiation” carries the connotation that a differentiated cell not only reverts to a precursor or progenitor state but that it also acquires the capacity for multipotency, in other words, to differentiate into cells of a different lineage. Currently, only a handful of studies have explicitly studied whether this type of strict dedifferentiation to multipotency occurs in the tissues we review here. However, we will make the case that most of the reprogramming events we describe here are, given the available evidence, more like “dedifferentiation” than they are like “transdifferentiation”. We believe our interpretation of the data reflects that of concomitant surveys of the literature (9, 10).
THE PROCESS OF REPROGRAMMING AND ITS ROLE IN REPAIR, METAPLASIA, AND CANCER IN THREE GI TISSUES
Pancreas
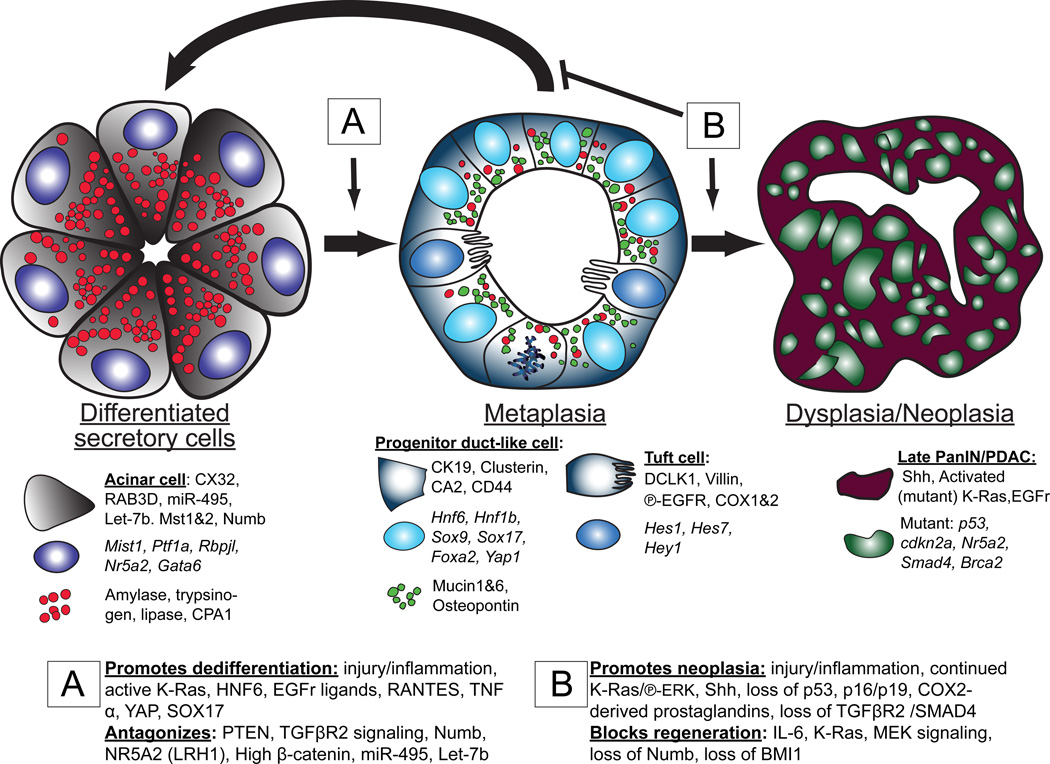
Fig. 1 illustrates the typical cellular changes differentiated secretory cells can undergo during a response to tissue injury or inflammation. Terminally differentiated, professional secretory acinar cells are normally post-mitotic. Their function is to secrete digestive enzymes into the intestine via the pancreatic ducts. The acinar cells store large secretory granules filled with these enzymes (e.g., Amylase, Trypsinogens, Lipases, and Carboxypeptidases) until stimulated to release them by regulated secretion. They constitutively express high levels of transcription factors like XBP1, which scales up and maintains an enormous rER network to produce large quantities of the enzymes (11), and the transcriptional target of XBP1, MIST1 (BHLHA15), which maintains the elaborate apical secretory granule architecture that packages and stores the enzymes until release (12, 13).
Fig. 1. Dedifferentiation, redifferentiation and metaplasia in the pancreas.
The figure diagrams how inflammation and/or tissue damage induces terminally differentiated acinar cells with large, abundant digestive enzyme containing granules (red) to dedifferentiate to mitotically active tubuloductal structures that co-express low levels of digestive enzyme granules along with abundant granules harboring mucins and other regeneration-promoting proteins like osteopontin (green). This reprogramming process, known as acinar to ductal metaplasia, also involves increased DCLK1-positive cells akin to tuft cells of the luminal GI tract, based on gene expression and morphology. If inflammation resolves, the dedifferentiated cells can redifferentiate to regenerate normal acini (and potentially serve as stem cells to regenerate ducts and islets, though this is still an area of controversy). ADM can persist indefinitely in the presence of mutations (such as those that lead to expression of KRAS that cannot be inactivated) and/or continued inflammation. With accumulation of additional mutations, ADM can progress to pancreatic ductal adenocarcinoma (PDAC). The proteins specifically expressed at each stage of the process are delineated in the cytoplasm, nucleus, and granules. The genes involved in promoting/blocking progression to ADM (boxed “A”) and blocking redifferentiation/promoting progression to PDAC (boxed “B”) are also shown.
Tissue injury or inflammation can trigger the acinar cells to reverse their post-mitotic, differentiated cell fate. They acquire morphological and molecular characteristics that are an approximate hybrid between the mature acinar cells they had been and those of duct epithelial cells, which are the cells that line the ducts that transport the acinar cell’s secreted enzymes toward the duodenum. Before genetic lineage tracing became available, histopathologists generally assumed such injury-induced duct-like complexes derived from the reactive proliferation of ducts themselves, because such complexes are organized histologically more like tubules or ducts. However, it is now clear that these proliferative, tubular, duct-like forms largely arise from mature acinar cells and not from mature ducts.(14–18) These lesions are designated Acinar-to-Ductal Metaplasias (ADM). They are metaplastic, because 1) they are morphologically and molecularly distinct from both the normal mature acinar and duct cells and so represent a new differentiation pattern not normally seen in the pancreas, yet 2) they are still composed of normal (i.e. non dysplastic, non neoplastic) epithelial cells. The term acinar-to-ductal metaplasia is somewhat misleading, however, because, although ADM cells express many genes characteristic of mature ducts (e.g., cytoplasmic and secretory products like Cytokeratin 19, Carbonic Anhydrase II, Mucins 1 and 6), they also often maintain (albeit attenuated) expression of many genes characteristic of mature acinar cells (e.g., digestive enzymes like amylase). Thus, in the ADM process, acinar cells are not becoming ducts (14). Rather, they adopt a pattern of gene expression that most closely resembles that of the embryonic progenitor cells that give rise to all the principal lineages of the adult pancreas(14, 19–21). Those progenitors are organized in duct-like complexes, similar to ADM lesions. Like pancreatic progenitor cells, ADM cells are proliferative, whereas mature acinar and ductal cells are largely mitotically quiescent.
Although acinar cells maintain expression of some mature acinar cell genes when converting to ADM, they do shut off specific transcription factors characteristic of the mature acinar cell fate (Fig. 1). Specifically, they downregulate transcription factors that control acinar cell maturation and architecture (MIST1, PTF1A RBPJL, NR5A2) (15, 21, 22). They re-initiate expression of transcription factors expressed both in mature duct cells and in embryonic pancreatic progenitors like HNF6, HNF1β, SOX9 (23–25), as well as transcription factors not expressed in mature ducts but in embryonic progenitors (SOX17, PDX1)(14, 26) (23, 24, 26, 27).
In sum, during ADM, acinar cells reprogram their acinar cell fate in favor of a proliferative cell population with features of embryonic pancreatic progenitors (in addition to previous citations, see also recent review in (10)). The process is evolutionarily conserved, as it happens in rodents and humans(23, 28–30), and, based on the reversion to a proliferative state with gene expression patterns characteristic of the progenitors for the acinar cells, many investigators refer to the process as a “dedifferentiation” of acinar cells(10, 14, 20–22, 28, 31–33). To meet the strictest definition of dedifferentiation, ADM lesions would have to re-acquire true progenitor features. Like embryonic pancreatic progenitors, they should – after the inflammation dies down – be able to differentiate back into acinar cells and, even into mature ducts or islet cells. Accordingly, there is good evidence that ADM may be a relatively commonplace way for the pancreas, following minor injuries, to regenerate acinar cells and potentially mature ducts (34). While it has been shown that dedifferentiated acinar cells can give rise to endocrine cells(25); many investigators think islet cells in the adult largely arise from existing islet cells, even during injury(35–37).
In any case, the preponderance of the evidence would indicate that ADM is formed from acinar cells that must scale down their existing cellular architecture and induce expression of genes that they had expressed during an earlier phase in their existence. Many of the acinar cells undergoing that process also re-enter the cell cycle. Thus, although ADM cells may be only bipotent (capable of regenerating mature acinar and ductal cells), the fact that acinar cells fueling ADM also become proliferative further favors terming the process of ADM as “dedifferentiation” and not as a “transdifferentiation” directly into another adult cell lineage. Eventually, as mentioned, acinar cells can give rise to normal, mature ducts, but this ultimate “transdifferentiation” likely occurs via an initial dedifferentiation to ADM. Clearly, though, as we study the various fate changes and understand the molecular bases of the reprogramming events better, we will be able to more confidently apply correct terminology.
A recent study proposed another, not mutually exclusive interpretation of ADM, which is that the lesions most closely resemble the normal adult ducts of the biliary tree, rather than pancreatic ducts; thus, they concluded ADM was a transdifferentiation to a biliary fate(26). Like the biliary tree, ADM also harbors tuft cells, which are uncommon in any compartment in the normal, adult pancreas. (26, 38)Tuft cells are morphologically distinct cells, scattered throughout the glandular, luminal GI tract, as well as in the extrahepatic biliary tree. They have extensive microvilli characterized by Villin expression (39). They also manifest microtubule networks characterized by acetylated tubulin and the enzymes used to promote tubulin acetylation (DCLK1, ATAT1) and are reported to exhibit enhanced expression of both receptors for EGF ligands and IGF (26, 38). It is not clear whether similar cells can be found during embryonic pancreatic development or if they are unique to ADM. Also unclear is whether DCLK1+ cells in ADM arise directly from acinar cells (via transdifferentiation) or from other ADM cells (in a dedifferentiation-redifferentiation process).
ADM frequently occurs in humans in the setting of pancreatitis of various etiologies (familial, alcohol-induced, spontaneous) (26, 40, 41). In mice, pancreatitis and ADM can be induced by drugs or by surgical resection (42). The most commonly used drug to induce ADM experimentally is caerulein, which is a decapeptide analog of the normal hormonal trigger for exocytosis of the acinar secretory granules, cholecystokinin (CCK). High doses of injected caerulein cause ADM in the setting of pancreatitis which is thought to result from the damage due to inappropriate, hypersecretion of digestive enzymes and, potentially, direct induction of pro-inflammatory mediators (43). The most common direct tissue injury mode used to study ADM is pancreatic ductal ligation wherein a duct from a pancreatic lobe is sutured closed, resulting in blockade of enzyme flow into the intestine and considerable tissue injury and inflammation in the affected lobe (44). Inflammatory cells, like macrophages (41, 44), are critical for ADM, no matter the experimental system, and the role of some cytokines in the process has been established (see below), but, overall, how cells initially sense damage and which upstream signals induce acinar cells to dedifferentiate have not been elucidated.
Given all the evidence presented above, we favor the interpretation that ADM is an evolutionarily conserved mechanism that allows for proliferation and expansion of cells that can eventually redifferentiate to replace tissue that was lost during injury. In mice, as discussed, it seems that ADM lesions largely redifferentiate into acinar cells(14, 28, 34), thereby restoring normal exocrine pancreatic architecture after an injury. However, ADM also seems to increase risk of progression to pancreatic ductal adenocarcinoma (PDAC), suggesting it may sometimes not resolve and, rather, serve as a precursor lesion initiating cancer in human patients. Thus, as has been clear for decades for esophageal and gastric adenocarcinomas, PDAC seems also to arise through a series of precursor lesions beginning with metaplasia. One reason why knowledge about the normal raison d’être for ADM is relatively limited (i.e., is it simply a means for the pancreas to regenerate damaged tissue via an evolutionarily conserved dedifferentiation-redifferentiation sequence?) is because the vast majority of studies on ADM are in the context of its role as a precursor lesion for PDAC, so investigators have focused on ADM progression rather than regression and recovery. Indeed if ADM is induced via ligation of the pancreatic duct, there is no chance for the affected lobe to regenerate fully, as enzymatic secretion will remain impaired, unless the ligature could be surgically removed without lasting damage to the duct. Mouse genetic lineage tracing studies show that continued severe inflammation and/or underlying mutations in key regulatory genes can cause ADM cells to further increase proliferation and eventually undergo non-normal (neoplastic) changes. The progression from ADM to pancreatic intraepithelial neoplasia (PanIN) has been well established both in mouse models by lineage tracing (first by Habbe, Shi et al. (17)) and in humans from pathology specimens (38, 45–47). Mouse models, supported by human histopathological studies, indicate that PanINs are the precursor for full-fledged, invasive PDAC and, accordingly, often already exhibit mutations characteristic of PDAC (48–50).
Thus, the decision ADM lesions make between redifferentiating to regenerate normal adult pancreas and progressing to an abnormal, neoplastic state is a critical one (Fig. 1). Emerging experimental evidence indicates that defects in certain signaling pathways simply trap cells in an ADM state but do not increase propensity for progression to PanIN. For example, loss of Numb causes rapid progression to ADM during pancreatitis and even towards early PanIN, but it also decreases proliferative capacity of PanINs thereby blocking progression towards neoplasia (32). Similarly, loss of IL-6 inhibits redifferentiation of ADM cells but also decreases proliferation and progression to PanIN, perhaps by decreasing inflammatory cells that promote tumorigenesis (SMA+ fibroblasts, myeloid suppressor cells) (51). Thus, there appears to be a functional difference between blocking redifferentiation and promoting progression to neoplasia.
Signaling networks regulating dedifferentiation
Fig. 1 details some of the genes known to mark each differentiation state as well as those that have been shown to promote dedifferentiation and block redifferentiation or promote progression to PDAC. The hub protein in dedifferentiation is clearly KRAS. Numerous studies have shown that inducing expression of constitutively active KRASG12D promotes dedifferentiation, and turning off expression of KRASG12D induces redifferentiation back to the acinar state (52). Active KRAS alone is not sufficient to induce dedifferentiation, however, as injury or inflammation must also occur (e.g., by treatment with caerulein(48, 53, 54)). Though the pro-dedifferentiation inflammatory signals are not particularly well understood, prostaglandins may be involved, as COX2 is increased during ADM(40, 41, 48, 55), and COX2 inhibition can block caerulein’s dedifferentiation inducing effects (56). Macrophages, NFκB signaling, and TNFα also have been implicated in the inflammation that induces dedifferentiation (41).
Once dedifferentiation occurs, KRAS is necessary and sufficient to maintain the ADM state. Multiple steps in the canonical Ras pathway have been shown to also be required and sufficient for promoting ADM; for example upstream of KRAS: EGF or TGFα signaling (22, 33, 47, 57); the EGF receptor, other ERBB receptors and ADAM17 which releases EGF, TGFα, and Amphiregulin (18, 40, 47, 58); and the key downstream mediator of the KRAS signal is Mitogen activated protein kinase kinase 1 and 2 (MAP2K1 and MAP2K2; aka MEK1/2) upstream of extracellular signal-regulated kinase 1 and 2 (ERK1/2) ((18, 26, 47)). Inhibitors at each stage can block or reverse ADM: Erlotinib and Cetuximab block EGFR interaction with its ligands (40), PD153035 blocks signaling from the EGFR (18), and the MEK inhibitors BAY 86–9766, PD325901, and U0126 (22, 30, 31, 40).
Roles for other important signaling pathways have been addressed in dedifferentiation and progression to PDAC (Hedgehog, Wnt, Akt-PTEN, Notch, TGFβ), though the literature on each of those is limited, perhaps because they either play auxiliary roles to EGFR-KRAS, (e.g., Notch (32) and PTEN (46), and/or may be more involved in promoting progression to PDAC than in causing dedifferentiation (e.g., Hedgehog (52) (56) and TGFβ (59, 60). A newly described, non-KRAS pathway sufficient in itself to induce ADM is Hippo. Induction of nuclear YAP1 activity (i.e., decreased signaling through the Hippo pathway) in adult mice is sufficient to cause ADM without manipulating KRAS (61). Activated Wnt-β-catenin may play a more complicated role, in part antagonizing KRAS’s dedifferentiation-promoting activity (62).
A role for BMI1-governed transcriptional targets has been shown during progression to PanIN and cancer. BMI1 suppresses its targets at the CDKN2A locus (p16 Ink Arf), which promote progression to PDAC; in other words, BMI1 is important for promoting redifferentiation and blocking progression to cancer (56, 63). Given BMI1 is expressed in rare acinar cells (63, 64) and promotes tissue repair, it could serve a parallel role to the one it plays in intestine (see below).
Finally, a role for the Scaling Factor MIST1(65) has also been posited, as MIST1 is one of the first genes expressed in differentiated acinar cells to be shut off during dedifferentiation (15, 21, 22), and Mist1−/− mice are prone to ADM (47). It may antagonize the KRAS→ERK1/2 signal, as forced expression slows dedifferentiation (18), though at what level MIST1 and KRAS may interact is not known.
Two recent studies implicate DCLK1 as playing a key role in ADM and progression to PDAC. DCLK1 is expressed in cells with substantial acetylated tubulin networks, like tuft cells, discussed above. DCLK1-positive cells are rare in the normal pancreas, and DCLK1 is at low levels in acinar cells. KRAS-driven ADM causes a large spike in DCLK1-positive cells with tuft cell morphology, and those cells can be isolated to grow spheroids in culture, suggesting they have stem cell and tumor initiating properties (38). They mark distinctively with Notch downstream targets, COX1 and COX2, and EGFR, and are lost in PDAC (26, 38). Thus far, the results in pancreas are intriguing with regards to similar roles for DCLK1-positive cells in the intestine.
An array of transcription factors that characterize the progenitor cells for the pancreas and biliary tree are also induced during ADM (e.g, SOX9, HNF1β; detailed in Fig. 1). As discussed above, induction of such duct-specific transcription factors at the expense of mature acinar transcription factors is a key feature of ADM and may be required for progression to PanIN. Again, we emphasize that it’s important to remember that mature ducts that normally express Sox9 and Hnf1β are resistant to progression to PanIN and that ADM lesions are more like Sox9-expressing progenitors present during pancreatic embryogenesis (23, 24) (66).
Stomach
The normal mammalian stomach differs from the pancreas in that there is a constitutively active stem cell population. Most of the proliferative activity of these stem cells appears to replace the rapidly turning over cells that line the surface of the stomach (67, 68). Likely as a consequence, in the bulk of the stomach (i.e., the proximal body or corpus portion vs. the more distal antrum and/or pylorus), the stem cell is located nearer the gastric lumen than the base of the gastric glands. Injury and inflammation can cause increased proliferation of these stem cells, located in the isthmus, but it also can induce metaplasia and proliferation in mature, post-mitotic digestive-enzyme secreting chief (zymogenic) cells deep in the base of the glands(69–71).
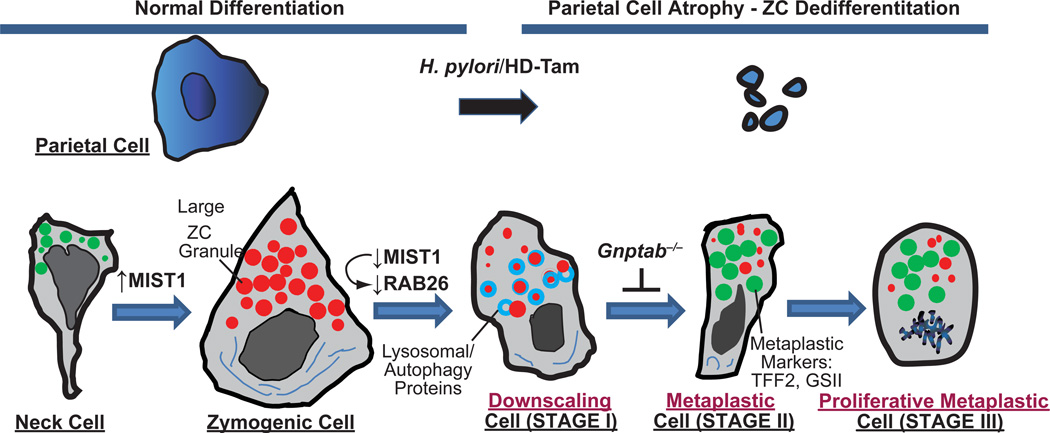
Though work on plasticity in the stomach is so far much more limited than in the pancreas, it is clear that the metaplasia that the digestive-enzyme-secreting gastric chief cells undergo parallels, in terms of general features, the reprogramming of pancreatic acinar cells in ADM. One aspect of chief cell metaplasia that is arguably clearer than ADM is the cellular trigger that induces reprogramming. In the stomach, death of another key functional secretory cell, the acid-secreting parietal cell, causes loss of normal chief cell differentiation (69, 72, 73) (Fig. 2). Loss or injury of parietal cells causes chief cells to downscale their large secretory granules containing digestive enzymes like Pepsinogen C and Carboxypeptidase B (74) and re-express markers of mucous neck cells (which are the precursors of chief cells in adult stomachs(75–77)), like TFF2 (Spasmolytic polypeptide), MUC6, the epitope for the lectin GS-II, and, in mice, Gastrokine 3 (69, 73, 78–80). Thus, tissue damage of the type that injures parietal cells causes a complete reorganization of the gastric unit, characterized by, in addition to parietal cell atrophy: expansion of a metaplastic lineage co-expressing mucous neck and chief cell markers and the loss of normal chief cells, as they change their differentiation state to this metaplastic lineage (70, 74, 81, 82). During parietal cell atrophy-induced metaplasia, the overall pattern of cell differentiation in the corpus resembles somewhat the organization of the more distal antral or pyloric region of the stomach. Accordingly, this aberrant differentiation has been called “antralization”, “pseudopyloric” metaplasia or, alternatively, based on the greatly increased expression of TFF2 (Spasmolytic Polypeptide) in the reprogrammed cells at the base of the glands, “Spasmolytic Polypeptide Expressing Metaplasia” or “SPEM” (73, 80, 83). The pathology term “atrophic gastritis” in the body of the stomach is largely synonymous with this type of metaplasia, as “atrophy” refers to the histological pattern of lost parietal and chief cells, and “gastritis” refers to the chronic inflammatory infiltrate associated with the insult that caused the parietal cell death. Though this type of metaplasia, seen in atrophic gastritis, is “pseudopyloric”, there may be differences between the adult antrum-pylorus and SPEM; for example, SPEM lesions are thought to lack gastrin-secreting G cells, which occur in adult antrum, though some have reported an increase in gastrin during atrophic gastritis(84). They have also been shown to lack the transcription factor PDX1(85), which is expressed in the normal epithelial cells of the antrum, not the body, and is involved in generating G-cells(85, 86), though another report did observe some PDX1 expression in human SPEM lesions(87). SPEM units also resemble the embryonic or pre-weaning stomach body, which similarly lacks mature, distinct neck, chief, and parietal cell populations(88–92).
Fig. 2. Proposed stages of reprogramming and metaplasia in the stomach.
Cartoon based on our work and that of the literature proposing that reprogramming during metaplastic response to parietal cell damage in the stomach proceeds via 4 distinct stages. Zymogenic “Chief” Cells normally differentiate from a secretory progenitor, known as the mucous neck cell. Atrophy (death) of acid-secreting parietal cells (caused, e.g., by chronic infection with Helicobacter pylori or, in mouse models, by high doses of tamoxifen “HD-Tam”) causes chief cells to reprogram. We propose that the first stage of the reprogramming response is for Chief Cells to “downscale” their secretory architecture (including decreased digestive enzyme-containing granules, red, and dismantling of the elaborate rER network, dark blue) in a process involving decreased expression of the bHLH transcription factor, MIST1, which is required for maintaining apical secretory granules. Once large enzyme-containing granules are recycled, cells re-express progenitor genes (green) with much scanter digestive enzyme granules and are thus, by definition, metaplastic (called “SPEM” based on greatly increased expression of TFF2, aka Spasmolytic Polypeptide). In the third stage, cells reenter the cell cycle, at which point they may, via poorly understood processes, give rise to other metaplasias (like intestinal metaplasia in humans) or progress directly to cancer. In a parallel to pancreatic ADM, metaplastic chief cells may also regenerate other cells again via processes that are yet to be determined. Genes expressed during mucous neck, zymogenic, and metaplastic stages are delineated below, along with secreted factors at each of those stages.
Thus, though it has become clear that chief cells fuel much of the metaplasia seen in atrophic gastritis (i.e., inflammatory injury with parietal cell atrophy), it is less clear whether this process represents a dedifferentiation or transdifferentiation event. The changes in gene expression follow a similar progression to that of ADM in that the first known event in chief cell reprogramming is downscaling of the secretory architecture with loss of MIST1 expression(69, 70, 74, 82, 93, 94). The cargo of the secretory granules (e.g., pepsinogen C) is decreased but not lost completely(82, 94), just as amylase is maintained in early ADM. The genes whose expression is induced and help define the metaplasia (e.g., Tff2, epitope for the lectin GS-II, Muc6) also characterize the mucous neck cell, the precursor that differentiates into the chief cell(75–77), and the pattern of gene expression showing a mixture of neck cell and chief cell secretory products is characteristic of gastric embryonic epithelial progenitor cells. In mouse models, at least a portion of the metaplastic cell population is also proliferative. Thus, the pattern of immature marker expression and proliferation is more consistent with a dedifferentiation event to a proliferative, regenerative state. Though there has been one study showing that mature chief cells can serve as stem cells(71), this was not done in the setting of metaplasia, and the dedifferentiation events were not common, perhaps, because parietal cells were not injured. Previous publications have referred to chief cell reprogramming in SPEM as a “transdifferentiation”.(81) Clearly, more work needs to be done to determine the eventual fate of the reprogramming of chief cells in response to atrophy.
Another confounding issue in the stomach, which does not have a parallel in the pancreas is that human stomachs, in particular in certain regions of the world, don’t only undergo pseudopyloric or SPEM-type metaplasia. They also rather commonly undergo intestinal metaplasia that histologically and molecularly resembles either small and/or large intestine; intestinal metaplasia has been studied as a precursor lesion for gastric cancer for many decades(95). The cellular origin of intestinal metaplasia lesions is not clear, but it has been proposed that chief cells, either by way of SPEM metaplasia or by direct reprogramming give rise to intestinal metaplasia(74, 96, 97). Given that stem cell activity in intestinal metaplasia is at the base of the gastric gland, whereas normal stem cell activity in the body of the stomach is nearer the lumen, it would make teleological sense if reprogrammed mature chief cells, which are also at the base, were the ultimate source of the intestine-like stem cells in intestinal metaplasia. One completely speculative way to explain how both antrum-like and intestine-like stem cell activity derives from a differentiated chief cell would be by a dedifferentiation event that resulted in reprogramming back to a stem cell roughly equivalent to that of an undifferentiated embryonic gastrointestinal tract progenitor prior to stomach and intestine specification. That would allow reprogrammed chief cells to redifferentiate as stem cells that generate units that characterize any of the glandular epithelium of the luminal GI tract.
In humans, chief cells reprogram most frequently in the setting of infection by the bacterium Helicobacter pylori (HP), especially in certain populations (e.g., in East Asians and in regions of Central and South Americans). In those populations, in particular, bacteria cause widespread parietal atrophy and chief cell metaplasia. The metaplastic events correlate with greatly increased risk for gastric carcinoma as patients age, and increasing portions of their stomachs are reprogrammed into SPEM and intestinal metaplasia (74, 83). Thus, depending on the relationship between chief cells, SPEM, intestinal metaplasia, and the subsequent cancers that arise in this setting of reprogramming and metaplasia, chief cells may be the root of many gastric cancers, just as acinar cell dedifferentiation may be the root of pancreatic ductal adenocarcinoma (74, 81, 97). Most gastric carcinomas exhibit molecular and histological features that are somewhere on a spectrum between those of stomach and intestine. One intriguing possibility for this variety in differentiation patterns is that the cancers might arise in cells that aberrantly dedifferentiate, developing mixed progenitor characteristics of the shared gastrointestinal embryonic progenitors, as discussed above (71).
Thus, unlike pancreas, in stomach there is a clear cell that sends the signal for reprogramming (the parietal cell), and there is a clear infectious agent (H. pylori) that can start the sequence off. On the other hand, we do not know how parietal cell atrophy signals to induce chief cell reprogramming. In addition to the variety of differentiation patterns from gastric-like to intestine-like, gastric cancers are also far more varied relative to PDAC in their molecular, morphological, and epigenetic patterns, making the relatively straightforward sequence of dedifferentiation of a digestive-enzyme-secreting cell to adenocarcinoma not as clear-cut. Interestingly, H. pylori not only induces reprogramming but can also cause considerable genetic instability with greatly increased mutation rate along with widespread epigenetic changes (98–102). The increased mutation rate could randomize somewhat the genotypes and phenotypes of cancers that might have arisen initially from a failed reprogramming event in mature gastric cells. McDonald and Wright and colleagues have shown that mutations in dysplastic lesions (i.e., precancerous lesions) rarely coincide with mutational state in the neighboring metaplastic lesions(103), so the development of cancer may ultimately originate from metaplasia but not directly, just as pancreatic cancer progresses via a PanIN stage which also involves additional mutations. Another factor to consider is that, though most cancers arise within or near regions of the stomach where chief cells are abundant, many also arise in the antrum where parietal and chief cells are normally far less abundant. Finally, though animal models, with human pathology correlation, have clearly shown that chief cells reprogram to SPEM cells, there are no animal models of gastric cancer that resemble human adenocarcinoma in terms of morphology, invasion and metastasis. In contrast, models of pancreatic cancer in mice show that acinar cells can be driven to undergo dedifferentiation, metaplasia, dysplasia, neoplasia and metastasis more or less the way it occurs in humans.
The early stages of cancer progression, reprogramming and metaplasia, are easy to study in mouse stomach. Mice can be infected with Helicobacter species themselves, which can cause reprogramming and metaplasia of chief cells in weeks to months (80, 102, 104). Several well-characterized drugs kill parietal cells directly and can induce near complete parietal atrophy and SPEM in mice within days. These include: the neutrophil elastase inhibitor DMP-777 and the drug L-635 (70), and, also, treatment with high doses of tamoxifen (94, 104). All the drugs seem to work by interfering with parietal cell proton gradients, as the proton pump inhibitor omeprazole partially rescues the death of parietal cells these agents induce (94, 105). The drugs cause such rapid, synchronous metaplasia that they make characterizing the molecular and cellular sequence of events during chief cell reprogramming relatively straightforward, and the field is ripe for an explosion of investigations in the coming years.
Signaling networks regulating reprogramming
The molecular mechanisms underlying metaplasia of chief cells are relatively uncharted (69, 74, 81, 93) (Fig. 2). In the absence of anterior gradient 2 (AGR2), chief cell differentiation from their neck cell progenitors is blocked, and SOX9-positive cells accumulate (106), but whether AGR2 is induced during chief cell reprogramming is not known. Sox9, like many other markers of SPEM, is normally expressed only in mucous neck cells in the adult (107). Inflammation may be key to induction of chief cell reprogramming, as depleting macrophages also reduces metaplasia (108), and a specific Shh-induced myeloid population has further been identified as required for metaplasia progression (109). Histamine-deficient (histidine decarboxylase null) mice showed increased induction of metaplasia (110). Global deletion of Amphiregulin, a gene encoding an EGFR ligand, caused spontaneous reprogramming and metaplasia of chief cells as they aged (96). A pERK→CD44→pSTAT3 signaling pathway was identified as being key to parietal cell-damage-induced proliferation during metaplasia, suggesting pERK in stomach may parallel the pancreas (104). As discussed above, in a clear parallel to ADM in the pancreas, MIST1 is also one of the first genes decreased during reprogramming of chief cells (70) (82). Goldenring and coworkers have identified many genes whose expression is increased in SPEM: Mal2, Wfdc2 (He4), Tacc3, Mcm3(110, 111)(Fig. 2). These genes are characteristic of SPEM but not normal adult stomach, though their expression during development should be investigated, as the parallel of chief cell reprogramming with ADM would suggest that these genes might be expressed in gastric embryonic progenitors.
Intestines
Even in the unstressed situation, the epithelium lining the intestines exhibits remarkably high rates of turnover relative to epithelia of other gastrointestinal organs. Following injury, the intestines show rapid regenerative capacity. Ex vivo, a single stem cell and a single Paneth cell in the correct culture conditions can rapidly induce an intestinal organoid structure that remains very faithful to the crypt of a normal small intestine (112). Organoids derived thus neither senesce nor accumulate mutation. Given the regenerative capacity of the resident stem cell, one might predict a much smaller need for dedifferentiation from mature cells to fuel injury-induced regeneration within this tissue. However, human expectations have little to do with the actual nature of things, and it turns out that, relative to other adult tissues like the hematopoietic system, the intestine can be defined in part by its high inherent plasticity(113). Recent studies have shown that multiple cell-types in the intestine, including differentiated cells, have the capacity to recover the intestinal epithelium and even repopulate the normal stem cell zones if the stem cells themselves are lost (114, 115).
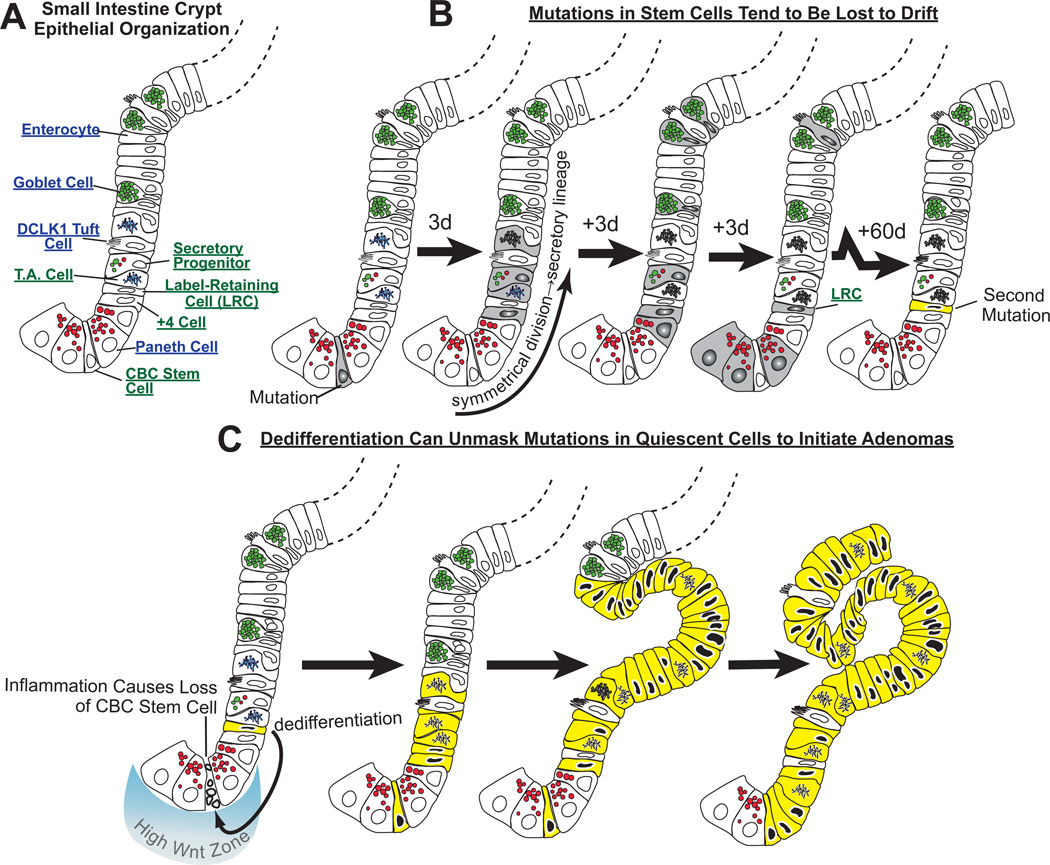
To understand how plasticity and reprogramming of differentiated cells to stem cells might work in the intestine, we must take some time to describe the normal stem cell niche and the normal hierarchy of cells with stem cell activity. The intestines are different from the pancreas, which lacks a constitutive stem cell, and the stomach which has both a clear constitutively active stem cell and a clearly identified reserve population that can be induced during injury (i.e. the chief cell). To understand stem cells and plasticity in the intestine, we must first discuss the central role that Wnt signaling plays (116). For example, stem cells, Paneth cells and transit- amplifying progenitor cells in the small intestine are all characterized by high levels of the Wnt signaling pathway (Fig. 3)(117). Intestinal stem cells have higher levels of nuclear β-catenin and express a different set of Wnt targets (e.g., LGR5) when compared to the transit amplifying(TA) cells higher in the crypt. It may be that the LGR5 receptor itself confers the higher levels of canonical Wnt signaling output within the stem cell zone culminating in the increased nuclear β-catenin (118, 119).
Fig. 3. Neoplastic growth in the intestines may occur because dedifferentiation of long-lived mature cells unmasks mutations.
A) Some of the key cell lineages in the cartoon of one side of a crypt are labeled. B) Mutations occurring in a crypt base columnar (“CBC”) stem cell may be lost by drift into differentiated cells, because CBC cells divide frequently and often symmetrically to produce two daughters that differentiate. Here, a CBC cell has acquired a mutation and has undergone symmetrical division such that both daughter cells differentiate along the secretory lineage. The progeny, depicted in time intervals (e.g., “3d” ≈3 days elapsed) differentiate into goblet, Paneth, tuft and enteroendocrine (not depicted) cells. With time, most of those cells die, at which point, the mutation will be lost, were it not for the fact that some longer lived cells (e.g., Paneth cells or perhaps even longer-lived “label-retaining cells”) could potentially harbor the mutation for months. In the case depicted, the Paneth cells are also lost to normal turnover, but the even longer living LRC in this unit acquires an additional mutation over the two months following the first mutation in the stem cell. C) Again, that mutation would likely be lost as even the LRC ages and dies, unless, as is shown at the bottom, inflammation causes death of the normal, constitutively active stem cell in the crypt. Here, CBC death induces the LRC, which has accumulated an additional mutation, to dedifferentiate, become exposed to high Wnt signaling and reenter the cell cycle. The mutations in the LRC are now unmasked as the cell is exposed to the high Wnt environment and begins to proliferate. The mutations prevent the cell from differentiating beyond the transit amplifying stage, and a clone of early neoplastic cells derived from this mutant stem cell develops an adenoma. The key aspect of this model is that the normal CBC divides too rapidly to accumulate mutations, whereas differentiated cells live longer and can store mutations that may affect proliferation or differentiation but won’t affect these quiescent cells until dedifferentiation unmasks them.
There have been numerous recent reviews on intestinal stem cells, so we will cover this topic only so that our discussion of reprogramming will have a conceptual foundation. Particularly pertinent to this review is the recent review by Vermeulen and Snippert (120), where they discuss the “clonogenic” capacity of different populations of intestinal cells. It is now clear that, rather than there being a single population of stem cells in the intestine, there are a number of different populations at any instant (120). One way to view the array of stem cells and cells with stem cell potential is using LGR5 expression, as it is an excellent marker of the crypt-base columnar (CBC) stem cells, which are at the very base of the crypt and nestle in amongst the Paneth cells. Such stem cells have a clear identity with the expression of multiple markers that can specifically identify these cells (including Olfm4, Aqp4, Cdca7, Cdk6, Tnfr19, Clca4, Kcnq1, Nav1, Smoc2, Soat1 ASCl2) (121). There is then another stem cell population that can be identified by a slightly broader set of markers including LRIG1 and BMI1. These markers such as LRIG1 will also be expressed within the LGR5 compartment but will have an broader expression so that they mark many more cells within the crypt (up to position 5–6) (122). Genetic lineage tracing techniques show that such cells can serve as a stem cell to replace all the differentiated lineages and even the LGR5 stem cells at the crypt base. However, relative to the LGR5 cells, the chance that they will serve as stem cells under normal homeostasis is much lower but increased following injury or inflammation (123). It is thought that most of the population expressing those markers will normally serve only as progenitors committed to specific differentiated lineages or act as rapidly dividing transit amplifying cells.
Another cell population with stem cell potential are the so-called Label Retaining Cells (LRC), which are slowly dividing and can repopulate the crypt following injury (Fig. 3). LRCs have not traditionally been characterized by their molecular markers; rather, this population has been defined by the capacity to incorporate labeled nucleotides (e.g., Brd-U, 3H-thymidine) during a single S-phase and then subsequently to divide so slowly over subsequent weeks to months that they retain that label in their nuclei. The recent studies by the Fodde and Winton laboratory have shown that LRCs do not act as stem cells in the normal crypt in the absence of injury or inflammation (114, 124). Some of the cells constituting the LRC population are the long-lived secretory cells at the base of the crypt. Although identified by a specific molecular marker – rather than their label-retaining capacity – DLL1 + cells, which normally give rise to secretory precursors but following injury can repopulate the intestine and then act as bona fide stem cells, are likely a LRC population (125). An interesting possibility is that some of these LRC’s may be long-lived, more differentiated cells with other, as yet undescribed normal functions. Thus, they, like pancreatic acinar or gastric chief cells, might maintain the capacity to reprogram to a stem cell state when necessary. Interestingly, Lund and co-workers showed that BMI1-expressing cells marked with high expression of EGFP driven by Sox9 promoter elements. Flow-sorted Sox9-EGFPHi cells had many characteristics of secretory cells, in particular, of the enteroendocrine lineage(123). The findings provide support for early work from Leiter et al., who showed that cells expressing the promoter for the endocrine-specific transcription factor, Ngn3, also showed surprising plasticity(126). Moreover, recent studies have shown that DCKL1+ Tuft cells contain a population of very long lived cells that have the capacity to repopulate the epithelium post injury (127). Together, the studies on the LRC cells as well the studies of Sox9Hi, Ngn3 precursors, DCKL+ and DLL1+ cells all indicate that a subset of the LRC or other differentiated population can clearly dedifferentiate to produce functional stem cells. And, thus, the intestine, like pancreas and stomach, has a long-lived population of secretory cells with stem cell capacity that must involve reprogramming, return to the cell cycle, and the capacity to serve as stem cells (i.e., these cells can “dedifferentiate”).
It should be noted during intestinal regeneration there is a marked increase in Wnt signaling that is functionally important (128). Inhibition of Wnt signaling or its targets suppresses intestinal regeneration, and it may be that in this case the increased Wnt signaling is required to recover the stem cell population. Remarkably, in the absence of other injury, specific ablation of the LGR5 cells alone does not perturb homeostasis or differentiation within the intestine (115). Thus, the other populations of cells with normally rare or infrequent stem cell activity can seamlessly take over full stem cell responsibility. Seemingly paradoxically, however, a LGR5Hi population of cells seems to be more critical for intestinal regeneration following injury, suggesting that response to injury might normally involve Wnt, so that population of cells recruited to reprogram to replace the normal stem cell may depend on the population’s ability to concentrate and coordinate a pronounced Wnt response, in part via LGR5 (129). One caveat here is that, in these specific experiments, there might be a cooperation of toxicity of irradiation and the diphtheria toxin killing of LGR5+ cells. Hence, it would be of interest to examine whether a mutation that causes the loss of the LGR5+ cells still stopped regeneration.
Given the normal plasticity of differentiated intestinal cells, and the role of reprogramming and dedifferentiation in other organs, it is probably not surprising that dedifferentiated cells might play a role in development of intestinal cancer. Within colorectal cancer, approximately 80% of tumors carry APC mutations. A key role of APC is to suppress the Wnt signaling pathway (130). Loss of the APC tumor suppressor immediately perturbs intestinal homeostasis in the mouse, with a crypt progenitor cell-like phenotype being bestowed upon APC-deficient cells (131, 132). Following APC loss: crypts are enlarged; differentiation is perturbed with increased numbers of LGR5Hi cells and Paneth cells; increased progenitor, transit amplifying cells; and reduced goblet and terminally differentiated enterocytes. Despite nuclear β-catenin in all cells along with the other numerous abnormalities in differentiation pattern, there is, remarkably, still a population of LGR5Hi cells based at the bottom of the crypt. The major cell type produced becomes a transit amplifying crypt progenitor cell. Thus, there is still a hierarchy retained following APC loss even in the adenomas derived from APC-deficient cells (133). Similar to expression of K-Ras alone in acinar cells, loss of APC alone in differentiated enterocytes on the villous of the intestine appear largely normal, despite abundant nuclear β-catenin. Thus, APC mutation alone, in at least one differentiated population is not sufficient to trigger adenomatous transformation.
Recent studies from us and others have suggested that a number of cellular processes manifest differential activity along the stem-differentiated cell axis and may help decide how differentiation occurs normally, following injury, and during carcinogenesis. Cells with the highest levels of Wnt signaling, the stem and Paneth cells, have the highest glycolytic activity and the most active protein translation and generate the most reactive oxygen species (ROS) (134, 135). Interestingly, increased glycolysis characterizes the “Warburg Effect” (the use of anaerobic glycolysis in favor of aerobic generation of ATP via mitochondria) that distinguishes most cancer cells from normal cells. Thus, the normal intestinal stem cells, in the high Wnt zone, can be distinguished from differentiated cells by their “Warburg-ean” characteristics(136). Importantly, following APC loss, all cells adopt a Warburgean phenotype; e.g., high levels of ROS now occur throughout the crypt and not just at the base. Deletion of RAC1 in APC-deficient intestines suppresses the upregulation of ROS and the consequent downstream activation of the NFκB pathway resulting in decreases in the hyperproliferative phenotype of APC deficient cells (90).
Recent studies inhibiting mTOR signaling in the intestine exemplify this ability to switch the altered differentiation-dedifferentiation homeostasis conferred by APC loss. Previous work had suggested that APC deficient cells require mTOR signaling (137, 138). Accordingly, we have shown recently that ApcMin/+ adenomas treated with rapamycin enter quiescence: differentiated Paneth cells eventually die off, after which, the adenomas shrink to lesions with high numbers of LGR5Hi cells but little proliferative activity. The mechanism of mTORC1 action seems to be by working to maintain high rates of translation elongation by the inhibition of the elongation suppressing factor EF2K by mTORC1’s target S6 Kinase. Cessation of mTORC1 inhibition allows tumors to regain proliferative capacity and “normal” differentiation (139). This role of mTOR in conferring proliferative capacity may not be restricted to the intestine, as muscle stem cells require mTOR to exit quiescence (140). In sum, differentiation states following APC loss can be modified by modulating basic cellular pathways, thereby offering proof of principle that the plasticity of the intestine, which may play a role in adenoma formation and generation of cancer, can be manipulated. That differentiation, dedifferentiation, and redifferentiation can be modified experimentally suggest that they can eventually be manipulated pharmaceutically to reduce risk for cancer. For example, a specific set of mutations in differentiated cells that allow those cells to dedifferentiate by re-acquiring stem and progenitor properties might be targeted (we will return to this later).
Is the stem cell or a dedifferentiating cell the cell of origin in intestinal carcinogenesis?
Cell of origin studies have shown that stem cells efficiently give rise to neoplasia in the mouse (141). Note the caveat that most models of intestinal carcinogenesis in the mouse are in small intestine, whereas small intestinal adenocarcinomas are rare in humans (the vast majority originate in the colorectal region), so there has to be some caution in extrapolating our understanding of carcinogenesis from mice to humans. Deletion of Apc using inducible Cre recombinase driven by promoters expressed in stem cell (e.g. Lgr5CreERT2) lines lead to rapid adenoma formation (141). Indeed, loss of both copies of Apc within a stem cell in a mouse appears sufficient for adenoma formation. Of course, it might be hard to exclude the possibility that the stem cells differentiate first into a more differentiated cell that then dedifferentiates to start the tumors, though that would be a far less economical interpretation of the data. The stem cell straight to tumor theory does not explain why APC mutations do not immediately cause adenomas and then cancer in humans, where it seemingly takes years for that to happen rather than days. Two potential explanations for the discrepancy are as follows. First, tumorigenesis takes so long in humans because it may take a second ‘hit’ (mutation) to occur within the same stem cell. Elegant studies by the Winton group have shown that although a single APC mutation confers a selective advantage to stem cells, there is still a high probability that a APC mutant clone will be lost through drift (120). Given the normal intestine has approximately 5–7 active stem cells per crypt, each stem cell has a stochastic chance of taking over the crypt. Even if a mutation has a selective advantage, given this number of stem cells, it is not guaranteed to be in a cell that takes over the crypt and therefore can be lost through drift. The relative increase in fitness of a single APC mutation in a stem cell is relatively small and thus can be lost. Therefore the increased time to tumorigenesis in sporadic cancer could be down to loss of the mutant stem cells. On the other hand, some of the progeny of stem cells with mutations may differentiate into longer-lived secretory cells and/or label retaining cells (Fig. 3), where the mutation could be stored for weeks or months, during which time those cells may acquire a second hit. If those cells are then induced into stem cell activity by dedifferentiation following inflammation-induced damage to the stem cells in the crypt base, then these now doubly mutant cells are driven into the cell cycle in the presence of high Wnt, which at that point could lead to adenomas as the cells expand but can only partially differentiate.
The other, non-mutually exclusive explanation for the decreased efficacy of APC loss in quickly causing tumors in humans is that mutations in APC may only rarely occur in stem cells. Differentiated cells may actually be the cells that develop the vast majority of mutations. As mentioned above, loss of APC may generate high levels of nuclear β-catenin, but it does not seem to cause differentiated cells to increase proliferative activity. Thus, another event needs to occur for those mutations to drive neoplastic lesions. It is possible that differentiated cells acquiring APC mutations must be also induced to dedifferentiate to unmask the mutation. From this point of view, a stem cell with mutant APC is still required to start a tumor, but the mutation driving the tumor occurs first in a differentiated cell that then dedifferentiates to become a stem cell. Note that, if it is true that the route to tumor is, according to that logic, differentiated cell→stem cell→tumor that does not mean that the direct stem cell→tumor route doesn’t also occur (i.e., these two routes are not mutually exclusive). It may just be that the direct route is far rarer because stem cells are somehow normally much more resistant to mutation than differentiated cells.
Human evidence supports either possible route. Familial Adenomatous Polyposis (FAP) patients, who carry a germline APC mutation, develop hundreds of intestinal polyps early in life and, if the bowel is not removed, some of these will progress into colorectal cancer (CRC). Analysis of resected bowels show multiple monocrypt adenomas where it is clear that an entire crypt is now composed of APC-deficient cells, more suggestive of direct stem cell transformation (142). Evidence also exists that these can spread via crypt fission into neighboring crypts. In contrast, there is also the “TOP DOWN” model of CRC which has been hypothesized due to the observation that adenomas often form on the surface of the colorectal lumen on top of a number of “normal” looking crypts (143). Importantly, sequencing of the tumor compared to the normal crypts underlying the tumor, showed that although the tumor carried an APC mutation, the underlying crypts did not. It should be noted that these studies were performed 10 years ago and it would be interesting to repeat with the latest sequencing technology.
As described above, there are now a multitude of studies that have shown that deletion of Apc within stem cells rapidly causes tumors. However there are relatively few where APC loss in other cell lineages within the intestine drives tumorigenesis. In part, this may be technical, given intestinal turnover is 3–5 days for the bulk of the differentiated cells (enterocytes and goblet cells, in other words). Thus, by the time it takes to drive Cre recombinase into the nucleus, find the loxP-flanked locus, mediate recombination and deletion of the gene to stop Apc transcription and turnover all extant protein (APC is an abundant, cytoskeleton-associated protein), many of these recombined cells would be lost from the tissue. However, interestingly, those same constraints (i.e. from mutation to loss of protein to phenotype) would also be imposed on human cells dedifferentiating to form a tumor too.
It should also be noted that it is possible that the initiating APC mutation may be in a stem cell producing an APC+/− crypt which would phenotypically be wild type. The key second APC mutation then would have to occur in a progeny cell that could thereafter form a tumor either 1) because the second APC mutation drives dedifferentiation alone or, 2) as we mentioned above, because the cell is stimulated to dedifferentiate by injury or chance alone, thereby unmasking the mutation because that cell has now become a functional stem cell that lacks APC (Fig. 3). Our initial studies suggested that this may be possible at least in mouse. Deletion of Apc in the non-stem compartment of mice through dietary Cre induction led to mice developing numerous lesions often at the crypt-villus junction (i.e., not in a differentiated cell zone, not in the crypt where the normal stem cells are) (141). In contrast to stem cell deletion, these did not rapidly form tumors but were able to form small lesions. Over a protracted timecourse, subsets of these lesions were able to form adenomas. These data were recently confirmed using a Cre recombinase which is inducible in all intestinal cells apart from intestinal stem cells (144). XBP1 is an enzyme responsible for responding to stress particularly within endoplasmic reticulum, but, under normal conditions, its expression is greatly induced during differentiation of secretory cells (like Paneth cells in the intestine, chief cells in the stomach, and acinar cells in the pancreas (11, 145, 146). Loss of XBP1 causes failure of stem cells to differentiate completely to Paneth cells in intestine and chief cells in the intestine. The generation of Xbp1CreERT2 thus allowed the Wnt pathway to be activated in the non-stem cell compartment, in particular in secretory cells. Wnt activation in Xbp1-expressing cells was incompatible with rapid tumor formation; however, when APC was lost in differentiated cells in the setting of activation of the NFκB pathway, it greatly increased capacity to form tumors from differentiated cells. The likely interpretation is that, similar to the inflammation-mediated induction of dedifferentiation in pancreas and stomach, NFκB signaling induced dedifferentiation of the mature cells with lost APC, thereby unmasking the constitutive Wnt activation in a stem cell(144). Thus, this study provided definitive in vivo proof that activating pathways in differentiated cells that can confer stem cell properties can facilitate dedifferentiation and cancer. A very recent study has shown a complementary finding when investigating the capacity of differentiated tuft cells to form tumors. Once again, loss of APC alone in this population was not sufficient to drive tumorigenesis (127). However, when these mice were treated with the inflammatory agent DSS (which would induce NF-KB signaling), the mice then developed colon tumors, showing that this population of cells could dedifferentiate to form tumors.
Signaling networks regulating dedifferentiation
That the NFκB pathway might be involved in dedifferentiation of cells en route to intestinal cancer may not be surprising. This pathway is often activated in colorectal, gastric, and pancreatic carcinoma by inflammation and other mutation events. NFκB can be activated by KRAS mutation, which information from the Cancer Genome Atlas (TCGA) reveals is mutated in up to 40% of CRC(147). Thus, there is a clear parallel between dedifferentiation and carcinogenesis of intestinal cells and acinar cells, because, as discussed, KRAS is a hub for ADM, and NFκB also plays a role. Also, the vast majority of PDACs have activating KRAS mutations. Mice carrying both APC and KRAS mutations often develop lesions that look like the “top-down” morphology and have villus lesions within the small intestine. Proof of principle studies in culture showed that villi extracted from wild type and, more importantly, APC deficient mice were unable to grow and form spheroids (144). However, when APC was deleted and KRAS mutated to its constitutively active, G12D form, villi from these mice dedifferentiated to produce spheroids, which could then be cultured unlimitedly and transplanted to form tumors in nude mice. Importantly, during the dedifferentiation process there was now expression of stem cell markers like OLFM4 and LGR5 which were completely absent from the villus. Taken together, these studies showed that dedifferentiation could occur to allow tumorigenesis in intestinal models. However, again, it should be noted these were all done predominantly using mouse models of small intestinal tumorigenesis, albeit with pathways that are altered in human colorectal carcinoma. It will be important for future work to elucidate markers of differentiated cells that remain on post dedifferentiation (if they exist) to aid assessment of whether this may occur in human CRC.
Thus far we have discussed only sporadic cancer driven by increased Wnt signaling, via APC loss, which, although the most common route to cancer (80%), does not account for all colorectal cancers. Recent data suggest that CRC developing potentially from adenomas with so-called serrated morphology have a poor prognosis but lack APC mutation. Interestingly, targeting either mutant, activated KRAS or BRAF into the mouse intestine cells (similar to the pancreas) does not lead to cancer until very long latencies so the cell of origin for this cancer is still unclear (148). However, we can learn much about the plasticity of the cell of origin of colorectal tumors from other mutations that confer familial polyposis. Foremost in these are the epithelial hamartomas formed in Peutz-Jeghers syndrome (PJS) and Cowden disease. Hamartomas contain many secretory cells and are often benign. Targeting the mutations that predispose to the disease LKB1 (PJS) or PTEN (Cowdens) specifically to the intestinal epithelium does not lead to rapid polyposis whilst constitutive heterozygotes develop polyps (149). Elegant work targeting LKB1 deletion to intestinal fibroblasts has shown that these are very efficient at forming intestinal tumors, remarkably suggesting that the cells of origin of these tumors may be mesenchymal cells that are transdifferentiating (150, 151). The most convincing data that dedifferentiation may occur in humans comes from studies investigating hereditary mixed polyposis syndrome. For many years the locus that conferred the disorder was unknown but this year it was mapped to a duplication in gremlin (GREM1). Gremlin was already of interest to sporadic CRC as it has been identified as leading to an increase risk of CRC in genomic wide association studies. Functionally, Gremlin is an inhibitor of BMP signaling which is a known negative regulator of both intestinal stem cells and intestinal crypt proliferation. A role for Gremlin in dedifferentiation was only discovered last year but it is of interest to note that a number of years ago, the Clevers laboratory had noticed that overexpression of another BMP antagonist Noggin produced a “crypt in villus” phenotype not dissimilar to the phenotype described above (152). Moreover, Noggin (or Gremlin) is required in crypt culture organoids for crypts to remain viable over several passages, again suggesting that inhibiting BMP signaling is required to maintain ‘stemness’ (112), which may also be observed in stomach, as chief cells reprogram during loss of BMP2 and BMP4 signaling (153, 154). However, the relevance of this to human tumorigenesis was unclear. Importantly, now with these recent studies, both the allele of Gremlin that increases risk and the duplication lead to high levels of epithelial Gremlin (normally it is only within the mesenchyme) (155, 156). Overexpression of Gremlin within murine intestinal epithelium leads to a “crypt in villus” structure, as following Noggin expression, and ultimately to tumors that resemble those in Hereditary Mixed Polposis Syndrome (HMPS) patients. Proof of dedifferentiation was again shown in culture where villi were cultured from Villin-Gremlin and Villin Gremlin APCMin/+ mice, and these showed the capacity to form organoids in vivo whilst other villi died. Thus, these studies all show the capacity of “non stem cells” to form tumors. One question that remains from these studies is whether these “crypts in the villus” phenomena (and crypt like structure at the top of colorectal crypts) is whether the formation of a new niche is a critical component required for tumorigenesis? Thus, by producing a crypt-like niche, there is almost a normal differentiation process occurring but in the incorrect position. How wild type intestinal enterocytes then interact with these niches and whether they are exposed to different environmental cues (e.g. closer to the gut microbiota so potentially different interactions with the immune system and/or inflammation) from those normal stem cells receive will need to be a subject of further investigation.
THOUGHTS AND QUESTIONS ABOUT POTENTIAL COMMON FEATURES OF DEDIFFERENTIATION AS A PROCESS IN MULTIPLE ORGANS
Are there genes or signaling pathways that are specific to reprogramming itself and are cell-lineage and tissue-independent?
The signaling pathway that may be the top a contender for a universal genetic reprogramming module is the one governed by KRAS. KRAS’s key role in acinar dedifferentiation has been well described, as detailed above. Chief cells in the stomach will also likely dedifferentiate following KRAS activation, and the metaplastic response in the stomach is already known to be p-ERK1/2 dependent(104). In intestine, KRAS mutation alone modifies differentiation: suppressing Paneth cell formation but increasing proliferative and goblet cell lineages. However, in combination with APC mutation, it can drive dedifferentiation (157).
Whether to undergo transdifferentiation or dedifferentiation, a reprogramming cell must first scale down its existing cellular architecture. That would seem to indicate that there will be processes that mediate scaling down that might be conserved in all cells that must reprogram. Given that there are gene cassettes like Xbp1→Mist1 that long-lived, protein-secreting cells of various embryonic origin and lineage globally use to scale up rER and the secretory apparatus(11, 65, 82, 145), it stands to reason that the downscaling is also conserved and might depend on decreased Mist1 and Xbp1 and other Scaling Factors. The cell-types that can dedifferentiate to cause adenomas in the small intestine are not entirely clear, though Paneth cells and intermediate, secretory cell progenitors, which could also be the DLL+ cells described above, also express MIST1. Wnt signaling may be key in promoting proliferation of intestinal cells with APC mutations, but it does not seem to be important in the dedifferentiation process in the intestine, and definitely does not seem to be in the stomach or pancreas. BMI1 may mark cells with the capacity to reprogram in both pancreas and intestine but hasn’t been explored in stomach. Finally, DCLK1-positive cells expand during injury or metaplasia in all three tissues (38, 158, 159). What their role is remains to be explored.
Must cells dedifferentiate to become stem cells before they can regenerate other mature lineages in the same tissue?
This question can be rephrased: Do mature cells have to dedifferentiate to give rise to cells of other lineages or can they directly transdifferentiate into those cells? It is clearly the case that mature cells can re-acquire progenitor features, but it isn’t clear that they all re-enter the cell cycle. As we perform more mathematically-temporally defined analyses of reprogramming and proliferation, it is possible we will discover that individual cells in a regenerating tissue may reprogram their gene expression patterns to match that of progenitor cells without actually proliferating. They might do this, for example, if the progenitor cell population expresses genes that are more appropriate for wound healing (like Tff2, e.g.). If mature cells convert directly to another, post-mitotic, adult cell-type, then that is certainly “transdifferentiation”, but what is it if they acquire embryonic progenitor markers but don’t proliferate? Is that dedifferentiation or transdifferentiation? Kinetic studies to track the fate of each reprogramming cell in a tissue have not been published. It is also not clear how much cells have to downscale to proliferate (e.g., for acinar cells in the pancreas with large rER networks and apical cytoplasms filled with secretory granules, how much of that elaborate secretory architecture must be dismantled for them to proliferate to regenerate damaged tissue?). Summing up these questions with the Waddington ball analogy, does the ball have to roll up the hill to come back down in a new groove, or can it simply stay on the bottom of the hill and migrate out of its groove directly into a neighboring groove?
Is dedifferentiation just differentiation in reverse?
Stages of reprogramming in the stomach
The literature to date suggests that there are three molecular stages that characterize how gastric chief cells respond to atrophy of parietal cells (Fig. 2). Clearly, they reprogram gene expression at some point, and that event is what we, by definition, term the Metaplasia Stage, given that that is when lineage marker expression changes occur to the extent that would allow a pathologist to identify the cells as metaplastic. Loss of MIST1 precedes metaplasia in mice and humans, as does scaling down of the secretory granules containing digestive enzymes and the secretory architecture of the cell(74, 82). Thus, the first stage must involve scaling down the secretory apparatus (termed the Downscaling Stage in Fig. 2). We are not aware of published studies determining the relationship between the Metaplasia Stage and another stage that at least some reprogramming chief cells undergo, which is re-entry into the cell cycle (“Proliferative Metaplastic Stage”). Proliferation of SPEM cells derived from mature chief cells is a clear feature of toxin and Helicobacter induced chief cell reprogramming in mice(70). Our unpublished work shows that this largely occurs only after metaplasia in mice, though the patterns of metaplasia are far more varied in humans, and it is possible that some metaplasias do not involve any increase in proliferation. (70) Summarizing, in the stomach (and likely in pancreatic ADM), there are at least three potentially overlapping stages to reprogramming (Fig. 2): 1) Downscaling, 2) Metaplasia and 3) Proliferation. The evidence that this is the case is also teleological: cells with elaborate rER and secretory granules containing digestive enzymes (like acinar and chief cells) must turn over the secretory apparatus if they are to avoid releasing their granule contents into the cytoplasm to damage themselves and other cells. Thus, the downscaling stage involves turning over these now superfluous cellular components so that the cells can become more like progenitor cells. The metaplastic stage is when dedifferentiating cells express genes of a new lineage, or, in the specific case of dedifferentiation, re-express progenitor markers (e.g., in the stomach, dedifferentiating chief cells re-express TFF2 that had been expressed in the neck cell precursor and in embryonic progenitors). The most controversial stage at this point in the stomach is Redifferentiation, which we have, with a “?”, designated Stage IV (Fig. 2). This would be expected to occur after proliferation, as the cells will have expanded to replace lost cells during the injury phase, and the injury, now cleared, would be permissive for recrudescence of cells lost. Chief cells do have the capacity to give rise to other lineages(71), but how broadly this occurs is even less well understood than redifferentiation in the pancreas, discussed at length above. Whether chief cells giving rise to other lineages, if this occurs frequently, do so always via dedifferentiation to a fully multipotent stem cell similar to the normal isthmal stem cell, or whether they do so via some other mechanism, again, can only be speculated about at this time.(71).
Are “reserve stem cells” mature cells that dedifferentiate to regenerate other lineages?
In pancreas, where there seems to be no stem cells dedicated to daily repopulation of the tissue, it makes sense that differentiated cells would have to play the stem cell role if called upon. In stomach, there is a constitutively active stem cell, but, at least in the main portion (body) of the stomach, it is close to the surface. Parietal cells lost deep in the gland might be more efficiently replaced by chief cells in the same deep zone. Thus, parietal cell atrophy could induce proliferation in two zones: one in the isthmus where the normal stem cell increases proliferation to make up for lost cells and one in the base, where chief cells re-enter the cell cycle to do the same. More work will need to be done to determine whether either proliferative center is required or sufficient to give the normal regeneration of lineages in reversibly models of atrophy. Within the intestine, outside of the stem cells, few cells have long life expectancy, and, thus, a “reserve” stem cell population would need to escape the rapid turnover of most intestinal epithelial cells (114). Secretory precursors and mature Paneth cells which reside at the base of the crypt are long-lived (upwards of 5 weeks) (160), and recent studies have shown that they represent an excellent candidate for a reserve stem population or an overlapping niche with the intestinal stem cells, it makes sense that, following the loss of the stem cells, they repopulate this niche and acquire stem cell properties.
Is the capacity to dedifferentiate restricted to certain lineages?
In stomach, the two principal, long-lived cells are parietal cells and chief cells. Mature parietal cells do not seem to have the capacity – at least not in any injury model system studied so far – to be capable of reprogramming (94). Rather, when there is damage, they seem to undergo apoptosis and are the cells that relay signals to chief cells to dedifferentiate (69, 72, 94, 105, 161). In pancreas, the acinar cell may be the principal lineage with dedifferentiation capacity: β-islet cells have the capacity to self renew (35), but β cells don’t seem to dedifferentiate to rapidly proliferate and serve as stem cells to repair the tissue. In intestine, the dedifferentiating cell population is not clear, so it is too early to speculate about which cells can and can’t do this. In short, there may be some cell lineages better equipped to downscale, undergo metaplasia, and then proliferate and expand to repair tissue damage. Perhaps cells that use a transcription factor like MIST1 that seems to be able to coordinate rapid scaling up or down of subcellular structures might be better equipped to dedifferentiate rapidly when needed.
Why might reprogramming increase risk for cancer?
The process of reprogramming or dedifferentiation may itself be dangerous. Perhaps the mechanics of all the chromatin remodeling to re-express progenitor- and proliferation- associated genes exposes cells to increased risk for mutation. Given we don’t understand how reprogramming occurs, we can only speculate at this point. Or perhaps, as discussed above and illustrated in Fig. 3, long-lived differentiated cells can harbor old mutations, whereas rapidly turning over cells, like constitutively active stem cells, would not keep a single mutant allele in a single cell for long before it would be apportioned to a differentiating daughter and lost to drift. In the case of genes that promote growth only with homozygous loss of function (e.g. p53, APC), it would be unlikely that such rapidly dividing cells would have the time to acquire a mutation in both alleles (Fig. 3). Differentiated cells, on the other hand, may acquire second mutations in tumor suppressors simply because they live longer. Or they might acquire activating, dominant mutations like G12D KRAS without triggering apoptosis because KRAS activation alone doesn’t cause dedifferentiation or increased proliferation. Thus, such mutations would be harmless, unless the cells were called to dedifferentiate and re-enter the cell cycle, at which point they would be unmasked. The possibility that differentiated cells might be better candidates for cells of origin for cancer was also recently discussed by Chaffer and Weinberg(162).
Gloss.
Over the past 5 years there has been increasing awareness that the canonical models of terminal differentiation need re-evaluation. First, it became clear that adult mammalian cells could be dedifferentiated in vitro into pluripotent cells demonstrating the plasticity of adult cells. Now, it is evident that mature epithelial cells can also reprogram in vivo to contribute to repair or regeneration, and, aberrantly, to cancer. This review compares three gastrointestinal epithelial tissues (pancreas, intestine and stomach) to draw insights into the molecular basis of reprogramming as a basic cellular process, akin to mitosis or apoptosis, and to highlight potential functional and dysfunctional roles reprogramming cells play in tissues.
Acknowledgments
OS is funded by a CRUK core grant and an ERC investigator grant COLONCAN. JCM is funded by RO1DK094989 from the NIH NIDDK, 7859-77 from the Barnes Hospital Foundation Cancer Frontier Fund, and RSG-12-171-01-LIB from the American Cancer Society. Thanks to Joseph Burclaff and Dr. Spencer Willet for their comments on manuscript draft.
References
- 1.Waddington CH. The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology. London: Geoge Allen & Unwin; 1957. [Google Scholar]
- 2.Hay ED. The fine structure of blastema cells and differentiating cartilage cells in regenerating limbs of Amblystoma larvae. J Biophys Biochem Cytol. 1958;4:583–591. doi: 10.1083/jcb.4.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashanov VS, Zueva OR, Garcia-Arraras JE. Expression of pluripotency factors in echinoderm regeneration. Cell Tissue Res. 2015;359:521–536. doi: 10.1007/s00441-014-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosher C. Observation on evisceration and visceral regeneration in the sea-cucumber, Actinopyga agassizi Selenka. Zoologica (NY) 1956;41:17–26. [Google Scholar]
- 5.Zipori D. The stem state: plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells. 2005;23:719–726. doi: 10.1634/stemcells.2005-0030. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 9.Murtaugh LC, Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol. 2015;77:229–249. doi: 10.1146/annurev-physiol-021014-071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri S, Folias AE, Hebrok M. Plasticity and dedifferentiation within the pancreas: development, homeostasis, and disease. Cell Stem Cell. 2015;16:18–31. doi: 10.1016/j.stem.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology. 2011;141:1463–1472. doi: 10.1053/j.gastro.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Direnzo D, Hess DA, Damsz B, Hallett JE, Marshall B, Goswami C, Liu Y, Deering T, Macdonald RJ, Konieczny SF. Induced Mist1 expression promotes remodeling of mouse pancreatic acinar cells. Gastroenterology. 2012;143:469–480. doi: 10.1053/j.gastro.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004;121:261–272. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi G, DiRenzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950–1958. doi: 10.1038/onc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folias AE, Penaranda C, Su AL, Bluestone JA, Hebrok M. Aberrant innate immune activation following tissue injury impairs pancreatic regeneration. PLoS One. 2014;9:e102125. doi: 10.1371/journal.pone.0102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iovanna JL, Lechene de la Porte P, Dagorn JC. Expression of genes associated with dedifferentiation and cell proliferation during pancreatic regeneration following acute pancreatitis. Pancreas. 1992;7:712–718. doi: 10.1097/00006676-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Pinho AV, Rooman I, Reichert M, De Medts N, Bouwens L, Rustgi AK, Real FX. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Sans MD, Hou Y, Ernst SA, Williams JA. c-Jun/AP-1 is required for CCK-induced pancreatic acinar cell dedifferentiation and DNA synthesis in vitro. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1381–G1396. doi: 10.1152/ajpgi.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J, Bertrand L, Heimberg H, Konieczny SF, Dor Y, Lemaigre FP, Jacquemin P. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut. 2012;61:1723–1732. doi: 10.1136/gutjnl-2011-300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a–expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, Wells JM, Crawford HC. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146:233–244. doi: 10.1053/j.gastro.2013.08.053. e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevot PP, Augereau C, Simion A, Van den Steen G, Dauguet N, Lemaigre FP, Jacquemin P. Let-7b and miR-495 Stimulate Differentiation and Prevent Metaplasia of Pancreatic Acinar Cells by Repressing HNF6. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Lechene de la Porte P, Iovanna J, Odaira C, Choux R, Sarles H, Berger Z. Involvement of tubular complexes in pancreatic regeneration after acute necrohemorrhagic pancreatitis. Pancreas. 1991;6:298–306. doi: 10.1097/00006676-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Bockman DE, Boydston WR, Anderson MC. Origin of tubular complexes in human chronic pancreatitis. Am J Surg. 1982;144:243–249. doi: 10.1016/0002-9610(82)90518-9. [DOI] [PubMed] [Google Scholar]
- 30.Houbracken I, de Waele E, Lardon J, Ling Z, Heimberg H, Rooman I, Bouwens L. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141:731–741. doi: 10.1053/j.gastro.2011.04.050. 741 e731-734. [DOI] [PubMed] [Google Scholar]
- 31.Collins MA, Yan W, Sebolt-Leopold JS, Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology. 2014;146:822–834. doi: 10.1053/j.gastro.2013.11.052. e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer RL, Staley BK, Liou A, Hebrok M. Numb regulates acinar cell dedifferentiation and survival during pancreatic damage and acinar-to-ductal metaplasia. Gastroenterology. 2013;145:1088–1097. doi: 10.1053/j.gastro.2013.07.027. e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 34.Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development. 2010;137:2289–2296. doi: 10.1242/dev.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 36.Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. beta-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62:1634–1645. doi: 10.2337/db12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, Paredes J, Welsh C, Wiersch J, Gittes GK. No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, Maitra A, Leach SD. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137:2179–2180. doi: 10.1053/j.gastro.2009.06.072. author reply 2180-2171. [DOI] [PubMed] [Google Scholar]
- 40.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, Pal D, Briel T, Herner A, Trajkovic-Arsic M, Sipos B, Liou GY, Storz P, Murray NR, Threadgill DW, Sibilia M, Washington MK, Wilson CL, Schmid RM, Raines EW, Crawford HC, Siveke JT. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liou GY, Doppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc. 2014;47:212–216. doi: 10.5946/ce.2014.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666–G676. doi: 10.1152/ajpgi.2000.279.4.G666. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe S, Abe K, Anbo Y, Katoh H. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol. 1995;58:365–374. doi: 10.1679/aohc.58.365. [DOI] [PubMed] [Google Scholar]
- 45.Baumgart M, Werther M, Bockholt A, Scheurer M, Ruschoff J, Dietmaier W, Ghadimi BM, Heinmoller E. Genomic instability at both the base pair level and the chromosomal level is detectable in earliest PanIN lesions in tissues of chronic pancreatitis. Pancreas. 2010;39:1093–1103. doi: 10.1097/MPA.0b013e3181dc62f6. [DOI] [PubMed] [Google Scholar]
- 46.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D–induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb Symp Quant Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- 50.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, Kloppel G, Lauwers GY, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Perez-Gallego L, Redston M, Tuveson DA. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–6374. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, Logsdon CD. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33:532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mallen-St Clair J, Soydaner-Azeloglu R, Lee KE, Taylor L, Livanos A, Pylayeva-Gupta Y, Miller G, Margueron R, Reinberg D, Bar-Sagi D. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012;26:439–444. doi: 10.1101/gad.181800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 58.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A. 2007;104:19327–19332. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao T, Zhou D, Yang C, Singh T, Penzo-Mendez A, Maddipati R, Tzatsos A, Bardeesy N, Avruch J, Stanger BZ. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–1553. doi: 10.1053/j.gastro.2013.02.037. e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukuda A, Morris JPt, Hebrok M. Bmi1 is required for regeneration of the exocrine pancreas in mice. Gastroenterology. 2012;143:821–831. doi: 10.1053/j.gastro.2012.05.009. e821-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci U S A. 2009;106:7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mills JC, Taghert PH. Scaling factors: transcription factors regulating subcellular domains. Bioessays. 2012;34:10–16. doi: 10.1002/bies.201100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 68.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 69.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. e2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 73.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 74.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The Transcription Factor MIST1 Is a Novel Human Gastric Chief Cell Marker Whose Expression Is Lost in Metaplasia, Dysplasia, and Carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 76.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 77.Hanby AM, Poulsom R, Playford RJ, Wright NA. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–337. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 78.Geahlen JH, Lapid C, Thorell K, Nikolskiy I, Huh WJ, Oates EL, Lennerz JK, Tian X, Weis VG, Khurana SS, Lundin SB, Templeton AR, Mills JC. Evolution of the human gastrokine locus and confounding factors regarding the pseudogenicity of GKN3. Physiol Genomics. 2013;45:667–683. doi: 10.1152/physiolgenomics.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menheniott TR, Peterson AJ, O’Connor L, Lee KS, Kalantzis A, Kondova I, Bontrop RE, Bell KM, Giraud AS. A novel gastrokine, Gkn3, marks gastric atrophy and shows evidence of adaptive gene loss in humans. Gastroenterology. 2010;138:1823–1835. doi: 10.1053/j.gastro.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 80.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 81.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Capoccia BJ, Jin RU, Kong YY, Peek RM, Jr, Fassan M, Rugge M, Mills JC. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest. 2013;123:1475–1491. doi: 10.1172/JCI65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PubMed] [Google Scholar]
- 84.Nookaew I, Thorell K, Worah K, Wang S, Hibberd ML, Sjovall H, Pettersson S, Nielsen J, Lundin SB. Transcriptome signatures in Helicobacter pylori-infected mucosa identifies acidic mammalian chitinase loss as a corpus atrophy marker. BMC Med Genomics. 2013;6:41. doi: 10.1186/1755-8794-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nomura S, Settle SH, Leys CM, Means AL, Peek RM, Jr, Leach SD, Wright CV, Coffey RJ, Goldenring JR. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier’s disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 86.Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- 87.Sakai H, Eishi Y, Li XL, Akiyama Y, Miyake S, Takizawa T, Konishi N, Tatematsu M, Koike M, Yuasa Y. PDX1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut. 2004;53:323–330. doi: 10.1136/gut.2003.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981;241:G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- 89.Johnson LR. Functional development of the stomach. Annu Rev Physiol. 1985;47:199–215. doi: 10.1146/annurev.ph.47.030185.001215. [DOI] [PubMed] [Google Scholar]
- 90.Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol. 1997;272:G1209–G1220. doi: 10.1152/ajpgi.1997.272.5.G1209. [DOI] [PubMed] [Google Scholar]
- 91.Osaki LH, Curi MA, Alvares EP, Gama P. Early weaning accelerates the differentiation of mucous neck cells in rat gastric mucosa: possible role of TGFalpha/EGFR. Differentiation. 2010;79:48–56. doi: 10.1016/j.diff.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–G1251. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 96.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE, Jr, Peek RM, Jr, Goldenring JR. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. 2210 e2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M, Muller A. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108:14944–14949. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsumoto Y, Marusawa H, Kinoshita K, Niwa Y, Sakai Y, Chiba T. Up-regulation of activation-induced cytidine deaminase causes genetic aberrations at the CDKN2b-CDKN2a in gastric cancer. Gastroenterology. 2010;139:1984–1994. doi: 10.1053/j.gastro.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 100.Machado AM, Figueiredo C, Seruca R, Rasmussen LJ. Helicobacter pylori infection generates genetic instability in gastric cells. Biochim Biophys Acta. 2010;1806:58–65. doi: 10.1016/j.bbcan.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Umeda M, Murata-Kamiya N, Saito Y, Ohba Y, Takahashi M, Hatakeyama M. Helicobacter pylori CagA causes mitotic impairment and induces chromosomal instability. J Biol Chem. 2009;284:22166–22172. doi: 10.1074/jbc.M109.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 103.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA, Wright NA. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 104.Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, Noto J, Peek RM, Jr, Stenson WF, Mills JC. The Hyaluronic Acid Receptor CD44 Coordinates Normal and Metaplastic Gastric Epithelial Progenitor Cell Proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 106.Gupta A, Wodziak D, Tun M, Bouley DM, Lowe AW. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J Biol Chem. 2013;288:4321–4333. doi: 10.1074/jbc.M112.433086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sashikawa Kimura M, Mutoh H, Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J Gastroenterol. 2011;46:1292–1299. doi: 10.1007/s00535-011-0443-5. [DOI] [PubMed] [Google Scholar]
- 108.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146:1727–1738. doi: 10.1053/j.gastro.2014.02.007. e1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Zaatari M, Kao JY, Tessier A, Bai L, Hayes MM, Fontaine C, Eaton KA, Merchant JL. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One. 2013;8:e58935. doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nozaki K, Weis V, Wang TC, Falus A, Goldenring JR. Altered gastric chief cell lineage differentiation in histamine-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1211–G1220. doi: 10.1152/ajpgi.90643.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weis VG, Petersen CP, Mills JC, Tuma PL, Whitehead RH, Goldenring JR. Establishment of novel in vitro mouse chief cell and SPEM cultures identifies MAL2 as a marker of metaplasia in the stomach. Am J Physiol Gastrointest Liver Physiol. 2014;307:G777–G792. doi: 10.1152/ajpgi.00169.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 113.Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511–515. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 115.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 117.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature cell biology. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 118.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 119.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 120.Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer. 2014;14:468–480. doi: 10.1038/nrc3744. [DOI] [PubMed] [Google Scholar]
- 121.Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. The EMBO journal. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature cell biology. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111–G1132. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, Fodde R. Paneth cells in intestinal homeostasis and tissue injury. PLoS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 127.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. The Journal of clinical investigation. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, Neufeld K, Clevers H, Brunton V, Winton DJ, Wang X, Sears RC, Clarke AR, Frame MC, Sansom OJ. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Developmental cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell stem cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 130.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 131.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, Romagnolo B. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 133.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 134.Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D, Timpson P, Vidal M, Murray GI, Greten FR, Anderson KI, Sansom OJ. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell stem cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stringari C, Edwards RA, Pate KT, Waterman ML, Donovan PJ, Gratton E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Scientific reports. 2012;2:568. doi: 10.1038/srep00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA, Edwards RA, Gratton E, Waterman ML. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. The EMBO journal. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hardiman KM, Liu J, Feng Y, Greenson JK, Fearon ER. Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS One. 2014;9:e96023. doi: 10.1371/journal.pone.0096023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, Jones C, Radulescu S, Huels DJ, Myant KB, Dudek KM, Casey HA, Scopelliti A, Cordero JB, Vidal M, Pende M, Ryazanov AG, Sonenberg N, Meyuhas O, Hall MN, Bushell M, Willis AE, Sansom OJ. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature. 2014 doi: 10.1038/nature13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 142.Preston SL, Wong WM, Chan AO, Poulsom R, Jeffery R, Goodlad RA, Mandir N, Elia G, Novelli M, Bodmer WF, Tomlinson IP, Wright NA. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer research. 2003;63:3819–3825. [PubMed] [Google Scholar]
- 143.Shih IM, Wang TL, Traverso G, Romans K, Hamilton SR, Ben-Sasson S, Kinzler KW, Vogelstein B. Top-down morphogenesis of colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2640–2645. doi: 10.1073/pnas.051629398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 145.Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139:2038–2049. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rad R, Cadinanos J, Rad L, Varela I, Strong A, Kriegl L, Constantino-Casas F, Eser S, Hieber M, Seidler B, Price S, Fraga MF, Calvanese V, Hoffman G, Ponstingl H, Schneider G, Yusa K, Grove C, Schmid RM, Wang W, Vassiliou G, Kirchner T, McDermott U, Liu P, Saur D, Bradley A. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer cell. 2013;24:15–29. doi: 10.1016/j.ccr.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, Clarke AR. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet. 2008;40:1436–1444. doi: 10.1038/ng.256. [DOI] [PubMed] [Google Scholar]
- 150.Katajisto P, Vaahtomeri K, Ekman N, Ventela E, Ristimaki A, Bardeesy N, Feil R, DePinho RA, Makela TP. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nature genetics. 2008;40:455–459. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- 151.Zac-Varghese S, Trapp S, Richards P, Sayers S, Sun G, Bloom SR, Reimann F, Gribble FM, Rutter GA. The Peutz-Jeghers kinase LKB1 suppresses polyp growth from intestinal cells of a proglucagon-expressing lineage in mice. Disease models & mechanisms. 2014;7:1275–1286. doi: 10.1242/dmm.014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 153.Takabayashi H, Shinohara M, Mao M, Phaosawasdi P, El-Zaatari M, Zhang M, Ji T, Eaton KA, Dang D, Kao J, Todisco A. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406. doi: 10.1053/j.gastro.2014.04.015. e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, Eaton KA, Samuelson LC, Merchant JL, Goldenring JR, Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. e2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C, Jaeger E, Lewis A, Freeman-Mills L, Giner FC, Rodenas-Cuadrado P, Mallappa S, Clark S, Thomas H, Jeffery R, Poulsom R, Rodriguez-Justo M, Novelli M, Chetty R, Silver A, Sansom OJ, Greten FR, Wang LM, East JE, Tomlinson I, Leedham SJ. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nature medicine. 2014 doi: 10.1038/nm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lewis A, Freeman-Mills L, de la Calle-Mustienes E, Giraldez-Perez RM, Davis H, Jaeger E, Becker M, Hubner NC, Nguyen LN, Zeron-Medina J, Bond G, Stunnenberg HG, Carvajal JJ, Gomez-Skarmeta JL, Leedham S, Tomlinson I. A polymorphic enhancer near GREM1 influences bowel cancer risk through differential CDX2 and TCF7L2 binding. Cell reports. 2014;8:983–990. doi: 10.1016/j.celrep.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feng Y, Bommer GT, Zhao J, Green M, Sands E, Zhai Y, Brown K, Burberry A, Cho KR, Fearon ER. Mutant KRAS promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology. 2011;141:1003–1013. doi: 10.1053/j.gastro.2011.05.007. e1001-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Li L, Sureban SM, Houchen CW. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 2014;32:822–827. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 160.Ireland H, Houghton C, Howard L, Winton DJ. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:1332–1336. doi: 10.1002/dvdy.20446. [DOI] [PubMed] [Google Scholar]
- 161.Nitsche H, Ramamoorthy S, Sareban M, Pausawasdi N, Todisco A. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G607–G614. doi: 10.1152/ajpgi.00194.2006. [DOI] [PubMed] [Google Scholar]
- 162.Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov. 2015;5:22–24. doi: 10.1158/2159-8290.CD-14-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]