Abstract
Three adjacent single nucleotide polymorphisms of the vitamin D receptor gene (VDR) BsmI (rs1544410), ApaI (rs7975232), and TaqI (rs731236) are commonly studied in several pathologies. We aimed to evaluate the distribution of VDR BsmI, ApaI, and TaqI allele, genotype, and haplotype frequencies in an Italian cohort of 266 patients with lumbar spine disorders assessed by Magnetic Resonance Imaging and 252 asymptomatic controls. The exposure to putative risk factors was evaluated by a questionnaire. Polymorphisms were detected by PCR-RFLP and TaqMan® SNP Genotyping Assay. The results were statistically adjusted for the identified conventional risk factors. The three SNPs were in linkage disequilibrium. For all cases BbAaTT was a 3-fold risk factor OR = 3.38), whereas bbAATT (OR = 0.22), and bbaaTT (OR = 0.47) genotypes were found to be protective. Specifically, for patients affected by disc herniation only (n = 88) and all lumbar pathologies excluding stenosis and/or spondylolistesis (n = 215) B allele, Bb, Aa, and BbAaTT genotypes were risky, whereas b allele, bb, aa, and bbaaTT genotypes were protective. In patients affected by osteochondrosis with or without disc hernation (n = 50), T allele, Aa, and bbAaTT genotypes were risky, whereas t allele, AA, tt genotypes were protective. In patients affected by stenosis and/or spondylolistesis (n = 51) no significant associations were found. This is the first study showing an association of the three genetic VDR variants BsmI, ApaI, and TaqI and lumbar spine pathologies. Our study contributes to delineate genetic risk factors for specific subgroups of patients with lumbar spine pathologies highlighting the importance of haplotype analysis, and of detailed clinical evaluation of the patients for identification of genetic biomarkers.
Introduction
BsmI (rs1544410), ApaI (rs7975232), and TaqI (rs731236) are adjacent restriction fragment length polymorphisms (RFLP) located in the region of intron 8/exon 9 of the vitamin D receptor gene (VDR), which has been commonly associated with several diseases [1]. Furthermore, they have been also associated with lumbar disc degeneration (LDD) [2–4]. These three single nucleotide polymorphisms (SNPs), BsmI (G>A), ApaI (T>G), and TaqI (T>C), are located close to the 3’ terminus of the gene, and do not determine structural modification in the receptor [1]. There is no clear evidence about the effect of BsmI and ApaI variants in affecting the VDR activity, whereas TaqI is a synonymous SNP [1]. Nevertheless, some authors hypothesized some effects on mRNA stability [1,5]. These 3 SNPs were frequently reported to be in linkage disequilibrium (LD). Moreover, it has been suggested that they could be in LD with other polymorphisms affecting the VDR function, this could probably explains their association with several pathological phenotypes [1].
The majority of previous studies dealing with LDD focused specifically on the TaqI polymorphism. The first association between TaqI SNP and intervertebral disc disease was reported in Finnish males [6]. Men with the tt genotype had the lowest prevalence of disc bulges and fewer lumbar disc herniation in comparison with those with the TT genotype [7]. On the contrary, other three Finnish studies found no significant differences in the genotype frequencies of VDR TaqI between pathological and asymptomatic men, no significant association of the SNP with Modic changes [8,9] and with lumbar spinal stenosis [10].
Other studies in white Caucasian subjects analyzing TaqI SNP were conducted in Turks [11,12], Norwegians [13], and Australians [14]. In young Turks no significant differences were observed in TaqI allele frequencies between patients with hernia or disc degeneration and controls. However, the TT genotype was associated with milder forms, while tt genotype with more severe forms of disc degeneration. Specifically, TT and Tt genotypes were associated with disc herniation protrusion, whereas the tt genotype was associated with extrusion/sequestration [11]. Another study in Turks showed that the presence of VDR TaqI mutation was associated with a worse disc degeneration score, and with the development of lumbar disc degeneration [12].
A decrease in the presence of spinal osteophytosis and disc space narrowing from tt to TT genotypes was observed in Australians [14]. On the contrary, in a Norway case-control study no association between TaqI SNP and LDD was reported [13].
Three studies on the association of TaqI SNP with LDD were found in Asians [15–17]. In young Japanese the Tt genotype was associated with an increased risk of severe, multilevel disc degeneration and herniation and with an early age of the disease onset [15]. In contrast, the frequencies of T and t alleles was not associated with the presence of disc degeneration and osteophyte in elderly Japanese females [16]. In a Chinese study, involving individuals younger than 40 years, the t allele was associated with degenerative disc features and disc bulging [17].
Taking together these results concerning TaqI SNP showed no association of this SNP with LDD in more than half studies (6/10). Moreover inconsistent findings between studies were reported, showing an association of TT and tt genotypes in Caucasians, Tt genotype and t allele in Asians with signs of disc degeneration.
To our knowledge, the great majority of studies concerning lumbar spine pathologies analyzed the association with TaqI and FokI VDR polymorphisms [18], only 4 studies analyzed ApaI [15,19–21] and only 2 studies BsmI polymorphism [20,22]. None of the above mentioned studies performed the analysis of all the 3 RFLP BsmI, ApaI and TaqI polymorphisms at the same time.
The A allele of VDR ApaI was significantly associated with LDD with synergistic interaction between the allele and spine bending/twisting in a Chinese case-control study involving low back pain patients [19]. Recently, Zawilla et al. found an OR = 3.1 for the association of mutant ApaI and LDD [21]. Moreover, B allele of VDR BsmI and birthweight were associated with increasing severity of osteophyte grade in British men with lumbar spine osteoarthritis [22].
Concerning haplotypes, the BsmI-ApaI haplotypes were evaluated in Japanese postmenopausal females affected by lumbar spondylosis [20]. This study observed an association of BsmI-ApaI VDR haplotypes with the severity of the pathology that was more significant in the older (>63.6 years) than in the younger subjects [20]. However, differently from the majority of the studies, in the Japanese study the BsmI and ApaI RFLPs were not in LD.
Differences in ethnicity, inclusion criteria when comparing the same ethnic group, and a not clear definition of the clinical phenotypes are likely responsible for the inconsistent results obtained so far. It is also to note that the extent of LD among BsmI, ApaI and TaqI varies in different studies [1], so that the choice to examine only one polymorphism (e.g., TaqI) as representative of the other two polymorphisms can possibly be misleading.
Detailed analysis of specific subgroups of LDD patients was performed in an Italian case-control study published in 2014 by our group [18]. In that study the FF genotype and F allele of the VDR FokI SNP were associated with an approximately 2-fold risk to develop discopathies, and particularly discopathies and/or osteochondrosis concomitant with disc herniation [18]. Further, major gender-related effects were observed [23,24].
By analyzing the same cohort of LDD subjects of our previous studies [18, 23], the aim of the present investigation was to evaluate the distribution of VDR BsmI, ApaI and TaqI allele, genotype, and haplotype frequencies in patients with specifically defined lumbar spine pathologies in comparison with asymptomatic controls. To our knowledge this is the first study evaluating at the same time all the 3 adjacent polymorphisms in relation to LDD.
Material and Methods
Ethics Statement
The study was approved by the Institutional Review Board ASL Città di Milano, protocol number GENODISC01. The methods used in this study were in accordance with the Helsinki Declaration of 1975 as revised in 1996.
Subjects and clinical assessment
Based on a case-control design, 266 patients (outpatients or hospitalized) with lumbar spine disorders recruited for the European Genodisc Project, and 252 asymptomatic controls (most were healthy volunteers, some were blood donors, few were subjects hospitalized for anterior cruciate ligament injuries or hallux valgus surgery) were enrolled at the I.R.C.C.S. Istituto Ortopedico Galeazzi (Milan, Italy) as previously described [18]. In respect to our previous investigation [18], the number of controls was increased including 32 additional subjects to have a total number of controls close to cases.
All subjects signed a written informed consent. Cases and controls were enrolled from May 2009 to December 2013. Exclusion criteria for both cases and controls were: pathologic condition such as cervical discopathies, scoliosis, fibromyalgia, pregnancy at study enrollment, and chronic diseases like diabetes, cardiovascular diseases, malignancies, lupus erythematosus, and rheumatoid arthritis. Assessment of lumbar spine disorders and patient’s classification into 4 different mutually exclusive subgroups (designed from 1 to 4 subgroup) based on detailed diagnosis were performed by a clinician expert in spine diseases by contrast-enhanced MRI 12 scans of the lumbar spine with a 1.5 T scanner (Avanto, Siemens, Erlangen, Germany) as described in our previous paper [18].
Subgroup 1 comprised patients affected by disc herniation only; Subgroup 2 comprised patients affected by osteochondrosis associated or not with disc hernation; Subgroup 3 comprised patients affected by discopathies, osteochondrosis or both without disc herniation; Subgroup 4 comprised patients affected by stenosis, lytic/isthmic spondylolisthesis, and degenerative spondylolisthesis.
Due to the close linkage between discopathies, disc herniation and osteochondrosis, a further subgroups division from A to D (not mutually exclusive) was performed: Subgroup A, comprising all herniation cases i. e. Subgroup 1 grouped with Subgroup 2; Subgroup B, including all discopathies and/or osteochondrosis regardless of herniation, i.e. Subgroup 2 grouped with Subgroup 3; Subgroup C, comprising all discopathies concomitant with disc herniation grouped with subjects with discopathies alone; and Subgroup D, comprising all osteochondrosis concomitant with disc herniation grouped with subjects with osteochondrosis alone.
Table 1 reported information for all study subjects including family medical history (parents, brothers or sisters) about spine disorders, smoking habit, job physical demand for the majority of the working years (evaluated by the following score: 0 = sedentary; 1 = light; 2 = medium; 3 = heavy), and hours/day spent driving or as a passenger in motorized vehicles (exposure to vibrations).
Table 1. Characteristics of the study subjects and comparison of conventional and non-genetic risk factors of lumbar spine pathologies between controls and all cases or subgroups.
| Factors | Controls | All Cases | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | |
|---|---|---|---|---|---|---|---|
| n = 252 | n = 266 (100%) | n = 88 (33.1%) | n = 87 (32.7%) | n = 40 (15.0%) | n = 51 (19.2%) | ||
| Age (Years) | mean ± SD | 39.42±10.61 | 44.18±9.12*** | 42.36±9.30* | 43.69±8.90*** | 42.80±8.33* | 49.22±8.16*** |
| Gender | Males n (%) | 127 (50.4) | 148 (55.6) | 47 (53.4) | 55 (63.2)* | 21 (52.5) | 25 (49.0) |
| Females n (%) | 125 (40.6) | 118 (44.4) | 41 (46.6) | 32 (36.8) | 19 (47.5) | 26 (51.0) | |
| BMI (kg/m2) | mean ± SD | 24.19±3.78 | 25.29±4.06*** | 24.91±4.07 | 25.27±3.59** | 24.29±3.91 | 26.75±4.61*** |
| Family history | n (%) | 37 (14.7) | 97 (36.5)*** | 33 (37.5)*** | 38 (43.7)*** | 11 (27.5)* | 15(29.4)* |
| Past and present smoker | n (%) | 104 (41.3) | 144 (54.1)** | 53 (60.2)** | 50 (57.5)** | 16 (40.0) | 25 (49.0) |
| Physical job demand1 (Score 0–3) | mean ± SD | 1.06±1.00 | 1.37±1.08*** | 1.39±1.07* | 1.38±1.11* | 1.40±1.13 | 1.33±1.05 |
| Exposure to vibrations (Hours/day) | mean±SD | 1.40±1.16 | 2.20±2.56** | 2.33±2.90 | 2.29±2.43** | 2.25±2.80 | 1.78±1.90 |
1 5 patients had missing information about intensity of physical demand at work, thus a total of 261 data were available. Physical job demand score used: 0 = sedentary; 1 = light; 2 = medium; 3 = heavy.
Subgroup 1 = patients with disc herniation alone; Subgroup 2 = patients with discopathies and/or osteochondrosis associated with disc herniation; Subgroup 3 = patients with discopathies and/or osteochondrosis without herniation; Subgroup 4 = patients with stenosis and/or spondylolisthesis.
* P<0.05,
** P<0.01,
*** P<0.001
Determination of genotypes
Blood samples were collected from the antecubital vein with evacuated ethylenediamine tetra acetic acid (EDTA) tubes (Vacutainer Tubes, Becton-Dickinson, Franklin Lakes, NJ, USA) from cases and controls. Genomic DNA was extracted from white blood cells according to the procedure of the DNeasy Midi kit (Qiagen, Duesseldorf, Germany) as described [18]. Polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) methods were applied to detect the BsmI, ApaI, and TaqI polymorphisms of VDR. Genomic DNA was amplified using PCR. At first DNA was denatured at 95°C for 5 minutes. Standard PCR conditions for DNA amplification using appropriate primers [25] were as follows: 94°C for 1 minute, annealing temperature 64°C for 1 minute and 72°C for 2 minutes for 32 cycles and finally 96°C for 1 minute and 72°C for 5 minutes.
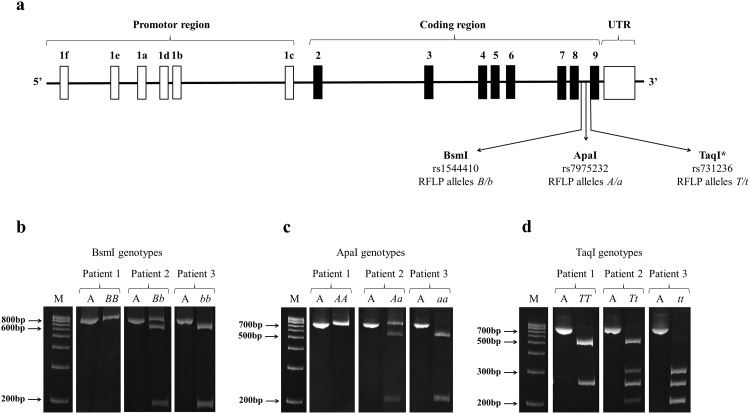
The BsmI, ApaI and TaqI polymorphisms of VDR were studied using previously tested primers [25] to amplify the first part of intron 8 for BsmI, and a fragment of intron 8/exon 9 for ApaI and TaqI (Fig 1). The allele digested by the restriction enzyme was denoted by a lower letter, whereas that not digested was indicated by a capital letter.
Fig 1.
Structure of the genomic region of the VDR and location of BsmI, ApaI and TaqI (*in the coding sequence, exon 9) SNPs located near the 3’ terminus of the gene (a). Representative gels for the determination of BsmI (b), ApaI (c) and TaqI (d) genotypes in three patients are showed. In the first lane there is a molecular weight DNA ladder (M) for size estimation of the DNA fragments. The letter “A” indicates the PCR amplicons which are of 825 bp for BsmI and 740 bp for ApaI and TaqI. After digestion of the PCR product with BsmI restriction enzyme an undigested 825 bp fragment (homozygous genotype BB), partially digested 825, 650 and 175 bp fragments (heterozygous genotype Bb), or totally digested 650 and 175 bp fragments (homozygous genotype bb) are present (b). After digestion of the PCR product with ApaI restriction enzyme an undigested 740 bp fragment (homozygous genotype AA), partially digested 740, 530 and 210 bp fragments (heterozygous genotype Aa), or totally digested 530 and 210 bp fragments (homozygous genotype aa) are present (c). After digestion of the PCR product with TaqI restriction enzyme, digested 495 and 245 bp fragments (homozygous genotype TT), partially digested 495, 290, 245 and 205 bp fragments (heterozygous genotype Tt), or totally digested 290, 245 and 205 bp fragments (homozygous genotype tt) are present (d). All the images of the original gels from which we cropped the representative images showed in Fig 1 are available as S1–S6 Figs.
To analyse the BsmI polymorphism, the resulting amplified 825 bp PCR fragment was digested with BsmI restriction enzyme (Euroclone, Milano, Italy) generating two fragments of 650 bp and 175 bp in presence of the b allele. Since a mismatched base at the primer binding region can lead to a drop out of b allele during PCR amplification and to the false higher prevalence of BB genotype [26], data obtained with PCR-RFLP were confirmed using TaqMan® SNP Genotyping Assay (Life Technologies, Carlsbad, CA, USA) for rs1544410. A higher number of Bb subjects with a proportionally smaller number of BB subjects were observed after application of the genotyping assay. Specifically, out of the 141 subjects having the BB genotype assigned with PCR-RFLP, 48 subjects were assigned as Bb, and 2 as bb after the analysis with the TaqMan® SNP Genotyping Assay.
ApaI digestion of the 740 bp amplified DNA used to determine both ApaI and TaqI RFLP, resulted in two fragments of 530 bp and 210 bp in presence of the a allele.
Digestion with TaqI of the 740 bp PCR fragment resulted in three fragments of 290 bp, 245 bp and 205 bp in presence of the t allele, and in two fragments of 495 bp and 245 bp in its absence (T allele) due to an additional monomorphic TaqI site.
DNA fragments were separated by electrophoresis on polyacrylamide gel.
Genotypes were designated by a lowercase letter (b, a, t alleles) for the presence of the restriction enzyme site, and by a capital letter (B, A, T alleles) for its absence.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess the normality of data distribution. Since data were not normally distributed, Mann Whitney test were used to assess the differences between continuous variables such as age, BMI, physical job demand, and exposure to vibration in all cases or subgroups and controls. For categorical variables, Odds ratios (ORs) and the 95% confidence interval (CI) were calculated to evaluate the risk of spine pathologies by comparing cases or subgroups of patients with controls, significance was evaluated by chi square Pearson or Fisher exact test, as appropriate (for clarity we showed in Table 1 only significance and not all ORs and CIs).
Tests for deviations from Hardy-Weinberg equilibrium (HWE) were performed using a chi-square distribution in all the subjects, and in cases and controls separately for each SNP.
ORs and the CI were calculated to set the association between alleles, genotypes, and haplotypes and risk of spine pathologies in cases, controls and subgroups of patients. Logistic regression was used to evaluate effects of confounders by obtaining adjusted ORs and 95% CIs for genotypes and alleles. Adjusted analysis included conventional risk factors previously observed [18] and confirmed in this cohort such as: age, BMI, smoke, physical job demand and exposure to vibrations (Table 1). Family history was not included as confounder to avoid overcorrection by considering that it includes genetic hereditary components. Throughout the text adjusted ORs for the genetic associations were reported.
Eight rare BsmI-ApaI-TaqI haplotypes estimated to be less than 3% in frequency in both cases and controls (including in total 11 subjects) were not reported. LD between the SNPs was determined using Haploview [27]. Haplotype frequencies were estimated with the program CHAPLIN [28].
Significance level was held at 0.05, and P values ≤ 0.10 were considered as a tendency. Statistical softwares used were GraphPad Prism version 5.00 (GraphPad software, La Jolla, CA, USA), and SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
VDR BsmI, ApaI, TaqI genotypes and alleles
In our total sample the frequency of BB was 17.6% (91/518), Bb was 51.3% (266/518), and bb was 31.1% (161/518). B allele had a frequency of 43.2% (448/1036), and the b allele had a frequency of 56.8% (588/1036). The observed BsmI genotype frequencies were in HWE (X2 = 1.102, P = 0.29). In particular, controls did not deviate (X2 = 0.943, P = 0.33), while cases (X2 = 6.043, P = 0.01) deviated from HWE. The frequency of AA genotype was 34.0% (176/518), Aa was 48.1% (249/518), and aa was 17.9% (93/518). A allele had a frequency of 58.0% (601/1036), and the a allele had a frequency of 42.0% (435/1036). The observed ApaI genotype frequencies were consistent with HWE (X2 = 0.091, P = 0.76). Neither controls (X2 = 3.67, P = 0.05) nor cases (X2 = 2.09, P = 0.15) deviated from HWE. The frequency of TT genotype was 42.5% (220/518), Tt was 43.6% (226/518), and tt was 13.9% (72/518). T allele had a frequency of 64.3% (666/1036), and the t allele had a frequency of 35.7% (370/1036). The observed TaqI genotype frequencies were consistent with HWE (X2 = 1.287, P = 0.26). Neither controls (X2 = 1.058, P = 0.30) nor cases (X2 = 0.33, P = 0.56) deviated from HWE.
In S1 Appendix were reported the frequencies of BsmI, ApaI and TaqI genotypes in cases and controls.
Tables 2 and 3, respectively, illustrated frequencies of BsmI, ApaI and TaqI genotypes and alleles in controls compared with overall LDD cases, and specific subgroups of patients.
Table 2. Association of lumbar spine pathologies and VDR BsmI, ApaI, and TaqI genotypes.
| BB | Bb | bb | AA | Aa | aa | TT | Tt | tt | |
|---|---|---|---|---|---|---|---|---|---|
| Controls n = 252 (%) | 45 (17.9) | 114 (45.2) | 93 (36.9) | 92 (36.5) | 108 (42.9) | 52 (20.6) | 106 (42.1) | 109 (43.2) | 37 (14.7) |
| Cases n = 266 (%) | 46 (17.3) | 152 (57.1)** | 68 (25.6)** | 84 (31.6) | 141 (53.0)* | 41 (15.4)^ | 114 (42.9) | 117 (44.0) | 35 (13.1) |
| Subgroup 1 n = 88 (%) | 15 (17.0) | 54 (61.4)** | 19 (21.6)** | 26 (29.5) | 51 (58.0)* | 11 (12.5)* | 37 (42.0) | 40 (45.5) | 11 (12.5) |
| Subgroup 2 n = 87 (%) | 12 (13.8) | 46 (52.9) | 29 (33.3) | 23 (26.4)^ | 50 (57.5)^ | 14 (16.1) | 43 (49.4) | 34 (39.1) | 10 (11.5) |
| Subgroup 3 n = 40 (%) | 9 (22.5) | 23 (57.5)^ | 8 (20.0)* | 16 (40.0) | 20 (50.0) | 4 (10.0) | 12 (30.0) | 21 (52.5) | 7 (17.5) |
| Subgroup 4 n = 51 (%) | 10 (19.6) | 29 (56.9) | 12 (23.5)^ | 19 (37.3) | 20 (39.2) | 12 (23.5) | 22 (43.1) | 22 (43.1) | 7 (13.8) |
| Subgroup 1+2+3 n = 215 (%) | 36 (16.7) | 123 (57.2)** | 56 (26.0)* | 65 (30.2) | 121 (56.3)** | 29 (13.5)* | 92 (42.8) | 95 (44.2) | 28 (13.0) |
| Subgroup A n = 175 (%) | 27 (15.4) | 100 (57.2)* | 48 (27.4)* | 49 (28.0)^ | 101 (57.7)** | 25 (14.3)* | 80 (45.7) | 74 (42.3) | 21 (12.0) |
| Subgroup B n = 127 (%) | 21 (16.5) | 69 (54.3)^ | 37 (29.1)^ | 39 (30.7) | 70 (55.1)^ | 18 (14.2) | 55 (43.3) | 55 (43.3) | 17 (13.4) |
| Subgroup C n = 64 (%) | 17 (26.6) | 34 (53.1) | 13 (20.3)* | 27 (42.2) | 28 (43.7) | 9 (14.1) | 22 (34.4) | 27 (42.2) | 15 (23.4)^ |
| Subgroup D n = 50 (%) | 3 (6.0)^ | 26 (52.0) | 21 (42.0) | 7 (14.0)** | 34 (68.0)** | 9 (18.0) | 29 (58.0)^ | 21 (42.0) | 0 (0)* |
Subgroup 1 = patients with disc herniation alone; Subgroup 2 = patients with discopathies and/or osteochondrosis associated with disc herniation; Subgroup 3 = patients with discopathies and/or osteochondrosis without herniation; Subgroup 4 = patients with stenosis and/or spondylolisthesis.
Subgroup A, Subgroup 1 grouped with Subgroup 2 (i.e. all hernia cases with or without concomitant additional conditions); Subgroup B, Subgroup 2 grouped with Subgroup 3; Subgroup C, all discopathies cases with or without concomitant disc herniation; Subgroup D, all osteochondrosis cases with or without concomitant disc herniation.
* P<0.05,
** P<0.01
^ P ≤ 0.10
Table 3. Association of lumbar spine pathologies and VDR BsmI, ApaI, and TaqI alleles.
| B | b | A | a | T | t | |
|---|---|---|---|---|---|---|
| Controls n = 252 (%) | 204/504 (40.5) | 300/504 (59.5) | 292/504 (57.9) | 212/504 (42.1) | 321/504 (63.7) | 183/504 (36.3) |
| Cases n = 266 (%) | 244/532 (45.9)^ | 288/532 (54.1)^ | 309/532 (58.1) | 223/532 (41.9) | 345/532 (64.8) | 187/532 (35.1) |
| Subgroup 1 n = 88 (%) | 84/176 (47.7)^ | 92/176 (52.3)^ | 103/176 (58.5) | 73/176 (41.5) | 114/176 (64.8) | 62/176 (35.2) |
| Subgroup 2 n = 87 (%) | 70/174 (40.2) | 104/174 (59.8) | 96/174 (55.2) | 78/174 (44.8) | 120/174 (69.0) | 54/174 (31.0) |
| Subgroup 3 n = 40 (%) | 41/80 (51.2)^ | 39/80 (48.8)^ | 52/80 (65.0) | 28/80 (35.0) | 45/80 (56.3) | 35/80 (43.7) |
| Subgroup 4 n = 51 (%) | 49/102 (48.0) | 53/102 (52.0) | 58/102 (56.9) | 44/102 (43.1) | 66/102 (64.7) | 36/102 (35.3) |
| Subgroup 1+2+3 n = 215 (%) | 195/430 (45.3) | 235/430 (54.7) | 251/430 (58.4) | 179/430 (41.6) | 279/430 (64.9) | 151/430 (35.1) |
| Subgroup A n = 175 (%) | 154/350 (44.0) | 196/350 (56.0) | 199/350 (56.9) | 151/350 (43.1) | 234/350 (66.9) | 116/350 (33.1) |
| Subgroup B n = 127 (%) | 111/254 (43.7) | 143/254 (56.3) | 148/254 (58.3) | 106/254 (41.7) | 165/254 (65.0) | 89/254 (35.0) |
| Subgroup C n = 64 (%) | 68/128 (53.1)* | 60/128 (46.9)* | 82/128 (64.1) | 46/128 (35.9) | 71/128 (55.5)^ | 57/128 (44.5)^ |
| Subgroup D n = 50 (%) | 32/100 (32.0) | 68/100 (68.0) | 48/100 (48.0)^ | 52/100 (52.0)^ | 79/100 (79.0)** | 21/100 (21.0)** |
Subgroup 1 = patients with disc herniation alone; Subgroup 2 = patients with discopathies and/or osteochondrosis associated with disc herniation; Subgroup 3 = patients with discopathies and/or osteochondrosis without herniation; Subgroup 4 = patients with stenosis and/or spondylolisthesis.
Subgroup A, Subgroup 1 grouped with Subgroup 2 (i.e. all hernia cases with or without concomitant additional conditions); Subgroup B, Subgroup 2 grouped with Subgroup 3; Subgroup C, all discopathies cases with or without concomitant disc herniation; Subgroup D, all osteochondrosis cases with or without concomitant disc herniation.
* P<0.05,
** P<0.01
^ P ≤ 0.10
By comparison of controls with all LDD cases, significant differences were observed for the heterozygous Bb and Aa genotypes (OR = 1.62, 95%CI = 1.11–2.36, P = 0.012, and OR = 1.60, 95%CI = 1.10–2.33, P = 0.014, respectively), while the homozygous bb genotype was protective (OR = 0.56, 95%CI = 0.37–0.84, P = 0.005).
By specific subgroups analysis, the Bb genotype was a risk factor for disc herniation (subgroup 1, OR = 1.97, 95%CI = 1.16–3.36, P = 0.013; subgroup 1+2+3, OR = 1.73, 95%CI = 1.16–2.56, P = 0.007; and subgroup A, OR = 1.67, 95%CI = 1.10–2.55, P = 0.017). On the contrary, the bb genotype was protective for almost all the subgroups (subgroup 1, OR = 0.40, 95%CI = 0.21–0.74, P = 0.004; subgroup 3, OR = 0.40, 95%CI = 0.17–0.95, P = 0.037; subgroup 1+2+3, OR = 0.54, 95%CI = 0.35–0.83, P = 0.005; subgroup A, OR = 0.57, 95%CI = 0.36–0.91, P = 0.018; and subgroup C, OR = 0.40, 95%CI = 0.20–0.82, P = 0.012). Patients with osteochondrosis with or without disc herniation showed an opposite trend, in fact, the BB genotype had a tendency to be protective (subgroup D, OR = 0.30, 95%CI = 0.09–1.08, P = 0.065).
The Aa genotype was a risk factor for disc herniation (subgroup 1, OR = 1.95, 95%CI = 1.15–3.32, P = 0.014; subgroup 1+2+3, OR = 1.76, 95%CI = 1.19–2.61, P = 0.005; and subgroup A, OR = 1.88, 95%CI = 1.23–2.88, P = 0.003). On the contrary, the aa genotype was protective for 2 subgroups (subgroup 1+2+3, OR = 0.52, 95%CI = 0.30–0.91, P = 0.021; subgroup A, OR = 0.55, 95%CI = 0.30–0.98, P = 0.043). For subgroup D (all osteochondrosis cases) the Aa genotype was a risk factor (OR = 2.70, 95%CI = 1.37–5.34, P = 0.004), while the AA genotype was protective (OR = 0.31, 95%CI = 0.13–0.73, P = 0.008). Finally, for the subgroup D only, the tt genotype was protective.
From the analysis of alleles distributions in pathological subgroups it was observed that B allele was a risk factor to develop discopathies with or without disc herniation (subgroup C, crude OR = 1.66, 95%CI = 1.10–2.51, P = 0.017), and the T allele was risky for all osteochondrosis (subgroup D, OR = 2.09, 95%CI = 1.21–3.61, P = 0.008).
For patients with stenosis and/or spondylolistesis no significant findings were obtained regarding genotypes and alleles.
VDR BsmI, ApaI, TaqI haplotypes
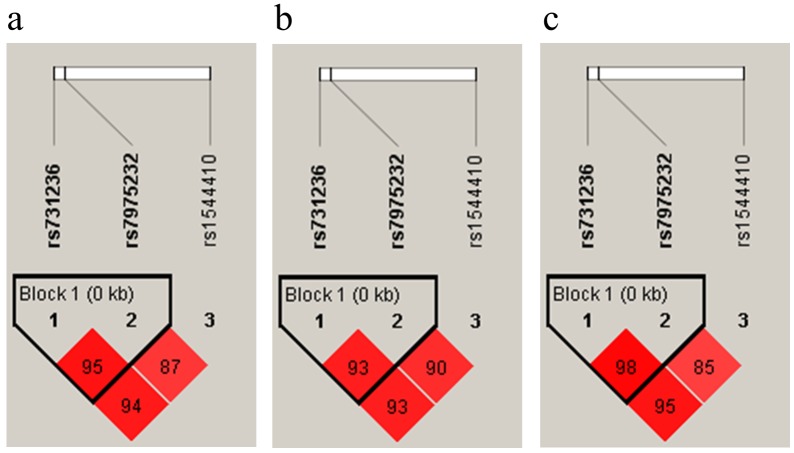
LD plots (r2) showed that all the three SNPs were in linkage disequilibrium (Fig 2). The strongest LD was observed between ApaI and TaqI (r2 = 0.95 in all the subjects, r2 = 0.93 in controls, and r2 = 0.98 in cases). TaqI and BsmI showed slightly lower values of LD (r2 = 0.94 in all the subjects, r2 = 0.93 in controls, and r2 = 0.95 in cases), while BsmI and ApaI showed the lowest values (r2 = 0.87 in all the subjects, r2 = 0.90 in controls, r2 = 0.85 in cases).
Fig 2. LD plots (r2) are shown for all subjects (a), controls (b) and cases (c).
Table 4 reported the frequencies of BsmI, ApaI and TaqI combined genotypes in controls compared with overall cases and specific subgroups of patients.
Table 4. Association of lumbar spine pathologies and VDR BsmI, ApaI, and TaqI combined genotypes.
| Genotypes | BbAaTt | bbaaTT | BBAAtt | BbAATt | bbAaTT | bbAATT | BBAATt | BbAaTT | BbAATT | BbaaTT |
|---|---|---|---|---|---|---|---|---|---|---|
| Controls n = 252 (%) | 66 (26.2) | 47 (18.6) | 34 (13.5) | 29 (11.5) | 28 (11.1) | 13 (5.2) | 10 (4.0) | 9 (3.6) | 5 (2.0) | 4 (1.6) |
| Cases n = 266 (%) | 79 (29.7) | 29 (10.9)* | 32 (12.0) | 23 (8.6) | 35 (13.2) | 3 (1.1)* | 13 (4.9) | 26 (9.8)** | 10 (3.8) | 11 (4.1)^ |
| Subgroup 1 n = 88 (%) | 29 (32.9) | 7 (7.9)* | 10 (11.4) | 6 (6.8) | 11 (12.5) | 1 (1.1) | 5 (5.7) | 11 (12.5)** | 3 (3.4) | 4 (4.5) |
| Subgroup 2 n = 87 (%) | 26 (30.0) | 12 (13.8) | 8 (9.2) | 4 (4.6)^ | 15 (17.2) | 2 (2.3) | 3 (3.4) | 8 (9.2)* | 4 (4.6) | 2 (2.3) |
| Subgroup 3 n = 40 (%) | 12 (30.0) | 2 (5.0)* | 7 (17.5) | 6 (15.0) | 5 (12.5) | 0 (0) | 2 (5.0) | 3 (7.5) | 1 (2.5) | 1 (2.5) |
| Subgroup 4 n = 51 (%) | 12 (23.5) | 8 (15.7) | 7 (13.7) | 7 (13.7) | 4 (7.8) | 0 (0) | 3 (5.9) | 4 (7.8)^ | 2 (3.9) | 4 (7.8)^ |
| Subgroup 1+2+3 n = 215 (%) | 67 (31.2) | 21 (9.8)** | 25 (11.6) | 16 (7.4) | 31 (14.4) | 3 (1.4)^ | 10 (4.6) | 22 (10.2)** | 8 (3.7) | 7 (3.2) |
| Subgroup A n = 175 (%) | 55 (31.4) | 19 (10.9)* | 18 (10.3) | 10 (5.7)^ | 26 (14.9) | 3 (1.7)^ | 8 (4.6) | 19 (10.9)** | 7 (4.0) | 6 (3.4) |
| Subgroup B n = 127 (%) | 38 (29.9) | 14 (11.0)^ | 15 (11.8) | 10 (7.9) | 20 (15.7) | 2 (1.6)^ | 5 (3.9) | 11 (8.7)* | 5 (3.9) | 3 (2.4) |
| Subgroup C n = 64 (%) | 16 (25.0) | 6 (9.4)^ | 14 (21.9)^ | 7 (10.9) | 6 (9.4) | 0 (0)^ | 2 (3.1) | 5 (7.8)^ | 3 (4.7) | 2 (3.1) |
| Subgroup D n = 50 (%) | 17 (34.0) | 8 (16.0) | 0 (0) | 1 (2.0)^ | 12 (24.0)* | 1 (2.0) | 3 (6.0) | 5 (10.0)^ | 2 (4.0) | 1 (2.0) |
Subgroup 1 = patients with disc herniation alone; Subgroup 2 = patients with discopathies and/or osteochondrosis associated with disc herniation; Subgroup 3 = patients with discopathies and/or osteochondrosis without herniation; Subgroup 4 = patients with stenosis and/or spondylolisthesis.
Subgroup A, Subgroup 1 grouped with Subgroup 2 (i.e. all hernia cases with or without concomitant additional conditions); Subgroup B, Subgroup 2 grouped with Subgroup 3; Subgroup C, all discopathies cases with or without concomitant disc herniation; Subgroup D, all osteochondrosis cases with or without concomitant disc herniation.
* P<0.05,
** P<0.01
^ P ≤ 0.10
From the frequency distributions of the 10 identified combined genotypes we observed that the BbAaTT was 3-fold more frequent in all LDD cases (OR = 3.38, 95%CI = 1.49–7.67, P = 0.004), and had significant OR = 4.44, 95%CI = 1.62–12.2, P = 0.004 for subgroup 1; OR = 3.16, 95%CI = 1.07–9.33, P = 0.038 for subgroup 2; OR = 3.44, 95%CI = 1.48–7.97, P = 0.004 for subgroup 1+2+3; OR = 3.85, 95%CI = 1.60–9.28, P = 0.003 for subgroup A; and OR = 2.81, 95%CI = 1.07–7.42, P = 0.036 for subgroup B. The bbaaTT was protective for all cases (OR = 0.47, 95%CI = 0.27–0.82, P = 0.008), and specifically for subgroup 1, OR = 0.24, 95%CI = 0.09–0.66, P = 0.006; subgroup 3, OR = 0.20, 95%CI = 0.04–0.90, P = 0.036; subgroup 1+2+3, OR = 0.39, 95%CI = 0.21–0.72, P = 0.003; and subgroup A, OR = 0.43, 95%CI = 0.23–0.83, P = 0.012. The bbAATT was protective for all cases (OR = 0.22, 95%CI = 0.06–0.80, P = 0.022), and showed a protective tendency for all the discopathies, i.e. subgroup 1+2+3, OR = 0.27, P = 0.051, and for subgroup A, OR = 0.32, P = 0.086.
Finally, the bbAaTT was risky (OR = 2.56, 95%CI = 1.10–5.97, P = 0.030) only for subgroup D, i. e., for all patients with osteochondrosis, a subgroup with a peculiar pathological phenotype.
For patients with stenosis and/or spondylolistesis no significant findings were obtained regarding haplotypes.
Estimated haplotype frequencies were shown in Table 5. The most represented haplotype in the whole cohort of controls and cases was baT, followed by BAt and bAT.
Table 5. Estimated frequencies of VDR BsmI, ApaI, and TaqI haplotypes.
| Haplotypes | baT | BAt | bAT | BAT | bAt | BaT |
|---|---|---|---|---|---|---|
| Controls n = 252 | 101 (40.4) | 86 (34.4) | 44 (17.6) | 12 (4.8) | 2 (1.1) | 2 (1.0) |
| Cases n = 266 | 103 (38.9) | 91 (34.3) | 38 (14.4) | 23 (8.8) | 1 (0.6) | 7 (2.8) |
| Subgroup 1 n = 88 | 34 (38.7) | 30 (34.6) | 11 (13.0) | 9 (10.3) | 0 (0.6) | 2 (2.8) |
| Subgroup 2 n = 87 | 37 (42.7) | 25 (29.8) | 13 (15.9) | 7 (8.3) | 1 (1.2) | 1 (2.2) |
| Subgroup 3 n = 40 | 12 (32.0) | 16 (42.4) | 6 (15.4) | 2 (7.2) | 0 (0) | 0 (1.6) |
| Subgroup 4 n = 51 | 19 (35.6) | 17 (35.3) | 6 (13.4) | 4 (8.2) | 0 (0) | 2 (4.6) |
| Subgroup 1+2+3 n = 215 | 83 (39.0) | 73 (34.1) | 31 (14.6) | 19 (8.9) | 1 (0.8) | 5 (2.3) |
| Subgroup A n = 175 | 71 (40.6) | 56 (32.2) | 25 (14.4) | 16 (9.3) | 1 (0.9) | 4 (2.5) |
| Subgroup B n = 127 | 49 (39.3) | 42 (33.7) | 19 (15.7) | 10 (8.0) | 1 (0.9) | 2 (2.0) |
| Subgroup C n = 64 | 20 (32.2) | 27 (42.8) | 8 (13.0) | 4 (7.4) | 0 (0.9) | 1 (2.9) |
| Subgroup D n = 50 | 25 (50.8) | 10 (21.0) | 8 (17.2) | 4 (9.8) | 0 (0) | 0 (1.2) |
Subgroup 1 = patients with disc herniation alone; Subgroup 2 = patients with discopathies and/or osteochondrosis associated with disc herniation; Subgroup 3 = patients with discopathies and/or osteochondrosis without herniation; Subgroup 4 = patients with stenosis and/or spondylolisthesis.
Subgroup A, Subgroup 1 grouped with Subgroup 2 (i.e. all hernia cases with or without concomitant additional conditions); Subgroup B, Subgroup 2 grouped with Subgroup 3; Subgroup C, all discopathies cases with or without concomitant disc herniation; Subgroup D, all osteochondrosis cases with or without concomitant disc herniation.
Discussion
To the best of our knowledge, this is the first study demonstrating an association between the three VDR BsmI, ApaI and TaqI variants and clearly defined lumbar spine pathologies, and the only one study performed in the Italian population.
Genotype and allele frequencies were closed to those of Italian subjects from Central Italy reported in HapMap (101 subjects: genotypes BB = 17.8%, Bb = 48.5%, bb = 33.7%, alleles B = 42.1%, b = 57.9%; 102 subjects: genotypes AA = 36.3%, Aa = 45.1%, aa = 18.6%, alleles A = 58.8%, a = 41.2%; 101 subjects: genotypes TT = 33.7%, Tt = 48.5%, tt = 17.8%, alleles T = 57.9%, t = 42.1%) [29].
The distribution of genotypes and alleles in our cohort of controls was in accordance also with data of controls reported in a meta-analysis, based on studies published till December 2005, that investigated the association between these VDR polymorphisms and the risk of osteoporosis in Caucasian, East Asian, Mexican, Latino and Turkish women [30].
The heterozygous Bb and Aa genotypes represented a risk factor, while the homozygous bb genotype was protective for the all subjects with lumbar spine pathologies recruited in our study.
Other studies showed an association between BsmI, ApaI and TaqI VDR genotypes or alleles and signs of disc degeneration such as bulges, osteophytosis, disc space narrowing or disc herniation. In particular B allele in British [22], genotypes TT in Finnish [7], Tt and tt in Turkish [11,12] and Australian [14], Tt in Japanese [15] and alleles t and A in Chinese [17,19] patients were reported as predisposing towards worse phenotypes.
The inconsistent findings between our study and the data reported in literature [31] are likely related to both the ethnic differences among the study populations and to the different inclusion criteria, as well as to the lack of a standardized approach to define pathological phenotypes [32,33].
In our study, after the analysis of the overall cohort of LDD patients, we classified our cases in subgroups of clinical relevance, according to specific pathological features revealed by MRI. This approach allowed us to better define whether the associations of variants observed in all the pathological subjects were true for all the subgroups or only for particular lumbar spine pathologies.
The results were confirmed after adjusting for previously identified conventional low back pain risk factors [18]. Data observed for the overall cohort of subjects were confirmed for particular subgroups of patients. Bb genotype and B allele were associated with a 1.66 to 1.97-fold increased risk to develop disc herniation and discopathies concomitant or not with disc herniation. On the contrary, bb genotype was protective. Aa genotype was also associated with a 1.76 to 1.95-fold increased risk to develop discopathies and/or osteochondrosis associated or not with disc herniation, while aa genotype was protective.
Interestingly, the subgroup of patients with osteochondrosis showed particular features. Aa genotype was a risk factor (2.70-fold increased risk), while AA genotype was protective for this condition. In this subgroup of patients an opposite trend for BsmI was observed with respect to other patients, with a protective tendency for BB genotype. In the same subgroup, the T allele represented a 2.09-fold increased risk to develop osteochondrosis, while tt genotype was highly protective.
The three SNPs analyzed in our study were in high LD, where the greatest degree of LD was found between ApaI and TaqI, followed by TaqI and BsmI, and then BsmI and ApaI. A different trend was reported in the meta-analysis of Thakkinstian et al. [34], where the greatest degree of LD was observed between the BsmI and TaqI polymorphisms, followed by BsmI and ApaI, and then ApaI and TaqI. These discrepancies are likely related to the absence of HWE for all the three sites in one study [35] and for BsmI in another one [36] described in the meta-analysis, while our observed genotype frequencies were consistent with HWE.
The frequency distributions of the combined genotypes in our controls were close to those of non-osteoporotic postmenopausal women from Belgium [35], Italy [37], Denmark [36] and the Netherlands [38], and our estimated haplotype frequencies showed that the most common haplotype was baT, followed by BAt and bAT as reported for Caucasian women [34].
By excluding too rare combined genotypes (estimated to be less than 3% in frequency), we identified ten combined genotypes, among which notably the BbAATT had not been described before. Of these combinations, four were particularly interesting since they were associated with specific clinical conditions. The BbAaTT was risky (OR ranging from 2.81 to 4.44), while the bbaaTT and bbAATT were protective for patients with disc herniation and discopathies. As observed for genotypes and alleles, even for combined genotype analysis, the subgroup of patients with osteochondrosis showed peculiar features; in fact, the bbAaTT combined genotype had a 2.56-fold risk to develop osteochondrosis, a degenerative process involving primarily the vertebral bodies structures limiting the disc [39].
No significant findings were obtained regarding alleles, genotypes and haplotypes for patients with stenosis and/or spondylolistesis.
Interesting results were found for all the three SNPs BsmI, ApaI and TaqI, when analyzed in the light of clinical relevant phenotypes. In summary, patients affected by disc herniation and discopathies and/or osteochondrosis associated or not with disc herniation showed risky B allele, Bb, Aa, BbAaTT genotypes, and protective bb, aa, bbaaTT, bbAATT genotypes. In patients with osteochondrosis, T allele, Aa, TT, bbAaTT genotypes were risky, while t allele, AA, tt genotypes were protective.
The bbaaTT genotype, the main protective combined genotype for spine pathologies observed in our study, was more prevalent also in 144 non-osteoporotic Italian postmenopausal women compared to 176 osteoporotic subjects [37].
It is to note that, in our cohort, the TT genotype was risky or protective depending on the specific haplotype to which it belonged. This could explain the discrepancies between studies which evaluated only the TaqI polymorphism without a further haplotype analysis.
In the future, it will be our interest to enlarge the group of controls and the pathological subgroups to confirm present results in a larger cohort of subjects. This is particularly important since in large studies no association between these 3 VDR polymorphisms and the risk of osteoporosis [30], vertebral fracture incidence in women [40] and osteoarthritis in European population [41] was observed.
Moreover, basic science studies should be performed to reach a deeper knowledge of the molecular mechanism regulated by vitamin D through its receptor in the spine tissues. This will help to understand the association between these 3 SNPs in non-coding VDR sequence and spine pathologies. Particularly, it will be useful to study the interaction of BsmI, ApaI and TaqI VDR variants and other SNPs in nearby functional genes supposed to be in LD, such as collagen type II alpha 1 (COL2A1) [42], whose gene product is abundantly present in the intervertebral disc [43], and whose expression is regulated by vitamin D active metabolites [44].
Nevertheless our results highlighted the importance to perform a clear clinical evaluation of the patients with lumbar spine pathologies to obtain specific phenotypes for the identification of genetic markers of pathology. Moreover, the presence of specific genotypes/alleles/haplotypes associated with defined phenotypes suggests the possibility of a personalized clinical approach aimed to prevent or at least to delay the development of these spine disorders.
Supporting Information
(PDF)
Lanes 2 and 3 reported in Fig 1b.
(TIF)
Lanes 12 and 13 and lanes 8 and 9, respectively, reported in Fig 1b.
(TIF)
Lanes 14 and 16, and lanes 11 and 13, respectively, reported in Fig 1c.
(TIF)
Lanes 13 and 14 reported in Fig 1c.
(TIF)
Lanes 11 and 12 and lanes 5 and 6, respectively, reported in Fig 1d.
(TIF)
Lanes 2 and 3 reported in Fig 1d.
(TIF)
Acknowledgments
The authors are grateful to Davide Rossi, Lucia Ferino, Marta Tibiletti and Patrizia Lanteri for helping in controls recruitment, and Patrizia Nacci and Martina Linussio for helping in experimental analysis. This work was supported by European Community’s Seventh 567 Framework Programme (FP7, 2007–2013 under Grant Agreement 568 No. HEALTH-F2-2008-201626) and by the Italian Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Underlying participant-level data cannot be made publicly available or be included in the supporting information for ethical reasons, due to patient’s privacy. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by European Community’s Seventh 567 Framework Programme (FP7, 2007–2013 under Grant Agreement 568 No. HEALTH-F2-2008-201626) and by the Italian Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338: 143–156. [DOI] [PubMed] [Google Scholar]
- 2.Colombini A, Cauci S, Lombardi G, Lanteri P, Croiset S, Brayda-Bruno M, et al. (2013) Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J Steroid Biochem Mol Biol 138: 24–40. 10.1016/j.jsbmb.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Mayer JE, Iatridis JC, Chan D, Qureshi SA, Gottesman O, Hecht AC (2013) Genetic polymorphisms associated with intervertebral disc degeneration. Spine J 13: 299–317. 10.1016/j.spinee.2013.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Zhu J, Gao C, Peng B (2015) Vitamin D receptor (VDR) genetic polymorphisms associated with intervertebral disc degeneration. J Genet Genomics 42: 135–140. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y, van Meurs JB, d'Alesio A, Jhamai M, Zhao H, Rivadeneira F, et al. (2005) Promoter and 3'-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: the Rotterdam study. Am J Hum Genet 77: 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, Peltonen L, et al. (1998) Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine (Phila Pa 1976) 23: 2477–2485. [DOI] [PubMed] [Google Scholar]
- 7.Videman T, Gibbons LE, Battie MC, Maravilla K, Vanninen E, Leppävuori J, et al. (2001) The relative roles of intragenic polymorphisms of the vitamin D receptor gene in lumbar spine degeneration and bone density. Spine (Phila Pa 1976) 26: E7–E12. [DOI] [PubMed] [Google Scholar]
- 8.Virtanen IM, Karppinen J, Taimela S, Ott J, Barral S, Kaikkonen K, et al. (2007) Occupational and genetic risk factors associated with intervertebral disc disease. Spine (Phila Pa 1976) 32: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 9.Karppinen J, Daavittila I, Solovieva S, Kuisma M, Taimela S, Natri A, et al. (2008) Genetic factors are associated with modic changes in endplates of lumbar vertebral bodies. Spine (Phila Pa 1976) 33: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 10.Noponen-Hietala N, Kyllonen E, Mannikko M, Ilkko E, Karppinen J, Ott J, et al. (2003) Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis 62: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eser B, Cora T, Eser O, Kalkan E, Haktanir A, Erdogan MO, et al. (2010) Association of the polymorphisms of vitamin D receptor and aggrecan genes with degenerative disc disease. Genet Test Mol Biomarkers 14: 313–317. 10.1089/gtmb.2009.0202 [DOI] [PubMed] [Google Scholar]
- 12.Toktas ZO, Eksi MS, Yilmaz B, Demir MK, Ozgen S, Kılıç T, et al. (2015) Association of collagen I, IX and vitamin D receptor gene polymorphisms with radiological severity of intervertebral disc degeneration in Southern European Ancestor. Eur Spine J 24: 2432–2441. 10.1007/s00586-015-4206-5 [DOI] [PubMed] [Google Scholar]
- 13.Omair A, Lie BA, Reikeras O, Brox JI (2012) An association study of Interleukin 18 receptor genes (IL18R1 and IL18RAP) in lumbar disc degeneration. Open Orthop J 6: 164–171. 10.2174/1874325001206010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones G, White C, Sambrook P, Eisman J (1998) Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann Rheum Dis 57: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T (2002) The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am 84-A: 2022–2028. [DOI] [PubMed] [Google Scholar]
- 16.Oishi Y, Shimizu K, Katoh T, Nakao H, Yamaura M, Furuko T, et al. (2003) Lack of association between lumbar disc degeneration and osteophyte formation in elderly Japanese women with back pain. Bone 32: 405–411. [DOI] [PubMed] [Google Scholar]
- 17.Cheung KM, Chan D, Karppinen J, Chen Y, Jim JJ, Yip SP, et al. (2006) Association of the Taq I allele in vitamin D receptor with degenerative disc disease and disc bulge in a Chinese population. Spine (Phila Pa 1976) 31: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 18.Colombini A, Brayda-Bruno M, Lombardi G, Croiset SJ, Vrech V, Maione V et al. (2014) FokI polymorphism in the vitamin D receptor gene (VDR) and its association with lumbar spine pathologies in the Italian population: a case-control study. PLoS One 9: e97027 10.1371/journal.pone.0097027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan HY, Tang Y, Liang YX, Lei L, Xiao GB, Wang S, et al. (2010) Matrix metalloproteinase-3 and vitamin D receptor genetic polymorphisms, and their interactions with occupational exposure in lumbar disc degeneration. J Occup Health 52: 23–30. [DOI] [PubMed] [Google Scholar]
- 20.Koshizuka Y, Ogata N, Shiraki M, Hosoi T, Seichi A, Takeshita K, et al. (2006) Distinct association of gene polymorphisms of estrogen receptor and vitamin D receptor with lumbar spondylosis in post-menopausal women. Eur Spine J 15: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 21.Zawilla NH, Darweesh H, Mansour N, Helal S, Taha FM, Awadallah M, et al. (2014) Matrix metalloproteinase-3, vitamin D receptor gene polymorphisms, and occupational risk factors in lumbar disc degeneration. J Occup Rehabil 24: 370–381. 10.1007/s10926-013-9472-7 [DOI] [PubMed] [Google Scholar]
- 22.Jordan KM, Syddall H, Dennison EM, Cooper C, Arden NK (2005) Birthweight, vitamin D receptor gene polymorphism, and risk of lumbar spine osteoarthritis. J Rheumatol 32: 678–683. [PubMed] [Google Scholar]
- 23.Colombini A, Brayda-Bruno M, Ferino L, Lombardi G, Maione V, Banfi G, et al. (2015) Gender differences in the VDR-FokI polymorphism and conventional non-genetic risk factors in association with lumbar spine pathologies in an Italian case-control study. Int J Mol Sci 16: 3722–3739. 10.3390/ijms16023722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansoni V, Perego S, Colombini A, Banfi G, Brayda-Bruno M, Lombardi G (2016) Interplay between low plasma RANKL and VDR-FokI polymorphism in lumbar disc herniation independently from age, body mass, and environmental factors: a case-control study in the Italian population. Eur Spine J 25:192–199. 10.1007/s00586-015-4176-7 [DOI] [PubMed] [Google Scholar]
- 25.Pani MA, Knapp M, Donner H, Braun J, Baur MP, Usadel KH, et al. (2000) Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes 49: 504–507. [DOI] [PubMed] [Google Scholar]
- 26.Zajickova K, Krepelova A, Zofkova I (2003) A single nucleotide polymorphism under the reverse primer binding site may lead to BsmI mis-genotyping in the vitamin D receptor gene. J Bone Miner Res 18: 1754–1757. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 28.Epstein MP, Satten GA (2003) Inference on haplotype effects in case-control studies using unphased genotype data. Am J Hum Genet 73: 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.HapMap (2016) HapMap SNP report for rs1544410, rs7975232 and rs731236.
- 30.Zintzaras E, Rodopoulou P, Koukoulis GN (2006) BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D receptor (VDR) gene and the risk of osteoporosis: a meta-analysis. Dis Markers 22: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu G, Mei Q, Zhou D, Wu J, Han L (2012) Vitamin D receptor gene and aggrecan gene polymorphisms and the risk of intervertebral disc degeneration—a meta-analysis. PLoS One 7: e50243 10.1371/journal.pone.0050243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasekaran S, Kanna RM, Senthil N, Raveendran M, Cheung KM, Chan D, et al. (2013) Phenotype variations affect genetic association studies of degenerative disc disease: conclusions of analysis of genetic association of 58 single nucleotide polymorphisms with highly specific phenotypes for disc degeneration in 332 subjects. Spine J 13: 1309–1320. 10.1016/j.spinee.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 33.Nakki A, Battie MC, Kaprio J (2014) Genetics of disc-related disorders: current findings and lessons from other complex diseases. Eur Spine J 23 Suppl 3: S354–363. 10.1007/s00586-013-2878-2 [DOI] [PubMed] [Google Scholar]
- 34.Thakkinstian A, D'Este C, Attia J (2004) Haplotype analysis of VDR gene polymorphisms: a meta-analysis. Osteoporos Int 15: 729–734. [DOI] [PubMed] [Google Scholar]
- 35.Vandevyver C, Wylin T, Cassiman JJ, Raus J, Geusens P (1997) Influence of the vitamin D receptor gene alleles on bone mineral density in postmenopausal and osteoporotic women. J Bone Miner Res 12: 241–247. [DOI] [PubMed] [Google Scholar]
- 36.Langdahl BL, Gravholt CH, Brixen K, Eriksen EF (2000) Polymorphisms in the vitamin D receptor gene and bone mass, bone turnover and osteoporotic fractures. Eur J Clin Invest 30: 608–617. [DOI] [PubMed] [Google Scholar]
- 37.Gennari L, Becherini L, Masi L, Mansani R, Gonnelli S, Cepollaro C, et al. (1998) Vitamin D and estrogen receptor allelic variants in Italian postmenopausal women: evidence of multiple gene contribution to bone mineral density. J Clin Endocrinol Metab 83: 939–944. [DOI] [PubMed] [Google Scholar]
- 38.Uitterlinden AG, Weel AEAM, Burger H, Fang Y, Van Duijn CM, Hofman A, et al. (2001) Interaction between the vitamin D receptor gene and collagen type I alpha 1 gene in susceptibility for fracture. J Bone Miner Res 16: 379–385. [DOI] [PubMed] [Google Scholar]
- 39.Leone A, Martino F (2008) Rachide degenerativo In: Leone A, Martino F, editors. Imaging del rachide: il vecchio e il nuovo. Italia: Springer-Verlag; pp. 27. [Google Scholar]
- 40.Horst-Sikorska W, Dytfeld J, Wawrzyniak A, Marcinkowska M, Michalak M, Franek E, et al. (2013) Vitamin D receptor gene polymorphisms, bone mineral density and fractures in postmenopausal women with osteoporosis. Mol Biol Rep 40: 383–390. 10.1007/s11033-012-2072-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu ZH, Jin XZ, Zhang W, Chen M, Ye DQ, Zhai Y, et al. (2014) Associations between vitamin D receptor gene polymorphisms and osteoarthritis: an updated meta-analysis. Rheumatology (Oxford) 53:998–1008. [DOI] [PubMed] [Google Scholar]
- 42.Hart DJ, Doyle DV, Spector TD (1995) Association between metabolic factors and Knee osteoarthritis in women: the Chingford Study. J Rheumatol 22: 1118–1123. [PubMed] [Google Scholar]
- 43.Colombini A, Lombardi G, Corsi MM, Banfi G (2008) Pathophysiology of the human intervertebral disc. Int J Biochem Cell Biol 40: 837–842. 10.1016/j.biocel.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 44.Colombini A, Lanteri P, Lombardi G, Grasso D, Recordati C, Lovi A, et al. (2012) Metabolic effects of vitamin D active metabolites in monolayer and micromass cultures of nucleus pulposus and annulus fibrosus cells isolated from human intervertebral disc. Int J Biochem Cell Biol 44: 1019–1030. 10.1016/j.biocel.2012.03.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Lanes 2 and 3 reported in Fig 1b.
(TIF)
Lanes 12 and 13 and lanes 8 and 9, respectively, reported in Fig 1b.
(TIF)
Lanes 14 and 16, and lanes 11 and 13, respectively, reported in Fig 1c.
(TIF)
Lanes 13 and 14 reported in Fig 1c.
(TIF)
Lanes 11 and 12 and lanes 5 and 6, respectively, reported in Fig 1d.
(TIF)
Lanes 2 and 3 reported in Fig 1d.
(TIF)
Data Availability Statement
Underlying participant-level data cannot be made publicly available or be included in the supporting information for ethical reasons, due to patient’s privacy. All other relevant data are within the paper and its Supporting Information files.