Abstract
Background
There is conflicting evidence regarding the association between decreased bone mineral density (BMD) and atherosclerosis. To this end, we performed a systematic review and meta-analysis to clarify the association.
Methods
To identify relevant studies, PubMed, Embase, and the Cochrane Library were systematically searched up to November 2015. All observational and comparative studies directly investigating the relationship between decreased BMD and clinical consequences of atherosclerotic vascular abnormalities, including carotid artery calcification (CAC), cardiovascular disease (CAD), and coronary artery disease (CAD) were obtained, without limitation of language or publication year.
Results
A total of 25 studies involving 10,299 patients were included. The incidence of atherosclerotic vascular abnormalities was significantly increased in low BMD patients, compared to patients with normal BMD (OR, 1.81, 95% CI [1.01, 2.19], p<0.00001)). Similar results were also observed for postmenopausal women (OR, 2.23, 95% CI [1.72, 2.89], p<0.00001). Subgroup analyses of osteopenia, osteoporosis, and normal BMD also revealed that the combined ORs for the incidence of atherosclerotic vascular abnormalities increased as BMD decreased. Of note, after adjusting for age, sex, body mass index (BMI) and other vascular risk factors, decreased BMD remained significantly associated with the incidence of atherosclerotic vascular abnormalities (OR, 2.96, 95% CI [2.25, 3.88], p < 0.00001).
Conclusions
Based on the results of this study, decreased BMD is an independent predictor for the development of atherosclerosis in elderly individuals. Moreover, the risk of atherosclerotic vascular abnormalities increased as BMD decreased. Future studies focusing on individuals with different severities of atherosclerosis and comorbidities are of interest.
Introduction
Osteoporosis (OP) and atherosclerosis are the two most common diseases in elderly individuals and are associated with significant morbidity, mortality, and disability. OP affects 44 million Americans (55%) over the age of 50 years [1, 2]. According to data from the World Health Organization (WHO), elderly individuals will comprise 20% of the population by 2050 [3–5], increasing the prevalence of OP and atherosclerosis.
Although mounting epidemiological evidence suggests an association between OP and atherosclerosis, these diseases are traditionally considered unrelated, with their co-existence being caused by independent age-related processes. Recently, a growing number of studies have linked atherosclerosis to OP in elderly individuals [3, 4, 6–13]. The association, however, is not fully understood and controversy still exists regarding whether it is gender-related. Moreover, the connection between bone mineral density (BMD) and atherosclerosis is also controversial. Several studies suggested that no relationship exists between decreased BMD and atherosclerosis [9, 14–18]. In another study, osteoporosis and atherosclerosis were independent processes in postmenopausal women, after adjusting for age, gender, BMI, hypertension, and other vascular risk factors [4]. Beer et al. also revealed that low BMD was not associated with coronary atherosclerosis in men [5].
In the absence of any studies that are sufficiently powered to examine the relationship between low BMD and atherosclerosis, a meta-analysis of previously conducted investigations is useful. Thus, we conducted a systematic review and meta-analysis of previous studies for the following purposes: (1) to investigate and compare the incidence of atherosclerotic vascular abnormalities in individuals with normal BMD, osteopenia, and osteoporosis; (2) to explore the relationships between low BMD and atherosclerotic vascular abnormalities among the complete population and in postmenopausal women; and (3) examine the relationships after adjusting for age, sex, body mass index (BMI) and other vascular risk factors.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19], and the Cochrane Handbook for Systematic Reviews of Interventions (ver. 5.0.2).
Literature search
An experienced medical librarian designed and performed the literature search. Three independent investigators (CY, MX, SW) searched electronic databases (PubMed, Embase, and the Cochrane Library) with no language restrictions through November 2015. The following keywords or corresponding Medical Subject Headings (MeSH) were used: “bone mineral density” or “BMD” or “osteoporosis” or “OP” or “osteopenia” and “atherosclerosis” or “atheroscleroses” or “calcium plaque” or “carotid artery calcification” or “CAC” or “cardiovascular disease” or “CVD” or “coronary artery disease” or “CAD” or “atherosclerotic vascular disease” or “AVD” or “coronary micro-vascular endothelial dysfunction” or “CMED”. Details of the search strategy are provided in S1 Table and S1 and S2 Texts. Reference lists were also searched manually for additional studies. In addition, we searched the National Institutes of Health, the Clinical Trial Registry, the Trials Central and the Center Watch, and the Current Controlled Trials for grey literature.
Inclusion criteria
Two reviewers (CY and MX) independently screened manuscript titles and abstracts, and implemented the following inclusion criteria: (1) observational studies that examined the association between low BMD and clinical consequences of atherosclerotic vascular abnormalities, including carotid artery calcification (CAC), cardiovascular disease (CAD), and coronary artery disease (CAD); (2) studies that enrolled individuals aged 18 years and older; and (3) studies that provided data pertaining to BMD in participants with and without atherosclerotic vascular abnormalities.
The following exclusion criteria were used in this study: (1) articles that did not satisfy the inclusion criteria; (2) animal studies, reviews, letters, abstracts, case reports, conference proceedings, and systematic reviews; and (3) studies that did not provide sufficient data on BMD values, including the means, medians, standard deviations (SDs), and/or standard errors for subjects with and without atherosclerotic vascular abnormalities. Any disagreements were evaluated using a kappa test and consensus was achieved by discussion with the corresponding author (RH).
Data extraction and quality assessment
Three independent reviewers (SJ, XC, XZ) obtained relevant data and assessed the accuracy. The following information was extracted from each study: first author’s family name, year of publication, country, study design, patient demographics (age, gender, sample size), BMD values, assessment methods, and definition of atherosclerotic vascular abnormalities. Contact with corresponding authors was also attempted to verify the accuracy of the data, as well as to obtain further data for the analysis. The quality of the methodological data included in the studies was assessed using the Newcastle-Ottawa Scale (NOS), as recommended by the Cochrane non-randomized studies methods working group [20]. All articles were scored according the three major categories of NOS (selection, comparability andassessment of outcome). The maximum scores for selection, comparability and exposure were 4, 2 and 3, respectively. Studies were considered of high quality if at least five of the nine criteria were met.
Statistical analysis
Revman software (ver. 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark) was used to pool the data. A p-value ≤ 0.05 was considered significant. Summary odds ratio (OR) and its 95% CI were calculated to assess the association between low BMD and the occurrence of atherosclerotic vascular abnormalities across studies. For continuous data, the SD was calculated using the method described by Walter et al. [21]. Fixed- and random-effects models were used to calculate the combined ORs. Q and I2 statistics were used to assess the heterogeneity of the ORs across multiple studies [22]. A p-value < 0.10 was considered significant. If no statistical heterogeneity was detected between studies (P > 0.10; I2 < 50%), a fixed-effect test (Mantel-Haenszel test) was used to pool the data. Otherwise, the random-effect (Der Simonian-Laird method) model was applied. A sensitivity analysis was also performed to explore possible explanations for heterogeneity.
Subgroup analyses were conducted according to the severity of low BMD and gender, to evaluate the potential effect on outcomes of modifying these variables. The symmetry of funnel plot was also employed to evaluate publication bias. Furthermore, Egger’s test and Begger’s test were performed to assess the publication bias.
Results
Literature search
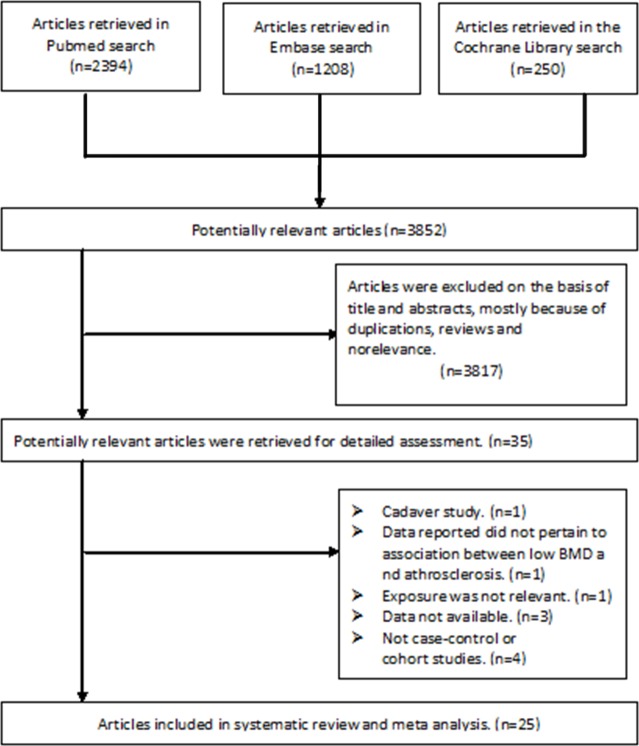
In total, the search identified 3,852 candidate publications; however, 3,817 were excluded due to duplications, nonrelevance, or because they were not observational or comparative studies. After assessing the 35 potentially-relevant articles, 25 articles (23 case-control studies and 2 cohort studies) involving 10,299 patients met the inclusion criteria [5, 15–18, 23–42]. The primary reasons for exclusion were as follows: one paper was a cadaver study [43]; one study failed to relate the data to low BMD and atherosclerotic vascular abnormalities [44]; one study was based on male patients with with type 2 diabetes mellitus, and the exposure was not relevant [45]; three articles were excluded because the study population size was unavailable and the association between low BMD and atherosclerosis was not presented [9, 46, 47]; and four studies were excluded because they were not case-control or cohort studies [12, 48–50]. The details of study selection are presented in Fig 1. The weighted kappa for the agreement on eligibility between the reviewers was 0.87 (95% CI [0.82–0.91]).
Fig 1. A PRISMA flowchart illustrated the selection of studies included in our systematic review.
Study characteristics
The characteristics and quality assessment results of the 25 studies are summarized in Table 1. Of those, two studies were performed in Italy, six in the US, three in China, three in Japan, two in Turkey, two in Korea, one in Morocco, one in Romania, one in Poland, one in Austria, one in Iran, one in Egypt, and one in Finland. Participants ranged in age from 46 to 89 years, and five of the studies included both males and females [5, 18, 24, 26, 37]. Nineteen studies included only post-menopausal individuals and one only consisted of male participants [15–17, 23, 25, 28–30, 32–35, 37, 39–42].
Table 1. Characteristics of subjects in eligible studies.
| Size | Gender | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Study design | Total | Normal BMD | Low BMD | Osteopenia | Osteoporosis | M | F | Age | Measurement of AS | Results | NOS Score |
| Montalcini, 2004 [15] | Italy | case-control | 157 | 100 | 57 | NA | NA | 0 | 157a | 56±8 | CAC | - | 5 |
| Tankó, 2005 [23] | America | case-control | 2576 | NA | 2442 | 1153 | 1289 | 0 | 2576a | 67±7 | CVD | + | 7 |
| Marcovitz, 2005[24] | America | case-control | 209 | 52 | 157 | 90 | 67 | 26 | 183 | 67±11 | CAD | + | 6 |
| Gupta, 2006 [25] | America | case-control | 101 | 50 | 51 | NA | NA | 0 | 101a | 77±12 | AVD | + | 6 |
| Varma, 2007 [26] | America | case-control | 198 | 66 | 132 | 79 | 53 | 52 | 146a | 66±6 | CAD | + | 6 |
| Tekin, 2008 [16] | Turkey | case-control | 227 | 57 | 159 | NA | NA | 0 | 227a | 60±9 | CAD | - | 7 |
| Sumino, 2008 [27] | Japan | case-control | 175 | 43 | 132 | 73 | 59 | 0 | 175a | 59±9 | CAC, cIMT | + | 7 |
| Tamaki, 2008 [28] | Japan | cohort | 609 | 71 | 88 | 66 | 22 | 0 | 609a | 56±10 | CAC | + | 5 |
| Reddy, 2008 [29] | America | case-control | 228 | 68 | 160 | 95 | 65 | 0 | 228a | 64±10 | CVD, BAC | + | 7 |
| Hmamouchi, 2009 [30] | Morocco | case-control | 72 | NA | NA | NA | 40 | 0 | 72a | 59 ± 8 | CAC, cIMT | ± | 7 |
| Mikumo, 2009 [31] | Japan | case-control | 143 | 17 | 21 | 12 | 9 | 0 | 143a | 58 ± 8 | AVD, PWV | + | 7 |
| Seo, 2009 [32] | Korea | case-control | 152 | 35 | 117 | 86 | 31 | 0 | 152a | 56 ± 6 | CAD, PWV | + | 5 |
| Pennisia, 2010 [33] | Italy | case-control | 100 | 20 | 72 | 32 | 40 | 0 | 100a | 75±10 | CAC, Femoral AS | ± | 6 |
| Fodor, 2011 [34] | Romania | case-control | 100 | 36 | 64 | 32 | 32 | 0 | 101a | 65±10 | CAC, cIMT | + | 7 |
| Bajew, 2011 [17] | Poland | case-control | 61 | 23 | 38 | 22 | 16 | 0 | 61a | 68±9 | CAD | - | 5 |
| Beer, 2011 [5] | Austria | case-control | 623 | 351 | 272 | 207 | 65 | 623 | 0 | 64±11 | CAD | - | 7 |
| Hajsadeghi, 2011 [18] | Iran | case-control | 119 | 25 | 94 | 39 | 55 | 58 | 61 | 59±8 | CAD | - | 5 |
| Shokry, 2012 [35] | Egypt | case-control | 100 | 30 | NA | NA | 30 | 0 | 100a | 63±4 | CAD, PAD | + | 7 |
| Yesil, 2012 [36] | Turkey | case-control | 2235 | 174 | 2061 | 766 | 1335 | 827 | 1408a | 73±6 | CVD | + | 7 |
| Teng, 2013 [37] | China | case-control | 481 | 163 | 318 | 155 | 163 | 0 | 481a | 72±6 | AVD, WBV | + | 6 |
| Liang, 2014 [38] | China | case-control | 385 | 206 | 179 | 130 | 49 | 163 | 222 | 59±12 | CVD, PWV, cIMT | ± | 5 |
| Prasad, 2014 [39] | America | cohort | 194 | NA | NA | NA | 39 | 0 | 194a | 61±7 | CAD, CMED | + | 7 |
| Värri, 2014 [40] | Finland | case-control | 290 | 122 | 168 | 148 | 20 | 0 | 290a | 74±3 | CAC, cIMT | ± | 6 |
| Seo, 2015 [41] | Korea | case-control | 252 | 75 | 177 | NA | NA | 0 | 252a | 55±6 | CAD | + | 7 |
| Yu, 2015 [42] | China | case-control | 512 | NA | NA | NA | 204 | 0 | 512a | 75±5 | AVD, PWV | + | 6 |
CAC: carotid artery calcification. CVD: cardiovascular disease. CAD: coronary artery disease. AVD: atherosclerotic vascular disease. IMT: intima-media thickness. BAC: breast arterial calcification. PWV: pulse wave velocity. AS: atherosclerosis. PAD: peripheral arterial disease. cIMT: Carotid IMT. WBV: whole blood viscosity. CMED: coronary microvascular endothelial dysfunction. Normal BMD: T-score>-1; Osteopenia: -1<T-score<-2.5; Osteoporosis: T-score<-2.5. + positive correlation between BMD and outcomes; − negative correlation between BMD and outcomes; ± both positive and negative correlation between BMD and outcomes were reported.
a postmenopausal.
The definition of arthrosclerotic vascular abnormalities was determined by the included studies, which referred to: carotid artery calcification (CAC); cardiovascular disease (CVD); and coronary artery disease (CAD). The pulse wave velocity (PWV), breast arterial calcification (BAC), intima-media thickness (IMT) and whole blood viscosity (WBV) were also recorded whenever available. The definition of normal BMD, osteopenia, and osteoporosis was made according to the criteria of the WHO. Osteoporosis was defined as BMD minus 2.5 standard deviations (SD) (or lower than the young adult mean), while osteopenia was defined as BMD between 1 and 2.5 SD below the young adult mean [51]. Low BMD was defined as a combination of osteoporosis and osteopenia. Accordingly, 1,784 individuals were included in the normal BMD group, while 6,959 individuals were included in the low BMD group. There were 3,185 individuals in the osteopenia group, and 3,683 individuals were included in the osteoporosis group.
Seven studies comprised of 5,850 participants were adjusted for age, gender, BMI, hypertension, and other vascular risk factors [23, 24, 29, 36, 39–41]. The adjusted ORs with 95% CIs are listed in Table 2. The overall quality of the studies averaged a score of 6 points (range: 5–7 points) on a scale of 0 to 9.
Table 2. Characteristics of eligible studies after adjusting for covariates.
| Study | Total Size | Gender | Comparison | Adjusted RR (95%CI) | Adjustment for covariates | Conclusion |
|---|---|---|---|---|---|---|
| Tankó, 2005 [23] | 2442 | F | Osteoporosis vs Osteopenia | 3.90 (2.00–7.70) | Age, Sex, diabetes, hypertension, hyperlipidemia, smoking, and prior CHD events. | Women with osteoporosis had increased risk for CV events. |
| Marcovitz, 2005 [24] | 209 | M/F | Osteoporosis vs Osteopenia | 5.58 (2.59–12.0) | Age, Sex, BMI, and hypertension, hyperlipidemia, smoking, and prior CHD events. | Low BMD predict significant CAD in women. |
| Reddy, 2008 [29] | 228 | F | Osteopenia vs Normal BMD | 2.70 (1.10–6.80) | Age, Sex, BMI, and other vascular risk factors (Menopause, Diabetes mellitus,Hypertension). | Osteoporosis was strongly associated with the presence of BAC. |
| Osteoporosis vs Normal BMD | 4.40 (1.60–12.0) | Age, Sex, BMI, and other vascular risk factors (Menopause, Diabetes mellitus,Hypertension). | Osteoporosis was strongly associated with the presence of BAC. | |||
| Yesil, 2012 [36] | 2235 | M/F | Low BMD vs Normal BMD | 1.64 (1.07–2.53) | Age, Sex, BMI, and Diabetes mellitus, Hypertension, Smoking, TC. | Significant negative correlation between CAD and OP/osteopenia. |
| Prasad, 2014 [39] | 194 | F | Osteoporosis vs Non- osteoporosis | 2.40 (1.10–5.60) | Age, hyperlipidemia, hypertension, BMI, estrogen use, and steroid use. | Women with CMED were twice as likely to develop osteoporosis. |
| Värri, 2014 [40] | 290 | F | Osteopenia vs Normal BMD | 1.70 (1.00–2.90) | Age, BMI, current smoking, HT use,and systolic blood pressure. | Maximum cIMT but not mean cIMT significantly associated with low BMD. |
| Osteoporosis vs Normal BMD | 4.20 (1.10–15.9) | Age, BMI, current smoking, HT use,and systolic blood pressure. | Maximum cIMT but not mean cIMT significantly associated with low BMD. | |||
| Seo, 2015 [41] | 252 | F | Low BMD vs Normal BMD | 3.35 (1.07–10.6) | Age, alcohol intake, exercise, and vascular risk factors. | Decreased BMD is associated with coronary atherosclerosis in healthy postmenopausal women. |
CV events: cardiovescular events. CMED: coronary microvascular endothelial dysfunction. Normal BMD: T-score>-1; Osteopenia: -1<T-score<-2.5; Osteoporosis: T-score<-2.5. CHD: coronary heart disease. BAC: breast arterial calcification. TC: total cholesterol. HT: hormone therapy.
Results of the population study
The pooled results are shown in Table 3.
Table 3. Summary of outcomes comparing BMD and the incidents of atherosclerosis.
| Outcomes | Gender | Studies | Participants | Odds Ratio, 95% CI | I2 for Heterogeneity | p value |
|---|---|---|---|---|---|---|
| 1. In male and female | ||||||
| Osteopenia vs Normal BMD | M/F | 9 | 1912 | 1.84 [1.45, 2.32] | 0% | p<0.00001 |
| Osteoporosis vs Normal BMD | M/F | 9 | 2491 | 2.05 [1.55, 2.71] | 41% | p<0.00001 |
| Low BMD vs Normal BMD | M/F | 14 | 4933 | 1.81 [1.01, 2.19] | 49% | p<0.00001 |
| Osteoporosis vs Non-osteoporosis | M/F | 11 | 2238 | 2.45 [1.90, 3.17] | 40% | p<0.00001 |
| 2. In postmenopausal women | ||||||
| Osteopenia vs Normal BMD | F | 5 | 651 | 2.07 [1.43, 3.00] | 41% | p = 0.00001 |
| Osteoporosis vs Normal BMD | F | 6 | 367 | 4.29 [2.37, 7.77] | 0% | p<0.00001 |
| Low BMD vs Normal BMD | F | 7 | 1305 | 1.23 [1.72, 2.89] | 39% | p<0.00001 |
| Osteoporosis vs Non-osteoporosis | F | 7 | 1009 | 2.05 [1.13, 3.72] | 61% | p = 0.02 |
| 3. Ajusted for Age, Sex, BMI and other vascular risk factors | ||||||
| Low BMD vs Normal BMD | M/F | 7 | 5641 | 2.96 [2.25, 3.88] | 29% | p<0.00001 |
Primary results
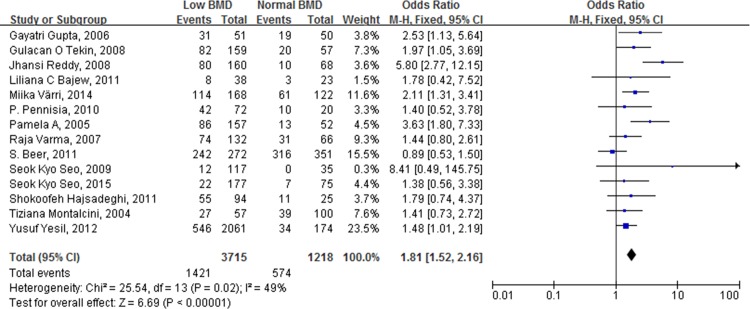
The ORs for each study and the combined OR for patients with low and normal BMD are shown in Fig 2. The combined OR for the incidence of atherosclerotic vascular abnormalities in patients with low BMD, compared to patients with normal BMD, was 1.81 (95% CI [1.01, 2.19], p < 0.00001).
Fig 2. Forest plot shows that the incidence of atherosclerotic vascular abnormalities is significantly higher in individuals (including male and female) with low BMD than those with normal BMD.
Subgroup and sensitivity analysis
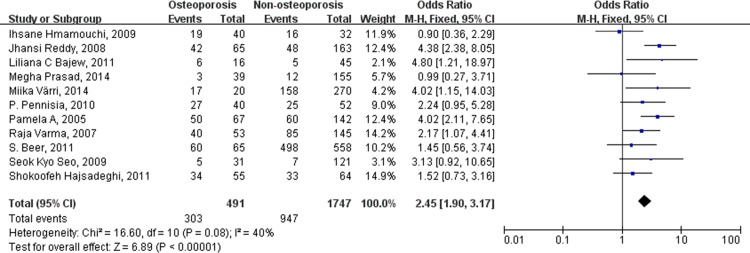
The results of the subgroup analyses that were based on the severity of the decreased BMD are shown in Table 3. The combined OR for the incidence of atherosclerotic vascular abnormalities in patients with osteopenia, compared to patients with normal BMD, was 1.84 (95% CI [1.45, 2.32], p < 0.00001). The combined OR for the incidence of atherosclerotic vascular abnormalities in patients with osteoporosis, compared to patients with normal BMD, was 2.05 (95% CI [1.55, 2.71], p < 0.00001). Finally, when comparing patients with and without osteoporosis, the combined OR for the incidence of atherosclerotic vascular abnormalities was 2.45 (95% CI [1.90, 3.17], p < 0.00001) (Fig 3).
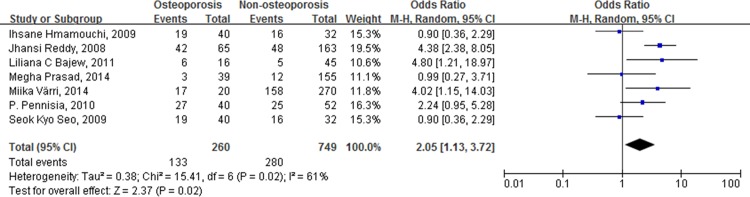
Fig 3. Forest plot shows that the incidence of atherosclerotic vascular abnormalities is significantly higher in individuals (including male and female) with osteoporosis than those without osteoporosis.
No evidence of significant heterogeneity was observed within any subgroup, while a significant association between incidence of atherosclerotic vascular abnormalities and decreased BMD was observed in all subgroups.
To test the robustness of the data, sensitivity analyses were performed by omitting one study at a time. These analyses yielded similar results to those obtained when all studies were analyzed simultaneously. The pooled ORs were all statistically significant.
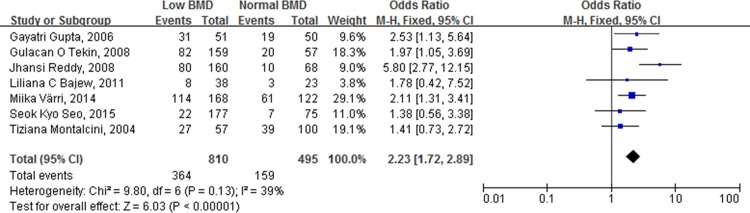
Results of postmenopausal women
When including only postmenopausal women, the combined ORs for the incidence of atherosclerotic vascular abnormalities in patients with low BMDs, versus patients with normal BMDs, was 2.23 (95% CI [1.72, 2.89], p < 0.00001) (Fig 4). When comparing patients with osteopenia to those with normal BMDs, the combined OR was 2.07 (95% CI [1.43, 3.00], p < 0.00001). For patients with osteoporosis versus patients with normal BMD, the combined OR was 4.29 (95% CI [2.37, 7.77], p < 0.00001). Comparing patients with osteoporosis to patients without, the combined OR for the incidence of atherosclerotic vascular abnormalities was 2.05 (95% CI [1.13, 3.72], p = 0.02) (Fig 5).
Fig 4. Forest plot shows that the incidence of atherosclerotic vascular abnormalities is significantly higher in postmenopausal women with low BMD than those with normal BMD.
Fig 5. Forest plot shows that the incidence of atherosclerotic vascular abnormalities is significantly higher in postmenopausal women with osteoporosis than those without osteoporosis.
Thus, the results revealed that under all circumstances, the combined OR for the incidence of atherosclerotic vascular abnormalities increased as BMD decreased.
Adjusted results
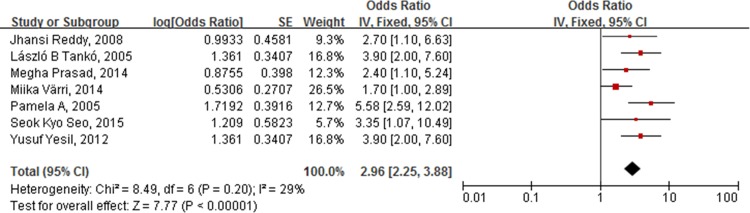
After adjusting for age, gender, BMI, hypertension, and other vascular risk factors, the combined OR for the incidence of atherosclerotic vascular abnormalities in patients with low BMD versus patients with normal BMD was 2.96 (95% CI [2.25, 3.88], p < 0.00001; I2 = 29%) (Fig 6).
Fig 6. Forest plot shows that the incidence of atherosclerotic vascular abnormalities is significantly higher in individuals with low BMD than those with normal BMD, after adjusting for age, gender, BMI, hypertension, and other vascular risk factors.
Publication bias
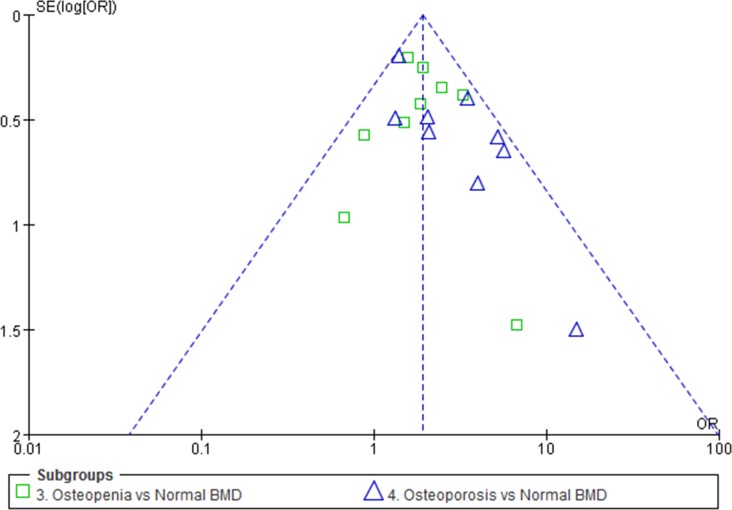
No substantial asymmetry was identified using Begg's rank correlation test (z = 1.25, p = 0.211) and Egger's regression test (t = 1.38, p = 0.187). Funnel plots of the combined ORs for patients with low BMDs, compared to patients with normal BMDs also showed no publication bias (Fig 7).
Fig 7. Funnel plot of combined OR for patients with low BMD compared with patients normal BMD showed no publication bias in visual.
Discussion
The systematic review and meta-analysis presented here revealed a strong link between decreased bone mineral density and the risk of atherosclerotic vascular abnormalities. This finding was independent of age, gender, BMI, hypertension, and other vascular risk factors. The combined ORs increased as BMD decreased. Moreover, the link between deceased bone mineral density and atherosclerotic vascular abnormalities was not only seen in postmenopausal women, but also in elderly men (aged above 50 years). These findings suggest that postmenopausal women and elderly men with decreased BMD have a higher risk of developing atherosclerotic vascular abnormalities. Our results not only confirmed a significant relationship between osteoporosis and atherosclerotic vascular abnormalities, as previous studies have suggested [3, 4, 6–13], but also a relationship between osteopenia and atherosclerotic vascular abnormalities. Based on the results of our study, patients who have decreased BMD scores, should be screened for cardiovascular risk, as it is an independent indicator of significant atherosclerotic burden. Besides, an enhanced understanding of the relationship between decreased BMD and atherosclerosis might lead to the development of therapeutic agents for the prevention and treatment of both osteoporosis and atherosclerotic vascular abnormalities.
In this study, no evidence of significant heterogeneity was observed between studies, except for the comparison between postmenopausal women with and without osteoporosis. The following reasons may account for this finding: the definition of atherosclerotic vascular abnormalities is clear and the severity of bone loss is definite, according to the standard of the WHO. Moreover, we included only high quality studies with a NOS score of five or more (S5 Text). In the subgroup analysis of postmenopausal women with and without osteoporosis, medium heterogeneity (I2 = 61%) was observed. When excluding the data of Reddy et al. [29], which used the breast arterial calcification (BAC) as a defined index of atherosclerotic vascular abnormalities, the heterogeneity reduced to 41% (p = 0.13).
The key pathology in atherosclerotic vascular abnormalities is the formation of calcified plaque and chronic inflammation on the endothelial surface, which progressively leads to plaque rupture and thrombosis (S3 Text)[52–55]. Superficial erosion and fibrous cap rupture are the two possible mechanisms of plaque disruption [54–56]. Inflammatory processes of the vascular wall should play a particularly important role in regulating the integrity of the fibrous cap. It is reported that inflammatory cytokines produced by leukocytes increase degradation of the structural components of the fibrous cap, which further increase the vulnerability of the plaque to rupture [57, 58]. Moreover, biomechanical studies showed that the increased circumferential stress also decrease the stability of the atherosclerotic lesion and increase the incidence of plaque rupture and thrombosis (S3 Text)[59].
Based on multiple biological observations, arterial vascular calcification is highly regulated and organized by mechanisms similar to those involved in bone mineralization [60, 61]. Additionally, several bone matrix proteins, including matrix Gla protein (MGP), osteoprotegerin (OPG), osteopontin (OPN), osteonectin (ON), bone morphogenetic protein (BMP)-2, collagen I, osteocalcin (OC), receptor-activated nuclear factor-kappa B ligand (RANKL), and the mineral hydroxyapatite, also exist in atherosclerotic arteries [62–66]. Inflammatory cytokines like interleukin-1 (IL-1), interleukin-1 (IL-6), tumor necrosis factor (TNF), and osteoclast-like cells (OLCs) have also been reported in calcified arteries (S3 Text)[67].
OPG/RANK/RANKL system is reported to play a key role in calcium deposition and bone formation [68]. RANKL is mainly expressed by osteoblast precursors. The combination between RANKL and RANK promote osteoclast formation and lead to bone resorption [69]. OPG is mainly produced by osteoblasts, and vascular smooth muscle cells (VSMCs), and is a potent inhibitor of osteoclast activation [70]. It is reported that in patients with atherosclerotic vascular abnormalities, OPG is over expressed lining the calcific deposits, while RANKL is expressed in the extracellular matrix around the calcific lesions. The OPG-knockout mice developed both osteoporosis and arterial calcification [71]. These findings suggest that the possible mechanism of OPG/RANK/RANKL on atherosclerosis may due to its differential and site-specific expression [72].
MGP and OC are both Gla-containing proteins that inhibit osteoid formation. It is reported that MGP and OC are both expressed in bone and vascular wall and are both up-regulated in atherosclerotic vessels. Furthermore, as key inhibitors of arterial calcification, MGP and OC inhibit BMP-induced chondrocyte differentiation and osteogenic differentiation of the vascular mesenchyme [73]. In animal experiments, knockout mice models of MGP, displayed both vascular calcification and osteoporosis [60]. In women with osteoporosis and atherosclerosis, serum MGP and OC are also elevated [74]. The possible mechanisms of other bone matrix proteins were shown in (S4 Text), including OPN, ON, BMP etc., which help providing an overview regarding the pathogenesis of concomitant osteoporosis and atherosclerosis.
Besides the potential mechanism introduced above, some characteristics may also influence the risk of atherosclerotic vascular abnormalities in postmenopausal women. Estrogen can prevent the development of atherosclerotic vascular abnormalities. For postmenopausal women, deficiency of estrogen not only impairs normal bone remodeling by breaking the balance of osteoblasts, but also increases the risk of cardiovascular diseases (CAD) [75]. Free iron deposits in tissues, which are responsible for iron overload, also play an important role in inhibiting osteoblast proliferation, which impairs bone remodeling [75]. Postmenopausal women showed a stronger correlation between low BMD and the development of atherosclerotic vascular abnormalities [3, 11, 76, 77]. In a study by Sung et al. [78], the authors showed that both premenopausal and postmenopausal women had a correlation between low BMD and atherosclerotic vascular abnormalities.
Uyl et al. conducted a systematic review in 2011 and indicated that persons with prevalent subclinical CV disease are at increased risk for bone loss, however, the association between different extent of low bone mineral density and increased cardiovascular risk was still not clear [7]. To clarify it, we included 25 studies with a NOS score ≥ 5 points and compared the incidence of atherosclerotic vascular abnormalities in people with different extent of decreased BMD. To our knowledge, this is the first systematic review and meta-analysis comparing the incidence of atherosclerotic vascular abnormalities in individuals with normal BMD, osteopenia and osteoporosis. Moreover, it is a relatively comprehensive and up-to-date summary of data on the topic. Male and female participants were included, and gender-specific analyses were performed in the subgroup analysis. Compared to the original studies, the large number of cases and participants increased the detection of significant associations and provided more precise estimates of their effects.
This systematic review and meta-analysis also has several limitations. Because of the paucity of randomized controlled trials, this study only evaluated case-control, cross-sectional and cohort studies, which decreases the robustness of the conclusions. Secondly, most of the included studies were based on healthy adults, which can limit the generalizability of the findings to patients with multiple atherosclerosis risk factors and comorbidities. Although we managed to conduct subgroup analyses regarding different severity of decreased BMD, we had limited ability to assess the relationship between different pathologic stages of atherosclerotic vascular abnormalities and decreased bone mineral density due to the lack of available studies. Future quantitative research focusing on individuals with different severities of atherosclerotic vascular abnormalities, and patients with different comorbidities, will be of interest.
Conclusion
Based on the results of this study, decreased BMD is an independent predictor for the development of atherosclerosis in elderly individuals. Additionally, the risk increases as BMD decreases. Patients who have decreased BMD scores, should be screened for cardiovascular risks. Future studies focusing on individuals with different severities of atherosclerotic vascular abnormalities and patients with different comorbidities would be of interest.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81572124).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81572124). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine 2004;23(1):1–10. Epub 2004/03/23. 10.1385/endo:23:1:01 . [DOI] [PubMed] [Google Scholar]
- 2.Ng KW, Martin TJ. New therapeutics for osteoporosis. Current opinion in pharmacology 2014;16:58–63. Epub 2014/04/05. 10.1016/j.coph.2014.03.004 . [DOI] [PubMed] [Google Scholar]
- 3.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcified tissue international 2001;68(5):271–6. Epub 2001/10/31. . [DOI] [PubMed] [Google Scholar]
- 4.Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcified tissue international 2006;78(4):195–202. Epub 2006/04/11. 10.1007/s00223-005-0244-z . [DOI] [PubMed] [Google Scholar]
- 5.Beer S, Saely CH, Hoefle G, Rein P, Vonbank A, Breuss J, et al. Low bone mineral density is not associated with angiographically determined coronary atherosclerosis in men. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2010;21(10):1695–701. Epub 2009/11/26. 10.1007/s00198-009-1103-y . [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke; a journal of cerebral circulation 2001;32(1):47–51. Epub 2001/01/04. . [DOI] [PubMed] [Google Scholar]
- 7.den Uyl D, Nurmohamed MT, van Tuyl LH, Raterman HG, Lems WF. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis research & therapy 2011;13(1):R5 Epub 2011/01/19. 10.1186/ar3224 ; PubMed Central PMCID: PMCPmc3241350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. The American journal of medicine 1999;106(3):273–8. Epub 1999/04/06. . [DOI] [PubMed] [Google Scholar]
- 9.Hyder JA, Allison MA, Criqui MH, Wright CM. Association between systemic calcified atherosclerosis and bone density. Calcified tissue international 2007;80(5):301–6. Epub 2007/05/17. 10.1007/s00223-007-9004-6 . [DOI] [PubMed] [Google Scholar]
- 10.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2006;21(12):1839–46. Epub 2006/09/28. 10.1359/jbmr.060903 . [DOI] [PubMed] [Google Scholar]
- 11.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcified tissue international 1998;62(3):209–13. Epub 1998/03/21. . [DOI] [PubMed] [Google Scholar]
- 12.Shaffer JR, Kammerer CM, Rainwater DL, O'Leary DH, Bruder JM, Bauer RL, et al. Decreased bone mineral density is correlated with increased subclinical atherosclerosis in older, but not younger, Mexican American women and men: the San Antonio Family Osteoporosis Study. Calcified tissue international 2007;81(6):430–41. Epub 2007/11/10. 10.1007/s00223-007-9079-0 . [DOI] [PubMed] [Google Scholar]
- 13.van der Klift M, Pols HA, Hak AE, Witteman JC, Hofman A, de Laet CE. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcified tissue international 2002;70(6):443–9. Epub 2002/04/27. 10.1007/s00223-001-2076-9 . [DOI] [PubMed] [Google Scholar]
- 14.Aoyagi K, Ross PD, Orloff J, Davis JW, Katagiri H, Wasnich RD. Low bone density is not associated with aortic calcification. Calcified tissue international 2001;69(1):20–4. Epub 2001/10/31. 10.1007/s002230020003 . [DOI] [PubMed] [Google Scholar]
- 15.Montalcini T, Emanuele V, Ceravolo R, Gorgone G, Sesti G, Perticone F, et al. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. The American journal of cardiology 2004;94(2):266–9. Epub 2004/07/13. 10.1016/j.amjcard.2004.03.083 . [DOI] [PubMed] [Google Scholar]
- 16.Tekin GO, Kekilli E, Yagmur J, Uckan A, Yagmur C, Aksoy Y, et al. Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography. International journal of cardiology 2008;131(1):66–9. Epub 2008/01/29. 10.1016/j.ijcard.2007.09.002 . [DOI] [PubMed] [Google Scholar]
- 17.Celczynska Bajew L, Horst Sikorska W, Bychowiec B, Wykretowicz A, Wesoly J, Michalak M. The effects of osteoprotegerin (OPG) gene polymorphism in patients with ischaemic heart disease on the morphology of coronary arteries and bone mineral density. Kardiologia polska 2011;69(6):573–8. Epub 2011/06/17. . [PubMed] [Google Scholar]
- 18.Hajsadeghi S, Khamseh ME, Larijani B, Abedin B, Vakili-Zarch A, Meysamie AP, et al. Bone mineral density and coronary atherosclerosis. Journal of the Saudi Heart Association 2011;23(3):143–6. Epub 2011/07/01. 10.1016/j.jsha.2011.03.001 ; PubMed Central PMCID: PMCPmc3801144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology 2009;62(10):e1–34. Epub 2009/07/28. 10.1016/j.jclinepi.2009.06.006 . [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology 2010;25(9):603–5. Epub 2010/07/24. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 21.Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. Journal of clinical epidemiology 2007;60(8):849–52. Epub 2007/07/04. 10.1016/j.jclinepi.2006.11.003 . [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMCPmc192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2005;20(11):1912–20. Epub 2005/10/20. 10.1359/jbmr.050711 . [DOI] [PubMed] [Google Scholar]
- 24.Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, et al. Usefulness of bone mineral density to predict significant coronary artery disease. The American journal of cardiology 2005;96(8):1059–63. Epub 2005/10/11. 10.1016/j.amjcard.2005.06.034 . [DOI] [PubMed] [Google Scholar]
- 25.Gupta G, Aronow WS. Atherosclerotic vascular disease may be associated with osteoporosis or osteopenia in postmenopausal women: a preliminary study. Archives of gerontology and geriatrics 2006;43(2):285–8. Epub 2006/01/24. 10.1016/j.archger.2005.11.003 . [DOI] [PubMed] [Google Scholar]
- 26.Varma R, Aronow WS, Basis Y, Singh T, Kalapatapu K, Weiss MB, et al. Relation of bone mineral density to frequency of coronary heart disease. The American journal of cardiology 2008;101(8):1103–4. Epub 2008/04/09. 10.1016/j.amjcard.2007.12.013 . [DOI] [PubMed] [Google Scholar]
- 27.Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H, et al. Relationship between carotid atherosclerosis and lumbar spine bone mineral density in postmenopausal women. Hypertension research: official journal of the Japanese Society of Hypertension 2008;31(6):1191–7. Epub 2008/08/22. 10.1291/hypres.31.1191 . [DOI] [PubMed] [Google Scholar]
- 28.Tamaki J, Iki M, Hirano Y, Sato Y, Kajita E, Kagamimori S, et al. Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2009;20(1):53–60. Epub 2008/05/23. 10.1007/s00198-008-0633-z . [DOI] [PubMed] [Google Scholar]
- 29.Reddy J, Bilezikian JP, Smith SJ, Mosca L. Reduced bone mineral density is associated with breast arterial calcification. J Clin Endocrinol Metab 2008;93(1):208–11. Epub 2007/11/01. 10.1210/jc.2007-0693 ; PubMed Central PMCID: PMCPmc2190738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hmamouchi I, Allali F, Khazzani H, Bennani L, El Mansouri L, Ichchou L, et al. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC public health 2009;9:388 Epub 2009/10/16. 10.1186/1471-2458-9-388 ; PubMed Central PMCID: PMCPmc2768707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikumo M, Okano H, Yoshikata R, Ishitani K, Ohta H. Association between lumbar bone mineral density and vascular stiffness as assessed by pulse wave velocity in postmenopausal women. Journal of bone and mineral metabolism 2009;27(1):89–94. Epub 2008/12/06. 10.1007/s00774-008-0014-x . [DOI] [PubMed] [Google Scholar]
- 32.Seo SK, Cho S, Kim HY, Choi YS, Park KH, Cho DJ, et al. Bone mineral density, arterial stiffness, and coronary atherosclerosis in healthy postmenopausal women. Menopause (New York, NY) 2009;16(5):937–43. Epub 2009/04/24. 10.1097/gme.0b013e3181a15552 . [DOI] [PubMed] [Google Scholar]
- 33.Pennisi P, Russo E, Gaudio A, Veca R, D'Amico F, Mangiafico RA, et al. The association between carotid or femoral atherosclerosis and low bone mass in postmenopausal women referred for osteoporosis screening. Does osteoprotegerin play a role? Maturitas 2010;67(4):358–62. Epub 2010/08/24. 10.1016/j.maturitas.2010.07.013 . [DOI] [PubMed] [Google Scholar]
- 34.Fodor D, Bondor C, Albu A, Muntean L, Simon SP, Poanta L, et al. Relation between intima-media thickness and bone mineral density in postmenopausal women: a cross-sectional study. Sao Paulo medical journal = Revista paulista de medicina 2011;129(3):139–45. Epub 2011/07/15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoud Shokry AAH GI, et al. Relation between postmenopausal osteoporosis and coronary and peripheral arterial disease. Middle East Fertility Society Journal 2012;17::181–6. [Google Scholar]
- 36.Yesil Y, Ulger Z, Halil M, Halacli B, Yavuz BB, Yesil NK, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Archives of gerontology and geriatrics 2012;54(3):473–6. Epub 2011/07/05. 10.1016/j.archger.2011.06.007 . [DOI] [PubMed] [Google Scholar]
- 37.Teng ZY, Pei LC, Zhang Y, Li Y, Wang RT. Whole blood viscosity is negatively associated with bone mineral density in postmenopausal women with osteoporosis. Bone 2013;56(2):343–6. Epub 2013/07/13. 10.1016/j.bone.2013.07.001 . [DOI] [PubMed] [Google Scholar]
- 38.Liang DK, Bai XJ, Wu B, Han LL, Wang XN, Yang J, et al. Associations between bone mineral density and subclinical atherosclerosis: a cross-sectional study of a Chinese population. The Journal of clinical endocrinology and metabolism 2014;99(2):469–77. Epub 2013/11/20. 10.1210/jc.2013-2572 . [DOI] [PubMed] [Google Scholar]
- 39.Prasad M, Reriani M, Khosla S, Gossl M, Lennon R, Gulati R, et al. Coronary microvascular endothelial dysfunction is an independent predictor of development of osteoporosis in postmenopausal women. Vascular health and risk management 2014;10:533–8. Epub 2014/09/12. 10.2147/vhrm.s63580 ; PubMed Central PMCID: PMCPmc4155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varri M, Tuomainen TP, Honkanen R, Rikkonen T, Niskanen L, Kroger H, et al. Carotid intima-media thickness and calcification in relation to bone mineral density in postmenopausal women-the OSTPRE-BBA study. Maturitas 2014;78(4):304–9. Epub 2014/06/24. 10.1016/j.maturitas.2014.05.017 . [DOI] [PubMed] [Google Scholar]
- 41.Seo SK, Yun BH, Noe EB, Suh JW, Choi YS, Lee BS. Decreased bone mineral density is associated with coronary atherosclerosis in healthy postmenopausal women. Obstetrics & gynecology science 2015;58(2):144–9. Epub 2015/03/24. 10.5468/ogs.2015.58.2.144 ; PubMed Central PMCID: PMCPmc4366867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu XY, Li XS, Li Y, Liu T, Wang RT. Neutrophil-lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Archives of gerontology and geriatrics 2015;61(1):76–80. Epub 2015/04/18. 10.1016/j.archger.2015.03.011 . [DOI] [PubMed] [Google Scholar]
- 43.Issever AS, Kentenich M, Kohlitz T, Diederichs G, Zimmermann E. Osteoporosis and atherosclerosis: a post-mortem MDCT study of an elderly cohort. European radiology 2013;23(10):2823–9. Epub 2013/06/01. 10.1007/s00330-013-2903-1 . [DOI] [PubMed] [Google Scholar]
- 44.Kong X, Jia X, Wei Y, Cui M, Wang Z, Tang L, et al. Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima-media in elderly patients with normal renal function. BMC nephrology 2012;13:37 Epub 2012/06/13. 10.1186/1471-2369-13-37 ; PubMed Central PMCID: PMCPmc3406990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Almeida Pereira Coutinho M, Bandeira E, de Almeida JM, Godoi ET, Vasconcelos G, Bandeira F. Low Bone Mass is Associated with Increased Carotid Intima Media Thickness in Men with Type 2 Diabetes Mellitus. Clinical medicine insights Endocrinology and diabetes 2013;6:1–6. Epub 2013/07/19. 10.4137/cmed.s11843 ; PubMed Central PMCID: PMCPmc3682693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YQ, Yang PT, Yuan H, Cao X, Zhu XL, Xu G, et al. Low bone mineral density is associated with increased arterial stiffness in participants of a health records based study. Journal of thoracic disease 2015;7(5):790–8. Epub 2015/06/24. 10.3978/j.issn.2072-1439.2015.04.47 ; PubMed Central PMCID: PMCPmc4454857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim NL, Jang HM, Kim SK, Ko KD, Hwang IC, Suh HS. Association of arterial stiffness and osteoporosis in healthy men undergoing screening medical examination. Journal of bone metabolism 2014;21(2):133–41. Epub 2014/07/10. 10.11005/jbm.2014.21.2.133 ; PubMed Central PMCID: PMCPmc4075267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. American journal of epidemiology 2004;160(6):549–56. Epub 2004/09/09. 10.1093/aje/kwh252 . [DOI] [PubMed] [Google Scholar]
- 49.Hyder JA, Allison MA, Barrett-Connor E, Detrano R, Wong ND, Sirlin C, et al. Bone mineral density and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis 2010;209(1):283–9. Epub 2009/10/13. 10.1016/j.atherosclerosis.2009.09.011 ; PubMed Central PMCID: PMCPmc4254856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada S, Inaba M, Goto H, Nagata-Sakurai M, Kumeda Y, Imanishi Y, et al. Associations between physical activity, peripheral atherosclerosis and bone status in healthy Japanese women. Atherosclerosis 2006;188(1):196–202. Epub 2005/12/01. 10.1016/j.atherosclerosis.2005.10.036 . [DOI] [PubMed] [Google Scholar]
- 51.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 1999;10(4):259–64. Epub 2000/02/29. . [DOI] [PubMed] [Google Scholar]
- 52.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). The New England journal of medicine 1992;326(5):310–8. Epub 1992/01/30. 10.1056/nejm199201303260506 . [DOI] [PubMed] [Google Scholar]
- 53.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. The New England journal of medicine 1987;316(22):1371–5. Epub 1987/05/28. 10.1056/nejm198705283162204 . [DOI] [PubMed] [Google Scholar]
- 54.Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996;94(8):2013–20. Epub 1996/10/15. . [DOI] [PubMed] [Google Scholar]
- 55.Libby P. Molecular bases of the acute coronary syndromes. Circulation 1995;91(11):2844–50. Epub 1995/06/01. . [DOI] [PubMed] [Google Scholar]
- 56.Welt FG, Simon DI. Atherosclerosis and plaque rupture. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 2001;53(1):56–63. Epub 2001/05/01. 10.1002/ccd.1130 . [DOI] [PubMed] [Google Scholar]
- 57.Ambrose JA, Winters SL, Arora RR, Haft JI, Goldstein J, Rentrop KP, et al. Coronary angiographic morphology in myocardial infarction: a link between the pathogenesis of unstable angina and myocardial infarction. Journal of the American College of Cardiology 1985;6(6):1233–8. Epub 1985/12/01. . [DOI] [PubMed] [Google Scholar]
- 58.Smith SC Jr. Risk-reduction therapy: the challenge to change. Presented at the 68th scientific sessions of the American Heart Association November 13, 1995 Anaheim, California. Circulation 1996;93(12):2205–11. Epub 1996/06/15. . [DOI] [PubMed] [Google Scholar]
- 59.Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arteriosclerosis, thrombosis, and vascular biology 1996;16(8):1070–3. Epub 1996/08/01. . [DOI] [PubMed] [Google Scholar]
- 60.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis—from clinical observation towards molecular understanding. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2007;18(3):251–9. Epub 2006/12/08. 10.1007/s00198-006-0282-z . [DOI] [PubMed] [Google Scholar]
- 61.Doherty TM, Detrano RC. Coronary arterial calcification as an active process: a new perspective on an old problem. Calcified tissue international 1994;54(3):224–30. Epub 1994/03/01. . [DOI] [PubMed] [Google Scholar]
- 62.Schmid K, McSharry WO, Pameijer CH, Binette JP. Chemical and physicochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis 1980;37(2):199–210. Epub 1980/10/01. . [DOI] [PubMed] [Google Scholar]
- 63.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. The Journal of clinical investigation 1994;93(6):2393–402. Epub 1994/06/01. 10.1172/jci117246 ; PubMed Central PMCID: PMCPmc294446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. The Journal of clinical investigation 1993;91(4):1800–9. Epub 1993/04/01. 10.1172/jci116391 ; PubMed Central PMCID: PMCPmc288161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arteriosclerosis, thrombosis, and vascular biology 2001;21(12):1998–2003. Epub 2001/12/18. . [DOI] [PubMed] [Google Scholar]
- 66.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. The Journal of clinical investigation 1993;92(4):1686–96. Epub 1993/10/01. 10.1172/jci116755 ; PubMed Central PMCID: PMCPmc288328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes & development 1998;12(9):1260–8. Epub 1998/06/06. ; PubMed Central PMCID: PMCPmc316769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2001;16(2):348–60. Epub 2001/02/24. 10.1359/jbmr.2001.16.2.348 . [DOI] [PubMed] [Google Scholar]
- 69.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93(2):165–76. Epub 1998/05/06. . [DOI] [PubMed] [Google Scholar]
- 70.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001;142(12):5050–5. Epub 2001/11/20. 10.1210/endo.142.12.8536 . [DOI] [PubMed] [Google Scholar]
- 71.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. The Journal of experimental medicine 2000;192(4):463–74. Epub 2000/08/22. ; PubMed Central PMCID: PMCPmc2193236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. The Journal of biological chemistry 1998;273(23):14363–7. Epub 1998/06/11. . [DOI] [PubMed] [Google Scholar]
- 73.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. The Journal of biological chemistry 2001;276(17):14044–52. Epub 2001/03/30. 10.1074/jbc.M008103200 . [DOI] [PubMed] [Google Scholar]
- 74.Bini A, Mann KG, Kudryk BJ, Schoen FJ. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 1999;19(8):1852–61. Epub 1999/08/14. . [DOI] [PubMed] [Google Scholar]
- 75.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. The Journal of steroid biochemistry and molecular biology 2014;142:155–70. Epub 2013/11/02. 10.1016/j.jsbmb.2013.09.008 ; PubMed Central PMCID: PMCPmc4187361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. Journal of internal medicine 2006;259(6):598–605. Epub 2006/05/18. 10.1111/j.1365-2796.2006.01640.x . [DOI] [PubMed] [Google Scholar]
- 77.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. The Journal of clinical endocrinology and metabolism 2004;89(9):4246–53. Epub 2004/09/10. 10.1210/jc.2003-030964 . [DOI] [PubMed] [Google Scholar]
- 78.Choi SH, An JH, Lim S, Koo BK, Park SE, Chang HJ, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clinical endocrinology 2009;71(5):644–51. Epub 2009/02/20. 10.1111/j.1365-2265.2009.03535.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.