Abstract
Although the gastrointestinal (GI) tract possesses intrinsic neural plexuses that allow a significant degree of autonomy over GI functions, the central nervous system (CNS) provides extrinsic neural inputs that regulate, modulate, and control these functions. While the intestines are capable of functioning in the absence of extrinsic inputs, the stomach and esophagus are much more dependent upon extrinsic neural inputs, particularly from parasympathetic and sympathetic pathways. The sympathetic nervous system exerts a predominantly inhibitory effect upon GI muscle and provides a tonic inhibitory influence over mucosal secretion while, at the same time, regulates GI blood flow via neurally mediated vasoconstriction. The parasympathetic nervous system, in contrast, exerts both excitatory and inhibitory control over gastric and intestinal tone and motility. Although GI functions are controlled by the autonomic nervous system and occur, by and large, independently of conscious perception, it is clear that the higher CNS centers influence homeostatic control as well as cognitive and behavioral functions. This review will describe the basic neural circuitry of extrinsic inputs to the GI tract as well as the major CNS nuclei that innervate and modulate the activity of these pathways. The role of CNS-centered reflexes in the regulation of GI functions will be discussed as will modulation of these reflexes under both physiological and pathophysiological conditions. Finally, future directions within the field will be discussed in terms of important questions that remain to be resolved and advances in technology that may help provide these answers.
Introduction
While the gastrointestinal (GI) tract possesses intrinsic neural plexuses that allow a significant degree of autonomy over functions including digestion, nutrient absorption and the elimination of waste, the central nervous system (CNS) provides extrinsic neural inputs that regulate, modulate, and control these functions. The small and large intestines display a considerable degree of independent neural control and are capable of functioning in the absence of extrinsic neural inputs. The stomach and esophagus, in contrast, are much more dependent upon extrinsic neural inputs, particularly from parasympathetic and sympathetic pathways that derive from, or are controlled by, nuclei located in the caudal brainstem. Removal of the extrinsic innervation to the stomach results in disorganized and dysregulated gastric activity that frequently results in symptoms such as nausea and vomiting, abdominal discomfort and pain, and diarrhea. Remarkably, however, gastric activity regains a degree of normality after a period of time suggesting that the role of the extrinsic neural inputs to the stomach are to co-ordinate and regulate the GI tract in a more widespread and integrated manner to maintain appropriate homeostatic control over digestive functions.
The sympathetic nervous system exerts a predominantly inhibitory effect upon GI muscle and provides a tonic inhibitory influence over mucosal secretion while, at the same time, regulates GI blood flow via neurally mediated vasoconstriction. The parasympathetic nervous system, in contrast, exerts both excitatory and inhibitory control over gastric and intestinal tone and motility (i.e., milling, absorption, secretion, and defecation), implying a more finely tuned and complex influence over GI activity. Although GI functions are controlled by the autonomic nervous system and occur, by and large, independently of conscious perception, it is clear that the sympathetic and parasympathetic regulation and modulation of the GI tract are modulated by higher CNS centers that influence homeostatic control as well as cognitive and behavioral functions.
This review will describe the basic neural circuitry of the parasympathetic and sympathetic neural inputs to the GI tract as well as the major CNS nuclei that innervate and modulate the activity of these pathways. The role of CNS-centered reflexes in the regulation of GI functions will be discussed as will modulation of these reflexes under both physiological and pathophysiological conditions. Finally, future directions within the field will be discussed in terms of important questions that remain to be resolved and advances in technology that may help provide these answers. A list of abbreviations can be found in Table 1.
Table 1.
Abbreviations
| AP | Area postrema |
| Arc | Arcuate nucleus of the hypothalamus |
| BNST | Bed nucleus of the stria terminalis |
| CCK | cholecystokinin |
| CNS | Central nervous system |
| CRF | Corticotropin releasing factor |
| CSF | Cerebrospinal fluid |
| DMV | Dorsal motor nucleus of the vagus |
| DVC | Dorsal vagal complex |
| ENS | Enteric nervous system |
| FD | Functional dyspepsia |
| GI | Gastrointestinal |
| GLP-1 | Glucagon-like peptide 1 |
| NANC | Nonadrenergic, noncholinergic |
| NPY | Neuropeptide Y |
| NTS | Nucleus tractus solitarius |
| LC | Locus coeruleus |
| PAG | Periaqueductal gray |
| PBN | Parabrachial nucleus |
| PVN | Paraventricular nucleus of the hypothalamus |
| PYY | Peptide YY |
| SCI | Spinal cord injury |
Parasympathetic innervation to the stomach and upper intestine
Parasympathetic innervation to the stomach, small intestine and proximal colon is supplied by the vagus nerve. Neuronal tracing techniques have shown that the preganglionic motoneurons that provide vagal innervation to the stomach and intestine originate in the dorsal motor nucleus of the vagus (DMV) within the brainstem (10, 11, 48, 49, 257, 437). The stomach receives an especially dense parasympathetic vagal innervation which decreases distally and becomes sparse as one progresses distally through the colon (11, 48, 49). The pharynx and larynx, in contrast, are innervated by preganglionic motoneurons located within the compact as well as the external formation of the nucleus ambiguus. The location of preganglionic neurons innervating the esophagus is species specific and dependent upon whether the esophagus is composed of smooth muscle (cat, rabbit, ferret, and human; originating in the DMV) or striated muscle (rat; originating in the nucleus ambiguus).
Vagal efferent motoneurons
Of the preganglionic parasympathetic motoneurons innervating GI organs, those located within the DMV have been studied most extensively. Rather than being organized in a viscerotopic or organotopic manner, they are organized in “columns” that extend throughout the rostro-caudal extent of the nucleus, and innervate the GI tract through each of the five subdiaphragmatic branches (anterior gastric, posterior gastric, hepatic, celiac, and accessory celiac) (168, 354, 437) which are organized in a medio-lateral arrangement within the brainstem nucleus. DMV neurons with axons projecting via the celiac and accessory celiac vagal branches, for example, are located within the lateral tips of the DMV whereas the cell bodies of axons projecting via the gastric branches are located within the medial left (anterior gastric branch) or right (posterior gastric branch) DMV. Such a level of organization is not maintained peripherally, however; the stomach is innervated by both gastric branches and the hepatic vagal branch, originating in the left DMV, while the duodenum is innervated by all vagal branches. The colon is innervated by both celiac and accessory celiac vagal branches although the density of this innervation decreases distally such that while approximately 65% of myenteric neurons within the cecum receive vagal efferent innervation, less than 20% of neurons in the descending colon are innervated by the efferent vagus (11, 48).
As preganglionic parasympathetic neurons, all DMV neurons are a priori cholinergic and release acetylcholine to activate nicotinic receptors present on postganglionic neurons within the target organ of interest [in this instance, Interstitial Cells of Cajal (ICC) and myenteric neurons within the stomach and upper intestine; reviewed in (422)]. Previously, it was thought that DMV neurons contacted specific “command neurons” within the enteric nervous system to modulate and influence hard-wired motor programs. Several morphological and functional studies have suggested, however, that most enteric neurons receive inputs from vagal fibers and that individual vagal fibers contact many enteric neurons (432, 526) suggesting that the role of the vagus is a more generalized modulation of existing activity levels within enteric neural circuits. Neurotransmitters other than acetylcholine have also been identified within vagal preganglionic neurons within the brainstem, including catecholamines and NO (207, 242, 256, 551); vagal neurotransmission is essentially prevented by nicotinic acetylcholine receptor antagonists, however, suggesting that these noncholinergic neurotransmitters play a modulatory role in cholinergic vagal transmission (432).
Postganglionic neurons within the stomach and upper intestine contacted by vagal efferent fibers form two distinct pathways, though, which allow a very precise, finely tuned control of GI functions. An excitatory cholinergic pathway allows smooth muscle contraction via activation of muscarinic cholinergic receptors on GI smooth muscle whereas a non-adrenergic, non-cholinergic (NANC) pathways allows smooth muscle relaxation via release of predominantly nitric oxide and/or vasoactive intestinal polypeptide (reviewed in (499). Technical constraints have meant that, to date, the vast majority of data on rodents gastric tone and motility has been generated in anesthetized animals; under these conditions, the excitatory cholinergic pathway appears to predominate. In fact, systemic administration of the muscarinic antagonist, atropine, reduces gastric tone and motility dramatically, whereas systemic administration of a NO synthase inhibitor has lesser effect to increase gastric tone and motility. Neurally mediated gastric relaxation, therefore, may be achieved via either withdrawal or inhibition of the tonic excitatory cholinergic pathway or activation of the inhibitory NANC pathway (499).
In contrast, gastric secretion is regulated via vagal inputs to postganglionic neurons that regulate parietal cell activity via postganglionic excitatory muscarinic pathway and a direct inhibition following vagal activation has not been observed (433, 434). The influence of the CNS over gastric secretion was famously first described by William Beaumont in 1833 [reprinted with editorial comments by Combe (40)] who noted that the acid secretion was affected by “fear, anger, and whatever depresses or disturbs the nervous system.” The role of the vagus nerve in gastric secretion was later confirmed by Pavlov in 1902 who noted that the cephalic phase of acid secretion is mediated entirely by the vagus nerve. In contrast, the gastric and intestinal phases of acid secretion involve the activation of both vagal and spinal reflexes in response to GI distention and/or the activation of mucosal receptors [reviewed in (270)]. Neurophysiological studies, notably electrical stimulation or lesion of discrete central nuclei, have highlighted the role of several areas within the brain in the control of gastric acid secretion, particularly the hypothalamus (lateral, ventromedial, and paraventricular nuclei), the locus coeruleus (LC), the caudal medullary raphe nuclei and the amygdala, as discussed in more detail below.
In contrast to the relatively extensive information regarding the central modulation of gastric acid secretion, far less information is available regarding the central control of other gastric secretions, particularly enzyme and mucus secretion. Activation of the caudal raphe nuclei increases gastric pepsin secretion in anesthetized animals in a vagally dependent manner (535). Similarly, stimulation of the DVC induces gastric prostaglandin release, which appears to play a significant role in gastric mucosal protection in response to stress (43, 107, 459).
Properties and organization of vagal efferent motoneurons
Despite the lack of strict viscerotopic organization within the brainstem, DMV neurons appear to exhibit a more function-specific configuration. DMV neurons involved in the inhibitory NANC pathway, for example, appear to be located more caudally within the brainstem, in contrast to DMV neurons within the excitatory cholinergic pathway which appear to be located in the medial and rostral areas of the nucleus (116, 275, 411, 412). Furthermore, Huang et al., have suggested that within the human brainstem, DMV neurons display distinctive morphological properties and are organized into functionally specific groups that innervate particular visceral organs (239). In support of this, studies in rodents have demonstrated that DMV neurons are morphologically heterogeneous (74, 169, 252, 317) and our studies have shown that the biophysical and morphological properties of DMV neurons are correlated with their target organ within the GI tract (74). In brief, it appears that DMV neurons projecting to the gastric fundus are smaller and have fewer dendritic branches as compared to other GI-projecting DMV neurons, with neurons projecting to the large intestine having the largest soma size (74,317). In addition, functional studies have also demonstrated that DMV neurons sensitive to gastric distention are morphologically different from those that respond to intestinal distention (164). It seems likely, therefore, that the properties of DMV neurons are influenced by, or even dependent upon, their function and efferent target.
DMV neurons are also heterogeneous with respect to their membrane properties; several studies have demonstrated that membrane currents are distributed unevenly within DMV neuronal populations (74, 420, 421, 494) and the presence or absence of these currents appears to play prominent roles in determining the excitability of DMV neurons. For example, we have shown that DMV neurons projecting to the stomach are more excitable and fire more action potentials in response to depolarizing current injections as compared to neurons projecting to the intestine (74). In part, this difference appears due to the presence of a smaller and faster action potential afterhyperpolarization in gastric-projecting neurons. While, in DMV neurons, this afterhyperpolarization is due primarily to activation of an apamin-sensitive (SK) calcium-dependent potassium current (74, 420, 421), we have shown recently that damage to DMV neurons, either by distal nerve section (vagotomy) or by perivagal capsaicin application induces the expression of a previously undetectable charybdotoxin-sensitive (BK) calcium-dependent potassium current (70). This would suggest that the intrinsic membrane properties of DMV neurons innervating the GI tract are not static but, rather, are open to expression changes in response to damage or insult.
The smaller and faster action potential afterhyperpolarization in gastric-projecting DMV neurons, coupled with their lower membrane input resistance (74) implies that, compared to intestinal-projecting neurons, they are more liable to respond to changes in synaptic inputs. This is of particular importance when one considers that DMV neurons are tonically active and fire action potentials spontaneously at a low frequency (~1 Hz) (22, 316, 494, 496). Minor changes in synaptic inputs or membrane currents, therefore, can have dramatic effects upon neuronal firing patterns hence vagal efferent outflow.
The activity of DMV neurons, and hence vagal efferent activity, is also regulated by synaptic inputs from several brainstem and higher CNS nuclei. As mentioned previously, gastric functions have been studied most commonly in anesthetized animals; under these “basal” conditions, the activity of DMV neurons innervating the GI tract is regulated by an ongoing, tonic inhibitory GABAergic input (22, 175, 447, 496). The majority of these inhibitory inputs arise from the adjacent nucleus tractus solitarius [NTS; (499)] although DMV neurons also receive innervation from several other brainstem and higher CNS nuclei (see below). While DMV neurons also receive excitatory glutamatergic and catecholaminergic inputs from the NTS, they do not appear to be as critical in regulating the tonic activity of vagal efferent motoneurons (412, 447). It is also important to note, at this point, that several morphological studies have shown that the dendritic projections of DMV neurons can extend into the NTS, the area postrema and even penetrate the ependymal layer of the fourth ventricle (407, 437) suggesting that vagal motoneurons are capable of responding to circulating factors directly. Additionally, the brainstem area containing the dorsal vagal complex (DVC, i.e., DMV, NTS, and area postrema) is essentially a circumventricular organ, lying ventral to the fourth ventricle and is highly vascularized with fenestrated capillaries (111, 171). An additional layer of neurons, termed subependymal cerebrospinal containing neurons (CSF-cNs) are positioned, strategically, between the CSF and neuronal parenchyma and potentially integrate CSF signaling with autonomic homeostatic signaling (361). Indeed, we have demonstrated previously that brainstem neurons can be excited following peripheral administration of the GI neurohormone, cholecystokinin (CCK) even after surgical removal of vagal afferent input to the brainstem (31) implying that the activity of central vagal neurons, including DMV neurons, may be modulated directly by circulating factors.
The DVC is innervated by several brainstem and higher CNS centers involved in autonomic regulation. NTS neurons, which provide the most prominent source of synaptic inputs to GI-projecting DMV neurons, play a critical role in shaping the activity of the efferent vagus nerve (Fig. 1). In addition to their involvement in vagal autonomic neurocircuits via their projections to vagal efferent motoneurons, NTS neurons are also the recipient of inputs from vagal afferent (sensory) fibers that innervate the thoracic and abdominal viscera. Indeed, while the vagus is a mixed sensory/motor nerve, it is predominantly sensory with 70% to 80% of vagal fibers being of sensory origin depending on the species (reviewed in (52)). Vagal afferents that transduce and relay information from the GI tract can be classified based upon their response to distention or pressure (principally low-threshold, although nociceptive high-threshold fibers also exist), the location of their receptive fields (mucosal, muscle, or serosal/mesenteric), their preferred stimulus modality (chemical, osmotic, mechanical, both in-series and in-parallel) or the region of GI tract innervated [reviewed in (52)]. Regardless of their sensory modality or function, the cell bodies of these vagal afferent fibers lie within the nodose ganglia (or nodose-petrosal-jugular ganglion complex) and their central terminals enter the brainstem via the tractus solitarius, and impinge, principally, onto NTS neurons. Within the NTS, neurons are organized in subnuclei which display a viscerotopic manner relative to their afferent inputs (10, 12, 36). Gastric vagal afferents, for example, innervate neurons within the NTS gelatinosus and commisuralis while esophageal afferents innervate neurons within the NTS centralis (10, 69). While cardiovascular and respiratory afferents innervate neurons within the medial and ventrolateral NTS (333, 400), it is important to recognize that the dendritic projections of NTS extent throughout the nucleus and intermingle within the various subnuclei [reviewed in (253)] providing a potential means by which homeostatic reflexes may be co-ordinated across autonomic organs.
Figure 1.
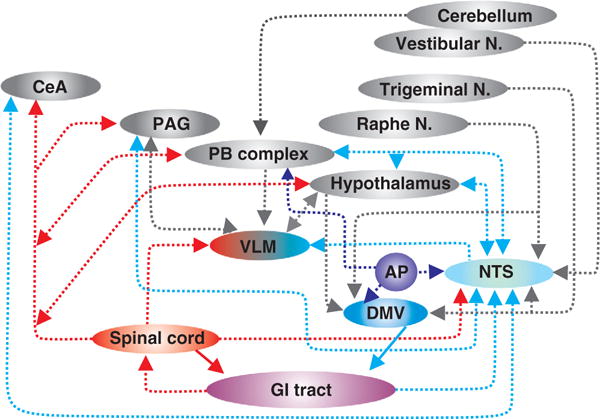
Schematic representation of neuroanatomical connections between the gastrointestinal (GI) tract and central nuclei involved in the regulation of gastrointestinal functions. Note that the location of nuclei is not intended to be anatomically accurate. AP—area postrema; DMV—dorsal motor nucleus of the vagus; NTS—nucleus of the tractus solitarius; PB Complex—parabrachial complex (i.e., parabrachial nucleus + Kölliker-Fuse nucleus); PAG—periaqueductal gray; CeA—central nucleus of the amygdala; Vestibular N—vestibular nucleus; Trigeminal N—trigeminal nucleus; Raphe N—raphe nuclei.
The central terminals of vagal afferent fibers innervate NTS neurons and use principally glutamate as a neurotransmitter (13–15,18,32,62). While activation of ionotropic glutamate receptors (NMDA and non-NMDA receptors) is critical in the transmission of visceral information to the NTS, several metabotropic receptors (mGluR) have also been identified within the DVC. Receptors from all three subtype groups of mGluR have been identified within the DVC (20,79,102,103, 186–189, 255). Group I mGluR are predominantly located postsynaptically (212) while Group II and group III mGluR are predominantly at presynaptic sites and modulate synaptic transmission (20, 79, 102, 186, 188, 211, 546). The presence of and location of mGluR within central vagal neurocircuits suggests that the glutamate released from vagal afferent terminals, in addition to relaying vagal afferent information, may also exert a longer term, more modulatory role over the activity of vagal neurocircuits.
Modulation of DMV neuronal activity
Studies from several groups, including our own, have shown that the excitability of DMV neurons can be modulated by numerous neurotransmitters, neurohormones, and neuromodulators that act either directly on the DMV neuronal membrane or indirectly, to modulate the activity of synaptic inputs, including several neuroactive substances known to be involved in the central modulation of GI functions such as serotonin (75,495), dopamine (90,550), norepinephrine (NE) (50, 318), opioid peptides (72, 73, 402), orexin (192), oxytocin (86, 229), corticotropin releasing factor (291, 460, 501), endocannabinoids (138, 185), neurokinins (281, 284, 292), CCK (32,83,231,549), thyrotropin-releasing hormone [TRH; (70,497)] and glucagon-like peptide 1 (71, 228).
The response of vagal motoneurons to neurotransmitters or neuromodulators does not appear to be static, however. Recent evidence from several laboratories, including our own, has shown that a significant degree of plasticity with vagal neurocircuits occurs in response to physiological or pathophysiological conditions. For example, while GABAergic projections from the NTS-DMV exert the most significant influence over DMV neuronal activity, hence vagal efferent output, only a few neurotransmitters or neuromodulators have been shown to modulate GABAergic synaptic transmission to GI-projecting DMV neurons while the majority of transmitters/modulators are able to regulate glutamatergic synaptic transmission (72, 75, 77, 128, 137, 138). Presynaptic inhibitory effects of many of these neurotransmitters or modulators can be induced, however, following activation of the cAMP-protein kinase A (PKA) pathway within brainstem neurocircuits either by direct activation of cAMP by forskolin or indirect activation via treatment with neurohormones such as TRH, CCK, or GLP-1, which are known to be involved in digestive functions and that act on receptors positively coupled to adenylate cyclase. Activation of the cAMP-PKA pathway appears to induce trafficking of receptors to GABAergic terminals allowing modulation of inhibitory synaptic transmission to be uncovered (73, 76, 80).
Further investigations revealed the mechanism behind this apparent disparity between glutamatergic and GABAergic synaptic transmission; GABAergic nerve terminals impinging upon GI-projecting DMV neurons contain group II mGluR that are tonically active via glutamate released from monosynaptic vagal afferent terminals (79). Activation of these presynaptic group II mGluR decreases cAMP levels within GABAergic nerve terminals, preventing the actions of neurotransmitters/modulators negatively coupled to adenylate cyclase (e.g., 5-HT1A, NPY/PYY Y1 and Y2, α-2-, and μ-opioid receptors) from exerting any modulation over inhibitory synaptic transmission. Preventing activation of these group II mGluR, either by surgically removing vagal afferent inputs to the brainstem, or by administration of selective group II mGluR antagonists, increases cAMP levels, activates a cAMP-PKA cascade, and uncovers presynaptic inhibitory actions of these neurotransmitters/modulators (79, 80, 84). In contrast, while excitatory glutamatergic terminals impinging upon GI-projecting DMV neurons display functional group II and group III mGluR, they are not active tonically, hence, cAMP levels within excitatory terminals are higher and the same neurotransmitters/modulators can regulate glutamatergic synaptic transmission (79).
This suggests that ongoing vagal afferent activity plays a prominent role in setting the “tone” or “state of activation” of vagal brainstem neurocircuits by regulating the ability of GABAergic neurotransmission to be modulated. Decreasing vagal afferent input, either by inhibiting their activation, or by damage to vagal fibers, would be expected to alter the “gain” of vagal efferent output by allowing modulation of the tonic GABAergic input controlling DMV neuronal activity. In addition, neurohormones and neuromodulators involved in the regulation of gastric functions such as CCK, GLP-1, and CRF, which act to activate adenylate cyclase, directly overcome the effects of group II mGluR to decrease cAMP levels within GABAergic terminals, will similarly alter the “gain” of vagal efferent output [reviewed in (78, 81, 82)].
To date, plasticity within central vagal neurocircuits in response to modulation of the cAMP-PKA second messenger system has certainly been studied in the greatest detail. To assume this is the only second messenger system that can modulate the response and activation of central vagal neurocircuits, however, would appear to be unreasonably simplistic. It would appear a far more realistic proposition to assume that other second messenger systems are equally capable of modulating the “state of activation” of central vagal neurocircuits, hence correspondingly capable of modulating vagal efferent control of GI functions.
Parasympathetic control of the colon
While the vagus nerve provides innervation to the proximal colon, the majority of parasympathetic innervation to the distal colon originates from preganglionic neurons within the lumbosacral spinal cord, prominently from S1–S4 regions (11, 51, 129, 348, 357, 525). These sacral preganglionic neurons form a continuous band of cells deep within the dorsal intermediolateral (IML) column and are smaller and spatially separate from those preganglionic neurons innervating the bladder [reviewed in (249)]. While preganglionic neurons innervating the colon are, as elsewhere, cholinergic, immunohistochemical studies have identified populations of preganglionic parasympathetic enkephalinergic neurons within the S2–S3 segments of the sacral spinal cord of the rat and cat (190, 262). While it is not known whether these enkephalinergic neurons are involved in parasympathetic regulation of colonic motility, opioid peptides have been shown to act presynaptically to modulate acetylcholine release from colonic-projecting preganglionic neurons (265) suggesting that neurally mediated release of enkephalins may modulate cholinergic transmission.
Regardless of their neurochemical phenotype, the axons of preganglionic parasympathetic neurons follow two pathways from the spinal cord to the colon; neurons either innervate postganglionic neurons within the myenteric plexus directly, or they innervate postganglionic neurons within ganglia of the pelvic (hypogastric) plexus (178, 282). Neurons of the pelvic ganglia then innervate neurons within the myenteric plexus of the distal colon and rectum via the rectal nerves. As reported for the parasympathetic innervation of the stomach and upper intestine, there appears to be a dual parasympathetic control of the colon. Stimulation of the pelvic or rectal nerves induces either (i) an increase in motility, via activation of an atropine-sensitive, cholinergic pathways, or (ii) an inhibition of motility, via activation of an atropine-insensitive, NANC pathway (155) that possibly involves release of nitric oxide or purines (267, 357, 518).
The parasympathetic neural innervation to the colon plays a significant role in regulating propulsive colonic motility, particular prior to defecation. Damage to parasympathetic nerves, or extrinsic denervation, frequently results in dysregulated colonic motility and constipation. As following extrinsic denervation of the stomach, however, over time colonic motility patterns may be somewhat restored suggesting the role of parasympathetic inputs is to enhance and modulate intrinsic motility patterns rather than initiate or terminate colonic functions.
Sympathetic control of the gastrointestinal tract
Sympathetic preganglionic neurons innervating the GI tract arise from the thoracic and lumbar spinal cord. The majority of neurons innervating the stomach arise from T6–T9 thoracic levels while neurons innervating the colon arise predominantly from spinal L2–L5 lumbar levels, depending on the species, although in humans some neurons may reside within the caudal thoracic segments. As with all preganglionic neurons, these sympathetic neurons are cholinergic, but several reports also describe the presence of small neuropeptides which may exert a modulatory influence of cholinergic transmission. Sympathetic preganglionic neurons activate postganglionic neurons that are contained within well-defined ganglia outside of the stomach and intestine. Most sympathetic postganglionic neurons innervating the GI tract are contained within prevertebral ganglia although some fibers can arise from neurons within paravertebral ganglia (sympathetic chain ganglia) [see (446) for review]. Postganglionic sympathetic neurons innervating the stomach are contained within the celiac ganglion, while neurons innervating the intestine reside in the superior mesenteric ganglion (small intestine), the inferior mesenteric ganglion (colon) or pelvic ganglion (colon). [NB—pelvic ganglia, therefore, are rather unique in that they contain the cell bodies of both postganglionic sympathetic and parasympathetic neurons. Cross-talk between these neurons allows for integration between sympathetic and parasympathetic regulation of the colon; (249).] Postganglionic sympathetic neurons use NE as their primary neurotransmitter although release of neuropeptides, including neuropeptide Y (NPY), somatostatin, and galanin, as well as purines have been described [reviewed in (247)].
Historically, Eduard Pflüger (1857) was the first to note that activation of the splanchnic sympathetic innervation to the GI tract inhibited motility but constricted sphincters [reviewed more recently in (247,305)]. While sympathetic fibers provide a dense innervation to sphincters, and induce constriction via a direct action on smooth muscle, innervation to circular and longitudinal smooth muscle is relatively sparse. Sympathetic fibers innervate principally enteric neurons (both myenteric and submucosal) and, with the advent of neural recording techniques, it was demonstrated that the sympathetic fibers can act pre- and postsynaptically to modulate the activity of enteric neurons either via direct actions on the enteric neuronal membrane (65, 355, 438, 467) or indirectly, via actions to modulate neurotransmitter release (226,438,467,524). The vascular bed of the entire GI tract is densely innervated by sympathetic fibers and regulation of GI vascular tone is an important modulator of blood pressure (195).
More recently, sympathetic innervation of gut-associated lymphoid tissue and modulation of GI immune function has been described. Sympathetic fibers innervate Peyer’s patches within the intestine (156) and macrophages (377) providing a potential means by which immune functions can be modulated. Enteric glial cells are also innervated by sympathetic fibers; while the precise role of enteric glia is unclear, they are known to respond to ATP released by sympathetic fibers which suggests a means by which the activity and functions of enteric neurons may be integrated and modulated (203–205).
Despite the dense sympathetic innervation to the GI tract, there appears to be little tonic sympathetic activity, at least with regard to GI motility although splanchnic nerve section does result in an moderate increase in peristaltic activity (38,39). Sympathetic fibers do provide a tonic level of activity to vasoconstrictor and secretomotor neurons, however; sympathectomy increases GI secretion (540) while stimulation of sympathetic nerves or exogenous application of NE increases fluid absorption, independently of alterations in vascular tone (197, 367), providing a means by which fluid secretion or absorption can be modulated, as required, by alterations in sympathetic tone. The activity of sympathetic motor and secretomotor neurons is modulated by inputs from submucosal and myenteric neurons, local viscerofugal neurons as well as inputs from spinal sensory afferent neurons [reviewed in (515, 516)]; the influence of the sympathetic nervous system over GI functions is modulated, therefore, in an ongoing manner, by local activity within the intestine. In contrast, sympathetic vasoconstrictor neurons regulate GI blood flow independently of viscerofugal enteric neurons and are regulated instead by descending CNS inputs.
CNS regulation of sympathetic and parasympathetic control of gastrointestinal functions
As mentioned earlier, vagal neurons within the hindbrain receive inputs from several other brainstem and higher CNS nuclei involved in the regulation of autonomic GI functions. The use of retrograde transneuronal viruses has enabled the identification of CNS nuclei that innervate preganglionic neurons involved in the regulation of GI targets, although distinguishing sympathetic from parasympathetic preautonomic pathways has been hampered by the close interaction between afferent projections from both pathways at the level of the brainstem, particularly the NTS. Further difficulties arise in separating innervation to the NTS from that of the DMV due to the close intermingling of dendrites from neurons of both nuclei. Nevertheless, studies in which transsynaptic virus labeling has been carried out in conjunction with selective denervation of sympathetic or parasympathetic afferents has allowed some delineation of parasympathetic versus sympathetic pathways (85,251,408; for similar studies investigating the central innervation of the pancreas).
Spinal Cord
Visceral organs, including the several regions of the GI tract (stomach, small intestine, and proximal large intestine), receive dual innervation from both the sympathetic and parasympathetic nervous systems. As described previously, sympathetic afferent fibers from the GI tract terminate in the thoracic (T1–T13) spinal cord. Several neuroanatomical tracing studies have demonstrated that ascending projections from dorsal horn of the spinal cord project directly to several CNS structures involved in the processing of GI autonomic information, including the NTS (spinosolitary tract), the hypothalamus (spinohypothalamic tract), the PB complex (spinoparabrachial tract) as well as the periaqueductal gray (PAG) and amygdala via the spinomesencephalic and spinotelencephalic tracts, respectively (87,88,99,302,329,330,481). Many of these CNS nuclei also receive parasympathetic sensory information, albeit indirectly via NTS efferent projections; this allows for a considerable degree of consolidation and integration of visceral (and somatic) information at multiple sites within the CNS.
The NTS, however, is the only central nucleus that is the recipient of both direct parasympathetic and sympathetic sensory information (Fig. 1). It is widely accepted that nociceptive information is relayed centrally via spinal afferent neurons while visceral reflex and integrative functions are transmitted by vagal afferents. Several lines of evidence, however, indicate that spinal visceral afferents are also activated by low pressure distention and innocuous chemical stimulation and therefore may also relay non-nociceptive information centrally (250,375,398) while vagal afferents also respond to noxious stimulation (490,491). This convergence of vagal and spinal afferent sensory inputs within the NTS allows a significant degree of overlap between the integration and processing of sympathetic and parasympathetic visceral information. As a consequence, vagal afferents are also able to modulate nociceptive processing (248, 387, 397) while spinal afferents can modulate vagal efferent motor control of the upper GI tract (234, 398).
Hindbrain nuclei regulating gastrointestinal functions
Area postrema
The area postrema is a circumventricular area located dorsal to the NTS that was identified as an emetic chemoreceptor trigger zone in the 1940 to 1950s (63, 64, 530). As with other circumventricular organs, the area postrema is highly vascularized with fenestrated capillaries and, as a result, is less isolated from the peripheral circulation by the blood brain barrier than other CNS nuclei (111, 171). Furthermore, the dendrites of area postrema neurons extend towards the basal lamina side of vascular endothelial cells allowing them to receive, and respond to, blood-borne information from the periphery (382). Additionally, the dendrites of NTS and even DMV neurons have been observed to project towards the ependymal layer of the fourth ventricle allowing them to also respond directly to circulating mediators (437). The majority of area postrema neurons are spontaneously active due to the presence of a hyperpolarization-activated cation current [IH, (442)] and primarily innervate the adjacent NTS but also send projections to the nucleus ambiguus, DMV and parabrachial nucleus (171, 382) (Fig. 1). Area postrema neurons contain receptors for several neurotransmitters, neuromodulators, and immune mediators [reviewed in (127,382)], hence, are ideally situated to allow modification of vagal efferent outflow to the GI tract, including emetic reflexes, in response to circulating factors.
Reticular formation
The reticular formation (or substantia reticulata), named for its mesh-like appearance, is a diffuse network of cells that surrounds the more compact, named nuclear structures of the brainstem. Although more recognized for its role in the integration of cardiovascular and respiratory functions, the reticular formation is also involved in several GI reflexes. Physiological as well as noxious gastric distention has been reported to induce vagal afferent-mediated reflex alterations in blood pressure and heart rate in rats, cats, and pigs (306, 356, 482, 504), effects that may involve the activation of neurons within the reticular formation (338,419). The integration of cardiovascular with GI reflexes within the reticular formation may provide a neuroanatomical basis for clinical findings that stimulation of gastric mechanoreceptors modulates hemodynamic reflexes, including, for example, decreased coronary blood flow and cardiac contractility following gastric distention (503). The integrative capacity of the reticular formation may also play a role in the co-ordination of several autonomic reflexes required for the generation of vomiting, which is associated with an increase in blood pressure, altered heart rate, and co-ordinated activity of several respiratory muscle groups in addition to the well-recognized modulation of GI and esophageal functions [reviewed in (19)].
Ventrolateral medulla
It has been known for some time that stimulation of abdominal vagal afferents increases arterial blood pressure (114, 184, 351) and that this response is abolished by vagotomy (114). Anatomical and functional studies have demonstrated a robust excitatory input from NTS neurons (the recipient of vagal afferent sensory information) to neurons within the caudal ventrolateral medulla and the rostral ventrolateral medulla (RVLM; premotor sympathoexcitatory neurons). Neurons within the RVLM then project directly to sympathetic preganglionic neurons within the IML column of the spinal cord to regulate vasomotor outflow [reviewed in (208)]. Vagal afferents are activated by a variety of stimuli [mechanical, chemical, and osmotic, for example; reviewed in (52)] and activation of RVLM neurons in response to more physiological stimuli have also been reported, including low intensity (i.e., non-noxious) gastric distention (419) and chemical stimulation, for example, bitter tastants (210). Systemic CCK, in contrast, inhibits the activity of RVLM neurons that regulate sympathetic vasomotor outflow to the splanchnic circulation. In this manner, CCK released from enteroendocrine cells in response to a mixed meal results not only in a vago-vagal reflex relaxation of the stomach (231, 392) but also mediates postprandial blood flow to the guts via a GI vagal-sympathetic vasomotor reflex (428, 521).
The NTS is the first relay network for GI, gustatory, cardiovascular, and respiratory sensory information from the viscera and projections from the NTS to the VLM allow for the integration of autonomic reflexes (Fig. 1). In addition, both the NTS and the VLM also have reciprocal connections with, and convey visceral afferent information to, the central nucleus of the amygdala (CeA) and the PAG. As described in more detail below, neural projections to and from these nuclei are important for the co-ordination of behavioral responses such as learning/memory and fear/anxiety with autonomic homeostatic control (522).
Trigeminal nucleus
While the trigeminal system is involved predominantly with processing sensory inputs from the head and face, neurons within the oral part of the spinal trigeminal nucleus are activated following high frequency/high intensity gastric distention (419). The existence of a nasogastric reflex in humans has been proposed; this reflex involves activation of trigeminal afferent fibers in response to nasal irritation resulting in gastric relaxation and increased acid secretion mediated via vagal efferent nerves (307, 444). Certainly, neuroanatomical studies using anterograde tracers have indicated that the anterior ethmoidal nerve within the nose, a major component of the afferent pathway of the diving reflex, innervates the trigeminal nucleus and NTS (365). The existence of a naso-gastric reflex is still somewhat controversial, however, and the GI symptoms observed following trigeminal stimulation may be the GI effects secondary to activation of the trigeminocardiac reflex (431).
Raphe nuclei
Several lines of evidence have suggested that the medullary raphe nuclei are an important site of vagally mediated modulation of gastric activity (237, 464). Microinjection of TRH into the DVC increases gastric motility and acid secretion in a vagally dependent manner (221, 452, 463, 535). Additionally, activation of neurons within the raphe pallidus, raphe obscurus, or parapyramidal regions induces a vagally dependent increase in gastric acid secretion that is attenuated by pretreatment with DVC microinjection of a TRH antibody (176, 222). TRH has also been shown to excite DMV neurons via direct actions on their neuronal membrane (497). Immunohistochemical studies showed a high density of TRH-immunoreactive fibers within the DVC (236, 310) that were eliminated following transection of the pathway from the medullary raphe nuclei to the DVC (364). Medullary raphe neurons have also been shown to contain and release 5-HT and substance P which have also been implicated in modulation of gastric functions including motility and secretion (273, 464, 476, 477) via actions at DVC neurons (7, 75, 76, 292, 378, 532).
Innervation of the DVC by TRH-containing medullary raphe neurons has been implicated in the increased gastric acid secretion in response to two distinct physiological reflexes. Firstly, the cephalic phase of digestion induces an increase in gastric acid secretion in response to the sight, sound, smell, taste, and swallowing of food; this vagally mediated response was subsequently demonstrated to be blocked by intracisternal application of TRH receptor antisense oligonucleotides [(319); reviewed in (381)]. Secondly, exposure to cold increases gastric motility and acid secretion in a vagally dependent manner, subsequent to activation of raphe pallidus, raphe obscurus and parapyramidal neurons (89, 464). These findings suggests that the medullary raphe nuclei play an important role in the regulation of vagally mediated gastric functions, particularly gastric acid secretion, and this neurocircuitry can be activated by both physiological and pathophysiological conditions.
Midbrain nuclei regulating gastrointestinal functions
Cerebellum and Vestibular System
While the cerebellum is traditionally considered as a subcortical motor center, it is clear that it also plays important roles in several GI-related autonomic functions. Claude Bernard, in 1858, famously described hyperglycemia resulting from cerebellar lesions. The cerebellum also certainly appears to be involved in the nausea and vomiting associated with motion sickness, although this appears to be a modulatory, rather than the principle, role (332). Recent behavioral studies have noted that cerebellar lesions results in altered food intake and decreased body weight in rodents (553).
Cerebellar neurons are activated following gastric distention in both humans (280) and rodents (336). Additionally, neurophysiological experiments have also shown that neurons within the DVC, including both NTS and DMV neurons, receive convergent inputs from the cerebellar or vestibular system and the GI tract (293, 453) (Fig. 1). The cerebellum is able to influence and modulate GI functions by way of indirect inputs to the brainstem; cerebellar neurons innervate the DVC via the vestibular nucleus (cerebellovestibular fibers) as well as from the hypothalamus (cerebellohypothalamic fibers) via the parabrachial and Kölliker-Fuse nucleus [see above; (27, 453)]. Following stimulation of the cerebellum, gastric, and intestinal motility, as well as gastric acid secretion, are modulated by both vagally dependent as well as vagally independent pathways (298, 314).
It appears, therefore, that the cerebellum does more than co-ordinate motor activity, playing an important role in the co-ordination of somato-visceral reflexes including the integration of several GI functions. The role of the cerebellum in the control of food intake as well as its involvement in several other autonomic pathways including cardiovascular, respiratory, emotional and cognitive [reviewed by (553)] suggests a more wide-spread integrative capability.
Parabrachial Complex
The parabrachial nucleus (PBN), within the brachium of the pons, is composed of several subnuclei; the medial PBN is part of the gustatory system while the lateral PBN is part of the visceral sensory system. The medial PBN receives gustatory information from the NTS whereas the lateral PBN, in contrast, receives GI viscerosensory information from both the NTS and the AP (218) (Fig. 1). The PBN, together with the Kölliker-Fuse nucleus, positioned within the ventrolateral extent of the nucleus, forms the parabrachial complex (PB complex). The PB complex integrates the gustatory and GI sensory information received from the NTS together with general visceral information (cardiovascular, respiratory, and nociceptive) received from the NTS and AP plus GI and seomatosensory information from the cerebellum (408). Indeed, studies have demonstrated that PB complex neurons may receive converging inputs from vestibular and visceral vagal afferents (453) and from orosensory and visceral vagal afferents (259) suggesting some potential for integration of afferent information.
PB complex neurons relay this sensory information to a number of structures including the thalamus, insular cortex, central nucleus of the amygdala (CeA) and lateral hypothalamus (172) (Figs. 1 and 2). Neurons of the PB complex also receive reciprocal connections from several of these higher ascending structures, including the cortex, basal forebrain and hypothalamus (337). Descending projection from the PB complex innervate the RVLM as well as the NTS (218) while descending projections from the Kölliker-Fuse nucleus innervate the sympathetic preganglionic column of the spinal cord, the nucleus ambiguus, the NTS and the rostro- and ventrolateral medulla (448). The PB complex, therefore, occupies an important position as an integrative relay between brainstem and forebrain integrative autonomic regulation and provides a neuroanatomical substrate for the involvement and integration of affective and emotional responses to visceral, including GI and gustatory, sensory information (259).
Figure 2.
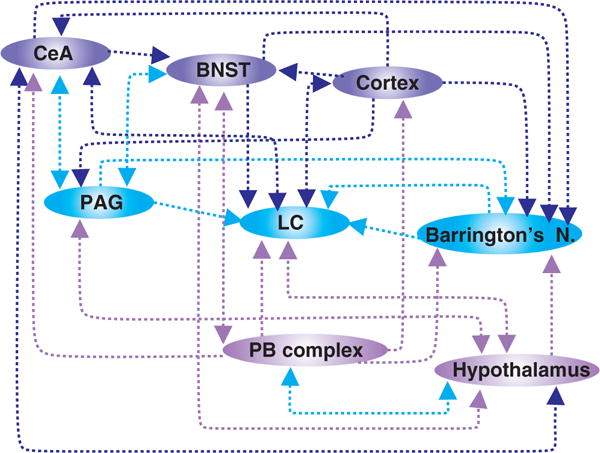
Schematic representation of the neuroanatomical connections between midbrain and forebrain structures involved in the regulation of gastrointestinal (GI) functions. Note that the location of nuclei is not intended to be anatomically accurate. CeA—central nucleus of the amygdala; BNST—bed nucleus of the stria terminals; PAG—periaqueductal gray; LC—locus coeruleus; Barrington’s N—Barrington’s nucleus; PB complex—parabrachial complex (i.e., parabrachial nucleus + Kölliker-Fuse nucleus).
Hypothalamus
The hypothalamus, located rostral to the brainstem, just below the thalamus, is composed of a number of nuclei that regulate a variety of autonomic functions including linking the nervous and endocrine systems via the pituitary gland. With regard to central regulation of GI functions, the paraventricular nucleus of the hypothalamus (PVN) and the arcuate nucleus are of particular importance.
The PVN receives both ascending and descending inputs from structures involved in the autonomic regulation of GI functions including the spinal cord, NTS, PB complex, bed nucleus of the stria terminalis (BNST), CeA [reviewed in (457) (Figs. 1 and 2)]. The PVN itself contains discrete subpopulations of neurons that subserve distinct functions; magnocellular neurons, for example, contain oxytocin (OXY) or vasopressin and release these neurotransmitters into the posterior pituitary gland (i.e., neurohypophysis). Parvocellular neurons, in contrast, contain hormones, particularly corticotrophin-releasing hormone (CRH; also known as corticotropin-releasing factor, CRF) and OXY and either release these neurohormones into the hypophysial portal system or project to other central nuclei involved in autonomic regulation and control, including the PAG, PB complex, DVC, ventrolateral medulla, and sympathetic preganglionic neurons in the spinal cord (308, 426, 457) (Figs. 1 and 2). Many studies have reported the importance these hypothalamic neuropeptides, particularly in the response of the GI tract to stress. CRF, the prototypical stress neuropeptide, acts as a neurotransmitter as well as a neurohormone involved in HPA axis activation in co-ordinating the autonomic response of the GI tract to stress [reviewed in (450)] and several lines of evidence suggest that altered central and peripheral CRF signaling plays a role in the stress-induced development and maintenance of functional bowel disorders [(460, 462); see below]. In vivo and in vitro studies have shown that CRF activates DMV neurons (directly as well as indirectly via actions to increase glutamatergic synaptic inputs) and decreases gastric motility via activation of the vagally dependent NANC pathway (224, 291). Indeed, central application of a CRF antagonist has been shown to prevent the postoperative ileus observed following abdominal surgery, suggesting involvement of a gastro-inhibitory CRF-dependent pathway.
In contrast to its effects to inhibit gastric motility and emptying, CRF acts centrally to accelerate colonic motility and transit [reviewed in (450)] via activation of both vagal and sympathetic pathways innervating the proximal and distal colon (245, 501). As discussed below, stress causes the activation of CRF-containing neurons within Barrington’s Nucleus and PVN that project to the LC (202, 289, 340, 342) that not only increases colonic transit but also increases arousal and anxiety-like behaviors which may be of importance in the pathophysiology of functional bowel disorders such as irritable bowel syndrome (IBS) associated with comorbid anxiety and depression (335, 461, 505).
In contrast to the prostress actions of CRF, OXY is the prototypical antistress neuropeptide. The PVN is the sole source of OXY-innervation to the DVC and OXY-containing axons and fibers have been shown to form close appositions to NTS neurons as well as gastric-projecting DMV neurons (304, 376). Electrophysiological studies support the role of OXY to modulate vagally mediated GI functions; OXY excites DMV neurons via a cAMP-sensitive current (6, 229, 385, 386) and increases glutamate release from vagal afferent terminals (376). OXY is released from the PVN following meal ingestion and acts within the DVC to stimulate gastric acid secretion and decrease gastric motility via a vagally dependent pathway (229). In fact, these OXY-releasing inputs to the DVC are tonically active since intracerebroventricular application of OXY antagonists increases baseline gastric motility (161). Furthermore, electrical stimulation of the PVN induces a vagally dependent decrease in gastric motility and increase in gastric acid secretion (404), actions that are blocked by DVC application of an OXY antagonist and mimicked by microinjection of an OXY agonist. As an antistress peptide, hypothalamic OXY plays an important role in the recovery of GI functions following adaptation to stress [(25, 26, 86, 548); see below)].
The arcuate nucleus (Arc) of the hypothalamus, located adjacent to the third ventricle in close apposition to the median eminence and the pituitary gland is connected to both via neuronal and humoral connections. The Arc is also a circumventricular organ (111,171) and Arc neurons have been shown to modulate their activity in response to several circulating neuropeptides and neurohormones which regulate GI functions (57). The Arc also has extensive afferent and efferent projections to several brain regions underscoring its important role in the modulation and regulation of a variety of autonomic homeostatic functions. With respect to its involvement in the regulation of GI functions, the Arc receives afferent inputs from other hypothalamic areas as well as from the LC, NTS, reticular formation, the BNST, and PVN (106, 134). In turn, Arc neurons send projections to other hypothalamic nuclei as well as to the limbic system, the thalamus and the brainstem, including the PAG and DVC, highlighting the role of this nucleus in the integration of endocrine and behavioral aspects of autonomic GI regulation (106).
In addition to its role in the modulation of feeding and satiety, stimulation of Arc has also been demonstrated to increase colonic motility and transit (471) and decrease gastric acid secretion (470). The promotility colonic effects were antagonized by application of CRF antagonists to the PVN while the effects on gastric acid secretion were blocked by a combination of CRF and NPY Y1 receptor antagonists, suggesting that an Arc-PVN neurocircuit may also regulate GI functions via vagally dependent efferent effects (469–471).
Barrington’s Nucleus
Barrington’s nucleus, located within the dorsolateral pontine tegmentum, often known as the pontine micturition center, is involved in the supraspinal regulation of both bladder and colonic functions (37,370). Transsynaptic tracing studies have demonstrated that an individual neuron within Barrington’s nucleus may innervate both the bladder and the colon, suggesting coregulation of viscera through projections to preganglionic parasympathetic neurons in the lumbosacral spinal cord (417). Consistent with this neuroanatomical evidence, functional studies have shown that most Barrington’s nucleus neurons respond to bladder distention with an increase in activity; approximately half of these neurons also respond to colonic distention (418). Furthermore stimulation of Barrington’s nucleus produces increases in colonic pressure via activation of lumbosacral parasympathetic preganglionic neurons (370). This suggests that information from the bladder and colon converges onto Barrington’s nucleus neurons which, in turn, can influence the parasympathetic outflow to both organs (418).
A significant proportion of Barrington’s neurons are immunoreactive for CRF (507), indeed, CRF-immunoreactivity within this pontine region has been suggested to be a definitive marker of Barrington’s nucleus (506). Additionally, the majority of colon-responsive Barrington’s neurons are CRF-immunopositive (418). CRF-containing neurons within Barrington’s nucleus also project to the lateral DMV (507), an area known to provide parasympathetic innervation to the proximal colon (11, 49, 74), to the spinal cord and to the LC (370). Several studies in many CNS areas have provided evidence for the important role of CRF in the co-ordinated physical and homeostatic response to anxiety and stress [reviewed in (28)]. Indeed, CRF levels within Barrington’s nucleus increase in response to both acute and chronic stress (243, 244) which may be of importance in stress and stress-related colonic dysfunction (454, 462, 505). While Barrington’s nucleus has been considered to be involved almost exclusively with the control of parasympathetic functions, neuronal tracing studies have also demonstrated that neurons within Barrington’s nucleus innervate sympathetic preganglionic neurons in the thoracic spinal cord, either directly or indirectly, suggesting a role in the integration of both sympathetic and parasympathetic autonomic functions (95).
Several neuroanatomical and functional studies have investigated the projections from the lumbosacral spinal cord to Barrington’s nucleus (139, 417, 506). In addition to these visceral sensory afferents, neurons within Barrington’s nucleus are also the recipients of inputs from a diverse range of brainstem, midbrain, and forebrain structures. Retrograde tracing studies have demonstrated that the greatest density of projections to Barrington’s nucleus arise from the PAG area, the lateral hypothalamus (LH) and the medial preoptic nucleus (506). Anterograde labeling studies further suggested that the PAG and LH neurons innervating Barrington’s nucleus target CRF neurons specifically (506). Retrogradely labeled neurons were also identified in the NTS, the PB complex, the dorsal and medial raphe nuclei, zone incerta, tuberomammillary and premammillary nuclei, BNST, dorsal, ventromedial, and PVN hypothalamic areas as well as motor, insular and infralimbic cortices (506). Such a variety of projections to and from Barrington’s nucleus, which includes connections with neurons at all supraspinal levels (brainstem, midbrain, pons, and forebrain) suggests involvement in a variety of integrative processes and may provide a neuroanatomical basis for assimilation of GI functions with emotional and cognitive behaviors.
Periaqueductal gray
The PAG refers to an area of the midbrain surrounding the cerebral aqueduct extending caudally from the dorsal tegmental nucleus to the most rostral level of the third nucleus at the level of the posterior commissure. The PAG contains prominent longitudinally arranged fiber systems connecting the forebrain with brainstem autonomic neurocircuits. In addition to its role in autonomic processing, the PAG is important in the regulation and processing of fear and anxiety, in vocalization and in the ascending transfer, as well as descending modulation, of nociceptive information [reviewed in (42)].
Neuroanatomical tracing studies have shown that the PAG receives a dense innervation of predominately catecholaminergic inputs from NTS and rostral ventrolateral reticular nucleus (RVL; involved in the integration of cardiovascular and respiratory reflexes) as well as inputs from neurons within lamina I and, to a lesser extent, lamina V of the spinal cord (Fig. 1) (219,263,276,458). Thus, both parasympathetic and sympathetic sensory information from the GI tract can be relayed to the PAG. Together with the inputs from the hypothalamus (96, 475), and inputs from forebrain regions including the prefrontal and cingulate cortex and, pre- and infra-limbic areas (Fig. 2) (160, 163, 240, 409), this allows for the PAG to integrate and modulate autonomic and emotional information from all supraspinal levels. The PAG provides reciprocal connections back to many of these regions, including the central nucleus of the amygdala (CeA), the hypothalamus, and spinal cord (295, 396, 409, 410) as well as projections to the raphe magnus, the LC and ventrolateral medulla (153, 509). Efferent projections to PB complex (272) potentially acts as filter system that depends on behavioral state to modulate ascending nociceptive or visceral information through the PB complex to forebrain areas, while efferent projections to the hypothalamus provides an additional, and significant, means by which sympathetic and parasympathetic autonomic homeostatic functions can be integrated.
Noxious gastric (104) and colonic (320, 492) stimulation activates supraspinal CNS regions, including PAG, that are involved in somatic pain processing suggestive either of a degree of overlap of nociceptive processing, or of a common central pain mechanism. In turn, PAG neurons activated by noxious stimulation of the GI tract project to the PVN (141). The critical role that the PAG plays in descending pain inhibition was confirmed by studies demonstrating that stimulation of the PAG induces profound analgesia (42, 401). While the mechanism is not completely understood, PAG stimulation appears to result in inhibition of dorsal horn nociceptive neurons (97, 374). The relatively sparse direct innervation of the spinal cord by PAG neurons (315) led to the suggestion that the analgesia resulting from PAG stimulation occurred indirectly via the medulla, particularly the nucleus raphe magnus (5, 396) although this medullary relay nuclei may not be solely responsible for the descending antinociceptive pathway activated from the PAG (179, 423).
Locus coeruleus
The LC (A6 noradrenergic region), located within the rostral pons at the lateral floor of the fourth ventricle, is the brain’s predominant source of noradrenergic innervation. LC neurons are spontaneously active (537); the activity and firing rate of neurons within the LC increase in response to sensory, autonomic and painful stimulation and decrease during sleep, suggesting the involvement of this nucleus in the maintenance of attention and arousal [reviewed in (17, 165)]. Historically, the identification of inputs to the LC has been controversial but more recent neuroanatomical tracing techniques have identified several inputs from autonomic-related nuclei (Fig. 2); inputs from the NTS provide a means by which vagal sensory information can be relayed to the LC (510). Inputs from the PVN [(399); see above], Barrington’s nucleus [(416, 454); see above], the PB complex [(309); see above], and PAG [(309); see above] provide a means by which vagal and spinal GI afferent information converge and can be integrated. Inputs from the BNST, central CeA, and from the prefrontal cortex (16, 309, 508) provide a means by which the autonomic components of emotional responses can be integrated with higher cognitive inputs (reviewed in (165). Both the location of the LC, in close proximity to the fourth ventricle and several large blood vessels, and the extensive dendritic projections, including toward the ependymal layer of the fourth ventricle, has led to speculation that they may be responsive to circulating neuroactive agents although this remains to be confirmed (201, 455).
The LC has extensive efferent connections including reciprocal connections with several of these areas mentioned above involved in autonomic GI regulation such as the brainstem (NTS; trigeminal) and hypothalamus, particularly the parvocellular region of the PVN (119,269,429). Efferent projections from the LC to the spinal cord have been found within the ventral columns, intermediate gray and ventral half of the dorsal column (4, 110, 162), although there may be regional and species variations. In the rat, for example, the LC has been estimated to provide 70% to 90% of noradrenergic inputs to some (but not all) levels of the spinal cord (260) and was found to be the sole source of noradrenergic inputs to the ventral horn (110). In contrast, Commissiong et al. (109) found that the LC does not innervate the dorsal commissural nucleus of the thoracic spinal cord and was, therefore, unlikely to influence the activity of sympathetic preganglionic neurons innervating the upper GI tract. Significant species differences appear to exist, however; studies by Westlund and Coulter in the monkey demonstrated that, in addition to projections to the sacral spinal cord, LC innervates the DVC and nucleus ambiguus suggesting an involvement in descending modulation of vagal parasympathetic regulation (534). Efferent projections from the LC to the cortex as well as projections to the cerebellum, thalamic relay network and amygdala provide a neuroanatomical basis for the involvement of the LC in attention and arousal [reviewed in (165)]. Multiple experimental approaches have demonstrated that NE exerts a profound influence over arousal and behavioral states; LC activity, in particular, acts as a gate on several modalities of sensory processing. By effectively increasing the signal to noise ratio of sensory inputs, the LC assists in enhancing and “tuning” the response to a specific stimulus [reviewed in (17, 427)].
Both immunohistochemical and electrophysiological studies have demonstrated that distention of the stomach or colon activates LC neurons in a pressure-dependent manner (152, 320, 416, 419). The colonic-distention induced increase in LC neuronal activity appears dependent upon CRF released from Barrington’s nucleus (507), although other nuclei (PVN, CeA, and BNST, in particular) innervate the LC with CRF-containing fibers (399). Microinjection of CRF into the LC stimulates colonic motility and accelerates colonic transit significantly but has no effect upon gastric motility or emptying (341, 462). Despite having no effect on gastric motility or emptying, microinjection of CRF into the LC inhibits gastric acid secretion; vagotomy had no effect upon this action of CRF suggesting it involves activation of a spinal pathway (342).
Interestingly, abdominal surgery activates LC neurons [in addition to activating neurons within the PVN, NTS, and supraoptic nucleus; (60)]. While this activation also involves CRF, it does not appear to involve a vagal pathway but rather activates a splanchnic afferent-spinosolitary tract-NTS-PVN-LC pathway that may be involved in the gastric ileus observed following abdominal surgery (35, 60). Stress also increases CRF levels within Barrington’s nucleus and alters CRF neurotransmission within the LC; acute stress attenuates LC neuronal response to CRF but repeated stress results in a sensitization of LC neurons (121) which may play a role in the well-described stress-induced alterations in GI functions (460, 505, 508).
Stress also activates the hypothalamic-pituitary-adrenal (HPA) axis resulting in the release of glucocorticoids from the adrenal gland. In addition to exerting multiple effects throughout the periphery, corticosteroids also exert profound effects on several CNS nuclei [reviewed in (220, 347)], including (i) activation of the CeA, BNST, PVN (regions which provide innervation to the LC) as well as the LC itself, (ii) the upregulation of CRF or CRF mRNA in CeA, BNST, PVN, and (iii) altered expression profiles of mineralocorticoid and glucocorticoid receptors in CeA, BNST, and LC. Together, these provide potential a neuroanatomical basis for the well-known effects of stress to induce colonic motor dysfunction and increase colonic pain sensitivity (120,121,294,485,486,520).
Cellular plasticity of LC neurons, particularly dependence and withdrawal in response to opioid drugs has been well described. Acutely, opioids inhibit LC neuronal activity (decreased adenylate cyclase activity as a consequence of increased potassium conductance) (493, 538, 539). In contrast, chronic opioid administration induces the development of opioid tolerance and dependence via upregulation of the cAMP-CREB pathway resulting in increased neuronal activity and firing rate [reviewed in (326)]. Opioid drug use, therefore, may result in bowel dysfunction via alterations in LC neuronal activity irrespective of actions at peripheral opioid receptors within the GI tract (474).
Forebrain nuclei regulating gastrointestinal functions
Central nucleus of the amygdala
The central CeA, rather than being a single anatomical or functional structure, is a relatively large, heterogeneous complex located within the anteromedial temporal lobe and is involved in learning and memory as well as the integration of aversive and emotional stimuli with learning and memory as well as autonomic, including GI, functions [reviewed in (217, 456)]. As an important structure within the limbic system, the CeA facilitates the processing of autonomic and emotional sensory information and appears to play a prominent role in the development of anxiety and stress-related GI disorders [reviewed in (345)].
The CeA receives inputs from structures at all supraspinal levels including the brainstem [NTS and ventrolateral medulla; (353,362,403)] the midbrain [PB complex, hypothalamus, and PAG; (47, 362, 425, 436)] and forebrain [insular cortex and thalamus; (285,424,542); (Fig. 2)]. In turn, the CeA sends reciprocal efferent connections to many of these nuclei including descending projections to several areas involved in autonomic regulation of GI functions such as the LC, Barrington’s nucleus, hypothalamus, PAG, NTS, DMV, and ventrolateral medulla (98, 196, 468, 505, 511, 517). The afferent and efferent connections of the CeA permit the integration and modulation both ascending and descending information allowing it to function as a major center for the altered perception of visceral and somatic information.
Several studies have demonstrated that the CeA modulates the effects of stress on the development of gastric pathology. Stimulation of the amygdala increases gastric acid secretion (216, 223, 266) while lesions of the amygdala attenuate the formation of stress-induced gastric ulcers (214); these effects were abolished by vagotomy (223, 266) illustrating the role descending pathways from the amygdala exert upon the modulation of vagal efferent activity. Indeed, studies have suggested that the integrity of the CeA is important for the maintenance and integrity of gastric mucosa (343). Multi-unit electrophysiological studies of CeA neurons have shown that the firing patterns and response profiles of neurons from rats susceptible to the development of stress-induced ulcers differs from those of resistant rats, suggesting that variations in emotional state influences the GI response to stressful events and that the behavior of CeA neurons plays a significant role in either adaptation to stress (215) or in the perception of visceral stimulation (345).
While the role of the amygdala to modulate gastric motility has not been studied to the same degree, nevertheless it has been known for some time that, in anesthetized animals, stimulation of the CeA alters gastric pressure (increased or decreased depending on the medial-lateral location of the stimulating electrode) in a vagally dependent manner (157, 303, 311). The role of the amygdala in regulation and modulation of GI functions is not restricted to the stomach, however. As a major efferent nucleus involved in the generation of fear and anxiety behaviors as well as the acquisition of emotional memories, the CeA plays a prominent role in linking stress and anxiety with the development of GI, particularly colonic, hypersensitivity (345).
Indeed, stimulation of the CeA increases anxiety-like behaviors and augments stress-induced responses via actions, at least in part, that activate the HPA axis. In contrast, lesions of the CeA decrease anxiety-like behaviors, decrease CRF mRNA expression and CRF levels within the hypothalamus and decrease the stress-induce release of ACTH and corticosteroids (91, 519); reviewed in (133). Functional magnetic resonance imaging (fMRI) and positron emission tomography techniques have demonstrated that patients exhibiting GI hypersensitivity show altered processing of visceral stimulation and increased activation of several CNS regions including the amygdala (322, 323) while rodent studies have shown an increased neuronal activation in CeA following colorectal distention (283, 533). Alteration of amygdala activity is also known to modulate the response to visceral stimulation; activation of glucocorticoid or mineralocorticoid receptors, for example, by exposure of the amygdala to corticosterone, increases the response to colorectal distention, supporting the hypothesis that the amygdala plays an important role in the perception and processing of visceral sensations (198, 345, 346). Similarly, central administration of the prototypical stress neuropeptide CRF is well-known to increase colonic activity; microinjection of CRF antagonists into the CeA attenuates these stress-induced effects. Furthermore, stress-susceptible species of rodents have been found to have increased levels of CRF within the CeA (206, 439) and CeA CRF levels are also increased in animal models of colonic hypersensitivity (199, 380). In addition to increasing anxiety- and stress-related behaviors, microinjection of corticosterone into the amygdala increases CRF levels (485); these actions are also attenuated by CRF antagonists (91). These, and other lines of evidence, have led to the suggestion that the sensitivity of the CeA to corticosterone may underlie the correlation between the stress and GI hypersensitivity observed in many IBS patients [reviewed in (345)].
Bed Nucleus of the Stria Terminalis
The BNST, as part of the extended amygdala within the forebrain, also plays a prominent role in the processing and consolidation of behavior and emotions. By relaying information from the CeA to the PVN, the BNST also plays a significant role in regulation of the HPA axis and in autonomic responses to stress (166). As with the amygdala, the BNST receives afferent inputs from nuclei at all supraspinal levels including the brainstem (NTS, ventrolateral medulla and PB complex; (441), midbrain [hypothalamus, PAG (8, 441)], and forebrain nuclei [cortex, rostral forebrain structures, and thalamus (424, 441, 542)] as well as the CeA itself (271, 383); (Fig. 2). The BNST projects to several areas involved in autonomic homeostatic regulation of GI functions including the NTS, DMV, PAG, hypothalamus, LC, and PB complex (46, 196, 232). As a result, the BNST is well placed to integrate physiological and behavioral responses.
The BNST, like the CeA, plays a role in the regulation and modulation of GI functions following stress. In contrast to the CeA, however, lesions of the BNST exacerbate gastric lesions induced by stressors (213, 343). The majority of BNST neurons display a GABAergic phenotype, and since these inhibitory neurons are the targets of projections from the CeA, the differential effects on gastric acid secretion may involve the engagement of different CeA output neurocircuits. By virtue of its projections to the hypothalamus, particularly the PVN, the BNST is also strategically positioned to influence HPA axis activity and responses to stress (115). Although some BNST inputs to the PVN are CRF-immunoreactive, anatomical studies indicate that the majority of the BNST inputs to the PVN are GABAergic (379), suggesting (i) that the BNST exerts a predominantly inhibitory influence over the HPA axis activity and (ii) that activation of the HPA axis may arise either from activation of CRF-containing BNST inputs or disinhibition of GABA-containing BNST inputs. Certainly, lesions of the BNST are known to increase PVN CRF mRNA levels, activate PVN neurons, and increase corticosterone release, suggesting a tonic inhibitory influence over the activity of PVN neurons (105).
The BNST is volumetrically and neurochemically sexually dimorphic in both humans and rodents (9, 225), particularly with respect to CRF and vasopressin-immunoreactivity (331, 334). Furthermore, both estrogen and androgen receptors are expressed within the BNST (445); activation of androgen receptors appears to enhance stress-induced PVN activation and may enhance HPA axis activation (55, 209). Further, the level of CRF within the BNST has been shown to fluctuate in concert with the estrous cycle (360) providing a neurophysiological basis for the differential influence of gender and sex hormones in the modulation of BNST, hence, HPA axis, activity.
Cortex
In addition to modulating GI functions via descending connections via the hypothalamus and the amygdala (see above), the cortex can modulate GI functions via direct descending connections from the infralimbic and prelimbic cortex to the dorsal vagal complex (363, 443, 472, 473, 511, 512). Stimulation of the infralimbic cortex induces gastric relaxation and decreases both gastric motility and tone in a vagally dependent manner (241,366,542). Several recent studies have highlighted the effects that cortical influences, including affect, motivation, and memory, exert over GI functions and the consequences that disturbances within this system exerts upon a variety of functional and inflammatory GI disorders (61,324).
Central regulation and modulation of gastrointestinal reflexes
The discussions above regarding the central modulation of GI functions have been written primarily from the point of “normal” or “basal” regulation. Increasing evidence indicates, however, that central regulation of GI functions does not occur in a static or inflexible manner but, rather, exhibits a remarkable degree of plasticity and adaptation.
During the intermeal interval, mammalian GI motor activity oscillates between periods of quiescence (Phase I) and different levels of activity (irregular, Phase II, or caudally migrating bursts of contractions, Phase III). During these phases, the proximal stomach experiences a (vagally mediated) tonic contraction, and the subsequent alternate periods of quiescence or activity occur in synchrony with the motor activity in the antrum and proximal small intestine. Meal ingestion and food transit from the stomach to the upper small intestine disrupt these cyclic patterns of activity and induce long-lasting relaxation of the proximal stomach [reviewed in (135)]. Many different types of vago-vagal reflexes (esophago-gastric, gastro-gastric, duodeno-gastric, and intestino-intestinal, for example) contribute to the CNS control of this gastric relaxation and it is not our intention to provide details of them all of them but, rather, we refer the reader to the numerous manuscripts and reviews that have dealt specifically with these functions [see, among others (132, 154, 498)]. Instead, we describe a few select GI reflexes briefly, as examples of feedback mechanisms that control and regulate meal transit through the GI tract to optimize digestive processes, and the modulation of these reflexes during pathophysiological conditions.
Functional Gastrointestinal Disorders and the Receptive Relaxation (or Esophago-Gastric) Reflex
First described by Walter Cannon in 1911 (94) reflex gastric accommodation to a meal is achieved via relaxation of the proximal stomach, allowing ingested food to be transported into the stomach without an accompanying increase in intragastric pressure. This reflex is mediated entirely by the vagus nerve (411); stimulation of low threshold mechanosensitive esophageal vagal afferents results in proximal gastric relaxation via either inhibition/withdrawal of tonic cholinergic vagal efferent pathways or activation of inhibitory NANC pathways [reviewed in (499)].
Functional GI motility disorders, including functional dyspepsia [FD; defined by the Rome III panel as “the presence of symptoms thought to originate in the gastroduodenal region, in the absence of any organic, systemic or metabolic disease that is likely to explain the symptoms” (466)], are frequently accompanied by symptoms such as impaired gastric emptying, reduced gastric compliance, early satiety and weight loss (93, 258, 465, 466, 479). While the pathophysiological mechanisms involved in the development of FD have not been elucidated completely, several studies suggest impairment of the vagal sensory-motor reflex loop connecting the GI tract and the brainstem. Almost 50% of FD patients have disturbed vagal efferent functions and abnormal intragastric meal distribution with preferential accumulation of food in the distal, rather than the proximal, stomach (233,500), suggesting that proximal gastric accommodation is abnormal. Stress-related alterations in gastric motility, along with food ingestion, are well-recognized as being among the main pathophysiological mechanisms involved in the generation of dyspeptic symptoms, and the majority of FD patients associate stressful life events with the initiation or exacerbation of symptoms (180, 465, 479, 514). Patients with FD exhibit gastric dysmotility following stress; anger, for example, inhibits gastric motility in FD patients but not in healthy volunteers. In healthy individuals, however, acute stress delays gastric emptying, supporting the idea of a vagal involvement in dyspeptic symptoms, while chronic stress results in symptoms consistent with the diagnosis of FD (92).
As described above, stress activates neurons within several CNS nuclei, including CRF containing neurons within the PVN, resulting in the release of CRF into brainstem nuclei including the DVC (28,41,296,462). Activation of brainstem CRF receptors decreases gastric motility and inhibits gastric emptying while stimulating colonic motility (460). When administered intracisternally, CRF shifts the motility of the duodenum from that of a fasted pattern towards that of a fed pattern with irregular contractions; in contrast, when administered to a fed animal, CRF maintains the fed pattern but reduces the amplitude of the antrum motility waves. These effects of CRF on gastric motility suggests its involvement in either the generation or the maintenance of stress-induced gastric dysmotility (177, 289, 291, 462). Application of CRF antagonists to the CNS does not alter basal gastric motility or vagal efferent output, suggesting that CRF pathways are not active tonically; the same antagonists, however, prevent stress-induced gastroinhibition (psychological, visceral, or chemical) (460). CRF receptors are positively coupled to adenylate cyclase and their activation increases intracellular cAMP levels (194). As described earlier, cAMP levels regulates the ability of GABAergic inputs onto gastric projecting DMV neurons to be modulated, hence controls vagal efferent outflow to the upper GI tract [reviewed in (78, 81)]. Stress, therefore, alters the tonic inhibitory input onto vagal efferent motoneurons, thus regulates their firing rate and vagal efferent outflow (Browning and Travagli, unpublished observations).
Acute stress may be best considered as inducing a stereotypical and protective homeostatic adaptation to an internal or external threat. This results in modulation of neural pathways in a time-limited adaptive manner. In this regard, we have demonstrated previously that the activation of the cAMP-PKA pathway (such as following CRF exposure) modulates GABAergic synaptic transmission to gastric projecting DMV neurons for approximately 30 to 60 min before returning to prestimulation levels (73, 80). Thus, the functional GI outcomes of acute exposure to CRF are relatively restricted and do not exacerbate the inhibitory gastric actions of the tonically active oxytocinergic pathway, hence, do not result in a prolonged, and potentially damaging, decrease in gastric functions.
In contrast to acute stress, chronic or prolonged stress presents a more serious challenge to homeostatic adaptive mechanisms and the inability to adapt to ongoing internal or external threats frequently results in GI dysfunction. While acute exposure to stress-related neuropeptides such as CRF results may modulate brainstem vagal neurocircuitry for a relatively short period of time, it is tempting to speculate that chronic exposure to stress results in a long-term, or even permanent, alteration in vagal neurocircuitry. Studies in rats have demonstrated adaptation in the hypothalamic corticotropinergic and oxytocinergic system in response to chronic homotypic stress whereas rats exposed to heterotypic stress fail to adapt (24–26, 86, 547). Such a potential dichotomy in neural rewiring presents a means by which adaptation to stress may be investigated, which may be of importance in understanding why some humans, but not others, show GI dysfunctions in response to ongoing stressful conditions.
Obesity and the Ileal Brake Reflex
As described in detail by Maljaars (313), the presence of unabsorbed or semi-digested fat in the ileum slows gastric emptying and the passage of ingested material through the small intestine, helping to prevent nutrient overload and increasing contact time with the absorptive intestinal epithelium (1, 235, 313, 394, 449). While this reflex was initially thought of as being operative in the diseased gut (i.e., activated only during malabsorbtion (449), later studies have shown that nutrients may reach the distal small intestine even under normal conditions, and re-establish the motility from the fed to the fasted pattern (264, 297).
While the precise mechanism underlying the ileal brake mechanism is still somewhat subject to debate, it appears to involve peptide YY (PYY) and GLP-1 release mainly from enteroendocrine L cells in the ileum and colon following a meal (159). Their release, however, occurs before nutrients have reached the lower gut, indicating a neural (including vagal)-mediated mechanism of release (1).
In addition to its prominent actions to induce insulin release from pancreatic β-cells (143, 144, 478), GLP-1 also has major effects at the level of the CNS, penetrating the blood brain barrier via simple diffusion (261). GLP-1 excites vagal afferent fibers and neurons (174,349,350), resulting in an activation of central vagal neurons and neurocircuitry (34,529). It is also important to note, however, that GLP-1 is also produced in preproglucagon neurons of the NTS and are also involved in the regulation of autonomic homoestatic functions [reviewed in (489)]. The exact contribution of peripheral versus central GLP-1 in the vagally dependent central regulation of GI functions remains to be elucidated fully. When administered centrally, GLP-1 acts via vagally dependent pathways to induce the release of insulin, decrease food intake, and delay gastric emptying as well as being involved in interoceptive stress (21, 143, 173, 230, 277, 405, 502, 513). GLP-1 receptors are seven transmembrane (7TM) proteins positively coupled to adenylate cyclase, activation of which increases intracellular cAMP levels and calcium concentration (325); as described above for other neuropeptides positively coupled to adenylate cyclase, activation of GLP-1 receptors would, therefore, be expected to alter the “state of activation” of inhibitory vagal neurocircuits, hence, modulate the activity and firing rate of vagal efferent motoneurons.
Once cleaved by dipeptidyl peptidase IV (DPP-IV), PYY 3–36 works preferentially on Y2 receptors (235); the presence of Y2 receptors on vagal afferent terminals suggests the involvement of a paracrine mechanism of action to induce gastric relaxation, while their location on hypothalamic neurons appears responsible for its anorexigenic effects. A CNS transport system exists, however, and peripherally injected peptide YY ([125I]PYY3–36) and pancreatic polypeptide ([125I]hPP) is transported into the CNS, particularly to the dorsal vagal complex (146, 352). Certainly, pancreatic polypeptide binding sites (Y1–Y5) have been identified within the DVC (312), and both NPY and PYY modulate the activity of vagal neurocircuits (33, 58, 77, 80).
Several studies have shown that the behavior, activity and responsiveness of vagal afferent neurons and fibers are altered by diet and obesity. The ability of GI neurohormones such as GLP-1 and CCK to activate vagal afferents and decrease feeding is attenuated in diet-induced obese animal models and humans (112, 113, 126, 142, 145, 299, 536) and exposure to a high fat diet alters the profile of neurotransmitter/neuromodulator receptors on vagal afferent neurons (131, 369). While little attention has been paid to the effects of diet or obesity on the properties of central neurons, a recent study demonstrated that the responsiveness of vagal motoneurons was also decreased in diet-induced obese rodents (71). This reduced responsiveness may contribute to diminished vagal signaling and attenuated vago-vagal reflex control of GI functions after exposure to a high fat diet and may contribute to the altered gastric motility and emptying observed in obese subjects (299).
Diabetes and Gastrointestinal Reflexes
Effective glucose sensing is of vital importance of the regulation and integration of a variety of physiological functions, including the optimal regulation of glycemic levels. Glucose levels fluctuate dramatically in response to food ingestion and a variety of autonomic reflexes are engaged to regulate gastric motility and emptying, in part to stabilize excessive fluctuations. An increase in plasma glucose levels decreases gastric motility and emptying which, in turn, delays the entry of food into the small intestine thus postponing further glucose absorption and preventing prolonged, and potentially damaging, increases in glycemic levels. Hypoglycemia, in contrast, increases gastric motility to accelerate nutrient delivery to the small intestine.
Within the intestine, glucose modulates the activity of enteric neurons directly (301,430,531) as well as modulating their response to GI neurohormones such as CCK and 5-HT (413). The principle action of glucose to modulate GI motility and gastric emptying, however, occurs via paracrine mechanisms of action. Intraluminal glucose induces the release of neurohormones, particularly 5-HT and GLP-1, from enteroendocrine cells. These released neurohormones activate receptors present on vagal afferent terminals and the resulting excitatory signal is relayed centrally (388–390, 523, 554). As described earlier, the central terminals of vagal afferent neurons use predominantly glutamate as their neurotransmitter hence their activation induces the excitation of second order NTS neurons within the brainstem. The subsequent vagal efferent motor response induces gastric relaxation and decreases motility thereby delaying gastric emptying (440, 552, 554, 555).
Once absorbed from the intestine, however, glucose enters the bloodstream where it continues to modulate GI functions via actions on vagal neurocircuits. Intravenous glucose has been shown to modulate the excitation of vagal afferent fibers to intestinal glucose suggesting that circulating glucose is able to modulate neuronal activity directly (327, 328). Despite the relatively tough capsule (nodose ganglion) surrounding vagal afferent neurons, they are appear accessible to circulating factors (278, 279). While glucose is a universal fuel for neurons, some neurons, particularly within the brainstem and hypothalamus, possess the additional ability to use variations in extracellular glucose levels to modulate their excitability (2, 148, 290). A subpopulation of vagal afferent sensory neurons also possess this ability; vagal afferent neurons innervating the stomach are more likely to exhibit excitatory responses to elevations in glucose while neurons innervating the portal vein are more likely to be inhibited suggesting that the effects of glucose on vagal sensory neurons are specialized as per the sensory information they transmit (193). As in other neurons excited by elevations in glucose levels, this appears to involve the closure of an ATP-sensitive potassium channel (30, 487, 488). The mechanism by which an elevation in glucose level inhibits vagal afferent neurons is not fully characterized although it may involve an ATP-insensitive potassium channel (193).
In addition to its direct actions to modulate vagal sensory neuronal activity, glucose also acts indirectly via modulation of neurotransmitter receptor density on the neuronal surface. In particular, an increase in extracellular glucose levels results in the trafficking of 5-HT3 receptors to the surface of GI vagal afferent neurons whereas a decrease in extracellular glucose results in their internalization (23). The physiological outcome of this glucose-induced trafficking of receptors is an increase or decrease in 5-HT-mediated inward current in response to an increase or decrease in extracellular glucose, respectively (23). This suggests that, in addition to inducing the release of 5-HT from enterochromaffin cells (170, 391), glucose may also increase the ability of vagal sensory neurons to respond to the released 5-HT, amplifying and prolonging the sensory signal. It appears, therefore, that the physiological responsiveness of vagal sensory neurons is altered by nutritional status (feeding, fasting, glucose level, etc.,) implying that the regulation, modulation, and outcome of GI vagal reflex activation may be dependent upon ongoing homeostatic demands.
Glucose also acts centrally to modulate the release of neurotransmitter from vagal afferent terminals (527). As with vagal sensory somata, glucose induces the trafficking of 5-HT3 receptors to the membrane of vagal sensory terminals, the tonic activation of which increases glutamate release onto NTS neurons (528). Earlier studies have also shown that glucose is able to modulate the activity of brainstem neurons; hepatic vagal afferent fibers that were inhibited by an increase in glucose levels, for example, innervate NTS neurons that are also inhibited by local application of glucose (3), whereas other NTS neurons are excited by an increase in glucose levels (2, 124, 125, 543, 544). As with other central and peripheral neurons, this increase in neuronal activity in response to elevated glucose concentrations appears to involve closure of ATP-sensitive potassium channels (29, 30, 125, 147); the mechanism responsible for the glucose-induced inhibition in neuronal activity remains to be elucidated although modulation of chloride conductances may be involved (30). The effects of glucose to modulate DMV neuronal activity is more contentions, however; a subpopulation of DMV neurons that project via the anterior gastric branch have been shown to modulate their activity in response to glucose levels (2, 268) and ATP-sensitive potassium channels have been identified on DMV neurons (30, 488). Other studies, in contrast, have failed to identify any direct effects of glucose on DMV neurons but, rather, demonstrated indirect effects via modulation of synaptic inputs, particularly GABAergic inputs originating likely from NTS neurons (158).
Since GI motility and emptying are modulated by, and dependent upon, physiological alterations in blood glucose levels (393), it is unsurprising that pathophysiological alterations in glucose levels induce profound GI dysfunction. Gastric hyperactivity has been observed in some rodent models of hyperglycemia and in some diabetic patients, whereas diabetic gastroparesis, defined as delayed gastric emptying accompanied by other upper GI symptoms including early satiety, nausea, fullness, bloating and abdominal pain, has been observed in others (100, 238, 435). While insulin certainly modulates the activity of central vagal motoneurons and alters vagally mediated gastric motility (56, 274), the finding that both Type 1 and Type 2 diabetic patients experience gastroparesis suggests that the hyperglycemia and/or disregulated glycemic control per se, plays an important role in the development of symptoms. Hyperglycemia and/or diabetes may alter GI functions via actions at multiple sites, both peripheral and central. Within the enteric nervous system itself, a loss of both enteric neurons and ICC have been reported (101, 136, 246, 358, 359), which may contribute to the observed disordered motility patterns and decreases NANC-mediated relaxation (254, 545). Furthermore enteroendocrine signaling is also disrupted in diabetes (286) as is expression and function of glucose transporters (53, 54, 149, 344). Centrally, the functioning of both sensory and motor vagal fibers is compromised in diabetes (287, 288, 395, 541) and recent evidence suggests that there is also impaired sensitivity of central vagal neurocircuits (556, 557). The reversibility of these hyperglycemia- and diabetes-induced effects on vagal neurocircuits is of particular importance for future investigations—is there a period of exposure to elevated glucose levels beyond which the damage to vagal neurocircuits is irreversible, or is the system sufficiently plastic to recover following subsequent tight glycemic control? The recent demonstration that Roux-en-Y gastric bypass surgery reverses the diet-induced obesity associated decreased in excitability and responsiveness of vagal efferent motoneurons suggests these neurocircuits are capable of a surprising degree of adaptation and that even long-term dysfunction does not necessarily result in permanent and irrecoverable rewiring of vagal neurocircuitry (71). The rapid remission of Type 2 diabetes following bariatric surgery, far in advance of weight loss, certainly implies that the recovery of glycemic regulation may be an important mechanism if the recovery of vagal afferent and efferent homeostatic control.
Spinal cord injury and Gastrointestinal Reflexes
GI dysfunction following spinal cord injury (SCI) is observed commonly in both patients and animal models at all levels of the GI tract including the esophagus (increased gastroesophageal reflux, esophagitis, and decreased esophageal contractility), stomach and upper GI tract (ulceration, dysregulated motility, altered accommodation, and delayed gastric emptying) and lower GI tract [constipation, distention; reviewed in (150,227)]. While the sympathetic innervation to the GI tract, as well as the parasympathetic innervation to the colon and rectum, is interrupted following SCI, vagal neural pathways to the stomach and upper GI are anatomically intact following spinal injury. This would suggest that the gastric and upper GI dysfunction observed following SCI occurs as the result of an indirect, rather than direct, effect upon vagal neurocircuit function.
As described above, the NTS is the recipient of both vagal and spinal sensory inputs; while the loss of spino-solitary inputs would certainly be expected to have some impact upon brainstem sensory information processing, several lines of evidence suggest that vagal afferent hypo-responsiveness following SCI may play a significant role in the observed upper GI dysfunction [reviewed in (227)] despite being anatomically intact following injury. Activation of NTS neurons in response to peripheral CCK administration is reduced following SCI and electrophysiological studies demonstrated a reduced activation of vagal sensory inputs onto NTS neurons (480). This decrease in responsiveness of vagal afferent sensory neurons may lead to chronic alterations in gut-brain signaling following SCI resulting in the observed GI and autonomic dysfunctions.
Cholinergic anti-inflammatory pathway
An increasing body of work supports the concept that the nervous and immune systems have a complex and intricate communication network, and that crosstalk between these systems is important in the regulation of immune responses [see reviews (66, 122, 123, 321, 372, 373)]. While neuroendocrine mechanisms certainly dampen and attenuate inflammatory responses via activation of the HPA axis (133, 347, 406), the idea that inflammatory responses are also modulated via the autonomic nervous system, however, is a more recent concept. The discovery that vagal nerve stimulation suppresses cytokine production potently lead to the recognition of a vagally mediated cholinergic anti-inflammatory pathway, whereby activation of vagal afferents by inflammatory mediators results in the reflex activation of vagal efferent pathways to dampen cytokine production and assist in the restoration of immune homeostasis [reviewed in (321, 371, 373, 415, 483, 484)].
In brief, local and systemic inflammation stimulates the brainstem directly, via activation of cytokine receptors in the dorsal vagal complex and indirectly, via activation of vagal afferent fibers. Following integration of these inflammatory inputs, the assimilated signal is relayed to the adjacent DMV resulting in vagal efferent activation [reviewed in (321)]. Vagal efferent motoneurons that innervate the GI tract have recently been demonstrated to terminate within close proximity of resident macrophages (377); release of acetylcholine following vagal nerve stimulation has been shown to attenuate the inflammation and ileus resulting from intestinal manipulation [reviewed in (59)]. Intestinal mucosal integrity is also known to be critically important in homeostasis, providing a defensive barrier between food antigens and intestinal microbiota. Decreased tolerance to microbiota is a hallmark of intestinal inflammatory conditions including Crohns disease and ulcerative colitis (368) while a decreased tolerance to food antigens is associated with food intolerance and allergies (45). Under both conditions, inappropriate and exaggerated activation of the immune system leads to tissue damage and, potentially, to sepsis. Immune cells within the intestinal mucosa and submucosa, including T cells, dendritic cell, mast cells and enteric glial cells, are known to contain nicotinic acetylcholine receptors and are, therefore, potential targets of the vagal anti-inflammatory pathway (321,368). Certainly, following vagotomy, mice have an increased susceptibility to inflammatory conditions such as colitis following mucosal irritation (181–183).
While the majority of studies have examined the important role of the cholinergic anti-inflammatory pathway under pathophysiological conditions, other investigations have demonstrated that enteral lipid suppresses mesenteric cytokine release (151) possibly via actions involving activation of vagal afferent CCK receptors (130) suggesting that this vagally dependent reflex may also be activated under physiological conditions.
The spleen is known to be the major source of several cytokines involved in the pathophysiology of inflammation. Until recently, the neuroanatomy of the parasympathetic innervation to the spleen was subject to controversy; lately, however, it has been demonstrated that the vagus nerve does not innervate the spleen directly but, rather, innervates the spleen indirectly via collateral projections to the celiac ganglion (67). Acetylcholine released from these vagal efferents activates nicotinic receptors, specifically α7 subunit-containing nicotinic receptors, on adrenergic neurons within this prevertebral ganglion, resulting in the release of NE within the spleen; activation of splenic adrenergic receptors stimulates the production of acetylcholine from splenic T cells which, in turn, suppresses the production of immune mediators (414).
From a therapeutic standpoint, the knowledge that the CNS, via the vagus nerve, may assist in the restoration of homeostasis via the modulation of inflammatory responses provides a means by which activation of the cholinergic anti-inflammatory pathway can be exploited to treat a variety of clinical conditions. Vagal nerve stimulation and activation of intestinal nicotinic cholinergic receptors have certainly been shown to be efficacious in the treatment of a variety of preclinical models of intestinal inflammation and sepsis [reviewed in (321)] although proof of concept in humans is still lacking.
Gut microbiome and CNS communication
The role of microbiota within the GI tract has become the focus of several recent investigations, particularly with regard to the growing awareness of the role that the microbiome plays in the development and function of the CNS and the acceptance that the microbiome plays a significant role in the modulation of the brains-gut axis [reviewed in (117,118,200)]. The pathways by which the GI microbiome modulates CNS structure and function still be remain to elucidated although, in addition to humoral and hormonal pathways, gut microbiota also appear to communicate with the CNS via neural, particularly vagal, pathways (167,191). The importance of vagal sensory (afferent) pathways in gut microbiota-brain communication have been demonstrated in several studies; for example, the ability of chronic probiotic treatment to alter GABA receptor expression in several CNS regions, including the cortex, amygdala, and LC, which modulate GI functions (see above) is dependent upon an intact vagus nerve (68) while vagotomy attenuates the anxiolytic actions of bifidobacteria in an animal model of colitis (44). Disorders of gut microbiota are associated with several CNS-dependent disorders including stress, anxiety and depression and appear to modulate cognition [see reviews by (108,118,339)]. As described above, the NTS is the recipient of vagal afferent sensory inputs; as detailed above, the extensive connections, many bidirectional, between the NTS and other hindbrain, midbrain, and forebrain structures provides a potential neuroanatomical substrate by which GI microbiota may modulate, regulate and alter “higher” CNS functions.
Conclusions
While great progress has been made regarding the role the CNS exerts upon the GI tract, there is still much to be learned regarding the interplay between different CNS areas with respect to the integration of GI homeostatic functions. Clearly, serious long-term pathophysiological consequences can result from the failure of appropriate autonomic assimilation but the precise mechanisms responsible for these outcomes remain to be discovered.
The dorsal vagal complex has a distinct and important role to play in the regulation of GI function; both vagal and some spinal sensory inputs terminate within the NTS and NTS neurons have direct and/or indirect connections with the majority of brainstem and higher CNS nuclei involved in the regulation of GI functions. The strategic location of some portions of brainstem vagal neurocircuits outside the blood-brain barrier makes them accessible to a variety of circulating hormones, neuromodulators, and cytokines which can alter the responsiveness of vagal neurons as well as alter their ability to integrate and process the vast volume of inputs they receive from the viscera as well as other CNS areas.
It is also evident that a significant degree of plasticity exists within CNS regions involved in the co-ordination and regulation of GI functions; vago-vagal reflexes in particular display a surprising degree of flexibility. While several disparate studies have suggested that altered vago-vagal reflexes may play a significant role in the development and maintenance of several pathophysiological conditions, there is much still to be learned regarding the normal physiology of vagal reflexes and the role that higher CNS centers exert upon their function. A large body of work has demonstrated that, peripherally, the activity and responsiveness of vagal sensory neurons varies according to nutritional status—the activation of vagal reflexes will depend, therefore, on ongoing metabolic and physiological conditions. Centrally, the majority of work to date investigating plasticity within central vagal neurocircuits has centered around the ability of activity within the cAMP-PKA pathway to affect modulation of inhibitory synaptic transmission—undoubtedly, it would be somewhat naïve to assume that this is the only second messenger system that can regulate the “gain” of vagal neurocircuitry and much remains to be investigated regarding the role of other neurotransmitter/neuromodulators and their second messenger systems in the manipulation and co-ordination of GI functions.
Embracing new technologies will unquestionably assist in the uncovering of the cellular mechanisms underlying the central control, regulation, and co-ordination of GI functions. The advent and continuous advancement of imaging, optogenetic, and pharmacogenetic techniques allows for the selective activation or inhibition of specific neuronal subpopulations or pathways; this may prove to be particularly useful and valuable when dealing with brainstem and other central neurocircuits, given their extensive, and often intermingled, afferent and efferent connections. The standardization of experimental techniques between groups of researchers would also be advantageous in comparing experimental outcomes; note, for example, the differential effects of anesthetics on gastric emptying rates (384). Species and strain differences may also be critically important in determining experimental outcomes; Sprague-Dawley, but not Fisher 344 rats, for example, show habituation to the GI effects of stress (25, 206). Results from several groups have suggested that GI responses may vary according to the time of the day and nutritional status [see reviews by (140, 451)]; consistent research outcomes may also benefit from uniform timing of experiments. Finally, the development of dependable and reliable animal models of GI diseases [e.g., FD (300)] will continue to be a priority in assisting basic and translational understanding of the central control and regulation of GI physiological, as well as pathophysiological, functions.
The vast body of currently available information serves to stress the complexity of CNS control of GI functions. To understand such a complex hierarchical control system almost certainly requires the wholehearted implementation of integrated approaches that consider the regulation of GI functions in toto, rather than as discrete anatomical, physiological, or neurological processes. While certainly challenging, such multi-faced, multi-disciplinary approaches may provide a means by which basic and translational approaches can combine research strategies to provide a variety of therapeutic options to treat a multitude of GI disorders.
Acknowledgments
The authors wish to thank the NIH grants NIDDK 78364 (to KNB) and NIDDK 55530 (to AT) for their support. We also thank Cesare M. and Zoraide Travagli and W. Nairn Browning for support and encouragement.
References
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Adachi A, Kobashi M, Funahashi M. Glucose-responsive neurons in the brainstem. Obes Res. 1995;3(Suppl 5):735S–740S. doi: 10.1002/j.1550-8528.1995.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 3.Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- 4.Ader JP, Postema F, Korf J. Contribution of the locus coeruleus to the adrenergic innervation of the rat spinal cord: A biochemical study. J Neural Transm. 1979;44:159–173. doi: 10.1007/BF01253060. [DOI] [PubMed] [Google Scholar]
- 5.Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: Mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 6.Alberi S, Dreifuss JJ, Raggenbass M. The oxytocin-induced inward current in vagal neurons of the rat is mediated by G protein activation but not by an increase in the intracellular calcium concentration. Eur J Neurosci. 1998;9:2605–2612. doi: 10.1111/j.1460-9568.1997.tb01690.x. [DOI] [PubMed] [Google Scholar]
- 7.Albert AP, Spyer KM, Brooks PA. The effect of 5HT and selective 5HT receptor agonists and antagonists on rat dorsal vagal preganglionic neurones in vitro. Br J Pharmacol. 1996;119:519–526. doi: 10.1111/j.1476-5381.1996.tb15702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: A PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- 9.Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- 10.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: Sensory Ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 11.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The Central Organization of the vagus nerve innervating the colon of the rat. Gastroenterol. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 12.Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol. 1991;304:261–274. doi: 10.1002/cne.903040209. [DOI] [PubMed] [Google Scholar]
- 13.Alywin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol. 1997;77:2539–2548. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- 14.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 15.Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- 17.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 18.Aylwin ML, Horowitz JM, Bonham AC. Non-NMDA and NMDA receptors in the synaptic pathway between area postrema and nucleus tractus solitarius. Am J Physiol. 1998;275:H1236–H1246. doi: 10.1152/ajpheart.1998.275.4.H1236. [DOI] [PubMed] [Google Scholar]
- 19.Babic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol. 2013;722:38–37. doi: 10.1016/j.ejphar.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babic T, Browning KN, Kawaguchi Y, Tang X, Travagli RA. Pancreatic insulin and exocrine secretion are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat. J Physiol. 2012;590:3611–3622. doi: 10.1113/jphysiol.2012.234955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babic T, Browning KN, Kawaguchi Y, Tang X, Travagli RA. Pancreatic insulin and exocrine secretion are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat. J Physiol. 2012;590:3611–3622. doi: 10.1113/jphysiol.2012.234955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21–G32. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babic T, Troy AE, Fortna SR, Browning KN. Glucose-dependent trafficking of 5-HT(3) receptors in rat gastrointestinal vagal afferent neurons. Neurogastroenterol Motil. 2012;24:e476–e488. doi: 10.1111/j.1365-2982.2012.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett. 2011;495:77–81. doi: 10.1016/j.neulet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci. 2012;167:56–60. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Babygirija R, Zheng J, Ludwig K, Takahashi T. Central oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in mice. Am J Physiol Regul Integr Comp Physiol. 2010;298:R157–R165. doi: 10.1152/ajpregu.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: Potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004;996:126–137. doi: 10.1016/j.brainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 29.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balfour RH, Trapp S. Ionic currents underlying the response of rat dorsal vagal neurones to hypoglycaemia and chemical anoxia. J Physiol. 2007;579:691–702. doi: 10.1113/jphysiol.2006.126094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baptista V, Browning KN, Travagli RA. Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1092–R100. doi: 10.1152/ajpregu.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol. 2005;94:2763–2771. doi: 10.1152/jn.00351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: The respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci. 2010;32:826–839. doi: 10.1111/j.1460-9568.2010.07318.x. [DOI] [PubMed] [Google Scholar]
- 34.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1011–R1024. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 35.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Tache Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 36.Barraco R, el-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- 37.Barrington FJF. The effect of lesions of the hind- and mid-brain on mictgurition in the cat. Q J Exp Physiol. 1925;15:81–102. [Google Scholar]
- 38.Bayliss WM, Starling EH. The movements and the innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1901;26:125–138. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaumont W. Experiments and observations on the gastric juice and the physiology of digestion. Neill & Co.; Old Fishmarket, Edinburgh: 1838. pp. 1–319. Maclachlan & Stewart, printed by. [Google Scholar]
- 41.Beglinger C, Degen L. Role of thyrotrophin releasing hormone and corticotrophin releasing factor in stress related alterations of gastrointestinal motor function. Gut. 2002;51(Suppl 1):I45–I49. doi: 10.1136/gut.51.suppl_1.i45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 43.Bennett A, Friedmann CA, Vane JR. Release of prostaglandin E-1 from the rat stomach. Nature. 1967;216:873–876. doi: 10.1038/216873a0. [DOI] [PubMed] [Google Scholar]
- 44.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berin MC. Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol. 2012;34:633–642. doi: 10.1007/s00281-012-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berk ML. Projections of the lateral hypothalamus and bed nucleus of the stria terminalis to the dorsal vagal complex in the pigeon. J Comp Neurol. 1987;260:140–156. doi: 10.1002/cne.902600111. [DOI] [PubMed] [Google Scholar]
- 47.Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 48.Berthoud HR, Jedrzejewska A, Powley TL. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J Comp Neurol. 1990;301:65–79. doi: 10.1002/cne.903010107. [DOI] [PubMed] [Google Scholar]
- 49.Berthoud H-R, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 50.Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic a2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol. 1997;272:G654–G661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- 51.Bessant AR, Robertson-Rintoul J. Origin of the parasympathetic preganglionic fibers to the distal colon of the rabbit as demonstrated by the horseradish peroxidase method. Neurosci Lett. 1986;63:17–22. doi: 10.1016/0304-3940(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 52.Beyak MJ, Grundy D. Vagal afferents innervating the gastrointestinal tract. In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 53.Bhutta HY, Deelman TE, Ashley SW, Rhoads DB, Tavakkoli A. Disrupted circadian rhythmicity of the intestinal glucose transporter SGLT1 in Zucker diabetic fatty rats. Dig Dis Sci. 2013;58:1537–1545. doi: 10.1007/s10620-013-2669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bihler I, Freund N. Sugar transport in the small intestine of obese hyperglycemic, fed and fasted mice. Diabetologia. 1975;11:387–393. doi: 10.1007/BF00429905. [DOI] [PubMed] [Google Scholar]
- 55.Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011;36:1433–1443. doi: 10.1038/npp.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blake CB, Smith BN. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol. 2012;303:R807–R814. doi: 10.1152/ajpregu.00276.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blevins JE, Baskin DG. Hypothalamic-brainstem circuits controlling eating. Forum Nutr. 2010;63:133–140. doi: 10.1159/000264401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blevins JE, Chelikani PK, Haver AC, Reidelberger RD. PYY(3–36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides. 2008;29:112–119. doi: 10.1016/j.peptides.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boeckxstaens GE, De Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 60.Bonaz B, Plourde V, Tache Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J Comp Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 61.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Bonham AC, Chen C-Y. Synaptic transmission in the nucleus tractus solitarius (NTS) In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 63.Borison HL, Brizzee KR. Morphology of emetic chemoreceptor trigger zone in cat medulla oblongata. Proc Soc Exp Biol Med. 1951;77:38–42. doi: 10.3181/00379727-77-18670. [DOI] [PubMed] [Google Scholar]
- 64.Borison HL, Wang SC. Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol. 1949;12:305–313. doi: 10.1152/jn.1949.12.5.305. [DOI] [PubMed] [Google Scholar]
- 65.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol. 1988;398:371–390. doi: 10.1113/jphysiol.1988.sp017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 67.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: No neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 68.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108:79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- 70.Browning KN, Babic T, Holmes GM, Swartz EM, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol. 2013;591:1563–1580. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591:2357–2372. doi: 10.1113/jphysiol.2012.249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Browning KN, Travagli RA. Characterisation of the in vitro effects of 5-Hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol. 2003;549:775–785. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: An overview. Auton Neurosci. 2006;126–127:2–8. doi: 10.1016/j.autneu.2006.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil. 2009;21:1309–1318. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil. 2010;22:1154–1163. doi: 10.1111/j.1365-2982.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13. doi: 10.1016/j.autneu.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Browning KN, Wan S, Baptista V, Travagli RA. Vanilloid, purinergic, and CCK receptors activate glutamate release on single neurons of the nucleus tractus solitarius centralis. Am J Physiol Regul Integr Comp Physiol. 2011;301:R394–R401. doi: 10.1152/ajpregu.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol. 2006;575:761–776. doi: 10.1113/jphysiol.2006.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buijs RM, Chun SJ, Nijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 86.Bulbul M, Babygirija R, Ludwig K, Takahashi T. Central oxytocin attenuates augmented gastric postprandial motility induced by restraint stress in rats. Neurosci Lett. 2010;479:302–306. doi: 10.1016/j.neulet.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 87.Burstein R, Cliffer KD, Giesler GJ., Jr Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci. 1987;7:4159–4164. doi: 10.1523/JNEUROSCI.07-12-04159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burstein R, Giesler GJ., Jr Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res. 1989;497:149–154. doi: 10.1016/0006-8993(89)90981-5. [DOI] [PubMed] [Google Scholar]
- 89.Cabral A, Valdivia S, Reynaldo M, Cyr NE, Nillni EA, Perello M. Short-term cold exposure activates TRH neurons exclusively in the hypothalamic paraventricular nucleus and raphe pallidus. Neurosci Lett. 2012;518:86–91. doi: 10.1016/j.neulet.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 90.Cai QQ, Zheng LF, Fan RF, Lian H, Zhou L, Song HY, Tang YY, Feng XY, Guo ZK, Wang ZY, Zhu JX. Distribution of dopamine receptors D1- and D2-immunoreactive neurons in the dorsal motor nucleus of vagus in rats. Auton Neurosci. 2013;176:48–53. doi: 10.1016/j.autneu.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Callahan LB, Tschetter KE, Ronan PJ. Inhibition of corticotropin releasing factor expression in the central nucleus of the amygdala attenuates stress-induced behavioral and endocrine responses. Front Neurosci. 2013;7:195. doi: 10.3389/fnins.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camilleri M, Malagelada JR, Kao PC, Zinsmeister AR. Gastric and autonomic responses to stress in functional dyspepsia. Dig Dis Sci. 1986;31:1169–1177. doi: 10.1007/BF01296514. [DOI] [PubMed] [Google Scholar]
- 93.Camilleri M, Saslow SB, Bharucha AE. Gastrointestinal sensation. Mechanisms and relation to functional gastrointestinal disorders. Gastroenterol Clin North Am. 1996;25:247–258. doi: 10.1016/s0889-8553(05)70374-5. [DOI] [PubMed] [Google Scholar]
- 94.Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267–273. [Google Scholar]
- 95.Cano G, Card JP, Rinaman L, Sved AF. Connections of Barrington’s nucleus to the sympathetic nervous system in rats. J Auton Nerv Syst. 2000;79:117–128. doi: 10.1016/s0165-1838(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 96.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: A Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 97.Carstens E, Yokota T, Zimmermann M. Inhibition of spinal neuronal responses to noxious skin heating by stimulation of mesencephalic periaqueductal gray in the cat. J Neurophysiol. 1979;42:558–568. doi: 10.1152/jn.1979.42.2.558. [DOI] [PubMed] [Google Scholar]
- 98.Cassell MD, Gray TS. The amygdala directly innervates adrenergic (C1) neurons in the ventrolateral medulla in the rat. Neurosci Lett. 1989;97:163–168. doi: 10.1016/0304-3940(89)90157-2. [DOI] [PubMed] [Google Scholar]
- 99.Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- 100.Chaikomin R, Rayner CK, Jones KL, Horowitz M. Upper gastrointestinal function and glycemic control in diabetes mellitus. World J Gastroenterol. 2006;12:5611–5621. doi: 10.3748/wjg.v12.i35.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–960. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen T, Dong YX, Li YQ. Fos expression in serotonergic neurons in the rat brainstem following noxious stimuli: An immunohistochemical double-labelling study. J Anat. 2003;203:579–588. doi: 10.1046/j.1469-7580.2003.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 107.Coceani F, Pace-Asciak C, Volta F, Wolfe LS. Effect of nerve stimulation on prostaglandin formation and release from the rat stomach. Am J Physiol. 1967;213:1056–1064. doi: 10.1152/ajplegacy.1967.213.4.1056. [DOI] [PubMed] [Google Scholar]
- 108.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 109.Commissiong JW. Evidence that the noradrenergic coerulospinal projection decussates at the spinal level. Brain Res. 1981;212:145–151. doi: 10.1016/0006-8993(81)90042-1. [DOI] [PubMed] [Google Scholar]
- 110.Commissiong JW, Hellstrom SO, Neff NH. A new projection from locus coeruleus to the spinal ventral columns: Histochemical and biochemical evidence. Brain Res. 1978;148:207–213. doi: 10.1016/0006-8993(78)90391-8. [DOI] [PubMed] [Google Scholar]
- 111.Cottrell GT, Ferguson AV. Sensory circumventricular organs: Central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000a;86:83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 113.Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000b;84:8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 114.Cragg BG, Evans DH. Some reflexes mediated by the afferent fibers of the abdominal vagus in the rabbit and cat. Exp Neurol. 1960;2:1–12. doi: 10.1016/0014-4886(60)90043-1. [DOI] [PubMed] [Google Scholar]
- 115.Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: A review. Curr Neuropharmacol. 2013;11:141–159. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R291–R307. doi: 10.1152/ajpregu.00863.2005. [DOI] [PubMed] [Google Scholar]
- 117.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 118.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 119.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 120.Curtis AL, Leiser SC, Snyder K, Valentino RJ. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62:1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 122.Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: The cholinergic anti-inflammatory pathway. J Endotoxin Res. 2003;9:409–413. doi: 10.1179/096805103225002755. [DOI] [PubMed] [Google Scholar]
- 123.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257:156–166. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- 124.Dallaporta M, Himmi T, Perrin J, Orsini JC. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport. 1999;10:2657–2660. doi: 10.1097/00001756-199908200-00040. [DOI] [PubMed] [Google Scholar]
- 125.Dallaporta M, Perrin J, Orsini J-C. Involvement of adenosine triphosphate-sensitive K+ channels in glucose-sensing in the rat solitary tract nucleus. Neurosci Lett. 2000;278:77–80. doi: 10.1016/s0304-3940(99)00898-8. [DOI] [PubMed] [Google Scholar]
- 126.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Darmani NA, Ray AP. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev. 2009;109:3158–3199. doi: 10.1021/cr900117p. [DOI] [PubMed] [Google Scholar]
- 128.Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: Implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 130.de Haan JJ, Hadfoune M, Lubbers T, Hodin C, Lenaerts K, Ito A, Verbaeys I, Skynner MJ, Cailotto C, van d V, De Jonge WJ, Greve JW, Buurman WA. Lipid-rich enteral nutrition regulates mucosal mast cell activation via the vagal anti-inflammatory reflex. Am J Physiol Gastrointest Liver Physiol. 2013;305:G383–G391. doi: 10.1152/ajpgi.00333.2012. [DOI] [PubMed] [Google Scholar]
- 131.de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Ponti F, Azpiroz F, Malagelada J-R. Reflex gastric relaxation in response to distention of the duodenum. Am J Physiol. 1987;252:G595–G601. doi: 10.1152/ajpgi.1987.252.5.G595. [DOI] [PubMed] [Google Scholar]
- 133.Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 134.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 135.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: Control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012;9:271–285. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- 136.Demedts I, Masaoka T, Kindt S, De HG, Geboes K, Farre R, Vanden Berghe P, Tack J. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol Motil. 2013;19:161–170. doi: 10.5056/jnm.2013.19.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–9672. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ. Direct projections from the lumbosacral spinal cord to Barrington’s nucleus in the rat: A special reference to micturition reflex. J Comp Neurol. 1997;389:149–160. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 140.Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97:531–536. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 141.Dong YX, Han ZA, Xiong KH, Rao ZR. Fos expression in serotonergic midbrain neurons projecting to the paraventricular nucleus of hypothalamus after noxious stimulation of the stomach: A triple labeling study in the rat. Neurosci Res. 1997;27:155–160. doi: 10.1016/s0168-0102(96)01143-1. [DOI] [PubMed] [Google Scholar]
- 142.Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92:969–974. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drucker DJ. Minireview: The glucagon-like peptides. Endocrinology. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 144.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 145.Duca FA, Sakar Y, Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes. 2013;62:2410–2415. doi: 10.2337/db12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dumont Y, Moyse E, Fournier A, Quirion R. Distribution of peripherally injected peptide YY ([125I] PYY (3–36)) and pancreatic polypeptide ([125I] hPP) in the CNS: Enrichment in the area postrema. J Mol Neurosci. 2007;33:294–304. doi: 10.1007/s12031-007-9007-9. [DOI] [PubMed] [Google Scholar]
- 147.Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 148.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 149.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 150.Ebert E. Gastrointestinal involvement in spinal cord injury: A clinical perspective. J Gastrointestin Liver Dis. 2012;21:75–82. [PubMed] [Google Scholar]
- 151.Eisner F, Jacob P, Frick JS, Feilitzsch M, Geisel J, Mueller MH, Kuper MA, Raybould HE, Konigsrainer I, Glatzle J. Immunonutrition with long-chain fatty acids prevents activation of macrophages in the gut wall. J Gastrointest Surg. 2011;15:853–859. doi: 10.1007/s11605-011-1431-z. [DOI] [PubMed] [Google Scholar]
- 152.Elam M, Thoren P, Svensson TH. Locus coeruleus neurons and sympathetic nerves: Activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- 153.Ennis M, Behbehani M, Shipley MT, Van Bockstaele EJ, Aston-Jones G. Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: Anatomic and physiologic studies. J Comp Neurol. 1991;306:480–494. doi: 10.1002/cne.903060311. [DOI] [PubMed] [Google Scholar]
- 154.Farre R, Tack J. Food and symptom generation in functional gastrointestinal disorders: Physiological aspects. Am J Gastroenterol. 2013;108:698–706. doi: 10.1038/ajg.2013.24. [DOI] [PubMed] [Google Scholar]
- 155.Fasth S, Hulten L, Nordgren S. Evidence for a dual pelvic nerve influence on large bowel motility in the cat. J Physiol. 1980;298:159–169. doi: 10.1113/jphysiol.1980.sp013073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 157.Fennegan FM, Puiggari MJ. Hypothalamic and amygdaloid influence on gastric motility in dogs. J Neurosurg. 1966;24:497–504. doi: 10.3171/jns.1966.24.2.0497. [DOI] [PubMed] [Google Scholar]
- 158.Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: Gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 160.Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res. 2000;859:83–95. doi: 10.1016/s0006-8993(00)01935-1. [DOI] [PubMed] [Google Scholar]
- 161.Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- 162.Fleetwood-Walker SM, Coote JH. The contribution of brain stem catecholamine cell groups to the innervation of the sympathetic lateral cell column. Brain Res. 1981;205:141–155. doi: 10.1016/0006-8993(81)90726-5. [DOI] [PubMed] [Google Scholar]
- 163.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 164.Fogel R, Zhang X, Renehan WE. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 165.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 166.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 167.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 168.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- 169.Fox EA, Powley TL. Morphology of identified preganglionic neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1992;322:79–98. doi: 10.1002/cne.903220107. [DOI] [PubMed] [Google Scholar]
- 170.Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 171.Fry M, Ferguson AV. The sensory circumventricular organs: Brain targets for circulating signals controlling ingestive behavior. Physiol Behav. 2007;91:413–423. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 172.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 173.Furuse M, Matsumoto M, Mori R, Sugahara K, Kano K, Hasegawa S. Influence of fasting and neuropeptide Y on the suppressive food intake induced by intracerebroventricular injection of glucagon-like peptide-1 in the neonatal chick. Brain Res. 1997;764:289–292. doi: 10.1016/s0006-8993(97)00623-9. [DOI] [PubMed] [Google Scholar]
- 174.Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil. 2010;22:470–9. e111. doi: 10.1111/j.1365-2982.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 175.Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol. 2010;103:904–914. doi: 10.1152/jn.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garrick T, Prince M, Yang Y, Ohning G, Tache Y. Raphe pallidus stimulation increases gastric contractility via TRH projections to the dorsal vagal complex in rats. Brain Res. 1994;636:343–347. doi: 10.1016/0006-8993(94)91035-9. [DOI] [PubMed] [Google Scholar]
- 177.Garrick T, Veiseh A, Sierra A, Weiner H, Tache Y. Corticotropin-releasing factor acts centrally to suppress stimulated gastric contractility in the rat. Regul Pept. 1988;21:173–181. doi: 10.1016/0167-0115(88)90101-2. [DOI] [PubMed] [Google Scholar]
- 178.Garry RC. The nervous control of the caudal region of the large bowel in the cat. J Physiol. 1933;77:422–431. doi: 10.1113/jphysiol.1933.sp002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gebhart GF, Sandkuhler J, Thalhammer JG, Zimmermann M. Quantitative comparison of inhibition in spinal cord of nociceptive information by stimulation in periaqueductal gray or nucleus raphe magnus of the cat. J Neurophysiol. 1983;50:1433–1445. doi: 10.1152/jn.1983.50.6.1433. [DOI] [PubMed] [Google Scholar]
- 180.Geeraerts B, Vandenberghe J, Van Oudenhove L, Gregory LJ, Aziz Q, Dupont P, Demyttenaere K, Janssens J, Tack J. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology. 2005;129:1437–1444. doi: 10.1053/j.gastro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 181.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 182.Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G711–G718. doi: 10.1152/ajpgi.00240.2007. [DOI] [PubMed] [Google Scholar]
- 183.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: A tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 184.Gieroba ZJ, Messenger JP, Blessing WW. Abdominal vagal stimulation excites bulbospinal barosensitive neurons in the rostral ventrolateral medulla. Neuroscience. 1995;65:355–364. doi: 10.1016/0306-4522(94)00509-4. [DOI] [PubMed] [Google Scholar]
- 185.Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol. 2004;93:2530–2540. doi: 10.1152/jn.00429.2004. [DOI] [PubMed] [Google Scholar]
- 186.Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci. 1992;12:2251–2258. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Glaum SR, Miller RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci. 1993a;13:1636–1641. doi: 10.1523/JNEUROSCI.13-04-01636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993b;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- 189.Glaum SR, Miller RJ. Presynaptic metabotropic glutamate receptors modulate omega-conotoxin- GVIA-insensitive calcium channels in the rat medulla. Neuropharmacology. 1995;34:953–964. doi: 10.1016/0028-3908(95)00076-i. [DOI] [PubMed] [Google Scholar]
- 190.Glazer EJ, Basbaum AI. Leucine enkephalin: Localization in and axoplasmic transport by sacral parasympathetic preganglionic neurons. Science. 1980;208:1479–1481. doi: 10.1126/science.6155697. [DOI] [PubMed] [Google Scholar]
- 191.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 192.Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549:37–56. doi: 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grabauskas G, Song I, Zhou SY, Owyang C. Electrophysiological identifications of glucose-sensing neurons in the rat nodose ganglia. J Physiol. 2010;588:617–632. doi: 10.1113/jphysiol.2009.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Granger DN, Richardson PD, Kvietys PR, Mortillaro NA. Intestinal blood flow. Gastroenterology. 1980;78:837–863. [PubMed] [Google Scholar]
- 196.Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- 197.Greenwood B, Tremblay L, Davison JS. Sympathetic control of motility, fluid transport, and transmural potential difference in the rabbit ileum. Am J Physiol. 1987;253:G726–G729. doi: 10.1152/ajpgi.1987.253.6.G726. [DOI] [PubMed] [Google Scholar]
- 198.Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- 199.Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA. Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res. 2006;1071:91–96. doi: 10.1016/j.brainres.2005.11.071. [DOI] [PubMed] [Google Scholar]
- 200.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grzanna R, Molliver ME. The locus coeruleus in the rat: An immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- 202.Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–970. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 203.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci. 2010;30:6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 205.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 206.Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van MB. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. 2000;69:379–382. doi: 10.1016/s0031-9384(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 207.Guo J, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol. 2001;280:G361–G367. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- 208.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 209.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2013;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2009;296:R528–R536. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hay M, Lindsley KA. Metabotropic glutamate receptor inhibition of visceral afferent potassium currents. Brain Res. 1995;698:169–174. doi: 10.1016/0006-8993(95)00889-x. [DOI] [PubMed] [Google Scholar]
- 212.Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- 213.Henke PG. The amygdala and restraint ulcers in rats. J Comp Physiol Psychol. 1980;94:313–323. doi: 10.1037/h0077670. [DOI] [PubMed] [Google Scholar]
- 214.Henke PG. Attenuation of shock-induced ulcers after lesions in the medial amygdala. Physiol Behav. 1981;27:143–146. doi: 10.1016/0031-9384(81)90311-5. [DOI] [PubMed] [Google Scholar]
- 215.Henke PG. Electrophysiological activity in the central nucleus of the amygdala: Emotionality and stress ulcers in rats. Behav Neurosci. 1988;102:77–83. doi: 10.1037//0735-7044.102.1.77. [DOI] [PubMed] [Google Scholar]
- 216.Henke PG. Recent studies of the central nucleus of the amygdala and stress ulcers. Neurosci Biobehav Rev. 1988;12:143–150. doi: 10.1016/s0149-7634(88)80006-x. [DOI] [PubMed] [Google Scholar]
- 217.Henke PG, Ray A, Sullivan RM. The amygdala. Emotions and gut functions. Dig Dis Sci. 1991;36:1633–1643. doi: 10.1007/BF01296409. [DOI] [PubMed] [Google Scholar]
- 218.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 219.Herbert H, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- 220.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hernandez DE, Emerick SG. Thyrotropin-releasing hormone: Medullary site of action to induce gastric ulcers and stimulate acid secretion. Brain Res. 1988;459:148–152. doi: 10.1016/0006-8993(88)90295-8. [DOI] [PubMed] [Google Scholar]
- 222.Hernandez DE, Jennes L, Emerick SG. Inhibition of gastric acid secretion by immunoneutralization of endogenous brain thyrotropin-releasing hormone. Brain Res. 1987;401:381–384. doi: 10.1016/0006-8993(87)91425-9. [DOI] [PubMed] [Google Scholar]
- 223.Hernandez DE, Salaiz AB, Morin P, Moreira MA. Administration of thyrotropin-releasing hormone into the central nucleus of the amygdala induces gastric lesions in rats. Brain Res Bull. 1990;24:697–699. doi: 10.1016/0361-9230(90)90010-w. [DOI] [PubMed] [Google Scholar]
- 224.Heymann-Monnikes I, Tache Y, Trauner M, Weiner H, Garrick T. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991;554:139–144. doi: 10.1016/0006-8993(91)90181-t. [DOI] [PubMed] [Google Scholar]
- 225.Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- 226.Hirst GD, McKirdy HC. Presynaptic inhibition at mammalian peripheral synapse? Nature. 1974;250:430–431. doi: 10.1038/250430a0. [DOI] [PubMed] [Google Scholar]
- 227.Holmes GM. Upper gastrointestinal dysmotility after spinal cord injury: Is diminished vagal sensory processing one culprit? Front Physiol. 2012;3:277. doi: 10.3389/fphys.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Holmes GM, Browning K, Tong M, Qualls-Creekmore E, Travagli RA. Vagally-mediated effects of glucagon- like peptide 1 (GLP-1): in vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol. 2013;591:3081–3100. doi: 10.1113/jphysiol.2013.253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: In vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Holmes GM, Tong M, Travagli RA. Effects of brain stem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol. 2009;296:G621–G631. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 233.Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998;42:501–506. doi: 10.1136/gut.42.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Holzer HH, Raybould HE. Vagal and splanchnic sensory pathways mediate inhibition of gastric motility induced by duodenal distension. Am J Physiol. 1992;262:G603–G608. doi: 10.1152/ajpgi.1992.262.4.G603. [DOI] [PubMed] [Google Scholar]
- 235.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hornby PJ, Rossiter CD, Pineo SV, Norman WP, Friedman EK, Benjamin S, Gillis RA. TRH: Immunocytochemical distribution in vagal nuclei of the cat and physiological effects of microinjection. Am J Physiol. 1989;257:G454–G462. doi: 10.1152/ajpgi.1989.257.3.G454. [DOI] [PubMed] [Google Scholar]
- 237.Hornby PJ, Rossiter CD, White RL, Norman WP, Kuhn DH, Gillis RA. Medullary raphe: A new site for vagally mediated stimulated of gastric motility in cats. Am J Physiol. 1990;258:G637–G647. doi: 10.1152/ajpgi.1990.258.4.G637. [DOI] [PubMed] [Google Scholar]
- 238.Horowitz M, Fraser R. Disordered gastric motor function in diabetes mellitus. Diabetologia. 1994;37:543–551. doi: 10.1007/BF00403371. [DOI] [PubMed] [Google Scholar]
- 239.Huang XF, Tork I, Paxinos G. Dorsal motor nucleus of the vagus nerve: A cyto- and chemoarchitectonic study in the human. J Comp Neurol. 1993;330:158–182. doi: 10.1002/cne.903300203. [DOI] [PubMed] [Google Scholar]
- 240.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 241.Hurley-Gius KM, Neafsey EJ. The medial frontal cortex and gastric motility: Microstimulation results and their possible significance for the overall pattern of organization of rat frontal and parietal cortex. Brain Res. 1986;365:241–248. doi: 10.1016/0006-8993(86)91635-5. [DOI] [PubMed] [Google Scholar]
- 242.Hyland NP, Abrahams TP, Fuchs K, Burmeister MA, Hornby PJ. Organization and neurochemistry of vagal preganglionic neurons innervating the lower esophageal sphincter in ferrets. J Comp Neurol. 2001;430:222–234. doi: 10.1002/1096-9861(20010205)430:2<222::aid-cne1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 243.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Imaki T, Vale W, Sawchenko PE. Regulation of corticotropin-releasing factor mRNA in neuroendocrine and autonomic neurons by osmotic stimulation and volume loading. Neuroendocrinology. 1992;56:633–640. doi: 10.1159/000126286. [DOI] [PubMed] [Google Scholar]
- 245.Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture elicits dual effects: Stimulation of delayed gastric emptying and inhibition of accelerated colonic transit induced by restraint stress in rats. Dig Dis Sci. 2006;51:1493–1500. doi: 10.1007/s10620-006-9083-7. [DOI] [PubMed] [Google Scholar]
- 246.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 247.Janig W. Integration of gut function by sympathetic reflexes. Baillieres Clin Gastroenterol. 1988;2:45–62. doi: 10.1016/0950-3528(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 248.Janig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res. 2000;122:273–287. doi: 10.1016/s0079-6123(08)62145-7. [DOI] [PubMed] [Google Scholar]
- 249.Janig W, McLachlan EM. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987;67:1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- 250.Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 251.Jansen ASP, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: A viral tracing study. Brain Res. 1997;766:29–38. doi: 10.1016/s0006-8993(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 252.Jarvinen MK, Powley TL. Dorsal motor nucleus of the vagus neurons: A multivariate taxonomy. J Comp Neurol. 1999;403:359–377. [PubMed] [Google Scholar]
- 253.Jean A. The nucleus tractus solitarius: Neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys. 1991;99:A3–52. doi: 10.3109/13813459109145916. [DOI] [PubMed] [Google Scholar]
- 254.Jenkinson KM, Reid JJ. Altered non-adrenergic non-cholinergic neurotransmission in gastric fundus from streptozotocin-diabetic rats. Eur J Pharmacol. 2000;401:251–258. doi: 10.1016/s0014-2999(00)00280-6. [DOI] [PubMed] [Google Scholar]
- 255.Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J Neurosci. 2004;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kalia M, Fuxe K, Goldstein M, Harfstrand A, Agnati LF, Coyle JT. Evidence for the existence of putative dopamine-, adrenanaline- and noradrenaline-containing vagal motor neurons in the brainstem of the rat. Neurosci Lett. 1984;50:57–62. doi: 10.1016/0304-3940(84)90462-2. [DOI] [PubMed] [Google Scholar]
- 257.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–264. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 258.Karamanolis G, Tack J. Nutrition and motility disorders. Best Pract Res Clin Gastroenterol. 2006;20:485–505. doi: 10.1016/j.bpg.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 259.Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- 260.Karoum F, Commissiong JW, Neff NH, Wyatt RJ. Biochemical evidence for uncrossed and crossed locus coeruleus projections to the spinal cord. Brain Res. 1980;196:237–241. doi: 10.1016/0006-8993(80)90730-1. [DOI] [PubMed] [Google Scholar]
- 261.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 262.Kawatani M, Shioda S, Nakai Y, Takeshige C, De Groat WC. Ultrastructural analysis of enkephalinergic terminals in parasympathetic ganglia innervating the urinary bladder of the cat. J Comp Neurol. 1989;288:81–91. doi: 10.1002/cne.902880107. [DOI] [PubMed] [Google Scholar]
- 263.Keay KA, Feil K, Gordon BD, Herbert H, Bandler R. Spinal afferents to functionally distinct periaqueductal gray columns in the rat: An anterograde and retrograde tracing study. J Comp Neurol. 1997;385:207–229. doi: 10.1002/(sici)1096-9861(19970825)385:2<207::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 264.Keller J, Runzi M, Goebell H, Layer P. Duodenal and ileal nutrient deliveries regulate human intestinal motor and pancreatic responses to a meal. Am J Physiol. 1997;272:G632–G637. doi: 10.1152/ajpgi.1997.272.3.G632. [DOI] [PubMed] [Google Scholar]
- 265.Kennedy C, Krier J. Delta-opioid receptors mediate inhibition of fast excitatory postsynaptic potentials in cat parasympathetic colonic ganglia. Br J Pharmacol. 1987;92:437–443. doi: 10.1111/j.1476-5381.1987.tb11340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kim MS, Jo YH, Yoon SH, Hahn SJ, Rhie DJ, Kim CC, Choi H. Electrical stimulation of the medial amygdala facilitates gastric acid secretion in conscious rats. Brain Res. 1990;524:208–212. doi: 10.1016/0006-8993(90)90692-5. [DOI] [PubMed] [Google Scholar]
- 267.King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- 268.Kobashi M, Adachi A. Effect of topical administration of glucose on neurons innervating abdominal viscera in dorsal motor nucleus of vagus in rats. Jpn J Physiol. 1994;44:729–734. doi: 10.2170/jjphysiol.44.729. [DOI] [PubMed] [Google Scholar]
- 269.Kobayashi RM, Palkovits M, Kopin IJ, Jacobowitz DM. Biochemical mapping of noradrenergic nerves arising from the rat locus coeruleus. Brain Res. 1974;77:269–279. doi: 10.1016/0006-8993(74)90790-2. [DOI] [PubMed] [Google Scholar]
- 270.Konturek PC, Konturek SJ, Ochmanski W. Neuroendocrinology of gastric H+ and duodenal HCO3− secretion: The role of brain-gut axis. Eur J Pharmacol. 2004;499:15–27. doi: 10.1016/j.ejphar.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 271.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 272.Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- 273.Krowicki ZK, Hornby PJ. Serotonin and Thyrotropin-Releasing Hormone do not augment their effects on Gastric Motility on their microinjection into the nucleus raphe obscurus of the rat. J Pharmacol Exp Ther. 1995;273:499–508. [PubMed] [Google Scholar]
- 274.Krowicki ZK, Nathan NA, Hornby PJ. Gastric motor and cardiovascular effects of insulin in dorsal vagal complex of the rat. Am J Physiol. 1998;275:G964–G972. doi: 10.1152/ajpgi.1998.275.5.G964. [DOI] [PubMed] [Google Scholar]
- 275.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 276.Kwiat GC, Basbaum AI. Organization of tyrosine hydroxylase- and serotonin-immunoreactive brainstem neurons with axon collaterals to the periaqueductal gray and the spinal cord in the rat. Brain Res. 1990;528:83–94. doi: 10.1016/0006-8993(90)90198-k. [DOI] [PubMed] [Google Scholar]
- 277.Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 278.Lacolley P, Owen JR, Sandock K, Lewis TH, Bates JN, Robertson TP, Lewis SJ. 5-HT activates vagal afferent cell bodies in vivo: Role of 5-HT2 and 5-HT3 receptors. Neuroscience. 2006;143:273–287. doi: 10.1016/j.neuroscience.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 279.Lacolley P, Owen JR, Sandock K, Lewis TH, Bates JN, Robertson TP, Lewis SJ. Occipital artery injections of 5-HT may directly activate the cell bodies of vagal and glossopharyngeal afferent cell bodies in the rat. Neuroscience. 2006;143:289–308. doi: 10.1016/j.neuroscience.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 280.Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–376. doi: 10.1053/gast.2001.21201. [DOI] [PubMed] [Google Scholar]
- 281.Ladic LA, Buchan AM. Association of substance P and its receptor with efferent neurons projecting to the greater curvature of the rat stomach. J Auton Nerv Syst. 1996;58:25–34. doi: 10.1016/0165-1838(96)00114-2. [DOI] [PubMed] [Google Scholar]
- 282.Langley JN, Anderson HK. On the innervation of the pelvic and adjoining viscera: Part I. The lower portion of the intestine. J Physiol. 1895;18:67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lazovic J, Wrzos HF, Yang QX, Collins CM, Smith MB, Norgren R, Matyas K, Ouyang A. Regional activation in the rat brain during visceral stimulation detected by c-fos expression and fMRI. Neurogastroenterol Motil. 2005;17:548–556. doi: 10.1111/j.1365-2982.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 284.Le Brun I, Dufour A, Crest M, Szabo G, Erdelyi F, Baude A. Differential expression of Nk1 and NK3 neurokinin receptors in neurons of the nucleus tractus solitarius and the dorsal vagal motor nucleus of the rat and mouse. Neuroscience. 2008;152:56–64. doi: 10.1016/j.neuroscience.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 285.LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657–R666. doi: 10.1152/ajpregu.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lee PG, Cai F, Helke CJ. Streptozotocin-induced diabetes reduces retrograde axonal transport in the afferent and efferent vagus nerve. Brain Res. 2002;941:127–136. doi: 10.1016/s0006-8993(02)02645-8. [DOI] [PubMed] [Google Scholar]
- 288.Lee PG, Hohman TC, Cai F, Regalia J, Helke CJ. Streptozotocin-induced diabetes causes metabolic changes and alterations in neurotrophin content and retrograde transport in the cervical vagus nerve. Exp Neurol. 2001;170:149–161. doi: 10.1006/exnr.2001.7673. [DOI] [PubMed] [Google Scholar]
- 289.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 290.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: What do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 291.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lewis MW, Travagli RA. Effects of substance P on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 2001;281:G164–G172. doi: 10.1152/ajpgi.2001.281.1.G164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li B, Guo CL, Tang J, Zhu JN, Wang JJ. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals. 2009;17:132–143. doi: 10.1159/000197913. [DOI] [PubMed] [Google Scholar]
- 294.Li M, Han F, Shi Y. Expression of locus coeruleus mineralocorticoid receptor and glucocorticoid receptor in rats under single-prolonged stress. Neurol Sci. 2011;32:625–631. doi: 10.1007/s10072-011-0597-1. [DOI] [PubMed] [Google Scholar]
- 295.Li YQ, Jia HG, Rao ZR, Shi JW. Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett. 1990;120:124–127. doi: 10.1016/0304-3940(90)90184-b. [DOI] [PubMed] [Google Scholar]
- 296.Lightman SL. Corticotropin-releasing factor. From stress to cognition. Nature. 1995;378:233–234. doi: 10.1038/378233a0. [DOI] [PubMed] [Google Scholar]
- 297.Lin HC, Zhao XT, Wang L. Fat absorption is not complete by midgut but is dependent on load of fat. Am J Physiol. 1996;271:G62–G67. doi: 10.1152/ajpgi.1996.271.1.G62. [DOI] [PubMed] [Google Scholar]
- 298.Lisander B, Martner J. Effects on gastric motility from the cerebellar fastigial nucleus. Acta Physiol Scand. 1975;94:368–377. doi: 10.1111/j.1748-1716.1975.tb05896.x. [DOI] [PubMed] [Google Scholar]
- 299.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. Am J Clin Nutr. 2007;86:531–541. doi: 10.1093/ajcn/86.3.531. [DOI] [PubMed] [Google Scholar]
- 300.Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterol. 2008;134:2070–2079. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 301.Liu M, Seino S, Kirchgessner AL. Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Liu RP. Laminar origins of spinal projection neurons to the periaqueductal gray of the rat. Brain Res. 1983;264:118–122. doi: 10.1016/0006-8993(83)91127-7. [DOI] [PubMed] [Google Scholar]
- 303.Liubashina O, Bagaev V, Khotiantsev S. Amygdalofugal modulation of the vago-vagal gastric motor reflex in rat. Neurosci Lett. 2002;325:183–186. doi: 10.1016/s0304-3940(02)00289-6. [DOI] [PubMed] [Google Scholar]
- 304.Llewellyn-Smith IJ, Kellett DO, Jordan D, Browning KN, Travagli RA. Oxytocin-immunoreactive innervation of identified neurons in the rat dorsal vagal complex. Neurogastroenterol Motil. 2012;24:e136–e146. doi: 10.1111/j.1365-2982.2011.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 306.Longhurst JC, Ibarra J. Sympathoadrenal mechanisms in hemodynamic responses to gastric distension in cats. Am J Physiol. 1982;243:H748–H753. doi: 10.1152/ajpheart.1982.243.5.H748. [DOI] [PubMed] [Google Scholar]
- 307.Lorber M. Results of simulated mastication suggest existence of a periodontogastric motility reflex. Can J Physiol Pharmacol. 2000;78:29–35. doi: 10.1139/cjpp-78-1-29. [DOI] [PubMed] [Google Scholar]
- 308.Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 309.Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 310.Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol. 1991;311:271–288. doi: 10.1002/cne.903110208. [DOI] [PubMed] [Google Scholar]
- 311.Lyubashina OA. Possible mechanisms of involvement of the amygdaloid complex in the control of gastric motor function. Neurosci Behav Physiol. 2004;34:379–388. doi: 10.1023/b:neab.0000018750.65372.18. [DOI] [PubMed] [Google Scholar]
- 312.Mahaut S, Dumont Y, Fournier A, Quirion R, Moyse E. Neuropeptide Y receptor subtypes in the dorsal vagal complex under acute feeding adaptation in the adult rat. Neuropeptides. 2010;44:77–86. doi: 10.1016/j.npep.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 313.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: A sensible food target for appetite control. A review. Physiol Behav. 2008;95:271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 314.Manchanda SK, Tandon OP, Aneja IS. Role of the cerebellum in the control of gastro-intestinal motility. J Neural Transm. 1972;33:195–209. doi: 10.1007/BF01245317. [DOI] [PubMed] [Google Scholar]
- 315.Mantyh PW, Peschanski M. Spinal projections from the periaqueductal grey and dorsal raphe in the rat, cat and monkey. Neuroscience. 1982;7:2769–2776. doi: 10.1016/0306-4522(82)90099-9. [DOI] [PubMed] [Google Scholar]
- 316.Marks JD, Donnelly DF, Haddad GG. Adenosine-induced inhibition of vagal motoneuron excitability: Receptor subtype and mechanisms. Am J Physiol. 1993;264:L124–L132. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- 317.Martinez de la Pena y Valenzuela I, Browning KN, Travagli RA. Morphological differences between planes of section do not influence the electrophysiological properties of identified rat dorsal motor nucleus of the vagus neurons. Brain Res. 2004;1003:54–60. doi: 10.1016/j.brainres.2003.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Martinez de la Pena y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 2004;286:G333–G339. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Martinez V, Barrachina MD, Ohning G, Tache Y. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1310–G1319. doi: 10.1152/ajpgi.00222.2002. [DOI] [PubMed] [Google Scholar]
- 320.Martinez V, Wang L, Tache Y. Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats. Brain Res. 2006;1086:168–180. doi: 10.1016/j.brainres.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 321.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: A Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 324.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: From basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 325.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 326.Mazei-Robinson MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harbor Perspect Med. 2012;2:1–16. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mei N. Intestinal chemosensitivity. Physiol Rev. 1985;65:211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- 329.Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: A possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 330.Menetrey D, de Pommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. Eur J Neurosci. 1991;3:249–259. doi: 10.1111/j.1460-9568.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 331.Micevych P, Akesson T, Elde R. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol. 1988;269:381–391. doi: 10.1002/cne.902690306. [DOI] [PubMed] [Google Scholar]
- 332.Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- 333.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 335.Million M, Wang L, Martinez V, Tache Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000;877:345–353. doi: 10.1016/s0006-8993(00)02719-0. [DOI] [PubMed] [Google Scholar]
- 336.Min DK, Tuor UI, Chelikani PK. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 2011;179:151–158. doi: 10.1016/j.neuroscience.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 337.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- 338.Molinari C, Sabbatini M, Grossini E, Mary DA, Cannas M, Vacca G. Cardiovascular effects and c-Fos expression in the rat hindbrain in response to innocuous stomach distension. Brain Res Bull. 2006;69:140–146. doi: 10.1016/j.brainresbull.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 339.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: Stress, health and disease. Mamm Genome. 2013;25:49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- 340.Monnikes H, Schmidt BG, Raybould HE, Tache Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 341.Monnikes H, Schmidt BG, Tebbe J, Bauer C, Tache Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res. 1994;644:101–108. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 342.Monnikes H, Tebbe J, Bauer C, Lauer G, Arnold R. Microinfusion of corticotropin-releasing factor into the locus coeruleus/subcoeruleus nuclei inhibits gastric acid secretion via spinal pathways in the rat. Brain Res. 1996;728:157–165. doi: 10.1016/0006-8993(96)00393-9. [DOI] [PubMed] [Google Scholar]
- 343.Morrow NS, Grijalva CV, Geiselman PJ, Novin D. Effects of amygdaloid lesions on gastric erosion formation during exposure to activity-stress. Physiol Behav. 1993;53:1043–1048. doi: 10.1016/0031-9384(93)90357-l. [DOI] [PubMed] [Google Scholar]
- 344.Morton AP, Hanson PJ. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1984;69:117–126. doi: 10.1113/expphysiol.1984.sp002772. [DOI] [PubMed] [Google Scholar]
- 345.Myers B, Greenwood-Van Meerveld B. Role of anxiety in the pathophysiology of irritable bowel syndrome: Importance of the amygdala. Front Neurosci. 2009;3:47. doi: 10.3389/neuro.21.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol. 2012;302:G260–G266. doi: 10.1152/ajpgi.00353.2011. [DOI] [PubMed] [Google Scholar]
- 347.Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: The many faces of feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: A horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 349.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 350.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 351.Neuman KO. The afferent fibres of the abdominal vagus in the rabbit and cat. J Physiol. 1914;49:34–37. doi: 10.1113/jphysiol.1914.sp001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther. 2003;306:948–953. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- 353.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 354.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 355.North RA, Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Nosaka S, Murase S, Murata K. Arterial baroreflex inhibition by gastric distension in rats: Mediation by splanchnic afferents. Am J Physiol. 1991;260:R985–R994. doi: 10.1152/ajpregu.1991.260.5.R985. [DOI] [PubMed] [Google Scholar]
- 357.Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ. Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J Comp Neurol. 2006;496:787–801. doi: 10.1002/cne.20965. [DOI] [PubMed] [Google Scholar]
- 358.Ordog T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/W mutant mice. Gastroenterology. 2002;123:2028–2040. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- 359.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 360.Oro AE, Simerly RB, Swanson LW. Estrous cycle variations in levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei. Evidence for a regulatory role for estrogen. Neuroendocrinology. 1988;47:225–235. doi: 10.1159/000124916. [DOI] [PubMed] [Google Scholar]
- 361.Orts-Del’immagine A, Wanaverbecq N, Tardivel C, Tillement V, Dallaporta M, Trouslard J. Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J Physiol. 2012;590:3719–3741. doi: 10.1113/jphysiol.2012.227959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- 363.Owens NC, Verberne AJ. An electrophysiological study of the medial prefrontal cortical projection to the nucleus of the solitary tract in rat. Exp Brain Res. 1996;110:55–61. doi: 10.1007/BF00241374. [DOI] [PubMed] [Google Scholar]
- 364.Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- 365.Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: The neural substrate for the diving response. Brain Res. 2000;874:48–65. doi: 10.1016/s0006-8993(00)02549-x. [DOI] [PubMed] [Google Scholar]
- 366.Panteleev S, Grundy D. Descending influences from the infralimbic cortex on vago-vagal reflex control of gastric motor activity in the rat. Auton Neurosci. 2000;86:78–83. doi: 10.1016/S1566-0702(00)00249-6. [DOI] [PubMed] [Google Scholar]
- 367.Parsons BJ, Poat JA, Roberts PA. Studies of the mechanism of noradrenaline stimulation of fluid absorption by rat jejunum in vitro. J Physiol. 1984;355:427–439. doi: 10.1113/jphysiol.1984.sp015428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Pastorelli L, De SC, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Paulino G, Barbier dlS, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington’s nucleus: Evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–361. doi: 10.1016/s0006-8993(97)01178-5. [DOI] [PubMed] [Google Scholar]
- 371.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 372.Pavlov VA, Tracey KJ. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochem Soc Trans. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 373.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 374.Peng YB, Lin Q, Willis WD. Effects of GABA and glycine receptor antagonists on the activity and PAG-induced inhibition of rat dorsal horn neurons. Brain Res. 1996;736:189–201. doi: 10.1016/0006-8993(96)00668-3. [DOI] [PubMed] [Google Scholar]
- 375.Perrin J, Crousillat J, Mei N. Assessment of true splanchnic glucoreceptors in the jejuno-ileum of the cat. Brain Res Bull. 1981;7:625–628. doi: 10.1016/0361-9230(81)90108-8. [DOI] [PubMed] [Google Scholar]
- 376.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci. 2012;169:12–27. doi: 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Plata-Salaman CR, Fukuda A, Minami T, Oomura Y. Substance P effects on the dorsal motor nucleus of the vagus. Brain Res Bull. 1989;23:149–153. doi: 10.1016/0361-9230(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 379.Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 380.Porcher C, Sinniger V, Juhem A, Mouchet P, Bonaz B. Neuronal activity and CRF receptor gene transcription in the brains of rats with colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G803–G814. doi: 10.1152/ajpgi.00135.2004. [DOI] [PubMed] [Google Scholar]
- 381.Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000;34:184–188. doi: 10.1006/appe.1999.0279. [DOI] [PubMed] [Google Scholar]
- 382.Price CJ, Hoyda TD, Ferguson AV. The area postrema: A brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14:182–194. doi: 10.1177/1073858407311100. [DOI] [PubMed] [Google Scholar]
- 383.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Qualls-Creekmore E, Tong M, Holmes GM. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol Motil. 2010;22:181–185. doi: 10.1111/j.1365-2982.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Raggenbass M, Dreifuss JJ. Mechanism of action of oxytocin in rat vagal neurones: Induction of a sustained sodium-dependent current. J Physiol. 1992;457:131–142. doi: 10.1113/jphysiol.1992.sp019368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Raggenbass M, Dubois-Dauphin M, Charpak S, Dreifuss JJ. Neurons in the dorsal motor nucleus of the vagus nerve are excited by oxytocin in the rat but not in the guinea pig. Proc Natl Acad Sci U S A. 1987;84:3926–3930. doi: 10.1073/pnas.84.11.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Randich A, Gebhart DF. Vagal afferent modulation of nociception. Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 388.Raybould HE. Does your gut taste? Sensory transduction in the gastrointestinal tract. News Physiol Sci. 1998;13:275–280. doi: 10.1152/physiologyonline.1998.13.6.275. [DOI] [PubMed] [Google Scholar]
- 389.Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol. 1999;277:G751–G755. doi: 10.1152/ajpgi.1999.277.4.G751. [DOI] [PubMed] [Google Scholar]
- 390.Raybould HE. Visceral perception: Sensory transduction in visceral afferents and nutrients. Gut. 2002;51(Suppl 1):I11–I14. doi: 10.1136/gut.51.suppl_1.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 392.Raybould HE, Roberts ME, Dockray GJ. Reflex decreases in intragastric pressure in response to cholecystokinin in rats. Am J Physiol. 1987;253:G165–G170. doi: 10.1152/ajpgi.1987.253.2.G165. [DOI] [PubMed] [Google Scholar]
- 393.Raymer CK, Park HS, Wishart JM, Kong M-F, Doran SM, Horowitz M. Effects of intraduodenal glucose and fructose on antropyloric motility and appetite in healthy humans. Am J Physiol. 2000;278:R360–R366. doi: 10.1152/ajpregu.2000.278.2.R360. [DOI] [PubMed] [Google Scholar]
- 394.Read NW, McFarlane A, Kinsman RI, Bates TE, Blackhall NW, Farrar GB, Hall JC, Moss G, Morris AP, O’Neill B. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterol. 1984;86:274–280. [PubMed] [Google Scholar]
- 395.Regalia J, Cai F, Helke C. Streptozotocin-induced diabetes and the neurochemistry of vagal afferent neurons. Brain Res. 2002;938:7–14. doi: 10.1016/s0006-8993(02)02456-3. [DOI] [PubMed] [Google Scholar]
- 396.Reichling DB, Basbaum AI. Collateralization of periaqueductal gray neurons to forebrain or diencephalon and to the medullary nucleus raphe magnus in the rat. Neuroscience. 1991;42:183–200. doi: 10.1016/0306-4522(91)90158-k. [DOI] [PubMed] [Google Scholar]
- 397.Ren K, Randich A, Gebhart GF. Modulation of spinal nociceptive transmission from nuclei tractus solitarii: A relay for effects of vagal afferent stimulation. J Neurophysiol. 1990;63:971–986. doi: 10.1152/jn.1990.63.5.971. [DOI] [PubMed] [Google Scholar]
- 398.Renehan WE, Zhang X, Beierwaltes WH, Fogel R. Neurons in the dorsal motor nucleus of the vagus may integrate vagal and spinal information from the GI tract. Am J Physiol. 1995;268:G780–G790. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- 399.Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- 400.Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: The effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- 401.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 402.Rhim H, Glaum S, Miller RJ. Selective opioid agonists modulate afferent transmission in the rat nucleus tractus solitarius. JPET. 1992;264:795–780. [PubMed] [Google Scholar]
- 403.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 404.Richar P, Moos F, Freund-Mercier M-J. Central effects of oxytocin. Physiol Rev. 1991;71:331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- 405.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 406.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural demonstration of a gastric monsynaptic vagal cirucit in the nucleus of the solitary tract in rat. Journal of Neuroscience. 1989;9:1985–1996. doi: 10.1523/JNEUROSCI.09-06-01985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 410.Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: A WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- 411.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Roosen L, Boesmans W, Dondeyne M, Depoortere I, Tack J, Vanden Berghe P. Specific hunger- and satiety-induced tuning of guinea pig enteric nerve activity. J Physiol. 2012;590:4321–4333. doi: 10.1113/jphysiol.2012.231134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: Neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Rouzade-Dominguez ML, Curtis AL, Valentino RJ. Role of Barrington’s nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- 417.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: Substrates for pelvic visceral coordination. Eur J Neurosci. 2003;18:3311–3324. doi: 10.1111/j.1460-9568.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 418.Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli in the rat: A juxtacellular labelling study. Eur J Neurosci. 2003;18:3325–3334. doi: 10.1111/j.1460-9568.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 419.Sabbatini M, Molinari C, Grossini E, Mary DA, Vacca G, Cannas M. The pattern of c-Fos immunoreactivity in the hindbrain of the rat following stomach distension. Exp Brain Res. 2004;157:315–323. doi: 10.1007/s00221-004-1845-x. [DOI] [PubMed] [Google Scholar]
- 420.Sah P. Role of calcium influx and buffering in the kinetics of a Ca2+-activated K+ current in rat vagal motoneurons. J Neurophysiol. 1992;65:2237–2247. doi: 10.1152/jn.1992.68.6.2237. [DOI] [PubMed] [Google Scholar]
- 421.Sah P. Different calcium channels are coupled to potassium channels with distinct physiological roles in vagal neurons. Proc R Soc Lond B. 1995;260:105–111. doi: 10.1098/rspb.1995.0066. [DOI] [PubMed] [Google Scholar]
- 422.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- 424.Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 425.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 426.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 427.Sara SJ, Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 428.Sartor DM, Verberne AJ. Cholecystokinin selectively affects presympathetic vasomotor neurons and sympathetic vasomotor outflow. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1174–R1184. doi: 10.1152/ajpregu.00500.2001. [DOI] [PubMed] [Google Scholar]
- 429.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 430.Sayegh AI, Covasa M, Ritter RC. Intestinal infusions of oleate and glucose activate distinct enteric neurons in the rat. Auton Neurosci. 2004;115:54–63. doi: 10.1016/j.autneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 431.Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90:215–220. doi: 10.3171/jns.1999.90.2.0215. [DOI] [PubMed] [Google Scholar]
- 432.Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992;263:G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- 433.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2003;19:519–525. doi: 10.1097/00001574-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 434.Schubert ML, Shamburek RD. Control of acid secretion. Gastroenterol Clin North Am. 1990;19:1–25. [PubMed] [Google Scholar]
- 435.Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 436.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol. 1988;270:416–419. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- 437.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 438.Shen KZ, Barajas-Lopez C, Surprenant A. Functional characterization of neuronal pre and postsynaptic alpha 2-adrenoceptor subtypes in guinea-pig submucosal plexus. Br J Pharmacol. 1990;101:925–931. doi: 10.1111/j.1476-5381.1990.tb14182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Shepard JD, Myers DA. Strain differences in anxiety-like behavior: Association with corticotropin-releasing factor. Behav Brain Res. 2008;186:239–245. doi: 10.1016/j.bbr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 440.Shi M, Jones AR, Ferreira M, Jr, Sahibzada N, Gillis RA, Verbalis JG. Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol. 2005;288:R742–R750. doi: 10.1152/ajpregu.00561.2004. [DOI] [PubMed] [Google Scholar]
- 441.Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Shinpo K, Hirai Y, Maezawa H, Totsuka Y, Funahashi M. The role of area postrema neurons expressing H-channels in the induction mechanism of nausea and vomiting. Physiol Behav. 2012;107:98–103. doi: 10.1016/j.physbeh.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 443.Shipley MT. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull. 1982;8:139–148. doi: 10.1016/0361-9230(82)90040-5. [DOI] [PubMed] [Google Scholar]
- 444.Shoja MM, Tubbs RS, Ansarin K, Farahani RM. Proposal for the existence of a nasogastric reflex in humans, as a potential cause of upper gastrointestinal symptoms. Med Hypotheses. 2007;69:346–348. doi: 10.1016/j.mehy.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 445.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 446.Simmons MA. The complexity and diversity of synaptic transmission in the prevertebral sympathetic ganglia. Prog Neurobiol. 1985;24:43–93. doi: 10.1016/0301-0082(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 447.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 448.Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Stengel A, Tache Y. Neuroendocrine control of the gut during stress: Corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Stenvers DJ, Jonkers CF, Fliers E, Bisschop PH, Kalsbeek A. Nutrition and the circadian timing system. Prog Brain Res. 2012;199:359–376. doi: 10.1016/B978-0-444-59427-3.00020-4. [DOI] [PubMed] [Google Scholar]
- 452.Stephens RL, Ishikawa T, Weiner H, Novin D, Tache Y. TRH analogue, RX 77368, injected nto dorsal vagal complex stimulates gatric secretion in rats. Am J Physiol. 1988;254:G639–G643. doi: 10.1152/ajpgi.1988.254.5.G639. [DOI] [PubMed] [Google Scholar]
- 453.Suzuki T, Sugiyama Y, Yates BJ. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R965–R975. doi: 10.1152/ajpregu.00680.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- 455.Swanson LW. The locus coeruleus: A cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 456.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 457.Swanson LW, Sawchenko PE. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 458.Swett JE, McMahon SB, Wall PD. Long ascending projections to the midbrain from cells of lamina I and nucleus of the dorsolateral funiculus of the rat spinal cord. J Comp Neurol. 1985;238:401–416. doi: 10.1002/cne.902380405. [DOI] [PubMed] [Google Scholar]
- 459.Tache Y. Brainstem neuropeptides and vagal protection of the gastric mucosal against injury: Role of prostaglandins, nitric oxide and calcitonin-gene related peptide in capsaicin afferents. Curr Med Chem. 2012;19:35–42. doi: 10.2174/092986712803414097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: Implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N Y Acad Sci. 1999;697:233–243. doi: 10.1111/j.1749-6632.1993.tb49936.x. [DOI] [PubMed] [Google Scholar]
- 463.Tache Y, Vale W, Brown M. Thyrotropin-releasing hormone–CNS action to stimulate gastric acid secretion. Nature. 1980;287:149–151. doi: 10.1038/287149a0. [DOI] [PubMed] [Google Scholar]
- 464.Tache Y, Yang H, Kaneko H. Caudal raphe-dorsal vagal complex peptidergic projections: Role in gastric vagal control. Peptides. 1995;16:431–435. doi: 10.1016/0196-9781(94)00212-o. [DOI] [PubMed] [Google Scholar]
- 465.Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol. 2005;39:S211–S216. doi: 10.1097/01.mcg.0000156109.97999.d1. [DOI] [PubMed] [Google Scholar]
- 466.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 467.Tack JF, Wood JD. Actions of noradrenaline on myenteric neurons in the guinea pig gastric antrum. J Auton Nerv Syst. 1992;41:67–77. doi: 10.1016/0165-1838(92)90128-4. [DOI] [PubMed] [Google Scholar]
- 468.Takeuchi Y, Matsushima S, Matsushima R, Hopkins DA. Direct amygdaloid projections to the dorsal motor nucleus of the vagus nerve: A light and electron microscopic study in the rat. Brain Res. 1983;280:143–147. doi: 10.1016/0006-8993(83)91182-4. [DOI] [PubMed] [Google Scholar]
- 469.Tebbe JJ, Dietze T, Grote C, Monnikes H. Excitatory stimulation of neurons in the arcuate nucleus inhibits gastric acid secretion via vagal pathways in anesthetized rats. Brain Res. 2001;913:10–17. doi: 10.1016/s0006-8993(01)02746-9. [DOI] [PubMed] [Google Scholar]
- 470.Tebbe JJ, Mronga S, Schafer MK, Ruter J, Kobelt P, Monnikes H. Stimulation of neurons in rat ARC inhibits gastric acid secretion via hypothalamic CRF1/2- and NPY-Y1 receptors. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1075–G1083. doi: 10.1152/ajpgi.00125.2003. [DOI] [PubMed] [Google Scholar]
- 471.Tebbe JJ, Pasat IR, Monnikes H, Ritter M, Kobelt P, Schafer MK. Excitatory stimulation of neurons in the arcuate nucleus initiates central CRF-dependent stimulation of colonic propulsion in rats. Brain Res. 2005;1036:130–138. doi: 10.1016/j.brainres.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 472.Terreberry RR, Neafsey EJ. Rat medial frontal cortex: A visceral motor region with a direct projection to the solitary nucleus. Brain Res. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- 473.Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- 474.Thomas J. Opioid-induced bowel dysfunction. J Pain Symptom Manage. 2008;35:103–113. doi: 10.1016/j.jpainsymman.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 475.Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: A PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 476.Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- 477.Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: Dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat. 1989;2:139–148. [PubMed] [Google Scholar]
- 478.Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabete Metab. 1995;21:311–318. [PubMed] [Google Scholar]
- 479.Thumshirn M. Pathophysiology of functional dyspepsia. Gut. 2002;51(Suppl 1):i63–i66. doi: 10.1136/gut.51.suppl_1.i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterol Motil. 2011;23:e69–e79. doi: 10.1111/j.1365-2982.2010.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956;106:51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- 482.Tougas G, Wang L. Pseudoaffective cardioautonomic responses to gastric distension in rats. Am J Physiol. 1999;277:R272–R278. doi: 10.1152/ajpregu.1999.277.1.R272. [DOI] [PubMed] [Google Scholar]
- 483.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 484.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Tran L, Greenwood-Van Meerveld B. Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behav Brain Res. 2012;234:380–385. doi: 10.1016/j.bbr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 486.Tran L, Wiskur B, Greenwood-Van Meerveld B. The role of the anterio-lateral bed nucleus of the stria terminalis in stress-induced nociception. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1301–G1309. doi: 10.1152/ajpgi.00501.2011. [DOI] [PubMed] [Google Scholar]
- 487.Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol. 1995;487:37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Trapp S, Ballanyi K, Richter DW. Spontaneous activation of KATP current in rat dorsal vagal neurones. Neuroreport. 1994;5:1285–1288. doi: 10.1097/00001756-199406020-00033. [DOI] [PubMed] [Google Scholar]
- 489.Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: Is this physiologically relevant? Curr Opin Pharmacol. 2013;13:964–969. doi: 10.1016/j.coph.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Traub RJ, Lim F, Sengupta JN, Meller ST, Gebhart GF. Noxious distention of viscera results in differential c-Fos expression in second order sensory neurons receiving ‘sympathetic’ or ‘parasympathetic’ input. Neurosci Lett. 1994;180:71–75. doi: 10.1016/0304-3940(94)90916-4. [DOI] [PubMed] [Google Scholar]
- 491.Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neurosci. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- 492.Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996;215:165–168. doi: 10.1016/0304-3940(96)12978-5. [DOI] [PubMed] [Google Scholar]
- 493.Travagli RA, Dunwiddie TV, Williams JT. Opioid inhibition in locus coeruleus. J Neurophysiol. 1995;74:519–528. doi: 10.1152/jn.1995.74.2.519. [DOI] [PubMed] [Google Scholar]
- 494.Travagli RA, Gillis RA. Hyperpolarization-activated currents Ih and IKIR in rat dorsal motor nucleus of the vagus neurons, in vitro. J Neurophysiol. 1994;71:1308–1317. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- 495.Travagli RA, Gillis RA. Effect of 5-HT alone and its interaction with TRH on neurons in rat dorsal motor nucleus of the vagus. Am J Physiol. 1995;263:G292–G299. doi: 10.1152/ajpgi.1995.268.2.G292. [DOI] [PubMed] [Google Scholar]
- 496.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 497.Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol. 1992;263:G508–G517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- 498.Travagli RA, Hermann GE, Browning KN, Rogers RC. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G180–G187. doi: 10.1152/ajpgi.00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Troncon LE, Thompson DG, Ahluwalia NK, Barlow J, Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut. 1995;37:17–22. doi: 10.1136/gut.37.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Tsukamoto K, Nakade Y, Mantyh C, Ludwig K, Pappas TN, Takahashi T. Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1537–R1541. doi: 10.1152/ajpregu.00713.2005. [DOI] [PubMed] [Google Scholar]
- 502.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 503.Vacca G, Mary DA, Vono P. The effect of distension of the stomach on coronary blood flow in anaesthetized pigs. Pflugers Arch. 1994;428:127–133. doi: 10.1007/BF00374849. [DOI] [PubMed] [Google Scholar]
- 504.Vacca G, Vono P. The primary reflex effects of distension of the stomach on heart rate, arterial pressure and left ventricular contractility in the anaesthetized pig. Pflugers Arch. 1993;425:248–255. doi: 10.1007/BF00374174. [DOI] [PubMed] [Google Scholar]
- 505.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: Pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- 506.Valentino RJ, Page ME, Luppi PH, Zhu Y, Van BE, Aston-Jones G. Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–143. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 507.Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington’s nucleus to the periaqueductal gray and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363:402–422. doi: 10.1002/cne.903630306. [DOI] [PubMed] [Google Scholar]
- 508.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Van Bockstaele EJ, Aston-Jones G. Distinct populations of neurons in the ventromedial periaqueductal gray project to the rostral ventral medulla and abducens nucleus. Brain Res. 1992;576:59–67. doi: 10.1016/0006-8993(92)90609-d. [DOI] [PubMed] [Google Scholar]
- 510.Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: Evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 511.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 512.van der Kooy D, McGinty JF, Koda LY, Gerfen CR, Bloom FE. Visceral cortex: A direct connection from prefrontal cortex to the solitary nucleus in rat. Neurosci Lett. 1982;33:123–127. doi: 10.1016/0304-3940(82)90238-5. [DOI] [PubMed] [Google Scholar]
- 513.Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, Bernstein IL, Woods SC, Seeley RJ. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol. 1996;271:R1096–R1100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- 514.Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:663–680. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 515.Vanner S, MacNaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):39–43. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 516.Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- 517.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: A combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- 518.Venkova K, Krier J. A nitric oxide and prostaglandin-dependent component of NANC off-contractions in cat colon. Am J Physiol. 1994;266:G40–G47. doi: 10.1152/ajpgi.1994.266.1.G40. [DOI] [PubMed] [Google Scholar]
- 519.Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pego JM. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Ventura-Silva AP, Pego JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012;36:3396–3406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- 521.Verberne AJ, Saita M, Sartor DM. Chemical stimulation of vagal afferent neurons and sympathetic vasomotor tone. Brain Res Brain Res Rev. 2003;41:288–305. doi: 10.1016/s0165-0173(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 522.Viltart O, Sartor DM, Verberne AJ. Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull. 2006;71:51–59. doi: 10.1016/j.brainresbull.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 523.Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gutbrain axis in awake rats. Neurogastroenterol Motil. 2011;23:e282–e293. doi: 10.1111/j.1365-2982.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Vizi ES, Knoll J. The effects of sympathetic nerve stimulation and guanethidine on parasympathetic neuroeffector transmission; the inhibition of acetylcholine release. J Pharm Pharmacol. 1971;23:918–925. doi: 10.1111/j.2042-7158.1971.tb09893.x. [DOI] [PubMed] [Google Scholar]
- 525.Vizzard MA, Brisson M, De Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- 526.Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Wan S, Browning KN. D-Glucose modulates synaptic transmission from the central terminals of vagal afferent fibers. Am J Physiol Gastrointest Liver Physiol. 2008;294:G757–G763. doi: 10.1152/ajpgi.00576.2007. [DOI] [PubMed] [Google Scholar]
- 528.Wan S, Browning KN. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5HT3 receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1050–G1057. doi: 10.1152/ajpgi.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007;28:2184–2191. doi: 10.1016/j.peptides.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 530.Wang SC, Borison HL. The vomiting center; a critical experimental analysis. Arch Neurol Psychiatry. 1950;63:928–941. doi: 10.1001/archneurpsyc.1950.02310240087005. [DOI] [PubMed] [Google Scholar]
- 531.Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095–1e92. doi: 10.1111/j.1365-2982.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 532.Wang Y, Jones JFX, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: An ionophoretic study. Br J Pharmacol. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Wang Z, Ocampo MA, Pang RD, Bota M, Bradesi S, Mayer EA, Holschneider DP. Alterations in prefrontal-limbic functional activation and connectivity in chronic stress-induced visceral hyperalgesia. PLoS ONE. 2013;8:e59138. doi: 10.1371/journal.pone.0059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: Axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]
- 535.White RL, Jr, Rossiter CD, Hornby PJ, Harmon JW, Kasbekar DK, Gillis RA. Excitation of neurons in the medullary raphe increases gastric acid and pepsin production in cats. Am J Physiol. 1991;260:G91–G96. doi: 10.1152/ajpgi.1991.260.1.G91. [DOI] [PubMed] [Google Scholar]
- 536.Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav. 2011;103:557–564. doi: 10.1016/j.physbeh.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Williams JT, Bobker DH, Harris GC. Synaptic potentials in locus coeruleus neurons in brain slices. Prog Brain Res. 1991;88:167–172. doi: 10.1016/s0079-6123(08)63806-6. [DOI] [PubMed] [Google Scholar]
- 538.Williams JT, Egan TM, North RA. Enkephalin opens potassium channels on mammalian central neurons. Nature. 1982;299:74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- 539.Williams JT, North RA, Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988;8:4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Wright RD, Jennings MA, Florey HW, Lium R. The influence of nerves an drugs on secretion by the small intestine and an investigation of the enzymes in intestinal juice. Q J Exp Physiol. 1940;30:73–120. [Google Scholar]
- 541.Yagihashi S, Sima AA. Diabetic autonomic neuropathy in BB rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733–743. doi: 10.2337/diab.35.7.733. [DOI] [PubMed] [Google Scholar]
- 542.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 543.Yettefti K, Orsini JC, El Ouazzain T, Himmi T, Boyer A, Perrin J. Sensitivity of the nucleus tractus solitarius neurons to induced moderate hyperglycemia, with special reference to catecholaminergic regions. JANS. 1995;51:191–197. doi: 10.1016/0165-1838(94)00130-c. [DOI] [PubMed] [Google Scholar]
- 544.Yettefti K, Orsini J-C, Perrin J. Characteristics of glycemia-sensitive neurons in the nucleus tractus solitarii: Possible involvement in nutritional regulation. Physiol Behav. 1997;61:93–100. doi: 10.1016/s0031-9384(96)00358-7. [DOI] [PubMed] [Google Scholar]
- 545.Yoneda S, Kadowaki M, Kuramoto H, Fukui H, Takaki M. Enhanced colonic peristalsis by impairment of nitrergic enteric neurons in spontaneously diabetic rats. Auton Neurosci. 2001;92:65–71. doi: 10.1016/S1566-0702(01)00317-4. [DOI] [PubMed] [Google Scholar]
- 546.Young RL, Cooper NJ, Blackshaw LA. Anatomy and function of group III metabotropic glutamate receptors in gastric vagal pathways. Neuropharmacology. 2008;54:965–975. doi: 10.1016/j.neuropharm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 547.Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1358–R1365. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]
- 549.Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1066–G1073. doi: 10.1152/ajpgi.00497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Zheng Z, Travagli RA. Dopamine effects on identified rat vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1002–G1008. doi: 10.1152/ajpgi.00527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neurosci. 1999;90:685–694. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- 552.Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1158–G1164. doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Zhu JN, Wang JJ. The cerebellum in feeding control: Possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Zittel TT, De GR, Sternini C, Raybould HE. Fos protein expression in the nucleus of the solitary tract in response to intestinal nutrients in awake rats. Brain Res. 1994;663:266–270. doi: 10.1016/0006-8993(94)91272-6. [DOI] [PubMed] [Google Scholar]
- 556.Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci. 2011;31:14024–14031. doi: 10.1523/JNEUROSCI.2081-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta. 2009;1792:423–431. doi: 10.1016/j.bbadis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


