Abstract
Although skeletal muscle is one of the most regenerative organs in our body, various genetic defects, alterations in extrinsic signaling, or substantial tissue damage can impair muscle function and the capacity for self-repair. The diversity and complexity of muscle disorders have attracted much interest from both cell biologists and, more recently, bioengineers, leading to concentrated efforts to better understand muscle pathology and develop more efficient therapies. This review describes the biological underpinnings of muscle development, repair, and disease, and discusses recent bioengineering efforts to design and control myomimetic environments, both to study muscle biology and function and to aid in the development of new drug, cell, and gene therapies for muscle disorders. The synergy between engineering-aided biological discovery and biology-inspired engineering solutions will be the path forward for translating laboratory results into clinical practice.
Keywords: regeneration, satellite cell, engineered muscle, volumetric muscle loss, dystrophy, iPSCs
1. INTRODUCTION
Skeletal muscle is the largest organ in the human body and of central importance to our daily activities, including all voluntary movements and respiration. Healthy muscle tissue consists of aligned bundles of multinucleated, striated contractile muscle cells, known as myofibers, and an intricate network of nerves, blood vessels, and the extracellular matrix (ECM). Upon neuronal activation and the release of acetylcholine at neuromuscular junctions (NMJs), the myofiber membrane, referred to as the sarcolemma, alters its polarization state and ion flow, eventually leading to the release of calcium ions from intracellular stores. Intracellular calcium, among other molecules in the cytoplasm [troponins, adenosine triphosphate (ATP)], regulates the binding and sliding of the filamentous proteins myosin and actin that result in myofiber contraction. During contraction, the generated force is transmitted through membrane-bound proteins, such as dystrophin, that transfer the energy generated by cytoskeletal motion to the ECM, tendons, and bones. Whole muscle is composed of multiple groups of myofibers (muscle fascicles) sheathed in connective tissue and the accompanying vasculature and nerves that support tissue viability and controlled movements (1).
A unique feature of healthy, adult skeletal muscle is its ability to fully recover the architecture and contractile function following small, everyday injuries or acute damage (2). This regenerative capacity is brought about by the orchestrated action of a diverse set of endogenous and exogenous (both local and systemic) factors, which enable the muscle to maintain its homeostasis and volume throughout most of a person’s lifetime. However, various genetic alterations in sarcolemmal, contractile, or ECM proteins; traumatic or surgical tissue loss; alterations in endocrine or metabolic signaling; cancer; starvation; or aging can all yield conditions in which muscle function and regeneration are severely impaired (2). The large size and distributed nature of skeletal muscle, as well as the variety of complex mechanisms contributing to its pathology, have made the development of efficient therapies for muscle-related disorders extremely challenging.
Over the past several decades, intensive efforts by biologists and physicians have unraveled the important contributions of molecular signaling, systemic and cellular factors, cell–matrix interactions, and stem cell niches to skeletal muscle function, regeneration, and disease (2–4). Although these studies have led to the discovery of various therapeutic targets and improved clinical outcomes, muscle-related diseases still impose significant health and economic burdens on society. To continue to make advances toward efficient clinical therapies, especially those involving the use of human stem cells, it will be essential to develop methods to better control in vitro and in vivo environments to enable accurate studies of cellular functions and interactions, re-create stem cell niches, and encourage the survival and integration of implanted cells. The integration of bioengineering principles and technologies into the field of muscle biology will help address these challenges and create new opportunities for improving the care of patients.
The utilization of engineering techniques in biological research has already shown promise by providing the ability to create and precisely manipulate 3D cellular environments, perform sophisticated in vitro and in situ imaging of live cells and their function, utilize microfluidic devices to isolate and analyze rare cell types, apply high-throughput platforms to study cell–cell and cell–material interactions, control cell responses using biomaterial-based delivery of therapeutic molecules, use body-on-a-chip platforms to model organ–organ interactions, and perform computer modeling to understand tissue growth and mechanics (5). Further, tissue-engineering technologies have been applied to re-create the structural complexity and functionality of native tissues in a dish (5) for use both in regenerative therapies and as a bridge between traditional studies of muscle biology in individual cells or groups of cells and physiological tests at the organ or whole-body level.
In this review, we first discuss the mechanisms and pathways involved in muscle development and regeneration, and the effects of various congenital and traumatic disorders on muscle function and repair. The application of tissue engineering to skeletal muscle replacement in vivo and the development of therapeutic screening platforms in vitro are reviewed in detail, followed by descriptions of recent progress made using human cells. Finally, we provide an overview of the current challenges facing the field and discuss how the synergy between traditional biological research and cutting-edge bioengineering can further advance our knowledge of skeletal muscle function and diseases, and lead to improved therapies.
2. SKELETAL MUSCLE DEVELOPMENT, REGENERATION, AND DISEASE
2.1. Muscle Development
During development, skeletal muscle is formed from myogenic progenitor cells in two major phases, referred to as primary and secondary myogenesis (6, 7). Primary myogenesis occurs during the late embryonic stages of development, when embryonic myoblasts fuse to form primary myofibers. The primary myofibers serve as founder scaffolds for the formation of the secondary myofibers that arise during fetal development, when most of secondary myogenesis occurs. Once the secondary myofibers have formed, they begin to grow by the continued fusion of fetal myoblasts. Multiple signaling pathways have been implicated in regulating proliferation and differentiation during development. Although members of the transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) families maintain fetal myoblasts in an undifferentiated state, embryonic myoblasts appear to be insensitive to this inhibition and can differentiate into primary myofibers even in the presence of these factors (8). The formed primary myofibers may, in turn, secrete factors, such as the fibroblast growth factor and platelet-derived growth factor (PDGF) family members, to induce proliferation of embryonic myoblasts and to maintain a pool of future stem cells (7). Notch signaling has also been implicated in multiple stages of developmental myogenesis. Early in the development of somites, Notch ligands and receptors are expressed and play a part in the process of segmentation (9). Later in development, Notch signaling has a role in muscle hypertrophy, as evidenced by the premature depletion of myogenic progenitors and by muscle hypotrophy when Notch signaling is reduced as a result of mutation of the ligand Delta-1 (10). Notch signaling has also been implicated in regulating the differentiation of both embryonic and fetal myoblasts, either directly, by inhibiting myogenic differentiation, or indirectly, via its crosstalk with the BMP pathway (11, 12). All of these developmental pathways are also important for maintaining and regenerating adult muscle (13–15).
2.2. Muscle Regeneration
In adult muscle, the normal process of muscle regeneration (Figure 1) in response to injury is complex, and this complexity is further increased in the setting of systemic or genetic disease. In response to various experimental injuries that lead to the degeneration of muscle fibers, there is a stereotypical pattern of tissue regeneration that is mediated by the stem cells resident in muscle, or the satellite cells (SCs), usually identified by their close apposition to muscle fibers and expression of the transcription factor Pax7. The SC response to injury is highly integrated with both the innate immune response and the responses of other resident cellular populations that contribute to the coordinated process of tissue repair. Unsurprisingly, the efficacy of the regenerative response declines with the severity of the injury. In particular, injuries that destroy or disrupt the ECM are more likely to lead to aberrant regeneration, in which muscle is replaced by adipose and connective tissue.
Figure 1.
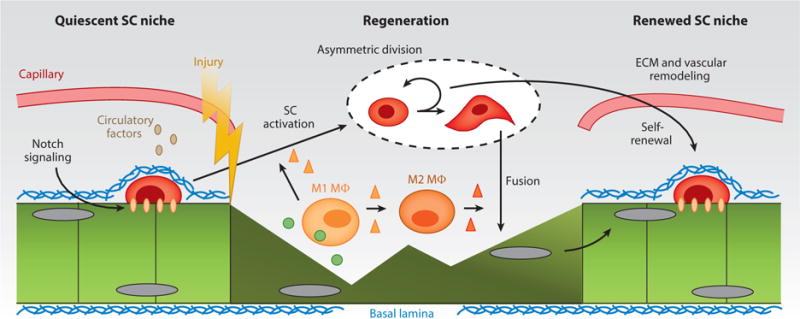
Regenerative response of injured skeletal muscle. A simplified schematic of the satellite cell (SC) niche at homeostasis and following an acute injury. Niche quiescence that is maintained via local juxtacrine and paracrine signaling is disturbed following injury. In response to injury-induced myofiber debris, M1 and M2 macrophages (MΦ) secrete factors that lead to SC activation, asymmetric division, and the formation of myogenic precursors primed for fusion that repair damaged myofibers and create new myofibers. After regeneration, the SC niche is reestablished along with its extracellular matrix (ECM) and vascular components.
If there is a hierarchy of importance of cell types that are essential for normal regeneration, SCs are at the pinnacle. Depletion of SCs leads to a complete absence of muscle regeneration (16). SCs exist in a quiescent state, a state that is actively regulated and maintained (17), and disruption of SC quiescence impairs their regenerative potential (18). In particular, Notch signaling is essential for maintaining SC quiescence (19, 20), and in the quiescent state, Notch signaling itself may be regulated by the actions of the FoxO3 transcription factor (21). In response to stimuli such as injury, fiber degeneration from genetic diseases, or eccentric muscle contractions, SCs respond to local cues to activate, enter the cell cycle, proliferate to generate sufficient progeny to regenerate the damaged tissue, and, ultimately, align, fuse, and differentiate into multinucleated myotubes. During the ensuing days and weeks, those myotubes grow to achieve the approximate size of the original myofibers.
All of the stages noted above, from SC activation to myotube hypertrophy, are modulated by other cellular components of the regenerative milieu. In terms of resident cells, an important population of mesenchymal stem cells, known as fibroadipogenic progenitors, appears to regulate SC differentiation, and may also be the source of the ECM that characterizes fibrotic changes during impaired regenerative responses (22, 23). The prevention of muscle fibrosis by inhibition of PDGF-receptor-α signaling is likely mediated by the effects on fibroadipogenic progenitors (24). For nonresident cells, those involved in innate immune responses are of the highest importance for regulating effective muscle regeneration. In particular, infiltrating macrophages have a critical role, both in terms of their phagocytic activity in areas of muscle necrosis and in terms of their role as secretory cells (25, 26). The timing of the switch from M1 proinflammatory to M2 anti-inflammatory macrophages appears to be a key regulator of the temporal sequences of events that occur during SC activation, proliferation, and differentiation (26).
Finally, the importance of the vascular and neural components of muscle to a normal regenerative process cannot be overemphasized. Regeneration would not occur without the ingrowth of new blood vessels into damaged muscle to provide oxygen and nutrients. Thus, angiogenesis and myogenesis are tightly coupled. Likewise, even if the early events of myogenesis can proceed without neural innervation, the maturation of myotubes to myofibers cannot. Therefore, a hospitable environment for axonal ingrowth and patterning is an essential feature of effective muscle regeneration.
Microenvironmental control of SC homeostasis and function in skeletal muscle relies on the existence of stem cell niches. The notion that stem cells exist in specialized compartments, or niches, dates back to more than 40 years ago when it was proposed, by analogy to species existing in distinct ecological systems, that stem cells maintain their potential by existing in specialized tissue compartments (27). These niches are composed of local cellular and extracellular support systems that are essential to allowing the stem cells that reside there to be held in reserve to support tissue homeostasis and repair over a lifetime. Niche components have been studied for a wide variety of stem cells. A common theme that has emerged is that niches tend to have a vascular component and local interstitial cells that are important for delivering homeostatic signals to stem cells, either by direct cellular contact or as secreted molecules (28). For SCs, the niche is only beginning to be understood. The muscle fiber is a major cellular component of the niche because each SC resides closely apposed to the sarcolemma of its parent myofiber (29). Given the importance of cell contact-dependent Notch signaling in maintaining SC quiescence (19, 20), it has been proposed, but not yet proven, that the relevant Notch ligand is expressed by the adjacent myofiber. Moreover, SCs lie beneath a basal lamina matrix that consists of laminin and collagen IV, among other ECM proteins. In addition, it is clear that SCs have a very close relationship with the capillary network that exists in muscles. Each quiescent SC tends to reside close to one of the few capillaries that run parallel to the myofiber’s longitudinal axis, and direct contact between SCs and capillary endothelial cells has been demonstrated (30). Since 2005, the concept of the stem cell niche has been broadened to include the systemic milieu. As exemplified by studies of stem cell aging and the decline in the regenerative potential of tissue with age, it became clear, first from the use of heterochronic parabiosis, and, more recently, by heterochronic plasma transfer, that stem cell function can be markedly affected by circulatory factors (31, 32). Using these approaches, it has been possible to enhance the function of aged stem cells by exposing them to factors present in the blood of young animals, and to suppress the function of young stem cells by exposing them to factors present in the blood of old animals (33–35).
2.3. Muscle Disorders
The majority of human muscular disorders have been shown to result from genetic abnormalities in muscle cells, neurological disorders, systemic diseases, or muscle trauma. Muscular dystrophies, for example, are rare genetic diseases that are more common in childhood but affect people of all ages. These diseases result from mutations in various genes that encode proteins ranging from ECM to nuclear proteins. They are commonly associated with muscle-fiber necrosis and fibrous and fatty infiltration and lead to weakness, loss of mobility or selective muscle group function, and, in severe cases, early death. The most common form of muscular dystrophy is Duchenne’s muscular dystrophy (DMD), which is an X-linked recessive disorder that occurs almost exclusively in males (36). The onset of symptoms typically occurs during the first 5 years of life, and boys with DMD are typically wheelchair-dependent by age 12. The numerous other forms of limb-girdle muscular dystrophy also typically have the onset of symptoms in childhood, but are less common and, generally, less severe than DMD. Common forms of muscular dystrophy with onset of symptoms in adulthood include the myotonic muscular dystrophies and facioscapulohumeral muscular dystrophy (FSHD). FSHD is notable for its characteristic pattern of weakness, reflected in the disease’s name. There are no cures for any of the dystrophies, although there is a burgeoning field of experimental therapeutics moving into clinical trials, with approaches ranging from pharmacological to viral gene therapies (37, 38). Stem cell therapies have been long envisioned, but are only in their infancy (39). The major therapeutic modalities for most of the dystrophies are limited to physical medicine approaches and surgical correction of consequences such as scoliosis and contractures. For DMD, the standard of care in the United States is the use of corticosteroids that improve function, but this is limited by the numerous adverse reactions that occur when they are administered chronically.
For muscle trauma, a major focus is on the acute loss of a large mass of muscle, referred to as volumetric muscle loss (VML). VML is associated with significant functional impairment and is a major source of disability among injured veterans who have experienced blast injuries. In addition to injuries among military combatants (40), muscle trauma can occur in civilians in a number of situations, ranging from automobile accidents to shark bites and surgical interventions. In general in VML, because of the significant loss of ECM, and nerve and vascular supply, the ensuing self-repair process does not yield sufficient new muscle in time to prevent an excessive fibrotic response (41). Excessive fibrosis, in turn, may prevent adequate revascularization and reinnervation at the injury site, thus posing additional challenges to the successful delivery of pharmacological, genetic, or cellular therapeutics. Overall, there is general agreement that the successful treatment of VML will likely involve timely surgical delivery of a large-volume implant to rapidly add or recruit new muscle before a chronic scar is formed (5). This may be a major challenge to treating VML in combatants, where more serious life-threatening conditions usually take precedence at the time of the injury.
A separate category of muscle disorders involves conditions that result in muscle atrophy and wasting. Examples include cachexia associated with chronic diseases, especially cancer, chronic obstructive pulmonary disease, chronic renal disease, and chronic infections such as AIDS. Muscle atrophy occurs in the setting of injury and diseases of the peripheral nerves and of motor neurons, such as amyotrophic lateral sclerosis, and there is prominent muscle wasting in the paralyzed limbs of patients with spinal cord injuries. Finally, age-related muscle loss, or sarcopenia, is a major source of disability in the elderly. In addition to the loss of strength and mobility, there are also the systemic effects of muscle loss related to the role of muscle as an endocrine tissue, a feature that is increasingly being recognized and studied (42). It is becoming clear that healthy muscle contributes to proper metabolic activity and the functional homeostatic maintenance of a large number of tissues, ranging from bone to brain. Although some of these benefits may be related to the biomechanical properties of healthy muscle, others appear to be related to the contraction-dependent secretion of anti-inflammatory molecules, referred to as myokines (42), that can have beneficial systemic effects. Therefore, the maintenance of muscle bulk in conditions of wasting and atrophy would be likely to have wide-ranging therapeutic effects.
3. BIOENGINEERING METHODS FOR SKELETAL MUSCLE RESEARCH AND REPAIR
For more than two decades, bioengineering efforts in the field have focused on developing biomimetic 3D cultures of skeletal muscle cells for studies of muscle physiology and disease. The functionality, complexity, and physiological accuracy of these in vitro models of native muscle have progressed with the development of advanced biomaterials that can act as cell-carrying scaffolds, 3D culturing techniques, and bioreactors for applying biophysical and biochemical stimulation. Recent progress in human pluripotent stem cell technologies and in vitro maintenance of muscle stem cells has presented the field with new opportunities for developing contractile human muscle tissues for use in disease-modeling and drug-discovery studies. Engineered muscle constructs have also been considered for use in regenerative therapy, prompting the development of methods to promote implant engraftment and vascular and neuronal integration into the host muscle.
3.1. Methods for Engineering Functional Skeletal Muscle
The traditional method of fabricating 3D muscle constructs, first developed more than 25 years ago (43), involves casting myogenic cells within a cylindrically shaped collagen-I gel that is anchored at the ends to porous felts (44). In this system, cell-mediated gel compaction and remodeling result in the generation of uniaxial passive stress within the gel, which, in turn, promotes the fusion of myoblasts into myotubes and also myotube alignment. Alternatively, myoblasts, or mixtures of myogenic precursors and fibroblasts, can be cultured on laminin- or hydrogel-coated dishes until spontaneous contractions of formed myotubes detach the entire cell layer, allowing it to self-assemble into a cylindrical tissue construct attached at the ends to premade suture anchors (45–47). Although cell alignment within 3D constructs is not required for the formation of contractile myotubes, it increases fusion efficiency (48), while passive stress promotes both cell survival and myogenesis (49). In addition to collagen I, different natural hydrogels (50, 51) and their chemically modified derivatives (52, 53) can support the 3D growth and fusion of myogenic cells; the most functional results have been achieved using fibrin-based gels. Carefully optimizing the composition of the fibrin gel to enhance cell–matrix interactions (54), as well as optimizing the starting cell population to improve myogenic fusion and SC maintenance (55), and providing dynamic culture conditions to improve cell survival and maturation (55), have enabled rodent skeletal muscle tissues to be engineered with contractile properties comparable to those of native muscle (e.g., twitch and tetanus-force amplitudes) (56). Rapid-prototyping techniques for hydrogel molding (57) can be further used to vary local myofiber alignment and to design complex muscle structures (58), and advanced biomaterials can deliver angiogenic, myogenic, and prosurvival factors to cells in a spatiotemporally controlled fashion (59–62). In addition to using biomaterial scaffolds, scaffold-free muscle tissue constructs have been generated using magnetic fields that allow the controlled assembly of magnetically labeled cells (63), as well as thermoresponsive polymers that allow controlled cell detachment from culture surfaces (64). Although hydrogels have been the dominant muscle-engineering scaffold in vitro, in vivo studies of muscle repair have mainly utilized acellular natural scaffolds (65–67), porous matrices made of degradable polymeric materials (68–70), or scaffold-free myoblast sheets (71).
In addition to using myogenic progenitors, nonmyogenic cells known to play important parts in muscle homeostasis and function can be included in engineered muscle tissues to further augment structural and contractile properties. Adding tissue-engineered tendons or motor neurons to muscle constructs results in the formation of, respectively, myotendinous junctions (72, 73) or NMJs (74–76); incorporating fibroblasts (77) or supplementing their paracrine factors (78) has been shown to augment engineered muscle density and protein expression. These and other studies in the field have led to the development of optimized culture conditions for engineering muscle constructs that mimic native muscle structure and function (55, 56, 79), and have enabled the initial success of tissue-engineering strategies for repairing muscle damage in rodents (79–83).
In addition to using rodent cells, a number of studies have shown that primary human myogenic cells can be isolated from muscle biopsies, expanded in vitro, and fused into multinucleated myotubes. When cultured in complex media, single human myotubes can contract in response to electrical stimulation (84). However, such a system is limited by the lack of native muscle architecture and the inability to study physiological cell–cell and cell–matrix interactions important for normal muscle function, as well their alterations in various pathologies, including dystrophies and muscle-wasting disorders (85). Tissue-engineering methods have been applied to generate 3D cultures of human myogenic cells (86–89), yet only recently have researchers been able to develop human muscle constructs with the ability to contract in response to electrical and chemical stimuli (90). These engineered tissues consist of aligned and striated myofibers, and they exhibit functional acetylcholine and β-adrenergic receptors, intact calcium handling, and physiological force–length and force–frequency relationships (90). Further optimization of myogenic cell expansion and differentiation by modifying substrate stiffness (91), cell-adhesion proteins (92), or biochemical factors (93, 94) are expected to enhance the contractile and regenerative properties of muscle constructs (Figure 2).
Figure 2.
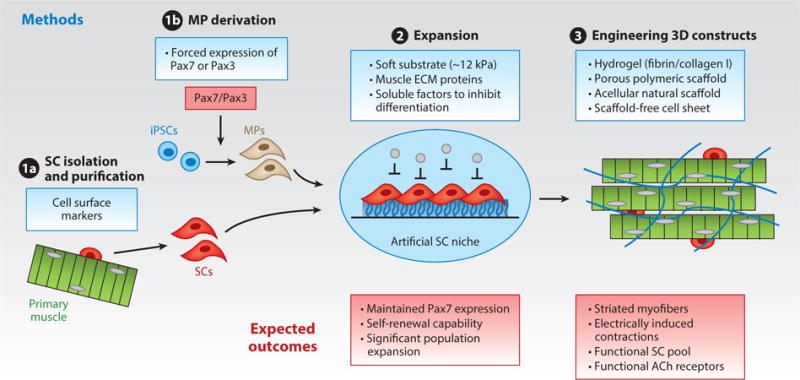
Methods for, and expected outcomes of, engineering functional, human skeletal muscle. Strategies are shown for (❶a) purifying, (❶b) deriving, and (❷) expanding functional satellite cells (SCs) or induced pluripotent stem cell (iPSC)-derived myogenic progenitors (MPs), and (❸) their use within 3D-engineered environments to form functional and self-regenerating human muscle tissues. Abbreviations: ACh, acetylcholine; ECM, extracellular matrix.
3.2. Methods for In Vitro Maintenance and Expansion of Functional Satellite Cells
It has been well established that the in vitro expansion of muscle stem cells results in the rapid loss of their self-renewing and regenerative properties due to the lack of niche signaling that is present in vivo (95, 96). Thus, the ability to re-create a niche environment in vitro may enable robust expansion of SCs while maintaining their stemness, which would be expected to increase their therapeutic capacity compared with the use of more committed myogenic cells (97, 98). Furthermore, systematic studies of the in vitro, reconstructed SC niche could help dissect how specific cellular and ECM components of the niche regulate myogenesis and self-repair of healthy, diseased, and aged muscle (97). During the past several years, the use of biomaterials and engineered muscle tissues has shown promise in re-creating important aspects of the SC niche in vitro to allow therapeutic expansion of SCs and study of their roles in muscle formation and regeneration.
In particular, soft, mechanically tunable polyethylene glycol hydrogel substrates, mimicking the stiffness of native muscle and crosslinked with the ECM protein laminin, a prominent niche constituent, have been used to enhance the self-renewal of mouse muscle stem cells in vitro, which enhanced the engraftment and therapeutic efficacy of these cells in vivo (99). The use of the same soft substrates combined with the pharmacological inhibition of the p38α/β mitogen-activated kinase pathway restored the potential of aged muscle stem cells to self-renew in vitro and repair muscle injury in vivo (100). The SC niche environment has been also been re-created in vitro using 3D-engineered muscle tissues made of a heterogeneous population of neonatal rat myogenic cells encapsulated in a fibrin-based hydrogel (55, 56). Within the engineered muscle, the rapid formation of myofibers after cell encapsulation provided a homing environment for SCs that abutted the myofiber membrane underneath a laminin- and collagen-IV-rich layer, and enabled the SCs to attain a quiescent Pax7+/MyoD− phenotype (55, 56). Upon injury of engineered muscle by cardiotoxin, the quiescent SCs underwent robust activation and myogenesis to support the regrowth of striated muscle fibers and recovery of their contractile function (56). Such a model may facilitate future in vitro studies of SC fate and function during simulated injury, exercise, or disease.
Despite the progress that has been made with rodent cells, the current methods used to expand primary, human muscle stem cells in vitro remain inefficient and, thus, the use of human induced pluripotent stem cells (iPSCs) holds the promise of providing unlimited numbers of patient-specific myogenic cells for disease modeling and autologous cell therapies (101). During the past 3 years, myogenic cells have been derived from human iPSCs from normal individuals and those with different congenital muscle diseases by forcing expression of muscle transcription factors PAX7 (102, 103) or MYOD (104–107) using doxycycline-inducible lentiviral and transposon vectors. These cells can be successfully expanded in vitro and induced to fuse into multinucleated myotubes; however, despite attaining the structural and calcium-handling properties of immature human muscle (103), the formed myotubes have small numbers of nuclei and do not appear capable of generating electrically induced contractions. Importantly, the human iPSC-derived myogenic cells can engraft in immunocompromised healthy and dystrophic mice, and, for cells obtained by Pax7+ overexpression, can incorporate into the host SC niche and participate in muscle regeneration (102). Nongenetic methods used to differentiate human iPSCs to myogenic cells have also been reported (108–112), but they are much less efficient compared with viral-based methods. This is to be expected because skeletal muscle cells, in contrast to other mesoderm-derived cells, such as cardiomyocytes or endothelial cells, are virtually absent from spontaneously differentiating embryonic stem cell and iPSC systems in vitro, a surprising observation considering that skeletal muscle is the single largest tissue in the body. In addition to iPSC technology, recent advances in generating immortalized myoblast lines from human muscle biopsies (113) hold promise for developing low-cost highly reproducible human myogenic cell sources for disease modeling and drug discovery.
3.3. Methods for Vascularizing Engineered Muscle
Creating thick, viable tissue implants that can readily connect to the host vasculature to ensure continued cell survival and function has been a long-standing goal of tissue engineering (114). In the muscle-engineering field, two general vascularization strategies have been pursued, either individually or in combination, the first involving in vitro tissue vasculogenesis (i.e., the formation of vascular structures within the construct during cell culture and prior to implantation), and the second involving in vivo tissue angiogenesis (i.e., the stimulation of rapid ingrowth of host vessels into the construct after implantation) (115). The vasculogenesis-based methods usually involve 3D coculture of myogenic and different types of vasculogenic cells to establish branched vascular structures within the engineered muscle. Compared with the biculture of endothelial cells and myoblasts, the triculture of endothelial cells, myoblasts, and embryonic fibroblasts has been shown to enhance the formation of lumen structures in vitro as the fibroblasts act to stabilize vessel networks, which, in turn, promotes cell survival and construct perfusion in vivo (69). The application of long-term construct culture to form well-developed vessel networks can further enhance the final density of the vessels in implants, and accelerate implant perfusion through inosculated host and donor vessels (83).
Methods for in vivo vascularization through angiogenesis rely on stimulating the host vascular system to rapidly invade an initially avascular muscle implant and establish perfusion. Without the need for coculture with vasculogenic cells, which may impede myoblast fusion, this method allows for the creation and implantation of highly dense muscle tissues with greater contractile strength (56). As recently shown, such avascular, engineered muscle implants undergo initial hypoxia and functional decline upon implantation; however, with the ingrowth of host blood vessels, their contractile function is rescued and further improved compared with preimplantation levels (56). Although promising, the use of this approach without further modifications may be limited to small implant sizes because large implants may undergo excessive and irreversible hypoxic damage after transition from the in vitro to in vivo environment. For larger muscle implants, vascular ingrowth in vivo can be accelerated by using biodegradable polymeric scaffolds that release proangiogenic growth factors such as vascular endothelial growth factor (VEGF) (59, 82, 116). Although sustained delivery of VEGF from a cell-free scaffold has been shown to enable full recovery of perfusion in an ischemic mouse muscle within 3 weeks (82), the addition of myoblasts and the release of insulin-like growth factor-1 (IGF-1) can further augment muscle mass and contractile function (59, 117). Similarly, the transient transfection of myoblasts with VEGF165 and stromal cell-derived factor-1 (SDF-1) before implantation has been shown to enhance vascularization and muscle repair (118). Alternatively, vascularization strategies involving the growth of 3D muscle constructs around larger arteries and veins shunted into an arteriovenous loop can provide means to enhance the perfusion of muscle tissues in vivo (119–121). Such vascularized tissues can then be excised along with the arteriovenous loop and surgically anastomosed to host vasculature at the site of muscle injury, as has been demonstrated for a tricultured muscle construct used to repair a full-thickness abdominal wall defect in mice (120).
3.4. Methods for Innervating Engineered Muscle
For efficient muscle repair, the restoration of functioning muscle and vascular tissue must be accompanied by successful innervation of the implant to promote its survival, synchronized contractions with the host muscle, and further functional maturation through the action of neurotrophic factors (122, 123). The formation of NMJs and improved force generation have been reported for engineered muscles that were implanted around a femoral nerve (123), and motor function in denervated muscle has been recovered through transplantation of a peroneal nerve (124) or stem cell–derived motor neurons (125). Although in vitro coculture of engineered rodent muscle and motor neurons can yield the formation of functional NMJs and enhance force production (74–76, 126), it is not clear that composite muscle–nerve constructs, although more complicated to produce, would add any benefit in vivo compared with the use of muscle-only constructs. Thus, recent studies have focused on treating engineered muscle in vitro with neuronal paracrine factors under the premise that such preconditioning may enhance myofiber expression of acetylcholine receptors (AChRs), the postsynaptic component of the NMJ (127), thereby facilitating the implant’s integration in vivo. In particular, treatment with agrin, a factor known to have a critical role in forming and stabilizing AChRs (128), has been shown to increase both contractile function (129) and the density or clustering of AChRs in engineered muscle in vitro (129, 130). Additionally, treatment with agrin has been shown to accelerate implant innervation in vivo (130). In an alternative or complementary approach, sustained delivery of VEGF from an implanted, biodegradable scaffold has been used to upregulate the expression of nerve growth factor and glial-derived neurotrophic factor to stimulate axonal regeneration and reinnervate ischemic muscle (116).
4. BIOENGINEERING STRATEGIES FOR CREATING CLINICALLY RELEVANT THERAPIES
Different types of muscle diseases and injuries present as diverse combinations of pathological changes in systemic factors, local niches, the ECM, and muscle and nonmuscle cells, all of which are involved in regulating muscle function, metabolism, and repair (2–4). Thus, efficient remedies for skeletal muscle disorders will have to be disease-specific and will require multidisciplinary approaches to address their high degree of complexity. Strong synergism between the understanding of the biology of cellular behaviors and the niche milieu, and the use of engineering solutions to re-create and control muscle environments in vitro and in vivo, will provide the foundation for developing new therapies for muscle diseases. In particular, the concept of merging bioengineering and stem cell biology for therapeutic purposes has been pursued experimentally primarily in two areas: hereditary, degenerative disorders of muscle and in muscle trauma.
4.1. Bioengineering for Hereditary Muscle Disorders
The direct utility of bioengineering technologies for treating hereditary diseases that affect all muscles in the body may be limited by the total tissue volume requiring treatment. However, localized repair of the most severely affected muscles (e.g., the diaphragm in DMD or facial muscles in FSHD) with engineered tissue grafts or gene-delivering biomaterials may still hold therapeutic promise. Additionally, the use of bioengineering techniques to re-create niche environments (99, 100) in vitro and to significantly expand muscle stem cells while maintaining their regenerative capacity could enable the generation of large numbers of autologous cells for use in cell-based therapies. In such an approach, SCs would, first, either be isolated from muscle biopsies or derived from patients’ iPSCs, then genetically or pharmacologically corrected, expanded in an engineered in vitro environment with maintained regenerative potential, and transplanted back into the diseased tissue. In recent proof-of-concept studies, similar strategies have been utilized to enhance aged mouse SCs (100) or to treat mice with DMD (131) or limb-girdle muscular dystrophy (132). During the past several years, rapid progress in genome-editing technologies (133, 134) has provided additional tools to genetically modify mouse and human iPSCs, but further advances will be needed to enable efficient genetic correction of postnatal somatic cells (135). Furthermore, the survival, migration, and differentiation of implanted cells may be hampered by host inflammatory and immune responses (136), important issues that could be addressed by engineering biomaterials to have immunomodulatory functions (137). In general, an improved understanding of the local and systemic pathological milieux is expected to yield more efficient cell-based therapies for congenital muscle diseases.
Currently, the most immediate utility of bioengineering methods for treating hereditary muscle diseases may lay in the further development of human in vitro assays that could be used for preclinical testing of candidate drug, gene, and cell therapeutics. In particular, tissue-engineered models of contractile human muscle hold potential for use as clinical trials in a dish to evaluate the safety and efficacy of candidate therapies in a patient-specific manner. Recently, constructs of contractile, human muscle tissue have been shown to exhibit functional and biochemical responses to statins, chloroquine, and clenbuterol that are similar to those that have been reported in vivo (90). Furthermore, the iPSC-based models of contractile, human engineered muscle are of particular interest for research into congenital muscle disorders where conventional mouse models [e.g., DMD (138, 139), glycogen storage disease type II (Pompe’s disease) (140), and others] are unable to re-create the severity of the human pathologies. The predictability and utility of these human-muscle platforms can be further enhanced by adding the relevant pathological features (e.g., a fibrotic environment with increased stiffness, ischemic or metabolic changes, inflammatory factors, aberrant vascular or neuronal components) or by integrating them with other human in vitro systems (e.g., liver, fat, heart) (141). In addition to measuring contractile force, the ability to tissue-engineer an in vitro, functional, human SC niche would allow the screening of therapies aimed at reducing human muscle weakness (142) and enhancing self-repair. Finally, controlling the cellular components of biomimetic, human engineered muscle would enable mechanistic and comparative studies of different candidate cell therapies for hereditary muscle diseases, including those utilizing circulating CD133+ cells (143) or iPSC-derived mesoangioblasts (132).
4.2. Bioengineering for Volumetric Muscle Loss
In addition to providing in vitro platforms for modeling and studies of hereditary muscle diseases, bioengineering methods hold promise for repairing large VML due to trauma. The main advantage of preformed, engineered muscle constructs as therapeutics for VML is their potential to provide rapid structural and functional repair of large areas of tissue damage. In theory, such a treatment would add new, functional muscle to a region in which the natural repair process is unable to generate sufficient muscle mass following trauma. In the case of chronic VML, successful treatment is likely to require more complex therapeutic approaches to also overcome the fibrotic and other degenerative changes that may accrue over time. VML therapies using contractile self-regenerating engineered muscle tissues or biomaterial scaffolds that are able to stimulate the growth of implanted or endogenous myogenic precursors, nerves, and blood vessels, are becoming increasingly closer to reality (56, 66, 120). Still, before potential clinical use in humans, a number of challenges need to be addressed, including the ability to produce large quantities of human myogenic cells and to promote their structural and functional maturation in vitro, as well as the development of biomaterials with adequate degradation times and growth factor-release profiles to support long-term survival and functional integration of implanted cells and tissues in vivo.
Defining the molecular and biophysical factors that can significantly advance the structural and functional maturation of engineered muscles in vitro (Figure 3) will be the key to facilitating efficient cell-based therapies for VML. Despite significant efforts in the field, in vitro engineered muscle constructs do not surpass neonatal levels of maturation (56), as reflected in their small myofiber size; lack of t-tubules; reduced expression of adult contractile protein isoforms; low levels of the primary glucose transporter, GLUT4; immature calcium handling; and weak contractile strength (144–146). Although longer culture times may result in some functional improvements, these changes progress at a much slower rate compared with normal postnatal maturation (56). During development, muscle contractions are required for the proper growth and maturation of muscle fibers and stabilization of NMJs (147, 148). In adult muscle, exercise has been shown to induce upregulation of endogenous IGF-1 (149) and stimulate autocrine-induced myofiber hypertrophy (150) by downstream activation of the mammalian target of rapamycin (mTOR), the controller of muscle mass (145). Exercise has been also shown to induce increased membrane expression of GLUT4 (151, 152), which, in turn, can enhance nutrient intake, a key determinant of muscle growth (153, 154). Consistent with the development of native muscle (146), the application of electrical stimulation to rodent engineered muscles, aimed at simulating neuronal input and muscle exercise, has been shown to cause a shift from fetal to adult isoforms of myosin heavy chain (MyHC), resulting in increased contractile strength (155–157). Similarly, applying progressive mechanical stretch to model muscle growth during development enhanced the alignment and fusion of myoblasts and elongation of myotubes (158, 159); conditioning by cyclic stretch stimulated myogenesis, hypertrophy, and the generation of contractile force in engineered muscle (86, 88).
Figure 3.
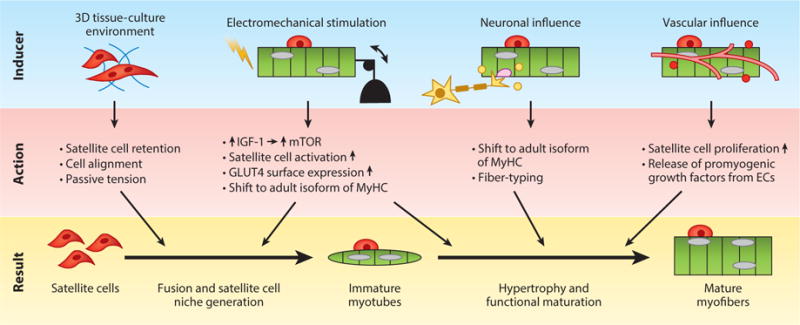
Methods to stimulate myogenic maturation in vitro. Bioengineering strategies to induce formation of the satellite cell niche and induce structural and functional maturation of myogenic cells in vitro, including creation of myomimetic 3D cell microenvironments, biophysical and biochemical stimulation, and coculture with other cell types. Abbreviations: EC, endothelial cell; IGF-1, insulin-like growth factor-1; mTOR, mammalian target of rapamycin; MyHC, myosin heavy chain.
Although biophysical stimulation is the most obvious strategy for promoting in vitro maturation of engineered muscle, the in vivo development of skeletal muscle is additionally enabled by the presence of the SC niche and neuronal and vascular inputs. Specifically, the differentiation of SCs and fusion to myofibers are required to increase the number of myonuclei and to support enhanced protein production and the growth of young muscle (153, 160, 161). The formation and maintenance of a functional SC niche, and its ability to support myogenesis within engineered muscle, have been shown for rodent cells (56) but not for human cells. In addition to the SC niche, neuronal influence, both direct and paracrine, could promote the maturation and plasticity of engineered muscle. During development, the formation of mature NMJs on individual myofibers and the loss of polyneuronal innervation have been shown to stimulate expression of adult MyHC isoforms (148, 162). Similarly, initial expression of embryonic MyHC in regenerating myofibers is known to shift toward more mature isoforms upon initiation of muscle reinnervation (148, 163). In addition to neuronal input, in vitro prevascularization of engineered muscle, even if it is unable to provide perfusion, can still promote myofiber hypertrophy and maturation: VEGF expressed by endothelial cells has been shown to stimulate the expression of myogenic markers, and the proliferation, fusion, and survival of myoblasts (164). Similarly, nitric oxide released by endothelial cells has been known to stimulate mitochondrial biogenesis, SC activation, myoblast fusion, and myogenic differentiation in skeletal muscle, but may require the presence of flow and shear stress (165). Proven biochemical stimulators of myogenic differentiation (2), such as IGF-1 and TGF-β, can further promote maturation and enhance the contractile function of engineered muscle tissues (166, 167). In addition, it is likely that various combinations of biophysical, biochemical, and heterocellular cues act synergistically to enhance the function and maturation of engineered muscle. The main progress, however, is expected to arise from improved understanding of the biological factors controlling postnatal muscle development, which, in turn, will enable the use of biomimetic rather than heuristic approaches for in vitro engineering of mature adult-like muscle tissues. Still, for all practical purposes, the design of engineered muscle should be made as simple as possible for a given application (in vitro or in vivo) to prevent logistical, financial, and regulatory hurdles from arising during subsequent translation or commercialization.
The road from bench to bedside use of engineered muscle technology to treat VML faces many additional challenges. Scaling up is one of the major obstacles when translating any drug, cell, or gene therapy from rodent to human systems. For adequate tissue replacement, constructs of human engineered muscle will need to provide tissue volume that is orders of magnitude larger than is currently achievable; the constructs will also need to rely on successful prevascularization to prevent hypoxia-induced cell death and neurotization to ensure rapid innervation in vivo (Figure 4). A robustly functioning SC pool within large constructs will be required to aid recovery from any hypoxia-induced cell death that could occur upon the transition from an in vitro to an in vivo environment. Because off-the-shelf availability is likely the only financially and logistically viable strategy for widespread clinical use in the future, methods to successfully preserve engineered muscle tissues over the long term will need to be developed for use in VML repair (168). The current techniques used to maintain cell or tissue viability in vitro and ex vivo suffer from numerous limitations. Hypothermic storage in optimal liquid environments or in circulation systems with oxygenation capability have limited shelf life and are prohibitively expensive (169). Tissue cryopreservation using traditional freeze–thaw techniques or vitrification are associated with a high risk of cell death, are less effective for larger tissue constructs, and are unproven in their ability to recover cellular function (170). Cellular desiccation and dry storage require additional research to ensure tolerance to oxidative damage, and have not yet been attempted on large tissue samples (169).
Figure 4.
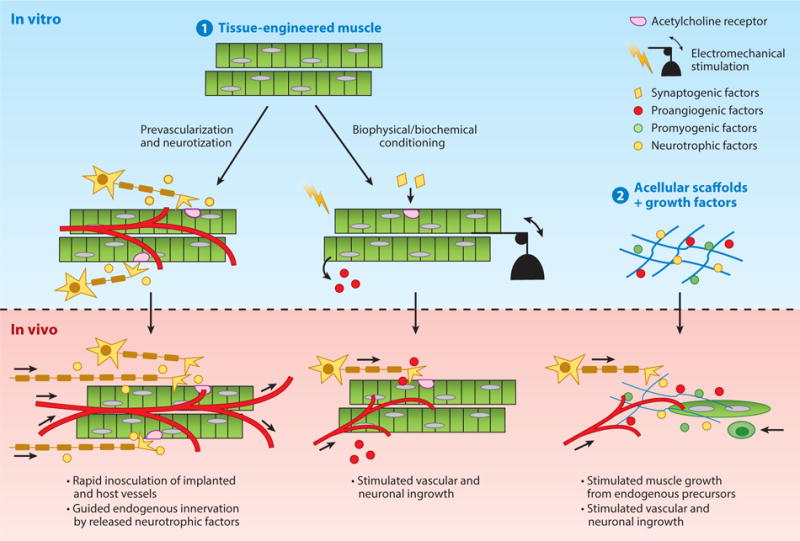
Strategies to enhance muscle repair following volumetric muscle loss. (❶) In vitro engineered muscles primed by coculture with vascular and neuronal cells, or biophysical or biochemical stimulation, and (❷) biomaterials tethered with myogenic, vasculogenic, and neurotrophic growth factors are used to accelerate muscle growth and neurovascular integration after implantation in vivo.
In addition to tissue preservation, methods for successful transplantation of engineered muscle will require surgical procedures to ensure that the implant initially remains under tension to promote cell survival and alignment (171); this may require additional engineering of tendon-like structures. Injecting muscle cells within photo- (172) or temperature-sensitive hydrogels (173) could avoid some of the issues; however, the ability to successfully reconstruct large, aligned muscle structures using these approaches remains to be proven. Until these daunting obstacles are overcome, the most promising therapeutic application of human engineered muscle would involve repairing small muscles, such as those affected by craniofacial injury or disease. For VML, a more feasible and simpler strategy would be to use cell-free scaffolds to stimulate muscle growth in situ. Specifically, smart biodegradable materials capable of controlling the release of antifibrotic, promyogenic, angiogenic, and neurotrophic factors could stimulate functional recovery of relatively large defects by preventing fibrotic response and promoting infiltration of the host cells needed to rebuild new muscle (66, 116, 117, 174). Recently, decellularized, porcine, urinary bladder scaffolds have been used to improve the functional capability of damaged muscle in patients (66), thus providing a foundation for future biomaterial-based treatments of VML.
Compared with traumatic muscle loss, the prospect of stem cell or bioengineering approaches for treating chronic muscle wasting and atrophy is much less clear. There is little evidence that SCs or transplanted muscle stem- or progenitor cells would have any beneficial effects as countermeasures to the processes leading to muscle loss. Rather, the evidence points to a central role for processes within the myofiber that underlie the pathogenesis of these diseases: SCs appear to be innocent and helpless bystanders at best and unwitting contributors at worst (175). Most importantly, there is little evidence that enhancing the function or activity of SCs, or transplanting exogenous muscle stem or progenitor cells, has any significant beneficial effect on the progression of muscle loss in these conditions. Although there has been little research into applying bioengineering approaches to the treatment of these disorders, the use of in vitro, human engineered muscle models could enhance our understanding of disease and provide a platform for discovering safe and efficient therapeutics.
5. SUMMARY
In this review, we have described the basic biological mechanisms underlying skeletal muscle homeostasis, repair, and disease; this was followed by an overview of the bioengineering approaches that have been used to replicate these processes in vitro and to develop cell- and biomaterial-based remedies for muscle disorders in vivo. The two main areas where bioengineering-aided strategies hold promise to significantly impact human muscle research and treatment include: (a) basic and preclinical studies in 3D-engineered muscle models, and (b) nonpharmacological therapies for VML.
In particular, the development of accurate, in vitro models of skeletal muscle is expected to promote systematic studies of the roles that the extracellular environment and cell–cell and cell–matrix interactions have in muscle function, regeneration, and disease. Importantly, 3D cultures of human myogenic cells could be utilized in disease modeling, drug discovery, and toxicology screening (90, 176), both to aid the development of new therapies and to help alleviate ethical, monetary, and predictability concerns associated with animal studies (177). Of particular interest is the modeling of human congenital muscle diseases that often lack clinically relevant large animal models beyond an often-inadequate mouse transgenic model. The need for predictive preclinical models of human skeletal muscle was signified by the market withdrawal of cerivastatin, which was well tolerated in mice but caused fatal myopathies in humans (178, 179). Furthermore, the in vitro integration of engineered muscle with other microtissue systems, such as liver or heart, may enable more predictive body-on-chip pharmacological studies (141, 176) and provide improved understanding of myokine-dependent interactions between muscle and other tissues and organs (42). Overall, the utility of such in vitro systems, designed from primary or stem cell-derived myogenic cells, will be highly dependent on their ability to faithfully replicate the cellular organization and contractile activity of native human muscle. Simulating exercise by using electrical or mechanical stimulation is one strategy to promote the functional output and maturation of human engineered muscle (180, 181). Mechanical stimulation, in particular, can additionally upregulate myotube expression of proangiogenic factors, including VEGF (182), to promote vascularization of the engineered muscle. The application of selected regimes of electrical stimulation (183, 184) to control the fraction of slow (type I) and fast (type II) fibers in engineered muscle can additionally allow study of fiber type-specific drug responses relevant to various muscle pathologies (85, 185, 186).
Moving toward therapies for VML, bioengineers have made progress in enhancing the maturation and functional output of 3D muscle constructs in vitro, and in stimulating their vascular and neuronal integration into the host muscle in vivo. The proof-of-concept studies in rodent models have been particularly encouraging. However, it remains to be seen whether similar results can be reproduced using human cell sources, considering that the expansion of cells in vitro and the generation of large volumes of functional muscle tissues, will be critical to the success of cell-based therapies in humans. As we move toward this goal, a large body of ongoing tissue-engineering work revolves around establishing perfusable capillary networks in vitro and inducing the ingrowth of functional vasculature in vivo (114, 187). Additionally, complex bidirectional interactions between myogenic and vascular cells are increasingly recognized as being critical for muscle function, disease, and regeneration (188–190). As such, the ability to engineer 3D skeletal muscle tissues with native-like architecture, mature contractile function, and a vascular bed—feats not yet accomplished—would promote both basic studies of muscle biology and the development of improved cell- and material-based therapies for VML. Conditioning with neurotrophic factors is expected to further enhance in vitro function and in vivo integration of engineered muscle. Currently, however, the most promising bioengineering therapies for VML involve the use of cell-free implantable biomaterials designed to rapidly stimulate antifibrotic, myogenic, vasculogenic, and neurogenic responses at the site of muscle damage.
6. CONCLUSIONS
Recent progress in the fields of muscle biology and bioengineering has increased our understanding of muscle development and disease, and improved our ability to model and control physiological and pathological tissue environments, and deliver therapeutic molecules and cells. Hypothesis-driven research, along with high-throughput technologies, are expected to drive future studies in the field, while the growing synergism between fundamental discovery and applied, biologically inspired solutions will create new therapeutic opportunities (Figure 5). The development of sophisticated, in vitro platforms to modify and study heterocellular interactions and the extracellular milieu will lead to advances beyond those achievable solely by the use of animals and traditional cell culture. As the field progresses, it may be essential that basic and applied research transition toward clinically relevant platforms involving the use of human cells and large-animal models. Critical to successful translation will be the development of methods to derive large numbers of human muscle progenitors and to efficiently deliver therapeutic molecules and cells. Technologies that allow real-time monitoring and active alteration of the in vitro culture environment based on developmental clues may address some of these challenges, along with the design of smart, environmentally responsive biomaterials. Continued work at the interface of bioengineering and biology holds exciting prospects for advancing basic knowledge and ameliorating human muscle disease.
Figure 5.
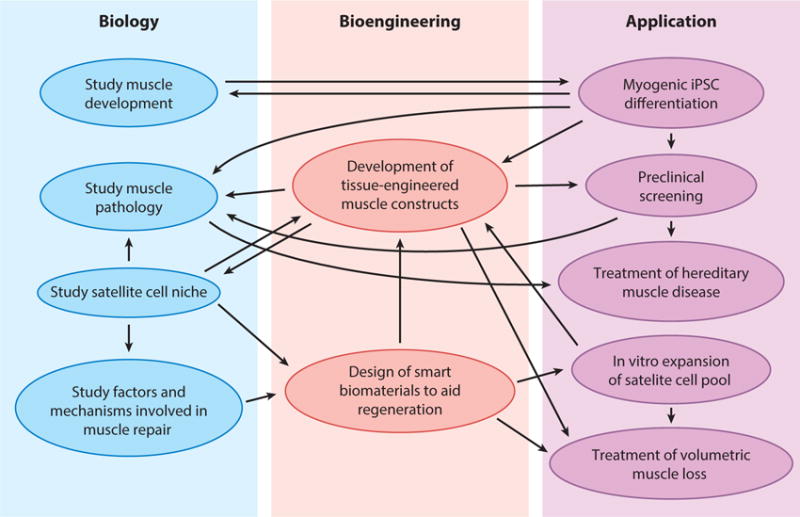
The synergy of biology and bioengineering for skeletal muscle research and repair. A large number of feedforward and feedback interactions between biology and bioengineering may lead to important scientific and therapeutic applications, including the ability to more efficiently study muscle development, physiology, and disease, as well as to develop new cell, gene, and biomaterial-based treatments for muscle disorders. Abbreviation: iPSC, induced pluripotent stem cell.
Acknowledgments
This work was supported by a National Science Foundation graduate research fellowship to M.J.; National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Disease grants AR055226 and AR065873, and NIH Common Fund and the Microphysiological Systems Initiative grant UH2TR000505 to N.B.; and by grants from the NIH (P01 AG036695, R01 AG23806, and R01 AR062185) and the Department of Veterans Affairs (Rehabilitation Research & Development Merit Review and SPiRE Award) to T.A.R. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or National Science Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–37. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 5.Juhas M, Bursac N. Engineering skeletal muscle repair. Curr Opin Biotechnol. 2013;24:880–86. doi: 10.1016/j.copbio.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly AM, Zacks SI. The histogenesis of rat intercostal muscle. J Cell Biol. 1969;42:135–53. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007;308:281–93. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Cusella-De Angelis MG, Molinari S, Le Donne A, Coletta M, Vivarelli E, et al. Differential response of embryonic and fetal myoblasts to TGF β: a possible regulatory mechanism of skeletal muscle histogenesis. Development. 1994;120:925–33. doi: 10.1242/dev.120.4.925. [DOI] [PubMed] [Google Scholar]
- 9.Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–78. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- 10.Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. PNAS. 2007;104:537–42. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, et al. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–73. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 12.Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, et al. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–99. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- 13.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16:612–22. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Sartori R, Gregorevic P, Sandri M. TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab. 2014;25:464–71. doi: 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Chakkalakal J, Brack A. Extrinsic regulation of satellite cell function and muscle regeneration capacity during aging. J Stem Cell Res Ther. 2012;2(Suppl 11):001. doi: 10.4172/2157-7633.S11-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–46. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–40. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung TH, Quach NL, Charville GW, Liu L, Park L, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–28. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–42. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–52. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 21.Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Rep. 2014;2:414–26. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joe AW, Yi L, Natarajan A, Le Grand F, So L, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, et al. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23:349–56. doi: 10.1016/j.nmd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–88. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–96. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 27.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 28.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 29.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–95. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–64. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 32.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–63. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–34. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha M, Jang YC, Oh J, Khong D, Wu EY, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emery AE. Duchenne muscular dystrophy—Meryon’s disease. Neuromuscul Disord. 1993;3:263–66. doi: 10.1016/0960-8966(93)90018-f. [DOI] [PubMed] [Google Scholar]
- 37.Goyenvalle A, Seto JT, Davies KE, Chamberlain J. Therapeutic approaches to muscular dystrophy. Hum Mol Genet. 2011;20(R1):R69–78. doi: 10.1093/hmg/ddr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Hamid H, Clemens PR. Pharmacological therapies for muscular dystrophies. Curr Opin Neurol. 2012;25:604–8. doi: 10.1097/WCO.0b013e328357f44c. [DOI] [PubMed] [Google Scholar]
- 39.Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- 40.Grogan BF, Hsu JR, Skelet. Trauma Res. Consort. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35–37. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 41.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–74. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 43.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol. 1988;24:166–74. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 44.Shansky J, Del Tatto M, Chromiak J, Vandenburgh H. A simplified method for tissue engineering skeletal muscle organoids in vitro. In Vitro Cell Dev Biol Anim. 1997;33:659–61. doi: 10.1007/s11626-997-0118-y. [DOI] [PubMed] [Google Scholar]
- 45.Strohman RC, Bayne E, Spector D, Obinata T, Micou-Eastwood J, Maniotis A. Myogenesis and histogenesis of skeletal muscle on flexible membranes in vitro. In Vitro Cell Dev Biol. 1990;26:201–8. doi: 10.1007/BF02624113. [DOI] [PubMed] [Google Scholar]
- 46.Dennis RG, Kosnik PE., 2nd Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2000;36:327–35. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Dennis RG, Kosnik PE, 2nd, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol. 2001;280:C288–95. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 48.Bian W, Juhas M, Pfeiler TW, Bursac N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng Part A. 2012;18:957–67. doi: 10.1089/ten.tea.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J. Skeletal muscle growth is stimulated by intermittent stretch-relaxation in tissue culture. Am J Physiol. 1989;256:C674–82. doi: 10.1152/ajpcell.1989.256.3.C674. [DOI] [PubMed] [Google Scholar]
- 50.Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater. 2012;7:055004. doi: 10.1088/1748-6041/7/5/055004. [DOI] [PubMed] [Google Scholar]
- 51.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol. 2005;98:706–13. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- 52.Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, et al. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng Part A. 2012;18:2453–65. doi: 10.1089/ten.tea.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuoco C, Salvatori ML, Biondo A, Shapira-Schweitzer K, Santoleri S, et al. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skeletal Muscle. 2012;2:24. doi: 10.1186/2044-5040-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575–83. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juhas M, Bursac N. Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials. 2014;35:9438–46. doi: 10.1016/j.biomaterials.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juhas M, Engelmayr GC, Jr, Fontanella AN, Palmer GM, Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. PNAS. 2014;111:5508–13. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4:1522–34. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bian W, Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401–12. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–14. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao IC, Leong KW. Efficacy of engineered FVIII-producing skeletal muscle enhanced by growth factor-releasing co-axial electrospun fibers. Biomaterials. 2011;32:1669–77. doi: 10.1016/j.biomaterials.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun YR, Lee S, Jeon E, Kang W, Kim KH, et al. Fibroblast growth factor 2-functionalized collagen matrices for skeletal muscle tissue engineering. Biotechnol Lett. 2012;34:771–78. doi: 10.1007/s10529-011-0812-4. [DOI] [PubMed] [Google Scholar]
- 62.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto Y, Ito A, Kato M, Kawabe Y, Shimizu K, et al. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J Biosci Bioeng. 2009;108:538–43. doi: 10.1016/j.jbiosc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi H, Shimizu T, Nakayama M, Yamato M, Okano T. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials. 2013;34:7372–80. doi: 10.1016/j.biomaterials.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 65.Teodori L, Costa A, Marzio R, Perniconi B, Coletti D, et al. Native extracellular matrix: a new scaffolding platform for repair of damaged muscle. Front Physiol. 2014;5:218. doi: 10.3389/fphys.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A. 2011;17:2291–303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed Mater Eng. 2001;11:275–81. [PubMed] [Google Scholar]
- 69.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 70.Ju YM, Atala A, Yoo JJ, Lee SJ. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater. 2014;10:4332–39. doi: 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, et al. Impaired myocardium regeneration with skeletal cell sheets—a preclinical trial for tissue-engineered regeneration therapy. Transplantation. 2010;90:364–72. doi: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 72.Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149–58. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A. 2014;20:2920–30. doi: 10.1089/ten.tea.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–88. doi: 10.1016/j.biomaterials.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2006;42:75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- 76.Morimoto Y, Kato-Negishi M, Onoe H, Takeuchi S. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials. 2013;34:9413–19. doi: 10.1016/j.biomaterials.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 77.Li M, Dickinson CE, Finkelstein EB, Neville CM, Sundback CA. The role of fibroblasts in self-assembled skeletal muscle. Tissue Eng Part A. 2011;17:2641–50. doi: 10.1089/ten.TEA.2010.0700. [DOI] [PubMed] [Google Scholar]
- 78.Hinds S, Tyhovych N, Sistrunk C, Terracio L. Improved tissue culture conditions for engineered skeletal muscle sheets. Sci World J. 2013;2013:370151. doi: 10.1155/2013/370151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, Musaro A. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET) Sci Rep. 2013;3:1420. doi: 10.1038/srep01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A. 2014;20:705–15. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A. 2012;18:1213–28. doi: 10.1089/ten.tea.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. PNAS. 2010;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, et al. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. PNAS. 2011;108:14789–94. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith AS, Long CJ, Pirozzi K, Najjar S, McAleer C, et al. A multiplexed chip-based assay system for investigating the functional development of human skeletal myotubes in vitro. J Biotechnol. 2014;185C:15–18. doi: 10.1016/j.jbiotec.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–99. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 86.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557–65. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- 87.Chiron S, Tomczak C, Duperray A, Laine J, Bonne G, et al. Complex interactions between human myoblasts and the surrounding 3D fibrin-based matrix. PLOS ONE. 2012;7:e36173. doi: 10.1371/journal.pone.0036173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moon DG, Christ G, Stitzel JD, Atala A, Yoo JJ. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A. 2008;14:473–82. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]
- 89.Martin NR, Passey SL, Player DJ, Khodabukus A, Ferguson RA, et al. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials. 2013;34:5759–65. doi: 10.1016/j.biomaterials.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife. 2015;4:e04885. doi: 10.7554/eLife.04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serena E, Zatti S, Reghelin E, Pasut A, Cimetta E, Elvassore N. Soft substrates drive optimal differentiation of human healthy and dystrophic myotubes. Integr Biol (Camb) 2010;2:193–201. doi: 10.1039/b921401a. [DOI] [PubMed] [Google Scholar]
- 92.Sengupta D, Gilbert PM, Johnson KJ, Blau HM, Heilshorn SC. Protein-engineered biomaterials to generate human skeletal muscle mimics. Adv Healthc Mater. 2012;1:785–89. doi: 10.1002/adhm.201200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koning M, Werker PM, van Luyn MJ, Harmsen MC. Hypoxia promotes proliferation of human myogenic satellite cells: a potential benefactor in tissue engineering of skeletal muscle. Tissue Eng Part A. 2011;17:1747–58. doi: 10.1089/ten.tea.2010.0624. [DOI] [PubMed] [Google Scholar]
- 94.Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, et al. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol. 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–67. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 96.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–94. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCullagh KJ, Perlingeiro RC. Coaxing stem cells for skeletal muscle repair. Adv Drug Deliv Rev. 2014;84:198–207. doi: 10.1016/j.addr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu X, Fu L, Yi F, Liu GH, Ocampo A, et al. Regeneration: making muscle from hPSCs. Cell Res. 2014;24:1159–61. doi: 10.1038/cr.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–19. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skoglund G, Laine J, Darabi R, Fournier E, Perlingeiro R, Tabti N. Physiological and ultrastructural features of human induced pluripotent and embryonic stem cell-derived skeletal myocytes in vitro. PNAS. 2014;111:8275–80. doi: 10.1073/pnas.1322258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abujarour R, Bennett M, Valamehr B, Lee TT, Robinson M, et al. Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl Med. 2014;3:149–60. doi: 10.5966/sctm.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, et al. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLOS ONE. 2013;8:e61540. doi: 10.1371/journal.pone.0061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, et al. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol Ther. 2012;20:2153–67. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yasuno T, Osafune K, Sakurai H, Asaka I, Tanaka A, et al. Functional analysis of iPSC-derived myocytes from a patient with carnitine palmitoyltransferase II deficiency. Biochem Biophys Res Commun. 2014;448:175–81. doi: 10.1016/j.bbrc.2014.04.084. [DOI] [PubMed] [Google Scholar]
- 108.Hosoyama T, McGivern JV, Van Dyke JM, Ebert AD, Suzuki M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl Med. 2014;3:564–74. doi: 10.5966/sctm.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borchin B, Chen J, Barberi T. Derivation and FACS-mediated purification of PAX3+/PAX7+skeletal muscle precursors from human pluripotent stem cells. Stem Cell Rep. 2013;1:620–31. doi: 10.1016/j.stemcr.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu C, Tabebordbar M, Iovino S, Ciarlo C, Liu J, et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell. 2013;155:909–21. doi: 10.1016/j.cell.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leung M, Cooper A, Jana S, Tsao CT, Petrie TA, Zhang M. Nanofiber-based in vitro system for high myogenic differentiation of human embryonic stem cells. Biomacromolecules. 2013;14:4207–16. doi: 10.1021/bm4009843. [DOI] [PubMed] [Google Scholar]
- 112.Hwang Y, Suk S, Shih YR, Seo T, Du B, et al. WNT3A promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment. Sci Rep. 2014;4:5916. doi: 10.1038/srep05916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skeletal Muscle. 2011;1:34. doi: 10.1186/2044-5040-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–69. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 115.Levenberg S, Langer R. Advances in tissue engineering. Curr Top Dev Biol. 2004;61:113–34. doi: 10.1016/S0070-2153(04)61005-2. [DOI] [PubMed] [Google Scholar]
- 116.Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF Signaling. Mol Ther. 2014;22:1243–53. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther. 2014;22:1441–49. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou W, He DQ, Liu JY, Feng Y, Zhang XY, et al. Angiogenic gene-modified myoblasts promote vascularization during repair of skeletal muscle defects. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1692. In press. [DOI] [PubMed] [Google Scholar]
- 119.Messina A, Bortolotto SK, Cassell OC, Kelly J, Abberton KM, Morrison WA. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J. 2005;19:1570–72. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- 120.Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, et al. An engineered muscle flap for reconstruction of large soft tissue defects. PNAS. 2014;111:6010–15. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tilkorn DJ, Bedogni A, Keramidaris E, Han X, Palmer JA, et al. Implanted myoblast survival is dependent on the degree of vascularization in a novel delayed implantation/prevascularization tissue engineering model. Tissue Eng Part A. 2010;16:165–78. doi: 10.1089/ten.TEA.2009.0075. [DOI] [PubMed] [Google Scholar]
- 122.Skouras E, Ozsoy U, Sarikcioglu L, Angelov DN. Intrinsic and therapeutic factors determining the recovery of motor function after peripheral nerve transection. Ann Anat. 2011;193:286–303. doi: 10.1016/j.aanat.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Dhawan V, Lytle IF, Dow DE, Huang YC, Brown DL. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 2007;13:2813–21. doi: 10.1089/ten.2007.0003. [DOI] [PubMed] [Google Scholar]
- 124.Kang SB, Olson JL, Atala A, Yoo JJ. Functional recovery of completely denervated muscle: implications for innervation of tissue-engineered muscle. Tissue Eng Part A. 2012;18:1912–20. doi: 10.1089/ten.tea.2011.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344:94–97. doi: 10.1126/science.1248523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo X, Das M, Rumsey J, Gonzalez M, Stancescu M, Hickman J. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods. 2010;16:1347–55. doi: 10.1089/ten.tec.2010.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Colomar A, Robitaille R. Glial modulation of synaptic transmission at the neuromuscular junction. Glia. 2004;47:284–89. doi: 10.1002/glia.20086. [DOI] [PubMed] [Google Scholar]
- 128.Bezakova G, Helm JP, Francolini M, Lomo T. Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. J Cell Biol. 2001;153:1441–52. doi: 10.1083/jcb.153.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bian W, Bursac N. Soluble miniagrin enhances contractile function of engineered skeletal muscle. FASEB J. 2012;26:955–65. doi: 10.1096/fj.11-187575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ko IK, Lee BK, Lee SJ, Andersson KE, Atala A, Yoo JJ. The effect of in vitro formation of acetylcholine receptor (AChR) clusters in engineered muscle fibers on subsequent innervation of constructs in vivo. Biomaterials. 2013;34:3246–55. doi: 10.1016/j.biomaterials.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 131.Filareto A, Parker S, Darabi R, Borges L, Iacovino M, et al. An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells. Nat Commun. 2013;4:1549. doi: 10.1038/ncomms2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med. 2012;4:140ra89. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 133.Li M, Suzuki K, Kim NY, Liu GH, Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. J Biol Chem. 2014;289:4594–99. doi: 10.1074/jbc.R113.488247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184–88. doi: 10.1126/science.1254445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maffioletti SM, Noviello M, English K, Tedesco FS. Stem cell transplantation for muscular dystrophy: the challenge of immune response. BioMed Res Int. 2014;2014:964010. doi: 10.1155/2014/964010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 138.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 2013;280:4177–86. doi: 10.1111/febs.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–71. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamphoven JH, Stubenitsky R, Reuser AJ, Van Der Ploeg AT, Verdouw PD, Duncker DJ. Cardiac remodeling and contractile function in acid α-glucosidase knockout mice. Physiol Genomics. 2001;5:171–79. doi: 10.1152/physiolgenomics.2001.5.4.171. [DOI] [PubMed] [Google Scholar]
- 141.Truskey GA, Achneck HE, Bursac N, Chan H, Cheng CS, et al. Design considerations for an integrated microphysiological muscle tissue for drug and tissue toxicity testing. Stem Cell Res Ther. 2013;4(Suppl 1):S10. doi: 10.1186/scrt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gilbreath HR, Castro D, Iannaccone ST. Congenital Myopathies and muscular dystrophies. Neurol Clin. 2014;32:689–703. doi: 10.1016/j.ncl.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 143.Meng J, Chun S, Asfahani R, Lochmuller H, Muntoni F, Morgan J. Human skeletal muscle-derived CD133+ cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22:1008–17. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fitts RH, McDonald KS, Schluter JM. The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. J Biomech. 1991;24(Suppl 1):111–22. doi: 10.1016/0021-9290(91)90382-w. [DOI] [PubMed] [Google Scholar]
- 145.Rüegg MA, Glass DJ. Molecular mechanisms and treatment options for muscle wasting diseases. Annu Rev Pharmacol Toxicol. 2011;51:373–95. doi: 10.1146/annurev-pharmtox-010510-100537. [DOI] [PubMed] [Google Scholar]
- 146.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 147.Ramachandran I, Terry M, Ferrari MB. Skeletal muscle myosin cross-bridge cycling is necessary for myofibrillogenesis. Cell Motil Cytoskelet. 2003;55:61–72. doi: 10.1002/cm.10113. [DOI] [PubMed] [Google Scholar]
- 148.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 149.DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259:E89–95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- 150.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154:557–68. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barnard RJ, Youngren JF. Regulation of glucose transport in skeletal muscle. FASEB J. 1992;6:3238–44. doi: 10.1096/fasebj.6.14.1426762. [DOI] [PubMed] [Google Scholar]
- 152.Aas V, Bakke SS, Feng YZ, Kase ET, Jensen J, et al. Are cultured human myotubes far from home? Cell Tissue Res. 2013;354:671–82. doi: 10.1007/s00441-013-1655-1. [DOI] [PubMed] [Google Scholar]
- 153.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr. 2009;12:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 155.Langelaan ML, Boonen KJ, Rosaria-Chak KY, van der Schaft DW, Post MJ, Baaijens FP. Advanced maturation by electrical stimulation: differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med. 2011;5:529–39. doi: 10.1002/term.345. [DOI] [PubMed] [Google Scholar]
- 156.Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16:711–18. doi: 10.1089/ten.TEC.2009.0125. [DOI] [PubMed] [Google Scholar]
- 157.Ito A, Yamamoto Y, Sato M, Ikeda K, Yamamoto M, et al. Induction of functional tissue-engineered skeletal muscle constructs by defined electrical stimulation. Sci Rep. 2014;4:4781. doi: 10.1038/srep04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheema U, Yang SY, Mudera V, Goldspink GG, Brown RA. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskelet. 2003;54:226–36. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 159.Vandenburgh HH, Karlisch P. Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell Dev Biol. 1989;25:607–16. doi: 10.1007/BF02623630. [DOI] [PubMed] [Google Scholar]
- 160.Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–13. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kawano F, Takeno Y, Nakai N, Higo Y, Terada M, et al. Essential role of satellite cells in the growth of rat soleus muscle fibers. Am J Physiol Cell Physiol. 2008;295:C458–67. doi: 10.1152/ajpcell.00497.2007. [DOI] [PubMed] [Google Scholar]
- 162.Slater CR. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev Biol. 1982;94:11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- 163.d’Albis A, Couteaux R, Janmot C, Roulet A, Mira JC. Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur J Biochem. 1988;174:103–10. doi: 10.1111/j.1432-1033.1988.tb14068.x. [DOI] [PubMed] [Google Scholar]
- 164.Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, et al. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19:994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tengan CH, Rodrigues GS, Godinho RO. Nitric oxide in skeletal muscle: role on mitochondrial biogenesis and function. Int J Mol Sci. 2012;13:17160–84. doi: 10.3390/ijms131217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weist MR, Wellington MS, Bermudez JE, Kostrominova TY, Mendias CL, et al. TGF-β1 enhances contractility in engineered skeletal muscle. J Tissue Eng Regen Med. 2013;7:562–71. doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve. 2008;37:438–47. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 168.Griffith LG, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 169.Acker JP. Biopreservation of cells and engineered tissues. Adv Biochem Eng Biotechnol. 2007;103:157–87. doi: 10.1007/b137204. [DOI] [PubMed] [Google Scholar]
- 170.Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243–56. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- 171.Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, et al. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29:75–84. doi: 10.1016/j.biomaterials.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25:2296–304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 173.Ding K, Yang Z, Zhang YL, Xu JZ. Injectable thermosensitive chitosan/β-glycerophosphate/collagen hydrogel maintains the plasticity of skeletal muscle satellite cells and supports their in vivo viability. Cell Biol Int. 2013;37:977–87. doi: 10.1002/cbin.10123. [DOI] [PubMed] [Google Scholar]
- 174.Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35:8605–12. doi: 10.1016/j.biomaterials.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 175.He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Investig. 2013;123:4821–35. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 177.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra47. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.von Keutz E, Schluter G. Preclinical safety evaluation of cerivastatin, a novel HMG-CoA reductase inhibitor. Am J Cardiol. 1998;82:11J–17J. doi: 10.1016/s0002-9149(98)00424-x. [DOI] [PubMed] [Google Scholar]
- 179.Furberg CD, Pitt B. Withdrawal of cerivastatin from the world market. Curr Control Trials Cardiovasc Med. 2001;2:205–7. doi: 10.1186/cvm-2-5-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rangarajan S, Madden L, Bursac N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann Biomed Eng. 2014;42:1391–405. doi: 10.1007/s10439-013-0966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng CS, Davis BN, Madden L, Bursac N, Truskey GA. Physiology and metabolism of tissue-engineered skeletal muscle. Exp Biol Med (Maywood) 2014;239:1203–14. doi: 10.1177/1535370214538589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van der Schaft DW, van Spreeuwel AC, van Assen HC, Baaijens FP. Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng Part A. 2011;17:2857–65. doi: 10.1089/ten.TEA.2011.0214. [DOI] [PubMed] [Google Scholar]
- 183.Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc. 2011;86:564–600. doi: 10.1111/j.1469-185X.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Westgaard RH, Lomo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. J Neurosci. 1988;8:4415–26. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Raben N, Fukuda T, Gilbert AL, de Jong D, Thurberg BL, et al. Replacing acid α-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 186.Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988;52:503–13. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- 187.Tian L, George SC. Biomaterials to prevascularize engineered tissues. J Cardiovasc Transl Res. 2011;4:685–98. doi: 10.1007/s12265-011-9301-3. [DOI] [PubMed] [Google Scholar]
- 188.Renault MA, Vandierdonck S, Chapouly C, Yu Y, Qin G, et al. Gli3 regulation of myogenesis is necessary for ischemia-induced angiogenesis. Circ Res. 2013;113:1148–58. doi: 10.1161/CIRCRESAHA.113.301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mounier R, Chretien F, Chazaud B. Blood vessels and the satellite cell niche. Curr Top Dev Biol. 2011;96:121–38. doi: 10.1016/B978-0-12-385940-2.00005-X. [DOI] [PubMed] [Google Scholar]
- 190.Lee SL, Pevec WC, Carlsen RC. Functional outcome of new blood vessel growth into ischemic skeletal muscle. J Vasc Surg. 2001;34:1096–102. doi: 10.1067/mva.2001.117889. [DOI] [PubMed] [Google Scholar]


