Abstract
Background
Current classification schemes for acute myocardial infarction (AMI) may not accommodate the breadth of clinical phenotypes in young women.
Methods and Results
We developed a novel taxonomy among young adults (<55 years) with AMI enrolled in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study. We first classified a subset of patients (n=600) according to the Third Universal Definition of MI using a structured abstraction tool. There was heterogeneity within Type 2 AMI, and 54 patients (9%; including 51 of 412 women) were unclassified. Using an inductive approach, we iteratively grouped patients with shared clinical characteristics, with the aims of developing a more inclusive taxonomy that could distinguish unique clinical phenotypes. The final VIRGO taxonomy classified 2,802 study participants as: Class 1, plaque-mediated culprit lesion (82.5% of women; 94.9% of men); Class 2, obstructive coronary artery disease with supply-demand mismatch (2a: 1.4% women; 0.9% men;) and without supply-demand mismatch (2b: 2.4% women; 1.1% men); Class 3, non-obstructive coronary artery disease with supply-demand mismatch (3a: 4.3% women; 0.8% men) and without supply-demand mismatch (3b: 7.0% women; 1.9% men); Class 4, other identifiable mechanism: spontaneous dissection; vasospasm; embolism (1.5% women; 0.2% men); and Class 5, undetermined classification (0.8% women; 0.2% men).
Conclusions
Approximately 1 in 8 young women with AMI are unclassified by the Universal Definition of MI. We propose a more inclusive taxonomy that could serve as a framework for understanding biological disease mechanisms, therapeutic efficacy and prognosis in this population.
Keywords: acute myocardial infarction, gender differences, classification, taxonomy
Introduction
Young women account for 40,000 hospitalizations for acute myocardial infarction (AMI) annually and have greater risks for morbidity and mortality compared with both young men and older women with AMI.1-3 The identification of distinct characteristics among young women with AMI, including genetic predisposition, non-traditional risk factors, and unique clinical features,4-6 raises the question of whether the current taxonomy for AMI will adequately characterize phenotypes of AMI in this population. Specifically, while all patients with AMI, by definition, have abnormal biomarkers and either an electrocardiogram or history consistent with ischemia, there is substantial variation in clinical presentation and mechanisms of injury in young women compared with similarly aged men, with important implications for clinical care and research.7, 8
Current classification schemes for AMI consider a diversity of disease mechanisms and clinical settings. The Global Myocardial Infarction (MI) Taskforce released the Third Universal Definition of MI in 2012, an updated classification system categorizing AMIs into 5 distinct types based on pathological and clinical differences, with the aims of facilitating immediate treatment strategies and informing prognosis.9 Yet, some presentations that are likely more prevalent in younger women do not fit into this widely accepted taxonomy.7, 10 For example, there is no category for patients with minimal or no coronary artery disease (CAD) who do not have an identifiable mechanism for increased myocardial oxygen demand or decreased myocardial oxygen supply. Furthermore, patients with different mechanisms (e.g., vasospasm and anemia), degrees of coronary atherosclerosis (e.g., non-obstructive versus obstructive) and myocardial function (e.g., abnormal versus preserved function) are classified together, such that groups in the current classification are likely to include patients with substantial phenotypic heterogeneity.7, 9
A more nuanced taxonomy that distinguishes these and other clinical phenotypes could be useful in comparing disease course and outcomes among similar AMI phenotypes, in guiding new therapies, and individualizing prognosis. While critically important for all patients with AMI, such a tool would be particularly important for young women with AMI – a population in which epicardial CAD and the classic mechanism of plaque rupture may be less common.6 Accordingly, we sought to develop a taxonomy that would be more useful in classifying young women with AMI by assessing the clinical presentations of young adults with a diagnosis of AMI, and examining whether there are sub-classes or new classes of phenotypes beyond those classified by the Third Universal Definition of MI.
Methods
Data source
Data were drawn from the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.11 VIRGO is a prospective cohort study that compared women (n=2,009) and men (n=976) with AMI. Eligible patients were aged 18-55 years and met criteria for AMI, defined as the elevation of cardiac biomarkers plus evidence of acute myocardial ischemia, including at least 1 of the following: symptoms of ischemia; electrocardiographic changes indicative of new ischemia (new ST-T changes; new or presumably new left bundle-branch block; or the development of pathological Q waves); or other evidence of myocardial necrosis (imaging, pathology). Importantly, patients had to be given a diagnosis of AMI by their primary team at the time of enrollment, so as to avoid discordance between the team's diagnosis (and the patient's understanding of the illness) and the study aims. For example, patients deemed to have Takotsubo cardiomyopathy (clinically determined by study sites) were considered not to have had an AMI and were excluded. As part of the protocol, medical records from the index hospitalization were sent to the Coordinating Center on a continuous basis. These records included admission and discharge summaries, the presenting or qualifying electrocardiograms, catheterization reports, and echocardiography reports. Additionally, trained site coordinators abstracted clinical information from the medical record using standardized definitions.
Approach to taxonomy development
We used an inductive approach consistent with accepted methods for building taxonomies from multifaceted, complex clinical information but with common conceptual domains and dimensions.12, 13 This approach was best suited for the goals of VIRGO for several reasons. First, we aimed to develop a taxonomy that could be used by clinicians and investigators to classify patients at the time of admission (or early in hospitalization) within the context of a current, standard evidence-based diagnostic and therapeutic evaluation. Hence, we did not consider information that was outside of routine management, such as novel genetic markers, or advanced imaging techniques. Second, we sought to build on the current understanding of myocardial ischemia and its clinical manifestations. For example, in this cohort of young adults with AMI, nearly all underwent a cardiac catheterization as part of the standard of care; thus, data on culprit lesions and coronary anatomy were readily available and acknowledged as clinically meaningful for inclusion. Third, the objective to explore a new taxonomy was motivated by the heterogeneity of AMI presentations among younger women as described in the literature.5, 7 Thus, an empiric, inductive approach had the particular advantage of potentially unveiling disease phenotypes previously not considered,14 which may be more relevant for young women with AMI. Fourth, we intended for the taxonomy to be used by clinical registries and other clinical studies; as such, we needed to define criteria that could easily be abstracted from the medical record by non-clinicians. Finally, we decided to include both women and men with AMI in the development of the taxonomy in order to capture the breadth of disease presentations, and to determine the extent to which sex was related to taxonomy categories.
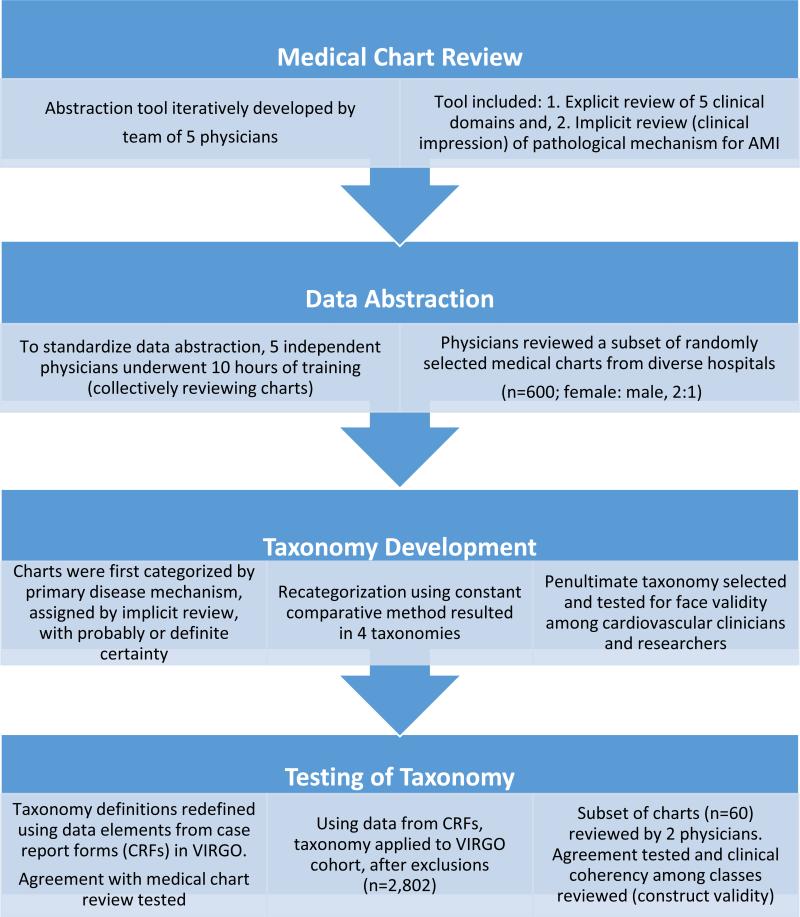
With this conceptual framework, we used a systematic, empirical approach to develop a novel taxonomy based on available clinical data from medical records during hospitalization for AMI (Figure 1). This process was conducted in 4 steps, described below.
Figure 1.
Four-step approach to taxonomy development.
1. Medical chart review
Through an iterative process, a team of 5 physicians (ESS, HMK, CPG, GD, BS), comprised of 2 cardiologists, 1 internist, and 2 emergency medicine physicians, developed an abstraction tool to enable both explicit and implicit medical chart review, methods commonly used to assess quality of clinical care.7, 15-17 The team identified conceptual codes (i.e., clinical domains) relevant to the diagnosis and treatment of AMI and established standard definitions and criteria for consistency (explicit review).18 Additionally, the tool was designed to accommodate the clinical judgment of the reviewer regarding disease mechanism and contributing comorbid states (implicit review).15, 16
The abstraction tool was iteratively developed in 3 cycles; the final tool was organized around the explicit review of clinical domains considered to be important for potential classification: (1) risk factors and clinical presentation; (2) electrocardiography; (3) angiographic findings; (4) left ventricular systolic function; and (5) magnitude of troponin elevation. Implicit review was operationalized as the reviewers’ impression of the primary and secondary pathophysiologic mechanisms underlying the AMI (e.g., plaque rupture, supply-demand mismatch (SDM), vasospasm) as well as the certainty of this adjudication: possible (hypothesized, no objective data); probable (≥1 supportive finding, but not definite); or definite (clear evidence).
2. Data abstraction
The review and development of a new classification system was based on a medical chart review of a subset of patients (n=600), randomly selected from different sites, preserving the 2:1 women to men ratio of enrollment. Four physician researchers used the abstraction tool described above to evaluate disease presentation (n= 150 charts each) – a sample size estimated to capture diversity in clinical presentation. As is standard with explicit review,15 physician reviewers participated in 10 hours of training, during which they collectively reviewed medical records selected for diversity in clinical presentation, to improve consistency in data interpretation and abstraction. Decision rules and operational definitions were refined to reduce ambiguity and facilitate standardized data abstraction. One author (ESS) re-reviewed 25% of the charts to ensure quality of data abstraction. Discrepancies were resolved during face-to-face meetings with all reviewers.
3. Taxonomy development: Constant comparative method and face validity
Charts were first classified based on the primary disease mechanism assigned with ‘probable’ or ‘definite’ certainty. We applied definitions from the Third Universal Definition of MI to categorize patients as: Type 1 (plaque rupture, ulceration, fissuring, erosion, dissection with resulting thrombus); Type 2 (condition other than CAD contributes to imbalance between myocardial oxygen supply or demand); and Type 4b (stent thrombosis). Other AMI Types 3 (cardiac death with symptoms suggesting ischemia), 4a (related to percutaneous coronary intervention (PCI)) and 5 (related to coronary artery bypass grafting (CABG)) were excluded from enrollment in VIRGO.
Given the heterogeneity among classification types and the high proportion of patients who remained unclassified (9%), we used the constant comparative method19, 20 to iteratively develop a new taxonomy. The primary author (ESS) presented 4 potential classification systems, based on the 5 clinical domains assessed with explicit review to 2 senior faculty cardiologists (HMK, FAM), both with extensive clinical and research experience, including guideline development for AMI.21 With their input, several clinical domains were refined and new classification strata emerged. For example, we considered several ways to distinguish the extent of CAD, including obstructive CAD: >50% versus >70%; and non-obstructive CAD: <50% versus <30% versus normal. Given the data source, i.e., catheterization reports as opposed to angiographic data from a core laboratory, there was variability in investigation (e.g., use of intravascular ultrasound) and reporting (e.g., threshold to describe luminal abnormalities). As such, we settled on a categorization germane to most clinicians and researchers: obstructive CAD (≥50%) versus non-obstructive CAD (<50%). As another example, with respect to left ventricular systolic function, we decided not to use this clinical domain given the variability in the timing of assessment during the hospitalization and inability to systematically compare myocardial function on presentation in all patients; this was consistent with our intention to use data in the manner that is routinely collected at the time of AMI. Non-traditional risk factors (e.g., autoimmune disease, emotional stress) and clinical presentation (e.g., triggers) did not distinguish angiographic findings or presumed mechanism of disease, and thus were not used for classification.
To assess face validity, a penultimate taxonomy was presented in 2 distinct venues: one was at a cardiology meeting for clinician scientists (n =20); the second was in a presentation at a scientific meeting, which received formal feedback from 2 senior faculty cardiologists assigned to provide feedback to presenters. Clinicians and researchers suggested the addition of a new taxonomy class to distinguish patients with unique but defined mechanisms of disease, e.g., spontaneous coronary artery dissection and vasospasm, in order to identify unique phenotypes for future investigation.
4. Testing of taxonomy: Reliability and construct validity
Consistent with the goal that the taxonomy be of use to both clinicians and researchers, we sought to assess the reliability of applying the taxonomy to the entire U.S. VIRGO population using data collected in the case report forms, typically abstracted by site coordinators who were not physicians and through patient interviews. We compared the agreement in classification using data abstracted by physician reviewers with data from the case report forms (n=598 out of 600; case report forms not available for 2 patients; agreement=86%). In cases of discordance (n=80; 14%), ESS reviewed the medical charts to resolve discrepancies. Most discrepancies were among charts reviewed early in the process, and involved data elements for which definitions had evolved to reflect new insights (e.g., restricting the criteria used to identify scenarios of potential myocardial oxygen SDM). In some cases (<5%), the case report form was incorrect; in these, the source data were corrected, though this is likely representative of the margin of error within clinical databases. Overall, we determined that the case report forms, with some additional review of catheterization reports, were sufficient for classifying patients.
The penultimate classification system was then applied to the entire VIRGO population in the U.S. (n=2,985), which ensured that all the charts would be in English, using data from the case report forms. Also, we excluded patients without cardiac catheterization (n=49), those missing medical records (n=3; 2 of whom had no cardiac catheterization), and patients who received fibrinolytic therapy because coronary anatomy in these patients could not be reliably distinguished (n=133). The final cohort was 2,802 patients of which 1,895 (67.6%) were women. This included the subset of 600 patients from which the taxonomy was developed. Including the full cohort in this analysis was necessary in order to fully characterize each class. To further assess construct validity, 10 cases from each taxonomy class were randomly selected for review. To finalize the taxonomy, 2 reviewers (ESS and KM) rated the type of AMI based on both the Universal Definition of MI classification system and the penultimate VIRGO classification system, assessing whether the taxonomy accommodated the distinct phenotypes commonly observed in young women, and whether the cases within each group were clinically coherent.
Analysis
We present the first-pass classification using the Third Universal Definition of MI, based on data abstracted from the subset of patients included in the medical chart review. We then present the classification of patients from the entire VIRGO cohort using the final VIRGO taxonomy based on data from the case report forms. In this cohort, we compare the distribution of women and men across the VIRGO taxonomy, and describe the baseline characteristics, clinical presentation and care management of patients in each VIRGO class. While not a primary aim of VIRGO, we also compare 1-year mortality among the VIRGO taxonomy classes and by gender. We provide case examples of each VIRGO class, comparing classification by the Third Universal Definition of MI with the new VIRGO taxonomy. VIRGO was approved by the Yale University Institutional Review Board, and all subjects provided informed consent.
Results
Classification by Third Universal Definition of MI
Applying the current classification system, 504 patients were classified as Type 1; 40 as Type 2; and 2 as Type 4b (Table 1). Among patients classified as Type 2 AMI, more than half had no significant CAD by cardiac catheterization. Additionally, patients with ‘probable’ or ‘definite’ disease mechanisms commonly described in young women with AMI, e.g., vasospasm (n=6); spontaneous coronary artery dissection (n=8); coronary embolism (n=2); and cocaine-related (n=2), were classified as either Type 1 or Type 2, as specified by the Universal Definition. Thrombophilia, as a risk factor, was present among patients with Types 1, 2 and unclassified AMI. We were unable to classify 54 patients, 51 of whom were women, who had no evidence of acute plaque disruption with thrombus or of myocardial oxygen SDM.
Table 1.
Taxonomy development: classification by the Third Global Definition of MI (chart review, n=600).
| Third Universal Definition of MI | Definition | Classification of VIRGO Patients (n) |
|---|---|---|
| Type I | Plaque rupture, ulceration, fissuring, erosion, dissection with resulting thrombus.* | 504 |
| Type 2 | Condition other than CAD contributes to imbalance between myocardial oxygen supply or demand.* | 40 |
| Type 3 | Cardiac death with symptoms suggesting ischemia | Excluded |
| Type 4a | Related to percutaneous coronary intervention | Excluded |
| Type 4b | Stent thrombosis | 2 |
| Type 5 | Related to coronary artery bypass graft | Excluded |
| Unclassified | 54* |
51 of the 54 unclassified patients were women.
MI, myocardial infarction; VIRGO, Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients
Final VIRGO taxonomy
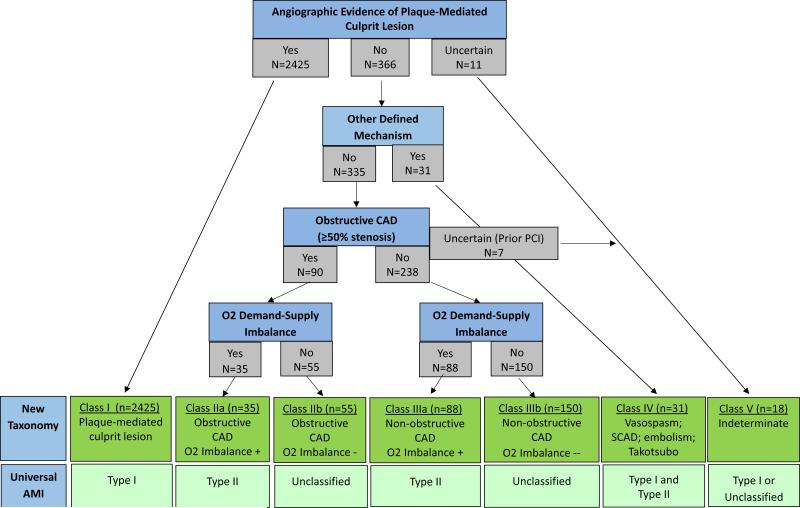
The algorithm for determining classification is presented in Figure 2. The final classification system (Figure 3) used data from the cardiac catheterization reports as well as clinical evidence supporting a scenario of increased myocardial oxygen demand or decreased myocardial oxygen supply. Data from the other 3 clinical domains (electrocardiogram, left ventricular systolic function and magnitude of troponin elevation) did not result in meaningful distinction of clinical phenotypes and were thus not included in the classification system.
Figure 2.
Algorithm used to determine the VIRGO classification system.
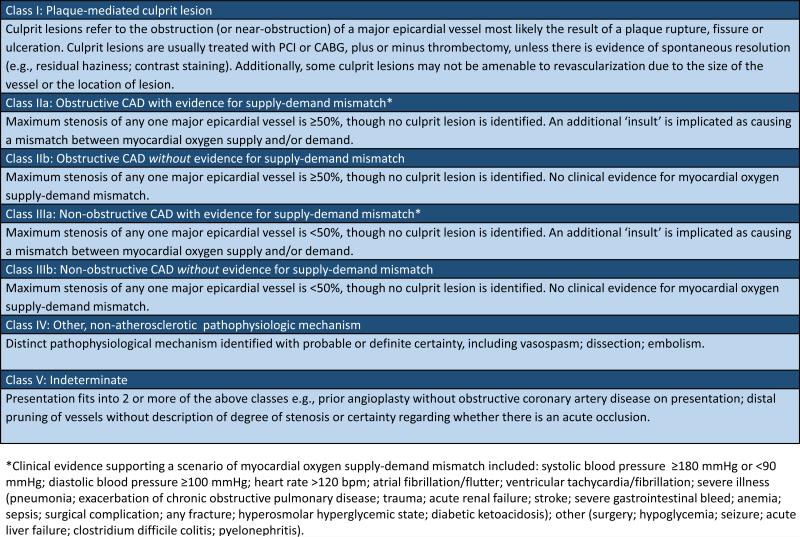
Figure 3.
VIRGO classification system.
Class I, plaque-mediated culprit lesion, refers to patients with angiographic evidence of either (a) thrombosis from plaque rupture, ulceration or fissure, or (b) critical plaque stenosis resulting in the receipt of or referral for PCI or CABG. Additionally, cases in which the clinical team reported ‘probable’ evidence for a culprit lesion (e.g., hazy lesions, slow-flow, contrast staining) were included in this class, despite some clinical uncertainty about the mechanism. Intravascular ultrasound was performed in 5 cases, of which 2 supported a diagnosis of plaque rupture, 1 supported a diagnosis of dissection, 1 revealed soft plaque, and the other was unremarkable. Consistent with clinician and researcher feedback, and in contrast with the Third Universal Definition of MI, patients with dissection were distinguished in a separate category.
Patients without evidence of a plaque-mediated culprit lesion were classified by whether there was another mechanism for the AMI identified with probable or definite certainty, and if not, by (1) the extent of coronary artery stenosis and, (2) whether there was supporting evidence for ischemia due to myocardial oxygen SDM. We considered the maximal degree of stenosis in any 1 epicardial artery, classifying patients as Class II if they had evidence of obstructive CAD (≥50% stenosis). Patients with non-obstructive CAD were classified as Class III. In Class III, patients either had no evidence of epicardial disease, luminal irregularities, or maximal luminal stenosis of <50%. Patients with isolated terminal branch disease, which was not determined to be the culprit and which was not quantified (e.g., disease of very small, distal branches), were also included in Class III.
Clinical evidence for myocardial oxygen SDM was used to sub-classify patients with Class II or Class III AMI into ‘a’ and ‘b.’ Patients in Class IIa or IIIa had evidence of either (a) increased myocardial oxygen demand, defined as systolic blood pressure ≥180mmg; diastolic blood pressure ≥100 mmHg; heart rate ≥120 bpm; atrial fibrillation or flutter, irrespective of heart rate; ventricular tachycardia or fibrillation; acute, life-threatening comorbid illness on presentation (e.g., severe pneumonia or exacerbation of chronic obstructive pulmonary disease; trauma; acute renal failure; stroke; sepsis; surgical complication; any fracture; diabetes ketoacidosis/hyperosmolar hyperglycemia syndrome/hypoglycemia; seizure; acute liver failure; and other acute, severe infections); or of (b) decreased myocardial oxygen supply, defined as systolic blood pressure <90 mmHg; hemoglobin <10 mg/dl; hypoxia; gastrointestinal bleed and other major bleeding. Patients without evidence for myocardial oxygen SDM were classified as either Class IIb or Class IIIb, depending on the degree of coronary artery stenosis.
Patients with other ‘probable’ or ‘definite’ pathophysiological mechanisms were categorized as Class IV. This class includes patients with angiographic evidence of spontaneous coronary artery dissection as well as patients with angiographic evidence of vasospasm, defined as non-catheter induced tubular stenosis that was responsive to intra-coronary vasodilators. Although vasospasm was often suspected despite a lack of response to vasodilators, in no cases was a pharmacological challenge with ergonovine or acetylcholine performed. Patients with AMI due to coronary embolism were also included as Class IV.
Finally, there were several cases that could not be reliably classified, rated as fitting >1 class; these were categorized as Class V – ‘Indeterminate.’ This class included patients with prior PCI but in whom no culprit lesion could be identified and in which there was no evidence of significant in-stent restenosis or other obstructive lesions. Also included in this class were patients with distal ‘pruning’ and/or ambiguous disease of ≥1 epicardial arteries as the only angiographic abnormality. These lesions were considered by the clinical teams to represent a possible culprit lesion with distal occlusion or small diseased arteries, possibly secondary to vasculopathy (n=3). Such cases were distinguished from other cases in which the clinical team identified a specific culprit, usually described as an occluded small, distal artery, not amenable to revascularization (Class I).
Sex differences, clinical characteristics and 1-year mortality
The baseline characteristics, clinical presentation, and care management for each VIRGO class are described in Supplemental Table 1. In comparing distributions by sex, 82.5% of women were classified as Class I compared with 94.9% of men. Among the other classes, the following sex distributions were observed: Class 2a: 1.4% women vs 0.9% men; Class 2b: 2.4% women vs 1.1% men; Class 3a: 4.3% women vs 0.8% men; Class 3b: 7.0% women vs 1.9% men; Class 4: 1.5% women vs 0.2% men; and Class 5: 0.8% women vs 0.2% men. The mean age ranged from 43.4 (Class IV) to 47.8 years (Class IIa). The race of patients varied between classes, with more Black/African American patients in the non-classic AMI groups (Classes II-V, except IIb) compared with Class I. The greatest proportion of Black/African American patients was in Class IIa. Otherwise, the findings were most notable for a lack of distinguishing clinical characteristics. For example, ST-segment elevation myocardial infarction accounted for 71-88% of AMIs in Classes II-V. A reduced ejection fraction was common among all classes, and though the mean troponin elevation was highest among Classes I and IV (classes with plaque-mediated and other culprit lesions), standard deviations around peak troponin for the other classes were large, signifying wide variation. The most common presenting symptom among all classes was typical chest pain. Similarly, triggers such as physical and mental stress were common among all classes, with cocaine and other illicit drugs accounting for a small proportion of AMI triggers. Cardiovascular risk factors were common among all classes, with patients in Class IIa (obstructive CAD with SDM) assuming the greatest burden of risk factors. Despite being a young population, prior AMI was common: 16% of patients in Class I; 44% of patients with obstructive CAD (Classes IIa and IIb); 10% of patients with non-obstructive CAD (Classes IIIa and IIIb); and 12% of patients in Class IV. Other non-cardiac diseases were common among all classes, including chronic renal failure (substantially more common among Class IIa); lung disease; cancer; depression; and venous thromboembolism. A history of autoimmune disease was noted in ~3-6% of Classes I and III-V, though was not observed at all among patients in Classes IIa and IIb.
One-year mortality was 2.3%. Of the 60 deaths, 47 (78%) occurred in women (75.9% of the population). There were 2 or fewer deaths in each of the other classes, all of them in women.
Final taxonomy and case examples
To assess the utility of the final taxonomy, we randomly selected cases from each VIRGO class (n=60) for review; a description of these cases, along with the adjudicated types of AMI using the Third Universal Definition of MI and the VIRGO classification systems, is included in the Appendix. Concordance between the 2 raters (ESS and KM) was 91%. Review of discordant cases revealed discrepancies in whether to classify patients as having a culprit lesion; these included cases with moderate angiographic coronary artery stenosis but electrocardiographic and echocardiographic findings that corresponded with the lesion, as well as small branch or distal artery disease that were ‘potentially’ occluded (n=4). In such cases, final adjudication was deferred to the judgment of the clinical team, and the certainty it ascribed to the lesion being the culprit. An additional 2 cases were identified as having possible Takotsubo cardiomyopathy. These patients were not distinguished since there was uncertainty as to the diagnosis and since other cases of Takotsubo cardiomyopathy had been excluded at the time of enrollment. Nevertheless, we recognize the occurrence of this phenomena and propose that they be included in Class IV, with other discrete pathophysiologic mechanisms.
Discussion
This study presents a new taxonomy for classifying diverse phenotypic presentations of young women with AMI. The taxonomy was developed empirically and considers the mechanism of infarction using clinical data routinely available at the time of presentation, in the context of a current, evidence-based diagnostic and therapeutic evaluation. As such, it is pragmatic for use in the clinical setting and can easily be derived for use with existing registries. Specifically, the taxonomy can act as a framework for understanding risk factors and pathophysiology, investigating the efficacy of current treatment strategies, and assessing outcomes among young women with AMI, a population for which there is limited data.22
While potentially applicable to all populations with AMI, the taxonomy was specifically developed for young women, a population in which the traditional mechanism of plaque rupture and thrombosis is less often detected.6 The methodological approach for taxonomy development utilized an inductive analysis to allow for the emergence of novel phenotypes that are not captured in the current classification system, and which may be overlooked, especially in patients without traditional risk factors. This methodology is well established in the literature to evaluate medical records for quality and patient safety.17, 23 For this study, we used a method known as structured implicit review to capture key clinical domains and clinical impressions; structured implicit review increases both face and construct validity and improves clinical utility.15-17, 24, 25 The final VIRGO taxonomy was derived using the constant comparative method, with frequent tests of face and construct validity, resulting in a classification system that can accommodate diverse phenotypes and serve as a basis for clinical practice and new discoveries.
The VIRGO taxonomy reflects the uncertainty that remains in identifying disease mechanism, reveals new insights into disease patterns, and challenges the generally accepted constructs for classifying AMI. The taxonomy adds to the formative work of the Third Global MI Taskforce.9 This classification system distinguished thrombus-mediated events from ischemic imbalance, as well as AMI related to revascularization and cardiac arrest. In applying that classification system to VIRGO patients, approximately 1 in 8 young women remained unclassified, and there was substantial heterogeneity in findings on cardiac catheterization among patients with Type 2 AMI, SDM. Patients who could not be classified using the Third Universal Definition of MI had no identifiable thrombus or culprit coronary lesion, or evidence of myocardial oxygen SDM. Moreover, we did not observe clinically apparent instances of isolated myocardial injury (e.g., myocardial contusion; myopericarditis) or other secondary mechanisms of troponin elevation (e.g., pulmonary embolism; congestive heart failure) frequently encountered in the clinical setting, though excluded from a prospective study based on a clinical diagnosis of AMI. As a consequence, even among patients with a classic presentation for myocardial ischemia, a substantial proportion do not fall into the current classification system, limiting our ability to effectively manage, study, and learn from patients who share similar phenotypic characteristics though the mechanism for their AMI remains unknown.
We attempted to determine the types and significance of alternate mechanisms for infarction in this population. We anticipated finding several cases of vasospasm, spontaneous coronary artery dissection, and coronary embolism, commonly implicated in young women. However, these clinical entities represented a very small proportion of AMI cases, which may reflect underuse of advanced diagnostic strategies, an inability to diagnose these clinical events during catheterization, or an overestimation of their occurrence in this population. Among a cohort of women with AMI from a single institution and with no evidence of obstructive CAD, intravascular ultrasound revealed a recent plaque rupture in 38%.26 It is unknown whether increased use of intravascular ultrasound among patients enrolled in VIRGO would have resulted in reclassification. There may also have been a bias not to screen patients with non-traditional findings for enrollment in a study of AMI. Moreover, the diagnosis of AMI may have been missed in women presenting with acute coronary syndrome who had troponin testing with conventional assays (as opposed to high-sensitivity assays) using thresholds for the diagnosis of AMI that do not account for patient sex.27 While we lacked data to evaluate troponin assays, women who have an AMI that is not detected with traditional biomarker assays may represent other phenotypes of AMI.
We also found that, as a distinct entity, myocardial oxygen SDM did not distinguish clinical presentation or coronary anatomy, raising the question of whether non-atherosclerotic epicardial disease, microvascular disease, or another mechanism made patients vulnerable to the effects of an ischemic imbalance; these concepts are supported in the literature, though may depart from clinical practice.28 For example, among classes with ischemic imbalance (Classes IIa and IIIa), 74% and 30% of patients received dual anti-platelet therapy, respectively, suggesting a lack of confidence in the mechanism of SDM.
The VIRGO taxonomy can be used as a construct to identify patterns in clinical presentation, while investigating differences in disease mechanism, therapeutic response, and prognosis of patients with AMI. Although the clinical significance of distinguishing coronary anatomy in this group of patients remains unknown, this classification system can facilitate research and knowledge sharing. For example, in distinguishing patients with non-obstructive CAD and those in whom the mechanism for AMI is unknown, we can prospectively and retrospectively identify those who may most benefit from advanced diagnostic evaluations29 (e.g., intravascular ultrasound,26 coronary flow reserve,30 computerized tomographic angiography,31 cardiac magnetic resonance imaging,26 nuclear imaging32). Ultimately, a more comprehensive classification system that distinguishes patients with shared phenotypes could facilitate the identification of patients for research and could inform sex-specific guideline recommendations for diagnosis and treatment.
There are some limitations to this study. First, our findings may underestimate the heterogeneity of disease presentations in this population. We analyzed charts of patients enrolled in a study about AMI. As such, if the diagnosis or interpretation of findings were ambiguous, despite meeting criteria for AMI, patients may not have been enrolled in the study. This approach is supported by the latest update to the Universal Definition of AMI, which also does not include AMIs as the result of non-ischemic myocardial injury. However, such clinical entities may exist on a spectrum, and it is not clear that we can reliably distinguish non-ischemic AMI. While we identified substantial heterogeneity in clinical presentation and disease pathology, it would be useful to apply the taxonomy in a more heterogeneous population, which would provide more information about the proportions of AMI classes by gender. A related limitation is that the number of patients in some of the groupings is small. For this reason, we did not test differences in patient characteristics and clinical outcomes between classes. Second, despite developing an iterative process for data abstraction and interpretation, the assignment of a primary mechanism of AMI may be subjective. For example, the assumption that revascularization with PCI or CABG during the time of AMI is consistent with a culprit lesion may not be judged similarly across clinicians. To address this, we deferred clinical judgment to the primary management team and considered only chart-reviewed diagnoses of probable or definite certainty for classification. Additionally, cases that could not be fit exclusively into one class or those involving differences in the interpretation of clinical data were classified as ‘indeterminate.’ Third, we utilized an inductive approach to identify clinical phenotypes using available clinical data. A potential tradeoff is that such a taxonomy is rendered outdated as our understanding of genetic risk and disease mechanisms advance, and as new technologies for disease detection are incorporated into clinical practice. However, we consider this a strength of our approach, as the VIRGO taxonomy can serve as a foundation for further investigations that may be used to refine the classification system. Fourth, this taxonomy was developed among a cohort of young patients with AMI; it is not yet known whether the taxonomy will be applicable to other populations, e.g., older patients, whose clinical presentation may also not fully be captured by the current classification system for AMI. Finally, the full value of this new taxonomy for classifying AMI among young women will depend on whether it is used to inform future scientific investigations and clinical management aimed at improving outcomes for patients.
A new taxonomy for AMI would allow for the identification and grouping of patients previously not captured by our current classification system. The taxonomy could and should be updated as new phenotypes emerge; however the basic framework would allow us to use clinical data available at the time of admission, as well as clinical trials and registries, to find these phenotypes and ultimately to determine optimal treatment strategies, prognosis, and outcomes. These efforts are particularly important for young women, a large proportion of whom do not fit into the traditional classification system.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Spatz was funded by grant 59973 from the Robert Wood Johnson Foundation Clinical Scholars Program in Princeton, NJ during the time the work was conducted. This work was funded by grant R01 HL081153 (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients [VIRGO]) and Dr. Krumholz is funded by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University), both from the National Heart, Lung, and Blood Institute in Bethesda, MD.
Footnotes
Conflict of Interest Disclosures: Dr. Curry is the recipient of a research grant, through Yale University, from The Medicines Company. Dr. Masoudi, as Senior Medical Officer of the National Cardiovascular Data Registry®, reports a contract with the American College of Cardiology. Drs. Gross and Krumholz are recipients of research agreements from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing. Dr. Krumholz chairs a cardiac scientific advisory board for UnitedHealth. Dr. Gross receives research funding from 21st Century Oncology. Dr. Safdar is the recipient of a research grant, through Yale University, from Gilead Sciences. The other authors report no potential conflicts of interest.
References
- 1.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochman JS, McCabe CH, Stone PH, Becker RC, Cannon CP, DeFeo-Fraulini T, Thompson B, Steingart R, Knatterud G, Braunwald E. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1997;30:141–148. doi: 10.1016/s0735-1097(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 5.Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155:375–381. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and non-obstructive coronary arteries (MINOCA). Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 7.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142:786–791. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 8.Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167:276–281. doi: 10.1001/archinte.167.3.276. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Kerensky RA, Wade M, Deedwania P, Boden WE, Pepine CJ. Revisiting the culprit lesion in non–Q-wave myocardial infarction. Results from the VANQWISH trial angiographic core laboratory. J Am Coll Cardiol. 2002;39:1456–1463. doi: 10.1016/s0735-1097(02)01770-9. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119:1442–1452. doi: 10.1161/CIRCULATIONAHA.107.742775. [DOI] [PubMed] [Google Scholar]
- 13.Patton MQ. Qualitative Research and Evaluation Methods. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 14.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758–1772. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein LV, Kahn KL, Harrison ER, Sherwood MJ, Rogers WH, Brook RH. Structured implict review of the medical record: a method for measuring the quality of inhospital medical care and a summary of quality changes following implementation of the Medicare prospective payment system. RAND; Santa Monica: 1991. [Google Scholar]
- 16.Hutchinson A, Coster JE, Cooper KL, McIntosh A, Bath P, Walters SJ, Pearson M. Creating and designing the healthcare experience. The International Ergonomics Association; 2008. Implicit and explicit case note review: two sides of the same coin? [Google Scholar]
- 17.Ashton CM, Kuykendall DH, Johnson ML, Wray NP. An empirical assessment of the validity of explicit and implicit process-of-care criteria for quality assessment. Med Care. 1999;37:798–808. doi: 10.1097/00005650-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Health Care Policy and Research Using clinical practice guidelines to evaluate quality of care. 1995:2. [Google Scholar]
- 19.Strauss AL, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage Publications; Thousand Oaks, CA: 1998. [Google Scholar]
- 20.Glaser BG, Strauss AL. The Discovery of Grounded Research: Strategies for Qualitative Research. Aldine De Gruyter; New York: 1967. [Google Scholar]
- 21.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 22.Pilote L, Karp I. GENESIS-PRAXY (GENdEr and Sex determInantS of cardiovascular disease: From bench to beyond-Premature Acute Coronary SYndrome). Am Heart J. 2012;163:741–746. e742. doi: 10.1016/j.ahj.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Dovey SM, Meyers DS, Phillips RL, Jr., Green LA, Fryer GE, Galliher JM, Kappus J, Grob P. A preliminary taxonomy of medical errors in family practice. Qual Saf Health Care. 2002;11:233–238. doi: 10.1136/qhc.11.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayward RA, McMahon LF, Jr., Bernard AM. Evaluating the care of general medicine inpatients: how good is implicit review? Ann Intern Med. 1993;118:550–556. doi: 10.7326/0003-4819-118-7-199304010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286:415–420. doi: 10.1001/jama.286.4.415. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, Collinson PO, Apple FS, Gray AJ, Fox KA, Newby DE, Mills NL. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoval Y, Smith SW, Thordsen SE, Apple FS. Supply/demand type 2 myocardial infarction: should we be paying more attention? J Am Coll Cardiol. 2014;63:2079–2087. doi: 10.1016/j.jacc.2014.02.541. [DOI] [PubMed] [Google Scholar]
- 29.Della Rocca DG, Pepine CJ. What causes myocardial infarction in women without obstructive coronary artery disease? Circulation. 2011;124:1404–1406. doi: 10.1161/CIRCULATIONAHA.111.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw LJ, Min JK, Narula J, Lin F, Bairey-Merz CN, Callister TQ, Berman DS. Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and nonobstructive coronary artery disease: an exploratory analysis. Circ Cardiovasc Imaging. 2010;3:473–481. doi: 10.1161/CIRCIMAGING.109.860981. [DOI] [PubMed] [Google Scholar]
- 32.Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, Brink I, Zhang X- L, Kreissl M, Magosaki N, Just H, Solzbach U. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45:1505–1512. doi: 10.1016/j.jacc.2005.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.