Abstract
Background
In 10% to 15% of individuals, inflammatory bowel disease (IBD) is difficult to classify as ulcerative colitis (UC) or Crohn’s disease (CD). Previous work has demonstrated that probe-based elastic scattering spectroscopy (ESS) can produce spectra, informed by parameters like tissue ultrastructure and hemoglobin content, capable of differentiating pathologies. This study investigates whether ESS is an in vivo optical biomarker for the presence, activity, and type of IBD in the colon.
Methods
Pilot study, a retrospective data analysis. ESS spectra of endoscopically normal and inflamed colon were obtained from 48 patients with IBD and 46 non-IBD controls. Measurements from patients with IBD were categorized as CD or UC based on clinical diagnosis. Spectra were analyzed using high-dimensional methods. Leave-one-patient-out cross-validation was used to obtain diagnostic performance estimates.
Results
Patients with IBD were distinguishable from non-IBD controls with a sensitivity of 0.93 and specificity of 0.91 based on readings from endoscopically normal mucosa, and 0.94 and 0.93 from inflamed mucosa. In patients with IBD, histologically normal and inflamed colon were distinguishable with per-class accuracies of 0.83 and 0.89, respectively; histologically normal from inactive inflammation with accuracies of 0.73 and 0.89, respectively; and inactive from active colitis with accuracies of 0.87 and 0.84, respectively. The diagnosis of CD versus UC was made with per-class accuracies of 0.92 and 0.87 in normal and 0.87 and 0.85 in inflamed mucosa, respectively.
Conclusions
ESS, a simple, low-cost clinically friendly optical biopsy modality, has the potential to enhance the endoscopic assessment of IBD and its activity in real time and may help to distinguish CD from UC.
Keywords: spectrum analysis, spectroscopy, endoscopy, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, biomarker
Inflammatory bowel disease (IBD) is a spectrum of disorders that leads to inflammation of the intestinal mucosa, classified clinically as ulcerative colitis (UC) or Crohn’s disease (CD). UC involves the large intestine and causes inflammation in a continuous pattern from the rectum to the cecum. CD, however, can involve the large or small intestines, generally spares the rectum, and can lead to fistulas, abscesses, and/or strictures. In the United States, it is estimated that between 1 million and 1.5 million people have IBD.1–3 There is no gold standard for classifying IBD as UC or CD, although this is crucial for prognostic and therapeutic reasons. In the majority of cases, UC can be distinguished from CD using clinical features, laboratory testing, routine white light endoscopy with associated biopsy histopathology, and radiological imaging. However, in approximately 10% to 15% of patients with disease limited to the colon, a definitive diagnosis can be difficult.4 IBD restricted to the colon that cannot be further classified as CD or UC is termed as IBD unclassified (IBD-U).5 Laboratory markers, such as fecal markers and serological antibody testing, may be used to aid in the diagnosis of IBD,6,7 and, in cases of IBD-U, to help and distinguish between IBD subtypes and identify high-risk individuals, which may have treatment implications.8,9 Although serological biomarkers alone are not useful in diagnosing IBD,10,11 they may have an adjunctive role in cases of IBD-U and in stratifying those at high-risk for disease-related complications.
Optical spectroscopy has been suggested as a promising tool for the management of IBD.12–14 Fluorescence spectroscopy has been reported to differentiate normal colon from IBD ex vivo in murine models15 and to increase the detection of invisible flat intraepithelial neoplasia.16 Recently, Raman spectroscopy has been proposed as an optical biomarker for distinguishing CD from UC in vitro in ex vivo tissue samples from patients with IBD.17
Elastic scattering spectroscopy (ESS) and related reflectance spectroscopies have shown promise in vivo in the gastrointestinal tract for detecting neoplasia in the colon,18–22 dysplasia in the esophagus,21,23–27 and colitis and dysplasia in patients with IBD.18,19 ESS has also been used to distinguish pathologies in other epithelially lined hollow organs, such as the urinary bladder,28 and in cystic and solid tissues, including breast and associated lymph nodes,29,30 pancreas,31 and thyroid.32,33
ESS, mediated by application-specific fiberoptic probes with specialized optical geometries, is sensitive to the absorption spectra of major chromophores (e.g., oxy-/deoxy-hemoglobin) and, more importantly, to the scattering spectra related to micromorphological features of tissues that are in contact with the tip of the probe. ESS spectra derive from the wavelength-dependent optical scattering efficiency (and the effects of changes in the scattering angular probability) caused by optical index gradients because of cellular and subcellular structures. Thus, unlike Raman and fluorescence spectroscopy, ESS provides largely microstructural, not biochemical, information. As such, ESS is sensitive to structural features such as nuclear size, crowding, chromaticity, and chromatin granularity, as well as to mitochondrial and organellar size and density. Such features are, to varying degrees, components of a histopathological assessment. ESS, however, is a real-time, point-source measurement that senses these types of morphological changes semiquantitatively without actually rendering a microscopic image, per se. In addition, because of its inherent simplicity and miniaturizability, ESS is extremely low cost, clinically robust, and impacts procedure flow minimally, especially when integrated into standard endoscopic biopsy tools.21
In this article, we investigate whether ESS has utility as an in vivo real-time endoscopic reporter of disease activity and an optical biomarker for distinguishing CD from UC.
METHODS
Instrumentation
The ESS system and probes have been described previously.20,21 Briefly, the ESS optical biopsy forceps consists of 2 identical adjacent fibers with 200 μm cores (one for illumination and the other for detection) with a numerical aperture of 0.22 in air. The center-to-center separation between the fibers is approximately 250 μm. With this probe configuration, a tissue depth of approximately 350 μm and a tissue volume ≤0.2 mm3 is interrogated. Biopsy forceps were constructed with a hollow central channel extending into the space between the jaws (ESCO Medical Instruments, Stony Brook, NY) capable of accommodating a 0.470 mm outer diameter of a hypotube encasing our probe (Fig. 1). As in previously described ESS designs, the forceps is connected to the ESS system, which consists of a broadband (320–850 nm) light source (pulsed Xenon arc lamp, LS-1130-3; Perkin Elmer, Waltham, MA), built-in computer with custom software, spectrometer (S2000; Ocean Optics, Inc., Dunedin, FL), microcontroller board, and power supply, all housed in a clinically friendly, compact enclosure (Optimum Technologies, Inc., Southbridge, MA) (Fig. 2). Immediately before each procedure, the ESS forceps and spectrometer were calibrated for system response by measuring a reflectance standard (Spectralon; Labsphere Inc, North Sutton, NH), a spectrally flat, diffuse-reflector, enabling correction of total system response, which includes effects of spectral variations in the light source, spectrometer, fiber transmission, and fiber coupling. It should be noted that all the endoscopes used have a continuous wave light source (Olympus 100 series with EVIS EXERA I & II systems, Olympus America, Melville, NY) that does not interfere with ESS readings because background light is measured separately and subtracted from the ESS measurement and all measurements occurring in less than 200 milliseconds. This step is sufficient to eliminate interference from background illumination in all widely available endoscopes, most of which use continuous wave illumination light sources.
FIGURE 1.
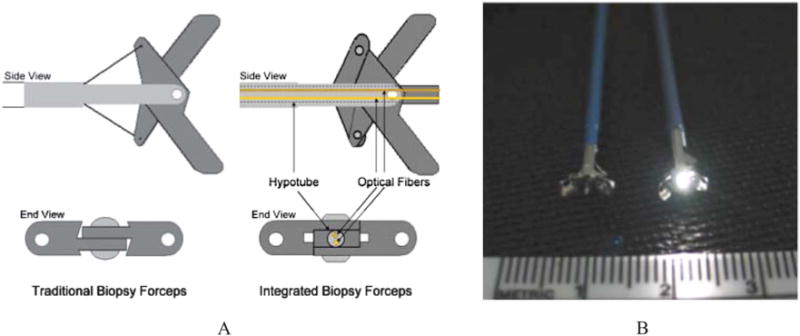
A 2-dimensional representation of the optical forceps tip is depicted in (A). The optical forceps is a modified traditional endoscopic jaw-type biopsy forceps (left) with a central channel through which our fiberoptic probe is introduced for tissue measurements while the jaws are held open (right). A photograph of a clinically usable unit (B) showing standard biopsy forceps (left) next to our ESS integrated optical forceps (right).
FIGURE 2.
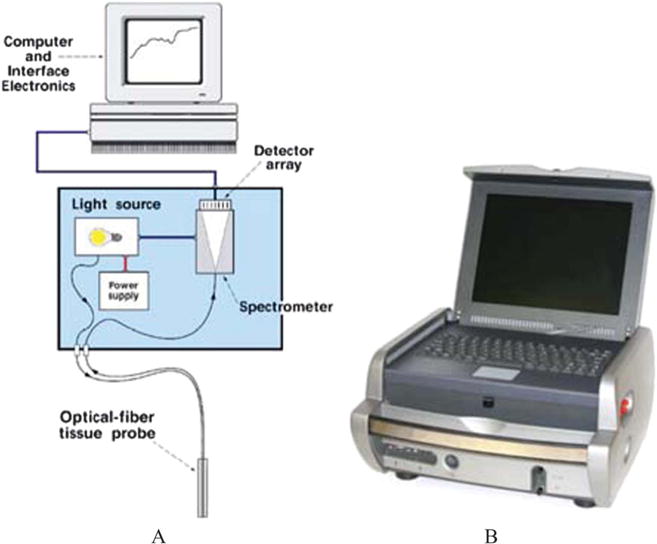
Schematic diagram of the ESS system (A) and a photograph of the clinical ESS optical biopsy box used for this study (B).
Clinical Measurements
Data collection was performed as part of IRB approved clinical studies at Boston Medical Center, Boston, MA. Patients used as controls were enrolled from our existing colorectal cancer screening pool and had no history or clinical diagnosis of IBD. Patients with IBD enrolled in the study were undergoing colonoscopy for clinical evaluation or surveillance of IBD. All endoscopic examinations and tissue sampling were clinically indicated for a given patient and were performed as scheduled by 1 of 3 operators (C.S.H., S.K.S., F.A.F.). In patients with IBD, a diagnosis of UC or CD was assigned based on clinical, radiographical, and histological information by an experienced IBD clinician (F.A.F.). In both control and IBD patients, noninvasive optical ESS readings of the mucosa were acquired by placing the optical forceps in gentle contact with the mucosa in question. Five ESS measurements were taken in rapid succession in at least 10 different random sites across all colonic segments. These ESS readings were labeled according to clinical diagnosis (control, CD, UC) for subsequent analysis. Patients with an indeterminate clinical IBD diagnosis were excluded from the analysis. In a subset of patients with IBD, several ESS readings were immediately followed by a pinch biopsy, using the optically integrated forceps and correlated to histology as assessed by two gastrointestinal pathologists (S.R.C., M.J.O.) and labeled as normal colon, inactive colitis, or active colitis. Data from 94 patients, including 46 controls and 48 patients with IBD (30 with CD, 18 with UC) were analyzed. Patient demographics are summarized in Table 1.
TABLE 1.
Patient Demographics
| Patient Demographics | |
|---|---|
| Non-IBD patients | 46 |
| Age, median (min–max), yrs | 56 (40–79) |
| Gender | |
| Male (%) | 24 (52) |
| Female (%) | 22 (48) |
| Race/ethnicity | |
| Caucasian (%) | 10 (22) |
| African American (%) | 21 (46) |
| Hispanic (%) | 10 (22) |
| Other (%) | 5 (10) |
| (+)IBD Patients | 48 |
| Age, median (min–max), yrs | |
| CD | 36 (19–67) |
| UC | 50 (18–80) |
| Gender | |
| CD | |
| Male (%) | 13 (43) |
| Female (%) | 17 (57) |
| UC | |
| Male (%) | 9 (50) |
| Female (%) | 9 (50) |
| Race/ethnicity | |
| CD | |
| Caucasian (%) | 25 (83) |
| African American (%) | 3 (10) |
| Hispanic (%) | 2 (7) |
| Other (%) | NA |
| UC | |
| Caucasian (%) | 14 (78) |
| African America (%) | 3 (17) |
| Hispanic (%) | NA |
| Other (%) | 1 (5) |
NA, not applicable.
Data Analysis
All ESS spectra were preprocessed before analysis, whereby the 5 measurements taken at each reading site were averaged, smoothed, and then cropped, resulting in spectra encompassing 126 wavelength bands from 330 to 760 nm. These spectra were then normalized to the intensity at 650 nm to enhance spectral shape, not relative intensities (Fig. 3). To classify measured spectra, we developed a diagnostic algorithm based on multidimensional pattern-recognition/machine learning. Given the high-dimensional nature of the data, we used a framework consisting of dimensionality reduction followed by classification. Principal component analysis was used for dimensionality reduction,34 followed by multidimensional classification using support vector machines.35,36 Leave-one-patient-out cross-validation was used to obtain classification performance estimates. We used sensitivity and specificity as a performance metric for tests involving classification between disease and nondisease classes and per-class accuracy to report performance of tests that distinguish disease states or subtypes. An algorithmic diagnosis for each individual was made based on all spectra from 1 subject, averaging the individual spectral classifications weighted by their distance from the decision boundary. Exact binomial confidence intervals of 95% are provided with the reported performance estimates.
FIGURE 3.
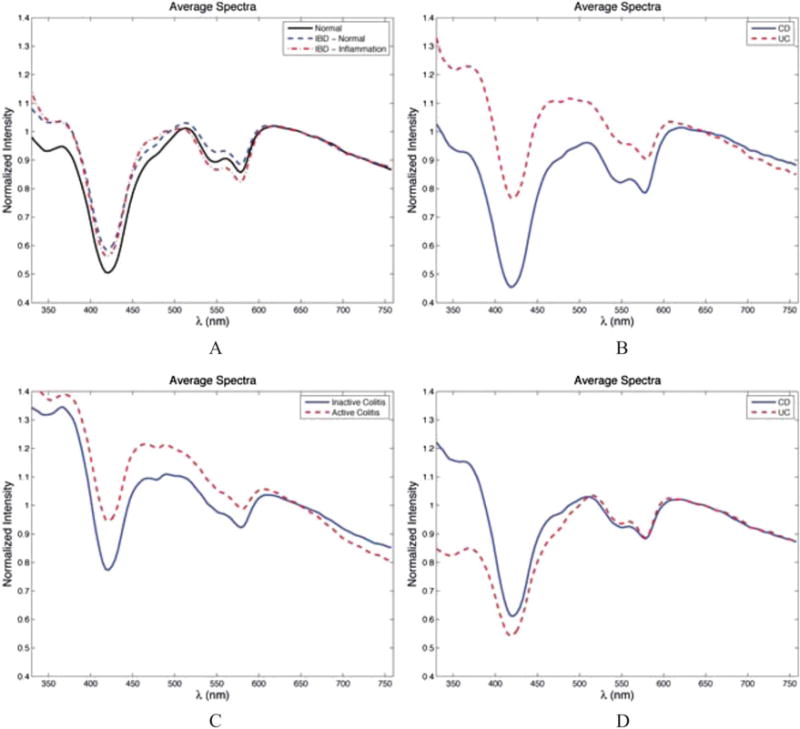
Representative ESS spectra for (A) non-IBD patients versus (+)IBD patients; (B) patients with CD versus UC, measurements from endoscopically inflamed mucosa in (+)IBD patients; (C) inactive versus active colitis in (+)IBD patients, measurements taken from areas of endoscopic inflammation; (D) CD versus UC, measurements from endoscopically normal mucosa in (+)IBD patients.
RESULTS
Our initial efforts were aimed at investigating whether ESS measurements could distinguish patients with and without IBD. As summarized in Table 2, we analyzed over 900 ESS spectra from 94 patients, encompassing 491 spectra from 46 controls (i.e., not diagnosed with IBD), and 490 spectra from 48 patients with IBD. ESS measurements of endoscopically inflamed mucosa were obtained from 36 of the 48 patients with IBD (181 spectra) and measurements of endoscopically normal mucosa from 41 of the 48 patients with IBD (309 spectra). Optical ESS readings from endoscopically normal appearing mucosa resulted in a sensitivity of 0.93 and specificity of 0.91 for distinguishing patients with IBD from those without IBD. As expected, endoscopic inflammation in patients with IBD was easily distinguishable from normal mucosa in non-IBD controls, yielding a sensitivity of 0.94 and a specificity of 0.93. When we limited the analysis solely to patients with IBD, not surprisingly, we found that spectra from endoscopically normal colon could be distinguished from endoscopic, visually inflamed mucosa with per-class accuracies of 0.83 and 0.89, respectively (Table 3).
TABLE 2.
Breakdown of ESS Mucosal Sites and Patients Used in the Analysis
| Patients | ESS Mucosal Sites | |
|---|---|---|
| Non-IBD controls | 46 | 491 |
| (+)IBD | 48 | 490 |
| (+)IBD, endoscopically inflamed mucosa | 36 | 181 |
| CD | 23 | 117 |
| UC | 13 | 64 |
| (+)IBD, endoscopically normal mucosa | 41 | 309 |
| CD | 26 | 192 |
| UC | 15 | 117 |
TABLE 3.
Classification Performance of ESS Comparing Endoscopic Mucosal Appearance in Non-IBD Controls and (+)IBD Patients
| Accuracy (95% CI) | |
|---|---|
| Non-IBD, endoscopically normal | 0.91 (0.79–0.98) |
| (+)IBD, endoscopically normal | 0.93 (0.80–0.98) |
| Non-IBD, endoscopically normal | 0.93 (0.82–0.99) |
| (+)IBD, endoscopically inflamed | 0.94 (0.81–0.99) |
| (+)IBD, endoscopically normal | 0.83 (0.68–0.93) |
| (+)IBD, endoscopically inflamed | 0.89 (0.74–0.97) |
Next, we analyzed data from a subset of patients with IBD (38 patients), where all ESS measurements were correlated with index histopathology. In this part of the study, we were interested in assessing the capability of ESS for detecting colitis and classifying inactive versus active inflammation. A total of 63 ESS measurements of distinct locations were found to be inactive inflammation by histopathology, 31 as active inflammation and 211 as normal colonic mucosa (Table 4). Inactive inflammation could be distinguished from active inflammation with per-class accuracies of 0.87 and 0.84, respectively. In addition, we found that normal colonic mucosa could be differentiated from inactive inflammation with per-class accuracies of 0.73 and 0.89, respectively and from active inflammation with per-class accuracies of 0.85 and 0.90, respectively, as summarized in Table 5.
TABLE 4.
Breakdown of Histopathology-correlated ESS Measurements in (+)IBD Patients (38 Patients in Total)
| Histology | ESS Mucosal Sites |
|---|---|
| Inactive colitis | 63 |
| Active colitis | 31 |
| Normal | 211 |
TABLE 5.
Classification Performance of ESS Measurements in (+)IBD Patients Correlated to Index Pathology
| Accuracy (95% CI) | |
|---|---|
| Inactive colitis | 0.87 (0.77–0.94) |
| Active colitis | 0.84 (0.66–0.93) |
| Inactive colitis | 0.89 (0.78–0.95) |
| Normal colon | 0.73 (0.66–0.79) |
| Active colitis | 0.90 (0.74–0.98) |
| Normal colon | 0.85 (0.79–0.89) |
Finally, we sought to assess whether ESS could serve as a biomarker for distinguishing CD from UC in patients with IBD. This analysis was initially performed using the ESS measurements of endoscopically inflamed mucosa collected from 36 of the 48 patients with IBD (Table 2). Measurements were grouped based on each patient’s clinical diagnosis of CD or UC. Using ESS measurements taken from areas of endoscopic inflammation, we were able to classify patients with CD (23 patients) with a per-class accuracy of 0.87 and patients with UC (13 patients) with a per-class accuracy of 0.85 (Table 6). Next, we restricted the subanalysis to ESS measurements from endoscopically normal colon, acquired from 41 of the 48 patients with IBD in our study. As seen in Table 6, as a result of this analysis, we obtained a per-class accuracy of 0.92 for classifying patients with CD (26 patients) and of 0.87 for patients with UC (15 patients).
TABLE 6.
Classification Performance in Differentiating CD from UC Based on ESS Measurements of Endoscopically Inflamed and Endoscopically Normal Mucosa of (+)IBD Patients
| Accuracy (95%, CI) | |
|---|---|
| Endoscopically inflamed mucosa | |
| CD | 0.87 (0.68–0.97) |
| UC | 0.85 (0.55–0.98) |
| Endoscopically normal mucosa | |
| CD | 0.92 (0.75–0.99) |
| UC | 0.87 (0.60–0.98) |
DISCUSSION
In this study, we sought to determine the feasibility of using ESS to identify the presence of IBD activity, with results suggesting that ESS may be sensitive to differences in the colonic mucosa of patients with IBD, independent of inflammation, compared with non-IBD controls. Furthermore, in patients with IBD, we sought to determine if particular spectral patterns could distinguish CD from UC. Our findings show that ESS may also serve as an optical biomarker that discriminates CD from UC. The data-driven methods used to analyze the spectral data relied on ESS measurements being associated with a corresponding “gold standard” for algorithm training and evaluation purposes. The assigned gold standard was obtained from the clinical diagnoses of IBD activity and subtype. As such, patients diagnosed as IBD-U were excluded from the analysis, given that their uncertain clinical diagnosis was not concordant with the goals of this pilot feasibility study.
Studies by other groups investigating factors associated with IBD activity and differences between CD and UC have produced outcomes that support our results and may lead to better understanding of the underlying factors that result in differences in ESS spectra. Riley et al found microscopic abnormalities in biopsies of endoscopically normal rectal mucosa in patients with IBD compared with a non-IBD control group. These abnormalities included a chronic inflammatory cell infiltrate, crypt architectural irregularities, epithelial surface breaches, acute inflammatory cell infiltrates, crypt abscesses, and mucin depletion.37 Allison et al38 described differences in the morphology and phenotypic expression of macrophage-like cells in normal colon in non-IBD controls versus the inflamed colon in IBD, with additional detectable differences between UC and Crohn’s colitis. Regarding the microvasculature, Danese et al39 described an intense process of inflammation-dependent angiogenesis that was not present in the histologically normal colon in both controls and patients with IBD. It has been suggested further that the colonic mucosa is always abnormal in CD even if macroscopic and histopathological examinations reveal an apparently normal mucosa.40,41 This was observed in rectal biopsies of patients with CD that spared the rectum. McCormick et al42 observed a significant difference in the mucosal gland content among patients with UC compared with both non-IBD controls and patients with CD. Finally, microbes are, in fact, microscale particles that can, in theory, affect scattering and absorption signatures as well. To this point, intramucosal bacteria have been found more frequently and in a wider distribution in patients with IBD compared with non-IBD, with the bacteria mostly found apposed to the epithelial surface and within the crypt lumen.43,44
The manner by which ESS distinguishes between these 2 forms of IBD, even in the setting of endoscopically normal mucosa, is not completely understood. The ESS spectrum itself is informed by the scattering and absorption properties of the mucosa,45–50 including nanoscale features.51,52 These changes are likely because of differences in morphology and/or function, which include micro- and nanoscale nuclear, organellar, and membrane changes, as well as differences in chromophore absorption because of microvascular structure, blood content, and/or blood flow. Because it relates to sensing IBD activity and subtypes with ESS, reported changes in cell infiltrates, crypt architecture, mucin content, macrophage morphology, microbiota, and angiogenesis could potentially affect the optical scattering and absorption properties of the mucosa. One or more of these changes could account for the differences observed in ESS measurements in endoscopically inflamed and normal mucosa of patients with IBD, especially when compared with non-IBD controls and may explain sensed differences in the measurements of CD and UC.
In summary, this study is the first to demonstrate that ESS may be an optical biomarker that can, at the time of colonoscopy, identify patients with IBD based on mucosal readings from both inflamed and normal appearing mucosa, and further classify inactive versus active colitis. Furthermore, ESS seems to discriminate accurately between UC and CD. Our overarching aim was to assess the feasibility of using ESS as an optical diagnostic technology in the setting of IBD, and we believe that feasibility and usability have been supported by these encouraging results. Nonetheless, our results should be observed in the context of a relatively small sample size and of the inherent limitations of the study design. A data-driven approach was used to retrospectively analyze collected ESS spectra and, as such, the approach will benefit from a larger cohort of patients, permitting algorithms to better learn and then test diagnostic patterns prospectively. In this context, additional studies will aim to build on the presently accrued number of subjects and move forward with a prospective study design, beyond the retrospective approach to the classification presently used. A future prospective study likely would include patients initially diagnosed as IBD-U and later reclassified as either CD or UC. In addition, inclusion of the terminal ileum may expand and improve the diagnostic capabilities of ESS in the setting of IBD. Ileal measurements were excluded from this study because this would introduce another epithelial subtype requiring another separate large ESS data set for training and evaluation, which would be beyond the scope of a pilot study. In addition, morphological and physiological factors associated with IBD including cell infiltrates, mucosal microstructure, and microbiota could be investigated to examine how each individually affects the ESS signal. This work would lead to a better understanding of the features that inform ESS optical diagnoses in IBD, customized instrumentation, expanded diagnostic algorithms, and improved performance. Additionally, associating spectral signatures with other clinical features like disease location, severity, fistulization, and treatment response may permit use of ESS as a risk stratification and/or prognostic tool.
In conclusion, our study indicates that ESS offers the potential to perform real-time classification of endoscopically apparent (and inapparent) IBD as normal mucosa, active colitis, or inactive colitis. Additionally, mucosal readings in patients with IBD, regardless of the presence of inflammation, seem to distinguish CD from UC, a feature of particular value as an additional biomarker in patients designated IBD-U. ESS has the potential to provide real-time, in situ assessment of the colonic mucosa and may prove to be an essential tool for the endoscopic diagnosis and classification of IBD. An optical diagnosis using ESS could serve as an adjunct to current diagnostic protocols and may provide additional information for the clarification of IBD-U.
Acknowledgments
Supported by VA CSR&D Merit Award (1I01CX000347), NIH/NCI (U54 CA10467), Wallace H. Coulter Foundation, and Center for the Integration of Medicine and Innovative Technology.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Loftus EV. The burden of inflammatory bowel disease in the United States: a moving target? Clin Gastroenterol Hepatol. 2007;5:1383–1384. doi: 10.1016/j.cgh.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–461. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
- 4.Price B. Overlap in the spectrum of non-specific inflammatory bowel disease—“colitis indeterminate”. J Clin Pathol. 1978;31:567–577. doi: 10.1136/jcp.31.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 7.von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 8.Quinton JF, Sendid B, Reumaux D, et al. Anti-saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joossens S, Reinisch W, Vermeire S, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–1247. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–1403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seow CH, Stempak JM, Xu W, et al. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am J Gastroenterol. 2009;104:1426–1434. doi: 10.1038/ajg.2009.79. [DOI] [PubMed] [Google Scholar]
- 12.Hommes DW, van Deventer SJH. Endoscopy in inflammatory bowel diseases. Gastroenterology. 2004;126:1561–1573. doi: 10.1053/j.gastro.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Leighton JA, Loftus EV. Evolving diagnostic modalities in inflammatory bowel disease. Curr Gastroenterol Rep. 2005;7:467–474. doi: 10.1007/s11894-005-0078-x. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Neurath MF, Mudter J. New endoscopic approaches in IBD. World J Gastroenterol. 2011;17:63–68. doi: 10.3748/wjg.v17.i1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri LP, Tumlinson AR, Wade NH, et al. Ex vivo optical coherence tomography and laser-induced fluorescence spectroscopy imaging of murine gastrointestinal tract. Comp Med. 2007;57:175–185. [PubMed] [Google Scholar]
- 16.Ortner MA, Fusco V, Ebert B, et al. Time-gated fluorescence spectroscopy improves endoscopic detection of low-grade dysplasia in ulcerative colitis. Gastrointest Endosc. 2010;71:312–318. doi: 10.1016/j.gie.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Bi X, Walsh A, Mahadevan-Jansen A, et al. Development of spectral markers for the discrimination of ulcerative colitis and Crohn’s disease using Raman spectroscopy. Dis Colon Rectum. 2011;54:48–53. doi: 10.1007/DCR.0b013e3181fcf68d. [DOI] [PubMed] [Google Scholar]
- 18.Dhar A, Johnson KS, Novelli MR, et al. Elastic scattering spectroscopy for the diagnosis of colonic lesions: initial results of a novel optical biopsy technique. Gastrointest Endosc. 2006;63:257–261. doi: 10.1016/j.gie.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Mourant JR, Bigio IJ, Boyer J, et al. Elastic scattering spectroscopy as a diagnostic tool for differentiating pathologies in the gastrointestinal tract: preliminary testing. J Biomed Opt. 1996;1:192–199. doi: 10.1117/12.231372. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Diaz E, Castanon DA, Singh SK, et al. Spectral classifier design with ensemble classifiers and misclassification-rejection: application to elastic-scattering spectroscopy for detection of colonic neoplasia. J Biomed Opt. 2011;16:67009. doi: 10.1117/1.3592488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Diaz E, Bigio IJ, Singh SK. Integrated optical tools for minimally invasive diagnosis and treatment at gastrointestinal endoscopy. Robot Comput Integr Manuf. 2011;27:249–256. doi: 10.1016/j.rcim.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zonios G, Perelman LT, Backman V, et al. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Appl Opt. 1999;38:6628–6637. doi: 10.1364/ao.38.006628. [DOI] [PubMed] [Google Scholar]
- 23.Amelink A, Haringsma J, Sterenborg HJ. Noninvasive measurement of oxygen saturation of the microvascular blood in Barrett’s dysplasia by use of optical spectroscopy. Gastrointest Endosc. 2009;70:1–6. doi: 10.1016/j.gie.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2001;120:1620–1629. doi: 10.1053/gast.2001.24842. [DOI] [PubMed] [Google Scholar]
- 25.Lovat L, Bown S. Elastic scattering spectroscopy for detection of dysplasia in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2004;14:507–517, ix. doi: 10.1016/j.giec.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Lovat LB, Johnson K, Mackenzie GD, et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett’s oesophagus. Gut. 2006;55:1078–1083. doi: 10.1136/gut.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Fearn T, Mackenzie G, et al. Elastic scattering spectroscopy for detection of cancer risk in Barrett’s esophagus: experimental and clinical validation of error removal by orthogonal subtraction for increasing accuracy. J Biomed Opt. 2009;14:044022. doi: 10.1117/1.3194291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourant JR, Bigio IJ, Boyer J, et al. Spectroscopic diagnosis of bladder cancer with elastic light scattering. Lasers Surg Med. 1995;17:350–357. doi: 10.1002/lsm.1900170403. [DOI] [PubMed] [Google Scholar]
- 29.Bigio IJ, Bown SG, Briggs G, et al. Diagnosis of breast cancer using elastic-scattering spectroscopy: preliminary clinical results. J Biomed Opt. 2000;5:221–228. doi: 10.1117/1.429990. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KS, Chicken DW, Pickard DC, et al. Elastic scattering spectroscopy for intraoperative determination of sentinel lymph node status in the breast. J Biomed Opt. 2004;9:1122–1128. doi: 10.1117/1.1802191. [DOI] [PubMed] [Google Scholar]
- 31.A’Amar OM, Liou L, Rodriguez-Diaz E, et al. Comparison of elastic scattering spectroscopy with histology in ex vivo prostate glands: potential application for optically guided biopsy and directed treatment. Lasers Med Sci. 2012;28:1323–1329. doi: 10.1007/s10103-012-1245-6. [DOI] [PubMed] [Google Scholar]
- 32.Rosen J, Suh H, Giordano N, et al. Preoperative discrimination of benign from malignant disease in thyroid nodules with indeterminate cytology using elastic light-scattering spectroscopy. IEEE Trans Biomed Eng. doi: 10.1109/TBME.2013.2267452. [published online ahead of print June 10 2013] [DOI] [PubMed] [Google Scholar]
- 33.Suh H, A’amar O, Rodriguez-Diaz E, et al. Elastic light-scattering spectroscopy for discrimination of benign from malignant disease in thyroid nodules. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 34.Duda RO, Hart PE, Stork DG. Pattern Classification. New York, NY: John Wiley & Sons; 2001. [Google Scholar]
- 35.Cortes C, Vapnik V. Support-vector networks. Machine Learning. 1995;20:273–297. [Google Scholar]
- 36.Vapnik VN. Statistical Learning Theory. New York, NY: John Wiley & Sons; 1998. [Google Scholar]
- 37.Riley A, Mani V, Goodman J, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison C, Cornwall S, Poulter W, et al. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988;29:1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Goodman MJ, Skinner JM, Truelove SC. Abnormalities in the apparently normal bowel mucosa in Crohn’s disease. Lancet. 1976;307:275–278. doi: 10.1016/s0140-6736(76)91404-5. [DOI] [PubMed] [Google Scholar]
- 41.Korelitz BI, Sommers SC. Rectal biopsy in patients with Crohn’s disease. JAMA. 1977;237:2742–2744. [PubMed] [Google Scholar]
- 42.McCormick A, Horton W, Mee S. Mucin depletion in inflammatory bowel disease. J Clin Pathol. 1990;43:143–146. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moussata D, Goetz M, Gloeckner A, et al. Confocal laser endomicroscopy is a new imaging modality for recognition of intramucosal bacteria in inflammatory bowel disease in vivo. Gut. 2011;60:26–33. doi: 10.1136/gut.2010.213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mylonaki M, Rayment NB, Rampton DS, et al. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:481–487. doi: 10.1097/01.mib.0000159663.62651.4f. [DOI] [PubMed] [Google Scholar]
- 45.Mourant JR, Boyer J, Hielscher AH, et al. Influence of the scattering phase function on light transport measurements in turbid media performed with small source-detector separations. Opt Lett. 1996;21:546–548. doi: 10.1364/ol.21.000546. [DOI] [PubMed] [Google Scholar]
- 46.Mourant JR, Fuselier T, Boyer J, et al. Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt. 1997;36:949–957. doi: 10.1364/ao.36.000949. [DOI] [PubMed] [Google Scholar]
- 47.Mourant JR, Bigio IJ, Jack DA, et al. Measuring absorption coefficients in small volumes of highly scattering media: source-detector separations for which path lengths do not depend on scattering properties. Appl Opt. 1997;36:5655–5661. doi: 10.1364/ao.36.005655. [DOI] [PubMed] [Google Scholar]
- 48.Mourant JR, Freyer JP, Hielscher AH, et al. Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics. Appl Opt. 1998;37:3586–3593. doi: 10.1364/ao.37.003586. [DOI] [PubMed] [Google Scholar]
- 49.Mourant JR, Hielscher AH, Eick AA, et al. Evidence of intrinsic differences in the light scattering properties of tumorigenic and nontumorigenic cells. Cancer. 1998;84:366–374. [PubMed] [Google Scholar]
- 50.Mourant JR, Canpolat M, Brocker C, et al. Light scattering from cells: the contribution of the nucleus and the effects of proliferative status. J Biomed Opt. 2000;5:131–137. doi: 10.1117/1.429979. [DOI] [PubMed] [Google Scholar]
- 51.Gomes AJ, Roy HK, Turzhitsky V, et al. Rectal mucosal microvascular blood supply increase is associated with colonic neoplasia. Clin Cancer Res. 2009;15:3110–3117. doi: 10.1158/1078-0432.CCR-08-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy HK, Turzhitsky V, Kim YL, et al. Spectral slope from the endoscopically-normal mucosa predicts concurrent colonic neoplasia: a pilot ex-vivo clinical study. Dis Colon Rectum. 2008;51:1381–1386. doi: 10.1007/s10350-008-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


