Abstract
Background
and objectives The Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 study demonstrated a significant beneficial effect of the vasopressin V2 receptor antagonist tolvaptan on rates of kidney growth and eGFR decline in autosomal dominant polycystic kidney disease (ADPKD). This post hoc analysis was performed to reassess the primary and secondary efficacy endpoints by CKD stage at baseline.
Design, setting, participants, & measurements
In a phase 3, multicenter, double-blind, placebo-controlled, 3-year trial, 1445 patients with ADPKD (age 18–50 years), with total kidney volume (TKV) ≥750 ml and estimated creatinine clearance ≥60 ml/min, were randomly assigned 2:1 to split-dose tolvaptan (45/15, 60/30, or 90/30 mg daily as tolerated) or placebo. The primary endpoint was annualized rate of TKV change. Secondary endpoints included a composite endpoint of time to multiple composite ADPKD-related events (worsening kidney function, kidney pain, hypertension, and albuminuria) and rate of kidney function decline.
Results
Tolvaptan reduced annualized TKV growth by 1.99%, 3.12%, and 2.61% per year (all P<0.001; subgroup–treatment interaction, P=0.17) and eGFR decline by 0.40 in CKD1 (P=0.23), 1.13 in CKD2 (P<0.001) and 1.66 ml/min per 1.73 m2 per year in CKD3 (P<0.001) with a trend for a positive subgroup-treatment interaction (P=0.07) across CKD1, CKD2 and CKD3. ADPKD-related events were less frequent in tolvaptan recipients than in placebo recipients among those with CKD1 (hazard ratio [HR], 0.83; 95% confidence interval [95% CI], 0.70–0.98; P=0.03) and those with CKD 3 (HR, 0.71; 95% CI, 0.57–0.89; P=0.003), but not among those with CKD2 (HR, 1.02; 95% CI, 0.85–1.21; P=0.86). Aquaresis-related adverse events (more frequent in the tolvaptan group) and ADPKD-related adverse events (more frequent in the placebo group) were not associated with CKD stage. Hypernatremia events in tolvaptan-treated patients with CKD3 and plasma aminotransferase elevations in tolvaptan-treated patients across CKD stages 1–3 occurred more frequently than in placebo recipients.
Conclusions
This post hoc analysis suggests clinically similar beneficial effects of tolvaptan in ADPKD across CKD stages 1–3.
Keywords: ADPKD; chronic kidney disease; albuminuria; glomerular filtration rate; humans; pain; polycystic kidney, autosomal dominant; receptors, vasopressin; renal insufficiency, chronic; tolvaptan
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic kidney disease and the fourth leading cause of ESRD in adults worldwide (1,2).
Studies in animal models implicated the antidiuretic hormone arginine vasopressin and its second messenger cAMP as promoters of kidney cyst cell proliferation and luminal fluid secretion (3). The suppression of vasopressin release by means of high water intake, genetic elimination of vasopressin, and vasopressin V2-receptor blockade all reduced cyst burden and protected kidney function in rodent models of the disease (4–12).
A randomized placebo-controlled clinical trial (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes [TEMPO] 3:4) in 1445 patients with ADPKD with an estimated creatinine clearance ≥60 mL=l/min and a total kidney volume (TKV) of ≥750 ml demonstrated a significant beneficial effect of the vasopressin V2 receptor antagonist tolvaptan on the rate of growth of TKV (−49%) and rate of eGFR decline (−26%) (13). Of the 1445 patients enrolled in this trial, 502 (35%), 689 (48%), and 248 (17%) of the 1445 patients had CKD stage 1 (CKD1), CKD stage 2 (CKD2), or CKD stage 3 (CKD3), respectively.
Tolvaptan was approved by the regulatory authorities in Japan (March 2014), Health Canada (February 2015), and the European Medicines Agency Committee for Medicinal Products for Human Use (May 2015) as a therapy for patients with ADPKD and CKD stages 1–3 with evidence of rapidly progressing disease (14,15). In the United States, the Food and Drug Administration (FDA) requested additional data to further evaluate the efficacy and safety of this drug in patients in later stages of ADPKD (16). Replicating Evidence of Preserved Renal Function: An Investigation of Tolvaptan Safety and Efficacy in ADPKD (NCT02160145), a large, multinational, placebo-controlled clinical trial to ascertain the efficacy and safety of tolvaptan in patients in CKD stages 2–4, is in progress.
The request for an additional trial by the FDA was in part due to concerns that, at more advanced stages of ADPKD, tolvaptan may lose pharmacodynamic efficacy and the destruction of renal parenchyma may reach a point of no return at which disease progression could occur by mechanisms not influenced by tolvaptan. Although these are legitimate concerns, other considerations may be reassuring. A recent study in patients with ADPKD who had a GFR >60, 30–60, and <30 ml/min per 1.73 m2 showed that the pharmacodynamic efficacy of tolvaptan is maintained in patients with advanced CKD, as reflected by a greater fractional free water clearance and a similar absolute change in TKV (17). Several experimental and clinical studies in various kidney diseases, hypertension, diabetes, and the metabolic syndrome support a role for vasopressin, via V2 receptors, in the progression of CKD in general and raise the possibility that the blockade of vasopressin V2 receptors may slow its progression (18,19). It is also possible that the effect of tolvaptan on the rate of decline of eGFR will be more easily demonstrable in patients with advanced rather than in early ADPKD, when GFR is still normal and not likely to change for many years. This post hoc exploratory analysis was conducted to reassess the primary and secondary efficacy endpoints from TEMPO 3:4 according to underlying CKD stage at baseline.
Materials and Methods
Trial Design
This is a post hoc, exploratory analysis of the TEMPO 3:4 trial (13,18) (ClinicalTrials.gov identifier: NCT00428948, January 26, 2007). Eligibility requirements included a diagnosis of ADPKD, a total kidney volume of ≥750 ml as measured by magnetic resonance imaging (MRI), a creatinine clearance of ≥60 ml/min as estimated by the Cockcroft–Gault formula and an age of 18–50 years. A total of 1445 patients were randomly assigned in a 2:1 ratio to receive tolvaptan (n=961) or placebo (n=484). Following randomization, patients received two doses of study drug per day (morning/afternoon) for 3 years, initiating with a 3-week titration period (45/15, 60/30, and 90/30 mg). Patients remained on the highest tolerated dose throughout the study period. Use of diuretics was avoided and water intake was encouraged.
Trial Assessments
Key assessments included standardized MRI scans of the kidney; measurements of serum creatinine, urine albumin, and BP; and evaluation of kidney pain. Most of these assessments were performed at baseline, at randomization, weekly during dose escalation, every 4 months (monthly in Japan) during treatment, and twice between 1 and 6 weeks after the completion of treatment at 36 months. MRI scans were obtained at baseline and at months 12, 24, and 36 (±2 weeks).
Outcome Measures
Subgroup analyses of the primary, composite secondary, and noncomposite secondary endpoints were prespecified in the protocol and reported previously (13,20). In this post hoc analysis, we reassessed the primary and secondary efficacy endpoints according to the underlying CKD stage at baseline. The primary endpoint was the annual rate of percentage change in TKV. The composite secondary endpoint was the time to multiple investigator-assessed ADPKD-related progression events. These events included worsening kidney function (a 25% reduction in the reciprocal of the serum creatinine level from the value at the end of the dose-adjustment period, reproduced after at least 2 weeks), clinically significant kidney pain (requiring medical intervention), worsening hypertension (changes in BP category or worsening of hypertension requiring an increase in hypertensive treatment), and worsening albuminuria (according to sex-specified categories) (13,20). The next secondary endpoint was the on-treatment slope of eGFR. An additional sensitivity analysis used the change in eGFR from pretreatment to post-treatment. Equations from the CKD-Epidemiology Collaboration adjusted for ethnic group were used to determine eGFR (21,22).
Statistical Analyses
Details of the analysis are available in the protocol (13,20). For the analysis of the primary endpoint, we compared individual slopes for TKV between the groups by fitting the log10-transformed data on TKV to a linear mixed-effects Laird–Ware model (23). Antilog (with a base of 10) of the treatment effect and 95% confidence intervals (95% CIs) derived from the model (in a log10 scale) provide a ratio of geometric means of the slope of TKV (i.e., 100% plus annual percentage change). A mixed-model repeated-measures analysis (MMRM), supported by extensive sensitivity analysis on missing data, was also applied to the repeated measures of change from baseline in log10-transformed data on total kidney volume as a sensitivity analysis.
Analysis of the rate of eGFR change from postdose baseline to last on-drug trial visit was similar to the analysis of the slope of the total kidney volume except the eGFR value, instead of log10 scale of the eGFR value, was used, and baseline was a covariate in the model. MMRM analysis was also applied to this efficacy variable as an exploratory analysis using off-treatment eGFR values.
The interactions of CKD stage and treatment group on the annual growth rate of TKV and the annual rate of change in eGFR were derived from a mixed model with the following factors: treatment, time, and CKD stage; two-variable interactions (treatment group and time, treatment group and CKD stage, CKD stage and time); and three-variable interaction (among CKD stage, treatment group, and time).
The primary analyses of all efficacy end points were prespecified as intention-to-treat analyses of data obtained during the treatment period, with sensitivity analyses that included pretreatment and post-treatment data in the intention-to-treat population. SAS software, version 9.4 (SAS Institute, Cary NC), was used for all statistical analyses reported here.
Results
Patients
At baseline for the TEMPO 3:4 trial, 502 (35%), 689 (48%), and 248 (17%) of the 1445 patients had CKD1, CKD2, and CKD3, respectively. Thirty-four percent, 49%, and 17% of patients randomly assigned to tolvaptan and 36%, 47%, and 17% of those randomly assigned to placebo had CKD1, CKD2, or CKD3, respectively. Mean baseline eGFRs were 105 (range, 90.0–132.8), 76 (range, 60.1–89.9), and 51 (range, 32.3–60.0) ml/min per 1.73 m2 for patients receiving tolvaptan and 107 (range, 90.0–186.7), 75 (range, 60.2–89.7), and 52 (range, 26.4–59.9) ml/min per 1.73 m2 for placebo recipients with CKD1, CKD2, and CKD3, respectively. Patients with CKD3 predominantly had stage 3a (n=135 [83%] in the tolvaptan group and n=70 [82%] in the placebo group). Demographic and clinical characteristics were well balanced (Table 1).
Table 1.
Demographic and clinical characteristics at baseline by CKD stage and treatment group
| Characteristics | CKD Stage 1 (n=502) | CKD Stage 2 (n=689) | CKD Stage 3 (n=248) | |||
|---|---|---|---|---|---|---|
| Tolvaptan (n=330) | Placebo (n=172) | Tolvaptan (n=465) | Placebo (n=224) | Tolvaptan (n=163) | Placebo (n=85) | |
| Men, % | 47.3 | 47.4 | 49.7 | 52.2 | 65.6 | 58.8 |
| Age, yr | 34±7 | 35±8 | 40±6 | 41±6 | 42±6 | 42±6 |
| Race, % | ||||||
| White | 91 | 94 | 82 | 80 | 78 | 75 |
| Asian | 7 | 4 | 15 | 17 | 17 | 20 |
| Other | 2 | 2 | 3 | 3 | 5 | 5 |
| Height, cm | 174±10 | 173±10 | 173±11 | 173±10 | 176±10 | 176±9 |
| Weight, kg | 78±19 | 77±19 | 78±18 | 77±18 | 86±17 | 85±17 |
| BP, mmHg | ||||||
| Systolic | 128±13 | 129±13 | 129±14 | 128±14 | 129±14 | 128±13 |
| Diastolic | 82±9 | 82±9 | 83±10 | 82±10 | 83±11 | 83±9 |
| History of hypertension, % | 73 | 71 | 84 | 90 | 95 | 93 |
| Treatment with ACEIs or ARBs, % | 73 | 69 | 84 | 88 | 95 | 93 |
| History of kidney pain, % | 56 | 51 | 48 | 50 | 53 | 42 |
| History of proteinuria, % | 19 | 20 | 26 | 25 | 30 | 31 |
| Estimated CrCl <80 ml/min, % | 2 | 2 | 25 | 28 | 66 | 71 |
| TKV, ml | 1375±681 | 1313±529 | 1713±861 | 1713±823 | 2353±1101 | 2266±1174 |
| Serum creatinine, mg/dl | 0.82(0.14) | 0.80±0.15 | 1.06±0.18 | 1.07±0.18 | 1.51(0.27) | 1.48±0.35 |
| Fasting urine osmolality, mOsm/kg | 572±180 | 592±197 | 483±165 | 491±154 | 413±119 | 444±199 |
| eGFR, ml/min per 1.73 m2 | 105±10 | 107±13 | 75±9 | 75±9 | 51±6 | 52±8 |
| Urinary ACR (mg/g)a | 24.0 (13.0–47.0) | 24.0 (14.7–53.0) | 28.3 (15.9–64.7) | 29.8 (14.0–56.1) | 37.5 (19.0–90.6) | 42.1 (21.0–113.7) |
Values expressed with a plus/minus sign are the mean±SD. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CrCl, creatinine clearance; TKV, total kidney volume; ACR, albumin-to-creatinine ratio.
Expressed as median (interquartile range).
Effect of Tolvaptan on Annualized Change in TKV by CKD Stage at Baseline
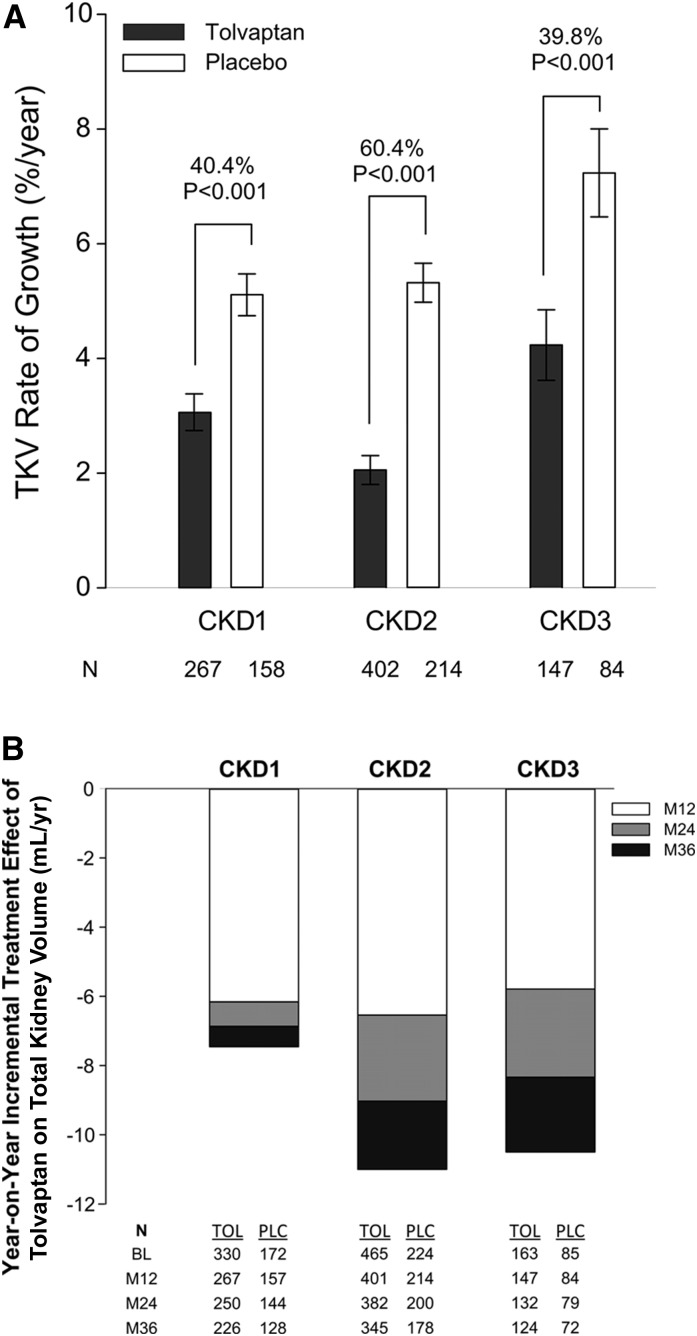
During the 3-year period of TEMPO 3:4, the administration of tolvaptan reduced the increase in TKV from a rate of 5.5% per year with placebo to 2.8% per year (13). The post hoc analysis by CKD stage showed that tolvaptan reduced the rates of kidney growth in the three CKD groups: by 1.99% per year (95% CI, −2.85% to −1.13%; P<0.001) for CKD1, by 3.12% per year (95% CI, −3.93% to −2.31%; P<0.001) for CKD2, and by 2.61 per year (95% CI, −4.08% to −1.17%; P<0.001) in CKD3 (Figure 1A and Table 2). These reductions were consistent across the CKD subgroups of interest as determined by a subgroup–treatment interaction analysis (P=0.17). Proportionate effect sizes were similar when patients with CKD3 were split into stages 3a and 3b (Supplemental Figure 1).
Figure 1.
Effect of tolvaptan on total kidney volume by CKD stage. (A) Annualized percentage changes in total kidney volume in the intention-to-treat population during the 3-year treatment period by CKD stage and treatment group (black bars refer to tolvaptan and white bars refer to placebo; mean ± SEM). Only the patients who had measurable baseline and at least one postbaseline magnetic resonance imaging scans were included in this analysis. The relative treatment effects (40.4%, 60.4%, and 39.8%) according to CKD stage subgroup are based on the between-group difference in the slopes, with variance approximated by means of the delta method under the assumption of independence between the slopes is indicated. (B) Year-on-year incremental treatment effect of tolvaptan on total kidney volume by CKD stage. Differences in least-squares mean total kidney volume changes for tolvaptan versus placebo on treatment by year. Annual treatment effects at months 12, 24, and 36 are represented by white, gray, and black/white dotted bars, respectively. CKD1, CKD stage 1; CKD2, CKD stage 2; CKD3, CKD stage 3; LS, least-squares; M, month; PLC, placebo; TOL, tolvaptan.
Table 2.
Rate of change in total kidney volume by CKD stage
| Variable | CKD Stage 1 (n=425) | CKD Stage 2 (n=616) | CKD Stage 3 (n=231) | |||
|---|---|---|---|---|---|---|
| Tolvaptan (n=267) | Placebo (n=158) | Tolvaptan (n=402) | Placebo (n=214) | Tolvaptan (n=147) | Placebo (n=84) | |
| Rate of TKV growth, %/yr | 3.1±5.2 | 5.1±4.6 | 2.1±5.0 | 5.3±5.0 | 4.2±7.5 | 7.2±7.0 |
| Treatment effect (95% CI), %/yr | −1.99 (−2.85 to −1.13) | −3.12 (−3.93 to −2.31) | −2.61 (−4.08 to −1.17) | |||
| P value | <0.001 | <0.001 | <0.001 | |||
| Relative treatment effect, % | 40.4 | 60.4 | 39.8 | |||
Values expressed with a plus/minus sign are the mean±SD. TKV, total kidney volume; 95% CI, 95% confidence interval.
A post hoc MMRM analysis of patients on-drug confirmed the beneficial effect of tolvaptan in the three CKD groups. Least-squares mean change in total kidney volume over the 3-year period for patients with CKD1, CKD2, and CKD3 were 5.9%, 7.2%, and 11.1% with tolvaptan and 13.4% (P<0.001), 18.2% (P<0.001), and 21.6% (P<0.001) with placebo, respectively. Treatment effects trended to be larger during the first year, but year-on-year differences in treatment effects were not significantly different for any CKD stage (P values ranging from 0.09 to 0.86) (Figure 1B).
Effect of Tolvaptan on the Composite Endpoint of Clinical Disease Progression Events by CKD Stage at Baseline
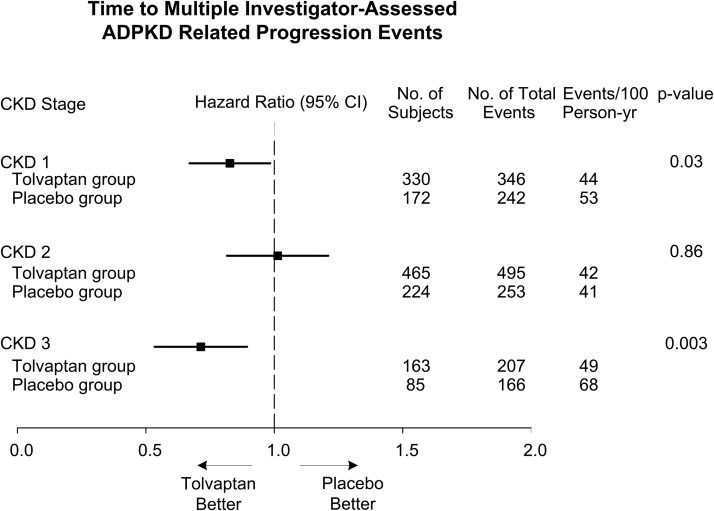
Analysis of the key secondary endpoint of time to multiple composite ADPKD-related events (worsening kidney function, kidney pain, hypertension, and albuminuria) showed fewer ADPKD-related events per 100 person-years of follow-up in tolvaptan- compared with placebo-treated patients (13). The post hoc analysis by CKD stage at baseline showed fewer ADPKD-related events per 100 person-years of follow-up in tolvaptan-treated compared with placebo-treated patients with CKD1 (hazard ratio [HR], 0.83; 95% CI, 0.70–0.98; P=0.03) and CKD 3 (HR, 0.71; 95% CI, 0.57–0.89; P=0.003), but not in in CKD2 (HR, 1.02; 95% CI, 0.85–1.21; P=0.86) (Figure 2). The positive results on the composite endpoint were driven primarily by events of renal function decline and renal pain (Supplemental Figure 2, A and B).
Figure 2.
Effects of tolvaptan on the time to multiple investigator-assessed autosomal-dominant polycystic kidney disease (ADPKD)-related progression events hazard ratios for the key secondary endpoint of multiple investigator-assessed ADPKD-related progression events with tolvaptan compared with placebo by CKD stage. 95% CI, 95% confidence interval.
Effect of Tolvaptan on Rate of eGFR Decline, by CKD Stage at Baseline
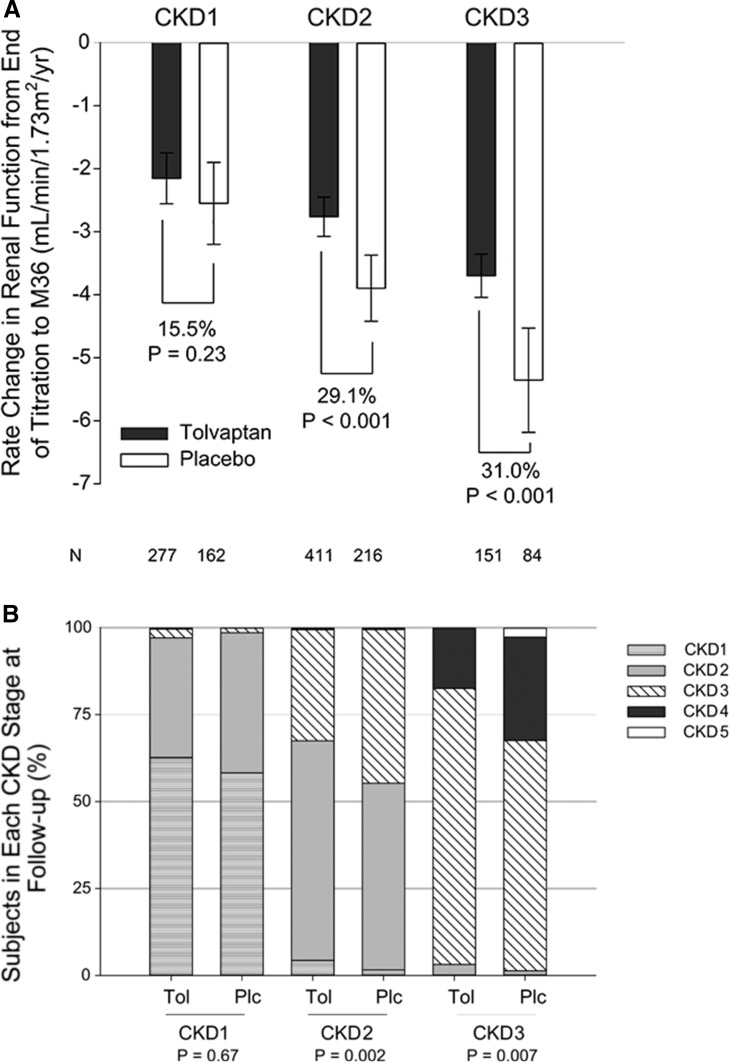
In TEMPO 3:4, tolvaptan reduced the decline in eGFR from −3.70 to −2.72 ml/min per 1.73 m2 per year (26.5%; P<0.001) (13). In the present post hoc analysis, tolvaptan decreased the rate of eGFR decline by 0.40 ml/min per 1.73 m2 per year (95% CI, −0.25 to 1.05; P=0.23) in patients with CKD1, by 1.13 (95% CI, 0.61–1.66; P<0.001) in those with CKD2, and by 1.66 (95% CI, 0.83–2.45; P<0.001) in those with CKD3, with a trend for a positive subgroup–treatment interaction (P=0.07) (Figure 3A and Table 3). Results for patients in CKD3 were similar when split into CKD3a and CKD3b (Supplemental Figure 3). Tolvaptan treatment effects on eGFR slope were confirmed by comparing the eGFR values before treatment (at baseline) and those after treatment (after discontinuation of study drug), which favored tolvaptan across CKD1 through CKD3 (Supplemental Figure 4). Consistent with the beneficial effects of tolvaptan on the rates of eGFR decline, patients with CKD2 and CKD3 randomly assigned to placebo were more likely to progress to a higher CKD stage at the last follow-up visit than those treated with tolvaptan (Figure 3B).
Figure 3.
Effect of tolvaptan on kidney function by CKD stage. (A) On-treatment slopes of eGFR (calculated by CKD-Epidemiology Collaboration [CKD-EPI]) with 95% confidence intervals by CKD stage and treatment group in the intention-to-treat population within the treatment period (black bars represent tolvaptan and white bars represent placebo). The relative treatment effects (15.5%, 29.1%, and 31.0%) according to CKD stage subgroup, based on the difference in annual change in slope, are shown. (B) Disease progression is plotted as a stacked bar chart for patients in CKD stages 1–3 at baseline compared with the last follow-up visit (eGFR estimated by CKD-EPI). P values were derived using Cochran–Mantel–Haenszel mean score statistic by baseline CKD Stage. EOT, end of titration; FU2, follow-up visit 2; M36, month 36; Plc, placebo; Tol, tolvaptan.
Table 3.
Rate of change in renal function by CKD stage
| Variable | CKD Stage 1 (n=389) | CKD Stage 2 (n=627) | CKD Stage 3 (n=235) | |||
|---|---|---|---|---|---|---|
| Tolvaptan (n=227) | Placebo (n=162) | Tolvaptan (n=411) | Placebo (n=216) | Tolvaptan (n=151) | Placebo (n=84) | |
| Rate of eGFR decline (95% CI), ml/min per 1.73 m2 per yr | −2.15 (−2.56 to −1.75) | −2.55 (−3.20 to −1.90) | −2.76 (−3.07 to −2.45) | −3.90 (−4.42 to −3.37) | −3.70 (−4.04 to −3.36) | −5.36 (−6.19 to −4.53) |
| Treatment effect (95% CI), ml/min per 1.73 m2 per yr | 0.4 (−0.25 to 1.05) | 1.13 (0.61–1.66) | 1.66 (0.83–2.45) | |||
| P value | 0.23 | <0.001 | <0.001 | |||
| Relative treatment effect, % | 15.5 | 29.1 | 31.0 | |||
95% CI, 95% confidence interval.
Adverse Events by CKD Stage at Baseline
In TEMPO 3:4, patients who received tolvaptan had more adverse events related to aquaresis (thirst, polyuria, and nocturia), whereas those who received placebo had more adverse events related to ADPKD (kidney pain, hematuria, and urinary tract infection) (Table 4). These increased risks were not associated with CKD stage.
Table 4.
Adverse events, potentially clinically significant laboratory values, and discontinuations related to adverse events or laboratory abnormalities by CKD stage and treatment group
| Variable | CKD Stage 1 (n=502) | CKD Stage 2 (n=689) | CKD Stage 3 (n=248) | |||
|---|---|---|---|---|---|---|
| Tolvaptan (n=330) | Placebo (n=172) | Tolvaptan (n=465) | Placebo (n=224) | Tolvaptan (n=163) | Placebo (n=85) | |
| AEs (% of patients with at least one event) | ||||||
| Thirst | 53.6 | 20.9 | 57.2 | 19.2 | 53.4 | 23.5 |
| Polyuria | 44.8 | 18.6 | 35.5 | 16.5 | 33.7 | 16.5 |
| Nocturia | 28.5 | 12.2 | 29.5 | 12.9 | 30.1 | 15.3 |
| Pollakiuria | 16.1 | 6.4 | 27.5 | 5.4 | 25.2 | 2.4 |
| Kidney pain | 27.9 | 37.2 | 27.7 | 32.1 | 30.7 | 44.7 |
| Hematuria | 7.2 | 14.5 | 8.0 | 12.4 | 10.4 | 17.6 |
| UTI | 9.4 | 14.0 | 12.3 | 14.2 | 4.3 | 12.9 |
| Potentially clinically significant laboratory values (% of patients with at least one value) | ||||||
| Serum sodium >150 mEq/L, % | 2.4 | 2.9 | 4.1 | 1.3 | 6.7 | 0 |
| Serum uric acid >7.5 mg/dl, % | 20.7 | 12.9 | 38.7 | 24.7 | 71.8 | 49.4 |
| Serum ALT >2.5 times ULN | 5.2 (2.18)a | 1.7 (0.66)a | 5.6 (2.21)a | 1.3 (0.48)a | 7.4 (2.87)a | 2.4 (0.82)a |
| Serum AST >2.5 times ULN | 3.9 (1.66)a | 0.6 (0.22)a | 3.7 (1.45)a | 0.9 (0.32)a | 4.9 (1.91)a | 1.2 (0.41)a |
| Serum bilirubin >2 times ULN | 0.3 (0.13)a | 0 (0)a | 0.2 (0.09)a | 1.3 (0.48)a | 0.6 (0.24)a | 0 (0)a |
| Discontinuations related to AEs or laboratory abnormalities (% of patients) | ||||||
| All AEs | 17.0 | 5.8 | 14.6 | 4.9 | 14.7 | 3.5 |
| Liver-related AEs or laboratory abnormalities | 1.5 | 2.9 | 1.5 | 0.9 | 1.8 | 1.2 |
AE, adverse events; UTI, urinary tract infection; ALT, alanine aminotransferase; ULN, upper limit of normal; AST, aspartate aminotransferase.
Events per 100 person years.
A greater proportion of patients who received tolvaptan had elevations in serum sodium, uric acid, and liver enzyme levels on at least one occasion. Episodes of laboratory-defined hypernatremia (serum sodium > 150 mEq/L) were more frequent in patients with CKD3 treated with tolvaptan than in those receiving placebo (P=0.01). Aminotransferase elevation (>2.5 times the upper limit of normal) events ranged from 1.45 to 2.87/100 patient-years of follow-up in the tolvaptan-treated patients compared with 0.22–0.82/100 patient-years of follow-up in the placebo recipients, without relation to CKD stage (Table 4). Two (one with CKD1 and one with CKD3) of the 958 patients taking tolvaptan (0.2%) met the definition of a Hy’s law case.
Seventeen percent, 14.6%, and 14.7% of patients with CKD1, CKD2, and CKD3 treated with tolvaptan compared with 5.8%, 4.9%, and 3.5% of those receiving placebo discontinued the trial treatment because of adverse events (Table 4).
Discussion
The TEMPO 3:4 trial demonstrated a significant beneficial effect of tolvaptan on the rates of TKV growth (−2.7 percentage points per year) and eGFR decline (−0.98 ml/min per 1.73 m2 per year) in patients with ADPKD.
Despite the effect of tolvaptan on TKV, concerns have been raised that in patients with more advanced disease this effect might not be observed or sustained or that it might be accompanied by a lesser, insignificant reduction in the rate of decline in renal function. Because the renal function criterion for entry into the trial was based on an estimated creatinine clearance of ≥60 ml/min by the Cockcroft-Gault equation rather than on an eGFR algorithm normalized to a body surface area of 1.73 m2, 17.2% of enrolled patients (248 of 1445) had a baseline eGFR by the CKD-Epidemiology Collaboration equation of <60 ml/min per 1.73 m2 (CKD3). Here we report the results of a post hoc analysis comparing the main outcomes of the trial by baseline CKD stages 1–3.
The results of the analysis of the primary endpoint trial suggest that tolvaptan was similarly effective in reducing the rate of increase in TKV in patients with ADPKD who had CKD1–CKD3 at baseline. Treatment effects were larger during the first year, but they were also significant during the second and third years, resulting in an incremental effect over time. This incremental effect was larger in patients with CKD2 and CKD3 than in those with CKD1, probably because the CKD1 group included more patients with milder, slowly progressive disease. The effect on kidney volume in the first year was likely due to deflation caused by slowing fluid secretion, while the effect during the second and third years was likely due to decreased cell proliferation as secretion is maintained at a reduced level. This is supported by studies in cultured ADPKD cystic cells in which low concentrations of tolvaptan inhibited arginine vasopressin–stimulated, chloride-driven fluid secretion and cell proliferation (24) and by clinical studies that demonstrated acute decreases in TKV as early as 1 and 3 weeks of treatment, which are probably not to be explained by effects on cell proliferation (17,25).
The reduction in the rate of kidney growth was accompanied by a slower rate in the decline of eGFR, reflected by lower on-treatment eGFR slopes in patients with CKD2 and CKD3. Tolvaptan and enhanced hydration are known to exert an acute, reversible renal hemodynamic effect, likely through inhibition of tubulo-glomerular feedback (17–19,25). Because this could have affected the eGFR slopes on treatment, a sensitivity analysis comparing the change in eGFR values from pretreatment baseline to post-treatment visits after discontinuation of the study drug was performed. This analysis showed a statistically significant, slower decline of eGFR not only in the patients with CKD2 and CKD3 but also in those with CKD1 treated with tolvaptan.
The large treatment effects of tolvaptan on TKV and eGFR in the patients with CKD3 are reassuring, at least as it relates to progression throughout CKD3, given the concern that tolvaptan could become less effective as the disease progresses. Consistent with its effects on kidney enlargement and eGFR decline, tolvaptan delayed the progression of patients with CKD2 and CKD3 at baseline to more advanced CKD stages at the last follow-up visit. Longer follow-up or a larger number of patients would have been required to detect a similar effect in patients with CKD1 who had less advanced and possibly less severe disease.
As previously reported, the administration of tolvaptan was associated with more frequent adverse events related to aquaresis, fewer adverse events related to ADPKD, and a higher frequency of discontinuation of the study drug, mostly related to aquaretic adverse events and elevation of aminotransferases in plasma. None of these, however, was affected by the CKD stage. The only laboratory abnormality that occurred more frequently in tolvaptan-treated patient and that was affected by CKD stage was hypernatremia, defined as an elevation of plasma sodium ≥150 mEq/L. Recently, an independent, blinded, expert hepatic adjudication committee examined data from TEMPO 3:4 using the five-point Drug-Induced Liver Injury Network classification and identified 16 tolvaptan-treated patients who experienced aminotransferase elevations that were deemed to be probably or highly likely due to study drug (26). Five of patients with CKD3 (3.1%) had an adjudicated hepatic event versus six patients with CKD1 (1.8%) and five with CKD2 (1.1%) (26).
The analysis presented here is post hoc, carrying the risk of a false-positive result, and should serve as hypothesis generating. While subgroup analyses of the primary, composite secondary, and noncomposite secondary endpoints were prespecified in the protocol and reported previously (13), TEMPO 3:4 was not powered to analyze these endpoints by CKD stage. Patients reported in CKD 3 were predominantly (∼75%) in CKD stage 3a; thus, results on each of the endpoints may not fully represent all patients across this spectrum of disease stage. Here we have analyzed secondary efficacy endpoints in a prespecified order with the strategy of preserving α. Once the significance of the analysis is lost, however, subsequent analyses are subject to type 1 error; thus, results of the secondary endpoints should be interpreted cautiously. Replicating Evidence of Preserved Renal Function: An Investigation of Tolvaptan Safety and Efficacy in ADPKD (NCT02160145), a multinational, multicenter, randomized-withdrawal, placebo-controlled, double-blind, parallel-group trial, will compare the efficacy and safety of tolvaptan in patients with CKD stages 2–4.
In summary, this post hoc analysis of the TEMPO 3:4 clinical trial suggests that the inhibitory effect of tolvaptan on the rate of kidney growth is similar in patients with ADPKD across CKD1, CKD2, and CKD3, while the beneficial effect on renal function decline is more easily demonstrable in patients with CKD2 or CKD3. This analysis also shows that these beneficial effects are achieved without an increased frequency of adverse events in more advanced stages of the disease except for an increased frequency of hypernatremia in CKD3. Because this is a post hoc analysis, these conclusions need to be interpreted cautiously.
Disclosures
V.E.T., E.H., O.D., A.B.C., R.T.G., R.D.P., J.O., and F.S.C. are members of the steering committee of the TEMPO 3:4 trial. V.E.T., O.D., A.B.C., R.T.G., and R.D.P. have received research funding from Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ); A.B.C. and J.J.G. have received consultancy fees from Otsuka Pharmaceutical Development & Commercialization, Inc. E.H. has received research funding and consultancy fees from Otsuka Pharmaceutical Co., Ltd. J.O., J.D.B. and F.S.C. are employees of Otsuka Pharmaceutical Development & Commercialization Inc.
Supplementary Material
Acknowledgments
We thank the patients involved in the TEMPO 3:4 trials for their participation and contribution, trial investigators, subinvestigators, radiologists, study coordinators and nurses, trial managers, trial monitors (Parexel International Corp.), data managers, programmers and statisticians, the University of Wisconsin Statistical Data Analysis Center, the members of the independent data monitoring committee and clinical events committee, and Dr. K. Bae (for assistance in the development of the Imaging Charter).
This study was funded by Otsuka Pharmaceuticals Co., Ltd. Tokyo, Japan, and Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, Maryland.
The investigators in the TEMPO 3:4 trial are listed in the Supplemental Appendix.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06300615/-/DCSupplemental.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC: Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25: 18–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP: Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Hopp K, Wang X, Ye H, Irazabal MV, Harris PC, Torres VE: Effects of hydration in rats and mice with polycystic kidney disease. Am J Physiol Renal Physiol 308: F261–F266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattone VH 2nd, Maser RL, Tian C, Rosenberg JM, Branden MG: Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev Genet 24: 309–318, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Gattone VH 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Gattone V 2nd, Harris PC, Torres VE: Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Meijer E, Gansevoort RT, de Jong PE, van der Wal AM, Leonhard WN, de Krey SR, van den Born J, Mulder GM, van Goor H, Struck J, de Heer E, Peters DJ: Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant 26: 2445–2453, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE: Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol 26: 39–47, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 14.Otsuka Pharmaceuticals: Otsuka Pharmaceutical’s Samsca® approved in Japan as the world’s first drug therapy for ADPKD, a rare kidney disease [News release]. March 24, 2014. Available at: http://www.otsuka.co.jp/en/company/release/2014/0324_01.html. Accessed February 15, 2016
- 15.Otsuka Pharmaceuticals: Now available in Canada: First-ever treatment for adults living with a life-threatening kidney disease (ADPKD). 2015. Available at: http://www.newswire.ca/news-releases/first-ever-treatment-approved-in-canada-for-adults-living-with-adpkd-a-life-threatening-kidney-disease-516975061.html. Accessed February 15, 2016
- 16.US Food and Drug Administration. Otsuka Pharmaceutical Development & Commercialization, Inc. New Drug Application 204441: Delaying progression of renal complication of autosomal dominant polycystic kidney disease by tolvaptan inhibition of arginine vasopressin. Issued July 3, 2013. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm363342.htm. Accessed February 15, 2016
- 17.Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, Kappert P, Ouyang J, Engels GE, van Oeveren W, Struck J, Czerwiec FS, Oberdhan D, Krasa HB, Gansevoort RT: Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis 65: 833–841, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Bankir L, Bouby N, Ritz E: Vasopressin: A novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Torres VE: Vasopressin in chronic kidney disease: an elephant in the room? Kidney Int 76: 925–928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS: Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 study. Am J Kidney Dis 57: 692–699, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S: Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: Accuracy and use for population estimates. Am J Kidney Dis 56: 32–38, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]
- 24.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP: Tolvaptan inhibits ERK-dependent cell proliferation, Cl⁻ secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, Krasa HB, Ouyang J, Czerwiec FS: Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 80: 295–301, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, Ouyang J, Torres VE, Czerwiec FS, Zimmer CA: Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: Analysis of clinical trials database. Drug Saf 38: 1103–1113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.