Abstract
Background and objectives
Poor linear growth is common in children with CKD and has been associated with higher mortality. However, recent data in adult dialysis patients have suggested a higher risk of death in persons of tall stature. In this study, we aimed to examine the risk of all-cause and cause-specific mortality in children at both extremes of height at the time of first RRT.
Design, setting, participants, & measurements
Using the US Renal Data System, we performed a retrospective analysis of 13,218 children aged 2–19 years, who received their first RRT (dialysis or transplant) during 1995–2011. We used adjusted Cox models to examine the association between short (<3rd percentile) and tall (>3rd percentile) stature and risk of death, compared with less extreme heights.
Results
Over a median follow-up of 7.1 years, there were 1721 deaths. Risk of death was higher in children with short (hazard ratio, 1.49; 95% confidence interval, 1.33 to 1.66) and tall stature (hazard ratio, 1.32; 95% confidence interval, 1.03 to 1.69) in adjusted analysis. In secondary analyses, there was a statistically significant interaction between height and body mass index categories (P=0.04), such that the association of tall stature with higher mortality was limited to children with elevated body mass index (defined as ≥95th percentile for age and sex). Children with short stature had a higher risk of cardiac- and infection-related death, whereas children with tall stature had a higher risk of cancer-related death.
Conclusions
Children with short and tall stature are at higher mortality risk, although this association was modified by body mass index at time of first RRT. Studies to further explore the reasons behind the higher risk of mortality in children with extremes of height at the time of first RRT are warranted.
Keywords: height, growth failure, pediatric ESRD, body height, body mass index, child, follow-up studies, humans, renal dialysis, renal replacement therapy
Introduction
Short stature is prevalent among children with CKD, affecting up to one in three patients (1). Studies performed over a decade ago demonstrated a higher risk of death associated with poor linear growth at the time of first RRT (2,3). Short stature in children with CKD has also been previously associated with higher rates of hospitalization and poor school performance (1–3). However, given overall improvements in the care of children with CKD (4), it is possible that the higher risk of mortality associated with poor linear growth in children with CKD has attenuated over time.
Interestingly, two recent studies of adults receiving dialysis treatment demonstrated that tall, as opposed to short, stature was associated with higher mortality risk (5,6). Tall stature in adults was also associated with a higher risk of cardiac-, infectious-, and cancer-related death (5). These observations differ from data reported in children, which have highlighted the risk associated with short stature. To our knowledge, the association between tall stature and mortality risk in children receiving RRT has not been examined.
In this study, we used data from the US Renal Data System (USRDS) to examine the association between short and tall stature at time of first RRT and mortality risk in children. We also examined the association between height at time of first RRT and cause-specific mortality. We hypothesized that short, but not tall, stature would be associated with higher risk of mortality in children at the time of first RRT, and that the association between height and mortality risk in children would differ from that found in adults.
Materials and Methods
Study Population, Data Source Population, and Data Source
We performed a retrospective cohort study of children between 2 and 19 years of age who started RRT between January 1, 1995, and December 31, 2011, using data from the USRDS (7). The Committee on Human Research at the University of California, San Francisco considers this study to be exempt from Institutional Review Board review.
In brief, patients with available demographic characteristics, cause of ESRD, Medicaid coverage (categorical variable as an indicator of lower income status), zip code, date of ESRD onset, height, weight, and body mass index (BMI) at time of first RRT from the Centers for Medicare and Medicaid 2728 (CMS-2728) Medical Evidence (MEDEVID) form and patient’s file in the USRDS were included for analysis. Zip code was used to determine the average median household income of patients’ neighborhoods using data from the American Community Survey between 2006 and 2010 (8). Initial ESRD treatment modality (pre-emptive transplant versus dialysis) was determined by comparing the first ESRD service date, dialysis service date, and first transplant date listed in the MEDEVID forms and patients’ files. eGFR was determined by the Schwartz Equation (9).
We determined transplant dates using USRDS patient and transplant files, which contain data reported by transplant centers to the United Network for Organ Sharing.
Height Definitions
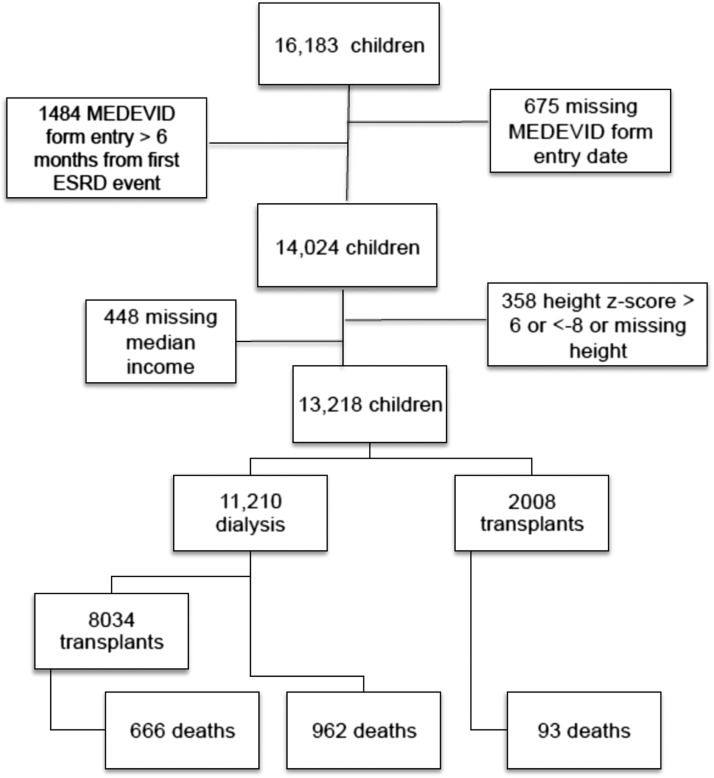
We converted height into age- and sex-standardized height z-scores using the 2000 Centers for Disease Control and Prevention (CDC) standards for children in the United States >2 years of age (10). We defined short stature as height <3rd percentile for age and sex (corresponding to z-score <−1.88), and tall stature as height >97th percentile for age and sex (corresponding to z-score >1.88) (11,12). We included only children whose first RRT fell within our study period, defined by availability of a CMS-2728 MEDEVID form filed within 6 months of the first RRT service date (n=14,024). We excluded patients whose forms had missing height (n=261) or persistent extremes of height (z-score <−8 and >6) that we thought were likely to be erroneous (n=97) (Figure 1). We also excluded patients with missing median income values from our analysis (n=448).
Figure 1.
Distribution of outcomes in children and adolescents with ESRD onset between 1995 and 2011. MEDEVID, Centers for Medicare and Medicaid 2728 Medical Evidence.
Primary Outcome
We abstracted death dates and primary causes of death (cardiac, infectious, cancer, and other) from the USRDS patient files. We ascertained all deaths through June 30, 2012.
Statistical Analyses
We examined temporal trends in height at time of first RRT using a multivariable linear regression model with calendar year of ESRD onset as the main predictor and height z-score at ESRD onset as the outcome. This model was adjusted for demographic factors including age at ESRD onset, sex, race, cause of ESRD, Medicaid status, and median neighborhood income.
We assessed the association between height category at time of first RRT and risk for all-cause mortality using a Cox proportional hazards model adjusted for sex, race, cause of ESRD, Medicaid status, median neighborhood income, calendar year (to account for temporal changes in height at time of first RRT over time and any potential secular trends in survival), transplant (as a time-dependent covariate), and age category at initial RRT onset. We categorized age at time of first RRT as 2–5 years, >5 years but <13 years, and ≥13 years, owing to known differences in risk of death by age from earlier literature (7,13,14). We did not censor patients at the time of transplantation during follow-up, given that transplantation occurs commonly in children, improves survival of children with ESRD, and may be a mediating factor that relates to all-cause mortality. This adjusted time-dependent Cox model was used as our primary model for all analyses. We did, however, explore the risk of death during the time attributed to transplantation versus dialysis in stratified adjusted Cox models during long-term follow-up.
To explore the U-shaped association between height z-score and risk of death given the results of our primary analysis, height z-score was further modeled as a spline term with four knots and used in a Cox model adjusted for sex, race, cause of ESRD, Medicaid status, median neighborhood income, calendar year, age category, and transplant as a time-dependent variable to evaluate hazard of death.
We chose a priori not to adjust for BMI or weight in our primary models because height is an integral component of BMI, and adjustment for weight would potentially reflect the association between weight-for-height (instead of height) and clinical outcomes. However, we did test for interaction between height and BMI categories in our primary Cox models. Owing to the presence of an interaction, we subsequently explored the association between height and weight status via subgroup analysis according to BMI categories as follows: low BMI (defined as BMI <5th percentile for age and sex), normal BMI (defined as BMI 5th to 94th percentile for age and sex), and elevated BMI (defined as BMI ≥95th percentile for age and sex) (12,15). We also tested for the presence of interaction between height and race categories.
Finally, we used Cox models (adjusted for the same covariates as above, with transplant as a time-dependent covariate) to examine the association between height category and risk of cause-specific mortality (cardiac-, infectious-, or malignancy-related death).
With the exception of conversion of height into z-scores, which was performed using a statistical analysis system tool provided by the CDC (16), all data analyses were conducted in Stata 13.
Results
Cohort Characteristics
We identified 13,218 children aged 2–19 years with available anthropometric measurements and other covariate information at the time of first RRT in the USRDS (Figure 1). Median age in this cohort was 14.5 years (interquartile range [IQR], 10.5–17.5), 55% were male, 67% were white, and 46% received Medicaid at the time of ESRD onset (Table 1). Median eGFR at the start of RRT in children with nonmissing creatinine (n=10,910) was 7.6 ml/min per 1.73 m2 (IQR, 5.6–10.2) and slightly higher in tall children (Table 1).
Table 1.
Characteristics of children at time of initial ESRD onset in the entire cohort and by height category
| Characteristics at ESRD Onseta | Overall (n=13,218) | Short Stature (n=3418) | Normal Stature (n=9430; Reference) | Tall Stature (n=370) | P Value |
|---|---|---|---|---|---|
| Median age, years (IQR) | 14.5 (10.5–17.5) | 12.5 (7.5–15.5) | 15.5 (11.5–17.5) | 14.5 (10.5–17.5) | <0.001 |
| Age category, % (n) | <0.001 | ||||
| 2–5 years | 7 (916) | 12 (425) | 5 (471) | 5 (20) | |
| >5 to <13 years | 29 (3860) | 40 (1350) | 25 (2391) | 32 (119) | |
| ≥13 years | 64 (8442) | 48 (1643) | 70 (6568) | 62 (231) | |
| Race, % (n) | <0.001 | ||||
| White | 67 (8877) | 71 (2436) | 66 (6255) | 50 (186) | |
| Black | 24 (3142) | 18 (602) | 25 (2372) | 45 (168) | |
| Asian | 6 (773) | 7 (250) | 5 (515) | 2 (8) | |
| Native American | 2 (208) | 1 (48) | 2 (155) | 1 (5) | |
| Other | 2 (218) | 2 (82) | 1 (133) | 1 (3) | |
| Male, % (n) | 55 (7224) | 56 (1904) | 54 (5119) | 54 (201) | 0.36 |
| Cause of ESRD, % (n) | <0.001 | ||||
| Congenital/cystic/hereditary diseases/pyelonephritis/interstitial nephritis | 39 (5121) | 55 (1867) | 34 (3188) | 18 (66) | |
| GN (primary and secondary) | 25 (3294) | 13 (447) | 29 (2723) | 34 (124) | |
| FSGS | 15 (1969) | 11 (377) | 16 (1517) | 20 (75) | |
| Hypertension | 4 (555) | 3 (105) | 4 (421) | 8 (29) | |
| Other | 17 (2279) | 18 (622) | 17 (1581) | 21 (76) | |
| eGFR (ml/min per 1.73 m2) at ESRD onset,b median (IQR) | 7.6 (5.6–10.2) | 7.4 (5.6–9.9) | 7.7 (5.6–10.3) | 8.2 (5.7–11.0) | 0.02 |
| Medicaid, % (n) | 46 (6047) | 54 (1831) | 43 (4074) | 38 (142) | <0.001 |
| Average median neighborhood income ($) | 52,362 | 52,553 | 52,299 | 52,192 | 0.58 |
| Body mass index,c % (n) | <0.001 | ||||
| <5th percentile | 10 (1357) | 13 (428) | 9 (868) | 18 (61) | |
| 5th to 94th percentile | 72 (9370) | 70 (2356) | 73 (6809) | 59 (205) | |
| ≥95th percentile | 17 (2256) | 17 (579) | 17 (1597) | 23 (80) | |
| Death rate per 100 person-years (95% CI) | 1.74 (1.66 to 1.83) | 2.04 (1.88 to 2.23) | 1.61 (1.52 to 1.70) | 2.40 (1.89 to 3.05) |
IQR, interquartile range; 95% CI, 95% confidence interval.
All ESRD characteristics are broken down by height category (column percentages).
Missing in n=2308 owing to missing serum creatinine values, especially in patients with pre-emptive transplantation.
Missing in n=235.
Median age- and sex-standardized height z-score at time of first RRT was −0.90 (IQR, −1.93–0.05), on the basis of CDC standards for children in the United States. In adjusted analysis, every 5-year study interval was associated with a 0.08-unit higher average height z-score (95% confidence interval [95% CI], 0.05 to 0.11; P<0.001) at the time of ESRD onset.
Overall, 26% of children were short and 3% were tall. Children with short stature were more likely to be white, have congenital anomalies of the kidney and urinary tract as cause of their ESRD, and have Medicaid as their health payer (Table 1). In contrast, a greater proportion of children with tall stature were black, had GN as the cause of their ESRD, and had a health payer other than Medicaid. By age at the time of first RRT, 46% of children with ESRD onset at 2–5 years of age had short stature, compared with 35% of children with ESRD onset at ages 5–13 years, and 19% of children with ESRD onset after age 13 years. Rates of death were higher in both short and tall children (Table 1).
Approximately three quarters of children (n=10,042) received at least one kidney transplant, and approximately 15% of the cohort received pre-emptive transplantation (Figure 1).
Height and All-Cause Mortality
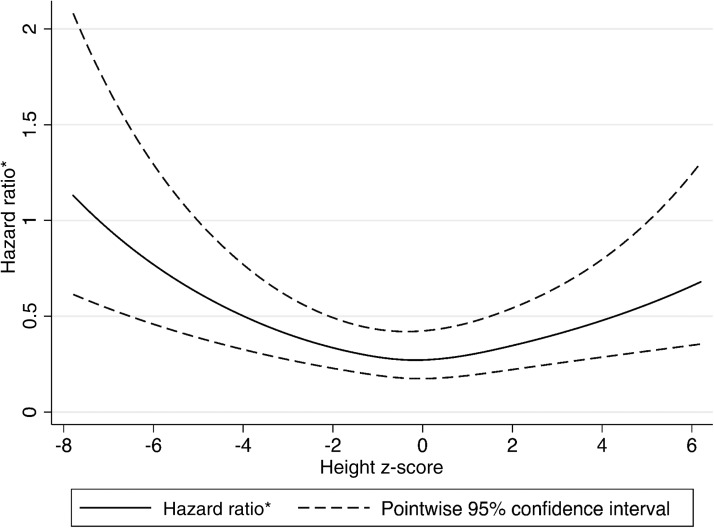
Approximately 13% of the cohort (n=1721) died during 98,786 person-years of follow-up (median follow-up period 7.1 years; IQR, 3.5–11.0). In primary analysis, the adjusted risk of death was 1.49 times higher in children who were short (95% CI, 1.33 to 1.66; P<0.001) and 1.32 times higher in children who were tall (95% CI, 1.03 to 1.69; P=0.03) compared with children with normal height at ESRD onset in an adjusted Cox model. The association between height z-score modeled as a spline and the hazard of death is shown in Figure 2.
Figure 2.
Adjusted spline of the association between height z-score and the risk of death. * Hazard ratio is the hazard ratio for a given height z-score, divided by the baseline hazard.
To evaluate whether the association between height and mortality may differ depending on modality of treatment, we stratified our primary models by time-updated RRT modality (transplant versus dialysis) in exploratory analysis. In this model, there were 39,235 at-risk person-years on dialysis and 59,551 at-risk person-years with a transplant. In children with short stature, we found the risk of death remained statistically significantly higher than that of children with normal stature, regardless of treatment modality (hazard ratio [HR], 1.48; 95% CI, 1.31 to 1.67 [during dialysis]; HR, 1.48; 95% CI, 1.16 to 1.88 [during transplant]). In contrast, the risk of death in children with tall stature was only statistically significantly higher than that of children with normal stature during dialysis (HR, 1.34; 95% CI, 1.02 to 1.76) but not transplant (HR, 1.16; 95% CI, 0.59 to 2.29).
Interaction between Height, BMI, and Race
In secondary analysis, BMI was found to modify the association between height and risk of all-cause mortality (P=0.04). Thus, we performed additional analyses to examine the risk of death by BMI category using our adjusted Cox models (Table 2). In children with short stature, risk of death was statistically significantly higher than that of children with normal stature if children had normal or elevated BMI, but not statistically significantly higher if children were underweight. In contrast, in children with tall stature, risk of death was higher than that of children with normal stature only for children with elevated BMI.
Table 2.
Hazard ratios for all-cause mortality by height category, according to BMI status in children at the time of ESRD onset
| Adjusted Cox Modela | Short Statureb | Tall Statureb | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Underweight (n=1357) | 1.33 (0.99 to 1.78) | 0.06 | 0.95 (0.47 to 1.89) | 0.88 |
| Normal BMI (n=9370) | 1.53 (1.33 to 1.75) | <0.001 | 0.99 (0.67 to 1.44) | 0.95 |
| Elevated BMI (n=2256) | 1.45 (1.10 to 1.90) | <0.01 | 1.78 (1.10 to 2.88) | 0.02 |
95% CI, 95% confidence interval; BMI, body mass index.
All models were adjusted for age (categorical variable), sex, race, cause of ESRD, Medicaid status, median neighborhood income, calendar year of ESRD onset, and transplant as a time-dependent covariate. BMI was missing in n=235 patients included in primary analysis.
Normal height is reference category, with short stature defined as <3rd percentile and tall stature defined as >97th percentile using Centers for Disease Control and Prevention age-and sex-standardized height z-scores.
We also tested for the presence of interaction between height and race categories and found a significant interaction (P=0.02) between black race and tallness. Black children who were tall did not have a statistically significantly different risk of death compared with black children with normal stature (HR, 1.00; 95% CI, 0.70 to 1.42). In contrast, white children who were tall had a significantly higher risk of death than white children with normal stature (HR, 1.89; 95% CI, 1.30 to 2.75).
Height and Cause-Specific Mortality
Of the children who died and had available cause of death (n=1355, 79% of total deaths), there were 466 cardiac-related deaths (34%), 232 infection-related deaths (17%), and 64 malignancy-related deaths (5%). Of the malignancy-related deaths, 30 (47%) were reported as cancer-related deaths in children previously on immunosuppression. The risk of cardiac- and infection-related death was statistically significantly higher in children with short stature compared with children with normal stature (Table 3). In contrast, the risk of cancer-related death was statistically significantly higher in children with tall stature compared with children with normal stature.
Table 3.
Hazard ratios for cause-specific mortality by height category in children at time of ESRD onset
| Adjusted Cox Modela Cause-Specific Mortality (n=13,218) | Short Stature (n=3418) | Tall Stature (n=370) | ||
|---|---|---|---|---|
| Hazard Ratiob (95% CI) | P Value | Hazard Ratiob (95% CI) | P Value | |
| Cardiac death | 1.52 (1.23 to 1.88) | <0.001 | 1.19 (0.73 to 1.95) | 0.48 |
| Infection-related death | 1.72 (1.29 to 2.29) | <0.001 | 1.25 (0.58 to 2.69) | 0.57 |
| Malignancy-related death | 0.79 (0.44 to 1.44) | 0.44 | 2.59 (1.05 to 6.38) | 0.04 |
95% CI, 95% confidence interval.
All models were adjusted for age (categorical variable), sex, race, cause of ESRD, Medicaid status, median neighborhood income, calendar year of ESRD onset, and transplant as a time-dependent covariate.
Normal height is reference category with short stature defined as <3rd percentile and tall stature defined as >97th percentile using Centers for Disease Control and Prevention age-and sex-standardized height z-scores.
Discussion
In this study, we examined the association between height and mortality risk at the time of first RRT, which should reflect growth during the CKD phase of disease. We found that, despite small improvements in height over the last 2 decades, over one quarter of children starting RRT still had short stature. Our study confirms and updates the association between short stature and higher risk of death that has previously been reported in both a prevalent USRDS cohort of children with ESRD followed between 1990 and 1995 and an incident cohort of children initiated on RRT before 2000 in a voluntary North American registry (2,3,13).
The elevated risk of death in the pediatric population with short stature may, in part, reflect illness severity or diminished access to nephrology care and is not entirely unexpected. However, the higher risk of death in children with short stature persisted despite adjustment for transplant as a time-dependent covariate in our primary model, especially if children were not underweight. Because improvements in height accrual are reported to occur after transplantation (17,18), we would have expected the higher mortality risk in children who may be short at the time of first RRT to attenuate with transplantation. However, our results demonstrated a persistently higher risk of death even when isolating the duration of follow-up to time with a transplant, although we were unable to account for the extent of renal function after transplantation. In our study, children with short stature had a higher prevalence of Medicaid use, which may be a marker of lower socioeconomic status that could partially explain the higher mortality risk in shorter children despite transplantation. Other factors such as nonadherence to therapy and lower access to care, which are commonly associated with short stature in children with CKD (19–21), may also be underlying factors that could contribute to the elevated mortality risk in children with short stature.
The higher risk of death in children with short stature was particularly evident for cardiac- and infection-related death. A higher risk of infectious complications is not unexpected because short stature may track with poor nutritional status, which may predispose children receiving RRT to infections (2,22). However, the reasons why short stature in children with kidney disease is associated with a higher risk of cardiac death are not entirely clear. In the general adult population, short stature has been linked genetically to a higher risk of atherosclerotic disease, but children with ESRD may not have genetic short stature (23,24).
Contrary to our hypothesis, we also found a higher risk of death among children with tall stature at the time of first RRT, especially if these tall children had elevated BMI or were white. It is unclear why the association between tall stature and higher mortality does not extend to black children, although our results are consistent with those found in black adult dialysis patients (6). We were also unable to address the reasons for the higher risk of mortality in children with tall stature, although we did find a higher risk of malignancy-related death. The higher risk of cancer in children with tall stature is consistent with previously reported associations described in the general adult population, where higher cell number and cell proliferation rates are suggested as potential explanations for the link between tall stature and cancer (5,25). In our study, taller children were more likely to have GN as the cause of their ESRD, and patients with GN may have been exposed to more lifetime immunosuppression and therefore be predisposed to a higher risk of cancer-related death. However, our study is limited by the lack of data on immunosuppressant exposure or exact type of malignancy (such as post-transplant lymphoproliferative disorder) both before and after the onset of ESRD.
Our novel finding of an association between tall stature at time of RRT and higher risk of death in children, especially those with elevated BMI, is consistent with results from two recent studies of adult dialysis patients (5,6). In a study by Shapiro and colleagues of over 117,000 prevalent adult hemodialysis patients, taller stature was associated with a higher risk of all-cause mortality and cause-specific (cardiac-, infectious-, and cancer-related) mortality (5). A more recent study performed in the USRDS of over one million adult dialysis patients also demonstrated a higher risk of death in taller persons (6). Adult studies have suggested that persons with tall stature may receive inadequate dialysis dosing (Kt/Vurea), which may be associated with a higher risk of death (5,26). However, the preferred modality of RRT in children is transplantation, and the majority of our cohort received at least one kidney transplant during the follow-up period. Thus, inadequate dialysis dosing seems less likely to be a contributor to the higher rates of death seen in taller children, although we did find that the higher mortality risk in tall children was attributable to the period of follow-up on dialysis.
The strengths of our study include the large size of our national cohort, the contemporary nature of the data, and a relatively large number of clinical outcomes. Limitations include the observational nature of these data, potential errors in data used for height and BMI determination (which may be influenced by presence of edema), and missing data in the USRDS, which may have caused some misclassification of height category, BMI category, transplant status, or excluded some children from our study. Additional limitations include the lack of data on growth hormone and immunosuppressant use both before and after onset of ESRD, exact type of malignancy (such as post-transplant lymphoproliferative disorder), and lack of data on height trajectory, although the focus of our study was on the relationship between achieved height at the time of ESRD onset and subsequent outcomes. Finally, it is unclear if height per se, versus factors that may track closely with height, including socioeconomic status, anemia, nutritional deficits, duration and treatment of CKD, or nonadherence, mediate the association identified between extremes of height and risk of mortality and transplantation.
In conclusion, the prevalence of short stature among children at time of first RRT remains high, and associations with higher mortality risk were still observed within this contemporary cohort of children treated with RRT. Tall stature is much less common but was associated with higher all-cause mortality, especially in children with elevated BMI, and with malignancy-associated mortality. Further studies are needed to determine reasons for the higher risk of mortality in children at both extremes of height.
Disclosures
None.
Acknowledgments
Because Kirsten Johansen is a Deputy Editor of the Clinical Journal of the American Society of Nephrology, she was not involved in the peer-review process for this manuscript. Another editor oversaw the peer-review and decision-making processes.
This work was supported by the American Kidney Fund and National Institutes of Health (NIH) (F32 DK098871 and KL2 TR00014 to E.K., K24 DK85153 to K.L.J., and K24 DK92291 to C.H.). This publication was also supported by the National Center for Advancing Translational Sciences, NIH, through University of California San Francisco Clinical and Translational Science Institute grant number UL1 TR000004.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Seikaly MG, Salhab N, Gipson D, Yiu V, Stablein D: Stature in children with chronic kidney disease: analysis of NAPRTCS database. Pediatr Nephrol 21: 793–799, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR: Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 17: 450–455, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA: Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 109: 909–913, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ: Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro BB, Streja E, Ravel VA, Kalantar-Zadeh K, Kopple JD: Association of Height with Mortality in Patients Undergoing Maintenance Hemodialysis. Clin J Am Soc Nephrol 10: 965–974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsayed ME, Ferguson JP, Stack AG: Association of height with elevated mortality risk in ESRD: variation by race and gender [published online ahead of print October 1, 2015]. J Am Soc Nephrol doi:10.1681/ASN.2014080821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku E, Glidden D, Hsu CY, Portale A, Grimes B, Johansen K: Association of body mass index with patient-centered outcomes in children with ESRD [published online ahead of print June 8, 2015]. J Am Soc Nephrol doi: 10.1681/ASN.2015010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michigan Population Studies Center: Zip Code Characteristics: Mean and Median Household Income, 2010. Available at: http://www.psc.isr.umich.edu/dis/census/Features/tract2zip/. Accessed September 5, 2015 [Google Scholar]
- 9.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2000 CDC Growth Charts for the United States: Methods and Development. Available at http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. Accessed March 3, 2014 [PubMed]
- 11.Nwosu BU, Lee MM: Evaluation of short and tall stature in children. Am Fam Physician 78: 597–604, 2008 [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 246: 1–190, 2002 [PubMed]
- 13.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C: Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36: 811–819, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hanevold CD, Ho PL, Talley L, Mitsnefes MM: Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 115: 352–356, 2005 [DOI] [PubMed] [Google Scholar]
- 15.CDC: About BMI for Children and Teens. Available at: http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html#interpreted. Accessed March 3, 2014
- 16.CDC: A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years), 2015. Available at: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed March 30, 2014
- 17.Franke D, Thomas L, Steffens R, Pavičić L, Gellermann J, Froede K, Querfeld U, Haffner D, Živičnjak M: Patterns of growth after kidney transplantation among children with ESRD. Clin J Am Soc Nephrol 10: 127–134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harambat J, Bonthuis M, van Stralen KJ, Ariceta G, Battelino N, Bjerre A, Jahnukainen T, Leroy V, Reusz G, Sandes AR, Sinha MD, Groothoff JW, Combe C, Jager KJ, Verrina E, Schaefer F; ESPN/ERA-EDTA Registry : Adult height in patients with advanced CKD requiring renal replacement therapy during childhood. Clin J Am Soc Nephrol 9: 92–99, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydın BK, Aycan Z, Sıklar Z, Berberoğlu M, Ocal G, Cetinkaya S, Baş VN, Kendirci HN, Cetinkaya E, Darcan S, Gökşen D, Evliyaoğlu O, Sükür M, Baş F, Darendeliler F: Adherence to growth hormone therapy: results of a multicenter study. Endocr Pract 20: 46–51, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Blydt-Hansen TD, Pierce CB, Cai Y, Samsonov D, Massengill S, Moxey-Mims M, Warady BA, Furth SL: Medication treatment complexity and adherence in children with CKD. Clin J Am Soc Nephrol 9: 247–254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenbaum LA, Hidalgo G, Chand D, Chiang M, Dell K, Kump T, Peschansky L, Smith HK, Boyle M, Kopf M, Metz LC, Kamel M, Mahan JD: Obstacles to the prescribing of growth hormone in children with chronic kidney disease. Pediatr Nephrol 23: 1531–1535, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Nelson CP, Hamby SE, Saleheen D, Hopewell JC, Zeng L, Assimes TL, Kanoni S, Willenborg C, Burgess S, Amouyel P, Anand S, Blankenberg S, Boehm BO, Clarke RJ, Collins R, Dedoussis G, Farrall M, Franks PW, Groop L, Hall AS, Hamsten A, Hengstenberg C, Hovingh GK, Ingelsson E, Kathiresan S, Kee F, König IR, Kooner J, Lehtimäki T, März W, McPherson R, Metspalu A, Nieminen MS, O’Donnell CJ, Palmer CN, Peters A, Perola M, Reilly MP, Ripatti S, Roberts R, Salomaa V, Shah SH, Schreiber S, Siegbahn A, Thorsteinsdottir U, Veronesi G, Wareham N, Willer CJ, Zalloua PA, Erdmann J, Deloukas P, Watkins H, Schunkert H, Danesh J, Thompson JR, Samani NJ; CARDIoGRAM+C4D Consortium : Genetically determined height and coronary artery disease. N Engl J Med 372: 1608–1618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takenaka T, Sato T, Hoshi H, Kato N, Sueyoshi K, Tsuda M, Watanabe Y, Takane H, Ohno Y, Suzuki H: Height constitutes an important predictor of mortality in end-stage renal disease. Cardiol Res Pract 242353: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR: Adult stature and risk of cancer. Cancer Res 48: 1658–1662, 1988 [PubMed] [Google Scholar]
- 26.Kuhlmann MK, König J, Riegel W, Köhler H: Gender-specific differences in dialysis quality (Kt/V): ‘big men’ are at risk of inadequate haemodialysis treatment. Nephrol Dial Transplant 14: 147–153, 1999 [DOI] [PubMed] [Google Scholar]