Abstract
Background and objectives
Guideline–recommended diagnostic criteria for hemodialysis (HD) catheter–related bloodstream infections (CRBSIs) are based on data from indwelling central catheters in patients not on HD and non-HD situations, and upon which peripheral vein cultures are the gold standard. We aimed to examine the validity of these criteria in patients on HD.
Design, settings, participants, & measurements
Adult patients on in-center HD using catheters were prospectively followed from 2011 to 2014 at a large academic–based HD facility (Toronto, Canada). When a CRBSI was suspected, blood culture sets were obtained from four sites (peripheral vein, both catheter hubs, and HD circuit) to determine the guideline–recommended differential time to positivity (DTTP). DTTP criteria were met when catheter hub cultures turned positive ≥120 minutes before peripheral vein cultures. The sensitivity, specificity, and accuracy were first calculated using peripheral vein cultures as the gold standard and then these same calculations were repeated with additional information, including exit site/catheter tip and HD circuit cultures, as the true gold standard. The feasibility of obtaining peripheral vein cultures was determined.
Results
Of 178 suspected CRBSIs, 100 had peripheral vein blood cultures. Using the true gold standard, sensitivity, specificity, and accuracy of blood culture results were highest in samples from the HD circuit (93.5%, 100%, and 95%, respectively). The guideline recommended combination of peripheral vein and arterial hub blood cultures was the least sensitive, specific, and accurate (91.7%, 93.1%, and 92.7%, respectively). The diagnostic criteria using measured DTTP were met in less than one third of events.
Conclusions
In patients on HD, blood culture results are the most sensitive, specific, and accurate for diagnosing CRBSIs when taken from the HD circuit and the venous catheter hub, and blood culture results are the least sensitive, specific, and accurate in any combination with peripheral vein cultures. The DTTP does not increase diagnostic accuracy, reducing the necessity for venipuncture and its potential vein damage. Future guidelines should consider the applicability of criterion on specific patient populations and tailor them accordingly.
Keywords: bacteremia; indwelling catheters; sensitivity and specificity; catheter-related infections; catheterization; catheters, indwelling; humans; phlebotomy; renal dialysis
Introduction
Catheter–related bloodstream infections (CRBSIs) are a feared hemodialysis complication, with increased patient morbidity, mortality, and associated financial burden for the health care sector (1–4). Timely and accurate diagnosis of CRBSI is crucial to target and treat the infecting microorganism with the most appropriate narrow–spectrum antibiotic, reduce unnecessary antibiotic exposure, prevent catheter removal when not indicated, and help anticipate infectious complications that may require additional investigations and interventions (5).
In 2009, the Infectious Disease Society of America (IDSA) published updated guidelines on the prevention and diagnosis of CRBSI, and for the first time, hemodialysis catheters were acknowledged as a separate entity (6). In these guidelines, the mainstay of the diagnosis is to obtain a set of peripheral blood cultures and compare them with blood cultures obtained from (1) the arterial or venous hemodialysis catheter hub meeting quantitative criteria (threefold higher count of CFU per milliliter in the catheter hub culture compared with the peripheral venous blood culture), (2) an arterial or venous catheter hub meeting criteria of differential time to positivity (DTTP: the blood culture from the catheter hub turning positive at least 2 hours before the peripheral blood culture), or (3) the hemodialysis catheter tip growing the same microorganism as the peripheral venous culture (which requires catheter removal for diagnosis).
These recommendations are in stark contrast to the current nonstandardized practices of diagnosing CRBSIs in most hemodialysis units, where one or more sets of blood cultures are obtained from the hemodialysis circuit, concurrent with the exclusion of other infectious sources and without attempting venipunctures for peripheral blood cultures (7–9).
The current recommendations are based on data from temporary central venous catheters used for medication and fluid administration in intensive care settings or deducted from permanent central venous catheters used for chemotherapy, total parenteral nutrition, or other nonhemodialysis uses. The generalizability of these guidelines to the hemodialysis population is questionable. For example, removing the hemodialysis catheter solely for diagnostic purposes is impractical, places patients at unnecessary risks associated with catheter intervention, and leaves the patient without access for dialysis. Also, unlike other central venous catheters used for fluid or other unidirectional administration of agents, hemodialysis catheters are part of a dynamic circuit that both receives and delivers blood during hemodialysis. Thus, blood circulating through an infected catheter during dialysis before cultures are obtained may dilute the density of microorganisms in the catheter, potentially rendering the DTTP criterion invalid for the diagnosis of CRBSI in patients on hemodialysis. Other factors that may affect the validity of these criterion are the variable transport and consequent processing times dictated by the locations and distances between freestanding dialysis units and receiving microbiology laboratories. Finally, peripheral venipuncture is often not possible, impractical, or purposefully avoided to preserve veins for future arteriovenous access creation.
In light of these considerations, there is a need to challenge an old paradigm, where the origins of many nephrology practices were borrowed and left largely unrefined to better suit the needs of our nephrology specialty and community. Thus, the main purposes of this study were to determine the applicability of IDSA criteria for the diagnosis of CRBSI in patients on hemodialysis and determine the diagnostic characteristics of an alternate real world approach to diagnosing CRBSI with blood cultures obtained from the dialysis circuit rather than the peripheral vein. We aimed to determine (1) the sensitivity, specificity, and accuracy of blood cultures obtained from the peripheral vein, dialysis circuit, and catheter hubs and (2) the feasibility of and barriers to peripheral vein blood cultures for the diagnosis of CRBSI. Importantly, we sought to validate the IDSA guideline’s recommendation to use DTTP to diagnose CRBSI in patients on hemodialysis.
Materials and Methods
This was a prospective study of an inception cohort within the University Health Network (UHN) hemodialysis program, and it was approved by the UHN nephrologists, infectious disease specialists, microbiologists, and research ethics review board. The program manages 250–300 patients on hemodialysis and has incorporated a multidisciplinary approach to vascular access and infection management (10). All adult patients on chronic hemodialysis with a central venous catheter and a suspected CRBSI (November of 2011 to April of 2014) were eligible for the study.
Hemodialysis nurses screened patients for vascular access–related infection as part of standard procedure during each hemodialysis session. The study protocol was implemented when the patient displayed signs or symptoms suspicious of CRBSI before or during the hemodialysis session, which included fever (>38.0°C before dialysis and >37.7°C during dialysis), chills, rigors, hypotension, and new unexplained malaise, with concurrent exclusion of catheter–unrelated infectious foci (determined by the nurse with or without evaluation by the rounding physician). When a patient became symptomatic during hemodialysis, hemodialysis was stopped for as long as necessary to obtain blood samples from both catheter hubs (typically ≤1 minute). Hemodialysis was not stopped to obtain peripheral vein or HD circuit blood cultures. Patients gave verbal consent for peripheral vein blood cultures, and written informed consent to participate in the study.
Four blood culture sets consisting of an aerobic and anaerobic adult blood culture bottle (10 ml blood each) from the dialysis circuit, the arterial and venous central catheter hubs, and a peripheral vein were obtained from the primary dialysis nurse using antiseptic technique (hubs and circuit port first cleansed with alcohol-based chlorhexidine) as per institutional policy and procedures. If the primary dialysis nurse was unsuccessful in obtaining the peripheral vein culture, another dialysis nurse or the dedicated UHN venipuncture team was asked to perform this procedure.
After bedside inoculation, the blood culture bottles were delivered to and processed at the University Health Network/Mount Sinai Hospital Microbiology Laboratory as per routine microbiology laboratory procedures. Blood culture bottles were placed in the BacT/ALERT 3D instrument (bioMérieux, Marcy l'Etoile, France) which continuously monitors for microbial growth records culture positivity every 15 minutes according to changes in fluorescence that relate to microbial growth. Time to positivity was determined for blood cultures with positive bacterial growth, and the DTTP was calculated. DTTP criteria were met when microbes were detected from catheter hub cultures >120 minutes earlier than in the concurrent peripheral vein culture (6). Colony counts were not measured.
To assess the feasibility of guideline recommendations to obtain peripheral vein blood cultures for the diagnosis of CRBSI, venipuncture was attempted in all patients in the study. The reasons for unsuccessful attempts were documented in the first 1 year of the study.
To assure the accuracy of blood culture draws, labeling, and processing, direct supervision and support were provided by the study team during the first 6 months of the study. Formal nursing in–services were held before the study started and then, yearly in addition to their regularly scheduled hemodialysis practice updates. The in–center dialysis physician also monitored the blood-drawing process.
A Hemodialysis Infection Control Subcommittee (HICS) independently confirmed the diagnoses of CRBSI and contamination (6,10,11). Contaminations were determined on the basis of the number of positive blood cultures and the identity of the organism (12). For example, one positive blood culture of four with Corynebacterium species as the organism would be determined to be a contamination. Partial positive events were blood cultures with more than one but less than four blood cultures growing the same organism, which is unlikely to be a contaminant (e.g., Enteroccocus species would not be considered a contaminant). Partial positives contributed to false negatives and true positives in the calculations on the basis of all available clinical and blood culture information as confirmed by the HICS (see below).
To assess the value of information gained from various blood culture sites, the sensitivity, specificity, and accuracy were calculated (13) using three approaches. The first was on the basis of the assumption that the peripheral vein culture is the gold standard—this approach considers the peripheral venous blood culture results and those from one other site (hubs or circuit). In the second approach, the real world peripheral hemodialysis circuit blood culture was assumed to be the gold standard. Finally, the third approach used all available information (a true gold standard; typically not obtained outside this research setting), including all blood cultures from one event (peripheral vein, arterial and venous hubs, and hemodialysis circuit) along with available exit site swabs and catheter tips to determine CRBSI by the HICS. All approaches considered the background clinical information of the suspected CRBSI.
Results
Patient Events
Two hundred ten patients were eligible to take part in this study, and all had cuffed, tunneled hemodialysis catheters. Patient and catheter characteristics are shown in Table 1.
Table 1.
Patient characteristics at the time of suspected catheter–related bloodstream infection
| Characteristics | Events, n=100 | Patients, n=62a |
|---|---|---|
| Demographics | ||
| Men (%) | 47 (51.6) | 35 (56.5) |
| Age, yr, mean (range, median) | 61 (26–88, 62) | 61 (26–88, 62) |
| Hemodialysis related | ||
| Hemodialysis vintage, mo, mean (range, median) | 71 (1–248, 54) | 63 (1–248, 47) |
| Central venous catheter, tunneled (%) | 100 (100) | 62 (100) |
| Central venous catheter location (%) | ||
| Right internal jugular | 70 (70) | 47 (75.8) |
| Left internal jugular | 24 (24) | 12 (19.4) |
| Right femoral | 4 (4) | 1 (1.6) |
| Left femoral | 1 (1) | 1 (1.6) |
| Right external jugular | 1 (1) | 1 (1.6) |
| Central venous catheter vintage, mean (range, median) d,b | 345 (1–1616, 172) | 338 (3–1475, 245) |
| Signs and symptoms on hemodialysis (%)c | Events, n=124 | Patients, n=74d |
| Fever (>37.8°C on hemodialysis) | 43 (34.7) | 27 (36.4) |
| Chills | 12 (9.7) | 9 (12.1) |
| Suspected exit site infection | 11 (8.9) | 10 (13.5) |
| Hypotension (SBP<100 mmHg or drop of SBP by >25 mmHg during hemodialysis) | 20 (16.1) | 9 (12.1) |
| Nausea or vomiting | 4 (3.2) | 1 (1.4) |
| Generalized pain | 4 (3.2) | 1 (1.4) |
| Weakness | 2 (1.6) | 0 |
| Feeling generally unwell | 8 (6.5) | 3 (4.1) |
| Shortness of breath | 2 (1.6) | 1 (1.4) |
| Undefined reason | 18 (14.5) | 13 (17.5) |
SBP, systolic BP.
Value related to the first event in the patients with multiple episodes of suspected hemodialysis catheter–related bloodstream infections.
Information on catheter vintage was not available for one patient, because catheter insertion occurred at another dialysis center.
Multiple signs and symptoms per event are reported.
Multiple signs and symptoms reported at the first event in the patients with multiple episodes of catheter–related bloodstream infections.
There were 178 events of suspected CRBSI in 87 patients, and in total, 654 blood cultures were drawn. Of these 178 events, 100 had blood cultures from a peripheral vein and either the hemodialysis circuit or both catheter hubs; these 100 events were included in this study. The most frequent signs or symptoms prompting the implementation of the study protocol were fever and chills (44.4%), hypotension or intradialytic hypotension (16.1%), and feeling generally unwell without an obvious cause (6.5%). In 14.5%, the specific reason was not documented (Table 1 shows details).
Blood Culture Events
The 100 events occurred in 62 patients (1.61 events per patient); of these, 55% had no bacterial growth. In five events, the patient had been on antibiotics before drawing blood cultures; 27 of these 100 events had the same microorganism growth in all of the blood cultures drawn. In six events, there was partial growth (i.e., bacteria was found in one or more but not all four blood cultures with an organism unlikely to represent merely a skin contaminant; e.g., Staphylococcus aureus). There were too few events in this subgroup to determine whether one site of blood draw underdetected bacterial growth compared with other sites (data not shown).
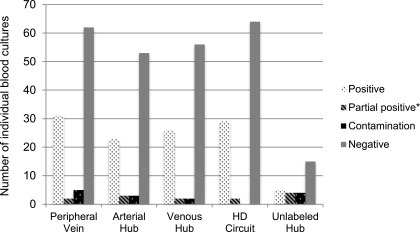
Contamination was determined (12) in 12 events: five from the peripheral vein, three from the arterial catheter hub, and two from the venous catheter hub. In two blood cultures, the source was a hub but without clear arterial or venous labeling. There was no contamination of blood cultures from the hemodialysis circuit (Figure 1).
Figure 1.
Distribution of bacterial growth depending on the site of blood culture draw. *The individual blood culture is negative for bacterial growth but parts of the blood culture set (peripheral vein, arterial hub, venous hub and HD circuit) from which it came had bacterial growth. HD, hemodialysis.
Blood cultures were drawn, on average, 2 hours and 18 minutes after hemodialysis initiation. One patient presented with clear CRBSI before starting hemodialysis (4 minutes before hookup), and one patient presented as long as 9 hours and 2 minutes after dialysis initiation. This latter patient was the only in-patient who was included at the beginning of the study and received sustained low–efficiency diafiltration in the intensive care unit. The average transit time of culture bottles to the microbiology laboratory and start of cultivation was 5 hours and 54 minutes. The time intervals between hemodialysis initiation, blood draws, and initiation of blood cultivation were similar in events with bacterial growth, no growth, and contamination.
Sensitivity, Specificity, and Accuracy of Blood Cultures
When the peripheral vein blood culture results are used as the gold standard as suggested by the IDSA 2009 guidelines, blood cultures obtained from the arterial catheter hub had sensitivity, specificity, and accuracy of 91.7%, 93.1%, and 92.7%, respectively. The venous catheter hub blood cultures had a sensitivity of 96.1%, a specificity of 95%, and an accuracy of 95.3%, and the hemodialysis circuit yielded a sensitivity of 89.7%, a specificity of 97.0%, and an accuracy of 94.7%. Using the real world peripheral sample obtained from the hemodialysis circuit as the gold standard, the sensitivity, specificity, and accuracy of blood cultures drawn from the arterial catheter hub were 95.8%, 94.8%, and 95.1%, respectively. The sensitivity, specificity, and accuracy of blood cultures obtained from the venous catheter hub were 96.3%, 95%, and 95.4%, respectively. Using results from all blood cultures available and adding clinical information from exit site swabs and catheter tips (the true gold standard), the peripheral vein had a sensitivity of 93.9%, a specificity of 92.5%, and an accuracy of 93%. The values for sensitivity, specificity, and accuracy for the arterial hub were 88.5%, 94.6%, and 92.7%, respectively; for the venous hub, the values for sensitivity, specificity, and accuracy were 92.9%, 96.6%, and 95.3%, respectively, and for the hemodialysis circuit, the values for sensitivity, specificity, and accuracy were 93.5%, 100%, and 95%, respectively.
Time to Positivity and DTTP
In total, 95 blood culture sets were assessed for time to positivity. The time to positivity was dependent on microorganism growth and registered earliest at 142 minutes (2 hours and 22 minutes) and as late as 3909 minutes (2.7 days) after blood cultures were received in the microbiology laboratory. The median times to positivity were 777, 744, 793, and 856 minutes (12 hours and 57 minutes, 12 hours and 24 minutes, 13 hours and 12 minutes, and 14 hours and 15 minutes, respectively) in the arterial hub, venous hub, hemodialysis circuit, and peripheral blood cultures, respectively.
DTTP was calculated from corresponding blood cultures of the individual sets in combinations of peripheral vein and arterial catheter hub, peripheral vein and venous catheter hub, and peripheral vein and hemodialysis circuit. Average DTTPs were −106, −176, and −107 minutes, respectively (with the minus sign before the number indicating earlier growth in arterial and venous hubs and hemodialysis circuit), with ranges of −1504 to +2787 minutes (arterial hub), −1710 to +1141 minutes (venous hub), and −1505 to +795 minutes (hemodialysis circuit). Criteria of time to positivity confirming CRBSI as per IDSA 2009 guidelines were met in 33% (arterial hub) and 29% (venous hub). Table 2 shows comparisons of time to positivity and DTTP.
Table 2.
Results of blood culture sets obtained (n=100)
| Bacterial Growth of Blood Culture Sets | All Positive | Partial Positive | Contamination | Negative |
|---|---|---|---|---|
| n | 27 | 6 | 12 | 55 |
| Antibiotics given before BC | 2 | 1 | 0 | 2 |
| Time from dialysis start to BC draw, h:min, mean (range, median) | 2:24 (0:03–7:10, 2:25) | 1:33 (0:41–2:09, 1:33) | 2:13 (0:26–4:57, 2:17) | 2:22 (−0:04–9:02, 2:09) |
| Time to sample cultivation, h:min, mean (range, median) | 4:27 (0:05–17:07, 3:43) | 8:54 (3:18–18:40, 8:11) | 7:40 (1:46–20:16, 4:21) | 5:48 (1:36–19:36, 4:56) |
| Mean TTP, min | Range, min | Median, min | ||
|---|---|---|---|---|
| Peripheral vein | 1092 | 292–2502 | 856 | |
| HD circuit | 966 | 163–2925 | 793 | |
| Arterial hub | 1025 | 142–3909 | 777 | |
| Venous hub | 947 | 280–2493 | 744 |
| Mean DTTP, min | Range, min | Median, min | Meeting Criteria of DTTP, % | |
|---|---|---|---|---|
| PV and HD circuit | −107 | −1505–795 | −9 | 28 |
| PV and arterial hub | −106 | −1504–2787 | −4 | 33 |
| PV and venous hub | −176 | −1710–1141 | −11 | 29 |
| HD circuit and arterial hub | 35 | −794–2799 | −2 | |
| HD circuit and venous hub | −33 | −795–1241 | −2 | |
| Arterial hub and venous hub | −106 | −2577–586 | 0 |
BC, blood culture; TTP, time to positivity; HD, hemodialysis; DTTP, differential time to positivity; PV, peripheral vein.
The ranges of DTTP were similar in blood culture sets taken <30, 60–120, and >120 minutes of hemodialysis initiation. Thus, taking blood cultures within the first 30 minutes of the dialysis session did not improve the likelihood of meeting DTTP criteria. Furthermore, the DTTP did not differ by type of microorganism (Table 3).
Table 3.
Microorganisms grown in culture sets
| Organisma | n | % | Time to Positivity Mean, h | Range (Median) |
|---|---|---|---|---|
| Gram-positive bacteria, total | 86 | 22.6 | ||
| Methicillin–sensitive Staphylococcus aureus | 36 | 9.5 | 13.2 | 2.4–25.8 (12.7) |
| Methicillin–resistant Staphylococcus aureus | 4 | 1 | 11.4 | 8–13.5 (11.9) |
| Coagulase-negative Staphylococcus | 13 | 3.4 | 25.9 | 7.7–84.3 (20.9) |
| Other Staphylococci | 6 | 1.6 | 21 | 14.8–32.6 (17.35) |
| Group B Streptococcus | 4 | 1 | 10.1 | 9.6–11.6 (9.61) |
| Enterococcus faecalis | 8 | 2.1 | 16 | 7.9–38.1 (10.8) |
| Otherb | 15 | 3.9 | 34.3 | 10.8–78.8 (35.5) |
| Gram-negative bacteria, total | 28 | 7.4 | ||
| Serratia marcescens | 20 | 5.3 | 22.2 | 10.6–65.2 (16.2) |
| Escherichia coli | 4 | 1 | 11.8 | 11.7–12.6 (11.8) |
| Proteus mirabilis | 4 | 1 | 15.9 | 15.8–15.9 (15.9) |
| No bacterial growth | 266 | 70 |
There were no blood cultures with fungus growth.
Exiguobacterium species, Kocuria kristinae, Micrococcus, Corynebacterium, Arcanobacterium haemolyticum, and Gemella species.
Microorganisms Cultivated
The majority of blood cultures (n=266 or 70%) had no bacterial growth after 5 days of cultivation. Of the positive blood cultures, the majority was Gram-positive bacteria and skin flora (Table 3). Bacteria detected in contaminated cultures are excluded from the breakdown of bacterial growth.
Feasibility of Peripheral Vein Blood Cultures during Hemodialysis
Within the first year of this study, 52 patients with suspected CRBSIs were assessed for feasibility of obtaining peripheral vein blood cultures during their hemodialysis sessions. In 39 (75%) patients, attaining peripheral vein blood cultures was successful. Poor vessel quality and patient refusal were the major reasons for unsuccessful attempts (Table 4). In the completed study, 56% had successful peripheral venous access.
Table 4.
Feasibility of obtaining peripheral blood cultures
| Characteristics | Successful Venipuncture | No Peripheral Blood Obtained |
|---|---|---|
| Total, n (%) | 39 (75) | 13 (25) |
| BC obtained without problems, n (%) | 30 (57.6) | N/A |
| BC obtained with problems, n (%) | 9 (17.3) | N/A |
| Poor veins, n (%) | 3a | 4 (7.7) |
| Collapsing veins, n | 3a | 0 |
| iv Nurse to draw BC, n | 3a | 0 |
| First attempt failed, n (%) | 7a | 4 (7.7) |
| Second attempt failed, n | 3a | 3b |
| Third attempt failed, n | 0 | 3b |
| Third attempt successful, n | 3a | N/A |
| Patient refused venipuncture, n (%) | N/A | 5 +1b,c (9.6) |
Data on feasibility of obtaining blood cultures (BCs) from peripheral veins during hemodialysis. In the first year of the study, additional data were collected on the ease and barriers to obtaining a peripheral BC. N/A, not applicable.
Multiple reasons for problems during venipuncture reported.
Patients already accounted for in the first failed attempt.
Five patients refused venipuncture immediately (and one after the first failed attempt).
Discussion
The main finding of this study is that the same bacterial microorganisms can be cultured from the hemodialysis circuit, the dialysis arterial and venous catheter hubs, and the peripheral vein during hemodialysis without loss of information. In fact, the sensitivity, specificity, and accuracy data indicate that the information gained from the dialysis circuit and the venous catheter hub is superior to that from any combination of a peripheral vein blood culture and the dialysis catheter hubs or the hemodialysis circuit. On its own, blood cultures from the hemodialysis circuit had the greatest sensitivity, specificity, and accuracy (93.5%, 100%, and 95%, respectively). This is an extremely important and clinically relevant finding that validates the day-to-day practice of a majority of dialysis units worldwide in diagnosing CRBSIs. These findings are unable to support the IDSA 2009 guideline recommendations on diagnosing CRBSI in patients on hemodialysis. This is not surprising, because these guidelines have never been tested in patients on hemodialysis but were deduced from infectious events in patients not on hemodialysis with indwelling catheters typically used for unidirectional administration of intravenous fluids: a contrast from the dynamic, continuous hemodialysis catheter-patient-hemodialysis blood circuit.
Furthermore, the IDSA guidelines make recommendations that are not practically feasible for hemodialysis units. For example, in many countries, such as the United States and Canada, the use of quantitative blood sampling is available in limited facilities for research purposes only and not for clinical diagnosis. The use of peripheral vein blood cultures for the diagnosis of CRBSI was found to be unnecessary in this study. Patients on hemodialysis and staff are educated to preserve their vessels for arteriovenous access; this study allows staff to comfortably and reliably attain the necessary blood cultures for diagnosing CRBSI without the conflict of potentially causing patient discomfort or damaging peripheral veins. Additionally, there was no association between microorganism type cultivated and ease of cultivation by location of blood draw. However, more contaminations were found in peripheral vein cultures, albeit the percentage remained below the facility’s acceptable rate. We speculate that the main reason for higher contaminations in peripheral vein cultures was venipuncture difficulties, because there were fewer contaminations in arterial and venous catheter hub cultures and none from the hemodialysis circuit. Contamination rates can be improved by using specially trained personnel familiar with proper antisepsis for this procedure (14), but despite our diligent access to the specialized institutional venipuncture team, the highest rate of contaminations was derived from peripheral vein blood cultures.
Peripheral vein blood cultures were possible in only 75% of patients in the first year of the study and 56% of patients overall. This can be explained by both patient and providers factors. For example, scarred or damaged vessels may collapse during dialysis (considering that approximately 200 ml blood is redistributed in the hemodialysis circuit with or without hypotension). Furthermore, staff may have lost competency to perform venipuncture in the absence of ongoing training and lack of practice because of the low frequency of suspected CRBSI and need for peripheral vein cultures. Finally, the real necessity of peripheral vein blood cultures in the absence of evidence that it improves accuracy in diagnosing CRBSI is questioned. Indeed, we present evidence to abandon peripheral vein blood cultures for the diagnosis of CRBSI. This highlights the importance of challenging old paradigms when nephrology practices adopt established processes created by other specialties without the necessary supporting evidence that such processes are truly applicable to nephrology. Perhaps a change in paradigm incited by this study will encourage alternate appropriate processes for greater CRBSI diagnosis. A large prospective study of nearly 800 outpatients on hemodialysis found that 68% of suspected CRBSIs were treated empirically without first obtaining cultures. In one center, only 3% of patients suspected of having CRBSI had blood cultures performed (15). It is likely that a more practical and efficient method of obtaining blood cultures from the hemodialysis circuit may remove barriers and improve the frequency of diagnosis of CRBSI in both the clinical and research settings.
Our study reveals that the DTTP is not helpful in diagnosing CRBSI as evidenced by the wide, highly variable, and nondiscriminating time intervals. The dynamic catheter-patient-hemodialysis blood circuit may play a role in diluting a potential differential in concentration of microorganisms by location. Because we do not have data on blood samples from a clearly defined noncatheter infectious source, such as pneumonia or urosepsis, we cannot compare or comment on the DTTP in these situations.
Our study has several limitations. First, the numbers of blood cultures are small because of our very low CRBSI rates (<1.0/1000 catheter-days) (10). However, data were collected over 2–3 years with multidisciplinary engagement, including the hospital venipuncture team, and this is the only study of such kind in patients on hemodialysis. Second, having four blood culture sets (eight bottles) could potentially confuse labeling. Labeling blood culture bottles immediately before drawing blood directly into the bottles mitigated this concern. Also, the stated time of blood culture draw was taken from the time of label printing (automatically provided), which reflects an approximate time rather than the actual time of blood draw. Third, the time to positivity measurement began when blood cultures arrived in the microbiology laboratory; however, the transit time from blood culture draw to the laboratory varied significantly but was, on average, below the threshold of 6 hours. Haimi-Cohen et al. (16) reported a decrease in time to positivity in blood cultures by 1.8 and 2.1 hours when incubation was delayed by 6 or 12 hours after inoculation, respectively. Our presented time intervals between inoculation and incubation are representative of the majority of dialysis units, which have regularly scheduled transports to the microbiology laboratory. We submit that this transport time may be even more variable in rural or remote, free–standing dialysis units because of their physically separate locations from microbiology laboratories. Fourth, we did not evaluate the IDSA criteria for diagnosing CRBSI using quantitative blood cultures. This would not have represented a practical approach to diagnosing CRBSI in the clinical setting, because this testing is only available for specific research purposes (above). The cost and logistics of such testing make it unfeasible and unsustainable for routine clinical diagnosis of CRBSI in patients on hemodialysis for most centers in North America.
For the diagnosis of CRBSI, blood culture results are most sensitive, specific, and accurate when taken from the hemodialysis circuit and the venous catheter hub and least sensitive, specific, and accurate when taken from any combination with peripheral vein blood cultures, making venipuncture unnecessary. The DTTP between the hemodialysis catheter and peripheral vein blood cultures is not maintained during dialysis and not useful for the diagnosis of CRBSI.
Disclosures
None.
Acknowledgments
We thank all of our patients on dialysis who agreed to have more than the standard two blood culture sets taken, the hemodialysis nurses for their patient assessments for potential catheter–related bloodstream infections, the University Health Network venipuncture team for providing help with venipunctures when it was thought to be impossible, and Pauline Lo and Alyssa Loughborough for their help in obtaining the time-to-positivity data from the microbiology laboratory.
This study was supported, in part, by peer–reviewed grant funding from the Physician’s Services Incorporated (PSI) Foundation.
The PSI did not have input into the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Optimal Approach for the Diagnosis of Hemodialysis Catheter–Related Bacteremia,” on pages 756–758.
References
- 1.Maraj S, Jacobs LE, Kung SC, Raja R, Krishnasamy P, Maraj R, Braitman LE, Kotler MN: Epidemiology and outcome of infective endocarditis in hemodialysis patients. Am J Med Sci 324: 254–260, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ishani A, Collins AJ, Herzog CA, Foley RN: Septicemia, access and cardiovascular disease in dialysis patients: The USRDS Wave 2 study. Kidney Int 68: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R, Lacson E Jr.: Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis 61: 123–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis SS, Sexton DJ: Metastatic complications of bloodstream infections in hemodialysis patients. Semin Dial 26: 47–53, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allon M: Dialysis catheter-related bacteremia: Treatment and prophylaxis. Am J Kidney Dis 44: 779–791, 2004 [PubMed] [Google Scholar]
- 8.Allon M: Treatment guidelines for dialysis catheter-related bacteremia: An update. Am J Kidney Dis 54: 13–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallen AJ: Identifying and classifying bloodstream infections among hemodialysis patients. Semin Dial 26: 407–415, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Battistella M, Bhola C, Lok CE: Long-term follow-up of the Hemodialysis Infection Prevention with Polysporin Ointment (HIPPO) Study: A quality improvement report. Am J Kidney Dis 57: 432–441, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Division of Nosocomial and Occupational Infectious Diseases, Bureau of Infectious Diseases, Laboratory Centre for Disease Control, Health Canada : Preventing infections associated with indwelling intravascular access devices. Can Commun Dis Rep 23[Suppl 8]: i-iii, 1–32, i-iv, 1–16, 1997 [PubMed] [Google Scholar]
- 12.Hall KK, Lyman JA: Updated review of blood culture contamination. Clin Microbiol Rev 19: 788–802, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kestenbaum B: Epidemiology and Biostatistics: An Introduction to Clinical Research, New York, Springer-Verlag, 2009 [Google Scholar]
- 14.Surdulescu S, Utamsingh D, Shekar R: Phlebotomy teams reduce blood-culture contamination rate and save money. Clin Perform Qual Health Care 6: 60–62, 1998 [PubMed] [Google Scholar]
- 15.Tokars JI, Light P, Anderson J, Miller ER, Parrish J, Armistead N, Jarvis WR, Gehr T: A prospective study of vascular access infections at seven outpatient hemodialysis centers. Am J Kidney Dis 37: 1232–1240, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Haimi-Cohen Y, Vellozzi EM, Rubin LG: Concentration of staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J Clin Microbiol 40: 898–901, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]