Abstract
Background and objectives
The effect of mammalian target of rapamycin (mTOR) inhibitors has never been tested in patients with autosomal dominant polycystic kidney disease (ADPKD) and severe renal insufficiency.
Design, setting, participants, & measurements
In this academic, prospective, randomized, open label, blinded end point, parallel group trial (ClinicalTrials.gov no. NCT01223755), 41 adults with ADPKD, CKD stage 3b or 4, and proteinuria ≤0.5 g/24 h were randomized between September of 2010 and March of 2012 to sirolimus (3 mg/d; serum target levels of 5–10 ng/ml) added on to conventional therapy (n=21) or conventional treatment alone (n=20). Primary outcome was GFR (iohexol plasma clearance) change at 1 and 3 years versus baseline.
Results
At the 1-year preplanned interim analysis, GFR fell from 26.7±5.8 to 21.3±6.3 ml/min per 1.73 m2 (P<0.001) and from 29.6±5.6 to 24.9±6.2 ml/min per 1.73 m2 (P<0.001) in the sirolimus and conventional treatment groups, respectively. Albuminuria (73.8±81.8 versus 154.9±152.9 μg/min; P=0.02) and proteinuria (0.3±0.2 versus 06±0.4 g/24 h; P<0.01) increased with sirolimus. Seven patients on sirolimus versus one control had de novo proteinuria (P=0.04), ten versus three patients doubled proteinuria (P=0.02), 18 versus 11 patients had peripheral edema (P=0.04), and 14 versus six patients had upper respiratory tract infections (P=0.03). Three patients on sirolimus had angioedema, 14 patients had aphthous stomatitis, and seven patients had acne (P<0.01 for both versus controls). Two patients progressed to ESRD, and two patients withdrew because of worsening of proteinuria. These events were not observed in controls. Thus, the independent data and safety monitoring board recommend early trial termination for safety reasons. At 1 year, total kidney volume (assessed by contrast–enhanced computed tomography imaging) increased by 9.0% from 2857.7±1447.3 to 3094.6±1519.5 ml on sirolimus and 4.3% from 3123.4±1695.3 to 3222.6±1651.4 ml on conventional therapy (P=0.12). On follow-up, 37% and 7% of serum sirolimus levels fell below or exceeded the therapeutic range, respectively.
Conclusions
Finding that sirolimus was unsafe and ineffective in patients with ADPKD and renal insufficiency suggests that mTOR inhibitor therapy may be contraindicated in this context.
Keywords: randomized controlled trials; sirolimus; kidney failure, chronic; adverse effects; proteinuria; Adult; Humans; Polycystic Kidney, Autosomal Dominant; Prospective Studies; Renal Insufficiency
Introduction
In total, 8%–10% of patients with ESRD have autosomal dominant polycystic kidney disease (ADPKD) (1), an inherited systemic disorder of relentless cyst enlargement caused by fluid transport into the cavities generated by uncontrolled renal tubular cell proliferation. cAMP accumulation and Ser/Thr kinase mammalian target of rapamycin (mTOR) activation mediate cyst expansion (2–5), whereas mTOR inhibition with sirolimus or everolimus slowed cyst growth and preserved renal function in a variety of animal models of polycystic kidney disease (4,6–8).
After observational findings that, in patients with ADPKD receiving a kidney transplant, cyst growth was slowed by sirolimus–based immunosuppressive therapy (4), a pilot, prospective, randomized, crossover trial found that 6-month sirolimus therapy, unlike conventional therapy, halted the growth of total cyst volume in 15 patients with normal renal function or mild to moderate renal dysfunction (9). However, two subsequent large clinical trials (10,11) failed to show a clear beneficial effect of either sirolimus or everolimus in patients with CKD stages 2–3b renal function.
To address whether mTOR inhibitors might have any therapeutic role in more advanced phases of the disease, we tested the effect of sirolimus on disease progression in patients with ADPKD and severe renal insufficiency (SIRENA 2 Study) in the context of a single–center, randomized, 3-year clinical trial registered in June of 2007 with the ClinicalTrials.gov number NCT01223755 (Supplemental Appendix 1).
Materials and Methods
Patients aged ≥18 years old with ADPKD and eGFR (by Modification of Diet in Renal Disease equation) =15–40 ml/min per 1.73 m2 and proteinuria ≤0.5 g/24 h were eligible. Those with concomitant glomerular or urinary tract disease, diabetes, cancer, psychiatric disorders, and any condition that might confound data interpretation or prevent full comprehension of the purposes and risks of the study were excluded as well as pregnant or breastfeeding women and women of childbearing potential without effective contraception (the protocol is at http://clintrials.marionegri.it/index.php/electronictrials/completed-electronic-trials.html). Eligible participants identified among patients referring to the Outpatient Clinic of the Unit of Nephrology of the Azienda Ospedaliera Papa Giovanni XXIII who provided written informed consent were randomized between September of 2010 and March of 2012. The study conformed to the principles of the Declaration of Helsinki and was approved by the local ethical committee. It was coordinated, monitored, and reported by the Clinical Research Center for Rare Diseases “Aldo e Cele Daccò,” IRCCS—Istituto di Ricerche Farmacologiche “Mario Negri” according to the Consolidated Statement of Reporting Trials guidelines (Supplemental Table 1). Data were recorded locally by an electronic case report form implemented by the Biomedical Technologies Laboratory of the Clinical Research Center. Locations of the source data were specified and listed at the center initiation visit.
Objectives
This single–center, academic, prospective, randomized, open label, blind end point, parallel group trial was organized into two phases. A core study primarily aimed to assess whether 12-month treatment with sirolimus added on to conventional treatment significantly reduced measured GFR decline (12,13) versus conventional treatment alone and was safe. Evidence that sirolimus may safely slow GFR decline would have provided the background for an extension phase to evaluate treatment effect on kidney and cystic growth and progression to ESRD over an additional 2-year follow-up. Because of the discouraging results of the core study, the extension phase was aborted.
Randomization, Allocation Concealment, and Follow-Up
An independent investigator (G. Giuliano) centrally randomized patients by telephone call to sirolimus (Rapamune; Pfizer Inc., New York, NY) or conventional treatment. A computer–generated randomization list (1:1 ratio and four or eight random block size) was created at the Laboratory of Biostatistics of the Clinical Research Center by using SAS software, version 9 (SAS Institute Inc., Cary, NC). Patients and their physicians were aware of treatment allocation, whereas outcome assessors were blinded. Sirolimus was started at 3 mg/d and subsequently titrated to target blood trough levels between 5 and 10 ng/ml. Drug levels were measured by HPLC (14).
BP (mean of three consecutive measurements) and laboratory parameters were evaluated at baseline and every 3 months thereafter. GFR was measured every 6 months by iohexol plasma clearance (12,13). Computed tomography images were acquired and analyzed at baseline and 12 months as previously reported (15,16) (Supplemental Appendix 2).
Stopping Rules
Interim analyses were preplanned at core study end to assess whether, on the basis of predefined safety and efficacy criteria, patients could enter the extension phase (Supplemental Table 2). Statistical stopping criteria were on the basis of analyses of the efficacy outcome. The critical value for the test was set to have a value of 0.005 (analysis 1) or 0.049 (analysis 2). The Data Safety and Monitoring Board (DSMB) (Supplemental Appendices 2 and 3), however, could also stop the study on the basis of clinical judgment of safety and efficacy outcome variables, including treatment–related side effects, new onset (urinary protein excretion >0.5 g/24 h in patients without preexisting proteinuria) or worsening (doubling of 24-hour urinary protein excretion compared with previous values) of proteinuria, and serum creatinine increases >25% compared with previous levels.
Sample Size Estimation
On the basis of data from patients with ADPKD and severe renal insufficiency maintained on conservative therapy in the context of the Ramipril Efficacy in Nephropathy Study (17), we predicted a 1-year mean (SD) GFR reduction versus baseline of 6.31 (±4.47) ml/min per 1.73 m2. Assuming a 65% reduction from 6.31 to 2.2 ml/min per 1.73 m2 by sirolimus treatment, we calculated that 20 patients per group had to complete the study to provide the analysis with an 80% power to detect a significantly (two-sided test; α=0.05) different change in GFR between treatment groups.
Statistical Analyses
Statistical analyses were performed according to a modified intention to treat approach (18) without replacing missing data (19) by using the SAS software, version 9 (SAS Institute Inc.) and the STATA software, version 13 (StataCorp., College Station, TX). Between-group changes in clinical and laboratory parameters before and after sirolimus or conventional treatment were assessed by analysis of covariance adjusted for baseline measurements (at randomization). Within-group changes in clinical and laboratory parameters were assessed by paired t test or Wilcoxon rank sum test (for continuous variables) and repeated measures ANOVA or McNemar test (for categorical variables) as appropriate. Relationships between continuous variables were assessed by means of Pearson r or Spearman rho correlation coefficient. Data were expressed as means±SDs or medians and interquartile ranges as appropriate. As per protocol, multiplicity adjustments were not planned for secondary efficacy and safety variables, subgroup analyses, supportive analyses, or sensitivity analyses. All tests were two sided, and P<0.05 was deemed statistically significant.
Results
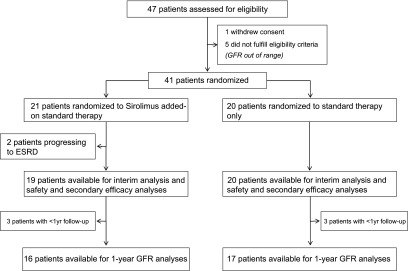
Of 47 assessed patients, one withdrew consent, and five had eGFRs out of range. Of 41 included participants, 21 were randomized to sirolimus added on to conventional treatment, and 20 were randomized to conventional treatment alone (Figure 1). Main patient characteristics were similar between groups (Table 1): 20 patients on sirolimus and 19 patients on conservative therapy only were on antihypertensive therapy, with average numbers of 2.2 and 2.0 medications per patient, respectively.
Figure 1.
Study flow diagram.
Table 1.
Demographic, anthropometric, clinical, laboratory, and kidney function parameters and concomitant medications at baseline according to randomization to sirolimus added on to conventional therapy (sirolimus) or conventional therapy only (conventional)
| Patients Parameters and Medications | Sirolimus, n=21 | Conventional, n=20 |
|---|---|---|
| Age, yr | 49.0 (7.1) | 47.6 (8.1) |
| Men, no. (%) | 9 (42.9) | 8 (40.0) |
| Height, cm | 168.7 (10.1) | 168.5 (10.3) |
| Weight, kg | 73.5 (14.3) | 73.8 (17.8) |
| BP, mmHg | ||
| Systolic | 136.3 (10.6) | 133.8 (14.4) |
| Diastolic | 86.1 (7.7) | 85.5 (8.4) |
| Mean | 102.8 (7.8) | 101.6 (9.8) |
| Laboratory parameters | ||
| AST, U/L | 18.5 (3.5) | 19.4 (5.1) |
| ALT, U/L | 16.7 (4.6) | 16.4 (4.9) |
| GGT, U/L | 24.0 (14.9) | 22.2 (9.7) |
| Alkaline phosphatase, U/L | 66.2 (14.4) | 58.5 (18.5) |
| Calcium, mg/dl | 9.3 (0.3) | 9.2 (0.5) |
| Phosphorus, mg/dl | 4.0 (0.5) | 3.7 (0.4) |
| Sodium, mEq/L | 139.9 (1.8) | 140.0 (1.6) |
| Potassium, mEq/L | 4.3 (0.4) | 4.1 (0.6) |
| Blood glucose, mg/dl | 89.8 (11.4) | 88.4 (12.2) |
| Uric acid, mg/dl | 6.6 (1.3) | 7.1 (1.5) |
| Total cholesterol, mg/dl | 201.7 (27.6) | 203.9 (25.7) |
| LDL cholesterol, mg/dl | 125.3 (23.9) | 127.5 (32.4) |
| HDL cholesterol, mg/dl | 47.5 (10.3) | 51.8 (14.3) |
| Triglycerides, mg/dl | 120.4 (37.2) | 105.8 (45.6) |
| Leukocytes, ×103/μl | 5.7 (1.5) | 5.6 (1.8) |
| Hemoglobin, g/dl | 12.3 (1.6) | 12.4 (1.2) |
| Hematocrit, % | 37.1 (5.0) | 37.5 (3.5) |
| Platelets, ×103/μl | 194.2 (56.4) | 188.1 (46.6) |
| Kidney function parameters | ||
| Serum creatinine, mg/dl | 2.89 (0.62) | 2.52 (0.49) |
| GFR, ml/min per 1.73 m2 | 26.8 (5.6) | 30.8 (6.6) |
| Albuminuria, μg/min | 43.0 (23.8–84.1) | 53.4 (42.8–131.7) |
| Proteinuria, g/24 h | 0.25 (0.16–0.36) | 0.24 (0.15–0.45) |
| Concomitant medications, no. (%) | ||
| ACE inhibitors | 12 (57.1) | 11 (55.0) |
| ARBs | 7 (33.3) | 8 (40.0) |
| CCBs | 9 (42.9) | 5 (25.0) |
| α-Blocking agents | 2 (9.5) | 6 (30.0) |
| β-Blockers | 7 (33.3) | 3 (15.0) |
| Diuretics | 6 (28.6) | 5 (25.0) |
| Statins | 2 (9.5) | 3 (15.0) |
| Anticoagulants | 0 (–) | 2 (10.0) |
| Iron | 1 (4.8) | 1 (5.0) |
| ESAs | 3 (14.3) | 1 (5.0) |
| Calcium | 1 (4.8) | 1 (5.0) |
| Vitamin D | 5 (23.8) | 6 (30.0) |
| Bicarbonate | 2 (9.5) | 2 (10.0) |
| PPIs | 3 (14.3) | 3 (15.0) |
Values are mean (SD), median (interquartile range), or number (percentage). GFR was by the iohexol plasma clearance technique. Mean BP = (systolic BP +2× diastolic BP)/3. AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl-transpeptidase; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; ESA, erythropoiesis-stimulating agent; PPI, proton pump inhibitor.
Safety and Tolerability
In >1 year of follow-up, proteinuria ensued de novo in seven patients (33.3%) on sirolimus versus one patient (5.0%) on conventional therapy (P=0.04). Ten patients on sirolimus (47.6%), including seven with new onset of proteinuria, doubled their proteinuria versus baseline compared with three patients (15.0%) on conventional therapy (P=0.02). Among patients on sirolimus, two were prematurely withdrawn because of worsening of proteinuria, and two progressed to ESRD. Serum creatinine increased by >25% versus baseline in ten patients on sirolimus and eight patients on conventional therapy (P=0.62).
Serious adverse events were observed in six patients on sirolimus and six patients on conventional treatment. One event in the sirolimus group (severe peripheral edema) was considered as treatment related (Table 2). There were 81 nonserious adverse events in the sirolimus group and 37 nonserious adverse events in the control group. Treatment-related events included aphthous stomatitis (n=14), acne (n=7; P<0.001 and P<0.01 versus conventional therapy, respectively), transient watery diarrhea (n=4), and angioedema (n=3). All patients with angioedema were on angiotensin–converting enzyme (ACE) inhibitor therapy. There were also significantly more cases of peripheral edema (18 versus 11; P=0.04) and upper respiratory tract infection (14 versus 6; P=0.03) in patients on sirolimus than in controls (Table 2). Other events were similarly distributed between groups.
Table 2.
Number (percentage) of patients with at least one serious or nonserious adverse event over the 1-year follow-up period according to randomization to sirolimus added on to conventional therapy (sirolimus) or conventional therapy only (conventional)
| Adverse Events | Sirolimus, n=21 | Conventional, n=20 |
|---|---|---|
| Serious | ||
| ESRD | 2 (9.5) | 0 |
| Acute diverticulitis | 1 (4.8) | 0 |
| Anal fissures, broncopneumoniaa | 1 (4.8) | 0 |
| Peripheral edemab | 1 (4.8) | 0 |
| Renal cyst rupture | 1 (4.8) | 0 |
| Inguinal hernia, gastroenteritis, pneumoniaa | 0 | 1 (5) |
| Chest pain | 0 | 1 (5) |
| Acute kidney function worsening | 0 | 1 (5) |
| Atrial fibrillation, ventricular extrasystolesa | 0 | 1 (5) |
| Acute bronchitis | 0 | 1 (5) |
| Hematuria | 0 | 1 (5) |
| Nonserious | ||
| Peripheral edema | 18 (85.7) | 11 (55.0)c |
| Aphthous stomatitisb | 14 (66.7) | 0d |
| Upper respiratory tract infections | 14 (66.7) | 6 (30.0)c |
| Acneb | 7 (33.3) | 0c |
| Dyspepsia | 5 (23.8) | 2 (10.0) |
| Diarrheab | 4 (19.0) | 0 |
| Dysmenorrhea | 4 (19.0) | 1 (5.0) |
| Arrhythmias | 4 (19.0) | 4 (20.0) |
| Dermatitis | 3 (14.3) | 1 (5.0) |
| Urinary tract infections | 3 (14.3) | 5 (25.0) |
| Hematuria | 2 (9.5) | 7 (35.0) |
| Angioedemab | 3 (14.3) | 0 |
Events observed in the same patient.
Treatment-related events according to the investigators’ judgment.
P<0.05 versus sirolimus.
P<0.001 versus sirolimus.
GFR Interim Analyses
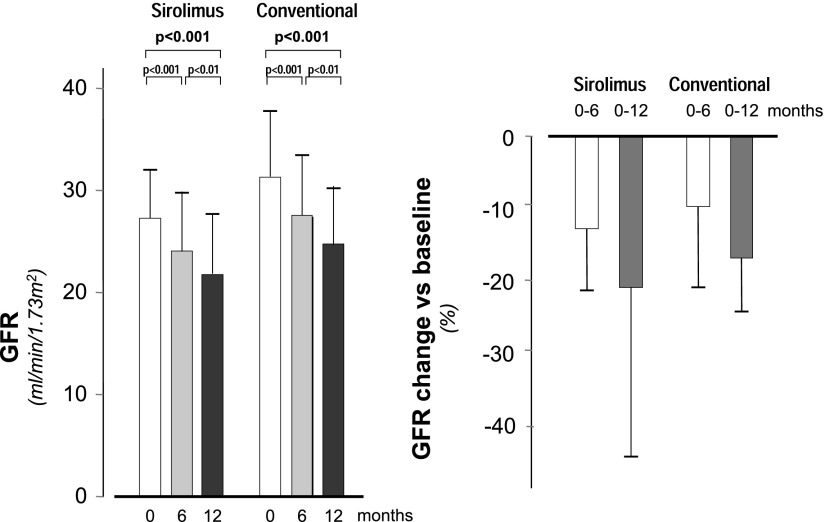
The above alarming safety parameters prompted the DSMB to anticipate the preplanned interim analyses of the primary efficacy variables with the prespecified decision to stop the study in the case that the analyses would not have detected a statistically significant benefit of sirolimus on the primary efficacy variable of the study. One-year data for interim GFR assessments were available from 16 participants on sirolimus and 17 controls (Figure 1). GFR fell from 26.7±5.8 ml/min per 1.73 m2 at baseline to 23.3±6.4 ml/min per 1.73 m2 at 6 months (−13.4±9.3%) and 21.3±6.3 ml/min per 1.73 m2 at 1 year (−20.7±13.5%) in the sirolimus group (P<0.001 versus baseline for both) and from 29.6±5.6 to 26.9±5.4 ml/min per 1.73 m2 at 6 months (−9.1±7.6%) and 24.9±6.2 ml/min per 1.73 m2 (−6.5±7.6%) at 1 year in controls (P<0.001 versus baseline for both) (Figure 2). At both time points, changes versus baseline did not differ significantly between groups (−0.68 ml/min per 1.73 m2; 95% confidence interval, −2.35 to 0.99 ml/min per 1.73 m2; P=0.25 at 6 months and −0.61 ml/min per 1.73 m2; 95% confidence interval, −2.57 to 1.35 ml/min per 1.73 m2; P=0.53 at 1 year). Over the whole observation period, the GFRs similarly declined by 0.4±0.3 and 0.4±0.2 ml/min per 1.73 m2 per month in the sirolimus and conventional treatment groups, respectively (between-group difference: 0.05 ml/min per 1.73 m2; 95% confidence interval, −0.99 to 2.35 ml/min per 1.73 m2 per month).
Figure 2.
GFR changes during the study according to treatment groups. Mean±SD. GFR at baseline and 6 and 12 months of follow-up (left panel) and percentage GFR changes at 6 and 12 months of follow-up versus baseline (right panel). GFR values at different time points and GFR changes versus baseline did not differ significantly between treatment groups.
Study Interruption
On the basis of observed adverse events and GFR data, the DSMB decided to stop the study because of safety and futility. The Steering Committee accepted this recommendation and on July 16, 2012, instructed the investigators to stop treatment but complete all of the planned evaluations at the 1-year follow-up whenever feasible.
Kidney Function
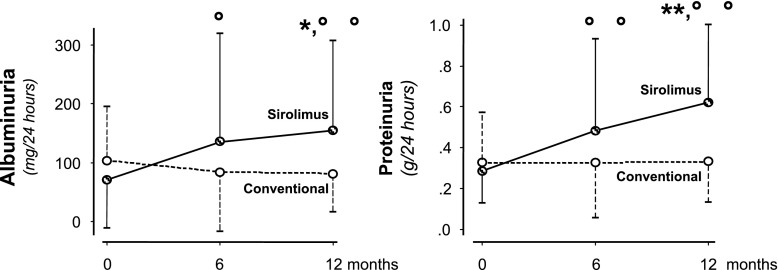
Albuminuria significantly increased in the sirolimus group at 6 (P<0.01) and 12 months (P<0.01) after randomization compared with baseline, whereas an opposite trend to decrease was observed in controls (Figure 3, left panel). At study end, changes between the two treatment groups were significantly different (P=0.003). Consistently, proteinuria progressively increased on sirolimus at 6 (P=0.04) and 12 months (P=0.01) versus baseline and did not change appreciably on conventional treatment (Figure 3, right panel). At 12 months, changes between groups were significantly different (P<0.01). Serum creatinine similarly increased in both groups (Table 3).
Figure 3.
Changes in twenty-four–hour albuminuria and proteinuria during the study according to treatment groups. Median (interquartile range) 24-hour albuminuria (left panel) and proteinuria (right panel) at baseline and 6 and 12 months of follow-up. Albuminuria and proteinuria both increased in the sirolimus group. At 12 months, changes in both parameters were significantly different between the two treatment groups. *P<0.05 versus conventional therapy (analysis of covariance); **P<0.01 versus conventional therapy (analysis of covariance); °P<0.05 versus baseline; °°P<0.01 versus baseline.
Table 3.
Renal function parameters at baseline and the end of the 1-year treatment period according to randomization to sirolimus added on to conventional therapy (sirolimus) or conventional therapy only (conventional)
| Sirolimus | Conventional | |||
|---|---|---|---|---|
| Renal Function Parameters | Baseline, n=16 | 1 yr, n=16 | Baseline, n=17 | 1 yr, n=17 |
| Diuresis, ml/24 h | 2268.8 (648.3) | 2245.6 (851.2) | 2287.7 (560.4) | 2508.4 (639.8) |
| Serum creatinine, mg/dl | 3.02 (0.59) | 4.03 (1.03)a | 2.63 (0.45) | 3.35 (0.83)a |
| GFR, ml/min per 1.73 m2 | 26.7 (5.8) | 21.3 (6.3)a | 29.6 (5.6) | 24.9 (6.2)b |
| Albuminuria, μg/min | 46.3 (26.0–80.9) | 101.7 (50.5–194.6)b | 53.7 (48.3–120.9) | 69.5 (33.6–103.1)a,c |
| Proteinuria, g/24 h | 0.28 (0.17–0.37) | 0.49 (0.39–0.70)a | 0.27 (0.15–0.45) | 0.29 (0.17–0.44)d |
Data are means (SDs) or medians (interquartile ranges). GFR was measured by the iohexol plasma clearance technique.
P<0.01 versus baseline (paired t test).
P<0.05 versus baseline (paired t test).
P<0.05 versus sirolimus adjusted for baseline value (analysis of covariance).
P<0.01 versus sirolimus adjusted for baseline value (analysis of covariance).
Other Parameters
Body weight significantly (P=0.04) increased in the conventional treatment compared with the sirolimus group (Table 4). Changes in BP did not significantly differ between groups (Table 4). At 1 year, all study participants were on antihypertensive therapy, with an average number of medications (2.4 in the sirolimus group and 2.3 in the conventional treatment group) that was similar between groups. HDL cholesterol, hemoglobin, and hematocrit values similarly decreased within each group compared with baseline. Changes in the other parameters were unremarkable in both groups, with the exception of serum calcium, which significantly decreased in patients on sirolimus compared with controls (P=0.002).
Table 4.
Clinical and laboratory parameters at randomization and the end of the 1-year treatment period according to randomization to sirolimus added on to conventional therapy (sirolimus) or conventional therapy only (conventional)
| Sirolimus | Conventional | |||
|---|---|---|---|---|
| Patients Parameters | Baseline, n=16 | 1 yr, n=16 | Baseline, n=17 | 1 yr, n=17 |
| Weight, kg | 73.5 (15.2) | 72.0 (13.9) | 74.2 (17.2) | 75.4 (19.0)a |
| SBP, mmHg | 138.3 (9.5) | 132.8 (12.7) | 134.1 (13.3) | 127.9 (11.6)b |
| DBP, mmHg | 87.1 (8.3) | 84.8 (7.3) | 85.9 (7.2) | 81.9 (6.4)b |
| MAP, mmHg | 102.8 (7.8) | 100.8 (8.8) | 101.6 (9.8) | 97.2 (7.1)b |
| AST, U/L | 19.1 (3.1) | 17.6 (2.8) | 19.6 (5.5) | 17.4 (4.6)c |
| ALT, U/L | 17.8 (4.4) | 17.3 (3.6) | 16.6 (5.2) | 15.1 (6.0) |
| GGT, U/L | 27.4 (14.4) | 24.6 (12.9) | 23.7 (9.8) | 21.5 (9.7) |
| Alkaline phosphatase, U/L | 67.0 (14.4) | 63.9 (11.3) | 61.4 (18.3) | 57.6 (17.7) |
| Calcium, mg/dl | 9.4 (0.3) | 8.9 (0.5)b | 9.2 (0.5) | 9.2 (0.5)d |
| Phosphorus, mg/dl | 3.9 (0.5) | 4.0 (0.8) | 3.7 (0.4) | 4.0 (0.6)b |
| Sodium, mEq/L | 140.3 (1.6) | 139.5 (1.1) | 140.0 (1.5) | 139.5 (2.0) |
| Potassium, mEq/L | 4.3 (0.4) | 4.2 (0.5) | 4.1 (0.7) | 4.3 (0.6) |
| Blood glucose, mg/dl | 90.1 (12.3) | 88.4 (8.7) | 89.4 (11.7) | 88.6 (8.9) |
| Uric acid, mg/dl | 6.4 (1.4) | 6.3 (1.0) | 7.3 (1.4) | 6.3 (1.0)c |
| Total cholesterol, mg/dl | 198.4 (23.2) | 189.9 (24.0) | 201.6 (26.8) | 188.7 (34.8) |
| LDL cholesterol, mg/dl | 123.6 (18.1) | 121.6 (22.6) | 124.4 (31.8) | 116.1 (28.5) |
| HDL cholesterol, mg/dl | 46.7 (10.4) | 42.9 (8.8)c | 51.3 (14.0) | 46.6 (13.5)c |
| Triglycerides, mg/dl | 116.3 (39.9) | 114.4 (25.3) | 108.1 (46.7) | 103.9 (43.9) |
| Leukocytes, ×103/μl | 5.3 (1.5) | 5.2 (1.3) | 5.1 (0.8) | 5.0 (1.0) |
| Hemoglobin, g/dl | 12.7 (1.5) | 12.0 (1.3)c | 12.4 (1.3) | 11.6 (1.3)b |
| Hematocrit, % | 38.5 (4.6) | 36.4 (3.5)c | 37.6 (3.7) | 34.6 (3.6)b |
| Platelets, ×103/μl | 188.1 (56.8) | 176.5 (53.2) | 182.8 (38.0) | 184.2 (37.7) |
Data are means (SDs). SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl-transpeptidase.
P<0.05 versus sirolimus adjusted for baseline value (analysis of covariance).
P<0.01 versus baseline (paired t test).
P<0.05 versus baseline (paired t test).
P<0.01 versus sirolimus adjusted for baseline value (analysis of covariance).
Volumetric Analyses
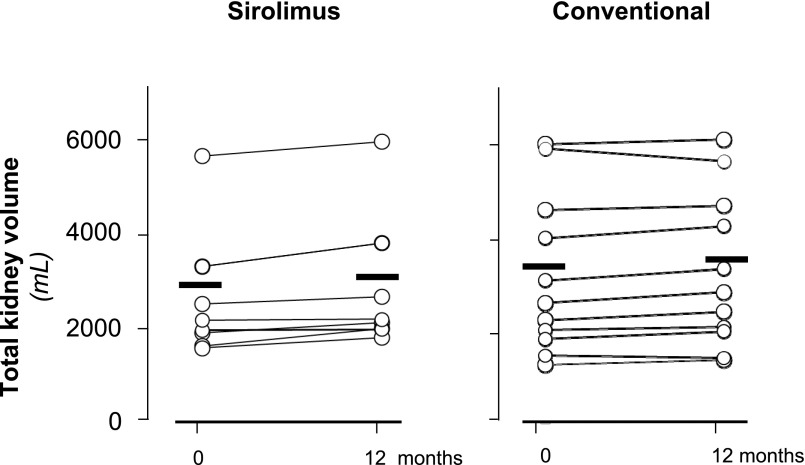
At study closure, total kidney volume (TKV) data were available from eight and 11 patients on sirolimus or conventional treatment, respectively. TKV slightly increased from 2857.7±1447.3 to 3094.6±1519.5 ml and from 3123.4±1695.3 to 3222.6±1651.4 ml in the sirolimus and conventional treatment groups, respectively (between-group difference: 137.6 ml; 95% confidence interval, −27.7 to 303.0 ml; P=0.12) (Figure 4). The percentage TKV increases (8.99±7.06% versus 4.30±5.01%) tended to be larger in the sirolimus group than in the conventional treatment group (P=0.13). Cystic volumes increased by 10.4±10.7% on sirolimus and 3.8±4.0% on conservative therapy (P=0.31), whereas parenchymal volumes were relatively stable in both groups (Supplemental Appendix 3).
Figure 4.
Individual patient total kidney values at baseline and 12 months of follow-up according to treatment groups. No significant change was observed in the sirolimus (left panel) and conventional therapy (right panel) groups. Circles identify single-patient data, and horizontal thick lines denote mean values.
Sirolimus Pharmacokinetic Parameters
Throughout 1 year of follow-up, the total and body weight–adjusted doses of sirolimus averaged 2.2±0.7 mg/d (range =0.5–3 mg/d) and 0.03±0.01 mg/kg per day (0.01–0.07 mg/kg per day), respectively. Trough blood levels averaged 6.1±2.8 ng/ml (2.5–20.7 ng/ml), whereas levels normalized for concomitant sirolimus dosages averaged 3.1±1.6 ng/ml per milligram (0.9–10.6 ng/ml per milligram). On follow-up, 37% and 7% of sirolimus trough blood levels fell below or exceeded the therapeutic range (5–10 ng/ml), respectively (Supplemental Figure 1). In the sirolimus treatment arm, we found no significant relationship between sirolimus trough levels—averaged throughout the whole treatment period or considered separately at each single time point—and different considered outcomes, including side effects and changes in GFR, albuminuria, and proteinuria.
Discussion
We primarily aimed to analyze whether sirolimus added on to conventional therapy allowed slowing of GFR decline and secondarily, kidney volume growth in ADPKD with severe renal insufficiency. The 1-year interim analysis had been planned to establish whether the trial could be continued or had to be stopped because of efficacy, futility, or safety reasons. Actually, the alarming cumulative incidence of treatment–related adverse events prompted the Safety Board to anticipate main efficacy analyses to assess whether an even initial benefit on GFR decline could be detected that could justify study prosecution, despite the excess of side effects in the sirolimus arm. Among major reasons of concern were progression to ESRD of two patients in addition to three patients with angioedema and two premature discontinuations from the study because of worsening of proteinuria in the sirolimus group. Moreover, proteinuria ensued de novo in seven patients on sirolimus but only one control. Thus, on the basis of safety outcomes and 1-year GFR data, the Safety Board decided to stop the study.
Safety
The three patients with angioedema were on concomitant treatment with ACE inhibitors. The excess risk of angioedema (ranging from minor facial edema up to life–threatening throat and mouth swelling) associated with mTOR and ACE inhibitor combination therapy could be explained by defective degradation of the vasoactive peptides bradykinin or substance P when ACE is inhibited (20,21). Bradykinin is inactivated by aminopeptidase P (22), whereas substance P is inactivated by dipeptidyl peptidase IV (23). Decreased dipeptidyl peptidase IV activity has been observed in patients with ACE inhibitor–associated angioedema. A 60% additional decrease can be observed with sirolimus (24), which might explain the increased risk of angioedema in patients receiving sirolimus and ACE inhibitor combination therapy. Thus, the possibility that sirolimus treatment might hinder the safe continuation of ACE inhibitor therapy might have major clinical implications in this context, because ACE inhibitors, in addition to exerting specific cardioprotective effects, have been reported to be renoprotective in children with ADPKD and glomerular hyperfiltration (25) as well as adults, particularly those with more severe proteinuria (26).
Increasing proteinuria led to premature withdrawal of two patients from the sirolimus group. Proteinuria doubled in ten patients on sirolimus versus three controls and ensued de novo in seven patients on sirolimus versus only one control. Proteinuria was reported to increase in renal transplant recipients with chronic allograft dysfunction who had been shifted to sirolimus treatment after withdrawal of a calcineurin inhibitor (27). Proteinuria was typically of glomerular origin (28) and could not be explained just by an increase in GFR associated with cyclosporin withdrawal (29). Finding that sirolimus exacerbated both proteinuria and different markers of podocyte damage in a model of severe puromycin–induced glomerular injury (30) can be taken to suggest that sirolimus may have a direct nephrotoxic effect, particularly in patients with advanced renal disease, such as our patients with ADPKD. Independent of the underlying mechanisms, worsening of proteinuria must be considered as a clinically relevant adverse effect, because proteinuria is a well established risk factor for the progression of chronic nephropathies, including ADPKD (31,32).
Finally, sirolimus therapy was associated with a series of nonserious but disturbing side effects, such as watery diarrhea, abdominal swelling, upper respiratory tract infections, and in particular, aphthous stomatitis, that caused consent withdrawal because of subjective distress for six patients. Down titration of the drug was often necessary to control symptoms. Consequently, in about 40% of measurements, sirolimus trough blood levels failed to fit the target range. This is a major limitation to sirolimus therapy, because underdosage or poor compliance to the drug dictated by its poor safety profile and tolerability is one of the possible explanations for treatment failure. The narrow therapeutic window of sirolimus might be an even more stringent limitation in everyday clinical practice, in particular in a fragile population of patients with ADPKD and severe renal insufficiency, such as those under consideration here.
Efficacy
At the 1-year interim analysis, sirolimus showed no appreciable protective effect against progressive GFR loss. GFR reduction even tended to be larger in the sirolimus group than in controls, particularly over the first 6 months after randomization. Previous large trials with mTOR inhibitors in patients with ADPKD and relatively preserved renal function (10,11) showed that sirolimus or everolimus did not affect renal function decline. Thus, available data converge to indicate that mTOR inhibitors have no appreciable protective effect against progressive renal function loss, independent of the level of initial GFR. Within the limitations of the small sample size, finding that sirolimus did not seem to appreciably affect TKV increase provided additional evidence that mTOR inhibition is devoid of any specific renoprotective effect, at least in this context.
Limitations and Strengths
Because of reduced exposure to radiocontrast agents, reliable data for subanalyses of different components of kidney volumes could be obtained only from a minority of patients (Supplemental Appendices 2 and 3). This limitation, however, did not affect TKV analyses as well as safety and GFR analyses. Failure to detect significant associations between sirolimus levels and considered outcomes was most likely explained by the relatively small sample size, the wide data fluctuations (particularly in sirolimus levels), and the confounding effect of changes introduced in sirolimus dosing to target therapeutic range and limit side effects. Direct measurement of GFR by a gold standard technique (12) was a major strength that allowed powerful analyses and avoided the limitations of estimation equations (13). In the context of the prospective, randomized, open label, blind end point design, the assessors of outcome variables were blinded to treatment. Moreover, all study participants were given the best available therapy. Finally, the two treatment regimens were evaluated in the context of daily clinical practice, which increases the generalizability of study findings to the average population of patients with ADPKD and advanced renal involvement.
Altogether, our findings and those from previous trials in patients with normal or mildly reduced renal function (10,11) can be taken to suggest that mTOR inhibitors do not seem to offer a valuable therapeutic option for patients with ADPKD, independent of their residual kidney function. Although some data suggest that low doses of sirolimus might offer some benefit (33) and ongoing trials are investigating whether pulsed oral administration may improve the drug risk/benefit profile (NCT02055079), future research efforts should probably focus on much safer medications with larger therapeutic windows and stronger evidence of efficacy in this context (34,35).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the participants of the SIRENA 2 Study; all trial investigators; the nephrologists, radiologists, and nurses of the participating centers for their invaluable assistance; the laboratory, medical imaging, and regulatory affairs staff; the trial monitors, data managers, and statisticians; and everyone at the Clinical Research Center for Rare Diseases “Aldo e Cele Daccò,” IRCCS—Istituto di Ricerche Farmacologiche “Mario Negri” for their efforts in making this study possible.
Part of this work was funded by TranCYST Marie Curie Initial Training Networks project within 7th European Community Framework programme EU-FP7/2007-2013 grant 317246 (TranCYST).
Pfizer Italia (Latina, Italy) and formerly, Wyeth Lederle (Aprilia, Italy) freely supplied sirolimus but did not fund the study, and they were not involved in the planning and conducting of the study or the data analyses and reporting.
Members of the SIRENA 2 Study organization are listed in Supplemental Appendix 1.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09900915/-/DCSupplemental.
References
- 1.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ: Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG: PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingar DC, Blenis J: Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Wu M, Arcaro A, Varga Z, Vogetseder A, Le Hir M, Wüthrich RP, Serra AL: Pulse mTOR inhibitor treatment effectively controls cyst growth but leads to severe parenchymal and glomerular hypertrophy in rat polycystic kidney disease. Am J Physiol Renal Physiol 297: F1597–F1605, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Tao Y, Kim J, Schrier RW, Edelstein CL: Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Zafar I, Belibi FA, He Z, Edelstein CL: Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol Dial Transplant 24: 2349–2353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G: Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21: 1031–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Gaspari F, Perico N, Matalone M, Signorini O, Azzollini N, Mister M, Remuzzi G: Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 9: 310–313, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ruggenenti P, Gaspari F, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Prandini S, Ene-Iordache B, Diadei O, Perico N, Ondei P, Pisani A, Buongiorno E, Messa P, Dugo M, Remuzzi G; GFR-ADPKD Study Group : Measuring and estimating GFR and treatment effect in ADPKD patients: Results and implications of a longitudinal cohort study. PLoS One 7: e32533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cattaneo D, Perico N, Gaspari F: Assessment of sirolimus concentrations in whole blood by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 774: 187–194, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Ibanez L, Schroeder W, Ng L, Cates J: The ITK SoftwareGuide, 2nd Ed., Albany, NY, Kitware Inc., 2005 [Google Scholar]
- 16.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Perna A, Gherardi G, Benini R, Remuzzi G: Chronic proteinuric nephropathies: Outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. Am J Kidney Dis 35: 1155–1165, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK: Intention-to-treat concept: A review. Perspect Clin Res 2: 109–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter JR, Kenward M: Missing Data in Randomised Controlled Trials—A Practical Guide. Birmingham: National Institute for Health Research, Publication RM03/JH17/MK, 2008. Available at: http://www.missingdata.org.uk. Accessed February 8, 2016
- 20.Burdese M, Rossetti M, Guarena C, Consiglio V, Mezza E, Soragna G, Gai M, Segoloni GP, Piccoli GB: Sirolimus and ACE-inhibitors: A note of caution. Transplantation 79: 251–252, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K: Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 5: 703–708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward PE, Chow A, Drapeau G: Metabolism of bradykinin agonists and antagonists by plasma aminopeptidase P. Biochem Pharmacol 42: 721–727, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Ahmad S, Wang L, Ward PE: Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther 260: 1257–1261, 1992 [PubMed] [Google Scholar]
- 24.Byrd JB, Woodard-Grice A, Stone E, Lucisano A, Schaefer H, Yu C, Eyler AE, Salloum NE, Brown NJ: Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy 65: 1381–1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4: 820–829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafar TH, Stark PC, Schmid CH, Strandgaard S, Kamper AL, Maschio G, Becker G, Perrone RD, Levey AS; ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group : The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int 67: 265–271, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kuypers DR: Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf 28: 153–181, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Rangan GK: Sirolimus-associated proteinuria and renal dysfunction. Drug Saf 29: 1153–1161, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Saurina A, Campistol JM, Piera C, Diekmann F, Campos B, Campos N, de las Cuevas X, Oppenheimer F: Conversion from calcineurin inhibitors to sirolimus in chronic allograft dysfunction: Changes in glomerular haemodynamics and proteinuria. Nephrol Dial Transplant 21: 488–493, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Torras J, Herrero-Fresneda I, Gulias O, Flaquer M, Vidal A, Cruzado JM, Lloberas N, Franquesa M, Grinyó JM: Rapamycin has dual opposing effects on proteinuric experimental nephropathies: Is it a matter of podocyte damage? Nephrol Dial Transplant 24: 3632–3640, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Chapman AB, Johnson AM, Gabow PA, Schrier RW: Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 1349–1354, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Braun WE, Schold JD, Stephany BR, Spirko RA, Herts BR: Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: An open-label randomized controlled pilot study. Clin J Am Soc Nephrol 9: 881–888, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 35.Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, Visciano B, Imbriaco M, Messa P, Cerutti R, Dugo M, Cancian L, Buongiorno E, De Pascalis A, Gaspari F, Carrara F, Rubis N, Prandini S, Remuzzi A, Remuzzi G, Ruggenenti P; ALADIN study group : Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): A randomised, placebo-controlled, multicentre trial. Lancet 382: 1485–1495, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.