Abstract
Quality improvement involves a combined effort among health care staff and stakeholders to diagnose and treat problems in the health care system. However, health care professionals often lack training in quality improvement methods, which makes it challenging to participate in improvement efforts. This article familiarizes health care professionals with how to begin a quality improvement project. The initial steps involve forming an improvement team that possesses expertise in the quality of care problem, leadership, and change management. Stakeholder mapping and analysis are useful tools at this stage, and these are reviewed to help identify individuals who might have a vested interest in the project. Physician engagement is a particularly important component of project success, and the knowledge that patients/caregivers can offer as members of a quality improvement team should not be overlooked. After a team is formed, an improvement framework helps to organize the scientific process of system change. Common quality improvement frameworks include Six Sigma, Lean, and the Model for Improvement. These models are contrasted, with a focus on the Model for Improvement, because it is widely used and applicable to a variety of quality of care problems without advanced training. It involves three steps: setting aims to focus improvement, choosing a balanced set of measures to determine if improvement occurs, and testing new ideas to change the current process. These new ideas are evaluated using Plan-Do-Study-Act cycles, where knowledge is gained by testing changes and reflecting on their effect. To show the real world utility of the quality improvement methods discussed, they are applied to a hypothetical quality improvement initiative that aims to promote home dialysis (home hemodialysis and peritoneal dialysis). This provides an example that kidney health care professionals can use to begin their own quality improvement projects.
Keywords: chronic kidney disease, clinical nephrology, end stage kidney disease, Delivery of Health Care, Hemodialysis, Home, Humans, Leadership, Quality Improvement, renal dialysis, Total Quality Management
Clinical Scenario
Local physicians, patients, and health care leaders have recently made home dialysis (home hemodialysis or peritoneal dialysis) a strategic priority at your health care facility. To promote home dialysis, they have instructed dialysis facilities that 30% of all patients on incident dialysis should initiate dialysis on a home dialysis modality within 6 months of dialysis initiation. Currently, the nephrology program in which you work is starting 15% of its new patients on a home dialysis option. You want to help your program improve its performance, but your quality improvement experience is limited. Although you know an improvement team is needed, you are unsure who to include. Most of all, you are overwhelmed by the number of different quality improvement frameworks and tools. Given these challenges, you wonder how you should even get started.
Enlist a Core Change Team
In quality improvement, the people who do the work need to be the ones to change the work. This usually requires an interdisciplinary improvement team, with representation from disciplines such as medicine, nursing, and administration. Health care professionals have experience working in teams, but determining the composition of a successful quality improvement team requires a different skillset (1). Stakeholder mapping and analysis are tools that may help identify appropriate team members.
Stakeholder Mapping and Analyses
A stakeholder is anyone who has an interest in a project and can influence its success or failure (2). Stakeholders are individuals and groups within (internal) or outside (external) your local setting. It is important to identify or map stakeholders who can affect your quality improvement project at an early stage when these relationships can be managed, including supporters and resistors of change. Inclusion of resistors may ultimately help to avoid conflict and administrative delays as the project evolves. There is no universally accepted strategy to identify stakeholders. In our experience, an effective technique is to brainstorm and list all stakeholders. This activity is followed by organizing the stakeholders into groups (physicians, patients, hospital leadership, etc.) and outlining the relationships among different groups. It is helpful to do this graphically using lines or arrows, with the quality problem remaining at the center of the map and the different stakeholder groups organized around it.
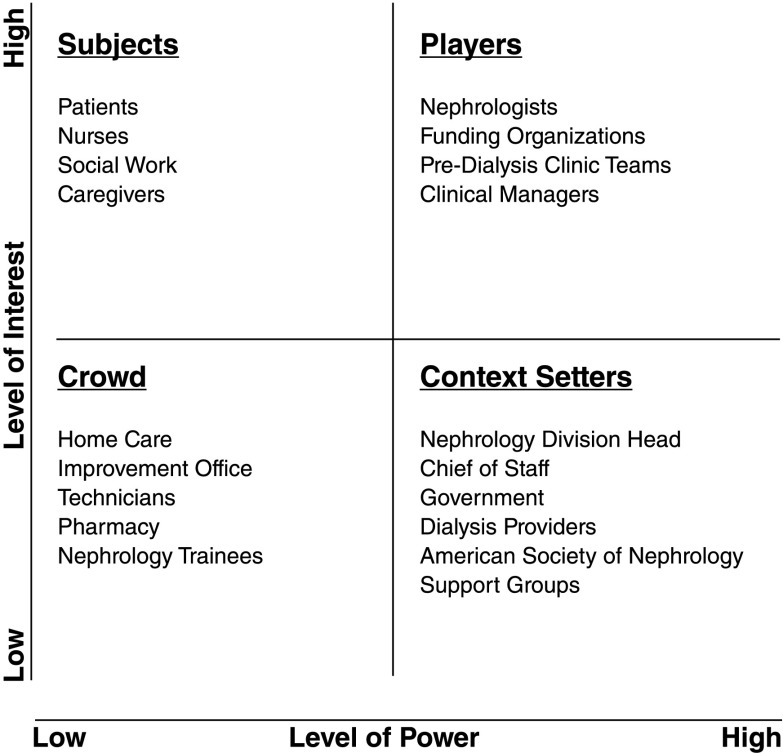
After stakeholders are identified, the stakeholder map requires analysis to determine which stakeholders to approach. Analysis requires prioritization of different stakeholders, because it is not feasible or necessary to engage all stakeholders with the same level of intensity. One useful tool is a power versus interest grid (3,4). These grids place stakeholders on a two-by-two matrix, where the y axis is the stakeholder’s political interest in the quality problem and the x axis is the stakeholder’s organizational power or control over the system. Four categories of stakeholders result: players who have both an interest and power, subjects who have an interest but little power, context setters who have power but little interest, and the crowd with little interest or power (3). In this way, power versus interest grids help identify stakeholders who should be involved in the project and considered for the improvement team (players) as well as the intensity of engagement strategies for other stakeholder groups (greater intensity for subjects and context setters than the crowd).
Stakeholder analysis also involves consideration of stakeholder motivations that may act as facilitators or barriers. These motivations may include patient care, finances, process efficiency, staff satisfaction, or staff recognition. Different stakeholders have different perspectives on a given quality improvement initiative, and therefore, stakeholder engagement strategies may have to be individualized to build a change team and overcome resistance to change. It is important to note that stakeholder mapping and analysis are dynamic processes, with new stakeholders emerging and stakeholder influence and interests changing as a quality improvement project evolves.
Improvement Team Roles and Characteristics
Stakeholder influence is not the only criterion to consider when forming an improvement team, which should consist of the following roles and characteristics (5–7).
Team lead: The individual responsible for day to day management of the quality improvement project, who is also part of the system that will undergo change.
Technical experts: Individuals who understand different components of the quality of care problem and are a major part of the system that needs to be improved.
Clinical/system leader: A manager who understands the implications of changes on other parts of the system, with sufficient authority to test changes that are recommended by the team lead and technical experts.
Improvement advisor: An individual with expertise in quality improvement methods to act as a resource and advisor for the team lead and technical experts.
Executive sponsor: An individual with power and leadership skill within the organization who can be approached when needed to secure resources and remove barriers.
Filling these roles will help ensure that an improvement team possesses the right balance of leadership, management, expertise, and power to succeed. When an improvement advisor is unavailable, resources and toolkits, such as those provided by the Forum of ESRD Networks, may be helpful (8,9). Although these team members can come from any area of health care, the role of physicians and patients/families in quality improvement has garnered the most attention.
Among health care providers, physicians have the most influence on variation in health care outcomes, and without physician engagement, it is very difficult to improve health care delivery (10,11). However, physicians participate in <35% of quality improvement efforts, and there are many barriers to physician involvement in quality improvement (12). These include lack of time, threats to physician autonomy, financial disincentives, and lack of quality improvement skills (10,13). Physician responsibilities are often spread across several areas, and the quality of care priorities faced by hospitals and other health care organizations seem misaligned with the quality of care issues faced by physicians (11). This discrepancy may be most evident when quality of care targets are not patient centered. Despite these obstacles, physicians are clearly interested in quality of care, particularly improvement of patient outcomes and reduction of inefficiencies. Quality improvement expertise is not necessary, because the physician role can be to focus improvement activities on patient outcomes, bring credibility to a project, and inspire colleagues (14). Effective physician participation in quality improvement depends on communication and teamwork (11). Physicians must resist the temptation to lead or control discussions and instead, defer to the team lead or improvement advisor when outside their area of clinical or system expertise. Strategies to engage physicians in quality improvement exist that address the aforementioned barriers, and they are recommended on the basis of the experiences of the Institute for Healthcare Improvement and leading health care institutions in the United States (10,11). These strategies include data transparency, strong organizational commitment to quality improvement, and showing physicians how quality improvement can lead to more time in their day for direct patient care. Compensation and incentives are more controversial strategies with mixed success (15,16). One option is for organizations to provide nurse practitioner and allied health support, which frees physician time for quality improvement and patient care (11). Effective stakeholder engagement depends on making quality improvement participation easy, and these strategies involve finding common purpose with physicians on quality of care initiatives that respects their time commitment and contributions.
Users of the health care system also possess unique knowledge and experiences that can inform quality improvement efforts and help design systems around the needs of the patient rather than the staff or organization (17). However, there is much debate over how to meaningfully involve patients and caregivers in quality improvement (18). Experience from the United Kingdom suggests that projects have a clear rationale and defined roles and responsibilities for patients and caregivers (18). Roles that patients and caregivers have played in quality improvement include:
Identifying improvement opportunities (19)
Creating a sense of urgency for change with storytelling (20,21)
Acting as an outlet to solicit other patient experiences (18,20)
Persuading health care providers that quality of care problems exist and need to be addressed (18).
Published experiences that involved patients and caregivers in quality improvement noted several advantages. These advantages largely focus on challenging assumptions that the current level of care is acceptable and lending credibility to system changes by showing that the proposed improvements are likely to be well received by patients and caregivers (18). In addition, patient and family involvement may energize staff and focus team members on improvement rather than longstanding organizational conflicts (20). Disadvantages include avoiding the appearance of tokenism and participation primarily to satisfy organizational requirements without concrete contributions (22). Barriers to patient and caregiver participation may have to be addressed, which include institutional culture, patient availability, and power indifferences (20,21). What constitutes a representative patient is also a matter of debate, although several published reports suggest that patient demographics are less important than the different experiences that any patient can offer (23,24). Patients and caregivers have helped to redesign several health care processes and systems across both inpatient and outpatient settings, with outcomes that include reducing missed appointments, medical errors, hospital length of stay, and costs (20,25,26). Although the literature on the role, effect, and strategies to engage patients and caregivers in quality improvement is evolving, their important stake and unique perspective on health care suggest that patients and caregivers can meaningfully contribute to an improvement team.
Select an Improvement Framework
After a team is in place, an improvement model can help provide guidance and structure for diagnosing and treating systems of care (27). Several models exist, which can make choosing the model for a specific project overwhelming (Table 1). Improvement teams should not spend valuable time ruminating over this decision. Often, the decision should be on the basis of the model with which the team, hospital, or organization has the most experience and resources. The most popular models are actually quite similar and not mutually exclusive; their differences should not delay the improvement process. To illustrate this, we will briefly describe Six Sigma, Lean, and the Model for Improvement. The articles in this Moving Points feature will focus on the Model for Improvement, because it is a simple yet powerful tool that can be applied to a variety of quality of care problems without advanced training (5). It also may be more familiar to nephrology health care providers, because it is used by the Forum of ESRD Networks (8,9). As this Moving Points progresses, tools from Six Sigma and Lean will be introduced, which reinforce that these different frameworks are often combined to achieve improvement (28).
Table 1.
Comparison of Six Sigma, Lean, and the Model for Improvement
| Six Sigma | Lean | Model for Improvement |
| Improvement philosophy | ||
| Continuous improvement | Continuous improvement | Daily improvement |
| Reduce unwanted variability | Staff respect and empowerment | Small–scale rapid cycle change with iterative learning |
| Approach | ||
| Five-phased process (define, measure, analyze, improve, and control) | Differentiate value- from nonvalue-added activities (waste) | Set an aim |
| Focus improvement efforts on eliminating waste | Create balanced measures | |
| Identify and test changes | ||
| Common tools | ||
| Mathematical modeling | Flow diagrams | Process mapping |
| PDSA cycles | Kaizen events | PDSA cycles |
| Control charts | Run charts | Run charts |
| PDSA cycles | Control charts | |
| Training | ||
| Experience with quantitative statistics | Lean apprenticeship | On the job improvement experience |
| On the job improvement experience | On the job improvement experience | |
| Project length for improvement | ||
| Months | Weeks to months | Days to weeks |
| Limitations | ||
| Complex and less accessible to frontline staff | Japanese terms can lead to confusion | Diagnostic tools and change ideas are adapted from other frameworks |
| Not ideal for projects focused on improving flow/speed | Not ideal for projects focused on statistical control and reducing variation | |
| Applications | ||
| Cause of variation is unknown | Problems that can be directly observed and managed visually | The main cause of the problem is already determined, and change ideas are easily identified |
| No immediate improvement solution exists | Increasing process flow and speed | Problems with established evidence–based solutions |
PDSA, Plan-Do-Study-Act.
Six Sigma
Six Sigma was developed by Motorola in the 1980s and focuses on understanding and controlling a process with quantitative tools (29). Improvement is on the basis of reducing unwanted variability. The term Six Sigma comes from the Greek symbol for SD, where six SDs from the mean (six sigma) represent 3.4 defects of 1 million. Six Sigma quality improvement projects use a five-phased process known as the define, measure, analyze, improve, and control approach (30).
Define: Determine the key metrics for measuring success.
Measure: Determine past levels of performance to act as a baseline for improvement.
Analyze: Identify the causes of the current quality problems and opportunities for improvement.
Improve: Develop solutions, test solutions, and redesign processes.
Control: Standardize the improvements so that they are sustained.
Any quality improvement tool can be used for these phases (31,32), but Six Sigma usually relies on quantitative methods, such as ANOVA, mathematical modeling, and control charts (30,33). Because of the time commitment required to apply these techniques (projects require several months), Six Sigma is best applied to quality of care problems in which the improvement team cannot identify an immediate cause or solution (29). In these circumstances, Six Sigma methods provide data to determine root causes that stimulate solutions and help reduce variance (29). Finally, Six Sigma methods are not ideal for improving the speed of a process.
Lean
Lean refers to lean thinking and can be traced back to Toyota in the 1940s (34). It is on the basis of two themes: continuous improvement through elimination of waste and respect for people (35). Continuous improvement is achieved by distinguishing value-added activities from nonvalue-added activities (waste). Value-added activities include any activity that contributes directly to satisfying the needs of a patient. Examples include a diagnosis, imaging test, or surgical procedure. Conversely, nonvalue-added activities do not contribute directly to satisfying the needs of a patient but require time, space, or resources. Examples include delayed physician consultation, ordering investigations that have been completed elsewhere, and searching for hand sanitizer. A typical process is 5% value added and 95% nonvalue added, and this perspective can create significant opportunities for improvement by focusing on the elimination of nonvalue-added activities (35). This method requires respect for people, because in lean thinking, the workers involved in the process are the most knowledgeable and in the best position to identify changes. Respect for people includes not only valuing the viewpoint and role of all participants (an important component of all improvement frameworks) but also, a commitment to staff retention, because people are more likely to engage in quality improvement if they are not worried about “improving away” their jobs (34).
Lean methods usually have Japanese names and focus on observing work, seeing waste, and eliminating waste. Examples include gemba (going to where the work is actually done to observe processes) and kaizen event (team–based rapid improvement cycle over a 2- to 5-day period) (35). Many tools are also common to Six Sigma and the Model for Improvement, such as Plan-Do-Study-Act (PDSA) cycles (34). However, training and apprenticeships in Lean are helpful to be facile with its methods. Lean does not address how to bring a process under statistical control and reduce variation (29). It is best applied to quality of care problems that are directly observable and where waste can be eliminated, particularly when flow, speed, and efficiency are priorities (35). Another major advantage is that Lean methods usually allow for improvement within weeks (29).
Model for Improvement
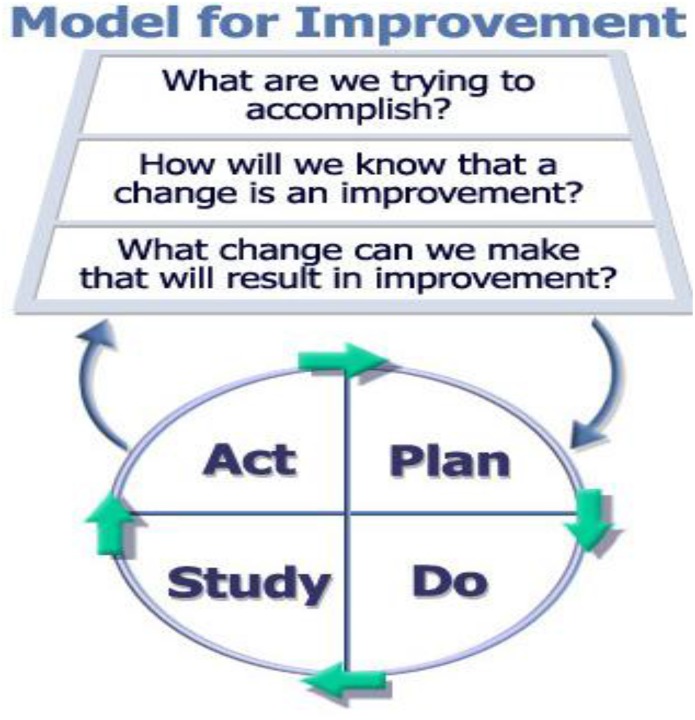
The Model for Improvement was developed by the Associates in Process Improvement in the 1990s and has been popularized by the Institute for Healthcare Improvement (27,34). It also has been adapted by other improvement frameworks, such as the Veterans Affairs Vision-Analysis-Team-Aim-Map-Measure-Change-Sustain approach (36). The Model for Improvement is designed as an algorithm for achieving an aim at any scale through learned experience and purposeful action. This model distills quality improvement into three questions (Figure 1) (27).
(1) What are we trying to accomplish?
(2) How will we know a change is an improvement?
(3) What changes can we make that will result in improvement?
Figure 1.
Model for improvement. Reprinted from ref. 27, with permission.
A key tenet is that not all change is improvement but that all improvement requires change (37). Accordingly, the model relies on the content knowledge of frontline staff to identify changes that are predicted to lead to improvement. Its main tools are PDSA cycles and rapid cycle testing (refer to other articles in this Moving Points feature), where teams develop a habit of immediate and sequential changes to learn which interventions in which contexts produce improvements (5,34). Although the Model for Improvement teaches teams to avoid starting change without thoughtful planning, it does not help identify the cause of a quality of care problem or change ideas (38). Therefore, the Model for Improvement is most appropriate for quality of care problems with clear causes and/or established evidence–based solutions (34).
Despite these limitations, the Model for Improvement is a simple and efficient framework applicable to small- and large-scale projects (34). It is accessible to not only specialists in quality improvement but also, frontline staff with minimal quality improvement training or experience and nephrology health care providers (8,9). Consultants are not usually required. For these reasons, we will focus on the Model for Improvement, with some Six Sigma and Lean tools described in subsequent articles. This overlap in methods and tools emphasizes that these three frameworks have many similarities, despite their differences (Table 1), and successful quality improvement usually requires the application of multiple tools from each of the different frameworks (39,40).
The Three Questions in the Model for Improvement
What Are We Trying to Accomplish (Aim)?
Starting quality improvement without a clear aim statement is similar to conducting research without a clear hypothesis (5). That is, lack of direction leads to wasted time and effort. There are multiple opinions on how to construct an effective aim statement (5,37). Most sources agree that aims should be patient centered in addition to the following principles.
Specific: Clearly state what will be improved in a given patient population by a certain date with responsibility assigned.
Measurable: Include a concrete numerical goal for assessing progress (increasing the use of home dialysis is not as effective as increase home dialysis use by 25% in 6 months).
Stretchable: Aim statements should be ambitious. This makes it clear that the current system is inadequate and that a new system is required, which stimulate creativity and innovation. Less ambitious goals can be achieved by stressing the current system and working harder, which is usually not sustainable.
How Will We Know a Change Is an Improvement (Measures)?
Measurement for improvement focuses on monitoring the outcomes of a system over time to learn if the processes are effective (5). The emphasis is on learning how potential interventions have affected processes and outcomes, so that subsequent improvement steps can be taken. Measurement for improvement should not be confused with measurement for research or accountability (41). Accountability measurement is often recommended or demanded by external organizations to compare performance (42,43). This process can help initiate improvement but is not sufficient (44). In addition, we require a balanced set of measures that accounts for health care system complexity. Areas to consider include not only clinical outcomes but also, the patient experience and smart resource use. These items constitute the Institute for Healthcare Improvement Triple Aim and are key components of the quality of care strategies for the Centers for Medicare and Medicaid Services and the National Quality Strategy (45–47). One common approach to quality improvement measurement involves the following types of measures.
Outcome Measures.
Evaluate the effect of the system on patients (e.g., central venous catheter infection rates).
Process Measures.
Evaluate system performance and potential changes (e.g., chlorhexidine swab to exit site).
Balancing Measures.
Monitor for unintended consequences of changes to a system (e.g., patient skin rash and dialysis nurse satisfaction).
It is important not to select too many measures (usually three to eight is sufficient), and they should be simple to collect, accurate, and reproducible. The goal is not perfect measurement but rather, to determine whether changes have improved the system (37). Measurement for improvement is a focus of another article in this Moving Points feature.
What Changes Can We Make That Will Result in Improvement (Changes)?
This final stage of the Model for Improvement consists of two parts. Clearly, changes need to first be identified. Smart ideas for change can come from many sources, including colleagues, evidence, theory, or experience. However, even the most clever and widely accepted change ideas may not work when adapted to a particular environment. Therefore, the PDSA cycle is used to design and test changes in quick succession, acting as the scientific method for action-oriented learning (5). Similar to measurement for improvement, the goal is not to identify perfect changes immediately or the perfect opportunity for change, which can significantly slow down improvement work (37). Rather, PDSA is intended to be performed as small imperfect tests of change with a few stakeholders who may even be able to start within days of their initial conception (37). The PDSA cycle allows for promising changes to be expanded and useless changes to be discarded. It is also a low-risk method to try new ideas that might encounter resistance, encouraging buy in as success is shown on a small scale before widespread implementation. Eventually, large-scale lessons are learned as the results from many small PDSA cycles accumulate. Although simple to describe, this final stage of the Model for Improvement requires planning, practice, and a systematic approach. We devote the next two articles in this Moving Points feature to change idea development and PDSA.
Scenario Resolution
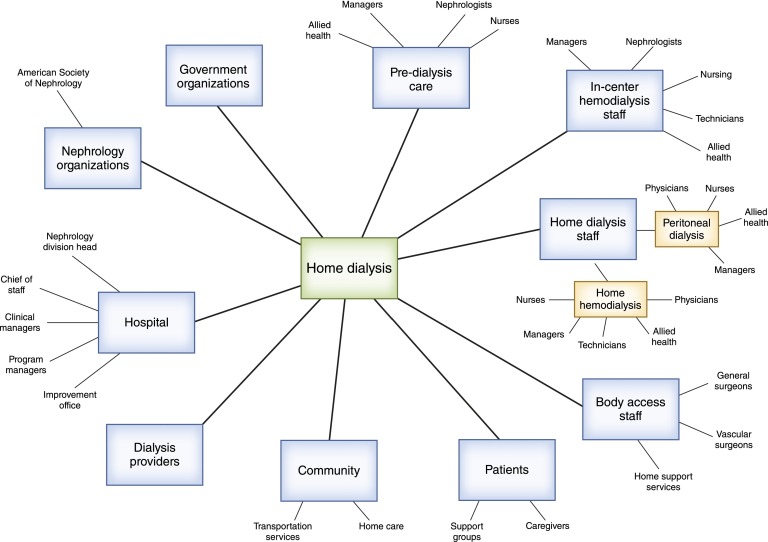
Along with one of your colleagues, home dialysis stakeholders are identified, and a stakeholder map is developed (Figure 2). A power versus interest grid is used to prioritize stakeholders for engagement and inclusion on the improvement team, considering their position within the organization to enact change (power) and political interest in the quality problem (Figure 3) (3,4). This list guides the creation of a 5–10 member interdisciplinary improvement team with knowledge of the process from CKD to ESRD.
Team lead: Nurse/physician from the home dialysis unit.
Technical experts: Nurse/physician from the predialysis clinic and in–center hemodialysis unit, social worker, home care worker, and patient/caregiver on home and in-center dialysis. All players should be invited to join the improvement team, but if unable, they should be updated on a regular basis.
Clinical/system leader: Manager from the ESRD program.
Improvement advisor: Clinical manager or hospital department with quality improvement experience.
Executive sponsor: Nephrology division head or hospital chief of staff.
Figure 2.
Stakeholder map for home dialysis. The home dialysis quality of care problem is located at the center of the map, and the different stakeholder groups are organized around it.
Figure 3.
Power versus interest grid for home dialysis. The y axis is the stakeholder’s political interest in the quality of care problem, and the x axis is the stakeholder’s organizational power or control over the system. Adapted from refs. 3 and 4, with permission.
The aim provided by the local physicians, patients, and health care leaders is measurable and stretchable. It should include a timeframe for the improvement efforts to increase specificity. Initially, it may be easier to focus on either home hemodialysis or peritoneal dialysis. The aim could be revised as follows: among patients on incident dialysis, we will increase the percentage treated with home hemodialysis (or peritoneal dialysis) within 6 months of starting dialysis from 15% to 25% within 1 year.
Measurement will depend partly on the change ideas that are developed (refer to other articles in this Moving Points feature). Measures that should be considered are included below.
Outcome Measures
The percentage of patients treated with a home dialysis option as their initial modality.
The percentage of patients treated with a home dialysis option within 6 months of starting dialysis.
Process Measures
The percentage of patients at the predialysis clinic referred for a home dialysis eligibility assessment.
The percentage of patients who received formal education on home dialysis at the predialysis clinic.
The mean number of training days required for patients successfully started on home dialysis.
Balancing Measures
Patient and family satisfaction with predialysis care.
Staff time spent on home dialysis assessments each week.
The percentage of patients who are not dialyzing at home within 90 days of home dialysis training completion.
These measures capture the intended patient goals, system changes (referral patterns and education), and unintended consequences. In addition, the balancing measures consider the patient perspective and staff opportunity costs.
What Are the Next Steps?
Now that the improvement team is formed along with an aim statement, you are ready to identify changes and begin testing their effects on the system of care for home dialysis. The most effective changes target the primary cause or source of the quality of care problem, and the next article will show how to analyze a system to identify these causes and stimulate change ideas.
Disclosures
None.
Acknowledgments
S.A.S. is supported by a Kidney Research Scientist Core Education and National Training Program Postdoctoral Fellowship (cofunded by the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institutes of Health Research). G.M.C. is supported by a K24 midcareer mentoring award from the National Institute of Diabetes and Digestive and Kidney Diseases.
Published online ahead of print. Publication date available at www.cjasn.org.
This article is part of a Moving Points Series on quality improvement tools.
References
- 1.DeOreo PB: Implementation of a continuous quality improvement process in a free-standing hemodialysis unit. Am J Kidney Dis 24: 355–361, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Brugha R, Varvasovszky Z: Stakeholder analysis: A review. Health Policy Plan 15: 239–246, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bryson JM: What to do when stakeholders matter. Public Manage Rev 6: 21–53, 2004 [Google Scholar]
- 4.Eden C, Ackermann F: Making Strategy: The Journey of Strategic Management, Thousand Oaks, CA, Sage Publications, 1998 [Google Scholar]
- 5.Ogrinc GS, Headrick LA, Moore SM, Barton AJ, Dolansky MA, Madigosky WS: Fundamentals of Health Care Improvement: A Guide to Improving Your Patient’s Care, Cambridge, MA, Institute for Healthcare Improvement, 2012 [Google Scholar]
- 6.Kotter JP: Leading Change, Boston, Harvard Business Review Press, 2012 [Google Scholar]
- 7.Lindenfeld S, Vlchek D: Engaging physicians in continuous quality improvement. Adv Ren Replace Ther 8: 120–124, 2001 [DOI] [PubMed] [Google Scholar]
- 8.DeOreo P, Duval L, Paul R, Kodali P, Rankin L, Molony D, Rodgers D, Murray B, Rosen S: Medical Director Toolkit. Forum of ESRD Networks, 2012. Available at: http://esrdnetworks.org/mac-toolkits-1/medical-director-toolkit/medical-director-toolkit/view. Accessed December 20, 2015
- 9.Dittrich M, Deane J, Gregory N: Quality Assessment and Performance Improvement. Forum of ESRD Networks, 2010. Available at: http://esrdnetworks.org/mac-toolkits-1/qapi-toolkit/qapi-toolkit/view. Accessed December 20, 2015
- 10.Taitz JM, Lee TH, Sequist TD: A framework for engaging physicians in quality and safety. BMJ Qual Saf 21: 722–728, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Reinertsen JL, Rupp W, Whittington JW: Engaging physicians in a shared quality agenda. IHI Innovation Series White Paper, Cambridge, MA, Institute for Healthcare Improvement, 2007
- 12.Audet AM, Doty MM, Shamasdin J, Schoenbaum SC: Measure, learn, and improve: Physicians’ involvement in quality improvement. Health Aff (Millwood) 24: 843–853, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Pronovost PJ, Miller MR, Wachter RM, Meyer GS: Perspective: Physician leadership in quality. Acad Med 84: 1651–1656, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lee TH: Turning doctors into leaders. Harv Bus Rev 88: 50–58, 2010 [PubMed] [Google Scholar]
- 15.Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, Young D: The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev 9: CD008451, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR: An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev 7: CD009255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards T, Montori VM, Godlee F, Lapsley P, Paul D: Let the patient revolution begin. BMJ 346: f2614, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Armstrong N, Herbert G, Aveling EL, Dixon-Woods M, Martin G: Optimizing patient involvement in quality improvement. Health Expect 16: e36–e47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent CA, Coulter A: Patient safety: What about the patient? Qual Saf Health Care 11: 76–80, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson B, Conway J, Simmons L, Edgman-Levitan S, Sodomka P, Schlucter J, Ford D: Partnering with Patients and Families to Design a Patient and Family-Centered Health Care System, Bethesda, MD, Institute for Family-Centered Care and Institute for Healthcare Improvement, 2008 [Google Scholar]
- 21.The Health Foundation: Involving Patients in Improving Safety. Available at: http://www.health.org.uk/publication/involving-patients-improving-safety. Accessed October 20, 2015
- 22.Entwistle VA, McCaughan D, Watt IS, Birks Y, Hall J, Peat M, Williams B, Wright J; Patient Involvement in Patient Safety Group: Speaking up about safety concerns: Multi-setting qualitative study of patients’ views and experiences. Qual Saf Health Care 19: e33, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Peat M, Entwistle V, Hall J, Birks Y, Golder S; PIPS Group: Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy 15[Suppl 1]: 17–25, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Craig GM: Involving users in developing health services. BMJ 336: 286–287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balik B, Zipperer L, Watson J: Achieving an exceptional patient and family experience of inpatient hospital care. IHI Innovation Series White Paper, Cambridge, MA, Institute for Healthcare Improvement, 2011
- 26.Conway J, Edgman-Levitan S, Schlucter J, Ford D, Sodomka P, Simmons L: Partnering with Patients and Families to Design a Patient and Family-Centered Health Care System: A Roadmap for the Future, Bethesda, MD, Institute for Family-Centered Care and Institute for Healthcare Improvement, 2006 [Google Scholar]
- 27.Langley GJ, Nolan KM, Nolan TW, Norman CL, Provost LP: The Improvement Guide: A Practical Approach to Enhancing Organizational Performance, San Francisco, CA, Jossey-Bass, 2009 [Google Scholar]
- 28.Ahmed S, Manaf NH, Islam R: Effects of Lean Six Sigma application in healthcare services: A literature review. Rev Environ Health 28: 189–194, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Antony J: Six Sigma vs Lean: Some perspectives from leading academics and practitioners. Int J Prod Perform Manag 60: 185–190, 2011 [Google Scholar]
- 30.Belson D: Quality Improvement Methods for Use in QUERI Research Proposals and Grant Projects, 2nd Ed., University of Southern California. Available at: http://www.queri.research.va.gov/implementation/quality_improvement/QI_Methods.pdf. Accessed October 28, 2015
- 31.Harel Z, Silver SA, McQuillan RF, Weizman AV, Thomas A, Chertow GM, Nesrallah G, Chan CT, Bell CM: How to diagnose solutions to a quality of care problem. Clin J Am Soc Nephrol 11: 901–907, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuillan RF, Silver SA, Harel Z, Weizman A, Thomas A, Bell C, Chertow GM, Chan CT, Nesrallah G: How to measure and interpret quality improvement data. Clin J Am Soc Nephrol 11: 908–914, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Koning H, Verver JP, van den Heuvel J, Bisgaard S, Does RJ: Lean Six Sigma in healthcare. J Healthc Qual 28: 4–11, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Scoville R, Little K: Comparing lean and quality improvement. IHI White Paper, Cambridge, MA, Institute for Healthcare Improvement, 2014
- 35.Bercaw R: Taking Improvement from the Assembly Line to Healthcare: The Application of Lean within the Healthcare Industry, Boca Raton, FL, Taylor & Francis, 2012 [Google Scholar]
- 36.Veterans Health Administration: Veterans Health Administration System Improvement Framework. Available at: http://www.paloalto.va.gov/docs/improvementguide.pdf. Accessed October 15, 2015
- 37.Berwick DM: A primer on leading the improvement of systems. BMJ 312: 619–622, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson EC, Batalden PB, Godfrey MM: Quality by Design: A Clinical Microsystems Approach, Lebanon, NH, Jossey-Bass/Wiley, 2007 [Google Scholar]
- 39.Bertolaccini L, Viti A, Terzi A: The statistical point of view of quality: The Lean Six Sigma methodology. J Thorac Dis 7: E66–E68, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens L, Goode G, Wold JF, Beck L, Martin G, Perings C, Stolt P, Baggerman L: Structured syncope care pathways based on lean six sigma methodology optimises resource use with shorter time to diagnosis and increased diagnostic yield. PLoS One 9: e100208, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solberg LI, Mosser G, McDonald S: The three faces of performance measurement: Improvement, accountability, and research. Jt Comm J Qual Improv 23: 135–147, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Rutherford WE, Gibney R: End-stage renal disease: A proving ground for quality improvement in health care. Semin Nephrol 17: 218–225, 1997 [PubMed] [Google Scholar]
- 43.Goldman RS: Continuous quality improvement in ESRD: The role of networks, the United States Renal Data System, and facility-specific reports. Am J Kidney Dis 32[Suppl 4]: S182–S189, 1998 [DOI] [PubMed] [Google Scholar]
- 44.DeOreo PB, Wilson R, Wish JB: Can better understanding and use of treatment center performance feedback improve hemodialysis care? A role for the medical director. Semin Dial 25: 290–293, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Institute for Healthcare Improvement: Institute for Healthcare Improvement Triple Aim Initiative. Available at: http://www.ihi.org/Engage/ Initiatives/TripleAim/pages/default.aspx. Accessed October 20, 2015
- 46.Quality Strategy CMS: CMS Quality Strategy. Available at: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/qualityinitiativesgeninfo/downloads/cms-quality-strategy.pdf. Accessed December 14, 2015
- 47.National Strategy for Quality Improvement in Health Care: Annual Progress Report to Congress, 2015. Available at: http://www.ahrq.gov/workingforquality/reports/annual reports/ nqs2015annlrpt.htm. Accessed December 20, 2015