Abstract
Background and objectives
The current allocation algorithm for deceased donor kidney transplantation takes into consideration HLA mismatches at the ABDR loci but not HLA mismatches at other loci, including HLA-DQ. However, the independent effects of incompatibilities for the closely linked HLA-DQ antigens in the context of HLA-DR antigen matched and mismatched allografts are uncertain. We aimed to determine the effect of HLA-DQ mismatches on renal allograft outcomes.
Design, setting, participants, & measurements
Using data from the Australia and New Zealand Dialysis and Transplant Registry, we examined the association between HLA-DQ mismatches and acute rejections in primary live and deceased donor kidney transplant recipients between 2004 and 2012 using adjusted Cox regression models.
Results
Of the 788 recipients followed for a median of 2.8 years (resulting in 2891 person-years), 321 (40.7%) and 467 (59.3%) received zero and one or two HLA-DQ mismatched kidneys, respectively. Compared with recipients who have received zero HLA-DQ mismatched kidneys, those who have received one or two HLA-DQ mismatched kidneys experienced greater numbers of any rejection (50 of 321 versus 117 of 467; P<0.01), late rejections (occurring >6 months post-transplant; 8 of 321 versus 27 of 467; P=0.03), and antibody-mediated rejections (AMRs; 12 of 321 versus 38 of 467; P=0.01). Compared with recipients of zero HLA-DQ mismatched kidneys, the adjusted hazard ratios for any and late rejections in recipients who had received one or two HLA-DQ mismatched kidneys were 1.54 (95% confidence interval [95% CI], 1.08 to 2.19) and 2.85 (95% CI, 1.05 to 7.75), respectively. HLA-DR was an effect modifier between HLA-DQ mismatches and AMR (P value for interaction =0.02), such that the association between HLA-DQ mismatches and AMR was statistically significant in those who have received one or two HLA-DR mismatched kidneys, with adjusted hazard ratio of 2.50 (95% CI, 1.05 to 5.94).
Conclusions
HLA-DQ mismatches are associated with acute rejection, independent of HLA-ABDR mismatches and initial immunosuppression. Clinicians should be aware of the potential importance of HLA-DQ matching in the assessment of immunologic risk in kidney transplant recipients.
Keywords: Epidemiology and outcomes, HLA-matching, registry, acute allograft rejection, kidney transplantation, Allografts, HLA Antigens, Humans, immunosuppression, renal dialysis
Introduction
Matching at the HLA-ABDR loci remains the cornerstone of deceased donor kidney allocation in Australia and worldwide because of the association between incremental HLA-ABDR mismatches and increased risk of rejection and/or graft loss after kidney transplantation (1–3). Although differences in HLA-DQ matching between donors and recipients have been shown to be associated with adverse graft outcomes, matching at the HLA-DQ locus is not explicitly considered in the allocation algorithm for deceased donor kidney transplantation (4,5). There is a general consensus suggesting that HLA-DQ mismatches are unlikely to have a major effect on graft survival, because serologic compatibility for HLA-DR usually ensures a corresponding compatibility for HLA-DQ (6–8). However, different HLA-DR alleles within an antigen group may be associated with different DQ antigens, resulting in HLA-DR antigen matched but HLA-DQ antigen mismatched grafts, which may result in a differential effect in graft outcomes.
In acute graft versus host disease after hematopoietic stem cell transplantation, donor-recipient incompatibility at the HLA-DQ locus is associated with almost a twofold greater risk of acute graft versus host disease, independent of compatibility at the HLA-DR locus (9,10). Recent studies have also shown that increasing numbers of HLA-DQ epitope mismatches and/or the development of donor–specific anti–HLA-DQ antibody after kidney transplantation may contribute to poorer graft outcomes, including the risk of developing transplant glomerulopathy and late graft loss (4,11,12). Despite these findings, the clinical importance of broad antigen HLA-DQ mismatches in predicting acute rejection after kidney transplantation independent of the effects of HLA matching at the ABDR loci has not been examined (7). The aims of this study are to examine the association between HLA-DQ mismatches and acute rejection and graft loss after kidney transplantation and assess whether HLA-DQ mismatches in the presence of compatibility for HLA-DR have any significant effect on graft outcomes.
Materials and Methods
Study Population
All primary live donor and deceased donor kidney transplant recipients in Australia and New Zealand between 2004 and 2012 were included in the analyses. We excluded recipients of multiple organ grafts, those with prior grafts, and those whose data on HLA-DQ matching were not available. Molecular HLA typing was introduced into clinical practice in 1997, with all HLA typing laboratories using this method (and therefore, reporting molecular HLA typing) from 2002. The number of HLA mismatches is provided to the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry from the National Organ Matching, a computerized system designed to allocate donor kidneys to potential kidney transplant recipients according to blood group and tissue compatibility. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Data Collection
The baseline data included donor characteristics of age and type; recipient characteristics of age, sex, race, cause of ESRD, preemptive transplantation, waiting time pretransplant, diabetes, coronary artery disease, and smoking history; and transplant-related characteristics, including use of induction antibody therapy, peak panel reactive antibody (PRA) expressed as a percentage, total ischemic time, type of initial immunosuppressive agents (categorized as calcineurin inhibitor, antimetabolite, and prednisolone), transplant era (categorized as 2004–2006, 2007–2009, and 2010–2012), and transplant state/country (New South Wales, Victoria, South Australia, Queensland, Western Australia, and New Zealand).
Clinical Outcomes
The primary clinical outcomes of this study were acute rejection (including early rejection occurring at ≤6 months, late rejection occurring at >6 months, any rejection, and humoral rejection [i.e., antibody-mediated rejection (AMR)]), overall graft loss (defined as death or returned to dialysis), death–censored graft loss (DCGL), and graft function (eGFR) at 1 and 5 years after transplantation. The various types of rejection are coded as the absence or presence of cellular, vascular, and humoral rejections. The diagnosis of AMR required at least two of the following three criteria: (1) positive C4d staining on immunofluorescence, (2) presence of donor–specific anti–HLA antibody, and (3) characteristic histologic changes of AMR. The dates of the first episode of acute rejection that occurred within the first 6 months after transplantation were available starting in 1997, whereas the dates of subsequent episodes were only consistently reported starting in 2004. As such, we have restricted the inclusion period from 2004 to 2012.
Statistical Analyses
Comparisons of the baseline characteristics between recipients stratified by HLA-DQ matched and mismatched kidney transplants were made by chi-squared test, ANOVA, and Mann–Whitney U test for categorical, parametric, and nonparametric continuous variables, respectively. Acute rejection, overall graft loss, and DCGL were examined using the adjusted and random effects Cox proportional hazard regression models, accounting for the potential intracluster correlation within transplant states and country. Linear regression was used to examine the association between HLA-DQ mismatches and eGFR at 1 and 5 years. The covariates included in the Cox and linear regression models were donor-, recipient-, and transplant-related characteristics outlined above. Results were expressed as hazard ratios (HRs) or mean differences with 95% confidence intervals (95% CIs). Effect modification was tested between the study factor and other covariates using two–way interaction terms in the adjusted models. Only covariates that were associated with rejection (overall and DCGL) and eGFR at 1 and 5 years with P values of <0.10 in the unadjusted analyses were included in the multivariable-adjusted analyses. All analyses were undertaken using the SPSS V10 statistical software program (SPSS Inc., Chicago, IL) and SAS statistical software 9.4 (SAS Institute Inc., Cary, NC).
Results
Study Population
Of 6107 recipients who were transplanted between 2004 and 2012, 5319 (87%) were excluded from the analyses because of missing HLA-DQ molecular typing. Apart from age and donor type, there were no significant differences in the sensitization status (peak PRA >50%; 5.4% versus 7.1%; P=0.06), the incidence of acute rejection (23.3% versus 21.2%; P=0.12), and other demographic characteristics, such as sex (men: 63.7% versus 61.9%; P=0.30), pretransplant diabetes (14.1% versus 11.7%; P=0.06), and race (white recipients: 79.6% versus 79.2%; P=0.45), among those who were excluded compared with those who were included in the analyses. Recipients excluded from the analyses were older (mean age ±SD: 46.6±15.2 versus 43.6±16.6 years old; P=0.001), had received kidneys from older donors (mean age ±SD: 47.3±15.0 versus 43.8±15.5 years old; P<0.001), and were more likely to have received live donor kidney transplants (44.6% versus 31.1%; P<0.001) compared with those included in the analyses. Initial types of immunosuppressive agents, era, and transplanting state/country were similar in both groups.
Table 1 shows the baseline characteristics of the study population stratified by recipients with HLA-DQ matched and mismatched kidneys. There were 788 kidney transplant recipients between 2004 and 2012 followed for a median of 2.8 years (interquartile range, 1.2–6.6 years), resulting in 2891 person-years. Of these, 321 (40.7%) received zero HLA-DQ mismatched kidneys, and 467 (59.3%) received one or two HLA-DQ mismatched kidneys (393 [49.9%] and 74 [9.4%] received one and two HLA-DQ mismatched kidneys, respectively). Compared with kidney transplant recipients who have received kidneys with one or two HLA-DQ mismatches, those who have received kidneys with zero HLA-DQ mismatches were more likely to be white and have received preemptive kidney transplants.
Table 1.
Baseline characteristics of kidney transplant recipients stratified by HLA-DQ mismatches (n=788)
| Characteristics and Outcomes | Zero HLA-DQ Mismatch, n=321 | One or Two HLA-DQ Mismatches, n=467 | P Value |
|---|---|---|---|
| Demographics | |||
| Median age (25%–75%), yr | 45 (32–56) | 47 (34–57) | 0.51 |
| Men, n (%) | 189 (38.7) | 299 (61.3) | 0.14 |
| White, n (%) | 257 (80.1) | 367 (78.6) | 0.004 |
| Preemptive, n (%) | 45 (14.0) | 36 (7.7) | 0.004 |
| Median BMI (25%–75%), kg/m2 | 24.8 (21.6–29.1) | 25.7 (22.4–29.3) | 0.13 |
| Diabetes, n (%) | 30 (9.3) | 61 (13.1) | 0.11 |
| Coronary artery disease, n (%) | 18 (5.6) | 36 (7.7) | 0.25 |
| Former/current smoker, n (%) | 121 (38.0) | 201 (42.3) | 0.33 |
| Cause of ESRD, n (%) | 0.22 | ||
| GN | 126 (39.3) | 184 (39.4) | |
| Diabetes | 22 (6.9) | 47 (10.1) | |
| Cystic | 48 (15.0) | 78 (16.7) | |
| Median waiting time (25%–75%), yr | 1.7 (0.6–3.0) | 1.9 (0.8–3.4) | 0.02 |
| Donor characteristics | |||
| Median age (25%–75%), yr | 47 (32–57) | 46 (33–55) | 0.57 |
| Live donor, n (%) | 104 (32.4) | 141 (30.2) | 0.51 |
| Immunology/transplant | |||
| HLA-A mismatches, n (%) | <0.001 | ||
| 0 | 86 (26.8) | 58 (12.4) | |
| 1 or 2 | 235 (73.2) | 409 (87.6) | |
| HLA-B mismatches, n (%) | <0.001 | ||
| 0 | 69 (21.5) | 30 (6.4) | |
| 1 or 2 | 252 (78.5) | 437 (93.6) | |
| HLA-DR mismatches, n (%) | <0.001 | ||
| 0 | 146 (45.5) | 26 (5.6) | |
| 1 or 2 | 175 (54.5) | 441 (94.4) | |
| Peak PRA >50%, n (%) | 19 (5.9) | 24 (5.1) | 0.64 |
| Median ischemic time (25%–75%), h | 9 (4–13) | 9 (4–13) | 0.94 |
| Induction, n (%) | 233 (72.6) | 319 (68.3) | 0.20 |
| Initial prednisolone, n (%) | 306 (95.3) | 437 (93.6) | 0.30 |
| Initial CNI, n (%) | 310 (96.6) | 440 (94.2) | 0.84 |
| Initial antimetabolite, n (%) | 0.88 | ||
| None | 22 (6.9) | 35 (7.5) | |
| Azathioprine | 2 (0.6) | 2 (0.4) | |
| MMF | 297 (92.5) | 430 (92.1) | |
| Outcomes | |||
| Mean±SD eGFR, ml/min per 1.73 m2 | |||
| 1-yr eGFR, n=585 | 63.9±57.0 | 58.3±45.0 | 0.18 |
| 5-yr eGFR, n=214 | 57.3±23.9 | 53.1±27.6 | 0.26 |
| Acute rejection, n (%) | |||
| First 6 mo | 42 (13.1) | 90 (19.3) | 0.02 |
| Late rejection | 8 (2.5) | 27 (5.8) | 0.03 |
| Any | 50 (15.6) | 117 (25.1) | 0.001 |
| AMR | 12 (3.7) | 38 (8.1) | 0.01 |
| Mean±SD total rejection episodes | 0.20±0.52 | 0.34±0.69 | 0.002 |
| Graft loss, n (%) | 42 (13.1) | 62 (13.3) | 0.94 |
| DCGL, n (%) | 20 (6.2) | 44 (9.4) | 0.11 |
Data are expressed as numbers (proportions), medians (25th–75th percentiles), or means±SDs. BMI, body mass index; PRA, panel reactive antibody; CNI, calcineurin inhibitor; MMF, mycophenolate; AMR, antibody-mediated rejection; DCGL, death–censored graft loss.
The proportion of recipients with HLA-DQ mismatches with zero to six HLA-ABDR mismatches is shown in Supplemental Figure 1. Only 26 (3%) recipients had no mismatches at the HLA-A, HLA-B, HLA-DR, and HLA-DQ loci. Of the recipients who received zero HLA-DQ mismatched kidneys (n=321), 235 (73%), 252 (78%), and 175 (54%) had mismatches at the HLA-A, HLA-B, and HLA-DR loci, respectively. Of those who had received one or two HLA-DQ mismatched kidneys (n=467), 58 (12%), 30 (6%), and 26 (6%) had zero mismatches at the HLA-A, HLA-B, and HLA-DR loci, respectively. Of those with zero HLA-DR mismatched kidneys, 15% were mismatched at the HLA-DQ locus.
A greater proportion of kidney transplant recipients who have received HLA-DQ mismatched kidneys experienced early acute rejection episodes (19.3% and 13.1%, respectively; P=0.02), late rejection (5.8% and 2.5%, respectively; P=0.03), any rejection episodes (25.1% and 15.6%, respectively; P=0.001), and AMR (8.1% and 3.7%, respectively; P=0.01) compared with recipients with zero HLA-DQ mismatched kidneys. The incidences of overall graft loss and DCGL were similar in the two groups.
HLA-DQ Mismatches, Overall Graft Loss, and DCGL
There were no statistically significant associations between HLA-DQ mismatches, overall graft loss, and DCGL in the unadjusted and adjusted Cox regression models (Table 2). In the random effects models, the adjusted HRs of one or two HLA-DQ mismatched grafts for overall graft loss and DCGL were 0.95 (95% CI, 0.64 to 1.40) and 1.26 (95% CI, 0.84 to 2.44), respectively, compared with zero HLA-DQ mismatched grafts. Of those who have experienced graft loss, the proportions of recipients who had received HLA-DQ matched and mismatched kidneys and had graft loss that was attributed to acute rejection were 4.8% and 16.1%, respectively (P=0.07).
Table 2.
Association between HLA-DQ mismatches and rejection, overall graft loss, and death–censored graft loss in adjusted Cox regression models
| Clinical Outcomes | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Timing of acute rejection | ||
| Any rejection | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 1.54 (1.08 to 2.19) | 0.02 |
| Waiting time per 1 yr longer | 1.07 (1.02 to 1.13) | 0.01 |
| Early rejection | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 1.38 (0.90 to 2.01) | 0.09 |
| Waiting time per yr longer | 1.08 (1.02 to 1.14) | 0.01 |
| HLA-B mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 2.31 (1.07 to 5.02) | 0.03 |
| Late rejection | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 2.85 (1.05 to 7.75) | 0.04 |
| Age per yr | 0.96 (0.94 to 0.98) | 0.001 |
| Types of acute rejection | ||
| Antibody-mediated rejection | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2a | 2.33 (1.19 to 4.58) | 0.01 |
| 1 or 2 with 0 HLA-DR mismatches | 2.50 (1.05 to 5.94) | 0.04 |
| 1 or 2 with 1 or 2 HLA-DR mismatches | 1.50 (0.20 to 9.99) | 0.46 |
| Peak PRA every 10% higher | 1.01 (1.00 to 1.02) | 0.07 |
| Graft loss | ||
| Overall graft loss | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 0.85 (0.55 to 1.35) | 0.51 |
| Ischemic time per h | 1.06 (1.01 to 1.12) | 0.02 |
| BMI per kg/m2 higher | 1.05 (1.01 to 1.09) | 0.02 |
| Race | ||
| White | 1.00 | |
| Nonwhite | 1.73 (0.80 to 3.76) | 0.16 |
| Indigenous | 3.45 (1.94 to 6.14) | <0.001 |
| Death–censored graft loss | ||
| HLA-DQ mismatches | ||
| 0 | 1.00 | |
| 1 or 2 | 1.16 (0.62 to 2.16) | 0.64 |
| Recipient age per yr | 0.97 (0.95 to 0.99) | <0.01 |
| Ischemic time per h | 1.09 (1.01 to 1.17) | 0.02 |
| BMI per kg/m2 higher | 1.07 (1.02 to 1.13) | 0.01 |
| Race | ||
| White | 1.00 | |
| Nonwhite | 2.09 (0.78 to 5.57) | 0.14 |
| Indigenous | 4.72 (2.40 to 9.28) | <0.001 |
Data presented are adjusted hazard ratios with 95% CIs for acute rejection and overall and death–censored graft loss. In the models adjusted for recipient age, donor age, donor type, BMI, HLA-ABDR mismatches, peak PRA, era, race, ischemic time, waiting time, induction therapy, and initial immunosuppression, only covariates (except HLA-DQ mismatches) remaining in the most parsimonious models are shown. 95% CI, 95% confidence interval; PRA, panel reactive antibody; BMI, body mass index.
Interaction between HLA-DQ mismatches and HLA-DR mismatches (P value for interaction =0.02), with adjusted hazard ratios (95% CIs) of one or two HLA-DQ mismatched kidneys shown for zero and one or two HLA-DR mismatched kidneys compared with zero HLA-DQ mismatched kidneys.
HLA-DQ Mismatches and the Timing of Acute Rejection
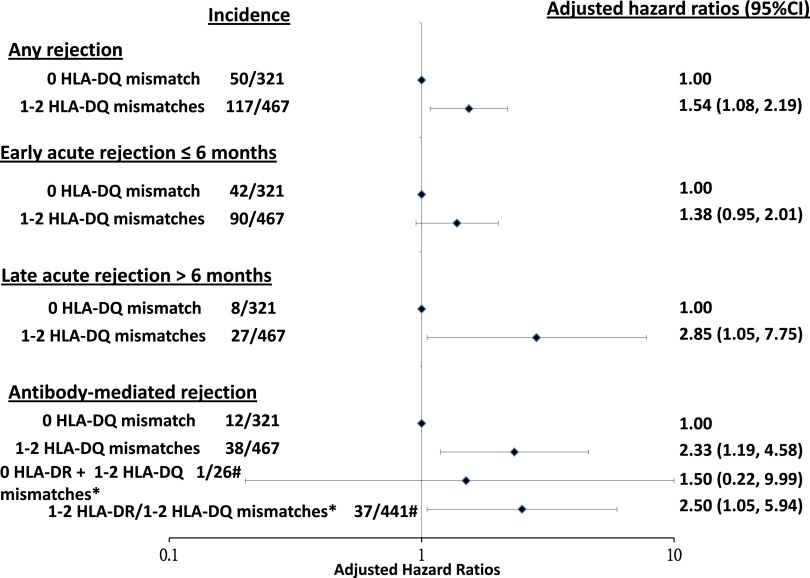
In the unadjusted models, one or two HLA-DQ mismatched kidneys were associated with a significantly greater risk of any rejection, early rejection, and late rejection, with unadjusted HRs of 1.71 (95% CI, 1.23 to 2.38; P=0.002), 1.53 (95% CI, 1.06 to 2.21; P=0.02), and 2.41 (95% CI, 1.09 to 5.31; P=0.03), respectively, compared with zero HLA-DQ mismatched kidneys. Compared with recipients who have received zero HLA-DQ mismatched kidneys, the adjusted HRs of recipients who have received one or two HLA-DQ mismatched kidneys for any rejection, early rejection, and late rejection were 1.54 (95% CI, 1.08 to 2.19; P=0.02), 1.38 (95% CI, 0.95 to 2.01; P=0.09), and 2.85 (95% CI, 1.05 to 7.75; P=0.04), respectively, independent of age, HLA mismatches at the ABDR loci, and era (Figure 1); similar adjusted HRs were observed in the random effects models (any rejection: HR, 1.51; 95% CI, 1.06 to 2.14; P=0.02; early rejection: HR, 1.42; 95% CI, 0.98 to 2.07; P=0.06; and late rejection: HR, 2.50; 95% CI, 1.06 to 5.86; P=0.03). Other covariates associated with any, early, and late rejections are shown in Table 2. The median (interquartile range) time to the occurrence of late rejection was 18 (10–44) months after transplant, with 40% of late rejections being AMR. HLA-A, HLA-B, and HLA-DR mismatches were not effect modifiers between HLA-DQ mismatches and any rejection, early rejection, and late rejection.
Figure 1.
Incidence and forest plots of the adjusted hazard ratios of timing and types of acute rejection after kidney transplantation stratified by HLA-DQ matched and mismatched kidney transplants adjusted for donor and recipient age, race, donor type, body mass index, era, number of HLA-ABDR mismatches, panel reactive antibody, waiting time, ischemic time, induction therapy, and initial immunosuppression. *Interaction between HLA-DQ and HLA-DR mismatches with adjusted hazard ratio of one or two HLA-DQ mismatches compared to zero HLA-DQ mismatch for zero and one or two HLA-DR mismatched kidneys; #incidence of antibody mediated rejection in recipients of zero HLA-DR and one or two HLA-DQ mismatched kidneys, and one or two HLA-DR and one or two HLA-DQ mismatched kidneys. 95% CI, 95% confidence interval.
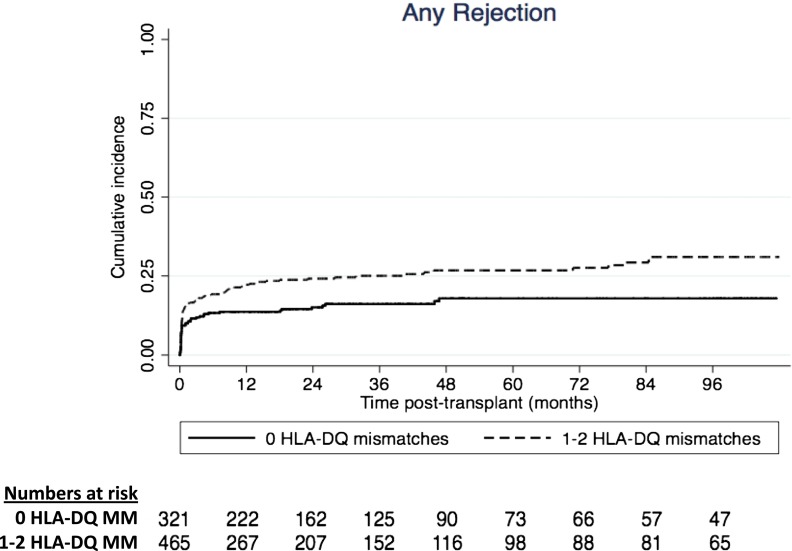
The adjusted cumulative incidence curve for any rejection is shown in Figure 2. The incidences of any rejection of recipients who have received zero and one or two HLA-DQ mismatched kidneys were 12% and 19%, respectively, at 3 months, 13% and 22%, respectively, at 12 months, 14% and 23%, respectively, at 2 years, and 15% and 23%, respectively, at 3 years after transplantation (log rank P=0.001).
Figure 2.
Adjusted cumulative incidence curves for any rejection after kidney transplantation according to HLA-DQ matched and mismatched kidney transplants (log rank P value <0.01). MM, mismatched.
HLA-DQ Mismatches and AMR
Compared with recipients who have received zero HLA-DQ mismatched kidneys, recipients of one or two HLA-DQ mismatched kidneys were associated with unadjusted and adjusted HRs of 2.22 (95% CI, 1.16 to 4.25; P=0.02) and 2.33 (95% CI, 1.19 to 4.58; P=0.01), respectively, independent of age, HLA mismatches at the other loci, and era (Figure 1). In the random effects model, the adjusted HR for AMR was 2.18 (95% CI, 1.14 to 4.16; P=0.02) for recipients of one or two HLA-DQ mismatched kidneys. Other covariates associated with AMR are shown in Table 2.
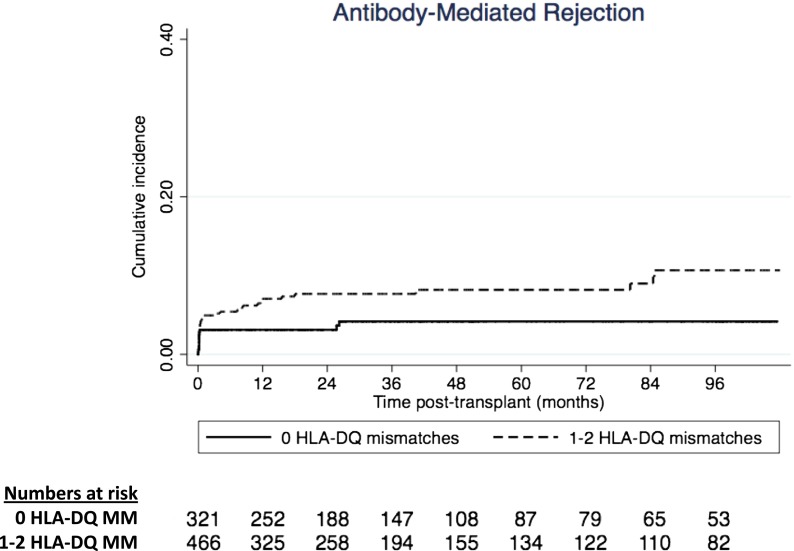
HLA-DR was an effect modifier between HLA-DQ mismatches and risk of AMR (P value for interaction =0.02). In the analyses stratified by HLA-DR mismatches, the association between HLA-DQ mismatches and AMR was only observed in recipients who have received one or two HLA-DR mismatched kidneys (n=616), with adjusted HR of 2.50 (95% CI, 1.05 to 5.94; P=0.04), but not observed in those who have received zero HLA-DR mismatched kidneys (n=172; adjusted HR, 1.50; 95% CI, 0.20 to 9.99; P=0.46). The adjusted cumulative incidence curves for AMR are shown in Figure 3. The incidences of AMR of recipients who have received HLA-DQ matched and mismatched kidneys were 3% and 5%, respectively, at 3 months, 3% and 7%, respectively, at 12 months, 3% and 7%, respectively, at 2 years, and 4% and 8%, respectively, at 3 years after transplantation (log rank P=0.01).
Figure 3.
Adjusted cumulative incidence curves for antibody-mediated rejection after kidney transplantation according to HLA-DQ matched and mismatched kidney transplants (log rank P value <0.01). MM, mismatched.
HLA-DQ Mismatches and Graft Function
The mean (SD) eGFRs at 1 and 5 years for recipients who have received HLA-DQ matched and mismatched kidneys are shown in Table 1. There were no associations between HLA-DQ mismatches and mean eGFR at 1 and 5 years in unadjusted and adjusted linear regression models. Compared with recipients of HLA-DQ matched kidneys, the mean eGFR was 4.20 ml/min per 1.73 m2 (95% CI, −11.17 to 2.76; P=0.24) lower at 1 year and 5.43 ml/min per 1.73 m2 (95% CI, −11.99 to 1.14; P=0.10) lower at 5 years in recipients of HLA-DQ mismatched kidneys when adjusted for sex, donor and recipient ages, and body mass index.
Discussion
In our study involving 788 kidney transplant recipients with a median follow-up time of 2.8 years, we have shown that the inclusion of HLA-DQ mismatches to conventional HLA-ABDR mismatches improved the risk stratification of acute rejection, particularly in those at risk of late rejection and AMR, independent of initial immunosuppression and sensitization status. We have also shown that HLA-DR was an effect modifier between HLA-DQ mismatches and risk of AMR, such that the association between HLA-DQ mismatches and risk of AMR may be more important in recipients who have received HLA-DR mismatched kidneys.
An association between HLA-ABDR mismatches and acute rejection risk in live and deceased donor kidney transplantation has been shown in multiple studies, with this association persisting even in the era of modern immunosuppression (3). In a retrospective study of 82 pediatric kidney transplant recipients with prior failed grafts, ≤30% of recipients developed de novo anti–HLA antibody predominantly against HLA-DQ (13). Similarly, it has been shown that increasing epitope mismatches at both HLA-DR and HLA-DQ loci was strongly predictive of developing de novo class 2 donor–specific antibody, suggesting that matching at the HLA-DQ locus is likely to improve the stratification of immunologic risk (and therefore, risk of developing rejection, de novo antibody, and/or long–term graft outcomes), particularly for recipients who may require retransplantation (4). Studies involving pediatric and adult kidney transplant only and kidney-pancreas transplant recipients have also shown that the development of de novo donor–specific anti–HLA-DQ antibody was associated with a ≤10-fold greater risk of developing early and late AMR, which was often associated with development of transplant glomerulopathy and early graft loss (14–16). In our study, we have highlighted the importance of class 2 mismatches and the risk of acute rejection. We have shown an independent association between HLA-DQ mismatches and acute rejection, including AMR. It is important to point out that the majority of acute rejection (approximately 80%) occurred within the first 6 months after transplantation, suggesting the potential contribution of pretransplant donor–specific anti–HLA-DQ antibody to the risk of early rejection. However, information pertaining to the development of antibodies before and after transplantation and histologic data on transplant glomerulopathy are not available within the registry.
The lack of direct association between HLA-DQ mismatches and longer–term graft outcomes is interesting and may imply that the causes of allograft failure are multifactorial. A large retrospective study of >12,000 primary deceased donor kidney transplant recipients showed that the addition of HLA-DQ mismatches does not improve the prediction of graft survival, independent of HLA-ABDR matching. However, these studies were using data from before 2000, when the utility of molecular HLA typing and the use of modern induction and maintenance immunosuppressive agents were not available (7,8). Similarly, our study did not report an association between HLA-DQ mismatches and graft loss and may reflect the shorter median follow-up time compared with that in previous studies. Long–term follow-up of this cohort will be important to establish the clinical significance of HLA-DQ mismatches.
There is also considerable variability in the immunogenicity of individual HLA, and matching at the epitope level may improve our understanding of otherwise unexplained sensitization status induced by a given HLA mismatch (17). Although HLA-DQ and -DR may be closely related at an antigen level, it has been shown that small differences in one or more epitopes between donors and recipients at either locus are sufficient to generate a humoral and/or T cell–mediated immune response (18,19). In a nested case-control study of a cohort of 52 kidney transplant recipients with established transplant glomerulopathy, an increasing number of HLA-DQ and HLA-DR eplet mismatches was associated with an increased risk of developing transplant glomerulopathy, suggesting that epitope mismatches at either HLA-DQ or HLA-DR locus are equally important in predicting graft outcomes (5). Future studies evaluating both broad antigen and epitope HLA-DQ mismatches are crucial in establishing a better understanding of the association between HLA-DQ mismatches and clinical outcomes.
Our study has several strengths and limitations. Using data from the ANZDATA Registry, we have established an association between HLA-DQ mismatches and rejection risk in kidney transplant recipients in the era of molecular HLA typing and modern immunosuppression. Selection bias may exist, because there may be systematic differences in the management of kidney transplant recipients between transplanting centers and clinicians. There were numerous recipients without donor-recipient HLA-DQ typing, likely reflecting variations in practices of HLA typing across the country and era. Although multiple confounding factors were adjusted for, there may be unmeasured residual confounders, such as the intensity of immunosuppression (i.e., therapeutic drug levels), the number of epitope mismatches, and the presence of pre– and post–transplant donor–specific anti–HLA antibodies, which are not collected by the ANZDATA Registry. In view of the lack of detailed descriptor within the ANZDATA Registry, misclassification bias of the outcomes could potentially occur, but the bias is likely to be random and nondifferential between the exposed and unexposed groups. We were unable to examine the effect of the incremental number of HLA-DQ mismatches and outcomes because of the small number of recipients who had received two HLA-DQ mismatched kidneys, and therefore, we would have had insufficient power to detect a significant difference between HLA-DQ matched and mismatched kidney transplants. The ANZDATA Registry does not collect data on HLA-DP mismatches, which has also been shown to be associated with rejection in case series and observational studies (20).
In the current deceased donor allocation algorithm used in Australia, additional points are assigned for patients who are highly sensitized (with peak PRA >80%) and those with zero to two HLA-ABDR mismatches, particularly zero HLA-DR mismatches, because lower numbers of HLA-ABDR mismatches are associated with improved graft outcomes. Currently, matching at the HLA-DQ locus is not considered in the allocation pathway for deceased donor kidneys. Our data have shown that improved HLA-DQ matching may reduce the overall risk of acute rejection but has no effect on the overall long–term graft survival. Although there is insufficient evidence to suggest that the current point allocation should be changed, we believe that our data add to the current debate as to whether adding bonus points to HLA-DQ and HLA-DR matching may improve access for highly sensitized and minority kidney transplant candidates, in whom the disparity in HLA matching occurs largely as a result of incompatibility to common class 1 HLA antigens found in the white donor population. An economic evaluation examining the addition of HLA-DQ matching to the current allocation model is prudent before considering potential changes to the current allocation pathway.
HLA-DQ mismatches are associated with an increased risk of any rejection, late rejection, and AMR, independent of HLA-ABDR mismatches, sensitization status, and initial immunosuppression. Our study findings highlight the importance of establishing HLA-DQ mismatches before transplantation to better define the immunologic risk of each potential kidney transplant candidate and potentially minimize sensitization against HLA-DQ antigen and subsequent development of de novo antibody, which may be particularly important in pediatric or younger patients who are likely to require future retransplantation. Future studies further defining the association between HLA-DQ mismatches, pretransplant and de novo HLA-DQ antibodies, and long–term graft outcomes could improve the understanding of the importance of HLA-DQ matching in kidney transplantation in addition to the conventional matching at HLA-ABDR loci.
Disclosures
W.H.L., P.T.C., and G.R.R. have participated in industry–sponsored clinical trials and symposia and received educational grants, travel support, or honoraria from companies producing immunosuppressant drugs, including Novartis (Basel, Switzerland), Alexion (New Haven, CT), Genzyme (Sanofi US, Bridgewater, NJ), and Pfizer (New York, New York).
Supplementary Material
Acknowledgments
The authors acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients), which provides information to and maintains the ANZDATA Database.
The data reported here have been supplied by the ANZDATA. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the ANZDATA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “HLA-DQ Mismatching: Mounting Evidence for a Role in Kidney Transplant Rejection,” on pages 759–760.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11641115/-/DCSupplemental.
References
- 1.Wissing KM, Fomegné G, Broeders N, Ghisdal L, Hoang AD, Mikhalski D, Donckier V, Vereerstraeten P, Abramowicz D: HLA mismatches remain risk factors for acute kidney allograft rejection in patients receiving quadruple immunosuppression with anti-interleukin-2 receptor antibodies. Transplantation 85: 411–416, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Beckingham IJ, Dennis MJ, Bishop MC, Blamey RW, Smith SJ, Nicholson ML: Effect of human leucocyte antigen matching on the incidence of acute rejection in renal transplantation. Br J Surg 81: 574–577, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Lim WH, Chadban SJ, Clayton P, Budgeon CA, Murray K, Campbell SB, Cohney S, Russ GR, McDonald SP: Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin Transplant 26: E428–E437, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Sapir-Pichhadze R, Tinckam K, Quach K, Logan AG, Laupacis A, John R, Beyene J, Kim SJ: HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: A nested case-control study. Am J Transplant 15: 137–148, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Bushell A, Higgins RM, Wood KJ, Morris PJ: HLA-DQ mismatches between donor and recipient in the presence of HLA-DR compatibility do not influence the function or outcome of renal transplants. Hum Immunol 26: 179–189, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Sasaki N, Idica A: The HLA-matching effect in different cohorts of kidney transplant recipients: 10 years later. Clin Transpl 2010: 261–282, 2010 [PubMed] [Google Scholar]
- 8.Freedman BI, Thacker LR, Heise ER, Adams PL: HLA-DQ matching in cadaveric renal transplantation. Clin Transplant 11: 480–484, 1997 [PubMed] [Google Scholar]
- 9.Petersdorf EW, Longton GM, Anasetti C, Mickelson EM, Smith AG, Martin PJ, Hansen JA: Definition of HLA-DQ as a transplantation antigen. Proc Natl Acad Sci U S A 93: 15358–15363, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Viña MA, Klein JP, Haagenson M, Spellman SR, Anasetti C, Noreen H, Baxter-Lowe LA, Cano P, Flomenberg N, Confer DL, Horowitz MM, Oudshoorn M, Petersdorf EW, Setterholm M, Champlin R, Lee SJ, de Lima M: Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood 121: 4603–4610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosmoliaptsis V, Gjorgjimajkoska O, Sharples LD, Chaudhry AN, Chatzizacharias N, Peacock S, Torpey N, Bolton EM, Taylor CJ, Bradley JA: Impact of donor mismatches at individual HLA-A, -B, -C, -DR, and -DQ loci on the development of HLA-specific antibodies in patients listed for repeat renal transplantation. Kidney Int 86: 1039–1048, 2014 [DOI] [PubMed] [Google Scholar]
- 12.DeVos JM, Gaber AO, Knight RJ, Land GA, Suki WN, Gaber LW, Patel SJ: Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int 82: 598–604, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Tagliamacco A, Cioni M, Comoli P, Ramondetta M, Brambilla C, Trivelli A, Magnasco A, Biticchi R, Fontana I, Dulbecco P, Palombo D, Klersy C, Ghiggeri GM, Ginevri F, Cardillo M, Nocera A: DQ molecules are the principal stimulators of de novo donor-specific antibodies in nonsensitized pediatric recipients receiving a first kidney transplant. Transplant Int 27: 667–673, 2014 [DOI] [PubMed]
- 14.Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, McLean A, Cook TH, Cairns T, Roufosse C, Taube D: De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 94: 172–177, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, Fontana I, Magnasco A, Nocco A, Tagliamacco A, Sementa A, Ceriolo P, Ghio L, Zecca M, Cardillo M, Garibotto G, Ghiggeri GM, Poli F: Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant 12: 3355–3362, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Devos JM, Gaber AO, Teeter LD, Graviss EA, Patel SJ, Land GA, Moore LW, Knight RJ: Intermediate-term graft loss after renal transplantation is associated with both donor-specific antibody and acute rejection. Transplantation 97: 534–540, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Lucas DP, Leffell MS, Zachary AA: Differences in immunogenicity of HLA antigens and the impact of cross-reactivity on the humoral response. Transplantation 99: 77–85, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duquesnoy RJ: A structurally based approach to determine HLA compatibility at the humoral immune level. Hum Immunol 67: 847–862, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dankers MK, Witvliet MD, Roelen DL, de Lange P, Korfage N, Persijn GG, Duquesnoy R, Doxiadis II, Claas FH: The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched human leukocyte antigens. Transplantation 77: 1236–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Jolly EC, Key T, Rasheed H, Morgan H, Butler A, Pritchard N, Taylor CJ, Clatworthy MR: Preformed donor HLA-DP-specific antibodies mediate acute and chronic antibody-mediated rejection following renal transplantation. Am J Transplant 12: 2845–2848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.