Abstract
Background
Long-term use of clozapine for individuals with schizophrenia carries a high risk for developing metabolic abnormalities, especially clozapine-induced weight gain. Previous studies suggest that metformin can decrease clozapine-induced weight gain, but the sample sizes of most of these studies are relatively small.
Methods
We identified randomized controlled trials (RCTs) published prior to December 15, 2015 about the use of metformin to treat clozapine-induced weight gain in adults with schizophrenia by searching several English-language and Chinese-language databases. Two independent researchers did the screening and data extraction. We used Revman 5.3 to conduct the meta-analyses, assessed the risk of bias (RoB), and assessed the strength of the evidence using the Cochrane Grades of Recommendation, Assessment, Development, and Evaluation (GRADE).
Results
Six studies with a pooled sample of 207 treatment-group patients and 207 control-group patients were included —— three double-blind, placebo-controlled RCTs and three RCTs that did not use placebo controls and were not blinded. The meta-analysis found that compared to the control condition, patients receiving metformin experienced significantly greater reductions in body weight (mean difference [MD]=-2.89 kg, 95% CI: -4.20 to -1.59 kg) and body mass index (BMI) (MD=-0.81, 95% CI: -1.16 to -0.45), but there was no significant difference between the groups in the prevalence of side effects. Based on the GRADE scale, the strength of the evidence for the change in weight outcome was ‘moderate’ and that for the change in BMI outcome was ‘high’, but the strength of evidence about differences in side effects between groups was ‘low’ or ‘very low’.
Conclusions
Adjunctive treatment with metformin appears to be effective for treating clozapine-induced weight gain and elevations in BMI in adult patients with schizophrenia. However, the quality of the evidence about the safety of this treatment is low, follow-up time in the available studies is relatively short, and half of the studies did not employ blinded assessment of outcome measures. Larger studies with placebo controls that follow patients for at least 24 weeks and that make blinded assessments of a range of relevant outcome measures (weight, BMI, blood lipids, insulin resistance, etc.) are needed to confirm these results.
Keywords: metformin, clozapine, schizophrenia, clozapine-induced weight gain, meta-analysis
Abstract
背景
精神分裂症患者长期使用氯氮平易出现代谢综合征,尤其是抗精神病药物引起的体重增加。既往研究表明,二甲双胍可以减少由氯氮平引起的体重增加,但大多数研究的样本量相对较小。
方法
通过检索多个中英文数据库,我们检索出在2015年12月15日之前发表的关于对成年精神分裂症患者使用二甲双胍治疗由氯氮平引起的体重增加的随机对照试验 (randomized controlled trial, RCT)。两位研究者独立进行筛选和数据提取。我们使用RevMan5.3软件进行Meta分析与偏倚风险评估,并采用Cochrane GRADE (Cochrane Grades of Recommendation, Assessment, Development, and Evaluation) 来评估证据质量。
结果
共纳入六项研究(合并样本量:2治疗组207例、对照组207例),其中三项研究是双盲、安慰剂对照的RCT,另三项RCT未在对照组中使用安慰剂,也未使用盲法。meta分析发现,与对照组相比服用二甲双胍的患者体重减轻更为显著 (MD=-2.89, 95% CI: -4.20 to-1.59),并且体质量指数 (body mass index, BMI) 的降低也更为显著 (MD=-0.81, 95% CI: -1.16 to -0.45),但两组不良反应的发生率之间无统计学差异。根据GRADE量表,关于体重结果变化的证据强度为“中等”,而关于BMI指数变化的证据强度是“高”,但关于组间不良反应差异的证据强度是“低” 或“非常低”。
结论
对成年精神分裂症患者使用二甲双胍辅助治疗可能对治疗由氯氮平引起的体重增加和BMI指数上升是有效的。然而,有关该治疗安全性的证据质量很低,现有研究的随访时间比较短,有一半的研究并没有对结果的进行盲法评估。我们需要进行更大样本量的研究,控制安慰剂效应并随访患者至少24周,且对一系列相关结果要进行盲法评估(体重、BMI 指数、血脂、胰岛素抵抗等),从而来证实这些结果。
中文全文
本文全文中文版从2016年04月25日起在http://dx. doi. org/10.11919/j. issn. 1002-0829.215071可供免费阅览下载
1. Introduction
Individuals with schizophrenia need long-term or even lifelong treatment with antipsychotic medication.[1] Clozapine is a highly effective and safe antipsychotic medication widely used for the treatment of schizophrenia, particularly among patients for whom other antipsychotic medications are ineffective. However, common side-effects of the long-term use of clozapine are weight gain and obesity,[2,3] problems that both lower patients' medication compliance and increase the risk of metabolic syndrome, diabetes, hypertension, and coronary heart disease.[4,5,6] Recent studies in China and elsewhere have assessed the effectiveness of adjunctive treatment with metformin,[7,8] memantine,[9] nizatidine,[10] and topiramate[11] to reduce or eliminate antipsychotic-induced weight gain and other metabolic changes in patients with schizophrenia. To our knowledge, there is no published meta-analysis on this issue that specifically focuses on clozapine-induced weight gain, so the present study identified randomized controlled trails (RCTs) published in English or Chinese about the adjunctive use of metformin to reduce clozapine-induced weight gain in adult patients with schizophrenia and then conducted a meta-analysis of the pooled results from these studies.
2.Methods
2.1 Search strategy
We searched the following databases for studies published in English or Chinese prior to December 15, 2015: PubMed, PsycINFO, EBSCO, EMbase, Cochrane Library, China Academic Journals Full-text Database (CJFD), WANFANG DATA, Chongqing VIP database for Chinese Technical Periodicals, and Chinese Biomedical Literature (CBM) Database. We used the keywords 'clozapine,' 'antipsychotics,' 'metformin,' 'body mass,' 'body weight,' 'BMI,' 'RCT', and 'randomized controlled trials' (and the Chinese equivalents) in the searches. The search strategy and keywords were modified to meet the requirements of the different databases. Reference lists of identified articles were hand-searched to locate relevant articles that may not have been identified in the electronic searches.
2.2 Inclusion and exclusion criteria
Included studies were randomized controlled trials (RCTs) published in either Chinese or English among adult patients with schizophrenia being treated with monotherapy clozapine (i.e., not including concurrent treatment with other antipsychotic medications) that compared changes in body weight or changes in body mass index (BMI) in an intervention group of patients receiving clozapine plus adjunctive treatment with metformin versus changes in a control group that received clozapine with an adjunctive placebo or in a control group that received clozapine alone (with no adjunctive treatment). Observational studies, nonhuman studies, systematic reviews, research proposals, case reports, and duplicate reports were excluded. Research participants had to be 18-65 years of age and meet the diagnostic criteria for schizophrenia specified in the 2nd or 3rd version of the Chinese Classification of Mental Disorders (CCMD-2, CCMD-3),[12,13] the 10th revision of the International Classification of Diseases (ICD-10),[14] or the 4th or 5th edition of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSM-IV, DSM-5).[15,16]
2.3 Screening of articles and data extraction
Two researchers (LZR and ZW) independently screened articles identified in the electronic databases using the inclusion criteria and abstracted information from selected articles. If they disagreed about the inclusion of an article or about abstracted data they jointly reviewed the article to identify the reason for the inconsistency and, if they could still not agree, they referred to a third author (GS) to make the final decision. Data extracted from the selected articles included year and language of the publication; location of the study; sample size, method of randomization, treatment in the control group, and duration of follow-up; dosage of clozapine and metformin; outcomes and side effects assessed; and methods for assessing these outcomes.
2.4 Evaluation of risk of bias
Two researchers (LZR and ZW) independently assessed the risk of bias for all included articles based on the Cochrane Risk of Bias (RoB) tool.[17] The items in this instrument are: (a) random assignment 'sequence generation'; (b) 'allocation sequence concealment'; (c) 'blinding of participants and personnel'; (d) 'blinding of outcome assessment'; (e) 'incomplete outcome data'; (f) 'selective outcome reporting'; and (g) 'other potential threats to validity'. A third author's (GS) opinion was sought when the two researchers disagreed. If necessary, study author(s) were contacted via e-mail to supply missing information.
2.5 Outcome measures
The main outcome measures were body weight in kilograms (kg) and body mass index (BMI, weight in kg divided by the square of height in meters). Secondary outcome measures included the treatment effect as evaluated by changes in the Brief Psychiatric Rating Scale (BPRS)[18] or the Positive and Negative Syndrome Scale (PANSS),[19] the dropout rate, and the prevalence of adverse events (which were based on patients' reports of side effects and routine blood chemistry tests, urinalysis tests, and electrocardiograms).
2.6 Evaluation of the strength of evidence
Two researchers (LZR and ZW) used the Cochrane Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) software[20] to evaluate the strength of evidence for each outcome measure of interest. Based on the study's design, the possibility of publication bias, indirect evidence, and the consistency, precision, and effect-size of the outcome measure, the level of evidence for each outcome was classified as 'high','moderate','low', or 'very low'.
2.7 Analysis
We used RevMan 5.3[21] to compute the mean difference (MD) and its 95% confidence interval (95% CI) for continuous outcomes (e.g., weight and BMI), the standardized mean difference (SMD) and its 95% CI for continuous measures assessed using different measures (e.g., treatment effect), and the risk ratio (RR) and its 95% CI for dichotomous outcomes (e.g., occurrence of each type of side effect). We assessed the Q-test and I2 to quantify heterogeneity:[22] if the p-value of the Q-test was >0.10 and I2<50%, this indicated that the study results were homogeneous and a fixed effect model was employed to meta-analyze the pooled sample; otherwise a random effects model was employed.[20] There were only 6 studies included in the meta-analysis, so it was not possible to assess publication bias (which requires a minimum of 10 studies[23]). All statistical tests were two-tailed and the level of statistical significance was set at p<0.05.
3.Results
3.1. Characteristics of included studies
As shown in Figure 1, we found 72 relevant articles in the various databases considered, 59 of which remained after removing papers that appeared in multiple databases. Six of these 59 studies met inclusion criteria;[7,24,25,26,27,28] three published in English[7,25,27] and three published in Chinese.[25,27,28]
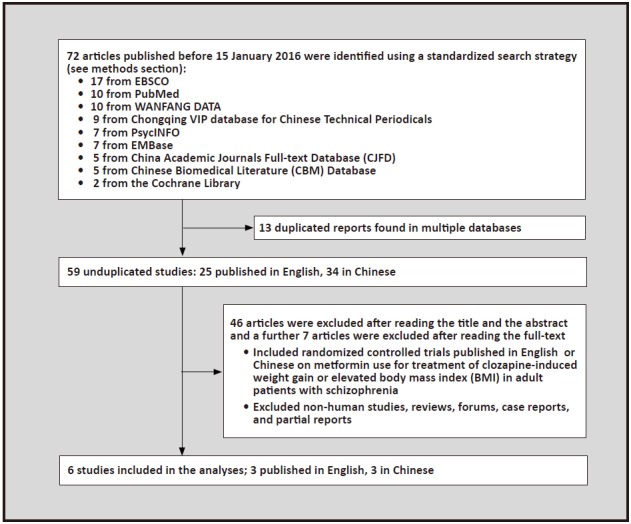
Figure 1. Identification of included studies
The characteristics of the six included studies are shown in Table 1 . The sample size ranged from 55 to 100 individuals with schizophrenia. The age and duration of illness of included patients varied considerably across studies. The oral dose of metformin used ranged from 250 to 1000 mg/d, and the duration of follow up ranged from 6 to 24 weeks. Three of the studies[7,24,26] provided clozapine plus an adjunctive placebo to patients in the control group; the other three studies[25,27,28] only provided clozapine to patients in the control group (i.e., without any adjunctive treatment).
Table 1. Characteristics of six randomized controlled trials included in the meta-analysis about adjunctive treatment of metformin for patients with schizophrenia being treated with clozapine
| study | diagnostic
criteria |
blind assessment
/control group |
sample
size (males, %) |
mean
age (years) |
mean duration
of illness (years) |
metformin
dosage (mg/day) |
duration of
follow-up (weeks) |
| N/A, no information available
CCMD-2, Chinese Classification of Mental Disorders, 2nd edition[12] CCMD-3, Chinese Classification of Mental Disorders, 3rd edition[13] DSM-IV, Diagnostic and Statistical Manual of the American Psychiatric Association, 4th edition[15] | |||||||
| Zhang 2004[28] | CCMD-2 | not blinded
no adjunctive treatment |
60
(45%) |
41 | N/A | 500 | 6 |
| Carrizo 2009[7] | DSM-IV | double-blind
placebo-controlled |
61
(N/A) |
38.8 | N/A | 1000 | 14 |
| Liu 2012[25] | CCMD-3 | not blinded
no adjunctive treatment |
100
(61%) |
N/A | 4.6 | 250-500 | 12 |
| Chen 2013[24] | DSM-IV | double-blind
placebo-controlled |
55
(51%) |
41.6 | N/A | 1500 | 24 |
| Wu 2014[27] | CCMD-3 | not blinded
no adjunctive treatment |
78
(59%) |
26.5 | 1.8 | 1000 | 12 |
| Hebrani 2015[26] | DSM-IV | double-blind
placebo-controlled |
60
(46%) |
46.5 | 20.2 | 1000 | 20 |
3.2 Evaluation of the risk of bias
As shown in Table 2 , three of the studies were not blinded,[25,27,28] so patients, the clinicians, and the evaluators of the outcome measures were all aware of which patients were and were not receiving metformin, greatly increasing the risk of biased reporting of the results. The other three studies[7,24,26] blinded the patients, clinicians, and evaluators to the treatment group of the participants, but two of them[7,26] did not indicate how blinding was maintained (i.e.,'allocation sequence concealment') and did not provide complete outcome data. Only one of the six studies[24] was classified as 'low risk of bias' on all seven items in the Cochrane Risk of Bias (RoB) tool.[17]
Table 2. Evaluation of risk of bias in the six included studies based on the Cochrane Risk of Bias (ROB) tool[17]
| study | sequence generation |
allocation sequence concealment | blinding of participants and personnel | blinding of outcome assessment | incomplete outcome data | selective outcome reporting | other potential threats to validity |
| N/A, no information available | |||||||
| Zhang 2004[28] | high | N/A | high | high | low | low | low |
| Carrizo 2009[7] | low | N/A | low | low | high | low | low |
| Liu 2012[25] | high | N/A | high | high | low | low | low |
| Chen 2013[24] | low | low | low | low | low | low | low |
| Wu 2014[27] | high | N/A | high | high | low | low | low |
| Hebrani 2015[26] | low | N/A | low | low | high | low | low |
3.3 Meta-analysis results for changes in weight and BMI after adjunctive treatment with metformin
As shown in Figure 2, five of the included studies with a pooled sample of 341 individuals compared changes in weight among adult patients with schizophrenia being treated with clozapine who were and were not treated with adjunctive metformin.[7,24,25,27,28] The results of the five studies were reasonably homogeneous (I2=40%, p=0.16), so a fixed effect model was used in the meta-analysis of the pooled sample. The meta-analysis shows a significantly greater decrease in weight over the treatment period among patients receiving metformin than among those who did not receive metformin: the mean difference between the two groups was -2.89 kg (95% CI: -4.20 to -1.59 kg, p<0.001).
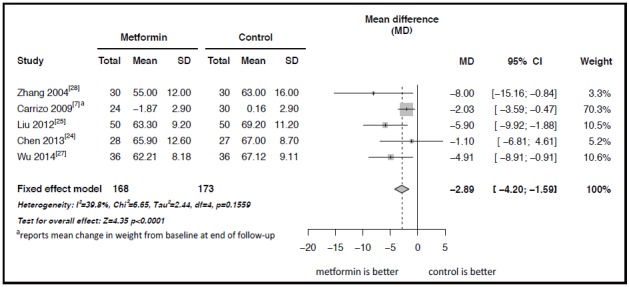
Figure 2. Forest plot of the meta-analysis of the effect of adjunctive treatment with metformin on the body weight (kg) of patients with schizophrenia being treated with clozapine
Figure 3 shows the Forest plot of the results of the meta-analysis of the four studies with a pooled sample of 265 individuals that compared changes in BMI after adjunctive treatment with metformin.[7,24,26,27] The results of these studies were quite homogeneous (I2=0% and p=0.83) so a fixed effect model was used in the pooled meta-analysis. The results indicate a significantly greater decrease in BMI over the course of treatment among patients who received metformin than in those who did not receive metformin: the mean difference in the change in BMI between groups was -0.81 (95% CI: -1.16 to -0.45, p<0.001).
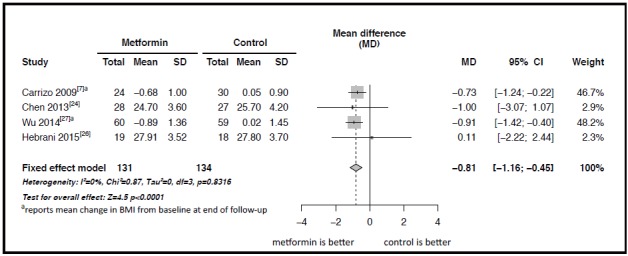
Figure 3. Forest plot of the meta-analysis of the effect of adjunctive treatment with metformin on the body mass index (BMI) of patients with schizophrenia being treated with clozapine
As shown in Table 3 , based on the GRADE measure, the strength of the evidence supporting the finding about reduction of clozapine-induced weight gain with adjunctive metformin treatment was classified as 'moderate' and the strength of the evidence supporting the finding about reduction in the clozapine-induced rise in BMI with adjunctive metformin treatment was classified as 'high'.
Table 3. Cochrane Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)[20] assessment of strength of evidence
| Outcome measure | number
of studies (pooled sample) |
heterogeneity | model of
analysis |
total effects | measure
compared |
95% CI | GRADE
result |
||
| I2 | p | Z | p | ||||||
| N/A, not applicable ALT, ratio | alanine aminotransferase | MD, mean difference | SMD, standardized mean difference | RR, risk | |||||
| Weight | 5(341) | 40% | 0.16 | fixed effect | 4.35 | <0.001 | MD=-2.89 | -4.20, -1.59 | moderate |
| Body Mass Index
(BMI) |
4(265) | 0% | 0.83 | fixed effect | 4.5 | <0.001 | MD=-0.81 | -1.16, -0.45 | high |
| Treatment effect | 3(146) | 40% | 0.19 | fixed effect | 1.36 | 0.17 | SMD=-0.23 | -0.56, 0.10 | high |
| Dropout rate | 3(241) | 59% | 0.09 | random
effects |
0.4 | 0.69 | RR=1.48 | 0.21, 10.30 | moderate |
| Any side effects | 2(172) | 0% | 0.75 | fixed effect | 0 | 1 | RR=1.00 | 0.77, 1.30 | low |
| constipation | 2(172) | 0% | 1 | fixed effect | 0.43 | 0.67 | RR=0.86 | 0.42, 1.74 | low |
| sinus tachycardia | 2(172) | 0% | 0.76 | fixed effect | 0.26 | 0.8 | RR=1.13 | 0.46, 2.78 | low |
| weakness | 2(172) | 0% | 0.6 | fixed effect | 0 | 1 | RR=1.00 | 0.39, 2.54 | low |
| ALT elevation | 2(172) | 0% | 0.93 | fixed effect | 0.86 | 0.39 | RR=1.60 | 0.55, 4.69 | low |
| orthostatic hypotension |
2(172) | 0% | 0.67 | fixed effect | 0.72 | 0.47 | RR=0.60 | 0.15, 2.43 | low |
| nausea | 2(172) | 0% | 0.85 | fixed effect | 0.72 | 0.47 | RR=1.67 | 0.41, 6.67 | low |
| dry mouth | 1(72) | N/A | N/A | fixed effect | 0.46 | 0.65 | RR=0.67 | 0.12, 3.75 | very low |
| hypersomnia | 1(72) | N/A | N/A | fixed effect | 0.58 | 0.56 | RR=0.50 | 0.05, 5.27 | very low |
3.4 Secondary results
As shown in Table 3 , three of the RCTs[7,24,26] compared the treatment effect (based on changes in BPRS or PANSS scores) in the intervention and control groups. The results of the three studies were reasonably homogenous (I2=40% and p=0.19) so a fixed effect model meta-analysis of the pooled results was conducted. The standardized mean difference (SMD) between the intervention and control conditions favored the metformin group (i.e., the treatment outcome was better), but the difference was not large enough to be statistically significant: the SMD between the two groups was -0.23 (95% CI: -0.56 to 0.10, p=0.17). The quality of the evidence for this outcome was classified as 'high'.
Only three of the studies[7,26,27] provided numbers of dropouts. A total of 31 (12.9%) dropouts occurred among the 241 individuals in the pooled sample from these three studies; the dropout rate in the pooled intervention group was 14.9% (18/121) and that in the pooled control group was 10.8% (13/120). The differences in the dropout rates between the intervention group and control group across the three studies were quite different (I2=59% and p=0.09), so a random effects model was used in the meta-analysis of the pooled sample. The result of the meta-analysis indicates that the difference in the dropout rate between the intervention group patients and control group patients was not statistically significant (RR=1.48, 95% CI: 0.21 to 10.30, p=0.69). The quality of the evidence for this outcome was classified as 'moderate'.
Only two studies with a pooled sample of 172 individuals (86 in each group) provided comparative data on side effects between the intervention and control groups.[25,26] A total of 96 separate episodes of side effects were reported in these two studies, 48 events in the pooled metformin group and 48 events in the pooled control group. The prevalence of the specific side effects in the two groups (in order of overall prevalence in the combined pooled sample) were as follows: constipation (14.0% in the intervention group v. 16.3% in the control group), tachycardia (10.5% v. 9.3%), weakness (9.3% v. 9.3%), elevated alanine aminotransferase (9.3% v. 5.8%), hypotension (3.5% v. 5.8%), nausea (5.8% v. 3.5%), dry mouth (5.6% v. 8.3% [only assessed in one study[27]]), and hypersomnia (2.8% v. 5.6% [only assessed in one study[27]]). As shown in Table 3 , meta-analysis of the pooled results found no statistically significant differences in the occurrence of these side effects between the intervention and control group. However, the data on side effects from these two studies was based on spontaneous patient reports and on routine laboratory tests and electrocardiograms - not on the regular administration of a standardized scale to assess side effects - so the quality of evidence supporting this conclusion about the relative safety of adjunctive treatment with metformin was classified as 'low' or 'very low'.
4.Discussion
4.1 Main findings
This meta-analysis finds that adjunctive treatment with metformin is effective in reducing clozapine-induced weight gain and clozapine-induced elevations in BMI. The mean difference in the change in weight over the treatment period between patients who do and do not receive adjunctive metformin (2.89 kg) is similar to the weight change reported in previous studies in China of adjunctive metformin treatment for olanzapine-induced weight gain[29] (3.6 kg) and of adjunctive metformin treatment for weight gain associated with the use of atypical antipsychotic medications[30] (3.1 kg). This result parallels that of a meta-analysis of 21 RCTs which assessed the effectiveness of metformin in reducing the weight gain induced by a wide variety of antipsychotic medications (including aripiprazole, clozapine, olanzapine, quetiapine, chloropromazine, risperidone and sulpiride).[31]
The proposed mechanisms of action of metformin that could result in reduced weight gain include: (a) activation of adenosine monophosphate (AMP) and protein kinases that suppress dysplasia of hepatic gluconeogenesis and the synthesis of lipids; (b) suppression of cells in the gastrointestinal wall that uptake glucose; and (c) an increase in insulin sensitivity.[25] There are also other agents that appear to have similar effects. For example, topiramate (an anticonvulsant drug) has been reported to be effective in the treatment of clozapine-induced weight gain in patients with schizophrenia.[32,33] More work is needed to clarify the mechanism of action (and potential negative effects) of these agents before they can be confidently recommended as standard adjunctive treatments in patients receiving long-term antipsychotic treatment. Moreover, most studies indicate that the risk profile for metabolic changes differs for different antipsychotic medications, so it is reasonable to assume that the effectiveness and mechanism of action of metformin and other agents in reducing these negative effects will vary depending on the type of antipsychotic medication being used. Future studies need to include more detailed comparisons of the effectiveness of adjunctive treatments for different types of antipsychotic medications.
4.2 Limitations
Several limitations need to be considered. (a) We only located 6 relevant RCTs, all of which were single-center studies with relatively small sample sizes (55 to 100 individuals), so the representativeness of the results for all patients with schizophrenia treated with clozapine is uncertain. And, given the small number of studies, it was not possible to assess publication bias. (b) Three of the six studies did not use placebo controls and, thus, the patients, clinicians, and evaluators were not blind to the group assignment of the patients. The main outcomes of interest (weight change and change in BMI) were fairly objective measures and the strength of the evidence for these results were classified as 'moderate' and 'high'; nevertheless, this lack of blinding could lead to biased reporting. (c) Only two of the six studies provided data on side effects and the quality of this evidence (which was not collected using standardized instruments) was classified as 'low' or 'very low', so it is impossible to determine whether or not adjunctive treatment with metformin increases the prevalence of side-effects in patients receiving clozapine. (d) Related studies[8] suggest that some of the beneficial effects of adjunctive metformin treatment in patients receiving antipsychotic medications do not occur until after 24 weeks of treatment, so the relatively short followup periods in the included studies may have led to an underestimate of the benefits of metformin. (e) Weight gain and increased BMI are the final outcome of a variety of metabolic changes related to dyslipidemia and insulin resistance. Effective use of adjunctive treatment with metformin (i.e., targeting the treatment on the most susceptible patients and determining the correct dosage and duration of treatment) will require more detailed assessment of the underlying mechanisms affecting metabolic changes in patients receiving antipsychotic medication and, importantly, the biochemical role metformin plays in halting or reversing these negative metabolic outcomes.
4.3 Importance
The present study pooled results from 3 RCTs published in English and 3 RCTs published in Chinese to assess the effectiveness of metformin in reducing clozapine-induced weight gain and clozapine-induced increases in BMI among adult patients with schizophrenia. The meta-analysis confirms the results of previous studies indicating that metformin is, indeed, effective in reducing antipsychotic-induced weight gain and increased BMI, and the quality of the evidence for these two outcomes was rated as 'moderate' and 'high', respectively. But the six included studies followed patients for relatively short periods (6 to 24 weeks) and they did not include a comprehensive assessment of potential side-effects that could complicate the long-term use of metformin as an adjunctive treatment with clozapine. Larger studies that are placebo controlled, that use double blind assessment of outcomes, that follow patients for much longer periods of time (up to 1 year), that include standardized assessments of the full range of potential side effects, and that include a wider range of outcome measures (including measures of dyslipidemia and insulin resistance) are needed before adjunctive metformin can be included in the standard treatment regimens of patients receiving long-term treatment with clozapine or other antipsychotic medications.
Acknowledgments
We thank the translators and reviewers of this analysis for their useful comments. We also thank Dr. Yutao Xiang for overall guidance and suggestions.
Biographies

Dr. Zhengrong Liu obtained a bachelor’s degree in clinical medicine from the University of South China in 2007. Since then she has been working as a chief physician at the Bureau of Civil Affairs Psychiatric Hospital in Guangzhou. Her main research intertest is psychiatric rehabilitation.

Dr. Wei Zheng obtained a bachelor’s degree from Hebei Medical University in 2012 and a master’s degree in psychiatry and mental health from the Capital Medical University in Beijing in 2015. Since then he has been working as a resident physician in the Department of Psychiatry in the Guangzhou Brain Hospital.
Funding Statement
None.
Footnotes
The authors Conflict of interest statement: report no conflict of interest related to this manuscript.
Authors’ contribution: LZR and ZW wrote the first draft. LZR and WZ selected studies and conducted the statistical analysis. GS checked all information and resolved disagreements in the classification of papers. All the authors contributed to the interpretation of data and approved the final manuscript.
References
- 1.Jääskeläinen E, Haapea M, Rautio N, Juola P, Penttilä M, Nordströmet T, et al. Twenty years of schizophrenia research in the Northern Finland birth cohort 1966: a systematic review. Schizophr Res Treatment. 2015;2015:524–875. doi: 10.1155/2015/524875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasnain M, Vieweg WV, Fredrickson SK. Metformin for atypical antipsychotic-induced weight gain and glucose metabolism dysregulation: review of the literature and clinical suggestions. CNS Drugs. 2010;24(3):193–206. doi: 10.2165/11530130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Jimenez M, Gonzalez-Blanch CFB, Hetrick S, Rodriguez-Sanchez J, Perez-Iglesias R, Vazquez-Barquero J. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs. 2008;22(7):547–562. doi: 10.2165/00023210-200822070-00002. [DOI] [PubMed] [Google Scholar]
- 4.Wu RR, Jin H, Gao KM, Twamley EW, Ou JJ, Shao F, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2012;169(8):813–821. doi: 10.1176/appi.ajp.2012.11091432. [DOI] [PubMed] [Google Scholar]
- 5.Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165(3):352–358. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- 6.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 7.Carrizo E, Fernandez VL, Connell L, Sandia I, Prieto D, Mogollón J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophrenia Res. 2009;113(1):19–26. doi: 10.1016/j.schres.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Wu RR, Zhang FY, Gao KM, Ou JJ, Shao P, Jin H, et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiaty. 2016 doi: 10.1038/mp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaefer M, Leopold K, Hinzpeter A, Heinz A, Krebs M. Memantine-associated reversal of clozapine-induced weight gain. Pharmacopsychiatry. 2007;40(4):149–151. doi: 10.1055/s-2007-984391. [DOI] [PubMed] [Google Scholar]
- 10.Reis de Sa A Jr, Elkis H. Comments on "Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine". Rev Bras Psiquiatr. 2007;29(1):90. doi: 10.1590/S1516-44462007000100025. [DOI] [PubMed] [Google Scholar]
- 11.Behdani F, Hebrani P, Rezaei Ardani A, Rafee E. Effect of topiramate augmentation in chronic schizophrenia: a placebo-controlled trial. Arch Iran Med. 2011;14(4):270–275. [PubMed] [Google Scholar]
- 12.Zhang YL, Zhang MY. [Field test report on the revision of the second edition of the Chinese Mental Disease Classification and Diagnostic Standard]. Zhong Hua Jing Shen Ke Za Zhi. 1996;1(1):27–30. Chinese. [Google Scholar]
- 13.Chinese Medical Association. Chinese Mental Disorders Classification and Diagnostic Criteria Third Edition (CCMD-3) Jinan: Shandong Science and Technology Press. 2001. Chinese. [Google Scholar]
- 14.World Health Organization. Diagnostic criteria for mental and behavioral disorders in ICD-10. Beijing: People's Medical Publishing House. 1995. Chinese. [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington: American Psychiatric Association; 1994 [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Association. 2013 [Google Scholar]
- 17.Higgins JPT, Green S(eds) Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK, John: Wiley & Sons. 2008 [Google Scholar]
- 18.Overall JE, Gorham DR. The Brief Psychiatric Rating-Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 19.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 20.David A, Dana B, Briss PA, Martin E, Yngve FY, Signe F, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5. The Cochrane Collaboration. 2011 [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Huang MC, Kao CF, Lin SK, Kuo PH, Chiu CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5) doi: 10.4088/JCP.12m08186. [DOI] [PubMed] [Google Scholar]
- 25.Liu HP. Effects of metformin hydrochloride on body mass and blood sugar of convalescence schizophrenics on clozapine. Lin Chuang Xin Shen Ji Bing Za Zhi. 2012;18(2):97–99. doi: 10.3969/j.issn.1672-187X.2012.02.001-0097-03. [DOI] [Google Scholar]
- 26.Hebrani P, Manteghi A A, Behdani F, Hessami E, Rezayat KA, Marvast MN, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J Res Med Sci. 2015;20(4):364–371. [PMC free article] [PubMed] [Google Scholar]
- 27.Wu W, Wang SR, Han L. [Effect of metformin hydrochloride on weight and blood glucose in the patients with refractory schizophrenia]. Zhongguo Min Kang Yi Xue. 2014;13:68–69. doi: 10.3969/j.issn.1672-0369.2014.13.032. [DOI] [Google Scholar]
- 28.Zhang JG, Wang ZX. [Effects of metformin on blood glucose, insulin, blood lipid and body weight in schizophrenia patients treated with clozapine]. Zhongguo Shen Jing Jing Shen Ji Bing Za Zhi. 2004;30(4):274. doi: 10.3969/j.issn.1002-0152.2004.04.043. Chinese. [DOI] [Google Scholar]
- 29.Wang Y, Zhang XL, Zhu DM, Chu ZX, Gong L, Wu Q. [Metformin for olanzapine-induced weight gain: a metaanalysis]. Zhongguo Jian kang Xin Li Xue Za Zhi. 2014;5:653–655. doi: 10.13342/j.cnki.cjhp.2014.05.006. Chinese. [DOI] [Google Scholar]
- 30.Yi F, Mei J, Su XJ, Mao JY, Zhang YY, Zhen LL. [Metformin for prevention of weight gain in patients with schizophrenia treated with second-generation antipsychotics: a metaanalysis]. Zhong Hua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2013;22(3):221–224. doi: 10.3760/cma.j.issn.1674-6554.2013.03.010. Chinese. [DOI] [Google Scholar]
- 31.Zheng W, Li XB, Tang YL, Xiang YQ, Wang CY, De Leon J. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: metaanalysis of randomized placebo-controlled trials. J Clin Psychopharmaco. 2015;35(5):499–509. doi: 10.1097/JCP.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 32.Zhen W, Tang LR, Weng YZ, Ma X, Xiang YQ. [Topiramate for prevention of gain in patients with schizophrenia treated with second-generation antipsychotics: a meta-analysis]. Zhongguo Yi Yao. 2015;10(1):71–76. doi: 10.3760/cma.j.issn.1673-4777.2015.01.018. Chinese. [DOI] [Google Scholar]
- 33.Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385–1403. doi: 10.1093/schbul/sbu030. [DOI] [PMC free article] [PubMed] [Google Scholar]


