Abstract
In recent years there has been an increase in the understanding of ethanol actions on the type A γ-aminobutyric acid chloride channel (GABAAR), a member of the pentameric ligand gated ion channels (pLGICs). However, the mechanism by which ethanol potentiates the complex is still not fully understood and a number of publications have shown contradictory results. Thus many questions still remain unresolved requiring further studies for a better comprehension of this effect. The present review concentrates on the involvement of GABAAR in the acute actions of ethanol and specifically focuses on the immediate, direct or indirect, synaptic and extra-synaptic modulatory effects. To elaborate on the immediate, direct modulation of GABAAR by acute ethanol exposure, electrophysiological studies investigating the importance of different subunits, and data from receptor mutants will be examined. We will also discuss the nature of the putative binding sites for ethanol based on structural data obtained from other members of the pLGICs family. Finally, we will briefly highlight the glycine gated chloride channel (GlyR), another member of the pLGIC family, as a suitable target for the development of new pharmacological tools.
Keywords: alcoholism, ethanol, GABA, GABAAR, GlyR
Introduction
Alcohol is a potent depressant drug and one of the most commonly used mind altering substance worldwide. The acute and chronic effects of alcohol abuse cause large medical, economic and social burdens. It is widely accepted that ethanol acts in the central nervous system (CNS) in a considerably complex manner as it affects several receptor-ion channel complexes such as the γ–aminobutyric acid type A receptor (GABAAR), glycine receptor (GlyR) and N-methyl-D-aspartate receptor (NMDAR) by different mechanisms that can be either direct (binding) or indirect (kinases, G proteins). This level of complexity presents a high threshold to overcome for the successful development of pharmacological tools to assist behavioral intervention in chronic alcohol abuse and treatment of life-threatening acute effects of excessive drinking. The therapeutic options that are currently available present limited efficacy, lack of adherence and serious side effects (Liang and Olsen, 2014; Spanagel et al., 2014). Therefore, it is crucial to further elucidate the mechanisms of action of ethanol in the CNS in order to meet the challenge of developing more specific and effective therapeutics. As the main inhibitory pentameric ligand gated ion channel (pLGIC) in the brain and one of the major targets of ethanol modulation, the GABAAR is one of the principal targets for pharmacotherapy. The evidence for the GABAmimetic effects of ethanol further supports the relevance of GABAAR. GABAAR can be potentiated by low to intermediate concentrations of ethanol (5–50 mM, corresponding to a few drinks up to complete intoxication). In recent years there has been an increase in the understanding of ethanol effects on GABAAR, however, the mechanism of action, whether it is direct or indirect, allosteric or not and which assembly of subunits are influenced in what way, is still not fully understood. In fact, a number of publications have shown differing results, and thus several fundamental questions on the mechanism of action still remain unanswered. The resolution of these questions will provide a more definitive picture on which GABAAR subunits are affected by ethanol in what way and in which regions of the CNS. This review aims to help put aside the conflicting available results and think about new key studies that will help answer these questions. For example, focusing the studies on extra-synaptic rather than on synaptic GABAARs.
The present review will focus on the involvement of GABAAR in the effects of alcohol and specifically the modulation of GABAAR by acute ethanol exposure. We will address a body of data linking GABAAR to ethanol effects and give an overview of evidence from human genetic studies and ethanol response in animal models, the effects of pharmacological compounds on the response to ethanol and the brain regions investigated in this context. Changes to the GABAergic system after chronic exposure to ethanol will be shortly reviewed and discussed in relationship to the acute effects. We will then focus on the immediate, direct and indirect, synaptic and extra or non-synaptic effects of ethanol on GABAAR. To elaborate on the immediate, direct modulation of postsynaptic GABAAR by acute ethanol, electrophysiological evidence from studies with different subunits, data from receptor mutants and structural information such as binding studies will be discussed. Presynaptic effects of ethanol will be mentioned briefly and we would like to refer the reader to the excellent recent review on this topic (Kelm et al., 2011). Finally, we will briefly highlight the glycine gated chloride channel (GlyR), another inhibitory member of the pLGIC family, as a more suitable target for the development of new pharmacological tools.
Functions and Distribution of GABAAR Subunits in Comparison to GABABR and GABACR
Three types of GABAR have been described up to now, two of those, the GABAAR and the GABACR are ligand gated ionic channels (LGIC), whereas GABABR is a G-protein coupled receptor (GPCR). Extensive research has been focused on the study of the functions, diversity and locations of GABAAR describing 19 subunits (α1–6, β1–3, γ1–3, δ, ε, π, ρ1–3 and θ). These receptors are broadly expressed in the CNS representing about 20% of the synapses in cortex, hippocampus, thalamus and cerebellum, where they possibly control cognitive functions such as memory, language and attention (Mody et al., 1994; Michels and Moss, 2007). Different receptor subunit composition has been shown of which the principal representation corresponds to: α1β2γ2 (43%), α2β2/3γ2 (18%), α3βnγ2/3 (17%), α2βnγ1 (8%), α5β3γ2/γ3 (4%), α6βγ2, α6βδ, α4βδ and others (2%). Also, receptors containing γ have been defined as synaptic while receptors containing α4, α5, α6 and δ are largely extra or non-synaptic (McKernan and Whiting, 1996; Cherubini and Conti, 2001). Furthermore, GABABR activation at presynaptic locations suppresses neurotransmitter (NT) release by inhibition of voltage sensitive Ca2+−-channels, while postsynaptic receptors induce a slow inhibitory postsynaptic current by gating KIR3-type K+-channels, which hyperpolarizes the membrane and shunts excitatory current (Bettler and Tiao, 2006). Two subtypes of GABABR have been described (B1 and B2), each featuring 2 isoforms (B1a, B1b and B2a and B2b) that are located pre- and post-synaptically and found in almost all brain neuronal populations (Bettler and Tiao, 2006). GABACR is composed of three subunits (ρ1–3) that originally belonged to the GABAAR subtype and has been associated to visual processing, regulation of sleep–waking rhythms, pain perception, memory, learning, regulation of hormones and neuroendocrine gastrointestinal secretion. These receptors are localized in the retina, thalamus, hippocampus, pituitary and gastro intestinal tract (Chebib, 2004). The potentiation mediated by ethanol is restricted to GABAAR.
Evidence for a GABAmimetic Action of Ethanol on the Central Nervous System
It is known that GABAAR are highly expressed throughout the brain, i.e., cerebellum, hippocampus, cerebral cortex, amygdala, VTA, nucleus accumbens and thalamus (Pirker et al., 2000; Schwarzer et al., 2001; for review see Faingold et al., 1998; Mihic, 1999). These areas of GABAAR expression represent potential sites for the GABAmimetic effect of ethanol. So far, most brain regions examined have shown similar ethanol actions at intoxicating concentrations. A concentration of 100–200 mM ethanol represents severe alcohol toxicity and is likely to be lethal (Lovinger and Homanics, 2007). Therefore, while used in some studies, these ranges of concentrations are unlikely to occur in vivo under physiological conditions. Some laboratories report significant potentiation of γ subunit-containing receptors with ethanol concentrations as low as 50 mM, but these concentrations are still equivalent to heavy intoxication (Ueno et al., 2001). For comparison, consuming a single drink on average results in a blood concentration corresponding to roughly 5 mM and already elicits mood changes, impaired cognition and reduced motor coordination, 20 mM would cause a strong intoxication, and a blood concentration corresponding to 100 mM will kill most individuals. The legal limit of blood ethanol concentration for drivers in many countries is 17 mM (Lovinger and Homanics, 2007). Consequently, relevant studies should always consider a range of ethanol concentration from low to high, starting from a concentration as low as 5–10 mM and testing intermediate and high concentrations up to 100 mM, to be of pharmacological interest.
As described later, a body of genetic and transgenic evidence supports the involvement of GABAAR in the behavioral response to ethanol (see Tables 1, 2). Additional supporting data comes from the effects that pharmacological substances interacting directly with GABAAR have on the response to ethanol. This notion is also supported by the similarities between the sedative and inhibitory effects of ethanol and drugs known to act on GABAAR, such as benzodiazepines and general anesthetics (Grobin et al., 1998; Breese et al., 2006). Pharmacological studies using drug discrimination choice support this GABAmimetic action of ethanol (Grant, 1999). Furthermore, it was shown that negative modulators of GABAAR reduced alcohol intake in animal models (Wegelius et al., 1993). Overall, these types of studies are strongly supportive of GABAergic actions for a range of ethanol concentrations.
Table 1.
Genetic evidence linking GABAAR to human alcoholism.
| Study | Affected alcohol related behavior |
|---|---|
| GABRA2 Human SNP | Increased responses to alcohol related cues in reward centers of the brain (Kareken et al., 2010; Villafuerte et al., 2012); changes in hedonic value of alcohol (Haughey et al., 2008) and subjective response to ethanol (Pierucci-Lagha et al., 2005; Haughey et al., 2008) |
| GABRG1 Human polymorphism | Linked to alcoholism independently of the impact of GABRA2 (Covault et al., 2008; Enoch et al., 2009) |
| GABRA6 Human association | Significant genome association with human alcoholism (Li et al., 2014) |
| GABRG2 Human association | Significant genome association with human alcoholism (Li et al., 2014) |
| GABRR1 and GABRR2 | Significant genome association with |
| Human association | human alcoholism (Xuei et al., 2010) |
Table 2.
Genetic animal models linking behavioral alterations to GABAAR.
| Model or Study | Affected alcohol related behavior |
|---|---|
| GABRA1 Mouse allelic noncoding variation* | Ethanol induced hypothermia and motor incoordination, ethanol-conditioned taste aversion and acute ethanol withdrawal (Ueno et al., 2001) |
| GABAAR α1 ethanol insensitive Mouse KI | Reduced sedative and increased anxiolytic effect of ethanol (Lobo and Harris, 2008) |
| GABAAR α2(S270H/L277A) ethanol insensitive Mouse KI | No taste aversion and hyperlocomotion, decreased hypnosis but still anxiolytic (Blednov et al., 2011) |
| GABAAR α2 Mouse KO | Reduction of conditioned taste aversion and faster recovery of ethanol induced incoordination (Blednov et al., 2013) |
| GABAAR α3 Mouse KO | Prolonged motor-incoordination (Blednov et al., 2013) |
| GABAAR α6 Mouse KO | Effect of ethanol unchanged, possibly due to compensatory adaptations (Homanics et al., 1997, 1998) |
| GABRA6 Mouse allelic non-coding variation* | Ethanol-induced hypothermia and motor incoordination, ethanol-conditioned taste aversion and acute ethanol withdrawal (Ueno et al., 2001) |
| GABRA6 ethanol sensitive rat allelic non-coding variation** | Increased motor impairment (Eriksson and Sarviharju, 1984; Korpi et al., 1993) |
| GABRA6 Ethanol sensitive rat R100Q SNP | Increased ethanol sensitivity in these rats seems to be independent of R100Q (Botta et al., 2007), despite previous suggestions (Korpi et al., 1993; Hanchar et al., 2005) |
| GABAAR β1 L285R mutant Mouse | Increased ethanol consumption without change in ethanol potentiation (Anstee et al., 2013) |
| GABAAR β3 N265M mutant Mouse KI | Increased tolerance and withdrawal but little change in acute ethanol sensitivity (Sanchis-Segura et al., 2007) |
| GABAAR α2 Mouse KO | Reduced anticonvulsant effects, ethanol consumption and withdrawal (Mihalek et al., 2001) |
| GABRG2 Mouse allelic coding variation* | Ethanol-induced hypothermia and motor incoordination, ethanol-conditioned taste aversion and acute ethanol withdrawal (Buck et al., 1997) |
| GABAAR γ2L Mouse KO | Normal ethanol response (Homanics et al., 1999) |
| GABAAR ρ Mouse KO | Reduced ethanol consumption and faster recovery of ethanol induced incoordination (Blednov et al., 2003) |
| PKCε Mouse KO | Increased hyperlocomotion and sedation, reduced voluntary alcohol consume (Hodge et al., 1999; Olive et al., 2000), increased alcohol aversion (Newton and Messing, 2007) and increased response to direct GABAAR antagonist muscimol (Ueno et al., 2001) |
| PKCδ Mouse KO | Resistance to ethanol intoxication and reduced or abolished potentiation of tonic GABAergic currents (Choi et al., 2008) |
| PKCγ Mouse KO | Shorter loss of righting reflex and less sensitive to ethanol-induced hypothermia (Harris et al., 1995) |
Cerebellar neurons have been critical for the study of ethanol effects on GABAAR. In the 90’s, in vivo recordings showed that ethanol depressed Purkinje neuron firing in rats, and this depression was antagonized by a benzodiazepine partial inverse agonist (RO15–4513), as well as by the competitive GABAAR antagonist bicuculline (Palmer et al., 1988; Palmer and Hoffer, 1990). Moreover, GABA-induced inhibition of Purkinje cell firing was enhanced by acute ethanol administration and most interestingly, it was reported that these cells were sensitized to the effects of ethanol by activation of β-adrenergic receptors, which suggests that the action of ethanol on GABAAR in these neurons could be affected by G protein activation (Lin et al., 1994; Freund and Palmer, 1997; Yang et al., 1998). The notion that G proteins are involved in mediating effects of ethanol is supported by other experiments showing that the potentiation of GABAAR in hippocampal neurons was dependent on the presence of guanine nucleotides that can affect the activation of G proteins (Weiner et al., 1997). A body of information of pre- and postsynaptic effects of ethanol is summarized in Figure 1.
Figure 1.
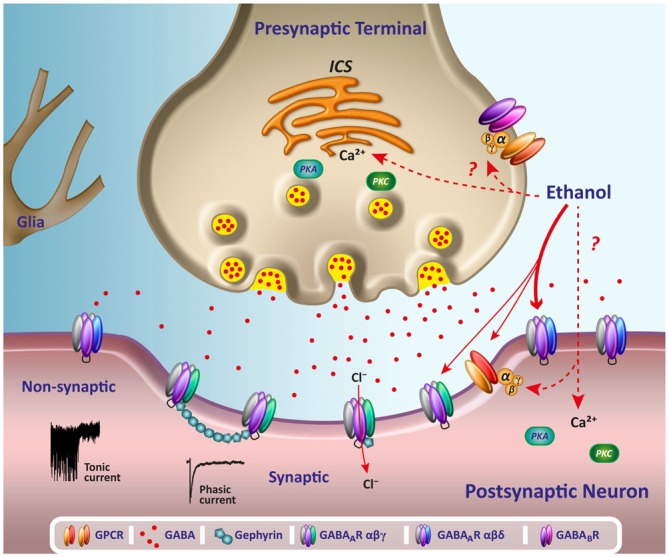
Acute effects of ethanol on GABAergic transmission. The scheme illustrates several potential acute pre and postsynaptic sites for the effects of ethanol on GABAergic neurotransmission. Reported changes on the release of presynaptic GABA might be mediated by changes on calcium release from intracellular calcium stores (ICS) following activation of G-protein coupled receptors (GPCR) or phosphorylation by protein kinase A (PKA) and C (PKC). Changes on the activation of GPCR, such as GABABR, could affect GABA release and alter the tonic Cl− current associated to non-synaptic GABAAR δ containing receptors through spillover of synaptically released GABA. At postsynaptic domains, acute low ethanol concentrations of alcohol appear to modulate primarily non-synaptic GABAAR (see thicker arrow) by a mechanism that might involve direct binding to the general anesthetics site of action or by intracellular signaling pathways, G protein, PKC and PKA or calcium release. The scheme also shows representative traces of a phasic current, activated by synaptic receptors, and a sustained small desensitizing tonic current, mediated by non synaptic receptors.
Low doses of ethanol were found to increase the frequency of spontaneous inhibitory post-synaptic currents (sIPSCs), and miniature inhibitory post-synaptic currents (mIPSCs) at higher doses, in synapses of cerebellar Golgi and granule cells (Carta et al., 2004). Additionally, local manipulation of GABAAR activity in distinct brain regions can have different effects on ethanol responses, i.e., reduction in GABAAR activity in the VTA reduces alcohol intake, but the same effect can be achieved by increased GABAAR activity in the nucleus accumbens (Vengeliene et al., 2008). A number of laboratories demonstrated that ethanol potentiates GABAAR-mediated synaptic transmission via an increase in GABA release from presynaptic terminals in several brain regions including the cerebellar cortex (Valenzuela and Jotty, 2015). Additionally, tonic currents associated with the activation of non-synaptic GABAAR in granule cells were found to be increased, but this effect was dependent on action potentials and therefore most likely mediated by the spill-over of synaptically released GABA (Carta et al., 2004; Siggins et al., 2005; Weiner and Valenzuela, 2006).
In terms of molecular characteristics for the potentiation of GABAAR, it is known that the ethanol sensitive receptor containing extra-synaptic δ-subunit responsible for the tonic current is expressed in the nucleus accumbens, hippocampus, thalamus, cortex and cerebellum (Pirker et al., 2000; Brickley et al., 2001; Schwarzer et al., 2001; Stell et al., 2003; Cope et al., 2005; Jia et al., 2005; Chandra et al., 2006). Interestingly, the knock down of GABAAR δ expression by RNAi in the dorsomedial shell region of the nucleus accumbens in mice caused a reduction in ethanol preference, probably by disinhibition of neuronal targets in the VTA, ventral pallidum or lateral hypothalamus (Nie et al., 2011; Tabakoff and Hoffman, 2013). Another line of support for involvement of the δ subunit comes from the use of a partial agonist of the benzodiazepine receptor, RO15–4513, that has high affinity for several GABAAR and acts more selectively on extrasynaptic α6/β containing receptors at the benzodiazepine binding site (Lüddens et al., 1990; Korpi et al., 1993, 1999). Its administration reduced voluntary ethanol intake, anxiolytic behavior and motor impairment, although clinical application was abolished after finding that it also increased withdrawal seizures (Suzdak et al., 1986a; Hoffman et al., 1987; Becker and Hale, 1991; June et al., 1991; Schmitt et al., 2002; Lovinger and Homanics, 2007). Also, application of the structural GABA analog Gabapentin in the central amygdala reversed alcohol operant behavior and increased IPSC amplitude in alcohol dependent rats, but had the opposite effect in naïve rats, and the effect on current amplitude was abolished by application of GABABR blockers (Roberto et al., 2008). Therefore, the effects of chronic alcohol exposure also need to be taken into consideration when evaluating acute effects.
The direct GABABR agonist baclofen reduced alcohol intake in mice, although it showed limited effects in human subjects (Colombo et al., 2004; Maccioni and Colombo, 2009; Tyacke et al., 2010; Addolorato et al., 2011, 2012; Brennan et al., 2013). GABA release in amygdala, VTA and hippocampus at high concentrations of ethanol appears to rely on activation of GPCRs, and the activation of the metabotropic GABABR can attenuate GABA release (Peris et al., 1997; Nie et al., 2004, 2009; Wu et al., 2005; Silberman et al., 2009; Kelm et al., 2011). Thus, the GABABR agonist GHB which also acts as a partial agonist for δ subunit containing GABAAR might affect alcohol intake by complex inhibitory mechanisms (Absalom et al., 2012). Additionally, increased expression of extra synaptic recombinant GABAAR containing α6 and δ subunits was positively modulated by alcohol (for review see Wallner et al., 2006).
Human and Animal Genetic Studies Support Effects of Ethanol on GABAARs
If GABAARs are important for alcohol use disorders (AUD), one would expect that gene variations would have an impact on alcohol abuse behaviors. In agreement with this idea, human genetic studies indicated a central role of GABAAR in alcoholism (Engin et al., 2012; see Table 1). For example GABRA2, the gene encoding the GABAAR α2 subunit, features small nucleotide polymorphisms (SNP) which do not affect the coding sequence, but correlate with mRNA levels in post-mortem prefrontal cortex (Haughey et al., 2008) of alcoholic patients. These SNPs were linked to alcohol dependence, specifically increased responses to alcohol related cues in reward centers of the brain, the hedonic value of alcohol, and subjective responses to ethanol in humans (Edenberg et al., 2004; Pierucci-Lagha et al., 2005; Haughey et al., 2008; Kareken et al., 2010; Villafuerte et al., 2012). Reduced expression of the α2 subunit in humans might have an effect similar to the α2 null mutant in mice discussed below, facilitating alcohol abuse by diminishing some of its negative side effects. In addition, GABRA6, GABRG1, and GABRG2 polymorphisms (GABAAR α6, γ1 and γ2 subunit) appear to be linked to alcoholism independent of GABRA2 (Covault et al., 2008; Enoch et al., 2009; Li et al., 2014). Furthermore, the genes coding for GABAA/CR Rρ1 and 2 were also implicated in human alcohol dependence (Xuei et al., 2010). While these studies suggest involvement of α6, γ1, γ2 subunits and especially α2 subunits in the effects of ethanol, they cannot elucidate the mechanism of action of ethanol.
On the other hand, a correlational analysis study of GABRG2 allelic variation in the GABAAR γ2 subunit in BXD recombinant inbred mouse strains indicated a link between allelic polymorphism in the protein coding sequence and ethanol-induced hypothermia, motor incoordination, ethanol-conditioned taste aversion and withdrawal (Hood and Buck, 2000; Ueno et al., 2001; see Table 2). However, differential expression levels of the GABAAR α1 and α6 subunits due to variation in the noncoding sequence of the GABRA1 and GABRA6 genes may also be responsible for these ethanol effects in the investigated mouse strains (Ueno et al., 2001). Another naturally occurring polymorphism in the gene coding for the α6 subunit may be responsible for increased motor impairment in selectively bred ethanol-sensitive rats, although it cannot be excluded that other co-segregated factors may account for these differences (Eriksson and Sarviharju, 1984; Korpi et al., 1993; Congeddu et al., 2003; Radcliffe et al., 2004). In agreement to the proposed role of GABAA/CR Rρ1 and 2 to alcoholism in humans, ρ1 KO mice show reduced ethanol consumption and faster recovery of ethanol-induced loss of motor coordination (Xuei et al., 2010). Although these studies have provided additional insights into the relative importance of individual subunits for specific effects of ethanol, caution is needed in the interpretation of behavioral responses in transgenic animals with global knock-out of gene products due to possible compensatory mechanisms.
Positive correlations between observations in vitro and transgenic mouse lines are not always evident making it difficult to draw conclusions about the role of genes in ethanol behaviors. For example, in vitro data from frog oocytes suggested a role of the long splice variant of the GABAAR γ2 subunit in the action of ethanol (Wafford et al., 1993), whereas studies in GABAAR γ2L knock-out mice, on the other hand, did not show a changed behavioral response to ethanol compared to wild-type mice (Homanics et al., 1999). Also, mutations in the GABAAR β3 subunit revealed effects on ethanol tolerance and withdrawal (Sanchis-Segura et al., 2007). In addition, GABAAR δ KO mice exhibited reduced anticonvulsant effects of ethanol, lower alcohol consumption and attenuated withdrawal, while other symptoms were not altered, supporting the involvement of extrasynaptic δ/α6 containing receptors in some responses to alcohol. KO of α6, on the contrary, did not show any behavioral changes upon ethanol exposure even though it was accompanied by loss of the δ subunit, indicating the need for α6 in δ subunit assembly (Homanics et al., 1997, 1998; Korpi et al., 1999). These KO mice also showed reduced α1 and β2 expression and exhibited an increased potassium leak current in cerebellar granule cells, which is mediated by activation of TASK-1 and 3 K2P channels (Uusi-Oukari et al., 2000). These changes might be compensatory for the loss of tonic inhibition that is produced by the non-synaptically located subunits (Brickley et al., 2001; Aller et al., 2005). Thus, it is important to consider that the observed differences might be in part due to compensatory adaptations as suggested previously in these transgenic models (Homanics et al., 1997, 1998). On the other hand, a GABRA6 R100Q SNP related to BDZ-binding properties was linked to increased ethanol sensitivity (Hanchar et al., 2005). However, this allele was found not to be conserved in the investigated line of selectively bred ethanol sensitive rats after 45 generations and further genetic studies did not support an important involvement of the R100Q polymorphism in the observed behavioral response to ethanol (Korpi and Uusi-Oukari, 1992; Crabbe et al., 1999; Radcliffe et al., 2004; Botta et al., 2007). GABAAR α2 subunit null mutant mice exhibited a reduction in conditioned taste aversion with ethanol and shorter recovery time from ethanol-induced motor incoordination, while deletion of GABAAR α3 led to a slower recovery (Blednov et al., 2013).
The results in GABAAR α2 subunit null mutants supporting the involvement of α2 are in agreement with data from α2(S270H/L277A) knock-in mice, which express ethanol insensitive GABAAR α2 subunit containing receptors. These KI animals did not develop conditioned taste aversion to ethanol and exhibited a decreased ethanol-induced hypnosis, as well as a complete loss of ethanol-induced motor stimulation, while the anxiolytic properties of ethanol were not abolished (Blednov et al., 2011). While knock-in models might reflect a more physiological condition than knock-out animals, it is important to consider that expression levels may still vary in these mutant animals as compared to wild type. Furthermore, the changes in mutant receptors can affect physiological receptor function. For example, ethanol consumption was increased in a knock-in mouse expressing L285R mutant GABAAR β1, although this mutation did not change ethanol potentiation in recombinant mutant receptors in vitro (Anstee et al., 2013). Taken together, these studies strongly implicate the α2 subunit in conditioned taste aversion, hypnotic, and motor stimulant effects of ethanol. The knock-in data, in particular, lends strong support to the idea that ethanol potentiation of α2-containing receptors mediates these aspects of ethanol response. The δ KO supports the involvement of GABAAR δ in promoting the anticonvulsant effects of ethanol, alcohol consumption and alcohol withdrawal, although KO of α6 does not support these findings. The results for the role of other subunits are even less clear cut. Overall, while these studies suggest a critical involvement of GABAAR in the expression of ethanol-induced behavior, they have not provided strong support for a specific molecular involvement in a particular ethanol related behavior. It is also largely unknown if these genotypic modifications on GABAAR in human and animals are associated to loss or gain of function, which might have a large impact on behavior, even in the absence of ethanol.
Studies with Recombinant Receptors Have Not Provided a Clearer Picture
To be able to deal with the large number of possible receptor subunit combinations that might be important for ethanol actions, recombinant receptors have been examined in several expression systems (McKernan and Whiting, 1996; Barnard et al., 1998). Similar to studies in native GABAAR, investigation of ethanol effects on recombinant GABAAR expressed in frog oocytes have provided conflicting results, in some cases even by the same laboratory (Wafford et al., 1991; Sigel et al., 1993; Marszalec et al., 1994; Mihic et al., 1994; McCool et al., 2003). A study on recombinant receptors in frog oocytes reported that the long γ2L, but not the short γ2S splice variant of GABAAR, controlled the ethanol potentiation (Wafford et al., 1991, 1993). Additionally, mutation of the putative phosphorylation S343 site (LLRMFSFK) in the splice insert in the large intracellular loop between TM3 and 4 of the γ2L variant, as well as the administration of kinase inhibitors, both abolished ethanol potentiation (Wafford et al., 1991; Wafford and Whiting, 1992). Thus, in vitro results from transiently transfected frog oocytes may not be transferable to mammalian neurons expressing native GABAAR, where posttranscriptional processing of the receptors and presynaptic mechanisms may play an additional important role (Harris et al., 1995; Aguayo et al., 2002).
In terms of other potentially important subunits for ethanol actions on the receptor, it was reported that while γ subunit containing receptors generally exhibit a low sensitivity to ethanol, GABAAR containing δ subunits exhibit a potentiation of up to 50% with ethanol concentrations of 10 mM in oocytes (Wallner et al., 2003, 2006; Lovinger and Homanics, 2007). These results, however, also raised some controversy because other studies did not report potentiation in similar experiments (Borghese et al., 2006; Yamashita et al., 2006). Thus, it is difficult to definitively conclude if the type of subunits that constitutes the GABAAR matters or not for the effect of ethanol on the protein complex (see Figure 2).
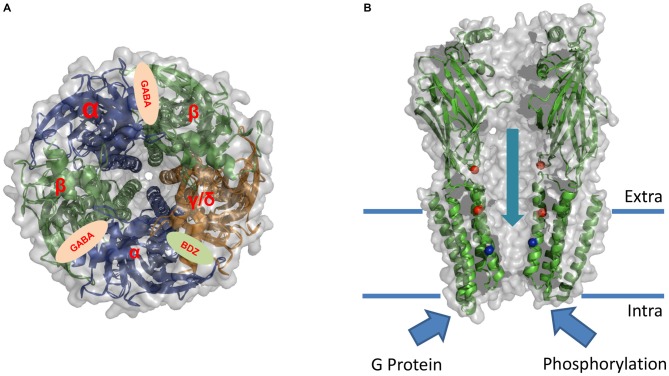
Figure 2.
Sites of action for allosteric modulation of GABAAR. Schematic illustration of the overall architecture and putative modulatory sites of action on GABAAR. The model is based in the X-ray structure of GABAAR β3 subunit (PDB: 4COF; see Miller and Aricescu, 2014). (A) Upper view of the putative subunit stoichiometry and global architecture of the αβγ/δ GABAAR. The cartoon shows the binding sites for GABA and Benzodiazepine (BDZ). (B) Lateral view showing the suggested binding sites for ethanol (red spheres) and Picrotoxin (blue spheres). This crystal structure lacks the intracellular domain between TM3 and TM4 which was shown to be critical for the modulation of the receptor by G protein and phosphorylation pathways.
Ethanol Binding Sites
While it has been suggested that ethanol can modulate the function of the GABAAR using a defined binding pocket (also crucial for the actions of other allosteric drugs), the relative position of the amino acids lining this pocket is still uncertain. So far, two regions of the GABAAR have been proposed as critical for ethanol effects: (1) an extracellular binding site located at the α+β3− subunit interface where both the α6R100 and β3Y66 residues contribute to form the ethanol/imidazobenzodiazepine binding site. This site, also important for benzodiazepine effects, is only functional in the α4/6β3δ receptor combination (Wallner et al., 2014); and (2) a binding pocket located in the transmembrane regions formed by residues in the TM2 and TM3 (see Figure 2). In the GlyR and possibly in GABAAR, corresponding residues S270 (α2) and S265 (β1) in the TM2 domain and A291 (α2) and M286 (β1) in the TM3 have been suggested to form an ethanol pocket between TM2 and TM3 (Mihic et al., 1997; Mascia et al., 2000; Ueno et al., 2000). Unfortunately, mutagenesis of key residues in the transmembrane regions of the α and β subunits of the GABAAR resulted in mutant receptors that displayed dramatic changes in GABAAR function, such as loss of function, or spontaneously open ion channels raising the question if the lack of ethanol effects was due to the existence of a site for ethanol or to a change in normal receptor physiology (see Table 3). In addition, these binding sites were not specific for ethanol, but rather water filled cavities able to accommodate a large number of drugs of different physical-chemical properties. On the other hand, comparative experimental data between all members of the pLGICs family showed opposing functional consequences (inhibition v/s potentiation by ethanol), even when the putative pocket-forming residues are conserved. Based on these observations, the hypothesis that the two effects of potentiation and inhibition can coexist in the same receptor gained more recognition in the last years. Molecular studies performed in GABAAR, GlyR and nAChR showed that one effect (inhibition or potentiation) could be masked by the other. For instance, the S270I mutation in the GABAAR α2 subunit resulted in inhibition by ethanol to a similar magnitude as potentiation in the α2 WT (Mihic et al., 1997). Moreover, changes in the volume of the cavity by the mutation T261W in α2 enhanced the potentiating effects of ethanol in GABAAR (Ueno et al., 2000; Johnson et al., 2012). Therefore, these studies suggest the presence of two independent (inhibiting and potentiating) binding sites inside the same receptor (see Figure 2).
Table 3.
Effects of GABAAR mutations in vitro.
| Receptor Mutation | Affected receptor functions |
|---|---|
| GABAAR α1 S270I TM2 substitution | Not potentiated by 200 mM ethanol* (Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR α1β1 S265I TM2 substitution | Reduced potentiation by ethanol (Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR α2 A291W TM3 substitution | Reduced potentiation by ethanol and spontaneously open state (Mihic et al., 1997; Ueno et al., 1999, 2001) |
| GABAAR α2 S265I β1 S265I TM2 substitution γ2L | Reduced potentiation by 200 mM ethanol (90% WT/15% MUT; Wafford et al., 1991; Wafford and Whiting, 1992; Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR α2β1 S265I TM2 substitution γ2L | Reduced potentiation by 200 mM ethanol (90% WT/20% MUT; Wafford et al., 1991; Wafford and Whiting, 1992; Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR α2β1 S270I TM2 substitution γ2L | Reduced potentiation by 200 mM ethanol (90% WT/34% MUT; Wafford et al., 1991; Wafford and Whiting, 1992; Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR α6 R100Q SNP substitution | Increased ethanol response with βδ but not βγ2 co-expression (Hanchar et al., 2005), but no effect in other studies (Botta et al., 2007) |
| in BDZ binding site | |
| GABAAR GABAAR ρ1 GlyR TM2 to TM3 | Potentiated by 200 mM ethanol (88% WT/48% C6; Mihic et al., 1997; Ueno et al., 1999) |
| 45 residue substitution | |
| GlyR α1 GABAAR γ-loop-2 chimera | No effect on ethanol sensitivity and general receptor function (Perkins et al., 2009) |
| GlyR α1 GABAAR δ-loop-2 chimera | Increased ethanol sensitivity but no effect on general receptor function (Perkins et al., 2009) |
| GlyR α1 GABAAR δ-loop-2 A52 chimera | Highly increased ethanol sensitivity but no effect on general receptor function (Perkins et al., 2009) |
| GlyR α1 GABAAR δ-loop-2 A52E chimera | Reduced ethanol sensitivity (Perkins et al., 2009) |
| GABAAR γ2L S265I | Unchanged potentiation by ethanol (Wafford et al., 1991; Wafford and Whiting, 1992; Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR γ2L S270I | Unchanged potentiation by ethanol (Wafford et al., 1991; Wafford and Whiting, 1992; Mihic et al., 1997; Ueno et al., 1999) |
| GABAAR γ2L TM2/3 loop phosphorylation site mutant | Effect of ethanol blocked (Wafford et al., 1991; Wafford and Whiting, 1992) |
| GABAAR TM2 cysteine residue mutant | Covalent binding of general anesthetics irreversibly increases apparent agonist affinity and prohibits subsequent further potentiation by ethanol (Mascia et al., 2000) |
*See also GABA hypersensitive and spontaneously open A2(S270I) mutant (Ueno et al., 2001).
The understanding of the molecular basis for alcohol modulation has been hampered by the lack of ethanol-bound crystal structures for mammalian pLGICs receptors. To date, a major insight on the structural basis of allosteric ethanol sites in pLGICs was the co-crystallization of the prokaryote receptor GLIC bound to ethanol (Sauguet et al., 2014). These crystal structures revealed that GLIC contains at least two different cavities (slightly different to what was previously predicted for mammalian pLGICs) able to accommodate propofol, desflurane and alcohols. The GLIC receptor, however, is normally insensitive to pharmacological concentrations of ethanol (1–100 mM). Introduction of the F14’A mutation inside the ethanol binding site in the pore-lining M2 helix, nevertheless, increased the ethanol sensitivity to similar levels as that observed in mammalian receptors (Howard et al., 2011). Comparison between the wild type and the F14’A mutant structures showed that the absence of the phenylalanine ring expanded the volume of the cavity, allowing the ethanol molecule to enter and stabilize the open conformation of the channel supporting the idea of two opposite binding sites coexisting in the same receptor (Sauguet et al., 2014). A homology model based on the structural data showed a homologous cavity in both GlyR and nAChR, suggesting a common mechanism of modulation in all members of the pLGIC family (Sauguet et al., 2014). In addition, the first X-ray crystal structure of a human β3 GABAAR has just been published (Miller and Aricescu, 2014). However, it is not possible to observe an ethanol-binding pocket in this structure perhaps because it is a homopentamer composed of only the β3 subunit, supporting the idea that at least in the GABAAR, the putative binding pocket is formed by the interaction of residues from different subunits.
Effects of Chronic Ethanol Exposure on the GABAergic System
Neuronal cell cultures, as well as animal models, show changes in mRNA levels for different GABAAR subunits after alcohol exposure (Kumar et al., 2004; Wafford, 2005). For example, alterations in GABAAR subunit expression levels, including changes in the levels of extra-synaptic subunits α6 and δ, have been detected in the cerebellum of rats chronically treated with ethanol (Mhatre and Ticku, 1992; Morrow et al., 1992; Vekovischeva et al., 2000; Sanna et al., 2004; Marutha Ravindran et al., 2007). In neonatal rats, repetitive exposure to ethanol increased expression of the δ subunit in cerebellar granule cells, but the properties of neither tonic nor phasic GABAergic currents were modified (Diaz et al., 2014). Accordingly, either the contribution of GABAAR δ is not highly relevant to inhibitory inputs, or compensatory homeostatic mechanisms maintained normal inhibitory input in the treated neurons. Chronic ethanol leads to increased PKCε expression in neuronal cell lines, which in turn stimulates neurite outgrowth and might impact on GABAAR function (Messing et al., 1991; Roivainen et al., 1994; Hundle et al., 1995, 1997). Interestingly, animal studies showed that prolonged exposure to alcohol gives rise to chronic effects, some of which countered the acute facilitation of ethanol on GABAergic transmission and could render the brain hyperexcitable during alcohol withdrawal (Diana et al., 2003; Lovinger, 2008; Steffensen et al., 2009; Roberto et al., 2012). Studies in primates and rats have reported that after extended ethanol exposure, expression levels of GABAAR α1, 2, 3 and 5 decreased, whereas α4, α6 and γ2 expression increased (Grobin et al., 2000; Cagetti et al., 2003; Anderson et al., 2007). Also, GABA release, IPSC frequency, and amplitude were augmented after chronic ethanol exposure in the central amygdala, while acute ethanol still increased IPSC frequency and amplitude similarly in alcohol naïve and dependent rats (Roberto et al., 2004). Postsynaptically, chronic ethanol seems to also decrease α4 expression in amygdala and nucleus accumbens (Papadeas et al., 2001). Furthermore, altered association of GABAAR with PKC changed protein phosphorylation in the cerebral cortex, which was shown to influence clatherin-mediated endocytosis of receptors, changing the ratio of GABAAR species that could mediate GABA responses at the cell surface (Kumar et al., 2002; Jacob et al., 2008; Gonzalez et al., 2012). Some of these changes are probably responsible for the changes in animal behavior after chronic ethanol exposure. Changes in subunit expression may for example affect the behaviors affected in corresponding KO models. The decreased expression of GABAAR α2 might contribute to the build-up of alcohol tolerance, while an increased expression of the δ subunit could facilitate increased alcohol intake and withdrawal, creating distinct experimental conditions from naïve animals. Therefore, the complex changes that occur after longer times of exposure need to be taken into consideration when evaluating the effect of acute ethanol application on GABAergic transmission. The large data dispersion suggest that ethanol actions on GABAAR are complex and depend on still unidentified factors.
Data that Support the Acute Action of Ethanol on GABAARs
Ethanol Potentiates GABAARs in a Complex Manner
After the original report of GABA-mediated neurotransmission modulation by ethanol in the cat brain, several ligand binding studies detected small effects of ethanol on GABAAR (Nestoros, 1980a,b; Ticku and Burch, 1980; Ticku et al., 1983; Ticku, 1989). Functional analysis examining 36Cl− efflux from synaptoneurosome preparations revealed potentiating effects of ethanol concentrations as low as 10 mM (Suzdak et al., 1986a,b; Allan and Harris, 1987; Mehta and Ticku, 1988; Harris, 1990; Engblom and Akerman, 1991). However, electrophysiological studies in brain slices and cultured neurons resulted in a considerable body of differing results, reporting ethanol potentiation in some brain areas, but not in others (Gage and Robertson, 1985; Barker et al., 1987; Siggins et al., 1987; White et al., 1990; Reynolds et al., 1992; Lovinger and Homanics, 2007). The first demonstration that ethanol caused a potentiation of GABA-induced Cl− current was in hippocampal and cortical neurons using whole cell patch clamp recordings (Aguayo, 1991). This study was able to show that ethanol affected some cells, but not others in the same group of neurons. Also, it showed that the effect was independent from that produced by diazepam and barbiturates and that ethanol was unable to directly gate the ion channel. Other studies have largely confirmed this neuronal variability using cultured and acutely dissociated Purkinje cells, supporting the idea of a large complexity in this phenomenon (Sapp and Yeh, 1998; Criswell et al., 2003, 2008). Opposing results were also found between species. For example, GABAAR in mouse hippocampus were about 300 times more sensitive to ethanol than those in rats (Aguayo et al., 1994). Furthermore, a number of studies, such as those showing that ethanol action was evident after sensitizing β-adrenergic receptor pathways, suggested that intracellular signaling played a role in the modulation of ethanol sensitivity (Lin et al., 1994; Freund and Palmer, 1997; Yang et al., 1998). Ethanol sensitivity may also change throughout development, similar to the sensitivity to other drugs acting on GABAAR (Kapur and Macdonald, 1999; Aguayo et al., 2002).
Extensive studies of ethanol effects at concentrations between 0.1–850 mM in hippocampal neurons found three populations of neurons falling into different groups of sensitivity. The majority of neurons (60%) responded to moderate concentrations of ethanol with a maximal response at about 40 mM, a small group of cells (5%) was sensitive to concentrations below 1 mM, while in the remaining neurons only application of more than 100 mM showed an effect (Aguayo, 1991). These results support the existence of two separate mechanisms of ethanol action: (1) high and (2) low-to-moderate concentration effects on GABAAR, of which the second would be the relevant mode of action for conditions that are more likely to be encountered in vivo (Aguayo et al., 2002). Alternately, these results suggest a wide range of intracellular modulation of GABAAR sensitivity to ethanol. The study of native receptors in mammalian neurons found that currents less affected by ethanol were desensitizing and exhibited a significantly larger current run-down. Both of these properties are highly modulated by signal transduction pathways, suggesting that the difference between ethanol-sensitive and insensitive receptors may depend on differential intracellular modulation (Aguayo, 1990, 1991; Aguayo et al., 2002). Certain similarities between ethanol modulation and calcium regulation of GABAAR suggest that the effect of low to medium doses of ethanol is mediated by soluble intracellular components (Aguayo et al., 2002). Calcium and ethanol modulation of GABAAR vary between whole-cell and cell-free outside-out patch modes (Mozrzymas and Cherubini, 1998; Aguayo et al., 2002). An increase in intracellular Ca2+ elicits a higher ethanol sensitivity in some cell types and brain regions, while in others it has no effect on ethanol sensitivity (Taleb et al., 1987; Llano et al., 1991; Kellenberger et al., 1992; Aguayo et al., 1994). Both effects also display a similar concentration-response curve that demonstrates a potentiation of receptor function at intermediate concentrations, but inhibition at higher concentrations (Llano et al., 1991; Mouginot et al., 1991; Aguayo et al., 1994). The finding that potentiation of GABAAR currents at a low GABA and intermediate ethanol concentration fades over time, and ethanol response is reversibly lost after repeated ethanol pulses within a short time span, further supports the involvement of intracellular signaling (Aguayo et al., 2002).
Post-Transcriptional Mechanisms Can Partly Explain the Variability of the Results
pLGICs are not the only proteins with a central role in the effects of ethanol on the brain. Several proteins involved in post-translational modification have also been reported as critical and might influence these receptors in a modulatory fashion. For instance, protein kinase C (PKC) activity has been associated with ethanol induced changes in vitro. Indeed, PKCε null mutant mice showed increased locomotion with low doses of ethanol and increased sedation with high doses of ethanol, while voluntary ethanol consumption was reduced (Hodge et al., 1999; Olive et al., 2000; Ueno et al., 2001). Interestingly, ethanol and flunitrazepam potentiation of GABAAR response to the direct agonist muscimol was increased in PKCε null mutants. Furthermore, treatment with a PKCε inhibitor mimicked the muscimol response of the PKCε null mutant in wild type animals, but had no additional effect in the mutant (Hodge et al., 1999). This change could be due to the lack of GABAAR γ2 phosphorylation at serine residue S327, which reduces the effect of benzodiazepines and ethanol on γ2 containing receptors (Qi et al., 2007). It would be an intriguing experiment to examine the effects of a phospho-mimetic or phosphorylation resistant GABAAR γ2 KI on the behavioral response to ethanol. This type of approach could help to determine which of the changes reported in PKCε null mutants rely on GABAAR γ2 S327 phosphorylation. KO of PKCδ resulted in an increased resistance to ethanol intoxication (Choi et al., 2008). PKCγ KO mice exhibited shorter loss of righting reflex and were less sensitive to ethanol-induced hypothermia (Harris et al., 1995). Also, there are several studies reporting that the sensitivity of GABAAR to ethanol can be affected by the activation of PKC, as well as signaling proteins such as G proteins (Weiner et al., 1997; Aguayo et al., 2002).
It is well accepted that protein kinases are able to modify GABAAR function, although the direction of this change, potentiating or depressing, depends not only on the type of kinase, but also on the neuron-type (Aguayo et al., 2002). The intracellular domain of the GABAAR γ2L subunit features a consensus sequence for PKC phosphorylation and it was reported that mutation of this region abolished PKC-dependent ethanol effects (Wafford et al., 1991; Wafford and Whiting, 1992). Contrary to this, phosphorylation of γ2 at residue S327 reduced the effect of benzodiazepines and ethanol on γ2 containing receptors (Qi et al., 2007). The differential influence of PKC on the action of ethanol was also reported in cultured hippocampal mouse neurons, where PKC activation reduced ethanol sensitivity and in rat hippocampal slices, where the activation of the kinase increased the effect of ethanol (Aguayo et al., 1994; Weiner et al., 1994, 1997). Interestingly, PKA activation was reported to positively influence ethanol sensitivity in cerebellar Purkinje neurons (Freund and Palmer, 1997). Furthermore, it appears that PKCε can facilitate alcohol-stimulated GABA release and regulate alcohol sensitivity of GABAAR (Olive et al., 2000; Qi et al., 2007; Bajo et al., 2008). Also, PKCδ may regulate the ethanol sensitivity of tonic GABAergic currents while PKCγ mediates ethanol-induced internalization of α1 containing GABAAR and increases the number of synaptic α4 containing receptors (Liang et al., 2007; Werner et al., 2011; Kumar et al., 2012). In addition, acute ethanol exposure also rapidly promotes the internalization of extra-synaptic GABAAR featuring the α4 and δ subunits (Liang et al., 2007; Shen et al., 2011).
Effects of Ethanol on GABAAR Depend on Synaptic or Non-Synaptic Location
While it appears that physiological concentrations of alcohol between 1 and 100 mM selectively potentiate some GABAAR conformations, it has proven highly difficult to unquestionably identify the relevant subunits composing the sensitive receptors (for review see Aguayo et al., 2002; Lovinger and Homanics, 2007). Also, another level of complication arises from the possibility that ethanol sensitivity might rely on post-transcriptional modifications and receptor phosphorylation (Aguayo, 1990, 1991; Mozrzymas and Cherubini, 1998; Aguayo et al., 2002). Despite these constraints, there is a wide consensus that synaptic GABAAR are only potentiated by relatively high doses of 100 mM ethanol (Aguayo et al., 2002; Cui et al., 2012; Roberto et al., 2012). Thus, ethanol might differently affect synaptic and extra-synaptic GABAAR and in turn cause complex effects on phasic and tonic inhibition. Moreover, acute ethanol triggers several GABAAR independent effects in neuronal tissue modulating GABAergic transmission by a combination of pre- and postsynaptic actions (Lovinger and Homanics, 2007; Fleming et al., 2009). Potential cross talk mechanisms might exist between pre- and post-synaptic actions of ethanol. For example, a study showed that ethanol-sensitivity of GABAAR in hippocampal pyramidal cells was significantly altered after blocking GABABR (Wan et al., 1996). These results are interesting since GABABR are associated to G proteins and they could add another degree of complexity to understand how ethanol affects GABAAR.
It is widely accepted that anesthetic concentrations of ethanol (50–100 mM) can increase the probability of GABA release via pre synaptic mechanisms (Roberto et al., 2003; Carta et al., 2004; Nie et al., 2004; Kelm et al., 2011). Thus, tonic currents can be enhanced by ethanol affecting action potential-dependent spill-over of GABA from synaptic sites as demonstrated in cerebellar granule cells (Carta et al., 2004). These results, however, cannot explain the lack of effect of 30 mM on the tonic current recorded in hippocampal and thalamic neurons of PKCδ KO mice (Choi et al., 2008; see Table 3).
In cerebellar slices, acute ethanol exposure was shown not only to increase the frequency of spontaneous inhibitory postsynaptic currents mediated by synaptic GABAAR, but also to potentiate tonic currents mediated by extra-synaptic GABAAR and in granule cells (for review see Cui et al., 2012). The tonic currents in cerebellar granule cells are mediated by GABAAR containing α6 and δ subunits associated with either β2 or β3 subunits (Pöltl et al., 2003). Therefore, these findings lend support to previous studies that showed ethanol potentiation of recombinant receptors with similar subunit conformation. Wallner and collaborators found potentiation of Cl− currents by concentrations of ethanol as low as 3 mM in Xenopus oocytes expressing recombinant GABAAR α6β3δ subunits (Wallner et al., 2003). Like most studies on the effect of ethanol on GABAAR, other laboratories have been unable to produce similar results. For example, additional studies with α6β3δ recombinant receptors in Xenopus oocytes failed to observe an effect of ethanol at concentrations between 7.5–30 mM (Baur et al., 2009). Moreover, studies in CHO cells expressing α6β3δ recombinant receptors reported no potentiating effect of 100 mM ethanol (Yamashita et al., 2006). More recent publications provided another plausible explanation for these conflicting findings by suggesting that the incorporation of the δ subunit into recombinant GABAAR is very dependent on DNA ratio and time of exposure (Meera et al., 2010). A concatenated receptor-construct fixing the GABAAR in the α6β3δ conformation was insensitive to ethanol as well (Baur et al., 2009). Furthermore, subunit compositions of extra-synaptic receptor populations at least in some investigated neuronal cell types, i.e., cerebellar granule neurons, seem to not be homogenous (Jechlinger et al., 1998; Sigel and Baur, 2000; Pöltl et al., 2003; Hanchar et al., 2005; Wallner et al., 2006). Taken together, these studies indicate that the elucidation of ethanol effects on GABAAR is far from resolved and more research is needed.
Final Remarks on the Effects of Ethanol on GABAARs
The large volume of data collected in search of the mechanisms involved in the potentiation of GABAAR by ethanol has allowed us to view a detailed, but far from complete picture. Considering the body of controversial evidence, we have to conclude that there is still no agreement on what makes a given conformation of GABAAR sensitive to physiologically relevant concentrations of ethanol. The large number of receptor subunits and possible combinations thereof, as well as the differences observed in various brain areas and cell types, together with conflicting results make it hard to comprehend the mechanism of acute ethanol action on the receptor. The important role of receptor phosphorylation, principally by PKC, and possibly other posttranscriptional modifications and intracellular signaling mechanisms, such as the activation state of G protein, further convolute the story. Changes in the GABAergic system after chronic alcohol exposure, as well as indirect effects of alcohol like increased GABA release, also make the interpretation of results difficult. While the potentiation of the GABAAR at a high ethanol concentration is widely accepted and reproducible, the effect of low concentrations has remained elusive and no convincing mechanism which is able to explain the many conflicting results has emerged. A direct action of ethanol on the receptor alone seems insufficient to explain this potentiation and the question whether ethanol potentiates GABAAR by direct binding or by intracellular signaling mechanisms remains largely unresolved. It is possible that both mechanisms exist in parallel, although they might not have the same relevance for low and high concentrations of ethanol.
GlyR as an Alternative Inhibitory Model of Study and Therapeutic Target
The existence of multiple potential conformations of GABAAR shifted the attention to another much simpler inhibitory member of the pLGIC family, the glycine receptor (GlyR). After the first demonstration that ethanol potentiates glycinergic Cl− current in central mammalian neurons, there is now universal agreement that GlyR is an important target for low ethanol concentrations (Aguayo et al., 1994; Burgos et al., 2015). Several physiological and pharmacological properties are shared between GlyR and GABAAR. Both receptor channels increase membrane permeability to anions, primarily chloride ions, leading to a fast and potent inhibition of neuronal firing (Lester et al., 2004; Miller and Smart, 2010). They share the overall pentameric architecture and function typical for all members of the family. In contrast to GABAAR which are predominantly expressed in the brain, GlyR are the main inhibitory receptors in the mammalian spinal cord and brain stem, although they are also found in the hippocampus and other brain regions both pre- and postsynaptically (Eichler and Meier, 2008; Eichler et al., 2008, 2009; Winkelmann et al., 2014). It has been described that 10 mM ethanol potentiates glycinergic currents in cultured spinal neurons by increasing the apparent affinity of GlyR, without changing the efficacy of the NT (Aguayo et al., 1996; Mihic et al., 1997; Crawford et al., 2007; Perkins et al., 2008). In a synaptic context, ethanol also positively modulates glycinergic synaptic events increasing inhibitory effects in mature neurons (Eggers and Berger, 2004). Subsequent studies have shown that the ability of physiological concentrations of ethanol below 100 mM to potentiate GlyR depends on the receptor conformation, demonstrating different responses according to subunit composition. Unlike GABAAR, the case of GlyR is simpler and it has been confirmed that the α1 subunit is essential for the effects observed in the presence of ethanol (Sebe et al., 2003; Aguayo et al., 2004).
The GlyR is a promising candidate for pharmacological intervention against the effects of alcohol in the CNS for various reasons. There are five genes encoding for GlyR subunits, only four of which are relevant for the formation of functional receptor channels in humans. GlyR α subunits can form functional homomeric receptors and heteromeric receptors are generally composed of the β subunit and only one of the α subunits giving rise to only six possible receptor conformations. While they are not as prevalent in supratentorial regions as GABAAR, GlyR (including the ethanol sensitive α1 subunit) have been reported in several regions of the brain. In fact, it has recently been shown that in neurons of the lateral orbitofrontal cortex, ethanol inhibition relies mostly on GlyR (Badanich et al., 2013). Also, there are far less conflicting results regarding GlyR and low concentrations of ethanol (5–10 mM), and the mechanism of action is better understood.
Similar to GABAAR, one proposed mechanism involves the interaction of ethanol with a group of amino acids, including alanine 52 (N-terminal), serine 267 (TM2) and alanine 288 (TM3), that form a binding pocket for direct ethanol interaction. Mutagenesis studies demonstrated loss of ethanol induced GlyR potentiation by alteration of the critical amino acid residues and more recently, the model has been refined by structural studies (Blednov et al., 2012; Howard et al., 2014; Trudell et al., 2014). As discussed for GABAAR, however, loss of an ethanol sensitive phenotype may not be strong enough evidence to support the contribution of a direct ethanol-binding site involved in the mechanism of potentiation. The three residues suggested to be involved in ethanol binding are likely critical for channel gating after ligand binding. The evidence suggests the existence of a hydrophobic binding pocket for general anesthetics and alcohol formed by the residues previously described. This site might be responsible for the potentiation of GlyR by ethanol, especially at higher concentrations. However, the lack of pharmacological specificity of this site and the dramatic alterations in physiological properties of the channel caused by the S267 mutation including desensitization, changes in apparent affinity, and pharmacological selectivity complicates the interpretation of these results (Lobo et al., 2004; Sine and Engel, 2006). Another alternative mechanism is based on the findings that the effect of ethanol on native and recombinant GlyR is blocked by GDP-β-S, sequestration of Gβγ using Gβγ-specific antibodies, Gα overexpression, and peptides that bind to Gβγ with high affinity (Burgos et al., 2015). In addition, mutant receptors that have reduced binding capacity to Gβγ are much less sensitive to low ethanol concentrations (Yevenes et al., 2008; Burgos et al., 2015). All of these studies support the idea that ethanol effects on GlyR are dependent on Gβγ activation. This hypothesis is supported by recent studies using a KI mouse model with mutations in the intracellular loop of the receptor. These animals displayed glycinergic currents insensitive to ethanol and Gβγ (Aguayo et al., 2014). The sedative effect of ethanol in these animals was likewise reduced (Burgos et al., 2015). These results indicate that ethanol can potentiate GlyR at low concentrations via G-protein interaction (Aguayo et al., 1996). According to this model, Gβγ binds to the large intracellular loop of the receptor, and the interaction can be interfered with by small molecules (Guzman et al., 2009). This indirect mode of ethanol action may more readily give rise to pharmacological intervention with higher specificity and fewer unwanted effects than interference with ethanol binding at the TM domain of the GABAAR. Moreover, further investigation on the mechanism of action of low ethanol concentrations on GlyR may help to elucidate the molecular mechanisms involved in the modulation of GABAAR by ethanol. Therefore, the GlyR could serve as a valuable model to learn about the effects of alcohol on the two most prevalent mediators of inhibition in the CNS with the aim of developing new pharmacological tools. Before this is accomplished, it will be most critical to determine the role of supratentorial GlyRs on the effects of ethanol, to identify the more abundant receptor conformation in key brain regions, and develop subunit specific ligands to modulate the actions of ethanol on these targets.
Author Contributions
BF contributed to all stages of manuscript preparation and editing. PAC and GM-C contributed with critical discussions and figure design. LGA contributed to all stages of manuscript preparation and final editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mrs. Lauren Aguayo for revising the article. This work was supported by National Institutes of Health Grant R01AA15150, Comisión Nacional de Investigación Científica Tecnológica Grant DPI 20140008, and Fondecyt Postdoctorado 2014 Grant number 3140194.
References
- Absalom N., Eghorn L. F., Villumsen I. S., Karim N., Bay T., Olsen J. V., et al. (2012). α4βδ GABAA receptors are high-affinity targets for γ-hydroxybutyric acid (GHB). Proc. Natl. Acad. Sci. U S A 109, 13404–13409. 10.1073/pnas.1204376109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G., Leggio L., Ferrulli A., Cardone S., Bedogni G., Caputo F., et al. (2011). Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 46, 312–317. 10.1093/alcalc/agr017 [DOI] [PubMed] [Google Scholar]
- Addolorato G., Leggio L., Hopf F. W., Diana M., Bonci A. (2012). Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology 37, 163–177. 10.1038/npp.2011.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo L. G. (1990). Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur. J. Pharmacol. 187, 127–130. 10.1016/0014-2999(90)90349-b [DOI] [PubMed] [Google Scholar]
- Aguayo L. G. (1991). Demonstration that ethanol potentiates the GABAA-activated Cl- current in central mammalian neurons. Alcohol Alcohol. Suppl. 1, 187–190. [PubMed] [Google Scholar]
- Aguayo L. G., Castro P., Mariqueo T., Muñoz B., Xiong W., Zhang L., et al. (2014). Altered sedative effects of ethanol in mice with α1 glycine receptor subunits that are insensitive to Gβγ modulation. Neuropsychopharmacology 39, 2538–2548. 10.1038/npp.2014.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo L. G., Pancetti F. C., Klein R. L., Harris R. A. (1994). Differential effects of GABAergic ligands in mouse and rat hippocampal neurons. Brain Res. 647, 97–105. 10.1016/0006-8993(94)91403-6 [DOI] [PubMed] [Google Scholar]
- Aguayo L. G., Peoples R. W., Yeh H. H., Yevenes G. E. (2002). GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr. Top. Med. Chem. 2, 869–885. 10.2174/1568026023393426 [DOI] [PubMed] [Google Scholar]
- Aguayo L. G., Tapia J. C., Pancetti F. C. (1996). Potentiation of the glycine-activated Cl- current by ethanol in cultured mouse spinal neurons. J. Pharmacol. Exp. Ther. 279, 1116–1122. [PubMed] [Google Scholar]
- Aguayo L. G., van Zundert B., Tapia J. C., Carrasco M. A., Alvarez F. J. (2004). Changes on the properties of glycine receptors during neuronal development. Brain Res. Brain Res. Rev. 47, 33–45. 10.1016/j.brainresrev.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Allan A. M., Harris R. A. (1987). Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol. Biochem. Behav. 27, 665–670. 10.1016/0091-3057(87)90192-4 [DOI] [PubMed] [Google Scholar]
- Aller M. I., Veale E. L., Linden A. M., Sandu C., Schwaninger M., Evans L. J., et al. (2005). Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J. Neurosci. 25, 11455–11467. 10.1523/JNEUROSCI.3153-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. J., Daunais J. B., Friedman D. P., Grant K. A., McCool B. A. (2007). Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABAA receptors. Alcohol. Clin. Exp. Res. 31, 1061–1070. 10.1111/j.1530-0277.2007.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee Q. M., Knapp S., Maguire E. P., Hosie A. M., Thomas P., Mortensen M., et al. (2013). Mutations in the Gabrb1 gene promote alcohol consumption through increased tonic inhibition. Nat. Commun. 4:2816. 10.1038/ncomms3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich K. A., Mulholland P. J., Beckley J. T., Trantham-Davidson H., Woodward J. J. (2013). Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38, 1176–1188. 10.1038/npp.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M., Cruz M. T., Siggins G. R., Messing R., Roberto M. (2008). Protein kinase C ε mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc. Natl. Acad. Sci. U S A 105, 8410–8415. 10.1073/pnas.0802302105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Harrison N. L., Lange G. D., Owen D. G. (1987). Potentiation of γ-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J. Physiol. 386, 485–501. 10.1113/jphysiol.1987.sp016547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Skolnick P., Olsen R. W., Mohler H., Sieghart W., Biggio G., et al. (1998). International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 50, 291–313. [PubMed] [Google Scholar]
- Baur R., Kaur K. H., Sigel E. (2009). Structure of α6β3δ GABAA receptors and their lack of ethanol sensitivity. J. Neurochem. 111, 1172–1181. 10.1111/j.1471-4159.2009.06387.x [DOI] [PubMed] [Google Scholar]
- Becker H. C., Hale R. L. (1991). RO15–4513 antagonizes the anxiolytic effects of ethanol in a nonshock conflict task at doses devoid of anxiogenic activity. Pharmacol. Biochem. Behav. 39, 803–807. 10.1016/0091-3057(91)90169-3 [DOI] [PubMed] [Google Scholar]
- Bettler B., Tiao J. Y. (2006). Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 110, 533–543. 10.1016/j.pharmthera.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Blednov Y. A., Benavidez J. M., Black M., Chandra D., Homanics G. E., Rudolph U., et al. (2013). Linking GABAA receptor subunits to alcohol-induced conditioned taste aversion and recovery from acute alcohol intoxication. Neuropharmacology 67, 46–56. 10.1016/j.neuropharm.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y. A., Benavidez J. M., Homanics G. E., Harris R. A. (2012). Behavioral characterization of knockin mice with mutations M287L and Q266I in the glycine receptor α1 subunit. J. Pharmacol. Exp. Ther. 340, 317–329. 10.1124/jpet.111.185124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y. A., Borghese C. M., McCracken M. L., Benavidez J. M., Geil C. R., Osterndorff-Kahanek E., et al. (2011). Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABAA receptors. J. Pharmacol. Exp. Ther. 336, 145–154. 10.1124/jpet.110.171645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y. A., Stoffel M., Alva H., Harris R. A. (2003). A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc. Natl. Acad. Sci. U S A 100, 277–282. 10.1073/pnas.012682399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese C. M., Stórustovu S., Ebert B., Herd M. B., Belelli D., Lambert J. J., et al. (2006). The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J. Pharmacol. Exp. Ther. 316, 1360–1368. 10.1124/jpet.105.092452 [DOI] [PubMed] [Google Scholar]
- Botta P., Radcliffe R. A., Carta M., Mameli M., Daly E., Floyd K. L., et al. (2007). Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol 41, 187–199. 10.1016/j.alcohol.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G. R., Criswell H. E., Carta M., Dodson P. D., Hanchar H. J., Khisti R. T., et al. (2006). Basis of the gabamimetic profile of ethanol. Alcohol. Clin. Exp. Res. 30, 731–744. 10.1111/j.0145-6008.2006.00086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J. L., Leung J. G., Gagliardi J. P., Rivelli S. K., Muzyk A. J. (2013). Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin. Pharmacol. 5, 99–107. 10.2147/CPAA.s32434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S. G., Revilla V., Cull-Candy S. G., Wisden W., Farrant M. (2001). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92. 10.1038/35051086 [DOI] [PubMed] [Google Scholar]
- Buck K. J., Metten P., Belknap J. K., Crabbe J. C. (1997). Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J. Neurosci. 17, 3946–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos C. F., Muñoz B., Guzman L., Aguayo L. G. (2015). Ethanol effects on glycinergic transmission: from molecular pharmacology to behavior responses. Pharmacol. Res. 101, 18–29. 10.1016/j.phrs.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E., Liang J., Spigelman I., Olsen R. W. (2003). Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63, 53–64. 10.1124/mol.63.1.53 [DOI] [PubMed] [Google Scholar]
- Carta M., Mameli M., Valenzuela C. F. (2004). Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 24, 3746–3751. 10.1523/jneurosci.0067-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D., Jia F., Liang J., Peng Z., Suryanarayanan A., Werner D. F., et al. (2006). GABAA receptor α 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U S A 103, 15230–15235. 10.1073/pnas.0604304103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M. (2004). GABAC receptor ion channels. Clin. Exp. Pharmacol. Physiol. 31, 800–804. 10.1111/j.1440-1681.2004.04083.x [DOI] [PubMed] [Google Scholar]
- Cherubini E., Conti F. (2001). Generating diversity at GABAergic synapses. Trends Neurosci. 24, 155–162. 10.1016/s0166-2236(00)01724-0 [DOI] [PubMed] [Google Scholar]
- Choi D. S., Wei W., Deitchman J. K., Kharazia V. N., Lesscher H. M., McMahon T., et al. (2008). Protein kinase Cδ regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 28, 11890–11899. 10.1523/JNEUROSCI.3156-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G., Addolorato G., Agabio R., Carai M. A., Pibiri F., Serra S., et al. (2004). Role of GABAB receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox. Res. 6, 403–414. 10.1007/bf03033315 [DOI] [PubMed] [Google Scholar]
- Congeddu E., Saba L., Porcella A., Sanna A., Marchese G., Lobina C., et al. (2003). Molecular characterization of new polymorphisms at the β2, α1, γ2 GABAA receptor subunit genes associated to a rat nonpreferring ethanol phenotype. Brain Res. Mol. Brain Res. 110, 289–297. 10.1016/s0169-328x(02)00660-5 [DOI] [PubMed] [Google Scholar]
- Cope D. W., Hughes S. W., Crunelli V. (2005). GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 25, 11553–11563. 10.1523/JNEUROSCI.3362-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Gelernter J., Jensen K., Anton R., Kranzler H. R. (2008). Markers in the 5’-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology 33, 837–848. 10.1038/sj.npp.1301456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe J. C., Wahlsten D., Dudek B. C. (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672. 10.1126/science.284.5420.1670 [DOI] [PubMed] [Google Scholar]
- Crawford D. K., Trudell J. R., Bertaccini E. J., Li K., Davies D. L., Alkana R. L. (2007). Evidence that ethanol acts on a target in Loop 2 of the extracellular domain of α1 glycine receptors. J. Neurochem. 102, 2097–2109. 10.1111/j.1471-4159.2007.04680.x [DOI] [PubMed] [Google Scholar]
- Criswell H. E., Ming Z., Griffith B. L., Breese G. R. (2003). Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J. Pharmacol. Exp. Ther. 304, 192–199. 10.1124/jpet.102.041590 [DOI] [PubMed] [Google Scholar]
- Criswell H. E., Ming Z., Kelm M. K., Breese G. R. (2008). Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J. Pharmacol. Exp. Ther. 326, 596–603. 10.1124/jpet.107.135418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W. Y., Seneviratne C., Gu J., Li M. D. (2012). Genetics of GABAergic signaling in nicotine and alcohol dependence. Hum. Genet. 131, 843–855. 10.1007/s00439-011-1108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M., Brodie M., Muntoni A., Puddu M. C., Pillolla G., Steffensen S., et al. (2003). Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol. Clin. Exp. Res. 27, 354–361. 10.1097/01.alc.0000057121.36127.19 [DOI] [PubMed] [Google Scholar]
- Diaz M. R., Vollmer C. C., Zamudio-Bulcock P. A., Vollmer W., Blomquist S. L., Morton R. A., et al. (2014). Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABAA receptor δ subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology 79, 262–274. 10.1016/j.neuropharm.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Dick D. M., Xuei X., Tian H., Almasy L., Bauer L. O., et al. (2004). Variations in GABRA2, encoding the α 2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 74, 705–714. 10.1086/383283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers E. D., Berger A. J. (2004). Mechanisms for the modulation of native glycine receptor channels by ethanol. J. Neurophysiol. 91, 2685–2695. 10.1152/jn.00907.2003 [DOI] [PubMed] [Google Scholar]
- Eichler S. A., Förstera B., Smolinsky B., Jüttner R., Lehmann T. N., Fähling M., et al. (2009). Splice-specific roles of glycine receptor α3 in the hippocampus. Eur. J. Neurosci. 30, 1077–1091. 10.1111/j.1460-9568.2009.06903.x [DOI] [PubMed] [Google Scholar]
- Eichler S. A., Kirischuk S., Jüttner R., Schäfermeier P. K., Legendre P., Lehmann T. N., et al. (2008). Glycinergic tonic inhibition of hippocampal neurons with depolarising GABAergic transmission elicits histopathological signs of temporal lobe epilepsy. J. Cell. Mol. Med. 12, 2848–2866. 10.1111/j.1582-4934.2008.00357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler S. A., Meier J. C. (2008). E-I balance and human diseases–from molecules to networking. Front. Mol. Neurosci. 1:2. 10.3389/neuro.02.002.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom A. C., Akerman K. E. (1991). Effect of ethanol on gamma-aminobutyric acid and glycine receptor-coupled Cl- fluxes in rat brain synaptoneurosomes. J. Neurochem 57, 384–390. [DOI] [PubMed] [Google Scholar]
- Engin E., Liu J., Rudolph U. (2012). α2-containing GABAA receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol. Ther. 136, 142–152. 10.1016/j.pharmthera.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M. A., Hodgkinson C. A., Yuan Q., Albaugh B., Virkkunen M., Goldman D. (2009). GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology 34, 1245–1254. 10.1038/npp.2008.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C. J., Sarviharju M. (1984). Motor impairment, narcosis and hypothermia by ethanol: separate genetic mechanisms. Alcohol 1, 59–62. 10.1016/0741-8329(84)90038-7 [DOI] [PubMed] [Google Scholar]
- Faingold C. L., N’Gouemo P., Riaz A. (1998). Ethanol and neurotransmitter interactions–from molecular to integrative effects. Prog. Neurobiol. 55, 509–535. 10.1016/s0301-0082(98)00027-6 [DOI] [PubMed] [Google Scholar]
- Fleming R. L., Manis P. B., Morrow A. L. (2009). The effects of acute and chronic ethanol exposure on presynaptic and postsynaptic γ-aminobutyric acid (GABA) neurotransmission in cultured cortical and hippocampal neurons. Alcohol 43, 603–618. 10.1016/j.alcohol.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund R. K., Palmer M. R. (1997). β adrenergic sensitization of γ-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. J. Pharmacol. Exp. Ther. 280, 1192–1200. [PubMed] [Google Scholar]
- Gage P. W., Robertson B. (1985). Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br. J. Pharmacol. 85, 675–681. 10.1111/j.1476-5381.1985.tb10563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Moss S. J., Olsen R. W. (2012). Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J. Neurosci. 32, 17874–17881. 10.1523/JNEUROSCI.2535-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K. A. (1999). Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol. Biochem. Behav. 64, 261–267. 10.1016/s0091-3057(99)00075-1 [DOI] [PubMed] [Google Scholar]
- Grobin A. C., Matthews D. B., Devaud L. L., Morrow A. L. (1998). The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 139, 2–19. 10.1007/s002130050685 [DOI] [PubMed] [Google Scholar]
- Grobin A. C., Papadeas S. T., Morrow A. L. (2000). Regional variations in the effects of chronic ethanol administration on GABAA receptor expression: potential mechanisms. Neurochem. Int. 37, 453–461. 10.1016/s0197-0186(00)00058-9 [DOI] [PubMed] [Google Scholar]
- Guzman L., Moraga-Cid G., Avila A., Figueroa M., Yevenes G. E., Fuentealba J., et al. (2009). Blockade of ethanol-induced potentiation of glycine receptors by a peptide that interferes with Gβγ binding. J. Pharmacol. Exp. Ther. 331, 933–939. 10.1124/jpet.109.160440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar H. J., Dodson P. D., Olsen R. W., Otis T. S., Wallner M. (2005). Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat. Neurosci. 8, 339–345. 10.1038/nn1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A. (1990). Distinct actions of alcohols, barbiturates and benzodiazepines on GABA-activated chloride channels. Alcohol 7, 273–275. 10.1016/0741-8329(90)90017-7 [DOI] [PubMed] [Google Scholar]
- Harris R. A., McQuilkin S. J., Paylor R., Abeliovich A., Tonegawa S., Wehner J. M. (1995). Mutant mice lacking the γ isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of γ-aminobutyrate type A receptors. Proc. Natl. Acad. Sci. U S A 92, 3658–3662. 10.1073/pnas.92.9.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey H. M., Ray L. A., Finan P., Villanueva R., Niculescu M., Hutchison K. E. (2008). Human γ-aminobutyric acid A receptor α2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 7, 447–454. 10.1111/j.1601-183x.2007.00369.x [DOI] [PubMed] [Google Scholar]
- Hodge C. W., Mehmert K. K., Kelley S. P., McMahon T., Haywood A., Olive M. F., et al. (1999). Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCε. Nat. Neurosci. 2, 997–1002. [DOI] [PubMed] [Google Scholar]
- Hoffman P. L., Tabakoff B., Szabó G., Suzdak P. D., Paul S. M. (1987). Effect of an imidazobenzodiazepine, Ro15–4513, on the incoordination and hypothermia produced by ethanol and pentobarbital. Life Sci. 41, 611–619. 10.1016/0024-3205(87)90415-2 [DOI] [PubMed] [Google Scholar]
- Homanics G. E., Ferguson C., Quinlan J. J., Daggett J., Snyder K., Lagenaur C., et al. (1997). Gene knockout of the α6 subunit of the γ-aminobutyric acid type A receptor: lack of effect on responses to ethanol, pentobarbital and general anesthetics. Mol. Pharmacol. 51, 588–596. [DOI] [PubMed] [Google Scholar]
- Homanics G. E., Harrison N. L., Quinlan J. J., Krasowski M. D., Rick C. E., De Blas A. L., et al. (1999). Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the γ2 subunit of the γ-aminobutyrate type A receptor. Neuropharmacology 38, 253–265. 10.1016/s0028-3908(98)00177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics G. E., Le N. Q., Kist F., Mihalek R., Hart A. R., Quinlan J. J. (1998). Ethanol tolerance and withdrawal responses in GABAA receptor α 6 subunit null allele mice and in inbred C57BL/6J and strain 129/SvJ mice. Alcohol. Clin. Exp. Res. 22, 259–265. 10.1111/j.1530-0277.1998.tb03647.x [DOI] [PubMed] [Google Scholar]
- Hood H. M., Buck K. J. (2000). Allelic variation in the GABA A receptor γ2 subunit is associated with genetic susceptibility to ethanol-induced motor incoordination and hypothermia, conditioned taste aversion and withdrawal in BXD/Ty recombinant inbred mice. Alcohol. Clin. Exp. Res. 24, 1327–1334. 10.1111/j.1530-0277.2000.tb02100.x [DOI] [PubMed] [Google Scholar]
- Howard R. J., Murail S., Ondricek K. E., Corringer P. J., Lindahl E., Trudell J. R., et al. (2011). Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. U S A 108, 12149–12154. 10.1073/pnas.1104480108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Trudell J. R., Harris R. A. (2014). Seeking structural specificity: direct modulation of pentameric ligand-gated ion channels by alcohols and general anesthetics. Pharmacol. Rev. 66, 396–412. 10.1124/pr.113.007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundle B., McMahon T., Dadgar J., Chen C. H., Mochly-Rosen D., Messing R. O. (1997). An inhibitory fragment derived from protein kinase Cε prevents enhancement of nerve growth factor responses by ethanol and phorbol esters. J. Biol. Chem. 272, 15028–15035. 10.1074/jbc.272.23.15028 [DOI] [PubMed] [Google Scholar]
- Hundle B., McMahon T., Dadgar J., Messing R. O. (1995). Overexpression of ε-protein kinase C enhances nerve growth factor-induced phosphorylation of mitogen-activated protein kinases and neurite outgrowth. J. Biol. Chem. 270, 30134–30140. 10.1074/jbc.270.50.30134 [DOI] [PubMed] [Google Scholar]
- Jacob T. C., Moss S. J., Jurd R. (2008). GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343. 10.1038/nrn2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M., Pelz R., Tretter V., Klausberger T., Sieghart W. (1998). Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J. Neurosci. 18, 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F., Pignataro L., Schofield C. M., Yue M., Harrison N. L., Goldstein P. A. (2005). An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 94, 4491–4501. 10.1152/jn.00421.2005 [DOI] [PubMed] [Google Scholar]
- Johnson W. D., 2nd, Howard R. J., Trudell J. R., Harris R. A. (2012). The TM2 6′ position of GABAA receptors mediates alcohol inhibition. J. Pharmacol. Exp. Ther. 340, 445–456. 10.1124/jpet.111.188037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June H. L., Lummis G. H., Colker R. E., Moore T. O., Lewis M. J. (1991). Ro15–4513 attenuates the consumption of ethanol in deprived rats. Alcohol. Clin. Exp. Res. 15, 406–411. 10.1111/j.1530-0277.1991.tb00538.x [DOI] [PubMed] [Google Scholar]
- Kapur J., Macdonald R. L. (1999). Postnatal development of hippocampal dentate granule cell γ-aminobutyric acidA receptor pharmacological properties. Mol. Pharmacol. 55, 444–452. [PubMed] [Google Scholar]
- Kareken D. A., Liang T., Wetherill L., Dzemidzic M., Bragulat V., Cox C., et al. (2010). A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcohol. Clin. Exp. Res. 34, 2169–2178. 10.1111/j.1530-0277.2010.01293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S., Malherbe P., Sigel E. (1992). Function of the α 1 β 2 γ 2S γ-aminobutyric acid type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J. Biol. Chem. 267, 25660–25663. [PubMed] [Google Scholar]
- Kelm M. K., Criswell H. E., Breese G. R. (2011). Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res. Rev. 65, 113–123. 10.1016/j.brainresrev.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi E. R., Kleingoor C., Kettenmann H., Seeburg P. H. (1993). Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature 361, 356–359. 10.1038/361356a0 [DOI] [PubMed] [Google Scholar]
- Korpi E. R., Koikkalainen P., Vekovischeva O. Y., Mäkelä R., Kleinz R., Uusi-Oukari M., et al. (1999). Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from α 6-subunit-deficient mice. Eur. J. Neurosci. 11, 233–240. 10.1046/j.1460-9568.1999.00421.x [DOI] [PubMed] [Google Scholar]
- Korpi E. R., Uusi-Oukari M. (1992). Cerebellar GABAA receptors and alcohol-related behaviors: focus on diazepam-insensitive [3H]Ro 15–4513 binding. Adv. Biochem. Psychopharmacol. 47, 289–299. [PubMed] [Google Scholar]
- Kumar S., Fleming R. L., Morrow A. L. (2004). Ethanol regulation of γ-aminobutyric acid(A) receptors: genomic and nongenomic mechanisms. Pharmacol. Ther. 101, 211–226. 10.1016/j.pharmthera.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Kumar S., Ren Q., Beckley J. H., O’Buckley T. K., Gigante E. D., Santerre J. L., et al. (2012). Ethanol activation of protein kinase A regulates GABAA receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Front. Neurosci. 6:44. 10.3389/fnins.2012.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Sieghart W., Morrow A. L. (2002). Association of protein kinase C with GABAA receptors containing α1 and α4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J. Neurochem. 82, 110–117. 10.1046/j.1471-4159.2002.00943.x [DOI] [PubMed] [Google Scholar]
- Lester H. A., Dibas M. I., Dahan D. S., Leite J. F., Dougherty D. A. (2004). Cys-loop receptors: new twists and turns. Trends Neurosci. 27, 329–336. 10.1016/j.tins.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Li D., Sulovari A., Cheng C., Zhao H., Kranzler H. R., Gelernter J. (2014). Association of γ-aminobutyric acid A receptor α2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology 39, 907–918. 10.1038/npp.2013.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Olsen R. W. (2014). Alcohol use disorders and current pharmacological therapies: the role of GABAA receptors. Acta Pharmacol. Sin. 35, 981–993. 10.1038/aps.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Suryanarayanan A., Abriam A., Snyder B., Olsen R. W., Spigelman I. (2007). Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J. Neurosci. 27, 12367–12377. 10.1523/jneurosci.2786-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. M., Freund R. K., Hoffer B. J., Palmer M. R. (1994). Ethanol-induced depressions of cerebellar Purkinje neurons are potentiated by β-adrenergic mechanisms in rat brain. J. Pharmacol. Exp. Ther. 271, 1175–1180. [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. (1991). Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6, 565–574. 10.1016/0896-6273(91)90059-9 [DOI] [PubMed] [Google Scholar]
- Lobo I. A., Harris R. A. (2008). GABAA receptors and alcohol. Pharmacol. Biochem. Behav. 90, 90–94. 10.1016/j.pbb.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo I. A., Mascia M. P., Trudell J. R., Harris R. A. (2004). Channel gating of the glycine receptor changes accessibility to residues implicated in receptor potentiation by alcohols and anesthetics. J. Biol. Chem. 279, 33919–33927. 10.1074/jbc.m313941200 [DOI] [PubMed] [Google Scholar]
- Lovinger D. M. (2008). Communication networks in the brain: neurons, receptors, neurotransmitters and alcohol. Alcohol Res. Health 31, 196–214. [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M., Homanics G. E. (2007). Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol 41, 139–143. 10.1016/j.alcohol.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., et al. (1990). Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature 346, 648–651. 10.1038/346648a0 [DOI] [PubMed] [Google Scholar]
- Maccioni P., Colombo G. (2009). Role of the GABAB receptor in alcohol-seeking and drinking behavior. Alcohol 43, 555–558. 10.1016/j.alcohol.2009.09.030 [DOI] [PubMed] [Google Scholar]
- Marszalec W., Kurata Y., Hamilton B. J., Carter D. B., Narahashi T. (1994). Selective effects of alcohols on γ-aminobutyric acid A receptor subunits expressed in human embryonic kidney cells. J. Pharmacol. Exp. Ther. 269, 157–163. [PubMed] [Google Scholar]
- Marutha Ravindran C. R., Mehta A. K., Ticku M. K. (2007). Effect of chronic administration of ethanol on the regulation of the δ-subunit of GABAA receptors in the rat brain. Brain Res. 1174, 47–52. 10.1016/j.brainres.2007.07.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia M. P., Trudell J. R., Harris R. A. (2000). Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc. Natl. Acad. Sci. U S A 97, 9305–9310. 10.1073/pnas.160128797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool B. A., Frye G. D., Pulido M. D., Botting S. K. (2003). Effects of chronic ethanol consumption on rat GABAA and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 963, 165–177. 10.1016/s0006-8993(02)03966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan R. M., Whiting P. J. (1996). Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 19, 139–143. 10.1016/s0166-2236(96)80023-3 [DOI] [PubMed] [Google Scholar]
- Meera P., Olsen R. W., Otis T. S., Wallner M. (2010). Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking δ subunit incorporation into functional receptors. Mol. Pharmacol. 78, 918–924. 10.1124/mol.109.062687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A. K., Ticku M. K. (1988). Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves γ-aminobutyric acidA-gated chloride channels. J. Pharmacol. Exp. Ther. 246, 558–564. [PubMed] [Google Scholar]
- Messing R. O., Petersen P. J., Henrich C. J. (1991). Chronic ethanol exposure increases levels of protein kinase C δ and ε and protein kinase C-mediated phosphorylation in cultured neural cells. J. Biol. Chem. 266, 23428–23432. [PubMed] [Google Scholar]
- Mhatre M. C., Ticku M. K. (1992). Chronic ethanol administration alters γ-aminobutyric acidA receptor gene expression. Mol. Pharmacol. 42, 415–422. [PubMed] [Google Scholar]
- Michels G., Moss S. J. (2007). GABAA receptors: properties and trafficking. Crit. Rev. Biochem. Mol. Biol. 42, 3–14. 10.1080/10409230601146219 [DOI] [PubMed] [Google Scholar]
- Mihalek R. M., Bowers B. J., Wehner J. M., Kralic J. E., VanDoren M. J., Morrow A. L., et al. (2001). GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol. Clin. Exp. Res. 25, 1708–1718. 10.1111/j.1530-0277.2001.tb02179.x [DOI] [PubMed] [Google Scholar]
- Mihic S. J. (1999). Acute effects of ethanol on GABAA and glycine receptor function. Neurochem. Int. 35, 115–123. 10.1016/s0197-0186(99)00053-4 [DOI] [PubMed] [Google Scholar]
- Mihic S. J., Whiting P. J., Harris R. A. (1994). Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: lack of subunit specificity. Eur. J. Pharmacol. 268, 209–214. 10.1016/0922-4106(94)90190-2 [DOI] [PubMed] [Google Scholar]
- Mihic S. J., Ye Q., Wick M. J., Koltchine V. V., Krasowski M. D., Finn S. E., et al. (1997). Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389, 385–389. 10.1038/38738 [DOI] [PubMed] [Google Scholar]
- Miller P. S., Aricescu A. R. (2014). Crystal structure of a human GABAA receptor. Nature 512, 270–275. 10.1038/nature13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Smart T. G. (2010). Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol. Sci. 31, 161–174. 10.1016/j.tips.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. (1994). Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 17, 517–525. 10.1016/0166-2236(94)90155-4 [DOI] [PubMed] [Google Scholar]
- Morrow A. L., Herbert J. S., Montpied P. (1992). Differential effects of chronic ethanol administration on GABAA receptor α1 and α6 subunit mRNA levels in rat cerebellum. Mol. Cell. Neurosci. 3, 251–258. 10.1016/1044-7431(92)90045-4 [DOI] [PubMed] [Google Scholar]
- Mouginot D., Feltz P., Schlichter R. (1991). Modulation of GABA-gated chloride currents by intracellular Ca2+ in cultured porcine melanotrophs. J. Physiol. 437, 109–132. 10.1113/jphysiol.1991.sp018587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas J. W., Cherubini E. (1998). Changes in intracellular calcium concentration affect desensitization of GABAA receptors in acutely dissociated P2–P6 rat hippocampal neurons. J. Neurophysiol. 79, 1321–1328. [DOI] [PubMed] [Google Scholar]
- Nestoros J. N. (1980a). Ethanol selectively potentiates GABA-mediated inhibition of single feline cortical neurons. Life Sci. 26, 519–523. 10.1016/0024-3205(80)90314-8 [DOI] [PubMed] [Google Scholar]
- Nestoros J. N. (1980b). Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science 209, 708–710. 10.1126/science.7394531 [DOI] [PubMed] [Google Scholar]
- Newton P. M., Messing R. O. (2007). Increased sensitivity to the aversive effects of ethanol in PKCε null mice revealed by place conditioning. Behav. Neurosci. 121, 439–442. 10.1037/0735-7044.121.2.439 [DOI] [PubMed] [Google Scholar]
- Nie H., Rewal M., Gill T. M., Ron D., Janak P. H. (2011). Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc. Natl. Acad. Sci. U S A 108, 4459–4464. 10.1073/pnas.1016156108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z., Schweitzer P., Roberts A. J., Madamba S. G., Moore S. D., Siggins G. R. (2004). Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303, 1512–1514. 10.1126/science.1092550 [DOI] [PubMed] [Google Scholar]
- Nie Z., Zorrilla E. P., Madamba S. G., Rice K. C., Roberto M., Siggins G. R. (2009). Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal 9, 68–85. 10.1100/tsw.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M. F., Mehmert K. K., Messing R. O., Hodge C. W. (2000). Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCε-deficient mice. Eur. J. Neurosci. 12, 4131–4140. 10.1046/j.1460-9568.2000.00297.x [DOI] [PubMed] [Google Scholar]
- Palmer M. R., Hoffer B. J. (1990). GABAergic mechanisms in the electrophysiological actions of ethanol on cerebellar neurons. Neurochem. Res. 15, 145–151. 10.1007/bf00972204 [DOI] [PubMed] [Google Scholar]
- Palmer M. R., van Horne C. G., Harlan J. T., Moore E. A. (1988). Antagonism of ethanol effects on cerebellar Purkinje neurons by the benzodiazepine inverse agonists Ro 15–4513 and FG 7142: electrophysiological studies. J. Pharmacol. Exp. Ther. 247, 1018–1024. [PubMed] [Google Scholar]
- Papadeas S., Grobin A. C., Morrow A. L. (2001). Chronic ethanol consumption differentially alters GABAA receptor α1 and α4 subunit peptide expression and GABAA receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol. Clin. Exp. Res. 25, 1270–1275. 10.1111/j.1530-0277.2001.tb02347.x [DOI] [PubMed] [Google Scholar]
- Peris J., Eppler B., Hu M., Walker D. W., Hunter B. E., Mason K., et al. (1997). Effects of chronic ethanol exposure on GABA receptors and GABAB receptor modulation of 3H-GABA release in the hippocampus. Alcohol. Clin. Exp. Res. 21, 1047–1052. 10.1111/j.1530-0277.1997.tb04252.x [DOI] [PubMed] [Google Scholar]
- Perkins D. I., Trudell J. R., Crawford D. K., Asatryan L., Alkana R. L., Davies D. L. (2009). Loop 2 structure in glycine and GABAA receptors plays a key role in determining ethanol sensitivity. J. Biol. Chem. 284, 27304–27314. 10.1074/jbc.M109.023598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. I., Trudell J. R., Crawford D. K., Alkana R. L., Davies D. L. (2008). Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J. Neurochem. 106, 1337–1349. 10.1111/j.1471-4159.2008.05476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A., Covault J., Feinn R., Nellissery M., Hernandez-Avila C., Oncken C., et al. (2005). GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology 30, 1193–1203. 10.1038/sj.npp.1300688 [DOI] [PubMed] [Google Scholar]
- Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. (2000). GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. 10.1016/s0306-4522(00)00442-5 [DOI] [PubMed] [Google Scholar]
- Pöltl A., Hauer B., Fuchs K., Tretter V., Sieghart W. (2003). Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. J. Neurochem. 87, 1444–1455. 10.1046/j.1471-4159.2003.02135.x [DOI] [PubMed] [Google Scholar]
- Qi Z. H., Song M., Wallace M. J., Wang D., Newton P. M., McMahon T., et al. (2007). Protein kinase C ε regulates γ-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of γ2 subunits. J. Biol. Chem. 282, 33052–33063. 10.1074/jbc.m707233200 [DOI] [PubMed] [Google Scholar]
- Radcliffe R. A., Hoffmann S. E., Deng X. S., Asperi W., Fay T., Bludeau P., et al. (2004). Behavioral characterization of alcohol-tolerant and alcohol-nontolerant rat lines and an f(2) generation. Behav. Genet. 34, 453–463. 10.1023/b:bege.0000023650.32243.39 [DOI] [PubMed] [Google Scholar]
- Reynolds J. N., Prasad A., MacDonald J. F. (1992). Ethanol modulation of GABA receptor-activated Cl- currents in neurons of the chick, rat and mouse central nervous system. Eur. J. Pharmacol. 224, 173–181. 10.1016/0014-2999(92)90802-b [DOI] [PubMed] [Google Scholar]
- Roberto M., Gilpin N. W., O’Dell L. E., Cruz M. T., Morse A. C., Siggins G. R., et al. (2008). Cellular and behavioral interactions of gabapentin with alcohol dependence. J. Neurosci. 28, 5762–5771. 10.1523/JNEUROSCI.0575-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Gilpin N. W., Siggins G. R. (2012). The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate and neuropeptides. Cold Spring Harb. Perspect. Med. 2:a012195. 10.1101/cshperspect.a012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Madamba S. G., Moore S. D., Tallent M. K., Siggins G. R. (2003). Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. U S A 100, 2053–2058. 10.1073/pnas.0437926100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Madamba S. G., Stouffer D. G., Parsons L. H., Siggins G. R. (2004). Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci. 24, 10159–10166. 10.1523/JNEUROSCI.3004-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen R., Hundle B., Messing R. O. (1994). Protein kinase C and adaptation to ethanol. EXS 71, 29–38. 10.1007/978-3-0348-7330-7_4 [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C., Cline B., Jurd R., Rudolph U., Spanagel R. (2007). Etomidate and propofol-hyposensitive GABAA receptor β3(N265M) mice show little changes in acute alcohol sensitivity but enhanced tolerance and withdrawal. Neurosci. Lett. 416, 275–278. 10.1016/j.neulet.2007.02.024 [DOI] [PubMed] [Google Scholar]
- Sanna A., Congeddu E., Saba L., Porcella A., Marchese G., Ruiu S., et al. (2004). The cerebellar GABAA α6 subunit is differentially modulated by chronic ethanol exposure in normal (R100R) and mutated (Q100Q) sNP rats. Brain Res. 998, 148–154. 10.1016/j.brainres.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Sapp D. W., Yeh H. H. (1998). Ethanol-GABAA receptor interactions: a comparison between cell lines and cerebellar Purkinje cells. J. Pharmacol. Exp. Ther. 284, 768–776. [PubMed] [Google Scholar]
- Sauguet L., Shahsavar A., Poitevin F., Huon C., Menny A., Nemecz A., et al. (2014). Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc. Natl. Acad. Sci. U S A 111, 966–971. 10.1073/pnas.1314997111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U., Waldhofer S., Weigelt T., Hiemke C. (2002). Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol. Clin. Exp. Res. 26, 457–462. 10.1097/00000374-200204000-00004 [DOI] [PubMed] [Google Scholar]
- Schwarzer C., Berresheim U., Pirker S., Wieselthaler A., Fuchs K., Sieghart W., et al. (2001). Distribution of the major γ-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J. Comp. Neurol. 433, 526–549. 10.1002/cne.1158 [DOI] [PubMed] [Google Scholar]
- Sebe J. Y., Eggers E. D., Berger A. J. (2003). Differential effects of ethanol on GABAA and glycine receptor-mediated synaptic currents in brain stem motoneurons. J. Neurophysiol. 90, 870–875. 10.1152/jn.00119.2003 [DOI] [PubMed] [Google Scholar]
- Shen Y., Lindemeyer A. K., Spigelman I., Sieghart W., Olsen R. W., Liang J. (2011). Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol. Pharmacol. 79, 432–442. 10.1124/mol.110.068650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Baur R. (2000). Electrophysiological evidence for the coexistence of α1 and α6 subunits in a single functional GABAA receptor. J. Neurochem. 74, 2590–2596. 10.1046/j.1471-4159.2000.0742590.x [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Malherbe P. (1993). Recombinant GABAA receptor function and ethanol. FEBS Lett. 324, 140–142. 10.1016/0014-5793(93)81380-I [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Pittman Q. J., French E. D. (1987). Effects of ethanol on CA1 and CA3 pyramidal cells in the hippocampal slice preparation: an intracellular study. Brain Res. 414, 22–34. 10.1016/0006-8993(87)91323-0 [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Roberto M., Nie Z. (2005). The tipsy terminal: presynaptic effects of ethanol. Pharmacol. Ther. 107, 80–98. 10.1016/j.pharmthera.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Silberman Y., Ariwodola O. J., Weiner J. L. (2009). Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology 56, 886–895. 10.1016/j.neuropharm.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Engel A. G. (2006). Recent advances in Cys-loop receptor structure and function. Nature 440, 448–455. 10.1038/nature04708 [DOI] [PubMed] [Google Scholar]
- Spanagel R., Vengeliene V., Jandeleit B., Fischer W. N., Grindstaff K., Zhang X., et al. (2014). Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology 39, 783–791. 10.1038/npp.2013.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen S. C., Walton C. H., Hansen D. M., Yorgason J. T., Gallegos R. A., Criado J. R. (2009). Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol. Biochem. Behav. 92, 68–75. 10.1016/j.pbb.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell B. M., Brickley S. G., Tang C. Y., Farrant M., Mody I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U S A 100, 14439–14444. 10.1073/pnas.2435457100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak P. D., Glowa J. R., Crawley J. N., Schwartz R. D., Skolnick P., Paul S. M. (1986a). A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science 234, 1243–1247. 10.1126/science.3022383 [DOI] [PubMed] [Google Scholar]
- Suzdak P. D., Schwartz R. D., Skolnick P., Paul S. M. (1986b). Ethanol stimulates γ-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc. Natl. Acad. Sci. U S A 83, 4071–4075. 10.1073/pnas.83.11.4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B., Hoffman P. L. (2013). The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol. Biochem. Behav. 113, 20–37. 10.1016/j.pbb.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb O., Trouslard J., Demeneix B. A., Feltz P., Bossu J. L., Dupont J. L., et al. (1987). Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflugers Arch 409, 620–631. 10.1007/bf00584663 [DOI] [PubMed] [Google Scholar]
- Ticku M. K. (1989). Ethanol and the benzodiazepine-GABA receptor-ionophore complex. Experientia 45, 413–418. 10.1007/bf01952022 [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Burch T. (1980). Alterations in γ-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J. Neurochem. 34, 417–423. 10.1111/j.1471-4159.1980.tb06612.x [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Burch T. P., Davis W. C. (1983). The interactions of ethanol with the benzodiazepine-GABA receptor-ionophore complex. Pharmacol. Biochem. Behav. 18, 15–18. 10.1016/0091-3057(83)90140-5 [DOI] [PubMed] [Google Scholar]
- Trudell J. R., Messing R. O., Mayfield J., Harris R. A. (2014). Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol. Sci. 35, 317–323. 10.1016/j.tips.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyacke R. J., Lingford-Hughes A., Reed L. J., Nutt D. J. (2010). GABAB receptors in addiction and its treatment. Adv. Pharmacol. 58, 373–396. 10.1016/s1054-3589(10)58014-1 [DOI] [PubMed] [Google Scholar]
- Ueno S., Harris R. A., Messing R. O., Sanchez-Perez A. M., Hodge C. W., McMahon T., et al. (2001). Alcohol actions on GABAA receptors: from protein structure to mouse behavior. Alcohol. Clin. Exp. Res. 25, 76S–81S. 10.1097/00000374-200105051-00014 [DOI] [PubMed] [Google Scholar]
- Ueno S., Lin A., Nikolaeva N., Trudell J. R., Mihic S. J., Harris R. A., et al. (2000). Tryptophan scanning mutagenesis in TM2 of the GABAA receptor α subunit: effects on channel gating and regulation by ethanol. Br. J. Pharmacol. 131, 296–302. 10.1038/sj.bjp.0703504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Wick M. J., Ye Q., Harrison N. L., Harris R. A. (1999). Subunit mutations affect ethanol actions on GABAA receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 127, 377–382. 10.1038/sj.bjp.0702563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M., Heikkilä J., Sinkkonen S. T., Mäkelä R., Hauer B., Homanics G. E., et al. (2000). Long-range interactions in neuronal gene expression: evidence from gene targeting in the GABAA receptor β2-α6-α1-γ2 subunit gene cluster. Mol. Cell. Neurosci. 16, 34–41. 10.1006/mcne.2000.0856 [DOI] [PubMed] [Google Scholar]
- Valenzuela C. F., Jotty K. (2015). Mini-review: effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex–recent advances. Cerebellum 14, 438–446. 10.1007/s12311-014-0639-3 [DOI] [PubMed] [Google Scholar]
- Vekovischeva O. Y., Uusi-Oukari M., Korpi E. R. (2000). Chronic ethanol treatment and GABAA receptor α6 subunit gene expression: a study using α6 subunit-deficient mice. Addict. Biol. 5, 463–467. 10.1111/j.1369-1600.2000.tb00216.x [DOI] [PubMed] [Google Scholar]
- Vengeliene V., Bilbao A., Molander A., Spanagel R. (2008). Neuropharmacology of alcohol addiction. Br. J. Pharmacol. 154, 299–315. 10.1038/bjp.2008.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S., Heitzeg M. M., Foley S., Yau W. Y., Majczenko K., Zubieta J. K., et al. (2012). Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol. Psychiatry 17, 511–519. 10.1038/mp.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford K. A. (2005). GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr. Opin. Pharmacol. 5, 47–52. 10.1016/j.coph.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Burnett D., Harris R. A., Whiting P. J. (1993). GABAA receptor subunit expression and sensitivity to ethanol. Alcohol Alcohol. Suppl. 2, 327–330. [PubMed] [Google Scholar]
- Wafford K. A., Burnett D. M., Leidenheimer N. J., Burt D. R., Wang J. B., Kofuji P., et al. (1991). Ethanol sensitivity of the GABAA receptor expressed in Xenopus oocytes requires 8 amino acids contained in the γ 2L subunit. Neuron 7, 27–33. 10.1016/0896-6273(91)90071-7 [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Whiting P. J. (1992). Ethanol potentiation of GABAA receptors requires phosphorylation of the alternatively spliced variant of the γ 2 subunit. FEBS Lett. 313, 113–117. 10.1016/0014-5793(92)81424-k [DOI] [PubMed] [Google Scholar]
- Wallner M., Hanchar H. J., Olsen R. W. (2003). Ethanol enhances α 4 β 3 δ and α 6 β 3 δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U S A 100, 15218–15223. 10.1073/pnas.2435171100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M., Hanchar H. J., Olsen R. W. (2006). Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15–4513. Proc. Natl. Acad. Sci. U S A 103, 8540–8545. 10.1073/pnas.0600194103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M., Hanchar H. J., Olsen R. W. (2014). Alcohol selectivity of β3-containing GABAA receptors: evidence for a unique extracellular alcohol/imidazobenzodiazepine Ro15–4513 binding site at the α+β- subunit interface in αβ3δ GABAA receptors. Neurochem. Res. 39, 1118–1126. 10.1007/s11064-014-1243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F. J., Berton F., Madamba S. G., Francesconi W., Siggins G. R. (1996). Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc. Natl. Acad. Sci. U S A 93, 5049–5054. 10.1073/pnas.93.10.5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegelius K., Halonen T., Korpi E. R. (1993). γ-vinyl GABA decreases voluntary alcohol consumption in alcohol-preferring AA rats. Pharmacol. Toxicol. 73, 150–152. 10.1111/j.1600-0773.1993.tb01554.x [DOI] [PubMed] [Google Scholar]
- Weiner J. L., Valenzuela C. F. (2006). Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol. Ther. 111, 533–554. 10.1016/j.pharmthera.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Weiner J. L., Valenzuela C. F., Watson P. L., Frazier C. J., Dunwiddie T. V. (1997). Elevation of basal protein kinase C activity increases ethanol sensitivity of GABAA receptors in rat hippocampal CA1 pyramidal neurons. J. Neurochem. 68, 1949–1959. 10.1046/j.1471-4159.1997.68051949.x [DOI] [PubMed] [Google Scholar]
- Weiner J. L., Zhang L., Carlen P. L. (1994). Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. J. Pharmacol. Exp. Ther. 268, 1388–1395. [PubMed] [Google Scholar]
- Werner D. F., Kumar S., Criswell H. E., Suryanarayanan A., Fetzer J. A., Comerford C. E., et al. (2011). PKCγ is required for ethanol-induced increases in GABAA receptor α4 subunit expression in cultured cerebral cortical neurons. J. Neurochem. 116, 554–563. 10.1111/j.1471-4159.2010.07140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G., Lovinger D. M., Weight F. F. (1990). Ethanol inhibits NMDA-activated current but does not alter GABA-activated current in an isolated adult mammalian neuron. Brain Res. 507, 332–336. 10.1016/0006-8993(90)90292-j [DOI] [PubMed] [Google Scholar]
- Winkelmann A., Maggio N., Eller J., Caliskan G., Semtner M., Haussler U., et al. (2014). Changes in neural network homeostasis trigger neuropsychiatric symptoms. J. Clin. Invest. 124, 696–711. 10.1172/JCI71472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Poelchen W., Proctor W. R. (2005). Differential GABAB receptor modulation of ethanol effects on GABAA synaptic activity in hippocampal CA1 neurons. J. Pharmacol. Exp. Ther. 312, 1082–1089. 10.1124/jpet.104.075663 [DOI] [PubMed] [Google Scholar]
- Xuei X., Flury-Wetherill L., Dick D., Goate A., Tischfield J., Nurnberger J., et al. (2010). GABRR1 and GABRR2, encoding the GABA-A receptor subunits rho1 and rho2, are associated with alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 418–427. 10.1002/ajmg.b.30995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Marszalec W., Yeh J. Z., Narahashi T. (2006). Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABAA receptors. J. Pharmacol. Exp. Ther. 319, 431–438. 10.1124/jpet.106.106260 [DOI] [PubMed] [Google Scholar]
- Yang X., Knapp D. J., Criswell H. E., Breese G. R. (1998). Action of ethanol and zolpidem on γ-aminobutyric acid responses from cerebellar Purkinje neurons: relationship to β-adrenergic receptor input. Alcohol. Clin. Exp. Res. 22, 1655–1661. 10.1097/00000374-199811000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes G. E., Moraga-Cid G., Peoples R. W., Schmalzing G., Aguayo L. G. (2008). A selective G βγ-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc. Natl. Acad. Sci. U S A 105, 20523–20528. 10.1073/pnas.0806257105 [DOI] [PMC free article] [PubMed] [Google Scholar]