Abstract
Angiogenic factor with G-patch and FHA domain 1 (AGGF1) is a novel angiogenic factor that was first described in Klippel-Trenaunay syndrome, a congenital vascular disease associated with capillary and venous malformations. AGGF1, similar to vascular endothelial growth factor (VEGF), has been shown to promote strong angiogenesis in chick embryos in vivo. Blocking AGGF1 expression prevented vessel formation, which suggests AGGF1 is a potent angiogenic factor linked to vascular malformations. So far, AGGF1 expression studies in human vascular lesions have not been performed. Here, we immunohistochemically investigated AGGF1 expression in venous, arteriovenous or capillary malformations, and infantile or congenital hemangioma. We found that AGGF1 was mostly expressed in endothelial cells with plump morphology. Moreover, the majority of mast cells strongly expressed AGGF1. Notwithstanding our incomplete knowledge of the molecular mechanism of AGGF1 in angiogenesis, our results show for the first time that AGGF1 is expressed in plump endothelial cells and mast cells.
Keywords: AGGF1, vascular malformation, vascular tumor, mast cell
I. Introduction
Angiogenesis, a process where new blood vessels sprout from a preexisting vascular network, is essential in integrated biological processes such as wound healing [8] and tumor growth [4, 9], which are tightly controlled by multifunctional molecules called angiogenic factors [17]. Angiogenic factors regulate vascular differentiation and maturation, and are vital for initiation of angiogenesis and maintenance of vascular networks [19]. Several angiogenic factors are derived from mast cells, which are involved in physiological and pathological angiogenesis [14].
Angiogenic factor with G-patch and FHA domain 1 (AGGF1) is a novel angiogenic factor that was first characterized as a susceptibility protein in Klippel-Trenaunay syndrome [2, 18], a congenital vascular disease associated with capillary and venous malformations in the absence of arterial defects [1, 11]. Consistent with this, a recent study by Chen et al. revealed that AGGF1 was involved in the establishment of venous identity in zebrafish embryos [5]. AGGF1 knockdown leads to vein loss but has no effect on arterial development [18]. The well-established role of AGGF1 in angiogenesis brought new insights to vascular lesion research, including vascular malformation and tumor research. Vascular malformations result from aberrant development of vascular elements in capillaries, arteries, veins or lymph vessels [6]. Even though the pathogenesis of vascular malformations is not clear, their formation and progression are closely related to angiogenesis [16]. To date, no studies have investigated AGGF1 expression in human vascular lesions. Here, we immunohistochemically investigated AGGF1 expression in various vascular lesions, such as venous, arteriovenous or capillary malformations, and infantile or congenital hemangioma. We found that AGGF1 was primarily expressed in endothelial cells with plump morphology. Moreover, we found that mast cells strongly expressed AGGF1.
II. Materials and Methods
Patients and cell line
From the database of Osaka University Hospital, Japan, during the period between 2010 and 2014, we identified 119 patients with a diagnosis of vascular malformation or hemangioma (62 venous malformations, 22 arteriovenous malformations, 14 capillary malformations, 7 infantile hemangiomas, 2 congenital hemangiomas, and 12 angiosarcomas). In total, 14 cases of granulation tissue and 7 cases of pyogenic granuloma were also included in our study. Clinicopathological features of included cases are summarized in Table 1. Specimens were fixed in 10% formalin, embedded in paraffin, and stored in a dark room at room temperature in the Department of Pathology at Osaka University Hospital. Then, 4-μm thickness sections were made and stained with the hematoxylin and eosin (H&E) and immunoperoxidase procedure. This study was approved by the Ethical Review Board of the Graduate School of Medicine at Osaka University (No. 13044). The human mastocytoma cell line, HMC-1, was cultured in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Central America Origin, Biosera, Kansas City, USA).
Table 1. .
Clinicopathological features of examined cases
| Number of cases |
Gender (male:female) |
Age (median) |
|
|---|---|---|---|
| Venous malformation | 62 | 23:39 | 1–78 (34) |
| Arteriovenous malformation | 22 | 12:10 | 7–63 (28) |
| Capillary malformation | 14 | 4:10 | 1–76 (30) |
| Infantile hemangioma | 7 | 1:6 | 0–4 (3) |
| Congenital hemangioma | 2 | 2:0 | 6–20 (13) |
| Angiosarcoma | 12 | 8:4 | 31–85 (72) |
| Pyogenic granuloma | 7 | 5:2 | 5–70 (27) |
| Granulation tissue | 14 | 6:8 | 38–66 (55) |
Immunohistochemistry
AGGF1 expression was immunohistochemically examined with polyclonal rabbit anti-AGGF1 antibody (×500, final 1 ng/mL, HPA043500, Sigma, St. Louis, MO, USA), using a Ventana Benchmark GX autostaining system (Roche Diagnostics, Tokyo, Japan) in accordance with the manufacturer’s instructions after extended heat-induced epitope retrieval in cell conditioning 1 (CC1) buffer (Roche Diagnostics). For the negative control, staining was carried out in the absence of primary antibody. In a second negative control, in addition to the absence of primary antibody, sections were incubated with rabbit immunoglobulin (DAKO, Glostrup, Denmark) instead of primary antibody. Moreover, sections were incubated with anti-AGGF1 antibody pre-incubated with AGGF1 antigen (APrEST80107, Atlas Antibodies AB, Stockholm, Sweden) to test the specificity of the anti-AGGF1 antibody. Briefly, 0.5 μg of anti-AGGF1 antibody was incubated with 10 μg of AGGF1 antigen for 10 min, and then immunohistochemistry was carried out as described above. Stained sections were independently evaluated by two pathologists (YH and EM).
Sections were also double stained for AGGF1 and c-Kit. After antigen retrieval with a Pascal pressurized heating chamber (DAKO), samples were simultaneously incubated with rabbit polyclonal anti-AGGF1 (Sigma) and mouse monoclonal anti-c-Kit (clone 1C5, Thermo Fisher Scientific, San Jose, CA, USA) antibodies, and then incubated with Alexa Fluor 488-conjugated anti-mouse IgG (Thermo Fisher Scientific) and Alexa Fluor 568-conjugated anti-rabbit IgG (Thermo Fisher Scientific). Images were taken with a Keyence Biozero microscope (Keyence Germany GmbH, Neu-Isenburg, Germany).
Immunocytochemistry
AGGF1 expression was immunocytochemically examined in HMC-1 cells. Cells were cytospun and then fixed with 10% formalin. After treatment with peroxidase blocking solution (DAKO), cells were incubated with anti-AGGF1 antibody. Then, cells were treated with ChemMate EnVision kit (DAKO) and DAB (DAKO) was used as a chromogen.
Immunoblotting
HMC-1 cells were lysed in buffer containing 10 mM HEPES, 10 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.1% Nonidet P-40 and 10% protease inhibitor cocktail (Sigma). Human vascular endothelial cell (HUVEC) lysate was kindly provided by Dr. S Kobayashi (Osaka University). Electrophoresis was performed with 10% sodium dodecyl sulfate polyacrylamide gels (ATTO, Tokyo, Japan), and proteins were transferred to polyvinylidene fluoride membranes (Merck Millipore, Billerica, MA, USA). Membranes were incubated with anti-AGGF1 (1:1000 dilution, Sigma) and anti-α-actin (1:1000 dilution, Sigma) primary antibodies. HRP-conjugated anti-rabbit IgG (H+L chain) (1:2000 dilution, MBL, Nagoya, Japan) was used as the secondary antibody. Data were analyzed with Image LabTM software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The Chi-square test was used to analyze the correlation of AGGF1 expression in plump endothelial cells. p-values <0.01 were considered to be statistically significant.
III. Results
AGGF1 expression in vascular malformations
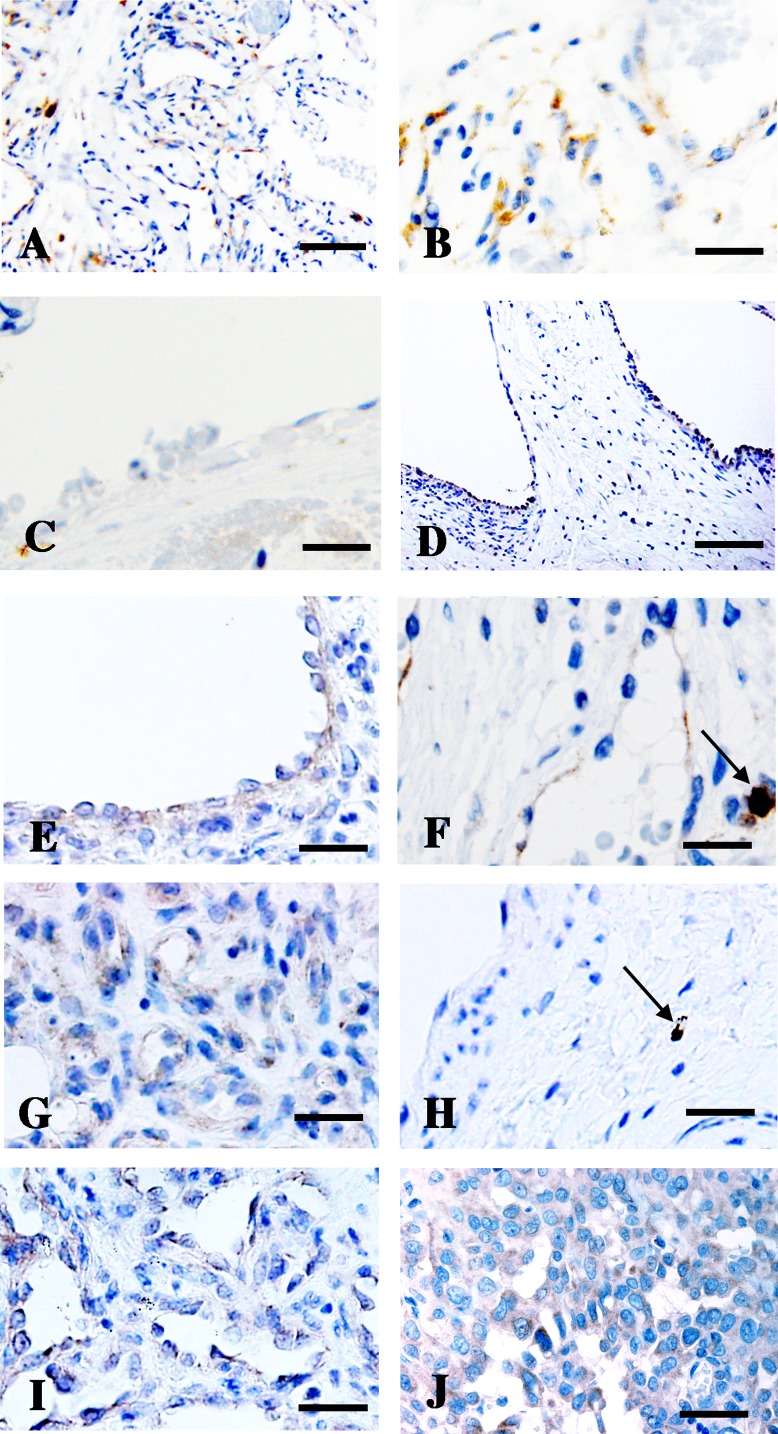
AGGF1 expression was immunohistochemically examined in vascular malformations. In venous malformations, AGGF1-positive signals were detected in a limited portion (Fig. 1A, upper half). Endothelial cells with plump morphology expressed AGGF1 (Fig. 1B), but endothelial cells with flat morphology did not (Fig. 1C). Among 62 cases of venous malformation, 45 contained both plump and flat endothelial cells, 13 contained only plump cells, and 4 contained only flat cells. Among 58 cases of venous malformation with plump endothelial cells, 50 showed AGGF1 expression in plump cells (Table 2). In total, 34 out of 49 samples did not show AGGF1 expression in flat endothelial cells (Table 2). Therefore, AGGF1 was preferentially expressed in plump endothelial cells but not in flat ones.
Fig. 1. .
Angiogenic factor with G-patch and FHA domain 1 (AGGF1) expression in vascular malformations, hemangiomas, and angiosarcomas. AGGF1 expression was patchy in venous malformation (A). AGGF1 was detected in plump endothelial cells (B) but not flat ones (C). AGGF1 expression was detected in plump endothelial cells from arteriovenous malformation (D and E), capillary malformation (F), infantile hemangioma (proliferating phase G and involuting phase H), congenital hemangioma (I), and angiosarcoma (J). Arrows in F and H indicate cells strongly expressing AGGF1 in connective tissues (Bar=200 nm in A, D; 100 nm in B, C; and 50 nm in E–J).
Table 2. .
Number of cases with plump and flat endothelial cells and the status of AGGF1 expression
| Number of cases | p-value | |||||
|---|---|---|---|---|---|---|
| Plump endothelial cells | Flat endothelial cells | |||||
| AGGF1 (+) | AGGF1 (−) | AGGF1 (+) | AGGF1 (−) | |||
| Venous malformation | 50 | 8 | 15 | 34 | <0.01 | |
| Arteriovenous malformation | 21 | 1 | 2 | 14 | <0.01 | |
| Capillary malformation | 14 | 0 | 2 | 8 | <0.01 | |
AGGF1 expression was examined in other vascular malformations. Of 22 cases of arteriovenous malformation, 21 showed AGGF1 expression in plump endothelial cells (Fig. 1D and 1E, Table 2). Flat endothelial cells were present in 16 out of 22 arteriovenous malformation cases; of these, only 2 showed AGGF1 expression. In capillary malformation, AGGF1 expression in plump endothelial cells was detected in all 14 cases (Fig. 1F, Table 2). In total, 10 out of 14 capillary malformation cases contained flat endothelial cells, and of these, only 2 showed AGGF1 expression. These findings indicated that preferential expression of AGGF1 in plump endothelial cells was applicable to arteriovenous and capillary malformation as well as venous malformation.
AGGF1 expression in hemangiomas
To examine whether the preferential expression of AGGF1 in plump endothelial cells of vascular malformations was applicable to vascular tumors, AGGF1 expression was immunohistochemically investigated in 7 cases of infantile hemangioma (six at involuting phase and one at proliferating phase), 2 cases of congenital hemangioma, and 12 cases of angiosarcoma. In all examined cases, plump endothelial cells expressed AGGF1, whereas flat cells did not (Fig. 1G–1I).
AGGF1 expression in other vessels
Since AGGF1 was expressed in plump endothelial cells of vascular malformations and hemangiomas, we next examined other vessels with plump morphology, such as those found in pyogenic granulomas and granulation tissue. In pyogenic granulomas, plump endothelial cells expressed AGGF1 (Fig. 2A). Granulation tissue consists of endothelial cells and many inflammatory cells such as macrophages and lymphocytes. AGGF1 was expressed in endothelial cells but not in inflammatory cells (Fig. 2B). AGGF1 expression was also examined in lymphatic endothelial cells but was not detected (data not shown).
Fig. 2. .
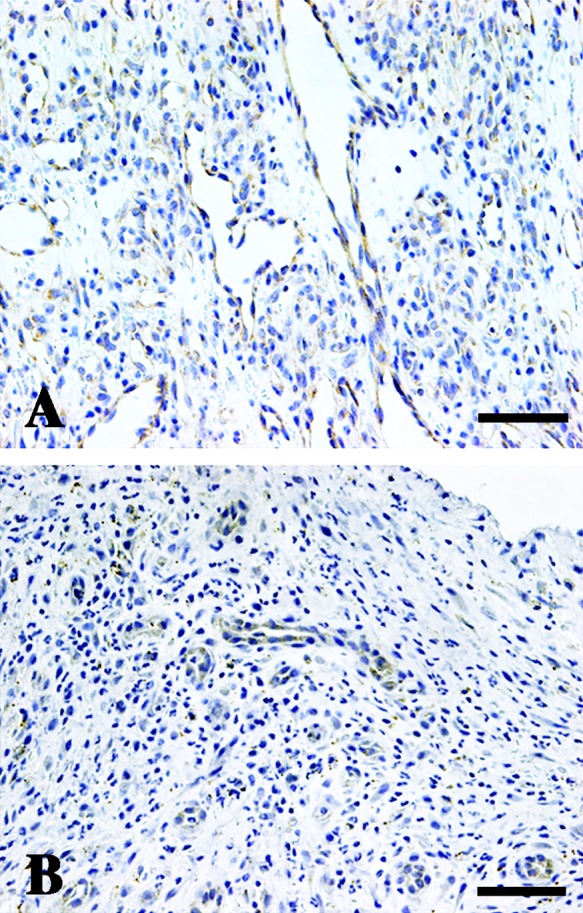
AGGF1 expression in pyogenic granuloma and granulation tissue. Plump endothelial cells in pyogenic granuloma expressed AGGF1 (A). In granulation tissue, plump endothelial cells, but not inflammatory cells, expressed AGGF1 (B) (Bar=200 nm).
AGGF1 expression in mast cells
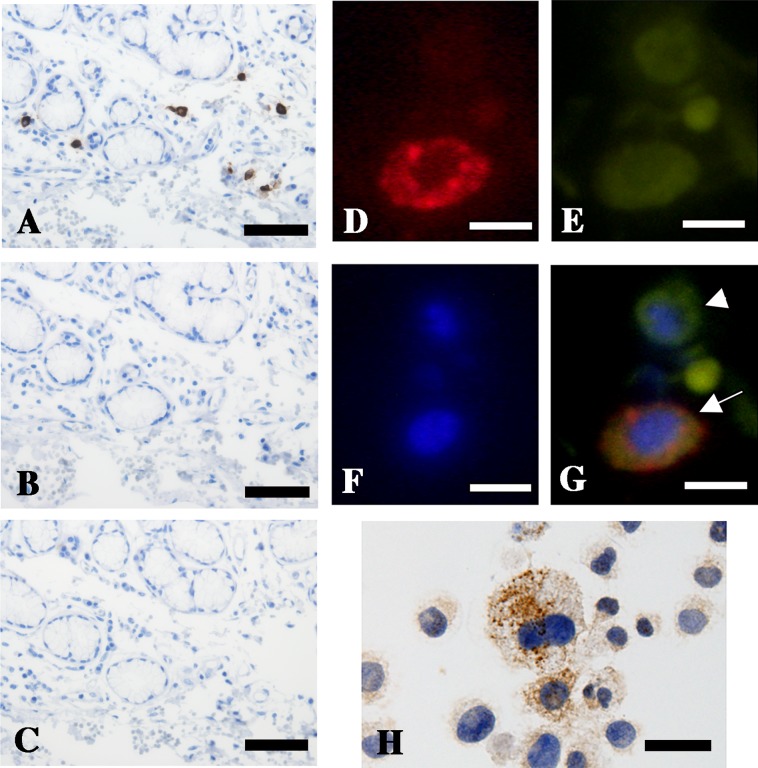
In addition to plump endothelial cells, several cells in connective tissue strongly expressed AGGF1 (arrows in Fig. 1F and 1H). Signals detected in Fig. 3A were abolished by the absorption of anti-AGGF1 antibody with AGGF1 antigen (Fig. 3B). The absence of primary AGGF1 antibody signals (Fig. 3C) indicated that the signals in Fig. 3A were specific for AGGF1. Double staining of AGGF1 and c-Kit revealed that AGGF1-expressing cells in connective tissues also expressed c-Kit (Fig. 3D–3G). This indicated that AGGF1-expressing cells in connective tissue were mast cells. Although most mast cells expressed AGGF1, some did not (Fig. 3G; arrow indicates AGGF1-positive mast cells and arrowhead indicates AGGF1-negative mast cells). The proportion of AGGF1-positive mast cells did not correlate to that of AGGF1-positive plump endothelial cells.
Fig. 3. .
AGGF1 expression in mast cells. A capillary malformation specimen was immunohistochemically stained. Strong signals were detected with rabbit anti-AGGF1 antibody (A). In contrast, no signals were detected when anti-AGGF1 antibody was absorbed with AGGF1 antigen (B) and when no primary antibody was used (C). Double staining of AGGF1 and c-Kit (D–G). AGGF1 was stained in red (D) and c-Kit in green (E). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, Sigma) (F). (G) Merged image. Arrow indicates mast cells expressing AGGF1, whereas arrowhead indicates mast cells with almost undetectable AGGF1. (H) Immunocytochemistry of HMC-1 cells to show AGGF1 expression. Most HMC-1 cells contained AGGF1-positive signals in their granules. Part of HMC-1 cells did not express AGGF1 (Bar=100 nm in A, B, C; 10 nm in D–H).
Next, AGGF1 expression was examined in HMC-1 cells. Strong granular staining was observed, indicating AGGF1 was contained in mast cell granules (Fig. 3H). Consistent with histology results, part of HMC-1 cells had almost undetectable AGGF1 expression. We further examined AGGF1 expression in HMC-1 cells with immunoblotting (Fig. 4). AGGF1 expression levels in HMC-1 cells were comparable to those previously reported in HUVEC [18].
Fig. 4. .
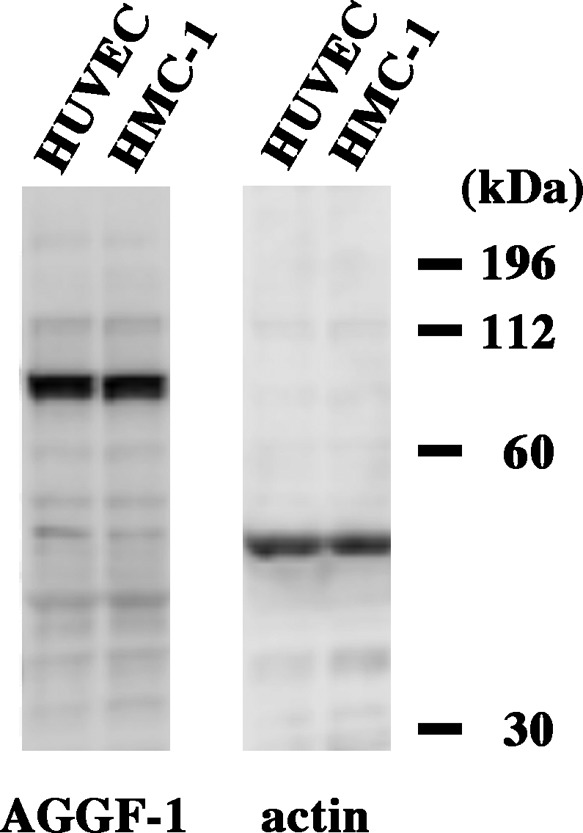
Immunoblotting for AGGF1. Chemiluminescent blot band for AGGF1 was detected with HUVEC and HMC-1 cells. Actin was detected by re-blotting with the same sample set.
IV. Discussion
In complex multicellular organisms, such as humans, angiogenesis leads to the development of a highly developed vascular system to precisely control the supply of oxygen to cells. Endothelial cell precursors differentiate to form a basic fetal network of veins, which subsequently remodel into a more mature network of large and small vessels [10]. During this process, angiogenesis becomes more complex and is controlled by local changes in angiogenic factors. When remodeling fails to occur normally, vessels may become deformed, resulting in vascular defects [3]. Some molecules, such as VEGF and basic fibroblast growth factor, have been implicated in vascular defects; however, most vascular malformations remain unexplained in terms of the genes and molecules involved. AGGF1 is a new angiogenic factor that was first described in Klippel-Trenaunay syndrome [2, 18], a congenital vascular disease associated with capillary and venous malformations [1, 11]. AGGF1, similar to VEGF, was shown to promote strong angiogenesis in chick embryos in vivo. Furthermore, blocking AGGF1 expression prevented vessel formation [18]. These results suggest that AGGF1 is a potent angiogenic factor linked to vascular malformations. In the present study, we found that AGGF1 was expressed in vascular lesions, including vascular malformations, infantile or congenital hemangiomas, pyogenic granulomas, and granulation tissue. These findings were consistent with AGGF1 being a potent angiogenic factor. However, AGGF1 did not seem to be a specific marker for vascular malformation, since vascular lesions other than malformations also expressed AGGF1.
AGGF1 was detected in endothelial cells with plump but not flat morphology. Endothelial cells become plump when activated; this indicates that AGGF1 expression is up-regulated during endothelial cell activation. This is consistent with a previous in vitro study that found AGGF1 expression in HUVEC was up-regulated by lipopolysaccharide, interleukin-1 and tumor necrosis factor-alpha [13].
In addition to plump endothelial cells, for the first time, we found that mast cells, which are often found near blood vessels [14], also express AGGF1. Many multifunctional cytokines and growth factors are produced in mast cells. These include VEGF [7] and bFGF [15], which are known to enhance angiogenic phenotypes. A previous report indicated that these factors could simultaneously recruit mast cells to these sites [12]. The local accumulation of mast cells is believed to facilitate new vessel formation through complex cell-to-cell interactions. We found that part of mast cells did not express AGGF1. Therefore, further studies will be needed to clarify the role of AGGF1 in mast cells.
Notwithstanding our incomplete knowledge of the molecular mechanisms of AGGF1 in angiogenesis, our results for the first time show that AGGF1 is expressed in plump endothelial cells and mast cells. Patients with vascular system defects face serious medical and social problems and we know little about the cause of these anomalies. Future studies on the function of AGGF1 may lead to the development of therapeutic approaches to modulate angiogenesis. The exact molecular mechanisms responsible for AGGF1 expression in various vascular malformations are still a matter of debate; therefore, further studies are warranted.
V. Acknowledgments
The authors thank Ms. Megumi Nihei-Sugano, Ms. Etsuko Maeno, Ms. Mitsuyo Tone, Ms. Yoko Tsuruta, and Ms. Takako Sawamura for their technical assistance, and Dr. S. Kobayashi for providing cell lysate of HUVEC.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (#T264604700 and #T15K083630).
VI. References
- 1.Berry S. A., Peterson C., Mize W., Bloom K., Zachary C., Blasco P. and Hunter D. (1998) Klippel-Trenaunay syndrome. Am. J. Med. Genet. 79; 319–326. [PubMed] [Google Scholar]
- 2.Callebaut I. and Mornon J. P. (2005) OCRE: a novel domain made of imperfect, aromatic-rich octamer repeats. Bioinformatics 21; 699–702. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9; 653–660. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. and Jain R. K. (2000) Angiogenesis in cancer and other diseases. Nature 407; 249–257. [DOI] [PubMed] [Google Scholar]
- 5.Chen D., Li L., Tu X., Yin Z. and Wang Q. (2013) Functional characterization of Klippel–Trenaunay syndrome gene AGGF1 identifies a novel angiogenic signaling pathway for specification of vein differentiation and angiogenesis during embryogenesis. Hum. Mol. Genet 22; 963–976. [DOI] [PubMed] [Google Scholar]
- 6.Chim H., Drolet B., Duffy K., Koshima I. and Gosain A. K. (2010) Vascular anomalies and lymphedema. Plast. Reconstr. Surg. 126; 55e–69e. [DOI] [PubMed] [Google Scholar]
- 7.Detoraki A., Staiano R. I., Granata F., Giannattasio G., Prevete N., de Paulis A., Ribatti D., Genovese A., Triggiani M. and Marone G. (2009) Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 123; 1142–1149. [DOI] [PubMed] [Google Scholar]
- 8.Findaly J. K. (1986) Angiogenesis in reproductive tissues. J. Endocrinol. 111; 357–366. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J., Long D. K. and Becker F. F. (1963) Growth and metastasis of tumor in organ culture. Cancer 16; 453–467. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. and D’Amore P. A. (1996) Blood vessel formation: what is its molecular basis? Cell 87; 1153–1155. [DOI] [PubMed] [Google Scholar]
- 11.George G., Kihiczak M. D. and Jon G. (2006) Klippel-Trenaunay syndrome: A multisystem disorder possibly resulting from a pathogenic gene for vascular and tissue overgrowth. Int. J. Dermatol. 45; 883–890. [DOI] [PubMed] [Google Scholar]
- 12.Gruber B. L., Marchese M. J. and Kew R. (1995) Angiogenic factors stimulate mast-cell migration. Blood 86; 2488–2493. [PubMed] [Google Scholar]
- 13.Hu F. Y., Wu C., Li Y., Xu K., Wang W. J., Cao H. and Tian X. L. (2013) AGGF1 is a novel anti-inflammatory factor associated with TNF-α-induced endothelial activation. Cell. Signal 25; 1645–1653. [DOI] [PubMed] [Google Scholar]
- 14.Meininger C. J. and Zetter B. R. (1992) Mast cells and angiogenesis. Semin. Cancer Biol. 3; 73–79. [PubMed] [Google Scholar]
- 15.Qu Z., Liebler J. M., Powers M. R., Galey T., Ahmadi P., Huang X. N., Ansel J. C., Butterfield J. H., Planck S. R. and Rosenbaum J. T. (1995) Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am. J. Pathol. 147; 564. [PMC free article] [PubMed] [Google Scholar]
- 16.Redondo P. (2007) Vascular malformations (I). Concept, classification, pathogenesis and clinical features. Actas Dermosifiliogr. 98; 141–158. [PubMed] [Google Scholar]
- 17.Risau W. (1997) Mechanisms of angiogenesis. Nature 386; 671–674. [DOI] [PubMed] [Google Scholar]
- 18.Tian X. L., Kadaba R., You S. A., Liu M., Timur A. A., Yang L., Chen Q., Szafranski P., Rao S., Wu L., Housman D. E., DiCorleto P. E., Driscoll D. J., Borrow J. and Wang Q. (2004) Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature 427; 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J. and Holash J. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407; 242–248. [DOI] [PubMed] [Google Scholar]