Abstract
Essential thrombocythemia (ET) is currently diagnosed either by the British Committee of Standards in Haematology (BCSH) criteria that are predominantly based on exclusion and not necessarily on bone marrow (BM) morphology, or the World Health Organization (WHO) criteria that require BM examination as essential criterion. We studied the morphological and clinical features in patients diagnosed according either to the BCSH (n=238) or the WHO guidelines (n=232). The BCSH-defined ET cohort was re-evaluated by applying the WHO classification. At presentation, patients of the BCSH group showed significantly higher values of serum lactate dehydrogenase and had palpable splenomegaly more frequently. Following the WHO criteria, the re-evaluation of the BCSH-diagnosed ET cohort displayed a heterogeneous population with 141 (59.2%) ET, 77 (32.4%) prefibrotic primary myelofibrosis (prePMF), 16 (6.7%) polycythemia vera and 4 (1.7%) primary myelofibrosis. Contrasting WHO-confirmed ET, the BCSH cohort revealed a significant worsening of fibrosis-free survival and prognosis. As demonstrated by the clinical data and different outcomes between WHO-diagnosed ET and prePMF, these adverse features were generated by the inadvertent inclusion of prePMF to the BCSH group. Taken together, the diagnosis of ET without a scrutinized examination of BM biopsy specimens will generate a heterogeneous cohort of patients impairing an appropriate clinical management.
Introduction
Accurate diagnosis of essential thrombocythemia (ET) is normally accomplished by applying either the recently updated British Committee for Standards in Haematology (BCSH) guidelines1, 2 or the World Health Organization (WHO) criteria.3 This, however, continues to be a controversial and challenging issue. The WHO classification places considerably more weight on bone marrow (BM) morphology as a major diagnostic criterion,3 which contrasts to the BCSH guidelines1, 2 that are mainly focused on an exclusion of the other subtypes of myeloproliferative neoplasm (MPN) or myelodysplastic syndromes (MDS). Consequently, the first set of BCSH diagnostic criteria (A1–A3) allows ET diagnosis without BM biopsy examination by the following criteria: A1-sustained platelet count >450 × 109/l; A2-presence of an acquired pathogenetic mutation; A3-no other myeloid malignancy, especially polycythemia vera (PV), primary myelofibrosis (PMF), chronic myeloid leukemia or MDS.1 This definition represents a major difference from the WHO classification.3 However, performance of a BM biopsy is included in the second set of the BCSH criteria (A1+A3−A5). In addition to the threshold value of the platelet count (A1) and exclusion of another myeloid malignancy (A3), these criteria require no reactive cause for thrombocytosis and normal iron stores (A4) and also BM morphology (A5) as diagnostic feature (‘BM aspirate and trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominantly large megakaryocytes with hyperlobulated nuclei and abundant cytoplasm. Reticulin is generally not increased (grades 0–2/4 or grade 0/3)').1, 2 Performance of a BM biopsy is recommended in cases where there are atypical features, if a change in management is planned during the course of treatment (such as change of cytoreductive therapy), or if transformation into myelofibrosis is suspected.1 In this context, the challenging differentiation of ET from major subtypes of MPN with presenting thrombocytosis is needed, and it is clinically important to be defined correctly already at diagnosis.4, 5, 6, 7 This concerns particularly PV that is excluded according to the BCSH by revealing a normal hematocrit (Hct) in an iron-replete patient8, 9 and PMF. Following the BCSH criteria,1, 2 PMF is defined as showing a significant BM fibrosis and palpable splenomegaly, blood film abnormalities (circulating progenitors and tear-drop cells) or unexplained anemia consistent with overt myelofibrosis with myeloid metaplasia (MMM).10, 11 Conversely, the prodromal stages, that is, prefibrotic PMF (prePMF), which often present with conspicuous thrombocytosis12, 13, 14, 15 but fail to meet the diagnostic signs and symptoms characterizing MMM,10 have to be addressed in context with MPN. Further, clinically, it is well known that a small fraction of PV patients may present initially with hemoglobin (Hb) and Hct levels that do not fulfill the 2008 threshold criteria,16, 17, 18 but a platelet count that is within BCSH- and WHO-defined ET criteria,1, 2 thus mimicking phenotypically ET at onset.13, 19, 20
The aim of this study was to investigate the clinical presentation and prognostic relevance of BM morphology for ET diagnosis by comparing those criteria as defined by the WHO classification3 with the first set (A1–A3) of the original and 2014 updated BCSH criteria that do not include BM evaluation.1, 2
Subjects and methods
A clinico-pathological database currently including 626 patients who were diagnosed and treated for MPN was created by clinicians and hematopathologists in the Departments of Hematology and Clinical Pathology at the Medical University of Vienna, Austria. Currently, the associated institutions are centers located in Vienna, Graz, Wels and Linz. Eligibility criteria for entry into this database include diagnosis between 1982 and 2015 with suspected MPN, well-documented clinical follow-up and mutation status (Table 1). Mutation analysis included allele-specific polymerase chain reaction techniques to screen for Janus kinase 2 (JAK2), calreticulin exon 9 (CALR) and myeloproliferative leukemia virus oncogene (MPL) mutations. A further essential aspect for entry was the availability of representative, initial, treatment-naive BM biopsies (hematoxylin-eosin staining and silver impregnation after Gomori). Iron stores were assessed either by clinical parameters (serum ferritin, mean corpuscular volume of red blood cells) and/or special staining (Prussian blue) of smears. The latter were also used in a very few cases with borderline to slight anemia to exclude MDS with ring sideroblasts, that is, refractory anemia with ring sideroblasts associated with marked thrombocytosis (RARS-T). In cooperation with the local hematopathologists, BM biopsies were centrally re-reviewed under a multi-headed microscope by three of the authors (JT, LM, C B-Sch) who were blinded to initial data (except for age and gender) at entry and outcome. Final diagnosis according to the 2008 WHO criteria was made based on the histopathology review and clinical data.
Table 1. Distribution of diagnoses in the Austrian database of 626 WHO-classified patients with MPN.
| WHO-classified cohort (n=626) | |
|---|---|
| ET | 259 (41.4%) |
| prePMF | 225 (36.0%) |
| PV | 116 (18.5%) |
| PMF | 22 (3.5%) |
| MPN-U | 4 (0.6%) |
Abbreviations: ET, essential thrombocythemia; MPN-U, myeloproliferative neoplasm-unclassifiable; PMF, advanced PMF; prePMF, prefibrotic primary myelofibrosis; PV, polycythemia vera.
For the purpose of the present study, we selected all patients with a sustained platelet count ⩾450 × 109/l, no evidence for a reactive cause for thrombocytosis and normal iron stores and BCR-ABL1 negativity. We then applied the WHO-defined ET criteria including BM biopsy evaluation as major diagnostic criterion,3 and the first set of the 2014 updated BCSH criteria for ET (A1–A3) that require no BM biopsy examination to these patients.1, 2 The WHO criteria consist of a platelet count ⩾450 × 109/l, BM biopsy examination, the exclusion of other myeloid neoplasm, and the presence of a clonal marker or the exclusion of reactive thrombocytosis. The first set of the BCSH guidelines (A1–A3) allow diagnosis of ET with the presence of a platelet count ⩾450 × 109/l, the presence of an acquired pathogenic mutation (for example, JAK2, CALR or MPL) and no other MPN or MDS.
These two cohorts were compared regarding their presenting clinico-pathological findings, prognosis and adverse events during follow-up.
The diagnosis of post-ET myelofibrosis was made using the IWG-MRT criteria21 and corresponding clinical and morphological features. These included worsening of anemia (at least a decrease by >2 g/dl from baseline Hb level), increase in splenomegaly either of newly palpable splenomegaly or >5 cm from baseline, overt leuko-erythroblastosis or anisopoikilocytosis with tear-drop erythrocytes, and an overt grade 2/3 reticulin/collagen BM fibrosis in sequential BM biopsies22 consistent with manifest MMM.11 Leukemic transformation met criteria for acute myeloid leukemia according to the WHO definition.23
Cytoreductive drugs included predominantly hydroxyurea, anagrelide and interferon-alpha or, very rarely, busulphan, pipobroman, P32 or other cytoreductive agents (for details, see Table 2). Many patients received more than one drug during treatment; however, only minor differences could be ascertained between WHO- versus BCSH-confirmed ET. Antithrombotic therapy with low-dose aspirin was applied in 160 patients of the WHO-confirmed ET and 189 patients of the BCSH-defined ET cohort.
Table 2. Clinical characteristics, molecular analysis and constitutional symptoms of patients with ET at presentation and treatment according to applied diagnostic criteria.
| BCSH-defined ET (criteria A1-A3)1, 2 | WHO-defined ET criteria3 | P-value | |
|---|---|---|---|
| General characteristics | |||
| n | 238 | 232 | |
| Age at diagnosis (years) | 61.3 (18.8–88.8) | 57.2 (17.5–88.8) | 0.073 |
| Sex male/female | 86/123 | 93/139 | 0.750 |
| Clinical characteristicsa | |||
| Platelets (× 109/l) | 769 (452–2530) | 754 (450–2490) | 0.539 |
| Hemoglobin (g/dl) | 14.2 (8.6–17.3) | 14.4 (8.6–17.3) | 0.826 |
| Hematocrit (%) | 42.9 (42.9–52.0) | 42.7 (29.9–52.6) | 0.630 |
| WBC (× 109/l) | 9.4 (2.21–31.32) | 8.82 (2.21–22.3) | 0.057 |
| LDH (U/l) | 221 (118–763) | 207 (104–763) | <0.001 |
| Palpable splenomegaly (218/238)b | 16.4% (39) | 11.9% (26) | 0.183 |
| Fibrosis grading ⩾1 | 8.4% (20) | 0.0% (0) | <0.001 |
| Molecular characteristics | |||
| Pathogenetic mutation present (169/238)b | 100% (238) | 72.8% (169) | — |
| JAK2 V617F (220/238)b | 72.7% (173) | 80.5% (136) | 0.016 |
| CALR (141/181)b | 32.0% (58) | 16.0% (27) | 0.011 |
| MPL (53/75)b | 9.3% (7) | 3.6% (6) | 0.771 |
| Symptoms at diagnosis | |||
| Constitutional symptoms (169/200)b | 16.0% (32) | 14.8% (25) | 0.774 |
| Weight loss | 4.5% (9) | 4.1% (7) | 1.000 |
| Night sweats | 8.5% (17) | 8.3% (14) | 1.000 |
| Fatigue | 5.0% (10) | 5.9% (10) | 0.818 |
| Pruritus (175/202)b | 2.0% (4) | 2.3% (4) | 1.000 |
| Cytoreductive Therapy (164/191)b | |||
| Hydroxurea | 42.9% (82) | 42.1% (69) | 0.494 |
| Interferon-alpha | 34.6% (66) | 30.5% (50) | 0.429 |
| Anagrelide | 30.4% (58) | 34.1% (56) | 0.494 |
| JAK1/2-Inhibitor | 4.7% (9) | 3.0% (5) | 0.586 |
| Busulfan | 2.6% (5) | 2.4% (4) | 1.000 |
| Othersc | 4.2% (8) | 0.6% (1) | 0.042 |
| Antithrombotic therapy with low dose aspirin (160/189)b | 90.5% (171) | 88.8% (142) | 0.602 |
Abbreviations: BCSH, British Committee of Standards in Haematology; CALR, calreticulin exon 9 mutations; ET, essential thrombocythemia; JAK2, Janus kinase 2; LDH, serum lactate dehydrogenase; MPL, myeloproliferative leukemia oncogene; WBC, white blood cell count.
Median, range.
Number evaluable in each cohort.
Pipobroman, P32 and other cytoreductive agents.
Statistical analysis regarded disease-relevant parameters considered at diagnosis. Differences in the distribution of continuous variables between categories were analyzed by the Mann–Whitney U-test. Patient groups with nominal variables were compared by Fisher's exact t-test. Survival curves were calculated using the Kaplan–Meier method, differences in survival were assessed using the log-rank test. Two-sided P-values <0.05 were considered significant.
The study protocol was approved by institutional research ethics committee of the Medical University of Vienna, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Results
From our Austrian MPN database, which at present includes 626 patients, we recruited 232 (37.1%) cases that according to the WHO criteria were diagnosed as ET and fulfilled the other eligibility criteria, whereas 238 (38.0%) patients met the BCSH criteria A1–A3. Patients that had a follow-up of <1 year (n=27) were not included. At diagnosis, both ET cohorts contained a higher proportion of female patients (Table 2). Patients in the BCSH group displayed higher serum lactate dehydrogenase levels (221 vs 207 U/l, P<0.001) and had palpable splenomegaly more frequently (16.4% vs 11.9%, P=0.183). Differences in the mutation status or therapeutic modalities were not present. The WHO-diagnosed ET cohort contained 18 (7.8%) triple-negative patients. Cytogenetic BCR-ABL1 testing as recommended by the WHO and BCSH was negative in all cases.
The re-classification of 238 patients with BCSH-confirmed ET diagnosis according to the WHO criteria revealed a heterogeneous population, including 77 (32.4%) prePMF and a small cohort of 16 (6.7%) PV cases (Table 3). BM biopsy specimens in the prePMF group showed reticulin fiber grade 1 on a three-graded scoring system in 20 (8.4%) cases, which was not found in the WHO-ET group. In the 16 PV cases, iron deficiency was excluded by showing normal levels of serum ferritin and a normal mean corpuscular volume of the red blood cells. In a few suspicious cases (6/16), an increased red cell mass was found in four confirming the diagnosis, and in 11 of the 16 PV patients the need for phlebotomy was documented in the follow-up. The four PMF patients displayed only fiber grade 2 in their BM associated with thrombocytosis, but no anemia or splenomegaly or blood film abnormalities and therefore were not compatible with MMM or the BCSH criteria for overt PMF.1, 2 Finally, the discrepancy in the number of WHO-confirmed ET cases in the BCSH versus WHO group (n=91) is due to the WHO criteria not requiring the presence of an acquired pathogenic mutation (n=28) and further included the group of triple-negative cases (n=18) and unknown mutation status (n=45) as well.
Table 3. Differentiation and comparison of the BSCH-defined cohort of ET patients 1, 2 by applying the diagnostic criteria of the WHO classification 3 .
| BCSH-defined ET (n=238) | WHO-defined ET (n=232) | |
|---|---|---|
| ET | 141 (59.2%) | 232 (100%) |
| prePMF | 77 (32.4%) | 0 |
| PV | 16 (6.7%) | 0 |
| PMF | 4 (1.7%) | 0 |
Abbreviations: BCSH, British Committee of Standards in Haematology; ET, essential thrombocythemia; PMF, advanced PMF; prePMF, prefibrotic primary myelofibrosis; PV, polycythemia vera.
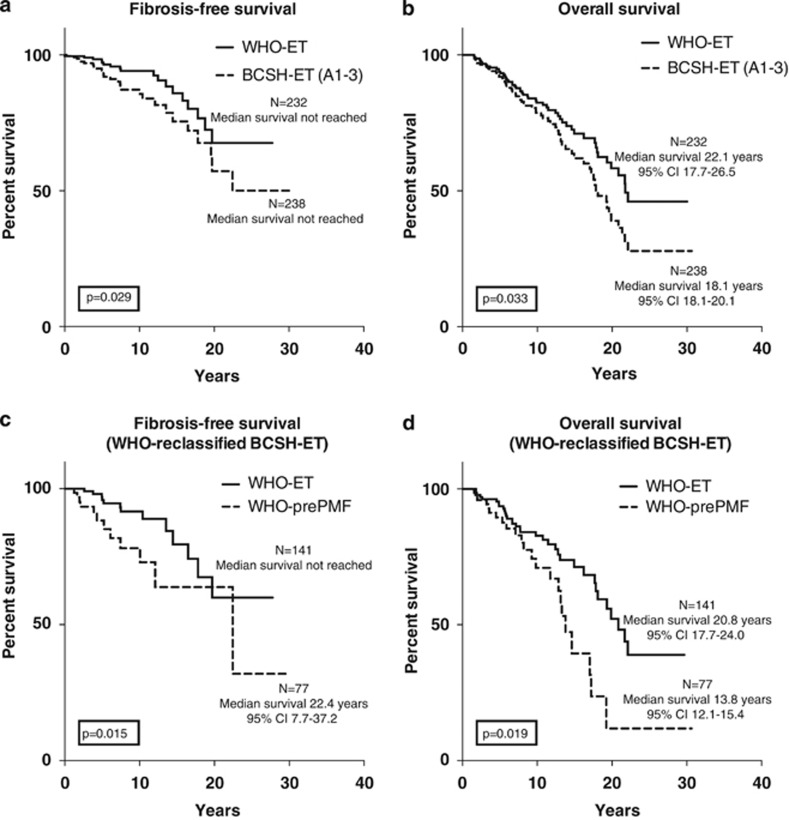
During follow-up of 3290 patient years (median 8.18 years per patient), fibrosis-free (Figure 1a) and overall survival (Figure 1b) were significantly more favorable (P=0.029/P=0.033) in the WHO-defined ET cohort. This finding is likely linked with the inclusion of many prePMF cases in the BCSH-confirmed ET group, which implies a worsening of hematological parameters and outcome. This can be demonstrated by comparison of the WHO-diagnosed ET with the prePMF group (Table 4), particularly concerning fibrosis-free survival (Figure 1c, P=0.015) and overall survival (Figure 1d, P=0.019). Cumulative risk rates for death were 6.0% vs 8.0%, 17.5% vs 21.9% and 27.3% vs 36.5% after 5, 10 and 15 years, respectively. The cumulative rates for post-ET MF was 2.1% vs 5.3%, 6.4% vs 13.3% and 13.4% vs 23.6% at years 5, 10 and 15 years, respectively. No acute leukemia was seen in the first 5 years of follow-up, after 10 years the rates were 0.9% vs 2.1% and 8.0% vs 4.4% after 15 years.
Figure 1.
(a–d) Kaplan–Meier estimates of fibrosis-free survival and overall survival with significant differences in 232 WHO-confirmed versus 238 BCSH-defined ET patients (Figures a and b). Separate analysis of the WHO-diagnosed prePMF fraction of 77 patients included in the BCSH-classified ET cohort of 238 patients reveals a significant worsening of fibrosis-free and overall survival (Figures c and d). Abbreviation: CI, confidence interval.
Table 4. Clinical characteristics of patients with WHO-defined ET compared with WHO-defined prefibrotic primary myelofibrosis (prePMF) at presentation as derived from the BCSH-confirmed ET cohort.
| WHO-defined ET3 | WHO-defined prePMF3 | P-value | |
|---|---|---|---|
| General characteristics | |||
| n | 141 | 77 | |
| Age at diagnosis (years) | 58.9 (18.8–88.8) | 64.6 (23.2–88.1) | 0.083 |
| Sex; male/female | 58/83 | 27/50 | 0.486 |
| Clinical characteristicsa | |||
| Platelets (× 109/l) | 725 (452–1836) | 840 (457–2530) | 0.012 |
| Hemoglobin (g/dl) | 14.5 (11.5–17.3) | 13.9 (8.6–16.6) | 0.007 |
| Hematocrit (%) | 43.0 (33.2–52.0) | 41.6 (27.5–48.9) | 0.036 |
| WBC (× 109/l) | 8.8 (2.2–21.1) | 10.3 (4.0–31.3) | 0.004 |
| LDH (U/l) | 209 (110–763) | 270 (136–598) | <0.001 |
| Palpable splenomegaly (141/77)b | 9.9% (14) | 23.4% (18) | 0.009 |
| Fibrosis grading ⩾1 | 0.0% (0) | 20.8% (16) | <0.001 |
| Molecular characteristics | |||
| Pathogenetic mutation present (141/77)b | 100% (141) | 100% (77) | — |
| JAK2 V617F (141/77)b | 70.9% (100) | 61.0% (47) | 0.011 |
| CALR (99/65)b | 27.3% (27) | 41.5% (27) | 0.064 |
| MPL (33/37)b | 12.1% (4) | 8.1% (3) | 0.699 |
| Symptoms at diagnosis | |||
| Constitutional symptoms (111/71)b | 15.8% (16) | 20.3% (10) | 1.000 |
| Weight loss | 3.6% (4) | 7.0% (5) | 0.315 |
| Night sweats | 8.1% (9) | 4.2% (3) | 0.372 |
| Fatigue | 5.4% (6) | 5.6% (4) | 1.000 |
| Pruritus (111/71)b | 1.8% (2) | 1.4% (1) | 1.000 |
| Cytoreductive therapy (108/63)b | |||
| Hydroxyurea | 45.4% (49) | 38.1% (24) | 0.423 |
| Interferon-alpha | 31.5% (34) | 34.9% (22) | 0.736 |
| Anagrelide | 33.3% (36) | 28.6% (18) | 0.610 |
| JAK1/2-inhibitor | 4.6% (5) | 6.3% (4) | 0.727 |
| Busulfan | 1.9% (2) | 3.2% (2) | 0.626 |
| Othersc | 0.9% (1) | 6.3% (4) | 0.062 |
| Antithrombotic therapy with low-dose aspirin (106/63)b | 89.6% (95) | 88.9% (56) | 1.000 |
Abbreviations: BCSH, British Committee of Standards in Haematology; CALR, calreticulin exon 9 mutations; ET, essential thrombocythemia; JAK2, Janus kinase 2; LDH, serum lactate dehydrogenase; MPL, myeloproliferative leukemia oncogene; WBC, white blood cell count.
Median, range.
Number evaluable in each cohort.
Pipobroman, P32 and other cytoreductive agents.
However, it may be argued that the comparison between the BCSH- versus WHO-defined ET cohorts should be restricted to the ET cases considered in both groups. Noting that the 91 surplus ET cases of the WHO group lacking mutation analysis or who were triple-negative would presumably be recommended to undergo a BM biopsy by following the diagnostic guidelines of the BCSH (A1+A3–A5), a corresponding re-calculation of these patients was performed. This showed that there is a trend for a more favorable overall survival (median difference 2.7 years) but no significance (P=0.185) and a comparable tendency regarding fibrosis-free survival (P=0.241), if we restrict our calculation to the identical ET cohorts. However, we have to keep in mind that this procedure is not strictly consistent with the first set of the BCSH diagnostic criteria (A1–A3) on which we focused in the present study. Interestingly, if we regarded only the 18 patients that were triple-negative according to their mutational status and compared these 189 WHO-defined ET cases with the BCSH group, overall survival turned out to be significantly different (P=0.028), whereas fibrosis-free survival revealed only a tendency (P=0.071).
Discussion
This comparative study elucidates differences between the two major classification systems for diagnosis of ET; the data presented here provide evidence that the first set of criteria proposed by the BCSH1, 2 fails to differentiate accurately between WHO-defined ET and prePMF.14, 24, 25 Controversy persists whether prePMF is an independent entity, which requires distinction from ET.1 However, overall application of the WHO-defined BM criteria23 on larger cohorts of patients, either blindly or explicitly in context with clinical data, has resulted in consensus rates ranging between 76% and >90% largely depending on study design (all subtypes of MPN, inclusion of control cases with reactive changes, restriction to single BM features or only ET versus PMF, blinded evaluation or consideration of clinical data).7, 14, 24, 25, 26, 27 Contrasting these supportive findings, several groups28, 29, 30, 31 failed to reproduce the WHO diagnostic guidelines, likely because of improper application of guidelines and/or small biopsy specimens.32 Taken together, ~10–15% of patients may present with unclassifiable MPN.7, 26
Although the second set of ET diagnostic criteria (A1+A3–A5) by the BCSH1, 2 includes BM morphology, it fails to recognize the other hematopoietic cell lineages besides megakaryocytes and fibers (‘A5 BM aspirate and trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominantly large megakaryocytes with hyperlobulated nuclei and abundant cytoplasm. Reticulin is generally not increased (grades 0–2/4 or grade 0/3)'). Although the corresponding description in the text is more detailed, the statement that reticulin is generally not increased (grade 0–2 in a four-graded scheme33 or grade 0 in a three-graded scheme22) may be the source of confusion. It applies only to score 0/3 but does not fully equal score 0–2/4, which is consistent with a minor increase. In overt PMF, reticulin fibrosis is explicitly defined as being increased (⩾grade 2/3 or grade 3/4) and may be accompanied by overt collagen and/or new bone formation.34, 35, 36 Patients with prePMF, however, present most frequently with thrombocytosis and normal or only minor accumulation of BM reticulin (score 0–2/4 or 0–1/3), and are probably not fully recognized by the BCSH1, 2 and thus presumably regarded as ET. This shortcoming is likely to be responsible for the adverse events and unfavorable outcome of this cohort. However, if the second set of diagnostic criteria by the BCSH (A1+A3–A5),1, 2 including BM aspirate and trephine biopsy examination, would have been regarded, it cannot be ruled out that a number of cases may have been recognized as being not consistent with ET.
The finding of a small group of 16 (~7%) PV patients in the BCSH group diagnosed according to the corresponding exclusion criteria1 and presenting with a normal Hct and no evidence of iron depletion8, 9 is not surprising and underscores the proposal to the WHO to enter BM morphology as a major diagnostic criterion for PV.37 Persuasive evidence has been provided that in patients not meeting the required Hct thresholds for the diagnosis of PV according to the BCSH,8, 9 the diagnosis of so-called masked PV can be established,17, 18 and that in this context BM morphology has an important role.12, 20, 38, 39 Determination of JAK2/CALR mutation status alone, without BM morphology examination, is not sufficient to differentiate PV from JAK2-mutant ET.40 It has been demonstrated that Hct threshold values in these patients were significantly higher than in JAK2-positive ET revealing a best cutoff for discrimination at 49% in males and 48% in females.41 Moreover, many of these patients developed signs and symptoms (raising Hct/Hb levels and need for phlebotomies) of overt PV during follow-up.
The distinction of WHO-ET and prePMF, specifically concerning clinical presentation, bleeding events and prognosis, has been shown to be of high clinical relevance.34, 35, 42, 43, 44, 45 In a multicenter study on 1104 patients, Barbui et al.26 validated the clinical relevance of a strict adherence to the WHO criteria, in particular BM morphology46 in the diagnosis of ET. They provided important information on presenting hematological features, disease complications and survival in ET versus prePMF. Contrasting these findings, a recent study on a small cohort of 20 young patients (age between 16 and 40 years) with prePMF versus 197 patients with WHO-defined ET failed to confirm these differences and questioned the central role of histological diagnosis for the clinical management and prognostication in young prePMF/ET patients.47 In this context, a conflicting opinion exists whether the differentiation between WHO-defined ET and prePMF has an impact on treatment modalities in these two entities. Although an only thromboreductive treatment or treatment with low-dose aspirin in WHO-confirmed ET may be successful in the prevention of thromboembolic and hemorrhagic complications,48 a more aggressive treatment approach using hydroxyurea seems necessary in BCSH-diagnosed ET to prevent thrombosis and transformation to overt myelofibrosis.49 This suggests that BCSH-defined ET diagnosed by the first set of criteria (A1–A3) includes a considerable fraction of misclassified patients with a more aggressive MPN, very similar to prePMF. This is also reflected by the presence of splenomegaly and elevated serum lactate dehydrogenase levels in our BCSH cohort, which are both features of WHO-classified prePMF. This entity is usually associated with an elevated white blood cell count, which constitutes a major risk factor for arterial thrombosis,50 increased bleeding tendency,43 transformation to overt myelofibrosis and a shorter survival.26
In conclusion, accurate diagnosis of WHO mandates a scrutinized examination of BM biopsy specimens as key feature. Classification schemes that fail to or do not precisely regard this postulate will end up with a heterogeneous, inadequately defined cohort of patients impairing an appropriate clinical management.
Acknowledgments
HG, JT and GJ designed the research, contributed patients, participated in data analysis and interpretation and wrote the paper. GJ performed the statistical analysis. JT reviewed all BM histopathology. All other authors either contributed patients or participated in reviewing bone marrow morphology. All the authors viewed the clinical data, and read and approved the final draft. HG and GJ contributed equally to this work.
The authors declare no conflict of interest.
References
- Harrison CN, Bareford D, Butt N, Campbell P, Conneally E, Drummond M et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 2010; 149: 352–375. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Butt N, Campbell P, Conneally E, Drummond M, Green AR et al. Modification of British Committee for Standards in Haematology diagnostic criteria for essential thrombocythaemia. Br J Haematol 2014; 167: 421–423. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka H, Orazi A, Tefferi A, Gisslinger H. Essential thrombocythemia. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon, France, 2008. [Google Scholar]
- Thiele J, Kvasnicka HM. Clinicopathological criteria for differential diagnosis of thrombocythemias in various myeloproliferative disorders. Semin Thromb Hemost 2006; 32: 219–230. [DOI] [PubMed] [Google Scholar]
- Abdulkarim K, Ridell B, Johansson P, Kutti J, Safai-Kutti S, Andréasson B. The impact of peripheral blood values and bone marrow findings on prognosis for patients with essential thrombocythemia and polycythemia vera. Eur J Haematol 2011; 86: 148–155. [DOI] [PubMed] [Google Scholar]
- Ejerblad E, Kvasnicka HM, Thiele J, Andreasson B, Björkholm M, Löfvenberg E et al. Diagnosis according to World Health Organization determines the long-term prognosis in patients with myeloproliferative neoplasms treated with anagrelide: results of a prospective long-term follow-up. Hematology 2013; 18: 8–13. [DOI] [PubMed] [Google Scholar]
- Gianelli U, Bossi A, Cortinovis I, Sabattini E, Tripodo C, Boveri E et al. Reproducibility of the WHO histological criteria for the diagnosis of Philadelphia chromosome-negative myeloproliferative neoplasms. Mod Pathol 2014; 27: 814–822. [DOI] [PubMed] [Google Scholar]
- McMullin MF, Bareford D, Campbell P, Green AR, Harrison C, Hunt B et al. Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br J Haematol 2005; 130: 174–195. [DOI] [PubMed] [Google Scholar]
- McMullin MF, Reilly JT, Campbell P, Bareford D, Green AR, Harrison CN et al. Amendment to the guideline for diagnosis and investigation of polycythaemia/erythrocytosis. Br J Haematol 2007; 138: 821–822. [DOI] [PubMed] [Google Scholar]
- Reilly JT, McMullin MF, Beer PA, Butt N, Conneally E, Duncombe A et al. Guideline for the diagnosis and management of myelofibrosis. Br J Haematol 2012; 158: 453–471. [DOI] [PubMed] [Google Scholar]
- Barosi G, Ambrosetti A, Finelli C, Grossi A, Leoni P, Liberato NL et al. The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol 1999; 104: 730–737. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Boeltken B, Zankovich R, Diehl V, Fischer R. Initial (prefibrotic) stages of idiopathic (primary) myelofibrosis (IMF) - a clinicopathological study. Leukemia 1999; 13: 1741–1748. [DOI] [PubMed] [Google Scholar]
- Kvasnicka HM, Thiele J. Prodromal myeloproliferative neoplasms: the 2008 WHO classification. Am J Hematol 2010; 85: 62–69. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Müllauer L, Buxhofer-Ausch V, Gisslinger B, Gisslinger H. Essential thrombocythemia versus early primary myelofibrosis: a multicenter study to validate the WHO classification. Blood 2011; 117: 5710–5718. [DOI] [PubMed] [Google Scholar]
- Barosi G, Rosti V, Bonetti E, Campanelli R, Carolei A, Catarsi P et al. Evidence that prefibrotic myelofibrosis is aligned along a clinical and biological continuum featuring primary myelofibrosis. PLoS One 2012; 7: e35631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Gisslinger H, Finazzi G, Carobbio A, Rumi E et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol 2013; 89: 52–54. [DOI] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Carobbio A, Gisslinger H, Finazzi G, Rumi E et al. Masked polycythemia vera diagnosed according to WHO and BCSH classification. Am J Hematol 2014; 89: 199–202. [DOI] [PubMed] [Google Scholar]
- Alvarez-Larrán A, Angona A, Ancochea A, García-Pallarols F, Fernández C, Longarón R et al. Masked polycythaemia vera: presenting features, response to treatment and clinical outcomes. Eur J Haematol 2015. doi:10.1111/ejh.12552. [DOI] [PubMed]
- Thiele J, Kvasnicka HM, Diehl V. Initial (latent) polycythemia vera with thrombocytosis mimicking essential thrombocythemia. Acta Haematol 2005; 113: 213–219. [DOI] [PubMed] [Google Scholar]
- Gianelli U, Iurlo A, Vener C, Moro A, Fermo E, Bianchi P et al. The significance of bone marrow biopsy and JAK2V617F mutation in the differential diagnosis between the ‘early' prepolycythemic phase of polycythemia vera and essential thrombocythemia. Am J Clin Pathol 2008; 130: 336–342. [DOI] [PubMed] [Google Scholar]
- Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008; 22: 437–438. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005; 90: 1128–1132. [PubMed] [Google Scholar]
- Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H et al (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn. IARC: Lyon, France, 2008. [Google Scholar]
- Florena AM, Tripodo C, Iannitto E, Porcasi R, Ingrao S, Franco V. Value of bone marrow biopsy in the diagnosis of essential thrombocythemia. Haematologica 2004; 89: 911–919. [PubMed] [Google Scholar]
- Gianelli U, Vener C, Raviele PR, Moro A, Savi F, Annaloro C et al. Essential thrombocythemia or chronic idiopathic myelofibrosis? A single-center study based on hematopoietic bone marrow histology. Leuk Lymphoma 2006; 47: 1774–1781. [DOI] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol 2011; 29: 3179–3184. [DOI] [PubMed] [Google Scholar]
- Madelung AB, Bondo H, Stamp I, Loevgreen P, Nielsen SL, Falensteen A et al. World Health Organization-defined classification of myeloproliferative neoplasms: morphological reproducibility and clinical correlations—the Danish experience. Am J Hematol 2013; 88: 1012–1016. [DOI] [PubMed] [Google Scholar]
- Wilkins BS, Erber WN, Bareford D, Buck G, Wheatley K, East CL et al. Bone marrow pathology in essential thrombocythemia: interobserver reliability and utility for identifying disease subtypes. Blood 2008; 111: 60–70. [DOI] [PubMed] [Google Scholar]
- Brousseau M, Parot-Schinkel E, Moles M-P, Boyer F, Hunault M, Rousselet M-C. Practical application and clinical impact of the WHO histopathological criteria on bone marrow biopsy for the diagnosis of essential thrombocythemia versus prefibrotic primary myelofibrosis. Histopathology 2010; 56: 758–767. [DOI] [PubMed] [Google Scholar]
- Buhr T, Hebeda K, Kaloutsi V, Porwit A, Van der Walt J, Kreipe H. European Bone Marrow Working Group trial on reproducibility of World Health Organization criteria to discriminate essential thrombocythemia from prefibrotic primary myelofibrosis. Haematologica 2012; 97: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Larrán A, Ancochea A, García M, Climent F, García-Pallarols F, Angona A et al. WHO-histological criteria for myeloproliferative neoplasms: reproducibility, diagnostic accuracy and correlation with gene mutations and clinical outcomes. Br J Haematol 2014; 166: 911–919. [DOI] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Vannucchi AM, Tefferi A. Problems and pitfalls regarding WHO-defined diagnosis of early/prefibrotic primary myelofibrosis versus essential thrombocythemia. Leukemia 2013; 27: 1953–1958. [DOI] [PubMed] [Google Scholar]
- Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol 2007; 139: 351–362. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. Grade of bone marrow fibrosis is associated with relevant hematological findings-a clinicopathological study on 865 patients with chronic idiopathic myelofibrosis. Ann Hematol 2006; 85: 226–232. [DOI] [PubMed] [Google Scholar]
- Vener C, Fracchiolla NS, Gianelli U, Calori R, Radaelli F, Iurlo A et al. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood 2008; 111: 1862–1865. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. Hematopathologic findings in chronic idiopathic myelofibrosis. Semin Oncol 2005; 32: 380–394. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia 2014; 28: 1407–1413. [DOI] [PubMed] [Google Scholar]
- Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood 2013; 122: 1881–1886. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. Diagnostic impact of bone marrow histopathology in polycythemia vera (PV). Histol Histopathol 2005; 20: 317–328. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014; 124: 2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Carobbio A, Guglielmelli P, Rambaldi A, Vannucchi AM et al. Discriminating between essential thrombocythemia and masked polycythemia vera in JAK2 mutated patients. Am J Hematol 2014; 89: 588–590. [DOI] [PubMed] [Google Scholar]
- Barosi G. Essential thrombocythemia vs. early/prefibrotic myelofibrosis: why does it matter. Best Pract Res Clin Haematol 2014; 27: 129–140. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia 2012; 26: 716–719. [DOI] [PubMed] [Google Scholar]
- Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011; 117: 5857–5859. [DOI] [PubMed] [Google Scholar]
- Kvasnicka HM, Thiele J. The impact of clinicopathological studies on staging and survival in essential thrombocythemia, chronic idiopathic myelofibrosis, and polycythemia rubra vera. Semin Thromb Hemost 2006; 32: 362–371. [DOI] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Curr Hematol Malig Rep 2009; 4: 33–40. [DOI] [PubMed] [Google Scholar]
- Palandri F, Latagliata R, Polverelli N, Tieghi A, Crugnola M, Martino B et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015; 29: 1344–1349. [DOI] [PubMed] [Google Scholar]
- Gisslinger H, Gotic M, Holowiecki J, Penka M, Thiele J, Kvasnicka H-M et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013; 121: 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005; 353: 33–45. [DOI] [PubMed] [Google Scholar]
- Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood 2010; 115: 778–782. [DOI] [PubMed] [Google Scholar]