Abstract
The leptin gene (LEP) plays a regulatory role in satiety, inflammation, and allergy. Prior findings linking leptin to asthma motivated us to investigate whether DNA methylation (DNA-M) of CpG (cytosine-phosphate-guanine) sites in concert with single nucleotide polymorphisms (SNPs) of LEP can explain the risk of asthma and lung function. Methylation of CpG sites was assessed using the Illumina Infinium Human Methylation 450 beadchip in blood samples collected from 10- and 18-year-old boys and girls from the Isle of Wight (IOW) birth cohort (UK). Four LEP SNPs were genotyped. Linear and log linear models were used for the analysis, adjusting for false discovery rate (FDR). The analyses were repeated in the BAMSE cohort (Sweden). In the IOW study, the interaction of cg00666422 and rs11763517 (CT vs TT and CC) was associated with FEV1 (FDR-adjusted p-value: 0.03), FEV1/FVC ratio (FDR-adjusted p-value: 0.0096), and FEF25-75% (FDR-adjusted p-value: 0.00048) such that they decreased with increasing DNA-M. The interaction of the same CpG-SNP pair was also associated with increased risk of asthma at age 18. We replicated the findings for FEV1/FVC and FEF25-75% in a smaller sample of 34 participants at age 10. Regarding the BAMSE cohort, although, the interaction of cg00666422 and rs11763517 on lung function were not significant, the direction of the effect was the same as in IOW cohort. Thus, penetrance of LEP genotype seems to be modified by methylation at cg00666422 and is linked to airway obstruction and asthma.
Keywords: Epigenetics, CpGs, SNPs, spirometry, FEV1, FEV1/FVC ratio, FEF25-75%, asthma
Introduction
Asthma represents a major public health burden. It is a chronic disease characterized by the airway inflammation and bronchial hyperresponsiveness [1]. Studies have shown that around 60% of asthma is heritable and that several genetic factors are associated with the heritability of asthma across generations [2]. The leptin (LEP) gene, encoding a 16 kDa pleiotropic adipokine, is one such gene that plays a regulatory role in inflammation in asthma and allergic responses [3]. In addition to being expressed in adipocytes, leptin and the leptin receptor are both expressed in bronchial epithelial cells and alveolar macrophages [4].
The relationship between asthma and leptin has been explored in several experimental and observational studies. Shore et al. found that serum leptin levels increased during allergic reactions [5]. Kattan et al. reported that leptin levels were positively correlated with asthma symptoms in girls [6]. In another study by Hickson, lung function was found to be inversely associated with serum leptin levels in African Americans [7]. Newson et al. have shown that leptin and leptin/adiponectin ratios are associated with asthma [8].
Though these studies indicate an association between leptin levels and asthma, the biological mechanisms underlying these relationships are unknown. The role of single nucleotide polymorphisms (SNPs) in LEP gene has been explored: Szczepankiewicz et al. reported the LEP 3’UTR A/G (rs13228377) and the -2549T/G (rs2167270) polymorphisms to be significantly associated with asthma. LEP is also a candidate gene for obstructive sleep apnea syndrome, which is an independent risk factor for asthma [9].
Apart from genetic polymorphisms, epigenetic mechanisms may also influence disease risk. DNA methylation (DNA-M) is one such epigenetic mechanism that alters gene expression without changing the DNA sequence [10]. It involves addition of methyl groups to cytosine bases in cytosine-phosphate-guanine (CpG) dinucleotides. DNA-M offers a potential mechanism through which the environment can affect gene expression [11], and so gene × environment interactions may be reflected at the DNA methylation level, especially for diseases such as allergy where there is a large environmental component to disease risk. While SNPs may affect outcomes at the population level, the effect of SNPs represents the average effect of a given genotype across a wide range of environments. It is therefore important to analyze interactions between SNP genotype and DNA-M, to detect different effects of genotype depending on environmental factors.
Findings that linked leptin to asthma and lung function [6-8] motivated us to investigate whether DNA-M of the LEP gene in concert with genetic polymorphisms of this gene can explain the risk of lung function and asthma. In particular, we hypothesize that the penetrance of the LEP gene variants for lung function and asthma is moderated by its methylation, as proposed for other genes [11,12]. To test this hypothesis we analyzed interactions of LEP SNPs and CpG sites on lung function and asthma outcomes in 10-year-old girls, and 18-year-old men and women in the Isle of Wight birth cohort. The significant CpG and SNP interactions were tested in an independent BAMSE cohort for replication of the results.
Methods
Isle of wight cohort
Study population
A whole population birth cohort was established on the Isle of Wight, UK, in 1989 to prospectively study the natural history of allergies and asthma. 1,456 of the 1,536 children born between January 1, 1989 and February 28, 1990 were enrolled in the study (F1 generation) after exclusion of adoptions, perinatal deaths, and refusals to participate. Ethical approvals were obtained from Local Research Ethics Committees (last: NRES Committee South Central-Hampshire B) and informed consents were obtained for all the participants at recruitment and at each follow up at ages of 1, 2, 4, 10, and 18 years (n=1,313). The birth cohort has been described in detail elsewhere [13]. Detailed interviews and examinations were completed for each child at follow up. A postal or telephone questionnaire was sent if a visit was not possible [14]. This study focusses on 125 men and 245 women at age 18 who had measurement of DNA-M, and a subset of these women at age 10 (n=34) randomly selected for DNA-M analysis.
SNP selection and genotyping
The genotype tagging system scheme used to tag the SNPs gave priority to the variants that showed strong association with asthma in the Isle of Wight birth cohort, or had been reported by others to be associated with asthma, or allergy and have functional importance (non-synonymous, located in conserved regions of DNA or present in regulatory regions). LEP SNPs (including 10 kb upstream and downstream of the gene) were determined using the tagger implemented in Haploview 3.2 based on Caucasian HapMap data. The threshold value for r2 was taken as 0.2 for tagging and one, two, and three SNP marker combination tests were used [10]. Four LEP SNPS were selected. DNA extracted from blood or saliva samples of 1,211 cohort subjects were interrogated using Golden Gate Genotyping Assays (Illumina Inc, San Diego, CA) on the Bead Xpress Veracode platform per Illumina’s protocol. DNA from each subject plus 37 replicate samples were analyzed. The quality threshold for allele determination was set at a GenCall score >0.25 with 98.3% retained for further analysis. Analysis of each locus included reclustering of genotyping data using our project data to define genotype cluster positions with additional manual reclustering to maximize both cluster separation and the 50th percentile of the distribution of the GenCall scores across all genotypes (50% GC score) [14].
DNA methylation analysis
DNA was extracted from peripheral blood samples using a salting out procedure [15] and its concentration was determined by Qubit quantitation. One microgram of DNA was bisulfite treated using the EZ 96-DNA methylation kit (Zymo Research, CA, USA), following the manufacturer’s standard protocol. Genome-wide DNA methylation was assessed using the Illumina Infinium Human Methylation 450 beadchip (Illumina, Inc., CA, USA), which interrogates >484,000 CpG sites associated with approximately 24,000 genes. Arrays were processed and imaged using the manufacturer’s recommendations. To estimate assay variability, multiple identical control samples were assigned to each bisulfite conversion batch. To control against batch effects the samples were randomly distributed on microarrays. The methylation level (β value) was calculated for each queried CpG locus using the methylation module of GenomeStudio software [10]. DNA methylation levels for each CpG were estimated as the proportion of intensity of methylated (M) over the sum of methylated (M) and unmethylated (U) probes, β=M/[c+M+U] with c being a constant to prevent dividing by zero [16]. Methylation data were preprocessed and batch-effect removed using Bioconductor packages IMA [17] and ComBat. DNA-M was assessed in 125 men and 245 women at age 18. Of these 245 women, 34 also provided a blood sample at age 10 for DNA-M analysis.
Clinical data collection and outcomes
Maternal and paternal histories of asthma, and maternal smoking status during pregnancy were obtained at birth. Height and weight of the child were measured and questionnaires were completed at follow-ups at ages 1, 2, 4, 10, and 18. Current smoking status was obtained at 18 years of age. Asthma at age 18 was determined using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire [18] defined as a physician diagnosed asthma plus current symptoms and/or asthma medications [10].
Serum leptin concentrations were measured in blood samples collected at 18 years of age using an enzyme-linked immunosorbent assay (Biokit, S.A.; Barcelona, Spain) following the manufacturer’s kit instructions. Each sample, standards, and blank were assayed in duplicates.
Lung function outcomes were assessed using the KoKo spirometry software package on a portable desktop device (PDS Instrumentation, Louisville, KY, USA) [19]. Tests were performed following the guidelines of the American Thoracic Society and European Respiratory Society [20]. Lung function outcomes and serum leptin levels were also measured at age 10. Participants were required to be free from respiratory infection for 14 days and not administering any systemic oral steroids. It was also necessary that they did not take any short-acting beta-2 agonist for at least 6 hours, long-acting beta-2 agonist for 12 hours and caffeine for at least 4 hours prior to testing. All the measurements were made with the participants in the standing position without using a nose clip. The best of three consecutive expiratory maneuvers of forced vital capacity (FVC), forced expiratory volume 1 (FEV1), the FEV1/FVC ratio, and forced expiratory flow 25-75% (FEF25-75%) were taken as final values. Participants who had symptoms of asthma or were on antibiotics or steroids were rescheduled for spirometry.
Statistical analysis
Genome Studio software was used to process the raw methylation intensities and the detection p-value for each CpG was used as a QC measure of probe performance. CpG sites that had detection P values >0.01 in >10% of the samples, and CpG sites with probe-SNPs, were excluded from all analyses. Statistical analyses were performed using the SAS statistical package (version 9.3; SAS Institute, Cary, NC, USA). Our analytical samples included 370 men and women at age 18, and 34 girls at 10 years, with DNA methylation data. We performed chi square analyses to assess whether these samples were representative of the total cohort. To identify haplotype blocks for the SNPs, linkage disequilibrium (LD) analysis was performed on 4 LEP SNPs with Haploview 4.2.
The statistical analysis was divided into two parts. First, via linear regressions, we examined whether the interaction of LEP SNPs and DNA methylation was associated with lung function outcomes (FVC, FEV1, FEF25-75%), exhaled nitric oxide (eNO), and serum leptin levels at 18 years of age. More women than men were included in this study due to an initial research focus on pregnancy. To account for this imbalance, all the linear regression models were weighted by sex proportions. Multiple testing was adjusted by controlling false discovery rate (FDR) of 0.05. This was followed by a replicatory step in which we repeated the analyses focussing on lung function outcomes and interaction of SNP and DNA-M in the 34 girls at 10 years of age. Since the interaction terms should be independent of each other, we also tested whether SNPs explained the methylation of CpG sites, also called genotype-dependent methylation [21]. If this was the case, we estimated the residuals of the CpG after the respective SNP was adjusted for. The methylation residuals are no longer dependent on the SNP and the combined effect of methylation residuals with SNPs then facilitates an unbiased assessment of the interaction.
In the second part of the analysis, we assessed whether the CpG and SNP pairs surviving FDR in the first step were associated with asthma at age 18. The analysis was controlled for maternal and paternal asthma, maternal smoking, and current smoking status, height, and weight of the participants.
The BAMSE cohort
Study population
The CpG and SNP interaction terms that were found to be significantly associated with lung function in the Isle of Wight birth cohort were tested in the data from BAMSE prospective birth cohort, Sweden. Parents of children (n=7,221) born between February 1994 to November 1996 in Northern and Central Stockholm were asked to participate in the study. After excluding non-responders and ineligible participants, a total of 4,089 children were enrolled. The cohort is described in detail elsewhere [22,23]. Epigenome-wide DNA-M was measured DNA extracted from blood samples collected from 472 children at 8 years of age. Bisulphite conversion of 500 ng DNA per sample was done by using the EZ-96 DNA Methylation kit (Shallow; Zymo Research Corporation, Irvine, USA). Samples were processed with the Illumina Infinium HumanMethylation450 BeadChip (Illumina Inc., San Diego, USA) [24]. Pre-processing of the DNA-M data, and quality control were performed by using standard criteria and samples with a call rate <99%, color balance >3, or low median intensity were excluded from the analysis. Lung function testing was performed at 8 years of age using the 2200 Pulmonary Function Laboratory (Sensormedics, Anaheim, CA, USA) and at 16 years with the Jaeger MasterScreen-IOS system (Carefusion Technologies, San Diego, California) [25]. The highest values of forced expiratory volume in 1 sec (FEV1) were extracted and used from analysis, provided that the subject’s effort was coded as being maximal by the test leader, the flow-volume curve passed visual quality inspection, and that the two highest FEV1 readings were reproducible according to ATS/ERS criteria. LEP genotypes were extracted from GWAS data (Illumina 610quad chip with imputation from the 1000 genomes project) available on 485 selected Caucasian subjects as described earlier [26]. In total, 343 and 294 BAMSE subjects had complete data for this study, i.e. lung function data at 8 and 16 years, respectively, as well as genotype and methylation data.
Statistical analysis
Linear regression models were used to test the significant interaction terms of CpGs (measured at age 8) and SNPs with lung function markers as dependent variables for both boys and girls at ages 8 and 16. The models were adjusted for maternal asthma, paternal asthma, height, weight, maternal smoking, current smoking, and were weighted by sex. Successful replication was defined as having the same direction of effect and a p-value of less than 0.05.
Results
The Isle of wight cohort
There were no significant differences in body mass index (BMI), current smoking status, maternal smoking status, maternal asthma or paternal asthma, between the participants of the whole cohort (n=1,313) and the subset of participants for whom DNA methylation data was collected (n=370) (Table 1). Methylation levels of CpG sites within LEP at age 18 are shown in Table 2. Seven CpG sites were removed after data cleaning and one CpG with a probe-SNP was excluded from the analysis. The eight remaining CpG sites have low, medium, and high levels of methylation (Table 2, median β: range 0.16 to 0.96).
Table 1.
Comparison of population characteristics of participants in the whole cohort with those with DNA methylation data in IOW cohort
| Parameter | Total cohort n=1313 | Participants with DNA-M data at age 18 n=370 | p-value |
|---|---|---|---|
|
|
|||
| Median (5%, 95% value); n | |||
| Height | |||
| Boys | 178 (167, 190); 483 | 177.3 (167, 187.3); 124 | 0.3 |
| Girls | 164 (154.5, 175); 511 | 165 (154, 176); 243 | 0.44 |
| Weight | |||
| Boys | 69 (55.6, 91.2); 468 | 70 (56, 97.2); 123 | 0.38 |
| Girls | 61.14 (48.5, 90.1); 502 | 61.45 (50.4, 89.1); 240 | 0.63 |
| BMI | |||
| Boys | 21.7 (18.1, 30.0); 465 | 21.85 (18.3, 29.1); 123 | 0.38 |
| Girls | 22.86 (18.2, 13.7); 499 | 22.88 (19.1, 32.9); 240 | 0.57 |
| FVC | |||
| Boys | 5.34 (4.21, 6.60); 395 | 5.34 (4.11, 6.70); 119 | 0.99 |
| Girls | 3.98 (3.1, 4.8); 443 | 4.05 (3.19, 5.02); 237 | 0.07 |
| FEV1 | |||
| Boys | 4.55 (3.64, 5.63); 396 | 4.57 (3.59, 5.62); 119 | 0.59 |
| Girls | 3.48 (2.72,4.17); 443 | 3.54 (2.81, 4.26); 237 | 0.08 |
| FEV1/FVC | |||
| Boys | 0.87 (0.74, 0.98); 396 | 0.87 (0.77, 0.99); 119 | 0.6 |
| Girls | 0.88 (0.75, 0.98); 443 | 0.88 (0.75, 0.98); 237 | 0.66 |
| FEF25-75% | |||
| Boys | 4.97 (3.23, 6.84); 396 | 5.12 (3.42, 6.94); 119 | 0.42 |
| Girls | 4.00 (2.5, 5.4); 443 | 4.05 (2.55, 5.42); 237 | 0.33 |
| ENO | |||
| Boys | 19.0 (7, 108); 387 | 18.00 (7, 78); 113 | 0.27 |
| Girls | 14.0 (5, 64); 435 | 13.00 (6, 64); 234 | 0.26 |
| Serum leptin (ng/ml) | |||
| Boys | 1.22 (0.40, 13.11); 288 | 1.2 (0.40, 16.04); 120 | 0.99 |
| Girls | 13.11 (2.4, 54.57); 265 | 13.11 (2.76, 54.57); 239 | 0.7 |
| Maternal asthma during pregnancy | 0.41 | ||
| Yes | 137 (10.51) | 44 (12.02) | |
| No | 1167 (89.49) | 322 (87.98) | |
| Paternal asthma | 0.23 | ||
| Yes | 128 (9.88) | 43 (11.88) | |
| No | 1168 (90.12) | 319 (88.12) | |
| Atopy Status | |||
| Boys | 194 (47.7) | 50 (40.6) | 0.17 |
| Girls | 159 (35.65) | 76 (31.4) | 0.26 |
| Maternal smoking status | 0.23 | ||
| Yes | 305 (23.34) | 75 (20.83) | |
| No | 1002 (76.66) | 293 (79.62) | |
| Current smoking status | 0.29 | ||
| Yes | 368 (28.79) | 95 (25.96) | |
| No | 910 (71.21) | 271 (74.04) | |
| Sex | <0.0001 | ||
| Male | 653 (49.73) | 125 (33.78) | |
| Female | 660 (50.3) | 245 (66.22) | |
Table 2.
Distributions of DNA-M at LEP CpG sites, in whole blood samples at age 18 in IOW cohort
| Subpopulation with methylation (n=370) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| CpG site | Location (genome v37) | Coordinate | Mean | Median | 5% | 95% | Std Dev |
| cg14734794 | 127879920 | TSS1500 | 0.96 | 0.96 | 0.95 | 0.97 | 0.01 |
| cg12782180 | 127880932 | TSS1500 | 0.43 | 0.43 | 0.33 | 0.52 | 0.06 |
| cg00840332 | 127881269 | TSS200 | 0.17 | 0.16 | 0.10 | 0.24 | 0.04 |
| cg00666422 | 127881440 | 5’UTR | 0.45 | 0.46 | 0.36 | 0.54 | 0.05 |
| cg12083122 | 127883819 | 5’UTR | 0.95 | 0.95 | 0.94 | 0.97 | 0.01 |
| cg25435800 | 127890193 | 5’UTR | 0.94 | 0.94 | 0.90 | 0.97 | 0.02 |
| cg25730670 | 127891366 | 5’UTR | 0.95 | 0.95 | 0.93 | 0.96 | 0.01 |
| cg24862443 | 127896859 | 3’UTR | 0.94 | 0.94 | 0.93 | 0.95 | 0.01 |
5’UTR=5’ untranslated region; TSS200=within 200 bp of the transcription start site; TSSS1500=within 1500 bp of the transcription start site.
Four SNPs were genotyped for the LEP gene. Out of these four, two were found to be in a single LD block when analyzed by Haploview 4.2. Thus, three uncorrelated SNPs were chosen for the analysis (Figure 1). The LEP gene SNPs rs4731429 and rs10954176 are located in the 3’UTR region, and the SNP rs11763517 is located in the first intron (Figure 4, Table 3).
Figure 1.
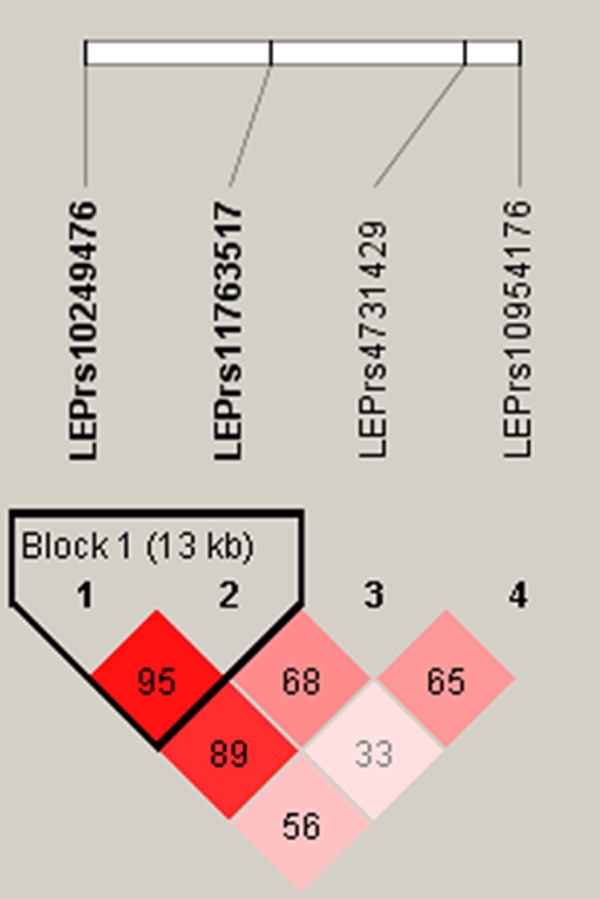
Linkage disequilibrium plot of LEP SNPs, standard (D’/LOD) color scheme; D’ LD values are displayed.
Figure 4.
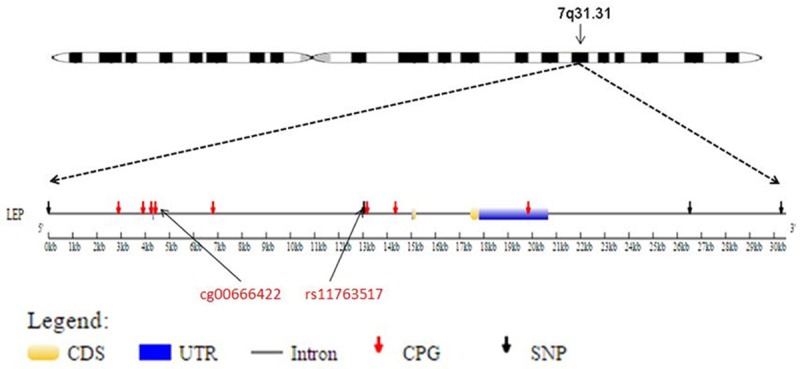
The positions of CpG sites and SNPs included in this study, relative to the LEP gene. CDS=coding sequence, UTR=untranslated region.
Table 3.
Location and comparison of LEP SNPs in whole cohort and participants with DNA methylation
| SNP | Genotype | Coordinate | Location | Whole cohort (n%) | Subpopulation with DNA-M data (n%) | p-value |
|---|---|---|---|---|---|---|
| rs10249476 | TT | 127877026 | Flanking | 147 (13.47) | 49 (14.2) | 0.16 |
| AC | 5’UTR | 512 (47.93) | 177 (51.3) | |||
| CC | 432 (39.60) | 119 (34.5) | ||||
| rs11763517 | TT | 127890062 | Intron | 277 (25.3) | 76 (21.9) | 0.26 |
| CT | 542 (49.6) | 179 (51.6) | ||||
| CC | 274 (25.1) | 92 (26.5) | ||||
| rs4731429 | TT | 127903539 | Flanking | 224 (20.51) | 76 (22.09) | 0.44 |
| CT | 3’UTR | 550 (50.37) | 174 (50.58) | |||
| CC | 318 (29.12) | 94 (27.33) | ||||
| rs10954176 | TT | 127907319 | Flanking | 319 (29.4) | 93 (27) | 0.43 |
| CT | 3’UTR | 508 (46.8) | 164 (48) | |||
| CC | 258 (23.8) | 86 (25.1) |
Effect of LEP SNPs, DNA-M, and their interaction on lung function, and leptin levels
We ran multiple linear regression models to analyze the effect of LEP CpGs in interaction with SNPs on lung function outcomes, focusing on FVC and on obstructive markers, namely FEV1, the FEV1/FVC ratio, and FEF25-75%. Additionally, we also investigated exhaled nitric oxide, and serum leptin levels as outcomes. All models were adjusted for height, weight, sex, current smoking status, maternal smoking status, and maternal and paternal asthma status. The significant associations that survived FDR were the interactions of CpG cg00666422 and SNP rs11763517 (CT vs CC) with FEV1, FEV1/FVC, and FEF25-75% at age 18. Exhaled nitric oxide and serum leptin levels were not found to be significantly related to this interaction term.
Since interaction terms should be independent of each other, we tested whether rs11763517 explained the methylation of cg00666422. Indeed we found that, compared to the CC genotype, the TT genotype had a 3.6% and CT a 1.5% lower methylation (p<0.0001 and p=0.026, respectively). In order to ensure that it is the DNA-M effect on the lung function, we removed the SNP effect from the methylation by running a regression model with cg00666422 as the dependent variable and rs11763517 as the independent variable, and extracted the residuals from this model. We then assessed whether the interaction of these residuals with SNP rs11763517 had an association with the lung function outcomes. We found that FEV1/FVC and FEF25-75% were significantly associated with the interaction of the residuals and rs11763517 in the same direction adjusting for the confounders indicating that original assessment was not biased. In addition, since for lung function the effect of the SNP followed a heterosis model (TT and CC were not different from one another), we combined the genotypes TT and CC and estimated the risk for the CT genotype. Applying a heterosis model, the methylation was no longer differently distributed on CT vs. TT and CC (p=0.74). Hence, also in a heterosis model the CpG site and the SNP were independent.
We repeated the analysis with all the eight CpG sites and three SNPs using the heterosis model. The analysis revealed that the interaction of the CpG cg00666422 and SNP rs11763517 (CT vs TT and CC) was significantly associated with FEV1, FEV1/FVC, and FEF25-75% at age 18 such that they decreased as methylation increased for the CT genotype compared to the TT and CC genotype of rs11763517 (Table 4). To demonstrate the effect of this interaction between a SNP and a continuous variable (linear association), we categorized the methylation values into six categories, ≤39%, 39% to 41%, 41% to 43%, 43% to 45%, 45% to 47%, 47% to 49% and assessed the effect of each category, not requiring a linear association. The plots indicate that, there is a decline in FEV1, FEV1/FVC ratio, and FEF25-75% among subjects with the CT genotype when the methylation level of cg00666422 was above 45% (Figure 2A-C).
Table 4.
Interaction effect of LEP CpG and SNP on FEV1, FEV1/FVC and FEF25-75% at 18 years of Age* (FDR adjusted) in IOW cohort
| Parameter | Genotype | Estimate | Standard Error | p-value | FDR p-value |
|---|---|---|---|---|---|
| FEV1 | |||||
| rs11763517 | CT | 1.14 | 0.39 | 0.0003 | |
| CT+TT | Ref. | Ref. | |||
| cg00666422 | 2.01 | 0.6 | 0.001 | ||
| cg00666422*rs11763517 | CT | -3.20 | 0.85 | 0.0002 | 0.03 |
| CC+TT | Ref. | Ref. | |||
| FEV1/FVC | |||||
| rs11763517 | CT | 0.25 | 0.06 | 0.0002 | |
| CT+TT | Ref. | Ref. | |||
| cg00666422 | 0.36 | 0.10 | 0.0005 | ||
| cg00666422*rs11763517 | CT | -0.57 | 0.14 | 0.000071 | 0.0096 |
| CC+TT | Ref. | Ref. | |||
| FEF25-75% | |||||
| rs11763517 | CT | 3.94 | 0.85 | 0.000006 | |
| CT+TT | Ref. | Ref. | |||
| cg00666422 | 5.72 | 1.32 | 0.00002 | ||
| cg00666422*rs11763517 | CT | -8.93 | 1.86 | 0.000003 | 0.00048 |
| CC+TT | Ref. | Ref. |
Adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, and weight at age 18, and weighted for sex.
Figure 2.
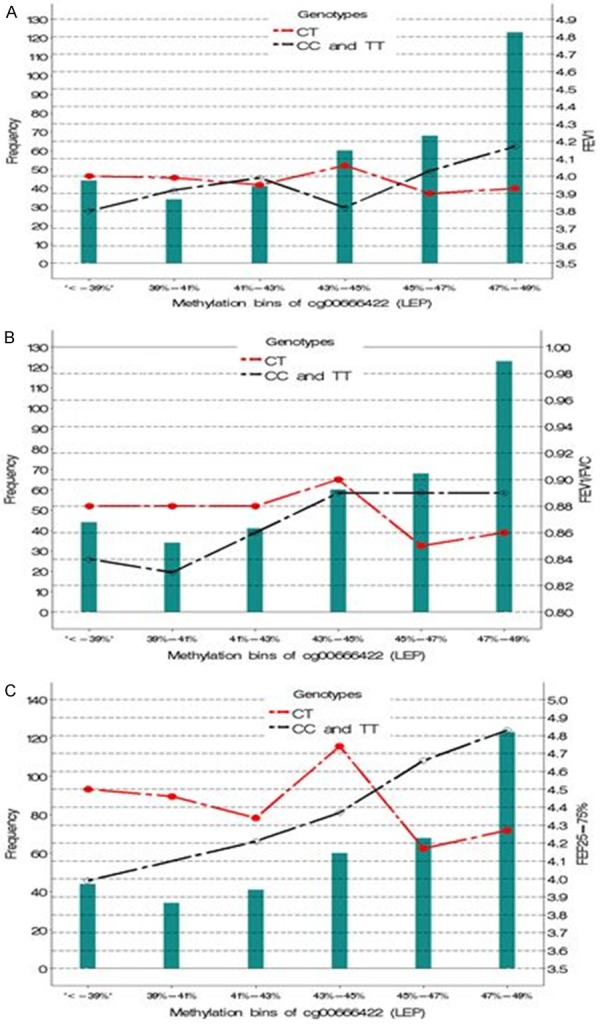
Comparison of rs11763517 genotypes in interaction with categorized methylation levels of cg00666422 on (A) FEV1 (B) FEV1/FVC and (C) FEF25-75% at age 18. The horizontal axis represents the bins of DNA methylation, and the green bars represent the frequency for the DNA methylation bins. For example, 34 individuals have a DNA methylation level of 39%-41%. The CT genotype (red dots) and the TT and CC (combined) genotype (black diamonds) are plotted against (A) FEV1 (B) FEV1/FVC and (C) FEF25-75%. The reference genotype was TT and CC combined.
None of the other associations of CpG and SNP interaction terms with markers of lung function nor eNO or leptin levels measured at age 18 survived FDR correction. However, the same CpG site and SNP interaction found at age 18 years (cg00666422 × rs11763517 CT vs TT and CC) was significantly associated with FEV1/FVC and FEF25-75% in the same direction detected at age 10 (Table 5).
Table 5.
Interaction effect of LEP CpG and SNP on FEV1, FEV1/FVC, and FEF25-75% in girls at 10 years of Age in IOW cohort
| Parameter | Genotype | Estimate | Standard Error | p-value |
|---|---|---|---|---|
| FEV1 | ||||
| rs11763517 | CT | 0.99 | 0.61 | 0.12 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 1.25 | 0.96 | 0.2 | |
| cg00666422*rs11763517 | CT | -2.23 | 1.33 | 0.11 |
| CC+TT | Ref. | Ref. | ||
| FEV1/FVC | ||||
| rs11763517 | CT | 0.27 | 0.13 | 0.045 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 0.47 | 0.20 | 0.03 | |
| cg00666422*rs11763517 | CT | -0.64 | 0.27 | 0.03 |
| CC+TT | Ref. | Ref. | ||
| FEF25-75% | ||||
| rs11763517 | CT | 3.25 | 1.39 | 0.03 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 4.62 | 2.18 | 0.04 | |
| cg00666422*rs11763517 | CT | -7.42 | 3.01 | 0.02 |
| CC+TT | Ref. | Ref. |
*Adjusted for maternal asthma, paternal asthma, maternal smoking, height, and weight at age 10.
Since circulating leptin hormone levels differ between men and women, we stratified our analysis for the CpG and SNP pairs showing significant associations with lung function, by sex. We found that SNPs and CpG sites were significantly associated with the FEV1/FVC ratio and FEF25-75% only in women. However, the effect size and the direction was the same for both men and women (Table 6). Thus, this CpG × SNP interaction, although not statistically significant in a smaller sample of men, has a similar effect in both men and women for the outcomes FEV1/FVC ratio and FEF25-75%.
Table 6.
Interaction of LEP CpGs and SNPs on FEV1, FEV1/FVC and FEF25-75% at 18 years of age (stratified by sex) in IOW cohort*
| Outcome | Genotype | Males | Females | ||
|---|---|---|---|---|---|
|
|
|||||
| Estimate (S.E.) | p-value | Estimate (S.E.) | p-value | ||
| FEV1 | |||||
| rs11763517 | CT | 0.75 (0.86) | 0.38 | 1.6 (0.44) | 0.0003 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | 2.20 (1.29) | 0.09 | 2.05 (0.69) | 0.0031 | |
| cg00666422*rs11763517 | CT | -1.72 (1.86) | 0.36 | -3.51 (0.95) | 0.0003 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| FEV1/FVC | |||||
| rs11763517 | CT | 0.16 (0.13) | 0.24 | 0.27 (0.08) | 0.0004 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | 0.28 (0.2) | 0.17 | 0.38 (0.12) | 0.0015 | |
| cg00666422*rs11763517 | CT | -0.30 (0.29) | 0.30 | -0.63 (0.16) | 0.0002 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| FEF25-75% | |||||
| rs11763517 | CT | 2.76 (1.97) | 1.16 | 4.25 (0.94) | <0.0001 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | 5.73 (2.97) | 0.06 | 6.06 (1.48) | <0.0001 | |
| cg00666422*rs11763517 | CT | -5.62 (4.27) | 0.19 | -9.66 (2.05) | <0.0001 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
Adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, and weight at age 18.
Effect of LEP SNPs, DNA-M, and their interaction on asthma
Next, we checked whether the cg00666422 × rs11763517 interaction had any effect on asthma at 18 years of age in men and women. We used a log-linear Poisson regression model with robust error variance to estimate the risk ratios for asthma. The model was weighted by sex and adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, weight, and sex of the participant. The interaction of cg00666422 and rs11763517 (CT vs. TT and CC) was significantly associated with the risk of asthma at age 18 (Table 7). To demonstrate the risk of asthma at specific levels of methylation, we estimated the risk ratios for 37%, 39%, 41%, 43%, 45%, 47% and 49% (Figure 3). For subjects of genotype CT, the risk of asthma steadily increased as methylation levels increased, relative to the combined TT and CC genotypes. The relative risk (RR) for asthma is lower (RR=0.3) if methylation is below 45% and higher thereafter (close to RR=2 if the methylation is 49%).
Table 7.
*Effect of the interaction term of the CpG site and SNP on asthma at age 18 in IOW cohort
| Asthma at age 18 | Genotype | Estimate | Standard error | p value |
|---|---|---|---|---|
| cg00666422 | -6.59 | 3.66 | 0.072 | |
| rs11763517 | CT | -6.28 | 2.20 | 0.004 |
| CC+TT | Ref. | Ref. | Ref. | |
| cg00666422 × rs11763517 | CT | 13.76 | 4.82 | 0.004 |
| CC+TT | Ref. | Ref. | Ref. |
Adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, and weight at age 18.
Figure 3.
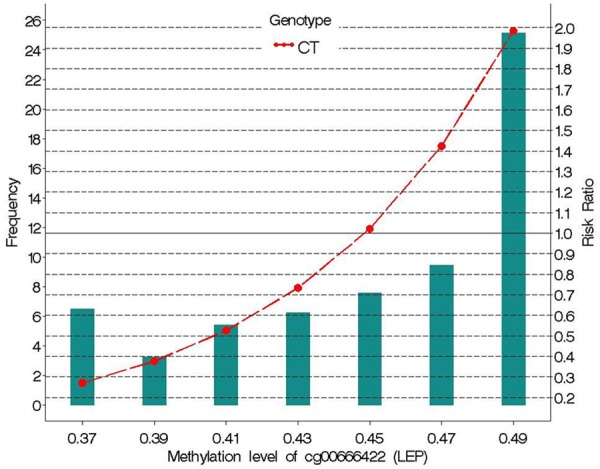
Risk ratio of asthma at age 18 versus methylation of the LEP CpG site cg00666422, for rs11763517 genotype CT relative to TT or CC (combined). The horizontal axis represents bins of DNA methylation and the green bars represent the frequency for those DNA methylation bins. The CT genotype (red dots) is plotted against asthma relative risk. The reference genotype was TT and CC combined.
The BAMSE cohort
The CpG site cg00666422 and the SNP rs11763517 that were found to be significantly associated with lung function and asthma in the Isle of Wight cohort were tested in the BAMSE cohort. The methylation level of this CpG site in BAMSE cohort ranged from 0.27 to 0.54 (median=0.42). In this cohort, cg00666422 × rs11763517 (CT vs. TT and CC) was not significantly associated with lung function markers. At age 8, DNA-M, FEV1, and FEV1/FVC measurements were available for boys and girls (Table 9). Although not significant, the results revealed same direction of association of cg00666422 × rs11763517 (CT vs. TT and CC) with FEV1/FVC as in Isle of Wight (Table 10). For boys and girls at age 16, this interaction term showed the same direction of association for FEV1/FVC, and FEF25-75% in both the cohorts (Table 11). The values of both these lung function markers decreased with increasing methylation for the CT genotype compared to the TT and CC genotype of rs11763517. However, opposite effect was found for the interaction term on FEV1 at ages 8 and 16.
Table 9.
Comparison of population characteristics of participants at ages 8 and 16 in BAMSE cohort
| Parameter | 8 Year (n=343) | 16 Year (n=294) |
|---|---|---|
| Height | 1.32 cm [1.16 cm-1.52 cm] | 1.74 cm [1.54 cm-2.04 cm] |
| Weight | 30.16 kg [21 kg-49.2 kg] | 67.41 kg [43.9 kg-107.4 kg] |
| FEV1 | 1768 [1060-2800] | 3885 [2400-6530] |
| FVC | 2075 [1240-3450] | 4761 [2670-7770] |
| FEF25-75% | NA | 4.084 [1.58-8.1] |
| Maternal asthma | ||
| Yes | 54 | 42 |
| No | 289 | 252 |
| Paternal asthma | ||
| Yes | 43 | 38 |
| No | 300 | 256 |
| Asthma doctor diagnosis up to | ||
| Yes | 178 | 163 |
| No | 165 | 131 |
| Atopy | ||
| Yes | 99 | 138 |
| No | 244 | 156 |
| Maternal smoking | ||
| Yes | 40 | 31 |
| No | 303 | 263 |
| Current smoking at 16 year | ||
| Yes | NA | 31 |
| No | NA | 263 |
| Sex | ||
| Male | 187 | 157 |
| Female | 156 | 137 |
Table 10.
Interaction effect of LEP CpG and SNP on FEV1, and FEV1/FVC in both boys and girls at 8 years of age in BAMSE cohort
| Parameter | Genotype | Estimate | Standard Error | p-value |
|---|---|---|---|---|
| FEV1 | ||||
| rs11763517 | CT | 0.321 | 0.1926 | 0.0967 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | -0.2451 | 0.2491 | 0.3257 | |
| cg00666422*rs11763517 | CT | 0.4786 | 0.4911 | 0.3305 |
| CC+TT | Ref. | Ref. | ||
| FEV1/FVC | ||||
| rs11763517 | CT | 0.000482 | 0.00601 | 0.93701 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | -0.00235 | 0.00785 | 0.76486 | |
| cg00666422*rs11763517 | CT | -0.09062 | 0.1555 | 0.56056 |
| CC+TT | Ref. | Ref. |
*Adjusted for maternal asthma, paternal asthma, maternal smoking, height, and weight at age 8, and weighted for sex.
Table 11.
Interaction effect of LEP CpG and SNP on FEV1, FEV1/FVC and FEF25-75% at 16 years of Age* in BAMSE cohort
| Parameter | Genotype | Estimate | Standard Error | p-value |
|---|---|---|---|---|
| FEV1 | ||||
| rs11763517 | CT | 0.01 | 0.05 | 0.83 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 0.064 | 0.63 | 0.92 | |
| cg00666422*rs11763517 | CT | 0.37 | 1.26 | 0.77 |
| CC+TT | Ref. | Ref. | ||
| FEV1/FVC | ||||
| rs11763517 | CT | 0.004 | 0.008 | 0.619 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 0.019 | 0.097 | 0.84 | |
| cg00666422*rs11763517 | CT | -0.18 | 0.19 | 0.347 |
| CC+TT | Ref. | Ref. | ||
| FEF25-75% | ||||
| rs11763517 | CT | 0.047 | 0.11 | 0.679 |
| CT+TT | Ref. | Ref. | ||
| cg00666422 | 0.567 | 1.39 | 0.68 | |
| cg00666422*rs11763517 | CT | -1.01 | 2.79 | 0.716 |
| CC+TT | Ref. | Ref. |
Adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, and weight at age 16, and weighted for sex.
Discussion
Our findings indicate that the interaction of genotype at LEP rs11763517 with DNA methylation at cg00666422 contributed to a decrease in FEV1, FEV1/FVC ratio, and FEF25-75%, and increased the risk of asthma at 18 years of age in the Isle of Wight birth cohort. The CpG site cg00666422 is located in the LEP 5’UTR and is 8622 base pairs (bp) away from the SNP rs11763517 located in the first intron (Figure 4). Additionally, this CpG is moderately methylated and has high variance in methylation (Table 2, Figure 5), suggesting that it may be more susceptible to changes in methylation triggered by environmental exposures. There have been prior studies suggesting a role of LEP SNPs on asthma [27], however, this is the first study to integrate the genetic and epigenetic factors and demonstrate that the methylation of the CpG site cg00666422 influences the penetrance of the SNP rs11763517 affecting lung function and asthma.
Figure 5.
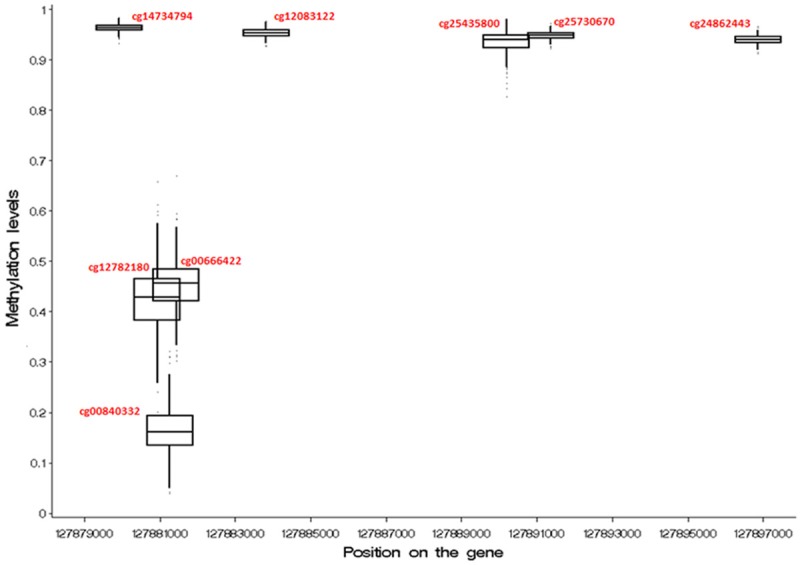
Methylation levels (β values) of the LEP CpG sites.
Participants for DNA-M analysis at age 18 were randomly selected from the cohort, limiting the chances of selection bias in the study. No differences other than sex were observed between the participants in the whole cohort and those in whom DNA-M was measured. We assessed more women than men at age 18 for DNA-M analysis due to a research focus on potential future pregnancy. To counter this imbalance, all regression models were weighted by sex. The probability of misclassification biases for asthma is low since asthma status was defined by clinical investigators based on accepted criteria.
A limitation of this study is that both lung function and DNA-M were measured at a single time point, which constrains our ability to determine if DNA-M is a consequence or a cause of the outcomes under study [28]. We addressed this with our study of a subset of participants at age 10 years and we found a high intra-class correlation coefficient (ICC=0.85) between the DNA-M of cg00666422 in a subset of n=34 girls between ages 10 and 18. Also, the mean, median, and 5th and 95th percentiles of methylation values for this CpG were similar between ages 10 and 18 (mean=0.46, median=0.47, 5th percentile=0.35, 95th percentile=0.55). This indicates that the DNA-M was probably established before age 18 and was stable before the study outcomes were measured, making reverse causation unlikely in this case. Additionally, the CpG site cg00666422 also shows a stable methylation from birth in the ARIES explorer dataset [29].
In the Isle of Wight study, we found that with increasing methylation of cg00666422, the interaction of the SNP (CT vs TT and CC) and categorized CpG leads to a decrease in FEV1, FEV1/FVC ratio, and FEF25-75% (Figure 2A-C). At 41%-43% methylation, we observed a cross-over in lung function outcomes for the CT vs CC levels of this SNP.
In the BAMSE cohort, we tested the effect of this cg00666422 × rs11763517 on lung function markers. Although the association was not significant in the BAMSE cohort, the direction of the effect was the same for cg00666422 × rs11763517 on FEV1/FVC for both ages 8 and 16, and FEF25-75% at age 16. However, FEV1 did not have the same direction of association as found in the Isle of Wight cohort. The partial replication and disagreement may be explained by different single nucleotide polymorphisms in linkage disequilibrium and/or correlated CpGs related to lung function in both cohorts. In the IOW birth cohort, we focused on rs11763517 and not rs10249476; both are in linkage disequilibrium (Figure 1). Also, in the IOW birth cohort we investigated the CpG site cg00666422, and not the two CpG sites next to this site: cg12782180 and cg00840332 (Figure 4). Additionally in the BAMSE cohort, methylation of cg00666422 was measured at a single time point at age 8. However, the methylation at age 8 may not predict the lung function at age 16 and this might lead to the difference in our results. Further investigation need to find out, whether similar interaction can be detected in the BAMSE cohort with slightly different SNPs or CpGs. We believe that it is unlikely that the IOW findings are spurious, since they could be replicated in the same cohort at a younger age.
Studies have shown that a decreased FEV1/FVC ratio and FEF25-75% indicates the presence of asthma [30-32], as marked by the presence of chronic airway obstruction [33]. In our study we identified that the same CpG and SNP interaction (cg00666422 × rs11763517) that affects lung function is also associated with the risk of asthma, such that the risk of asthma increases with increases in DNA-M. We also estimated the risk at each level of DNA-M, and found that risk of asthma is low (RR: 0.7) if the methylation level is below 45% and it increases steadily with increasing methylation thereafter (RR: 2.0 for 49% methylation) (Figure 3).
The LEP gene not only plays a vital role in regulating metabolism, but is also responsible for pro-inflammatory systemic effects and is expressed in lung tissue [34]. For the LEP gene, we did not find that the SNP x CpG interaction (cg00666422 × rs11763517) is associated with serum leptin levels. Also none of the LEP SNPs were associated with the leptin levels. However, in a prior study we reported that genetic variants at the leptin receptor genes (LEPR, LEPROT) are related to serum leptin levels [14] . Hence, it is possible that the levels of leptin in the serum are less regulated by the LEP gene but determined more by leptin receptor genes. In the present analysis, we adjusted all models with BMI at age 18, to see whether BMI influences the association of the SNP and the CpG sites with lung function or asthma. However, the cg00666422 × rs11763517 interactions with FEV1/FVC, FEF25-75%, and asthma were not diminished (data not shown). Thus, it is possible that DNA-M of the LEP modifies the penetrance of its SNPs to affect lung function and asthma independent of BMI and leptin.
Regarding exhaled nitric oxide, no association was detected. Exhaled nitric oxide has been shown to be strongly associated with allergic sensitization (atopy) [35]. However, in the Isle of Wight study, we found that the combined effect of the LEP SNP and the LEP CpG site was stronger among non-atopic participants (Table 8), which may indicate why we did not find an association with exhaled nitric oxide. Hence, our results are in concordance with findings that non-atopic inflammation and asthma may be explained by involvement of the leptin gene [36]. However, in the BAMSE cohort, no difference between atopic and non-atopic children was detected (Table 12).
Table 8.
*Effect of the interaction term of the CpG site and SNP on FEV1, FEV1/FVC, FEF25-75%, and asthma at age 18, stratified by atopy in IOW cohort
| Outcome | Genotype | Atopic individuals (n=239) | Non-atopic individuals (n=126) | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (S.E.) | p-value | Estimate (S.E.) | p-value | ||
| FEV1 | |||||
| rs11763517 | CT | 1.61 (0.74) | 0.033 | 1.19 (0.51) | 0.02 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | 2.14 (1.12) | 0.06 | 1.95 (0.79) | 0.015 | |
| cg00666422*rs11763517 | CT | -3.86 (1.58) | 0.02 | -2.65 (1.13) | 0.019 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| FEV1/FVC | |||||
| rs11763517 | CT | 0.17 (0.12) | 0.18 | 2.28 (0.08) | 0.0008 |
| CC+TT | Ref. | Ref. | |||
| cg00666422 | 0.27 (0.19) | 0.15 | 0.38 (0.13) | 0.0029 | |
| cg00666422*rs11763517 | CT | -0.39 (0.26) | 0.14 | -0.63 (0.18) | 0.0006 |
| CC+TT | Ref. | Ref. | |||
| FEF25-75% | |||||
| rs11763517 | CT | 2.99 (1.69) | 0.08 | 4.12 (1.08) | 0.0002 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | 4.51 (2.55) | 0.08 | 6.17 (1.68) | 0.0003 | |
| cg00666422*rs11763517 | CT | -7.18 (3.59) | 0.05 | -9.14 (2.39) | 0.0002 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| Asthma at age 18 | |||||
| cg00666422 | -3.25 (4.77) | 0.50 | -8.27 (4.03) | 0.04 | |
| rs11763517 | CT | -1.94 (2.82) | 0.49 | -10.30 (4.80) | 0.03 |
| rs11763517 | CC+TT | Ref. | Ref. | Ref. | Ref. |
| cg00666422*rs11763517 | CT | 5.08 (6.02) | 0.40 | 20.68 (10.06) | 0.04 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
Table 12.
Effect of the interaction term of the CpG site and SNP on FEV1, FEV1/FVC, and FEF25-75%, at age 16, stratified by atopy in BAMSE cohort
| Outcome | Genotype | Atopic individuals (n=138) | Non-atopic individuals (n=156) | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (S.E.) | p-value | Estimate (S.E.) | p-value | ||
| FEV1 | |||||
| rs11763517 | CT | 0.05 (0.08) | 0.52 | -0.01 (0.07) | 0.89 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | -0.94 (0.95) | 0.32 | 1.46 (0.87) | 0.097 | |
| cg00666422*rs11763517 | CT | 1.79 (1.87) | 0.34 | -0.95 (1.74) | 0.584 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| FEV1/FVC | |||||
| rs11763517 | CT | 0.02 (0.011) | 0.069 | -0.01 (0.01) | 0.308 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | -0.14 (0.14) | 0.33 | 0.22 (0.14) | 0.110 | |
| cg00666422*rs11763517 | CT | -0.25 (0.28) | 0.37 | -0.05 (0.27) | 0.848 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| FEF25-75% | |||||
| rs11763517 | CT | 0.29 (0.16) | 0.07 | -0.16 (0.16) | 0.340 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
| cg00666422 | -2.21 (1.97) | 0.26 | 3.96 (2.0) | 0.050 | |
| cg00666422*rs11763517 | CT | -0.33 (3.86) | 0.93 | -1.12 (3.98) | 0.779 |
| CC+TT | Ref. | Ref. | Ref. | Ref. | |
*Adjusted for maternal asthma, paternal asthma, maternal smoking, current smoking, height, and weight at age 16, and weighted for sex.
In summary, our results demonstrate the importance of interactions between genetic variants and DNA methylation of the LEP gene on spirometric lung function markers and asthma, particularly in non-atopic participants. Further studies should explore these epigenetic mechanisms and investigate the biological mechanism by which changes in LEP expression lead to asthma development. The involvement of a modifiable CpG site in the risk related to a single nucleotide polymorphisms (SNP) of the LEP gene suggests the SNP-related gene can be altered.
Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases under Award Number R01 AI091905-01 (PI: Wilfried Karmaus) and R01 AI061471 (PI: Susan Ewart). National Asthma Campaign, UK (Grant No 364) funded 10-year follow-up of this study and NIH/NHLBI R01 HL082925-01 funded the 18-year follow-up (PI: S. Hasan Arshad). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors gratefully acknowledge the participation and cooperation of the children and parents of Isle of Wight and appreciate the hard work of Mrs. Sharon Matthews and the Isle of Wight research team in collecting data and Nikki Graham for technical support. We thank the High-Throughput Genomics Group at the Welcome Trust Centre for Human Genetics (funded by Welcome Trust grant reference 090532/Z/09/Z and MRC Hub grant G0900747 91070) for the generation of the methylation data. The BAMSE study was supported by grants from the Swedish Foundation for Strategic Research (SSF), the Swedish Research Council, the Swedish Heart-Lung Foundation, Stockholm County Council (ALF), and the Strategic Research Programme (SFO) in Epidemiology at Karolinska Institutet.
Disclosure of conflict of interest
None.
References
- 1.Zhou E, Fu Y, Wei Z, Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-kappaB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014;5:2106–2112. doi: 10.1039/c4fo00384e. [DOI] [PubMed] [Google Scholar]
- 2.Holloway JW, Koppelman GH. Identifying novel genes contributing to asthma pathogenesis. Curr Opin Allergy Clin Immunol. 2007;7:69–74. doi: 10.1097/ACI.0b013e328013d51b. [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 4.Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M. Leptin and leptin receptor expression in asthma. J Allergy Clin Immunol. 2009;124:230–237. 237.e231–234. doi: 10.1016/j.jaci.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickson DA, Burchfiel CM, Petrini MF, Liu J, Campbell-Jenkins BW, Bhagat R, Marshall GD. Leptin is inversely associated with lung function in African Americans, independent of adiposity: the Jackson Heart Study. Obesity (Silver Spring) 2011;19:1054–1061. doi: 10.1038/oby.2010.240. [DOI] [PubMed] [Google Scholar]
- 8.Newson RB, Jones M, Forsberg B, Janson C, Bossios A, Dahlen SE, Toskala EM, Al-Kalemji A, Kowalski ML, Rymarczyk B, Salagean EM, van Drunen CM, Bachert C, Wehrend T, Kramer U, Mota-Pinto A, Burney P, Leynaert B, Jarvis D. The association of asthma, nasal allergies, and positive skin prick tests with obesity, leptin, and adiponectin. Clin Exp Allergy. 2014;44:250–260. doi: 10.1111/cea.12221. [DOI] [PubMed] [Google Scholar]
- 9.Szczepankiewicz A, Breborowicz A, Sobkowiak P, Popiel A. Are genes associated with energy metabolism important in asthma and BMI? J Asthma. 2009;46:53–58. doi: 10.1080/02770900802460514. [DOI] [PubMed] [Google Scholar]
- 10.Soto-Ramirez N, Arshad SH, Holloway JW, Zhang H, Schauberger E, Ewart S, Patil V, Karmaus W. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clin Epigenetics. 2013;5:1. doi: 10.1186/1868-7083-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockett GA, Patil VK, Soto-Ramirez N, Ziyab AH, Holloway JW, Karmaus W. Epigenomics and allergic disease. Epigenomics. 2013;5:685–699. doi: 10.2217/epi.13.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karmaus W, Ziyab AH, Everson T, Holloway JW. Epigenetic mechanisms and models in the origins of asthma. Curr Opin Allergy Clin Immunol. 2013;13:63–69. doi: 10.1097/ACI.0b013e32835ad0e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto-Ramirez N, Ziyab AH, Karmaus W, Zhang H, Kurukulaaratchy RJ, Ewart S, Arshad SH. Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: measures of change and stability. J Epidemiol. 2013;23:399–410. doi: 10.2188/jea.JE20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefi M, Karmaus W, Zhang H, Ewart S, Arshad H, Holloway JW. The methylation of the LEPR/LEPROT genotype at the promoter and body regions influence concentrations of leptin in girls and BMI at age 18 years if their mother smoked during pregnancy. Int J Mol Epidemiol Genet. 2013;4:86–100. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuan PF, Wang S, Zhou X, Chu H. A statistical framework for Illumina DNA methylation arrays. Bioinformatics. 2010;26:2849–2855. doi: 10.1093/bioinformatics/btq553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Yan L, Hu Q, Sucheston LE, Higgins MJ, Ambrosone CB, Johnson CS, Smiraglia DJ, Liu S. IMA: an R package for high-throughput analysis of Illumina’s 450K Infinium methylation data. Bioinformatics. 2012;28:729–730. doi: 10.1093/bioinformatics/bts013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 19.I FR. Koko spirometer & koko digidoser windows operations guide. 2002. appendix a: Normal equations. [Google Scholar]
- 20.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 21.Gertz J, Varley KE, Reddy TE, Bowling KM, Pauli F, Parker SL, Kucera KS, Willard HF, Myers RM. Analysis of DNA methylation in a threegeneration family reveals widespread genetic influence on epigenetic regulation. PLoS Genet. 2011;7:e1002228. doi: 10.1371/journal.pgen.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kull I, Melen E, Alm J, Hallberg J, Svartengren M, van Hage M, Pershagen G, Wickman M, Bergstrom A. Breast-feeding in relation to asthma, lung function, and sensitization in young schoolchildren. J Allergy Clin Immunol. 2010;125:1013–1019. doi: 10.1016/j.jaci.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(Suppl 15):11–13. doi: 10.1034/j.1399-3038.13.s.15.10.x. [DOI] [PubMed] [Google Scholar]
- 24.Gruzieva O, Merid SK, Melen E. An update on epigenetics and childhood respiratory diseases. Paediatr Respir Rev. 2014;15:348–354. doi: 10.1016/j.prrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Hallberg J, Thunqvist P, Schultz ES, Kull I, Bottai M, Merritt AS, Chiesa F, Gustafsson PM, Melen E. Asthma phenotypes and lung function up to 16 years of age-the BAMSE cohort. Allergy. 2015;70:667–673. doi: 10.1111/all.12598. [DOI] [PubMed] [Google Scholar]
- 26.Melen E, Granell R, Kogevinas M, Strachan D, Gonzalez JR, Wjst M, Jarvis D, Ege M, Braun-Fahrlander C, Genuneit J, Horak E, Bouzigon E, Demenais F, Kauffmann F, Siroux V, Michel S, von Berg A, Heinzmann A, Kabesch M, Probst-Hensch NM, Curjuric I, Imboden M, Rochat T, Henderson J, Sterne JA, McArdle WL, Hui J, James AL, William Musk A, Palmer LJ, Becker A, Kozyrskyj AL, Chan-Young M, Park JE, Leung A, Daley D, Freidin MB, Deev IA, Ogorodova LM, Puzyrev VP, Celedon JC, Brehm JM, Cloutier MM, Canino G, Acosta-Perez E, Soto-Quiros M, Avila L, Bergstrom A, Magnusson J, Soderhall C, Kull I, Scholtens S, Marike Boezen H, Koppelman GH, Wijga AH, Marenholz I, Esparza-Gordillo J, Lau S, Lee YA, Standl M, Tiesler CM, Flexeder C, Heinrich J, Myers RA, Ober C, Nicolae DL, Farrall M, Kumar A, Moffatt MF, Cookson WO, Lasky-Su J. Genome-wide association study of body mass index in 23 000 individuals with and without asthma. Clin Exp Allergy. 2013;43:463–474. doi: 10.1111/cea.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Borst B, Souren NY, Loos RJ, Paulussen AD, Derom C, Schols AM, Zeegers MP. Genetics of maximally attained lung function: a role for leptin? Respir Med. 2012;106:235–242. doi: 10.1016/j.rmed.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Black M. Why Cannot an Effect Precede its Cause? Analysis. 1956;16:49–58. [Google Scholar]
- 29. http://www.ariesepigenomics.org.uk/ariesexplorer.
- 30.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167:917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 31.Alberts WM, Ferris MC, Brooks SM, Goldman AL. The FEF25-75% and the clinical diagnosis of asthma. Ann Allergy. 1994;73:221–225. [PubMed] [Google Scholar]
- 32.Tavakol M, Gharagozlou M, Afaride M, Movahedi M, Tavakol Z. Asthma diagnosis and treatment - 1002. FEF25-75%: a more sensitive indicator in the early detection of asthma. World Allergy Organ J. 2013;6(Suppl 1):P2. doi: 10.1186/1939-4551-6-S1-P2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang F, del-Rio-Navarro BE, Alcantara ST, Ontiveros JA, Cienfuegos DR, Bello Gonzalez SA, Villafana S, Bravo G, Hong E. Plasminogen activator inhibitor-1, fibrinogen, and lung function in adolescents with asthma and obesity. Endocr Res. 2012;37:135–144. doi: 10.3109/07435800.2012.654555. [DOI] [PubMed] [Google Scholar]
- 34.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol (1985) 2010;108:744–753. doi: 10.1152/japplphysiol.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, Arshad SH, Roberts G. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65:258–262. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mai XM, Chen Y, Krewski D. Does leptin play a role in obesity-asthma relationship? Pediatr Allergy Immunol. 2009;20:207–212. doi: 10.1111/j.1399-3038.2008.00812.x. [DOI] [PubMed] [Google Scholar]


