Abstract
AIM: To investigate the role of diarylpropionitrile (DPN), a selective agonist of estrogen receptor β (ERβ), in liver cirrhosis with portal hypertension (PHT) and isolated hepatic stellate cells (HSCs).
METHODS: Female Sprague-Dawley rats were ovariectomized (OVX), and liver cirrhosis with PHT was induced by CCl4 injection. DPN and PHTPP, the selective ERβ agonist and antagonist, were used as drug interventions. Liver fibrosis was assessed by hematoxylin and eosin (HE) and Masson’s trichrome staining and by analyzing smooth muscle actin expression. Hemodynamic parameters were determined in vivo using colored microspheres technique. Protein expression and phosphorylation were determined by immunohistochemical staining and Western blot analysis. Messenger RNA levels were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). Collagen gel contraction assay was performed using gel lattices containing HSCs treated with DPN, PHTPP, or Y-27632 prior to ET-1 addition.
RESULTS: Treatment with DPN in vivo greatly lowered portal pressure and improved hemodynamic parameters without affecting mean arterial pressure, which was associated with the attenuation of liver fibrosis and intrahepatic vascular resistance (IHVR). In CCl4-treated rat livers, DPN significantly decreased the expression of RhoA and ROCK II, and even suppressed ROCK II activity. Moreover, DPN remarkedly increased the levels of endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS, and promoted the activities of protein kinase G (PKG), which is an NO effector in the liver. Furthermore, DPN reduced the contractility of activated HSCs in the 3-dimensional stress-relaxed collagen lattices, and decreased the ROCK II activity in activated HSCs. Finally, in vivo/in vitro experiments demonstrated that MLC activity was inhibited by DPN.
CONCLUSION: For OVX rats with liver cirrhosis, DPN suppressed liver RhoA/ROCK signal, facilitated NO/PKG pathways, and decreased IHVR, giving rise to reduced portal pressure. Therefore, DPN represents a relevant treatment choice against PHT in cirrhotic patients, especially postmenopausal women.
Keywords: Portal hypertension, Estrogen receptor, Rho-kinase signaling, Nitric oxide, Hepatic stellate cells
Core tip: Liver cirrhosis and portal hypertension (PHT) are subject to gender and estrogen levels. The aim of the present study was to investigate whether estrogen receptor β selective agonists could ameliorate intrahepatic resistance and mitigate PHT in rats with CCl4-induced cirrhosis, and uncover the underlying mechanism by investigating RhoA/ROCK and NO/PKG signaling. The authors propose that treatment with an estrogen receptor β selective agonist could improve cirrhotic PHT via regulating RhoA/ROCK and NO/PKG signaling.
INTRODUCTION
Increased intrahepatic vascular resistance (IHVR) to portal blood flow is a major contribution to portal hypertension (PHT) in liver cirrhosis[1,2], and decreased splanchnic vascular resistance worsens and maintains the increased portal pressure (PP)[2,3]. Over the past 20 years, with a keen grasp on hepatic microcirculation, a dynamic component involving changes in hepatic vascular tone has been demonstrated to contribute to IHVR; hence, increased vascular tone augments IHVR[4,5]. Apart from structural changes (fibrosis, vascular remodeling, vascular occlusion, and nodule formation), activated hepatic stellate cells (HSCs), contraction of intrahepatic vascular smooth muscle cells (VSMCs) and decreased levels of NO vasodilator, all play a critical role in contributing to increased IHVR[4,6]. It is well known that the intrahepatic upregulation of RhoA/ROCK signaling, as well as the inhibition of NO/PKG signaling, contributes to increased IHVR[7-9]. Furthermore, the two pathways regulate each other, maintaining the balance between phosphorylation and dephosphorylation of myosin light chains (MLC)[9-11]. Thus the two pathways are crucial therapeutic targets to inhibit the increased IHVR and PP occurring in cirrhosis.
Epidemiological studies have reported the male to female ratio among patients with cirrhosis is in the range of 2.3:1-2.6:1; moreover, menopause increases the susceptibility to cirrhosis and PHT[12,13]. Animal experiments and clinical trials have provided consistent evidence for the protective effect of endogenous and exogenous estrogen on liver fibrosis[12-16]. However, the administration of exogenous estrogen had its potential risks, causing their clinical use[17] to be impeded. Fortunately, previous studies regarding estrogen receptor (ER) subtypes have indicated that estrogen receptor β (ERβ) gives rise to few side effects of estrogen[18], while ERα mediates the majority effects of estrogen on classic estrogen target tissues, as well as their associated side effects[19]. Interestingly, high ERβ expression levels and low ERα expression levels were observed in both men’s and women’s normal and fibrotic livers, and HSCs had functional ERβ, rather than ERα, which responded directly to estradiol (E2) exposure[20]. ERβ selective agonists hold the key to producing protective effects of estrogens on liver cirrhosis and PHT, while reducing undesired side effects[21].
Therefore, this study investigated the effect of diarylpropionitrile (DPN), an ERβ selective agonist, on the intrahepatic RhoA/ROCK and NO/PKG pathways, and on hepatic hemodynamics systemically as well.
MATERIALS AND METHODS
Animals
Female SD rats - initially weighing 180-200 g - were acquired from the Laboratory Animal Center of School of Medicine, Shanghai Jiao Tong University, China. Under the constant temperature of 21 °C, rats were exposed to a light/darkness cycle of 12 h/12 h, and accessed to water and standard rat chow. All animal experiments conformed to guidelines on caring and using lab animals which were reviewed by the Research Ethics Committee of Renji Hospital (No. RJ-20151211).
Treatment regimens
Rats were assigned to a sham-operated control group (n = 15) or an ovariectomized (OVX) group (n = 45) in a random way. The rats were intraperitoneally injected with ketamine (100 mg/kg per body weight) and xylazine (12 mg/kg per body weight) for anesthesia. The surgical procedure was performed from a midline back incision and both ovaries were removed. The control group received the same incisions and the two ovaries were explored but not excised. The animals were allowed 2 wk for recovery. OVX rats were divided into three groups with 15 in each, as below: OVX + CCl4 group, OVX + CCl4 + DPN group and OVX + CCl4 + DPN + PHTPP group.
CCl4 administration
The rats needed to weigh and administer mixed food on a daily basis. For the OVX + CCl4 group, the subcutaneous injection at a dose of 4 mL/kg was conducted twice a week while doubling dosage for the first injection, as 400 mL/L CCl4 with olive oil needs to be done. After 14-16 mo, this procedure led to micro nodular cirrhosis with PHT. In addition to this, the OVX + CCl4 + DPN group was treated subcutaneously with 30 nmol/100 g DPN in 1 mL dimethyl sulfoxide (DMSO), twice weekly. Along with CCl4 and DPN, the OVX + CCl4 + DPN + PHTPP group also received 30 nmol/100 g PHTPP in 1 mL DMSO, twice weekly. The control group was injected with 1 mL DMSO, twice weekly. After 14 to 16 mo, CCl4 and drug injections were stopped within 6 d prior to the start of experiments. Although there were 15 rats in each group at the beginning of the study, the number of the rats decreased to 11, 13 and 12 in the OVX + CCl4, OVX + DPN + CCl4 and OVX + DPN + PHTPP + CCl4 groups, respectively, due to death caused by illness. Five rats from each group were sacrificed for tissue harvesting. Sample livers were kept in formaldehyde or snap-frozen by liquid nitrogen under the temperature of -80 °C. The mesenteric arteries were used to detect mesenteric arteriole reactivity. The other rats were used for hemodynamic studies and their blood was used to analyze biochemical parameters.
Hemodynamic studies
When rats were given ketamine anesthesia (100 mg/kg, imp), in a median laparotomy, a PE-50 catheter was inserted into a small ileocaecal vein and guided to the portal vein to measure PP. A PE-50 catheter was introduced to a left femoral artery to measure mean arterial pressure (MAP). An additional PE-50 catheter was inserted from a right carotid artery leading to the left ventricle, which was used for microsphere injection. The femoral artery catheters and the portal vein were in connection with a pressure transducer (M100613, United States Philips Corporation). The PP and MAP were recorded on a multi-channel recorder (COLIN, BP508, Japan). The zero reference point referred to the spot of 1 cm above the operating table.
The Dye-Trak microsphere technique was performed as per previous description. Briefly, the 1-min withdrawal (0.65 mL/min) of a reference sample was conducted with a continuous extraction pump (ALC-IP900, shanghai, china). Suspending in the solution of 0.3 mL saline with 0.05% Tween, approximately 300000 yellow microspheres of 15.5 μm in diameter (Triton Technologies, San Diego, California, United States) were injected into the left ventricle within 10 s of starting blood withdrawal. Suspending in a solution as same as yellow ones, 150000 blue microspheres was injected into an ileocaecal vein within 30 s to evaluate mesenteric portal-systemic shunt volume. Ten minutes later, the rats were sacrificed by injecting KCl intravenously. The blood and tissue samples were assimilated by a portion of 3.8 mL of 5.3 mol/L KOH and 0.5 mL Tween 80, and were subsequently boiled for 1 h. Then ready samples were processed by vortex and filtered with Whatman Nucleopore filters (Whatman International, Maidstone, United Kingdom). Colors were extracted from the filtered microspheres by using 0.2 mL dimethyl formamide and measured by absorption spectrophotometry. Hemodynamic parameters were measured and calculated according to standard methods[22,23]. Afterwards, with the software of Triton Technologies, cardiac output and organ blood flow were calculated and expressed based on 100 g per body weight. Splanchnic perfusion pressure was obtained by deducting PP from MAP. Splanchnic vascular resistance was rated by the splanchnic perfusion pressure to the splanchnic blood flow. Mesenteric portal-systemic shunt flow was derived from the fraction in the lung out of total injected blue microspheres. Hepatic portal-vascular resistance was estimated as PP divided by the sum of gastrointestinal and splenic perfusion minus mesenteric portal-systemic shunt flow. Systemic vascular resistance (SVR) was defined as the ratio of MAP to cardiac output.
Histological and immunohistochemical assessment
HE, immunohistochemical, and trichrome collagen staining were applied to examine liver sections (4 mm) on glass slides with silane coating. Liver sections were evaluated randomly by an accomplished liver pathologist yet unfamiliar with animal groups.
For immunohistochemistry, primary antibodies (Cell Signaling Technology, Danvers, MA) were used at a dilution of 1:200 (phosphor-Thr18/Ser19-PML) or 1:100 (α-SMA) to incubate liver sections, after the incubation in the streptavidin-peroxidase complex. Peroxidase conjugates were then viewed in diaminobenzidine (DAB) solution. Afterwards, the prepared livers were processed by hematoxylin counterstaining and then covered with a plate.
Collagen contents were quantified using Masson’s trichrome collagen stain, and the Masson-stained areas are reported to be its ratio to the total area. The positive areas were analyzed using Image J software. The liver sections were then averagely valued among five rats from each group.
Quantitative RT-PCR
An array of processes need to be done, including separating RNA from 30 mg shock-frozen hepatic tissue with TRIzol (Invitrogen), using MMLV reverse transcriptase (Invitrogen) to perform reverse transcription, conducting quantitative RT-PCR (qRT-PCR) with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen), and preparing primers and probes for RT-PCR with Primer Express Software (Applied Biosystems, Foster City, CA). 18S rRNA was used for the endogenous control. The used primer sequences were the following: GAPDH, (Forward) GGAGTCCACTGGCGTCTTC and (Reverse) GGCATTGCTGATGATCTTGAGG; RhoA, (Forward) GGCAGAGATATGGCAAACAGG and (Reverse) TCCGTCTTTGGTCTTTGCTGA; ROCK-II, (Forward) CCCGATCATCCCCTAGAACC and (Reverse) TTGGAGCAAGCTGTCGACTG.
Western blot and antibodies
Shock-frozen section samples were processed by homogenization in buffer containing 25 mmol/L Tris/HCl, 5 mmol/L ethylenediamine tetraacetic acid, 10 μmol/L phenylmethanesulfonyl fluoride, 1 mmol/L benzamidine, and 10 μg/mL leupeptin. Liver samples were put in the buffer for dilution. Homogenate protein concentrations were determined using the BCA Protein Assay kit (Beyotime, Haimen, China). Samples (40 μg of protein/lane) were assayed by SDS-PAGE (15% gels for RhoA and p-MLC; 8% for ROCK, iNOS, eNOS, and p-eNOS; and 10% for moesin, p-moesin, VASP, p-VASP, and α-SMA). After electrophoresis, protein was shifted under the effect of a 250 mA current for 1.5 h to a polyvinylidene difluoride membrane which was stemmed by 5% BSA for 2 h and treated as below in primary antibodies at the temperature of 4 °C overnight: DAPDH, ERβ, α-SMA, iNOS, eNOS, and p-eNOS (Ser1177) from Abcam (Cambridge, United Kingdom); RhoA, ROCKII, moesin, p-moesin (Thr558), VASP, p-VASP (Ser239), MLC, and p-MLC (Thr18/Ser19) from Santa Cruz Biotechnology (Santa Cruz, CA). The membrane was then processed for 1 h with appropriate secondary antibodies (Abcam) at a 1:5000 dilution. Fluorescent signals were detected with an Odyssey Imaging System (Li-Cor Biosciences, Lincoln, NE). GAPDH served as an internal control.
Assessment of PKG, ROCK and MLC activities
PKG activity was assessed and indicated by the phosphorylation level of endogenous substrate VASP at Ser239[8,22]. ROCK activity was evaluated by measuring the phosphorylation of endogenous substrate, moesin, at Thr558[8,22]. MLC activity was assessed by measuring the phosphorylation of MLC at Thr18/Ser19[24]. The analysis was performed by Western blot with site- and phosphor-specific antibodies.
Cell culture
HSCs were separated from male SD rats with the weights of 300-400 g as per previous description[24]. Technically, after in situ sequential perfusion of the solutions of collagenase IV (Sigma, St. Louis, United States) and pronase E (Merck, Darmstadt, Germany) to hepatic samples, decentralized cells were separated by density gradient centrifugation with Optiprep (Nycomed, Sweden). Cells were obtained at a density less than 1.053 (9% Optiprep). Viability and purity were determined to be higher than 95% as per Trypan blue exclusion and morphological characteristics. Then cells were plated onto uncovered plastic culture plates, being cultivated with Dulbecco’s Modified Eagle Medium without Phenol Red (DMEM; Invitrogen), which was complemented with 10% fetal bovine serum, 0.6 IU/mL insulin, 2 mmol/L glutamine, and 1% antibiotic-antimycotic solution (Invitrogen), and was renewed every 48-72 h.
Collagen gel contraction assay
Collagen gel contraction experiments were conducted upon slight modifications[25] as per previous descriptions. Briefly, hydrated collagen gels were produced with rat tail tendon collagen I (Becton Dickinson Labware, Bedford, MA) and made adjustments with 0.1 N NaOH and 10 × PBS, to a final collagen concentration of 1.2 mg/mL and pH 7.4 at 4 °C. A 500 μL portion of collagen solution was put into wells of a 24-well tissue culture dish for 1 h incubation at 37 °C. Then HSCs were layered on top of the collagen lattice of 5 × 105 cells/mL. Twenty-four hours after adding 1 mL/well of serum free culture medium and the starvation process, lattices in stability were flushed twice with 1 × PBS. After pretreatment with DPN (10-7 mol/L), DPN (10-7 mol/L) + PHTPP (10-7 mol/L), or Y-27632 (10-5 mol/L) for 30 min, HSCs were exposed to ET-1 (10-8 mol/L, Roche Diagnostics, Brussels, Belgium). Buffer without ET-1 was used as a control. Gels were immediately detached with the tip of a 100 μL pipette from the plates in the pattern of gentle circumferential dislodgment. Digital photos were obtained to monitor the change in lattice area 4 h after addition of the contractile agonist. All information was drawn from studies of more than three sets of triple collagen lattices by cultured HSCs out of three different rat HSC separations.
Analysis of Rho kinase and MLC activity in HSCs
Activated HSCs grown on culture dishes were starved for 24 h. HSCs were pretreated with DPN (10-7 mol/L), DPN (10-7 mol/L) + PHTPP (10-7 mol/L), or Y-27632 (10-5 mol/L) for 30 min, prior to exposure to ET-1 (10-8 mol/L) for 4 h. Buffer without ET-1 was used as a control. ROCK and MLC activities were assessed by monitoring the phosphorylation levels of moesin at Thr558 and MLC at Thr18/Ser19, respectively[22,26].
Statistical analysis
Data are expressed as mean ± SE. After the Bonferroni/Dunn or Mann-Whitney U test, ANOVA was applied to compare among groups (SPSS 21 for Windows, SPSS Inc., Chicago, IL). A P-value < 0.05 had great statistical significance. With regards to analyzing concentration response curves, the information was seated by nonlinear regression in the Prism computing project (Graph Pad Software Inc., San Diego, CA).
RESULTS
Biochemical parameters
CCl4 caused a great increase of the analyzed biochemical parameters, including ALT, AST and bilirubin, and a significant decrease in albumin. However, treatment with DPN decreased the ALT, AST and bilirubin levels, and increased the albumin levels in PHT rats. PHTPP counteracted the effect of DPN (Table 1).
Table 1.
Biochemical parameters of the different treatment groups
| Group | ALT (U/L) | AST (U/L) | Bilirubin (mg/dL) | Albumin (g/L) | BUN (mmol/L) | Scr (μmol/L) |
| Control (n = 8) | 43.3 ± 7.7 | 124 ± 14 | 0.3 ± 0.1 | 34.1 ± 3.4 | 8.7 ± 1.2 | 22.4 ± 4.0 |
| OVX + CCl4 (n = 6) | 166 ± 10.1ab | 439 ± 19ab | 3.4 ± 0.3ab | 21.5 ± 2.8ab | 16.7 ± 1.8ab | 35.0 ± 4.7ab |
| OVX + CCl4 + DPN (n = 7) | 86.1 ± 8.7a | 211 ± 15a | 1.7 ± 0.2a | 31.0 ± 3.3 | 9.6 ± 1.5 | 24.9 ± 4.5 |
| OVX + CCl4 + DPN + PHTPP (n = 6) | 168 ± 10.2ab | 436 ± 23ab | 3.3 ± 0.4ab | 20.8 ± 2.0ab | 16.5 ± 2.1ab | 37.5 ± 4.6ab |
P < 0.05 vs control group;
P < 0.05 vs DPN group. OVX: Ovariectomized; SVR: Systemic vascular resistance; DPN: Diarylpropionitrile.
Morphological characteristics of the rat livers
CCl4 caused significant hepatocyte steatosis, fibrous proliferation of interlobular portal areas, and formation of pseudolobules and tubercles, whereas DPN attenuated these phenomena (Figure 1A). This could be verified by trichrome staining (Figure 1B) from a histological perspective. Collagen content quantification demonstrated that the collagen volume fraction increased in CCl4-induced cirrhotic rats (24.8% ± 4.8%) in comparison with the control group (2.1% ± 0.5%), however, DPN treatment played a significant role in inhibiting the secretion of collagen (17.0% ± 4.0%). There was no statistically significant difference in collagen content between the PHTPP group (22.9% ± 4.9%) and the CCl4-induced group (Figure 1C).
Figure 1.
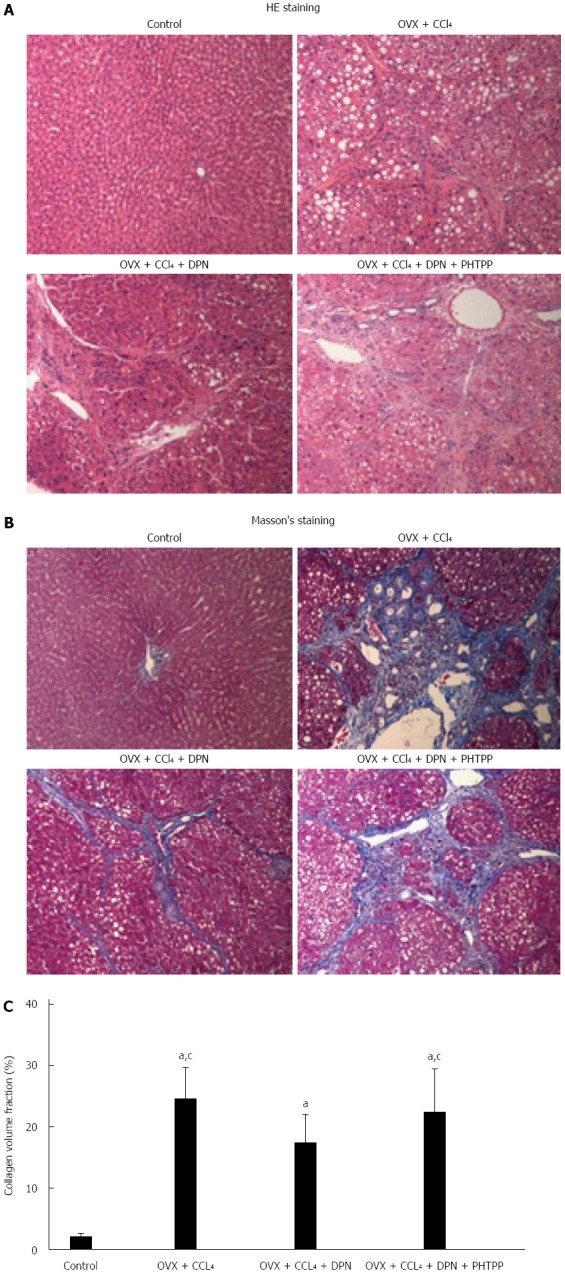
Therapeutic effects of diarylpropionitrile on hepatic fibrosis in CCl4-treated rats. Histological images of rat livers stained with HE (A) or Masson’s staining (B) (magnification × 100) and semi-quantitative measurement of Masson’s staining (C). aP < 0.05 vs control group; cP < 0.05 vs DPN group. DPN: Diarylpropionitrile.
Expression of α-SMA in rat livers
The expression of α-SMA correlated with the activity of HSCs and the degree of cirrhosis. Immunohistochemical staining and Western blot analysis against α-SMA consistently showed a significant increase in α-SMA expression in the OVX + CCl4 and OVX + CCl4 + DPN + PHTPP groups, while DPN treatment greatly decreased the expression of hepatic α-SMA in CCl4-treated rats (Figure 2A-D).
Figure 2.
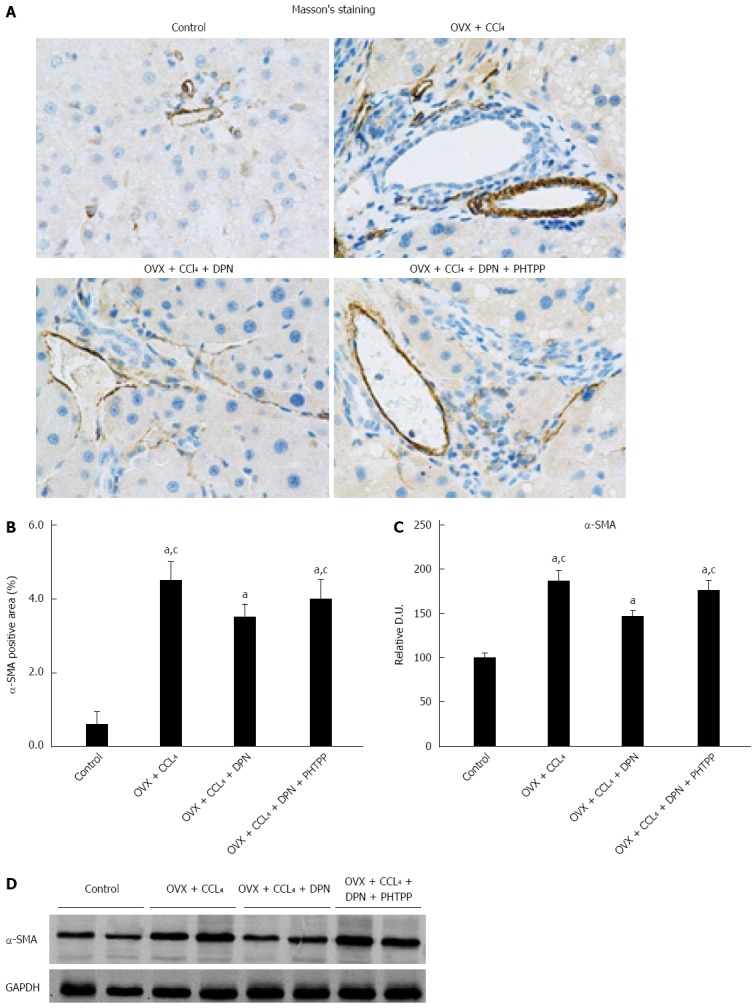
Diarylpropionitrile downregulates α-SMA expression in the livers of CCl4-treated rats. A and B: Immunohistochemical staining for α-SMA (magnification × 400); C and D: Analysis of α-SMA protein expression by Western blot (each group n = 5). aP < 0.05 vs control group; cP < 0.05 vs DPN group. DPN: Diarylpropionitrile.
Expression of ERβ in rat livers
ERβ expression was present in the rat livers of all of four groups. The expression of ERβ in DPN treated rats was slightly higher than that in the other three groups, although there was no statistically significant difference between all groups (Figure 3A-B)
Figure 3.
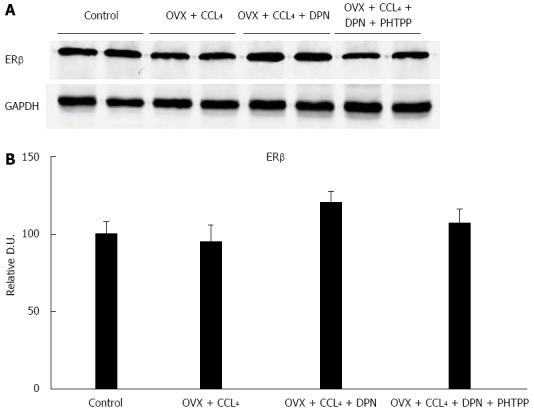
There were no statistically significant differences between the hepatic estrogen receptor β protein expression levels of all groups, as determined by Western blot analysis (A-B) (each group n = 5). aP < 0.05 vs control group; cP < 0.05 vs DPN group. DPN: Diarylpropionitrile; ERβ: Estrogen receptor β.
In vivo hemodynamic studies
In the OVX + CCl4 group there were markedly increased PP and IHVR compared to the control group. However, treatment with DPN significantly decreased PP and IHVR, while PHTPP counteracted the effect of DPN (Table 2). With regards to hyperdynamic circulation, the OVX + CCl4 treated rats presented markedly increased CO and PVI, and decreased MAP, TPR and SVR. Nevertheless, DPN significantly decreased CO and PVI, and increased SVR, but did not affect MAP and TPR. PHTPP counteracted the effect of DPN on CO, PVI and SVR (Table 2).
Table 2.
In vivo hemodynamic data of the four treatment groups
| Parameter | Control | OVX + CCl4 | OVX + CCl4 + DPN | OVX + CCl4 + DPN + PHTPP |
| PP (mmHg) | 7.5 ± 1.1 | 14.9 ± 1.6a,c | 11.0 ± 1.3a | 14.6 ± 1.5a,c |
| CO (mL/min per 100 g) | 19.3 ± 2.4 | 32.2 ± 5.0a,c | 25.7 ± 4.0a | 31.4 ± 5.4a,c |
| MAP (mmHg) | 120.6 ± 14.2 | 82.1 ± 12.2a | 95.7 ± 14.0a | 89.5 ± 13.3a |
| TPR (mmHg/mL/min per 100 g) | 6.0 ± 0.9 | 2.6 ± 0.3a | 3.1 ± 0.7a | 2.9 ± 0.6a |
| PVI (mL/min per 100 g) | 2.1 ± 0.3 | 4.8 ± 0.8a,c | 3.0 ± 0.4a | 4.4 ± 0.6a,c |
| SVR (mmHg/mL/min per 100 g) | 55.5 ± 8.5 | 15.0 ± 2.3a,c | 31.7 ± 3.7a | 16.8 ± 1.5a,c |
| PSS (%) | 0.2 ± 0.1 | 50.5 ± 6.3a,c | 27.9 ± 4.9a | 48.1 ± 6.1a,c |
| IHVR (mmHg/mL/min per 100 g) | 1.5 ± 0.2 | 3.2 ± 0.6a,c | 2.2 ± 0.3a | 3.0 ± 0.5a,c |
P < 0.05 vs control group; bP < 0.05 vs DPN group. OVX: Ovariectomized; SVR: Systemic vascular resistance; DPN: Diarylpropionitrile.
Effect of DPN on the RhoA/ROCK pathway in rat livers
In comparison with those sham-operated non-cirrhotic rats, both RhoA and ROCK protein levels were dramatically improved in the OVX + CCl4 rat livers. Treatment with DPN significantly down-regulated RhoA and ROCK protein levels, though they remained higher than those in sham-operated rats. In contrast, treatment with PHTPP counteracted DPN (Figure 4A-B). In all four groups, the mRNA expression levels of both RhoA and ROCK were consistent with RhoA and ROCK protein levels (Figure 4C). As a ROCK activity indicator, the moesin phosphorylation was examined by Western blot. Western blot analysis showed that p-moesin (Thr558) levels were significantly enhanced in OVX + CCl4 rat livers. DPN treatment significantly decreased the level of p-moesin (Thr558), while PHTPP treatment counteracted the effect of DPN. These differences were not in association with variation of overall moesin levels, alike in all groups (Figure 4D-E).
Figure 4.
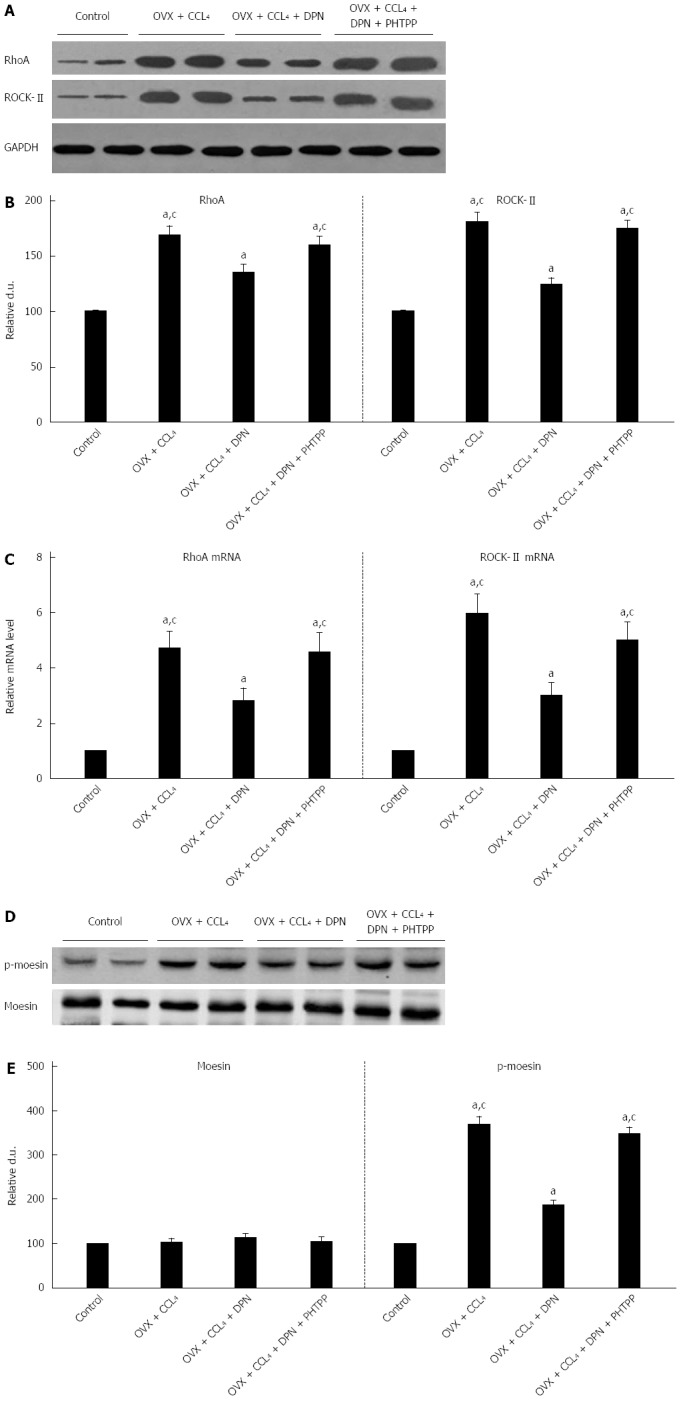
Diarylpropionitrile inhibits the protein (A, B) and mRNA (C, D) expression of RhoA and ROCKII, and even suppresses the site-specific phosphorylation of moesin (Thr558) in CCl4-treated rats (D, E). Shown are the relative densitometric quantifications of all experiments (mean ± SE), with values from the sham-operated controls set to 100 DU. aP < 0.05 vs control group; cP < 0.05 vs DPN group. DPN: Diarylpropionitrile.
Effect of DPN on the NO/PKG pathway in rat livers
There was no distinction between the intrahepatic eNOS expression levels of the OVX + CCl4 group and the control group, but there was an important decrease in intrahepatic eNOS phosphorylation amount. Treatment with DPN not only markedly increased the expression of eNOS, but also significantly up-regulated p-eNOS (Ser1177) levels in the cirrhotic livers of OVX rats (Figure 5A-B).
Figure 5.
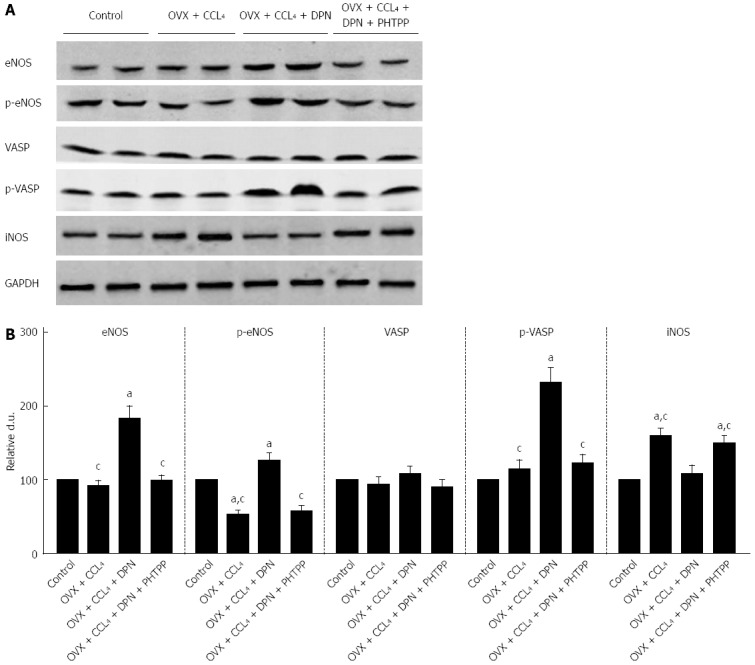
Diarylpropionitrile increases the hepatic expression of NO/PKG pathway proteins and increases their activity but inhibits hepatic iNOS expression in CCl4-treated rats. A: Western blot analysis of eNOS, p-eNOS, VASP, p-VASP, and iNOS protein expression; B: Relative densitometric quantifications of all experiments (mean ± SE), with the values from the controls set to 100 DU (each group n = 5). aP < 0.05 vs control group; cP < 0.05 vs DPN group.
PKG activity was evaluated by measuring the phosphorylation of its endogenous substrate, VASP at Ser239. Western blot analysis using p-VASP (Ser239) antibodies revealed that p-VASP (Ser239) levels remained unchanged in the OVX + CCl4 and PHTPP treated rats, compared to the control group. However, DPN treatment significantly enhanced intrahepatic p-VASP (Ser239) levels. No differences in total VASP expression were found between treatment groups. Therefore, it can be said that DPN increases PKG activity in the livers of OVX + CCl4 rats (Figure 5A-B).
Regarding the expression of iNOS, Western blot analysis revealed that the marked increase in iNOS expression seen in the cirrhotic livers OVX rats could be inhibited by DPN (Figure 5A-B).
MLC activity
The RhoA/ROCK and NO/PKG pathways maintain the balance between phosphorylation and dephosphorylation of MLC[9-11]. Thus, we investigated the level of p-MLC (Thr18/Ser19) using Western blot analysis. The results revealed that p-MLC (Thr18/Ser19) levels greatly increased in the OVX + CCl4 rat livers compared to control rats. However, treatment with DPN significantly decreased the level of p-MLC (Thr18/Ser19), while the addition of PHTPP increased the level of p-MLC (Thr18/Ser19) once more (Figure 6A-B). Therefore, DPN inhibited MLC activity in the OVX + CCl4 rat livers, which reduced the contraction of intrahepatic VSMCs.
Figure 6.
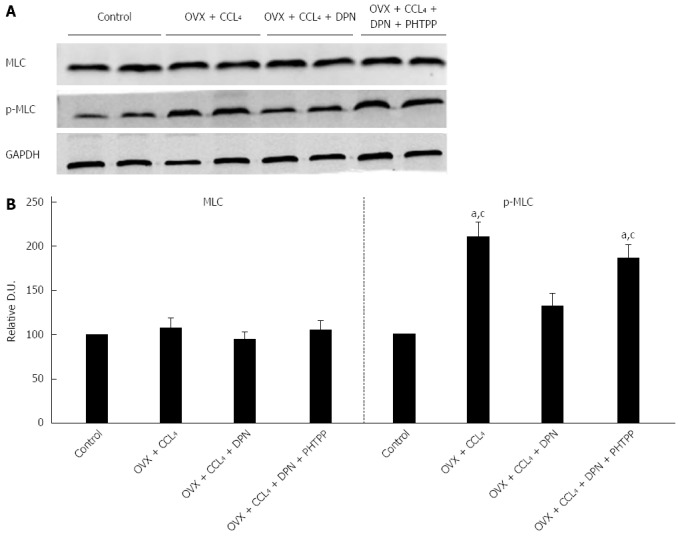
Diarylpropionitrile inhibits the phosphorylation of MLC in the livers of CCl4-treated rats. A: Western blot analysis of total MLC and p-MLC; B: Relative densitometric quantifications of all experiments (mean ± SE), with the values of the controls set to 100 DU (each group n = 5) aP < 0.05 vs control group; cP < 0.05 vs DPN group. DPN: Diarylpropionitrile.
Collagen gel contraction assay
The contraction was assessed using a model in which subcultured cells were grown on top of gel lattices composed of type 1 collagen. The lattice contraction in ET-1 processed cells registered stronger than in normal cells, producing gels by 51.8% ± 7.1% and 82.6% ± 8.9% of the original size, respectively. Pretreatment with 10-7 mol/L DPN or 10-5 mol/L Y-27632 significantly reduced the contraction of HSCs, reducing the gel areas to only 73.8% ± 8.3% and 77.7% ± 9.6%, respectively. However, pretreatment with both 10-7 mol/L DPN and 10-7 mol/L PHTPP produced HSC contraction similar to that of the ET-1 control, 54.4% ± 7.2% of the initial gel area (Figure 7A-B). Thus, DPN inhibited the ET-1-induced HSC contraction, and the inhibitory capacity of DPN was similar to that of the ROCK inhibitor Y-27632.
Figure 7.
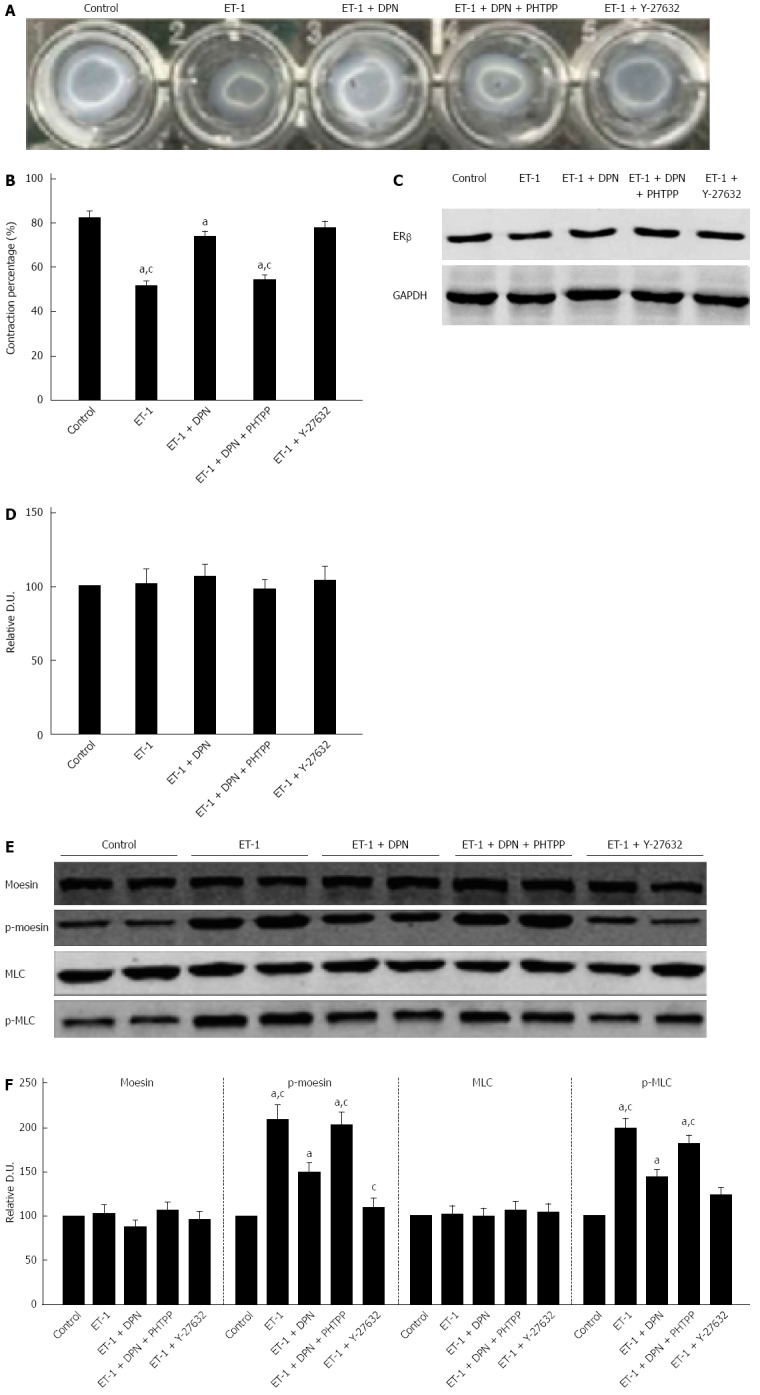
Diarylpropionitrile inhibits collagen lattice contraction in hepatic stellate cells and decreases ET-1 induced moesin and MLC phosphorylation. A: Appearance of collagen lattices 4 h after drug treatment; B: The percentage of remaining lattice area 4 h after drug treatment (each group n = 12); C, D: Western blot analysis of ERβ protein expression in hepatic stellate cells (HSCs) (each group n = 5); E: Western blot analysis of the total and phosphorylated moesin and MLC in HSCs; F: Relative densitometric quantifications of moesin and MLC experiments (mean ± SE), with the values of the controls set to 100 DU (each group n = 5). aP < 0.05 vs control group; cP < 0.05 vs DPN group. OVX: ovariectomized; SVR: Systemic vascular resistance; DPN: Diarylpropionitrile.
Expression of ERβ in HSCs
Expression of ERβ existed in all of the five cell treatment groups. Furthermore, there were no statistically significant differences in the ERβ expression of the five groups (Figure 7C-D).
Effect of DPN on ROCK activity in HSCs
Western blot analysis showed that ET-1-induced activation significantly increased the level of p-moesin (Thr558) in HSCs compared to the control group. Treatment with DPN reduced the phosphorylation of moesin in HSCs, while addition of PHTPP returned the p-moesin (Thr558) level to that seen in the ET-1 group. Treatment with Y-27632 was more effective than DPN in inhibiting the level of p-moesin (Thr558) (Figure 7A-B). Thus, the results revealed that DPN could inhibit the activity of ROCK in HSCs, but its capacity was less than that of the classic ROCK inhibitor Y-27632 (Figure 7E-F).
Effect of DPN on MLC activity in HSCs
We also investigated the phosphorylation of MLC in the five cell treatment groups. As a result, DPN played a more powerful role in inhibiting p-MLC (Thr18/Ser19) than inhibiting p-moesin (Thr558). The role of DPN in inhibiting p-MLC (Thr18/Ser19) was similar to that of Y-27632 (Figure 7E-F).
DISCUSSION
Our study shows that DPN, an ERβ selective agonist, not only postpones the development of liver cirrhosis, but also decreases the PP and IHVR in OVX CCl4-induced cirrhotic rats. Furthermore, our data demonstrate that DPN inhibits the RhoA/ROCK pathway and activates the NO/PKG pathway, leading to the inactivation of p-MLC in the cirrhotic livers of OVX rats. Furthermore, the in vitro studies demonstrate that DPN suppresses the contraction of HSCs, which is associated with the inhibition of ROCK and phosphorylation of MLC.
In the previous studies, estrogen therapy was indicated to improve hepatic fibrosis[12-15]. Thus, we first examined the effect of DPN on CCl4-induced liver cirrhosis in OVX rats by histological and immunochemical assessments. Furthermore, we examined α-SMA expression. HE staining and computerized collagen volume fraction analysis using trichrome staining indicated that DPN significantly inhibited CCl4-induced liver cirrhosis in OVX rats (Figure 1). α-SMA expression markedly activated HSCs, which play a critical role in liver fibrogenesis[26]. Immunohistochemical staining and Western blot analysis against α-SMA confirmed that DPN downregulated α-SMA expression in CCl4-induced cirrhotic livers. However, an ERβ antagonist, PHTPP, counteracted the effect of DPN (Figure 2). This indicates that DPN may improve liver cirrhosis in an HSC-dependent manner. The results of the liver and renal examinations affirmed the conclusion above (Table 1).
To investigate the effect of DPN on PP, IHVR and hyperdynamic circulation, we measured in vivo hemodynamic parameters using the microspheres technique. Interestingly, our data indicated that DPN markedly decreased the PP and IHVR in CCl4-induced cirrhotic OVX rats, but PHTPP counteracted the effects of DPN. This suggested that DPN played an important role in decreasing the PP and IHVR via ERβ in the liver. Moreover, DPN improved the hyperdynamic circulation of cirrhotic rats without effecting the MAP or TPR (Table 2). In this regard, DPN is clearly different from other vasodilators, such as nitrates and ROCK antagonists, as these medicines have a risk of decreasing MAP and TRP[27,28]. This characteristic of DPN is worth intensively exploring.
Although the liver did not work as the estrogen’s classic target organ, in our present experiment, Western blot analysis showed that there indeed existed ERβ expression in the livers of all of four rat groups. This result is consistent with a previous study[20], and is the basis of DPN having an effective role in the liver (Figure 3).
More and more evidence indicates that the high responsiveness of the intrahepatic vascular bed is closely related to increased expression and activation of ROCK in cirrhotic livers, which leads to increased IHVR[7]. Furthermore, ROCK antagonists, such as Y-27632 and fasudil, significantly decrease the IHVR[28]. As described in previous studies[29-31], estrogen could attenuate vascular contraction through inhibition of the RhoA/ROCK pathway. Thus, we further investigated the effect of DPN on the RhoA/ROCK pathway in the CCl4-induced cirrhotic livers of rats. Our data indicate that treatment with DPN greatly reduced the mRNA and protein expression levels of RhoA and ROCKII (Figure 4A-C). Moreover, DPN inhibited the increase in moesin phosphorylation typically seen in the cirrhotic livers, without altering the levels of total moesin (Figure 4D-E). This means that DPN not only inhibited the expression of RhoA and ROCK, but also blocked hepatic ROCK activity as seen by the suppression of moesin phosphorylation, which is a common measure of ROCK activity. Therefore, it can be said that besides improving liver fibrosis, DPN can decrease intrahepatic vasoconstriction, thus decreasing intrahepatic vascular tone.
RhoA/ROCK signaling and NO/PKG signaling regulated each other and maintained the balance between intrahepatic vasocontraction and vasodilatation[9-11]. Hence, we explored the effect of DPN treatment on NO/PKG signaling in the cirrhotic livers of OVX rats. It has been reported that although eNOS protein levels may appear unchanged, eNOS activity and NO production can decrease in sinusoidal endothelial cells (SECs)[16,32]. It was also been reported that estrogen stimulated eNOS expression in SECs and increased NO production in both normal and cirrhotic rats[32,33]. Our experiment revealed unchanged eNOS expression in cirrhotic OVX rats compared to the control group, although the levels of p-eNOS (Ser1177) were noticeably lower (Figure 5). However, DPN might not only increase eNOS expression, but also increase p-eNOS (Ser1177) levels (Figure 5). Hence, DPN could not only increase the expression of eNOS in SECs, but also upregulate the activation of eNOS, ultimately augmenting eNOS-derived NO generation. In addition, we investigated iNOS expression, and found that the markedly increased iNOS expression seen in the cirrhotic livers of OVX rats could be inhibited by DPN, which could in turn be blocked by PHTPP (Figure 5). It was reported that estrogen, which has potent antioxidant properties, could significantly attenuate cytokine-induced iNOS production in rat hepatocytes[34]. Thus, we speculate that DPN might negatively regulate protein nitrosylation and enhance NO bioavailability via inhibiting iNOS, which is closely associated with oxidative stress and cytokines[35,36]. To further understand the role of DPN in the activation of PKG, we detected phosphorylation levels of VSAP, an endogenous PKG substrate[8,22]. Our data indicate that DPN upregulates p-VASP (Ser239) and ultimately mediates NO-induced vasodilatation (Figure 5). There is evidence that defective eNOS signaling is mediated by ROCK activation in rats with secondary biliary cirrhosis[12], and that PKG-dependent RhoA deactivation would lead to a perpetuating loop in the effect of statins on RhoA/ROCK activity[37]. In our current study, we also found that inhibition of the RhoA/ROCK pathway might contribute to the activity of NO/PKG pathway in CCl4-induced cirrhotic OVX rats, and vice versa. Taken together, we concluded that DPN could simultaneously play an important role in the inhibition of the RhoA/ROCK and the activation of the NO/PKG pathways in the intrahepatic vascular system of cirrhotic rats. In VSMCs, both inhibition of the RhoA/ROCK pathway and activation of the NO/PKG pathways commonly attribute to the activity of myosin light chain phosphatase (MLCP), causing inhibited myosin light chain phosphorylation and vasodilatation[38]. As both pathways converge at this step, the level of MLC phosphorylation was detected. The results indicated that treatment with DPN significantly decreased the level of p-MLC (Thr18/Ser19), however, PHTPP can offset this effect (Figure 5). In conclusion, it can be said that DPN attenuates vasoconstriction in the cirrhotic livers of OVX rats by inhibiting MLC activity via regulation of the RhoA/ROCK and NO/PKG pathways.
HSCs have crucial importance in the development of liver cirrhosis[26]. Activated HSCs transform into myofibroblast-like cells, and acquire contractility. Their shrinkage is reported to be conditioned mainly through a Ca2+-sensitization mechanism which is analogous to the contraction in VSMCs[39,40]. Therefore, HSCs were considered the key cells in IHVR regulation. Recently, selective inhibition of the RhoA/ROCK pathway in activated HSCs has been regarded as a potential novel therapeutic target to reduce PHT[41,42]. Our in vivo studies suggest that DPN treatment in OVX cirrhotic rats significantly reduced hepatic α-SMA expression, thus we hypothesized that DPN negatively regulates the activation of HSCs. Nonetheless, the effect of DPN on the contraction of HSCs required more extensive investigation. First, we verified that ERβ expression was truly present in HSCs (Figure 7C-D). With regard to the physiological role of DPN in the regulation of HSC contractility, a collagen gel contraction assay was performed. We observed that 10-7 mol/L DPN could significantly inhibit the 10-7 mol/L ET-1-induced contraction of the HSC containing gel lattices. This was also observed with 10-5 mol/L Y-27632, while 10-7 mol/L PHTPP counteracted the effect of DPN (Figure 7A-B). Hence, it can be said that 10-7 mol/L DPN has a similar efficacy to 10-5 mol/L Y-27632 in blocking the HSC contraction.
To further specify the role of DPN in the regulation of ROCK activity in HSCs, the phosphorylation of moesin was examined. Although 10-7 mol/L DPN was less effective than 10-5 mol/L Y-17632 in downregulating p-moesin (Thr558), treatment with 10-7 mol/L DPN still significantly decreased p-moesin (Thr558) levels compared to HSCs stimulated with 10-7 mol/L ET-1 (Figure 7D-E). We therefore concluded that DPN could be a novel ROCK inhibitor used to block the contraction of activated HSCs. To our knowledge, in HSCs the inactivation of MLCP is mainly regulated by the RhoA/ROCK pathway[43]. So we investigated the effect of DPN on the phosphorylation of MLC in HSCs. Interestingly, 10-7 mol/L DPN distinctly inhibited the ET-1-induced overexpression of p-MLC (Thr18/Ser19), and 10-7 mol/L DPN was equally effective in inhibiting p-MLC (Thr18/Ser19) as 10-5 mol/L Y-27632 (Figure 7D-E). Although DPN was less potent than Y-27632 in blocking the moesin phosphorylation (Thr558), its final effect on p-MLC (Thr18/Ser19) and HSC contraction was no different than that of Y-27632. Further research is needed to fully explore the mechanisms involved. As a final note, it has been shown that HSCs contain functional ERβ but no ERα[20]; therefore, DPN could be used as a targeted ROCK inhibitor without causing severe systemic side effects.
In summary, treatment with DPN was effective in lowering PHT in CCl4-induced cirrhotic OVX rats, which was attributed to its anti-hepatic fibrosis effect and its ability to decrease IHVR via inhibition of the RhoA/ROCK and activation of the NO/PKG signaling pathways. DPN also significantly reduced HSC contractility by inhibiting ROCK activation and downstream MLC phosphorylation. This study suggests that DPN could be a potential candidate for estrogen replacement therapy, benefiting menopausal women with liver cirrhosis and PHT.
COMMENTS
Background
Increased intrahepatic vascular resistance (IHVR) is a major cause for portal hypertension (PHT), and activated hepatic stellate cells (HSCs), contraction of intrahepatic vascular smooth muscle cells, and reduced vasodilator nitric oxide levels all play a critical role in contributing to increased IHVR. NO is of great significance in increasing IHVR levels. Animal experiments and clinical trials provide consistent evidence for the protective effect of endogenous and exogenous estrogens on liver fibrosis. However, exogenous estrogens give rise to a number of potential risks, which restrain them from clinical uses.
Research frontiers
Evidence indicates the intrahepatic upregulation of RhoA and Rho-kinase signaling and inhibition of NO/PKG signaling from increasing IHVR. For these reasons, the two pathways serve as the crucial therapeutic target to ameliorate PHT. High estrogen receptor (ER) β expression levels and low ERα expression levels were observed in livers, moreover, HSCs have functional ERβ, rather than ERα. Therefore, this paper studied the effect of DPN - an ERβ selective agonist - on the two pathways, and also on hepatic hemodynamics systemically.
Innovations and breakthroughs
DPN treatment is effective in lowering PHT in CCl4-induced cirrhosis of the OVX rats, contributes to its anti-hepatic fibrosis effect, and is capable of decreasing IHVR by the inhibition of RhoA/ROCK and activation of the NO/PKG signaling pathways. DPN also significantly reduced HSC shrinkage by restraining ROCK activation and down-streaming MLC phosphorylation via ERβ.
Applications
The ERβ selective agonist may be a potential therapeutic approach to managing PHT and liver fibrosis, particularly for those menopausal women and patients with low estrogen levels.
Terminology
ER subtypes play a distinct role in exerting different biological effects with tissue-specific responses. ERβ selective agonists may produce biological effects without causing any classic side effects of estrogens.
Peer-review
This is a well-done experimental study concerning the efficacy of ER agonist against cirrhosis-related portal hypertension. Clinically practical benefits will not come into effect within a short time. However, the study suggests that the ERβ selective agonist be a potential therapeutic method to manage PHT and liver fibrosis.
Footnotes
Supported by the National Natural Science Foundation for the Youth of China, No. 81400630.
Institutional review board statement: The study was approved by the Research Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (No. RJ-20151211).
Institutional animal care and use committee statement: All of the animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No 80-23, revised in 1996). All experimental animal procedures conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict-of-interest statement: No conflict of interest exists whatsoever.
Data sharing statement: The data referred to in this manuscript have been generated solely by the authors. No other party has been involved. Therefore, no additional unpublished data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 29, 2015
First decision: January 28, 2016
Article in press: March 18, 2016
P- Reviewer: Dumitrascu DL, Ikura Y S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Ekataksin W, Kaneda K. Liver microvascular architecture: an insight into the pathophysiology of portal hypertension. Semin Liver Dis. 1999;19:359–382. doi: 10.1055/s-2007-1007126. [DOI] [PubMed] [Google Scholar]
- 2.Gatta A, Bolognesi M, Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med. 2008;29:119–129. doi: 10.1016/j.mam.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300–1314. doi: 10.1136/gut.2007.144584. [DOI] [PubMed] [Google Scholar]
- 4.García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461. doi: 10.1016/j.jhep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558–567. doi: 10.1016/j.jhep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Bosch J, García-Pagán JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220–227. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Hennenberg M, Trebicka J, Jochem K, Leifeld L, Biecker E, Sauerbruch T, Heller J. Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut. 2006;55:1296–1305. doi: 10.1136/gut.2005.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 9.Luo W, Meng Y, Ji HL, Pan CQ, Huang S, Yu CH, Xiao LM, Cui K, Ni SY, Zhang ZS, et al. Spironolactone lowers portal hypertension by inhibiting liver fibrosis, ROCK-2 activity and activating NO/PKG pathway in the bile-duct-ligated rat. PLoS One. 2012;7:e34230. doi: 10.1371/journal.pone.0034230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anegawa G, Kawanaka H, Yoshida D, Konishi K, Yamaguchi S, Kinjo N, Taketomi A, Hashizume M, Shimokawa H, Maehara Y. Defective endothelial nitric oxide synthase signaling is mediated by rho-kinase activation in rats with secondary biliary cirrhosis. Hepatology. 2008;47:966–977. doi: 10.1002/hep.22089. [DOI] [PubMed] [Google Scholar]
- 11.Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol. 2009;587:3587–3603. doi: 10.1113/jphysiol.2009.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63–69. doi: 10.1034/j.1600-0676.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res. 2007;37:239–247. doi: 10.1111/j.1872-034X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol. 2007;13:4295–4305. doi: 10.3748/wjg.v13.i32.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell M, Yang YX, Reddy KR. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2005;41:939; author reply 939–940. doi: 10.1002/hep.20611. [DOI] [PubMed] [Google Scholar]
- 16.Shah V. Molecular mechanisms of increased intrahepatic resistance in portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S259–S261. doi: 10.1097/MCG.0b013e318150d0e1. [DOI] [PubMed] [Google Scholar]
- 17.Anghel A, Narita D, Seclaman E, Popovici E, Anghel M, Tamas L. Estrogen receptor alpha polymorphisms and the risk of malignancies. Pathol Oncol Res. 2010;16:485–496. doi: 10.1007/s12253-010-9263-9. [DOI] [PubMed] [Google Scholar]
- 18.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Dahlman-Wright K, Gustafsson JÅ. Estrogen signaling via estrogen receptor {beta} J Biol Chem. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M, Ito S. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059–1065. doi: 10.1006/bbrc.2001.5479. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Wu ZY. Estrogen derivatives: novel therapeutic agents for liver cirrhosis and portal hypertension. Eur J Gastroenterol Hepatol. 2013;25:263–270. doi: 10.1097/MEG.0b013e32835ab5dc. [DOI] [PubMed] [Google Scholar]
- 22.Moleda L, Trebicka J, Dietrich P, Gäbele E, Hellerbrand C, Straub RH, Sauerbruch T, Schoelmerich J, Wiest R. Amelioration of portal hypertension and the hyperdynamic circulatory syndrome in cirrhotic rats by neuropeptide Y via pronounced splanchnic vasoaction. Gut. 2011;60:1122–1132. doi: 10.1136/gut.2010.226407. [DOI] [PubMed] [Google Scholar]
- 23.Trebicka J, Leifeld L, Hennenberg M, Biecker E, Eckhardt A, Fischer N, Pröbsting AS, Clemens C, Lammert F, Sauerbruch T, et al. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264–1276. doi: 10.1002/hep.22170. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka M, Murata T, Hori M, Ozaki H. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1010–G1021. doi: 10.1152/ajpgi.00350.2010. [DOI] [PubMed] [Google Scholar]
- 25.Perri RE, Langer DA, Chatterjee S, Gibbons SJ, Gadgil J, Cao S, Farrugia G, Shah VH. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–G542. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 26.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piscaglia F, Donati G, Gaiani S, Gramantieri L, Leoni S, Mancini M, Bolondi L. Different haemodynamic effects of a single dose of long-acting isosorbide-5-mononitrate in healthy subjects and patients with cirrhotic portal hypertension. Dig Liver Dis. 2004;36:594–602. doi: 10.1016/j.dld.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda T, Narahara Y, Kanazawa H, Matsushita Y, Kidokoro H, Itokawa N, Kondo C, Atsukawa M, Nakatsuka K, Sakamoto C. Effects of fasudil on the portal and systemic hemodynamics of patients with cirrhosis. J Gastroenterol Hepatol. 2014;29:325–329. doi: 10.1111/jgh.12360. [DOI] [PubMed] [Google Scholar]
- 29.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35:2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 30.Yang E, Jeon SB, Baek I, Chen ZA, Jin Z, Kim IK. 17beta-estradiol attenuates vascular contraction through inhibition of RhoA/Rho kinase pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:35–44. doi: 10.1007/s00210-009-0408-x. [DOI] [PubMed] [Google Scholar]
- 31.Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326:154–159. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44:209–216. doi: 10.1016/j.jhep.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto M, Ueno T, Nakamura T, Sakata R, Hasimoto O, Torimura T, Sata M. Improvement of portal hypertension and hepatic blood flow in cirrhotic rats by oestrogen. Eur J Clin Invest. 2005;35:220–225. doi: 10.1111/j.1365-2362.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 34.Nweze IC, Smith JW, Zhang B, Klinge CM, Lakshmanan J, Harbrecht BG. 17β-Estradiol attenuates cytokine-induced nitric oxide production in rat hepatocyte. J Trauma Acute Care Surg. 2012;73:408–412. doi: 10.1097/TA.0b013e31825a789b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Fernández M, Bosch J, García-Pagán JC. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47:1248–1256. doi: 10.1002/hep.22166. [DOI] [PubMed] [Google Scholar]
- 36.Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707–1717. doi: 10.3748/wjg.v19.i11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1006–G1016. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- 38.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 39.Melton AC, Datta A, Yee HF. [Ca2+]i-independent contractile force generation by rat hepatic stellate cells in response to endothelin-1. Am J Physiol Gastrointest Liver Physiol. 2006;290:G7–13. doi: 10.1152/ajpgi.00337.2005. [DOI] [PubMed] [Google Scholar]
- 40.Saiman Y, Agarwal R, Hickman DA, Fausther M, El-Shamy A, Dranoff JA, Friedman SL, Bansal MB. CXCL12 induces hepatic stellate cell contraction through a calcium-independent pathway. Am J Physiol Gastrointest Liver Physiol. 2013;305:G375–G382. doi: 10.1152/ajpgi.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beuge MM, Prakash J, Lacombe M, Post E, Reker-Smit C, Beljaars L, Poelstra K. Increased liver uptake and reduced hepatic stellate cell activation with a cell-specific conjugate of the Rho-kinase inhibitor Y27632. Pharm Res. 2011;28:2045–2054. doi: 10.1007/s11095-011-0430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, Heidari I, Huss S, Sauerbruch T, Poelstra K, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 43.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G407–G416. doi: 10.1152/ajpgi.00398.2004. [DOI] [PubMed] [Google Scholar]


