Abstract
AIM: To investigate serum interleukin (IL)-38 level and its clinical role in predicting virological response (VR) to telbivudine (LdT) in patients with chronic hepatitis B (CHB).
METHODS: The study participants were divided into two groups; one group consisted of 43 healthy controls (HCs) and the other group consisted of 46 patients with hepatitis B e antigen-positive CHB. All patients were administered 600 mg of oral LdT daily for 52 wk, and they visited physicians every 12 wk for physical examination and laboratory tests. Serum IL-38 levels were determined using ELISA. The concentrations of serum Th1- and Th2-type cytokines were measured using the cytometric bead array (CBA) method.
RESULTS: Serum levels of IL-38 at baseline in all patients were higher than those in HCs [306.97 (123.26-492.79) pg/mL vs 184.50 (135.56-292.16) pg/mL, P = 0.019]; the levels returned to normal after the first 12 wk of treatment with LdT [175.51 (103.90-331.91) pg/mL vs 184.50 (135.56-292.16) pg/mL, P > 0.05]. Serum IL-38 levels at baseline were positively associated with serum aspartate aminotransferase levels in patients with CHB (r = 0.311, P = 0.036). Higher levels of serum IL-38 at baseline were associated with a greater probability of VR to LdT treatment at 24 wk (48.15% vs 15.79%, P = 0.023) and 52 wk (66.67% vs 36.84%, P = 0.044). The levels of serum IL-38 in patients with primary non-response at week 12 after treatment initiation were lower than those in patients with primary response [64.44 (49.85-172.08) pg/mL vs 190.54 (121.35-355.28) pg/mL, P = 0.036]. Serum IL-38 levels were correlated with serum IL-6 and IL-12 levels in patients with CHB during treatment with LdT.
CONCLUSION: Elevated serum IL-38 levels in untreated CHB patients reflect ongoing liver injury. Higher serum IL-38 levels before treatment indicate a greater probability of VR to LdT treatment.
Keywords: Alanine aminotransferase, Aspartate aminotransferase, Interleukin-6, Interleukin-12, Interleukin-38, Chronic hepatitis B, Primary non-response, Virological response
Core tip: This is the first study detailing kinetic changes in serum interleukin-38 (IL-38) levels during chronic hepatitis B (CHB). Higher pretreatment serum IL-38 levels are associated with a greater probability of virological response to telbivudine treatment. Elevated levels of serum IL-38 in untreated patients with CHB reflect ongoing liver injury, which is an indirect indicator of vigorous endogenous clearance of hepatitis B virus infection. Our findings suggest that clear signs of viral clearance at baseline may predict a favorable response to treatment of CHB using nucleoside analogs.
INTRODUCTION
Hepatitis B virus (HBV) infection is a serious global health concern, and approximately 240 million people have been chronically infected with HBV[1]. Patients with chronic hepatitis B (CHB) have a high risk of developing liver cirrhosis and hepatocellular carcinoma[2].
Previous studies have revealed that HBV infection may suppress the immune system, and that Th1 and Th2 cells and their respective cytokines are possibly involved in the pathogenesis of CHB[3,4]. Telbivudine (LdT), a nucleoside analog (NA), not only inhibits viral replication, but also increases cytokine production by the Th1 cell subpopulation, which could contribute to its antiviral efficacy[5]. Th2 cells secrete interleukin (IL)-10 and IL-6, which are involved in liver inflammation in CHB[6,7]. Members of the IL-1 cytokine family are also associated with acute and chronic inflammation, and they facilitate the differentiation and function of polarized innate and adaptive lymphoid cells[8,9]; they likely participate in CHB infection. For example, it has been reported that IL-37, an anti-inflammatory cytokine, was a part of the immune response in patients with CHB who showed hepatitis B e antigen (HBeAg) seroconversion, and that both serum IL-33 and IL-37 levels are associated with liver injury in these patients[10,11].
IL-38 (IL-1F10) is a newly characterized cytokine of the IL-1 family, and it is expressed in the basal epithelia of the skin and in the proliferating tonsillar B cells[12]. It was suggested that IL-38 may have anti-inflammatory properties, since it shares some sequence homology with IL-1Ra (41%) and IL-36Ra (43%)[8]. This assumption was first supported by a study that showed that a recombinant IL-38 bound to the IL-36 receptor and inhibited the expression of IL-17, IL-22, and IL-8 in human peripheral blood mononuclear cells (PBMCs) under inflammatory conditions. It was further suggested that IL-38 might function as a partial IL-36 receptor antagonist[13]. Another study observed a marked increase in lupus-associated pro-inflammatory mediators following silencing of endogenous IL-38 in PBMCs, which supported the assumed anti-inflammatory function of IL-38[14]. Furthermore, IL-38 was upregulated in patients with primary Sjogren’s syndrome, and it was suggested to counteract the imbalanced activation of IL-36[15]. Genetic association studies indicated that IL-38 polymorphisms are linked with a high frequency of psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis in the population, suggesting that IL-38 is a likely component in the pathogenesis of these inflammatory diseases[8,16,17].
NAs and interferons (IFNs) are the currently available antiviral agents for treating CHB[18]. LdT is a synthetic thymidine NA that shows potent inhibition of HBV replication[19]. Recent studies have shown that treatment with LdT not only suppresses HBV replication, but also modulates the immune response in treated CHB patients[20].
To our knowledge, there are no available data regarding the expression or function of IL-38 in CHB. In this study, we quantified the kinetic changes in serum IL-38 level and investigated the potential correlation between the levels of HBV-related biochemical markers, Th1/Th2 type cytokines, and serum IL-38 levels during LdT treatment of patients with CHB. In addition, we discussed the implications of our findings.
MATERIALS AND METHODS
Patients
A total of 46 patients with CHB were recruited at the First Hospital of Jilin University from September 2012 to October 2014. Patients with CHB showed positive results for hepatitis B surface antigen (HBsAg), HBeAg, and HBV DNA for at least 12 mo. Another 43 gender-, age- and ethnicity-matched healthy controls (HCs) were recruited from the Physical Examination Center of our hospital during the same period. Individuals who showed seropositivity of hepatitis C, G, or D virus, those infected by HIV-1 or those with autoimmune liver disease were excluded from the study. None of the participants had received immunosuppressive or antiviral therapy during the 12 mo preceding their inclusion in the study, and none of them reported a history of exposure to known hepatotoxins. The demographic and clinical characteristics of the patients are summarized in Table 1. Written informed consent was obtained from each study participant; the experimental protocol was established according to the guidelines of the 1975 Declaration of Helsinki and was approved by the Human Ethics Committee of Jilin University, China.
Table 1.
Demographic and clinical characteristics of study subjects at baseline
| Parameters | Patients with CHB (n = 46) | Healthy controls (n = 43) |
| Age (yr) | 34.5 (23.75-38.25) | 34 (22-40) |
| Sex (M/F) | 38/8 | 34/9 |
| HBV DNA load (log10 IU/mL) | 7.71 (6.98-8.10) | NA |
| ALT level (U/L) | 117.35a (74.50-241.48) | 14 (6-22) |
| AST level (U/L) | 96.75a (57.25-176.25) | 12 (10-30) |
| HBsAg level (IU/mL) | 7821.43 (3337.23-20737.14) | NA |
| HBsAg, pos/neg | 46/0 | 0/43 |
| HBeAg, pos/neg | 46/0 | 0/43 |
Data are represented as median and interquartile range (IQR) or real case number. Normal values: ALT ≤ 50 IU/L; AST ≤ 40 IU/L; HBV DNA ≤ 1.78 log10 IU/mL (60 IU/mL).
P < 0.05 vs the HCs. The undetectable HBV DNA loads were recorded as those ≤ 60 IU/mL. CHB: Chronic hepatitis B; HBV: Hepatitis B virus; NA: Not applicable; ALT: Alanine transferase; AST: Aspartate transferase; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; pos: Positive; neg: Negative.
Patients were administered 600 mg LdT (Novartis Pharmaceuticals, Beijing, China) daily for 52 wk, and they visited the outpatient department of the hospital every 12 wk for physical examination and laboratory tests. All patients were followed up for one year. Peripheral blood samples were obtained and serum samples were separated; the samples were stored at -80 °C until use.
Measurement of serum IL-38 by enzyme-linked immunosorbent assay
Serum concentrations of IL-38 in patients and HCs were determined by enzyme-linked immunosorbent assay [human IL-38 enzyme-linked immunosorbent assay (ELISA) kit; CUSABIO Life Sciences, Wuhan, Hunan province, China]. Briefly, individual sera were subjected to ELISA, and the concentrations of serum IL-38 in individual samples were calculated using the standard curve established with the recombinant IL-38 provided. The detection limit of IL-38 ranged between 31.25 and 2000 pg/mL.
Cytometric bead array of serum Th1- and Th2-type cytokines
The concentrations of serum Th1- and Th2-type cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-12, IL-10, and IL-6) were determined by cytometric bead array (CBA) according to the manufacturer’s protocol (BD Biosciences, San Jose, CA, United States), with minor modifications. Briefly, 25 μL of individual serum was used in duplicate for analysis. The concentrations of serum cytokines were quantified using the CellQuest Pro and CBA software (Becton Dickinson, San Jose, CA, United States) on an FACSAria II flow cytometer (BD Biosciences).
Serological analysis of HBV markers
Serum HBsAg, hepatitis B surface antibody (HBsAb), HBeAg, and hepatitis B e antibody (HBeAb) were detected by chemiluminescent microparticle immunoassay (CMIA), using an Abbott i2000 automated chemiluminescent immunoassay analyzer (Abbott Laboratories, Abbott Park, IL, United States). The levels of serum alanine transferase (ALT) and aspartate transferase (AST) were detected using a Biochemistry Automated Analyzer (Roche Diagnostics, Branchburg, New Jersey, United States). Serum HBV DNA was measured by quantitative PCR using a luciferase-based detection kit, following the manufacturer’s protocols (Roche). The detection limit of viral DNA was 60 IU/mL (HBV DNA ≤ 60 IU/mL was considered undetectable).
Statistical analysis
Quantitative data were expressed as median and interquartile range (IQR). Differences between independent groups were analyzed by Mann-Whitney U test or Student’s t-test when appropriate and differences between related groups were analyzed by Wilcoxon signed ranks test. The correlation between variables was evaluated using Spearman’s rank correlation test. Receiver operating characteristic curves were constructed to identify optimal cutoff values for predicting virological response (VR) to treatment. Fisher’s exact tests were carried out to compare the rates of VR, HBeAg loss, ALT normalization and HBsAg loss. Binary logistic regression was used to determine predictors of VR. All statistical analyses were performed by the SPSS software (version 18.0). P-value < 0.05 was considered as statistically significant.
RESULTS
HBV-related biochemical markers in patients with CHB before and during treatment with LdT
There was no significant difference in the distribution of age or gender between patients with CHB and HCs (Table 1).
HBV DNA and HBsAg levels gradually decreased in response to the LdT treatment and were lower than the levels before treatment. Both ALT and AST levels also decreased during treatment (Table 2). VR was defined as undetectable HBV DNA (≤ 60 IU/mL). Primary non-response (PNR) was defined as a decrease in HBV DNA of less than 1 log10 IU/mL from baseline at 12 wk after treatment initiation[21]. Of the 46 patients, 5 showed PNR and the remaining 41 patients showed primary response (PR) at week 12. At weeks 24 and 52 of LdT therapy, 16 (35%) and 25 (54%) patients with CHB showed VR, respectively.
Table 2.
Dynamics of clinical parameters in patients with chronic hepatitis B during LdT treatment
| Parameters | Baseline | 3 mo | 6 mo | 9 mo | 13 mo |
| HBV DNA load (log10 IU/mL) | 8.41 (7.68-8.80) | 3.79a (3.20-5.14) | 3.04a (2.48-3.98) | 2.81a (2.48-3.10) | 2.48a (2.48-3.50) |
| ALT level (U/L) | 117.35 (74.50-241.48) | 36.00a (23.93-85.50) | 27.00a (22.00-38.00) | 27.50a (23.00-34.25) | 29.50a (24.00-55.00) |
| AST level (U/L) | 96.75 (57.25-176.25) | 34.00a (28.00-55.25) | 26.00a (22.00-31.25) | 24.50a (19.75-30.00) | 27.00a (22.75-43.25) |
| HBsAg level (IU/mL) | 7821.43 (3337.23-20737.14) | 4214.99a (2034.48-10170.46) | 5153.19a (2039.45-10671.30) | 4414.88a (2317.96-10084.21) | 4210.48a (1664.73-9823.78) |
Data are represented as median and interquartile range (IQR). Normal values: ALT ≤ 50 IU/L; AST ≤ 40 IU/L; HBV DNA ≤ 1.78 log10 IU/mL (60 IU/mL).
P < 0.05 vs baseline value. The undetectable HBV DNA loads were recorded as those ≤ 60 IU/mL. CHB: Chronic hepatitis B; HBV: Hepatitis B virus; ALT: Alanine transferase; AST: Aspartate transferase; HBsAg: Hepatitis B surface antigen.
Kinetic changes in serum IL-38 levels of patients with CHB during treatment with LdT and association of serum IL-38 with serum AST prior to treatment
The kinetic changes in serum IL-38 levels during treatment were determined. Serum IL-38 levels at the baseline and at 12, 24, 36, and 52 wk were 306.97 (123.26-492.79) pg/mL, 175.51 (103.90-331.91) pg/mL, 226.53 (84.33-346.47) pg/mL, 205.91 (103.48-387.16) pg/mL, and 194.79 (90.71-356.48) pg/mL, respectively. As shown in Figure 1, IL-38 levels were higher in patients with CHB compared with HCs at baseline [306.97 (123.26-492.79) pg/mL vs 184.50 (135.56-292.16) pg/mL, P = 0.019, Figure 1A], and they were reduced at week 12, 24, 36, and 52 of LdT treatment (P < 0.001, P = 0.003, P = 0.027, P = 0.019, Figure 1A). In addition, the levels of serum IL-38 in patients with CHB were no longer different from that of the HCs after 12 wk of LdT treatment, since a quick reduction was observed only during the first 12 wk.
Figure 1.
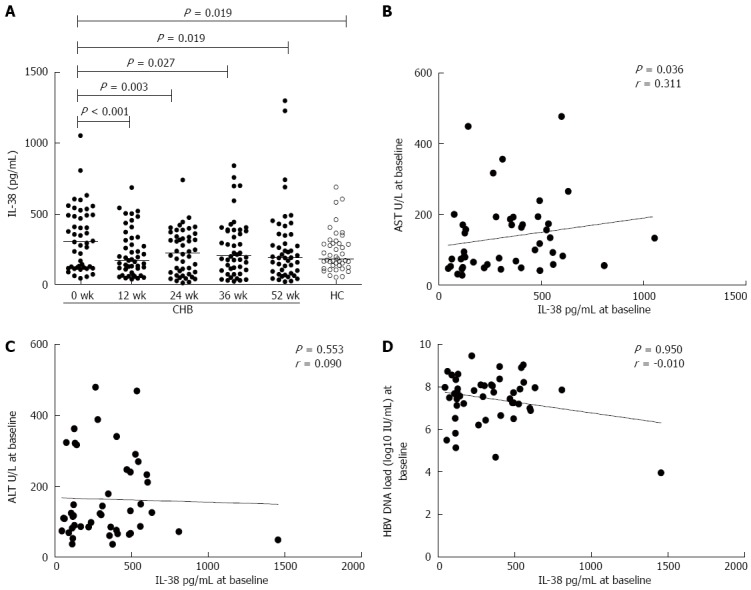
Kinetic changes in serum interleukin-38 levels of patients with chronic hepatitis B during treatment with telbivudine and association of serum interleukin-38 with serum aspartate transferase, alanine transferase, hepatitis B virus DNA loads prior to treatment. A: Kinetic changes in the levels of serum IL-38 in HCs and patients with CHB at baseline and at 12, 24, 36, and 52 wk of LdT treatment. IL-38 levels were higher in patients with CHB compared with HCs at baseline [306.97 (123.26-492.79) pg/mL vs 184.50 (135.56-292.16) pg/mL; P = 0.019]. They were reduced at week 12, 24, 36, and 52 of LdT treatment (P < 0.001, P = 0.003, P = 0.027, P = 0.019), and the levels of serum IL-38 in patients with CHB were no longer different from that of the HCs after 12 wk of LdT treatment. B: Serum AST levels correlated with serum IL-38 levels at baseline. C and D: Serum IL-38 levels did not correlate with serum ALT levels or with serum HBV DNA loads at baseline. IL-38: Interleukin-38; HCs: Healthy controls; CHB: Chronic hepatitis B; LdT: Telbivudine; ALT: Alanine transferase; AST: Aspartate transferase; HBV: Hepatitis B virus.
Correlation analysis of serum IL-38 with ALT and AST levels showed that serum IL-38 levels at baseline were positively associated with serum AST levels (P = 0.036, r = 0.311, Figure 1B) but not with serum ALT levels (P > 0.05, Figure 1C) in patients with CHB. No significant correlation was found between serum IL-38 and serum AST or ALT values during LdT therapy (data not shown).
In addition, there was no significant association between serum IL-38 and HBV DNA levels prior to LdT treatment (P > 0.05, Figure 1D), and no significant correlation during LdT therapy (data not shown).
Pretreatment serum IL-38 levels in patients with CHB were associated with antiviral therapy outcomes
We generated receiver operating characteristic curves to decide optimal cut-off values of serum IL-38 at baseline to predict VR. Using the serum IL-38 levels at baseline, we divided the 46 patients with CHB into two groups: high level group (HG; serum IL-38 ≥ 250 pg/mL, n = 27) and low level group (LG; serum IL-38 < 250 pg/mL, n = 19). The pretreatment characteristics of the two groups are shown in Table 3. There was no significant difference in the distribution of age or gender, and serum HBV DNA, ALT, and HBsAg levels between HG and LG. However, the serum AST levels differed between the groups. Interestingly, the serum HBV DNA loads in HG were lower than those in LG at week 12, 24, 36, and 52 post initial LdT treatment (P = 0.041, 0.003, 0.003, 0.020, Figure 2A), suggesting a better response in patients with high serum levels of IL-38. No significant difference in serum HBsAg levels was noted between the groups during LdT therapy (P > 0.05 at week 12, 24, 36, and 52, Figure 2B).
Table 3.
Comparison of demographic and clinical characteristics of patients with high and low interleukin-38 levels before treatment
| Parameter | High IL-38 levelbefore treatment (n = 27) | Low IL-38 levelbefore treatment (n = 19) |
| Age (yr) | 35 (23-38) | 34 (25-40) |
| Sex (M/F) | 22/5 | 16/3 |
| HBV DNA load (log10 IU/mL) | 7.74 (6.89-8.10) | 7.69 (7.14-8.36) |
| ALT level (U/L) | 130.00 (71.00-246.50) | 108.00 (81.00-147.00) |
| AST level (U/L) | 156.00c (76.00-193.00) | 74.00 (49.00-147.00) |
| HBsAg level (IU/mL) | 6641.55 (4437.10-18631.33) | 10222.92 (2657.06-24877.19) |
Data are represented as median and interquartile range (IQR) or real case number. Normal values: ALT ≤ 50 IU/L; AST ≤ 40 IU/L; HBV DNA ≤ 1.78 log10 IU/mL (60 IU/mL).
P < 0.05 vs the group of patients with low serum IL-38 levels before LdT treatment. The undetectable HBV DNA loads were recorded as those ≤ 60 IU/mL. High IL-38 level: Serum IL-38 ≥ 250 pg/mL; Low IL-38 level: Serum IL-38 < 250 pg/mL; IL-38: Interleukin-38; HBV: Hepatitis B virus; ALT: Alanine transferase; AST: Aspartate transferase; HBsAg: Hepatitis B surface antigen; M: Male; F: Female.
Figure 2.
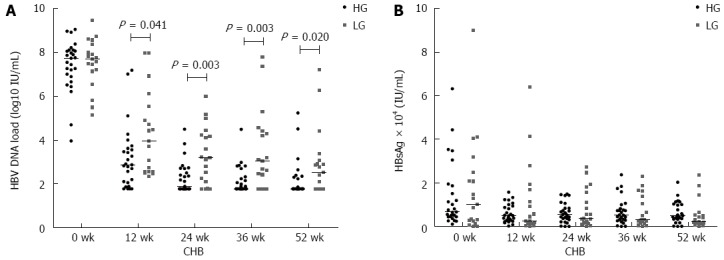
Comparison of serum hepatitis B virus DNA loads (A) and hepatitis B surface antigen levels (B) between high level group and low level group at baseline and at 12, 24, 36, and 52 wk of LdT treatment. A: The serum HBV DNA loads in HG were lower than those in LG at week 12, 24, 36, and 52 post initial LdT treatment (P = 0.041, 0.003, 0.003, 0.020). B: No significant difference in serum HBsAg levels was noted between the groups during LdT therapy. LdT: Telbivudine; HBV: Hepatitis B virus; HG: High level group; LG: Low level group; HBsAg: Hepatitis B surface antigen.
The VR rates were compared between HG and LG, and it was observed that at 24 wk, VR was achieved in 13 patients (48.15%) in HG and 3 patients (15.79%) in LG. Higher serum IL-38 levels at baseline were associated with greater VR (OR = 4.95, 95%CI: 1.17-21.03, P = 0.023). At 52 wk, 18 patients (66.67%) in HG and 7 patients (36.84%) in LG achieved VR. The IL-38 levels at baseline elicited different responses to therapy (OR = 3.43, 95%CI: 1.00-11.71, P = 0.044). Treatment outcomes, including HBeAg elimination, ALT normalization and HBsAg loss, between patients with high and low baseline IL-38 levels were also compared. As shown in Table 4, there were no significant differences in HBeAg elimination, ALT normalization and HBsAg loss between HG and LG. According to our data, an elevated serum IL-38 level at baseline could predict better virological response.
Table 4.
Comparison of treatment outcomes between patients with high and low baseline interleukin-38 levels
| Parameters |
Week 24 |
Week 52 |
||||
| HG (n = 27) | LG (n = 19) | P value | HG (n = 27) | LG (n = 19) | P value | |
| HBV DNA ≤ 60 IU/mL | 13 (48.1) | 3 (15.8) | 0.023 | 18 (66.7) | 7 (36.8) | 0.044 |
| HBeAg seroconversion | 4 (14.8) | 2 (10.5) | 0.516 | 8 (29.6) | 4 (21.1) | 0.382 |
| ALT normalization | 25 (92.6) | 15 (78.9) | 0.182 | 21 (77.8) | 12 (63.2) | 0.225 |
| HBsAg loss | 0 (0.0) | 0 (0.0) | NA | 1 (3.7) | 1 (5.3) | 0.661 |
Normal values: ALT ≤ 50 IU/L; AST ≤ 40 IU/L; HBV DNA ≤ 1.78 log10 IU/mL (60 IU/mL). The undetectable HBV DNA loads were recorded as those ≤ 60 IU/mL. HG: High IL-38 level group, basement serum IL-38 ≥ 250 pg/mL; LG: Low IL-38 level, basement serum IL-38 < 250 pg/mL; IL-38: Interleukin-38; HBV: Hepatitis B virus; ALT: Alanine transferase; AST: Aspartate transferase; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; NA: Not applicable.
The serum AST, ALT, HBsAg, and HBV DNA levels at baseline were analyzed by binary logistic regression and they were not associated with VR at week 24 or 52 of LdT treatment (data not shown).
Primary response was associated with high serum IL-38 levels
The levels of serum IL-38 in patients with CHB who showed PR were higher than those of CHB patients who showed PNR at 12 wk after LdT treatment [190.54 (121.35-355.28) pg/mL vs 64.44 (49.85-172.08) pg/mL, P = 0.036, Figure 3], while they did not differ at baseline.
Figure 3.
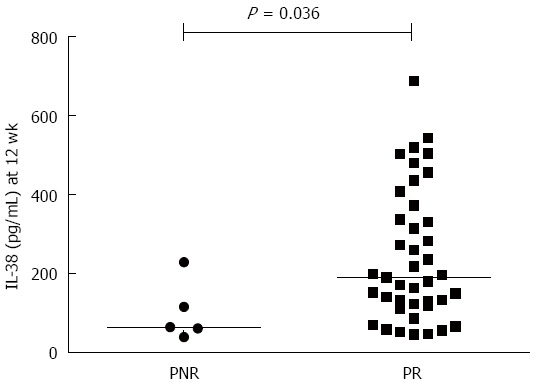
Comparison of serum interleukin-38 levels between patients with primary non-response and primary response at 12 wk post initial telbivudine treatment. The levels of serum IL-38 in patients with CHB who showed PR were higher than those of CHB patients showed PNR at 12 wk after LdT treatment [190.54 (121.35-355.28) pg/mL vs 64.44 (49.85-172.08) pg/mL, P = 0.036]. IL-38: Interleukin-38; CHB: Chronic hepatitis B; LdT: Telbivudine; ALT: Alanine transferase; AST: Aspartate transferase; HBV: Hepatitis B virus; PNR: Primary non-response; PR: Primary response.
Serum IL-38 levels correlated with IL-6 and IL-12 levels in patients with CHB during LdT treatment
As shown in Figure 4, the serum levels of IL-38 were positively associated with the levels of serum IL-6 at baseline, week 12, 24, 36, and 52 after starting LdT treatment (P = 0.003, r = 0.435; P = 0.002, r = 0.436; P = 0.001, r = 0.481; P < 0.001, r = 0.552; P = 0.008, r = 0.386; Figure 4A-E). The levels of serum IL-38 were positively associated with the levels of serum IL-12 at week 24, 36, and 52 of LdT treatment (P = 0.042, r = 0.301; P < 0.001, r = 0.515; P = 0.002, r = 0.451; Figure 4F-H).
Figure 4.
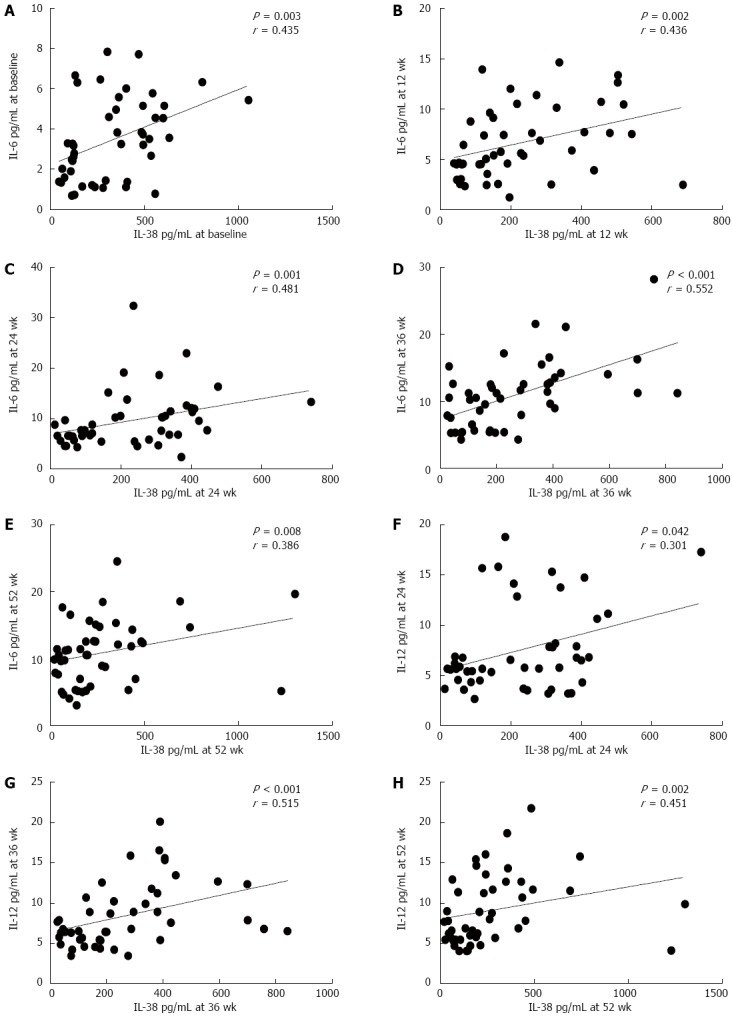
Correlation of serum interleukin-38, Th1- and Th2-type cytokines levels. A-E: Serum IL-38 levels correlated with serum IL-6 levels in patients with CHB during LdT treatment at baseline, and at 12, 24, 36, and 52 wk, respectively; F-H: Serum IL-38 levels correlated with serum IL-12 levels in patients with CHB during LdT treatment at 24, 36, and 52 wk, respectively. IL-38: Interleukin-38; CHB: Chronic hepatitis B; LdT: Telbivudine.
DISCUSSION
To our knowledge, this is the first study detailing kinetic changes in serum IL-38 levels during CHB. We found that the levels of serum IL-38 at baseline in patients with CHB were higher than in HCs, which declined and returned to normal after 12 wk of LdT treatment. There was a positive correlation between IL-38 and AST levels at baseline. Patients with high serum levels of IL-38 showed a higher probability of VR than patients with low serum levels of IL-38. Further, the serum HBV DNA level in patients with high baseline IL-38 levels was reduced after 12 wk of LdT treatment as compared to patients with low baseline IL-38 levels.
Our findings indicated that IL-38 level may be a marker reflecting liver injury, since elevated IL-38 at baseline was in parallel with elevated AST, an indicator of liver injury[22]. However, we could not confirm whether elevated IL-38 level was a primary trigger for the liver injury or a response to it. Nevertheless, the elevated IL-38 level indicates hepatic necroinflammation to a certain extent. An active hepatic necroinflammation is a result of destruction of infected hepatocytes, and it can be viewed as viral clearance. Based on this information, we can explain why patients with high IL-38 and AST levels at baseline showed much better VR to LdT treatment, because these patients showed more vigorous endogenous clearance of HBV infection in addition to the antiviral effect of LdT. In contrast, patients with lower IL-38 and AST levels likely indicated a weak endogenous clearance of HBV infection. NAs such as LdT may not show potent antiviral efficacy without a strong endogenous clearance.
PNR suggests failure of antiviral treatment. Thus, in a compliant patient with PNR, more potent antiviral therapy might be necessary[21]. At 12 wk of LdT therapy, serum IL-38 levels of patients with PNR were lower than those of patients with PR, suggesting that patients with high IL-38 levels at baseline show a more effective clearance of HBV infection, which supports our speculation of more vigorous endogenous viral clearance in patients with a high serum level of IL-38.
Previous studies indicated that cytokines were involved in the noncytopathic suppression of HBV replication[23]. IL-6 is reported to be responsible for early suppression of HBV in infected hepatocytes[24]. IL-6 exerted its inhibitory effect through reducing HBV transcripts/core protein and decreasing the level of HBV genome-containing nucleocapsids[25]. IL-6 also inhibits HBV entry through down regulation of sodium taurocholate cotransporting polypeptide[26]. IL-12 plays an important role in the defense against viral infections through promoting naive T cells differentiation into Th1 cells and inhibiting viral replication[27]. Cytokine IL-12 can rescue the anti-viral function of exhausted HBV-specific CD8 T cells[28]. Our data showed positive correlations between IL-38 and IL-6 or IL-12, suggesting the functional association between cytokine IL-38 and Th1/Th2 type cytokines IL-6 and IL-12. Further studies are required to uncover the underlying molecular links between these cytokines.
Accurate prediction of response to antivirals in treating chronic HBV infection remains challenging. However, our findings strongly suggest that a favorable response does not completely depend on the antiviral efficacy of the agent, but also relies on the vigorous endogenous viral clearance. Thus, our study provides a clue for predicting VR by looking for signs of endogenous viral clearance.
In conclusion, our study demonstrated that higher pretreatment serum IL-38 levels are associated with a greater probability of VR to LdT treatment. Elevated levels of serum IL-38 in untreated patients with CHB reflect ongoing liver injury, which is an indirect indicator of vigorous endogenous clearance of HBV infection. A favorable response may be observed if the LdT antiviral effect is combined with a strong endogenous viral clearance. Therefore, our findings suggest that clear signs of viral clearance at baseline may predict a favorable response to treatment of CHB using NAs.
ACKNOWLEDGMENTS
The authors would like to thank the members of Department of Central Laboratory, the Second Part of First Hospital of Jilin University for their technical support. The authors also thank Rui-Hong Wu for her biostatistics assistance.
COMMENTS
Background
Hepatitis B virus (HBV) infection is a serious global health concern. Interleukin (IL)-38 is a new anti-inflammatory cytokine of the IL-1 family, and its polymorphisms are associated with inflammatory diseases.
Research frontiers
The clinical outcome of HBV infection is a complex process between viral replication and host immune response. Cytokines are involved in the regulation of immune responses against HBV infection. Many investigators attempt to find ideal immunological indicators for response to antiviral therapy of chronic hepatitis B (CHB).
Innovations and breakthroughs
To date, there are no available data regarding the expression or function of IL-38 in CHB. This study demonstrated that higher serum IL-38 levels at pretreatment were associated with a greater probability of VR to LdT treatment. Elevated levels of serum IL-38 in untreated patients with CHB reflect ongoing liver injury, which is an indirect indicator of vigorous endogenous clearance of HBV infection. A favorable response could be observed if the LdT antiviral effect is combined with a strong endogenous viral clearance.
Applications
The findings suggest that clear signs of viral clearance at baseline may predict a favorable response to NAs treatment of CHB.
Peer-review
This manuscript demonstrated that higher pretreatment serum IL-38 levels are associated with a greater probability of VR to LdT treatment. The authors suggested that elevated serum IL-38 levels reflect ongoing liver injury, which is an indicator of endogenous clearance of HBV infection. These findings are interesting because it might provide novel predictors for good response to antiviral therapy.
Footnotes
Institutional review board statement: The study was reviewed and approved by the First Hospital of Jilin University Institutional Review Board.
Conflict-of-interest statement: The authors declare no financial or commercial conflicts of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 28, 2015
First decision: January 13, 2016
Article in press: March 2, 2016
P- Reviewer: Inoue K, Kim IH, Yoshioka K S- Editor: Gong ZM L- Editor: O’Neill M E- Editor: Zhang DN
References
- 1.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Ma Z, Xin G, Yan H, Li W, Xu H, Hao C, Niu J, Zhao P. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm. 2010;2010:143026. doi: 10.1155/2010/143026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertino G, Ardiri AM, Calvagno GS, Bertino N, Ruggeri MI, Malaguarnera M, Malaguarnera G, Toro A, Di Carlo I. Telbivudine on-treatment HBsAg loss in naive HBeAg negative chronic hepatitis B: a case report and brief review of the literature. Clin Ter. 2012;163:e429–e434. [PubMed] [Google Scholar]
- 6.Wang K, Wu ZB, Ye YN, Liu J, Zhang GL, Su YJ, He HL, Zheng YB, Gao ZL. Plasma Interleukin-10: A Likely Predictive Marker for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Hepat Mon. 2014;14:e19370. doi: 10.5812/hepatmon.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 8.van de Veerdonk FL, Netea MG. New Insights in the Immunobiology of IL-1 Family Members. Front Immunol. 2013;4:167. doi: 10.3389/fimmu.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Ji H, Cai Y, Ayana DA, Lv P, Liu M, Jiang Y. Serum interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. J Interferon Cytokine Res. 2013;33:612–618. doi: 10.1089/jir.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, Jiang Y. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res. 2012;32:248–253. doi: 10.1089/jir.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Ho AS, Haley-Vicente D, Zhang J, Bernal-Fussell J, Pace AM, Hansen D, Schweighofer K, Mize NK, Ford JE. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J Biol Chem. 2001;276:20597–20602. doi: 10.1074/jbc.M010095200. [DOI] [PubMed] [Google Scholar]
- 13.van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci USA. 2012;109:3001–3005. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudloff I, Godsell J, Nold-Petry CA, Harris J, Hoi A, Morand EF, Nold MF. Brief Report: Interleukin-38 Exerts Antiinflammatory Functions and Is Associated With Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015;67:3219–3225. doi: 10.1002/art.39328. [DOI] [PubMed] [Google Scholar]
- 15.Ciccia F, Accardo-Palumbo A, Alessandro R, Alessandri C, Priori R, Guggino G, Raimondo S, Carubbi F, Valesini G, Giacomelli R, et al. Interleukin-36α axis is modulated in patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2015;181:230–238. doi: 10.1111/cei.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman P, Sun S, Peddle L, Snelgrove T, Melay W, Greenwood C, Gladman D. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum. 2006;54:2321–2325. doi: 10.1002/art.21928. [DOI] [PubMed] [Google Scholar]
- 17.Chou CT, Timms AE, Wei JC, Tsai WC, Wordsworth BP, Brown MA. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann Rheum Dis. 2006;65:1106–1109. doi: 10.1136/ard.2005.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enomoto M, Tamori A, Nishiguchi S, Kawada N. Combination therapy with a nucleos(t)ide analogue and interferon for chronic hepatitis B: simultaneous or sequential. J Gastroenterol. 2013;48:999–1005. doi: 10.1007/s00535-012-0742-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang GQ, Ding YP, Dong YH. Telbivudine treatment is associated with high hepatitis B e antigen seroconversion and immune modulatory effects in chronic hepatitis B patients. J Viral Hepat. 2013;20 Suppl 1:9–17. doi: 10.1111/jvh.12059. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Huang Z, Chen X, Tian Y, Tang J, Zhang Y, Zhang X, Zhou J, Mao Q, Ni B, et al. Effects of telbivudine treatment on the circulating CD4⁺ T-cell subpopulations in chronic hepatitis B patients. Mediators Inflamm. 2012;2012:789859. doi: 10.1155/2012/789859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, Forouzanfar MH, Abedian S, Tavangar SM, Mohamadkhani A, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol. 2006;101:2537–2545. doi: 10.1111/j.1572-0241.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 24.Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 25.Kuo TM, Hu CP, Chen YL, Hong MH, Jeng KS, Liang CC, Chen ML, Chang C. HBV replication is significantly reduced by IL-6. J Biomed Sci. 2009;16:41. doi: 10.1186/1423-0127-16-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34–42. doi: 10.1016/j.virol.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]


