Abstract
AIM: To investigate the expression and clinical significance of B7-H4 and hepatitis B virus X (HBx) protein in hepatitis B virus-related hepatocellular carcinoma (HBV-HCC).
METHODS: The expression of B7-H4 in the human HCC cell lines HepG2 and HepG2.2.15 were detected by western blot, flow cytometry, and immunofluorescence. The expression of B7-H4 and HBx in 83 HBV-HCC was detected by immunohistochemistry, and the relationship with clinicopathological features was analyzed. Paraffin sections were generated from 83 HBV-HCC patients (22 females and 61 males) enrolled in this study. The age of these patients ranged from 35 to 77 years, with an average of 52.5 ± 11.3 years. All experiments were approved by the Ethics Committees of the Second Affiliated Hospital, Zhejiang University School of Medicine.
RESULTS: B7-H4 was significantly upregulated in HepG2.2.15 cells compared to HepG2 cells. Specifically, the protein expression of B7-H4 in the lysates of HepG2 cells was more than that in HepG2.2.15 cells. In addition, HBx was expressed only in HepG2.2.15 cells. Similar data were obtained by flow cytometry. The positive rates of B7-H4 and HBx in the tissues of 83 HBV-HCC patients were 68.67% (57/83) and 59.04% (49/83), respectively. The expression of HBx was correlated with tumor node metastases (TNM) stage, and the expression of B7-H4 was positively correlated with HBx (rs = 0.388; P < 0.01). The expression level of B7-H4 in HBx-positive HBV-HCC tissues was substantially higher than that in HBx-negative HBV-HCC tissues. The expression level of B7H4 was negatively related to tumor TNM stage.
CONCLUSION: Higher expression of HBx and B7-H4 was correlated with tumor progression of HBV-HCC, suggesting that B7-H4 may be involved in facilitating HBV-related hepatocarcinogenesis.
Keywords: Hepatocellular carcinoma, Hepatitis B virus, Hepatitis B virus X, B7-H4, Immunohistochemistry
Core tip: Hepatitis B virus (HBV) is a major public health problem, and HBV-related hepatocellular carcinoma (HBV-HCC) has an extremely poor prognosis due to the lack of effective treatments. B7-H4 is a newly characterized member of the B7 superfamily that is actively involved in regulating the pathogenesis of tumors. However, the intrahepatic expression of B7-H4 in HBV-HCC patients has not been described. In this study, we found that the higher expression of HBx and B7-H4 was correlated with tumor progression of HBV-HCC. Therefore, B7-H4 may be involved in facilitating HBV-related hepatocarcinogenesis.
INTRODUCTION
More than 350 million people worldwide suffer from a persistent hepatitis B virus (HBV) infection and are at an increased risk of developing hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)[1]. HCC is the fifth most common cancer and the third most common cause of death due to cancer worldwide[2]. No systemic therapy has been shown to improve survival, and recurrence is common, even for curative treatment. Therefore, there is a need to identify prognostic biomarkers and new therapeutic strategies. It has been shown that the majority of HBV-related HCC tissue samples contain integrated viral DNA[3]. Hepatitis B virus X (HBx) originates from the HBV genome and is a multifunctional regulatory protein. Although HBx does not bind directly to DNA, it can trans-activate gene transcription through multiple cis-acting elements[4,5]. HBx may be associated with the development of human HCC[6,7], but the precise mechanism of HBx in tumorigenic transformation of hepatocytes remains unclear.
The coregulatory molecules of the B7 family have an indispensable role in the immune regulation of several pathologies[8]. It has been reported that B7-H1 and B7-H4 are expressed in human tumors and that they may act as coregulatory inhibitors to inhibit T cell activation or to induce T cell apoptosis[9-11]. Furthermore, the expression of B7-H1 in hepatocarcinoma cells can be initiated by HBx, leading to T cell apoptosis and potentially facilitating the genesis of HCC[12].
B7-H4 (also named B7S1 or B7x), a member of the B7 superfamily[13,14], has been demonstrated to inhibit T cell activation, proliferation, and differentiation and to be inversely correlated with tumor T cell infiltration[15,16]. B7-H4 is widely prevalent in a variety of tumor tissues[10,17-21], but it is absent on the cell surface of most human normal somatic tissues[13]. Multiple studies have revealed that B7-H4 can downregulate tumor-reactive cytotoxic T lymphocyte (CTL) function[13] and that inhibition of B7-H4 with small interference RNA (siRNA) led to antitumor immunity and inhibition of tumor growth[17,22]. Therefore, B7-H4 has been suggested to play a key role in tumor progression and immune escape. Interestingly, recent data have demonstrated that B7-H4 expression was enhanced in cells infected with a virus, such as Epstein-Barr virus (EBV)[23] and HBV[24]. These results indicate that B7-H4 signaling may affect the pathogenesis of a viral infection, and a clear understanding of its functional role may further elucidate the disease process. Although the presence of B7-H4 in human tumors appears to be a general phenomenon, there are no clinical data available on the expression levels of B7-H4 in human HBV-HCC.
It is evident that both HBx and B7-H4 are involved in the pathogenesis of HBV-HCC. However, the relationship between these two proteins remains unknown. Therefore, we speculated that both HBx and B7-H4 would promote the survival of hepatocytes transfected with HBV malignant transformation and tumor development. To test this hypothesis, HepG2 cells, a human HCC cell line, were transfected with HBV (HepG2.2.15). We investigated the prognostic significance and clinical relevance of HBx and B7-H4 expression in a cohort of 83 HCC patients treated by a curative resection.
MATERIALS AND METHODS
Cell lines
HepG2 cells derived from human HCC were purchased from American Type Culture Collection (ATCC, Manassas, VA, United States) and were grown in Dulbecco’s Modified Eagle’ Medium (DMEM) culture media (Life Technologies Corporation, Gaithersburg, MD, United States) containing 100 IU/mL penicillin, 100 μg/mL streptomycin (Life Technologies Corporation), and 10% fetal bovine serum (FBS, PAA, Morningside, Australia) at 37 °C in a humidified incubator with 5% CO2.
HepG2.2.15 cells transfected with the complete HBV DNA and stably HBV-expressing hepatoma cells derived from HepG2 cells (firstly described by Sells et at 1987 and 1988)[25,26] were purchased from the Chinese Center For Type Culture Collection (CCTCC, Wuhan, China) and were grown in DMEM culture media containing 300 μg/mL G418 (Promega, Madison, WI, United States), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% FBS at 37 °C in a humidified incubator with 5% CO2.
Antibodies
Mouse monoclonal antibody (3E8 mAb) specific for human B7-H4 was previously described[27]. Rabbit anti-HBx antibody was purchased from Abcam (Cambridge, MA, United States). Horseradish peroxidase (HRP)-conjugated rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody was purchased from Cell Signaling Technology (Danvers, MA, United States). HRP-conjugated goat anti-mouse IgM, goat anti-mouse IgG, and goat anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, United States). Alexa Fluor® 488 goat anti-mouse IgM antibody was purchased from Invitrogen (Life Technologies Corporation).
Cell lysis
Cells were lysed using cell lysis buffer containing 2 mmol/L Tris-HCl, pH 7.5, 15 mmol/L NaCl, 0.1 mmol/L Na2EDTA, 0.1 mmol/L EGTA, 0.1% Triton X-100, 0.25 mmol/L sodium pyrophosphate, 0.1 mmol/L beta-glycerophosphate, 0.1 mmol/L Na3VO4, and 0.1 μg/mL leupeptin (Cell Signaling Technology). Samples containing 1 × 106 cells were lysed in 200 μL cell lysis buffer for 30 min at 4 °C and then clarified by centrifugation at 8000 g for 10 min. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, United States), with bovine serum albumin (BSA) as the standard.
Western blot analysis
Cell lysate preparation and western blot were performed as described previously[27]. Briefly, cell lysates were denatured for 10 min at 95 °C with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, electrophoresed on 10% SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with Tween [TBST 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.05% (v/v) Tween 20] and then incubated with specific antibodies at 4 °C overnight. After thoroughly washing, blots were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Protein band intensity was analyzed using enhanced chemiluminescence (ECL) reagents (Millipore, Billerica, MA, United States) and a VersaDoc MP5000 imaging system (Bio-Rad, Hercules, CA, United States).
Flow cytometry analysis
Each sample of cells (2 × 106) was incubated with 3E8 mAb for 1 h at 4 °C, followed with Alexa Fluor® 488 goat anti-mouse IgM antibody for 30 min at 4 °C. Normal mouse IgM was used as an antibody control. Cells were washed twice, and samples were analyzed using a flow cytometer (FACScan, San Jose, CA, United States) by flowjo7.6.
Immunofluorescence detection
Cell samples were fixed in ice-cold 3%-4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.4) for 15 min at room temperature, incubated for 10 min with PBS containing 0.25% Triton X-100, and then incubated with 1% BSA in PBS with Tween (PBST) for 30 min. Cells were then incubated in the diluted 3E8 mAb in 1% BSA in PBST in a humidified chamber overnight at 4 °C. Normal mouse IgM was used as an antibody control. After thorough washing, cells were incubated with Alexa Fluor® 488 goat anti-mouse IgM antibody in 1% BSA for 1 h at room temperature in the dark and then rinsed with PBS. Cells were incubated with 3 ng/mL 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen, Life Technologies Corporation) for 10 min, mounted with ProLong Gold Antifade Reagent (Invitrogen, Life Technologies Corporation), and observed under an Olympus 1X81 fluorescence microscope (Center Valley, PA, United States) microscopy using filters for fluorescein isothiocyanate (FITC) and DAPI.
Immunohistochemical staining
HBV-HCC tissue microarrays were obtained from Alenabio Biotechnology Co., LTD (Shanxi, China) for immunohistochemical (IHC) staining. Clinical and pathological information for individual cancer samples was provided by the array manufacturers (for details see www.alenabio.com). Paraffin sections of tumors were obtained from 83 HBV-HCC patients (22 females and 61 males) enrolled in this study. Informed consent for this study was obtained from each patient. The age of these patients ranged from 35 to 77 years, with an average of 52.5 ± 11.3 years. Tumor grade was divided into three categories: tumor grade I is well-differentiated, low grade malignant; tumor grade II is moderately-differentiated, intermediate grade malignant; tumor grade III is poorly-differentiated, high grade malignant. TNM stage refers to the Tumor Node Metastasis stage. All of these experiments were approved by the Ethics Committees of the Second Affiliated Hospital, Zhejiang University School of Medicine. IHC staining was performed on mouse tumor tissues as previously described[27]. Briefly, tissues were labeled using primary antibodies specific for B7-H4 and HBx, and antibody binding was detected using EnVision System reagents (DAKO, Glostrup, Denmark). Semi-quantitative measurements of staining intensity (0-3, least intense to most intense), and the proportion of stained cells (0-4, no cells stained to more than 70% cells stained) were determined as previously described[27]. A combined score of ≥ 2 was considered to indicate positive expression. All slides were scored by two researchers blinded to the pathological and clinical features.
Statistical analysis
All experiments were performed in triplicate. Differences in positive rates between groups were determined using a χ2 test (SPSS 18, IBM Corporation, New York, NY, United States). Differences of B7-H4 expression between groups were determined using a Student’s t-test. A correlation analysis was determined using Spearman correlation coefficients. Statistical significance was set at P < 0.05.
RESULTS
Detection of B7-H4 expression by Western blot and flow cytometry
The expression of B7-H4 in lysates of HepG2 and HepG2.2.15 cells was determined by western blot (Figure 1A). The results revealed that HBx was expressed in HepG2.2.15 cells but not in HepG2 cells. Moreover, the expression level of B7-H4 was significantly higher in HepG2.2.15 cells compared with HepG2 cells, consistent with results from the flow cytometry analysis (P < 0.01, Figure 1B).
Figure 1.
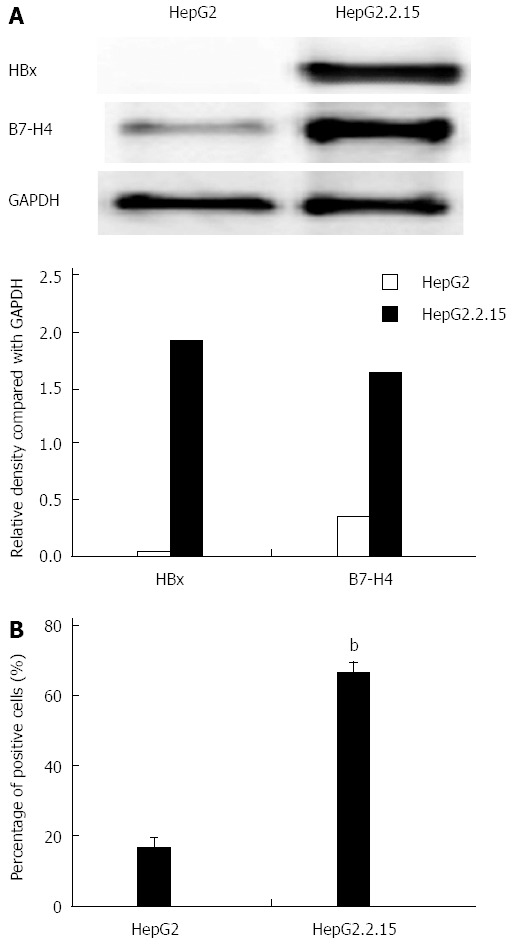
Expression of B7-H4 in HepG2 and HepG2.2.15 cells. A: Detection of B7-H4 expression by western blot. The expression level of B7-H4 and HBx was shown by the relative density ratio of B7-H4 and GAPDH; B: Detection of B7-H4 expression by flow cytometry. bP < 0.01, HepG2.2.15 vs HepG2 cells. HBx: Hepatitis B virus X; GAPDH: Reduced glyceraldehyde-phosphate dehydrogenase.
Detection of B7-H4 expression by immunofluorescence staining
The immunofluorescence assay confirmed that the immunoreactivity for B7-H4 was strong in HepG2.2.15 cells. In contrast, the immunoreactivity for B7-H4 was virtually absent in HepG2 cells (Figure 2). Moreover, B7-H4 was diffusely expressed either in the membrane or the cytoplasm of HepG2.2.15 cells.
Figure 2.
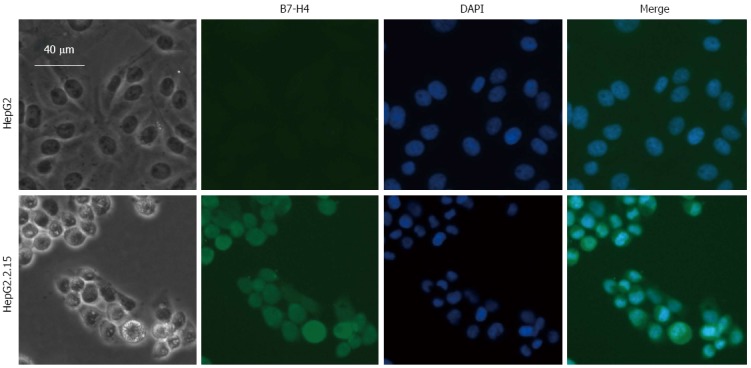
Expression of B7-H4 in HepG2 and HepG2.2.15 cells determined by immunofluorescence staining. Immunofluorescence detection of B7-H4 in HepG2 and HepG2.2.15 cells was stained with 3E8 mAb followed with Alexa Fluor® 488 goat anti-mouse IgM antibody (green). Nuclei were stained with DAPI (blue). Changes in cell morphology were recorded with a light microscope at 200 × magnification.
Expression and clinical significance of B7-H4 and HBx in HBV-related hepatocellular carcinoma
Immunohistochemical analysis detected the expression of B7-H4 and HBx in HBV-HCC tissues. Among the 83 HBV-HCC tissue samples examined, the positive rates of B7-H4 and HBx were 68.67% (57/83) and 59.04% (49/83), respectively. Statistical analysis revealed that the positive rates of HBx were correlated with the tumor TNM stage (P < 0.01) but not with patient’s age, sex, or tumor grade. The positive rate of HBx expression in TNM stage III/IV tissues was 75.56% (34/45), substantially higher than the 39.47% in TNM stage I/II tissues (15/38, P < 0.01; Table 1). The positive rates of B7-H4 expression in HBV-HCC tissues were not correlated with patient’s age, sex, tumor grade, or tumor TNM stage.
Table 1.
Expression and clinical significance of B7-H4 and hepatitis B virus X in hepatitis B virus-related hepatocellular carcinoma
|
HBx |
χ2/P |
B7-H4 |
χ2/P | |||
| No. of positive | No. of negative | No. of positive | No. of negative | |||
| Gender | ||||||
| Male | 39 | 22 | χ2 = 2.28 | 42 | 19 | χ2 = 0.00 |
| Female | 10 | 12 | P > 0.05 | 15 | 7 | P > 0.05 |
| Age (yr) | ||||||
| ≤ 50 | 21 | 21 | χ2 = 2.87 | 25 | 17 | χ2 = 3.31 |
| > 50 | 28 | 13 | P > 0.05 | 32 | 9 | P > 0.05 |
| Tumor grade | ||||||
| I/II | 36 | 24 | χ2 = 0.08 | 39 | 21 | χ2 = 1.36 |
| III | 13 | 10 | P > 0.05 | 18 | 5 | P > 0.05 |
| TNM stage | ||||||
| I/II | 15 | 23 | χ2 = 11.09 | 23 | 15 | χ2 = 2.16 |
| III/IV | 34 | 11 | P < 0.01 | 34 | 11 | P > 0.05 |
HBx: Hepatitis B virus X; HBV: Hepatitis B virus.
Correlation between B7-H4 and HBx in HBV-related hepatocellular carcinoma
The positive rate of B7-H4 in HBx-positive HBV-HCC tissues 83.67% (41/49) was significantly higher than the 47.06% in HBx-negative HBV-HCC tissues (16/34, P < 0.01). The expression of B7-H4 was positively correlated with HBx (rs = 0.388, P < 0.01).
Furthermore, the effect of HBx on the expression level of B7-H4 in HBV-HCC tissues was analyzed (Table 2). B7-H4 expression with scores of 3.43 ± 1.68 in HBx-positive HBV-HCC tissues was substantially higher than that in HBx-negative HBV-HCC tissue scores of 1.81 ± 1.30 (P < 0.01). The expression level of B7-H4 in HBx-positive HBV-HCC tissues was not related to patient’s age, sex, or tumor grade. However, a slight decrease was seen in TNM III/IV samples (3.12 ± 1.63), and this difference was statistically significant in comparison with that of the TNM I/II samples (4.13 ± 1.51, P < 0.05; Figure 3).
Table 2.
Expression and clinical significance of B7-H4 in hepatitis B virus X-positive hepatitis B virus X-related hepatocellular carcinoma
|
HBx-positive |
P value | ||
| B7-H4 expression scores | Case No. | ||
| Gender | |||
| Male | 3.28 ± 1.65 | 39 | > 0.05 |
| Female | 4.00 ± 1.56 | 10 | |
| Age (yr) | |||
| ≤ 50 | 3.14 ± 1.62 | 21 | > 0.05 |
| > 50 | 3.64 ± 1.66 | 28 | |
| Tumor grade | |||
| I/II | 3.33 ± 1.67 | 36 | > 0.05 |
| III | 3.69 ± 1.60 | 13 | |
| TNM stage | |||
| I/II | 4.13 ± 1.51 | 15 | < 0.05 |
| III/IV | 3.12 ± 1.63 | 34 | |
HBx: Hepatitis B virus X; HBV: Hepatitis B virus.
Figure 3.
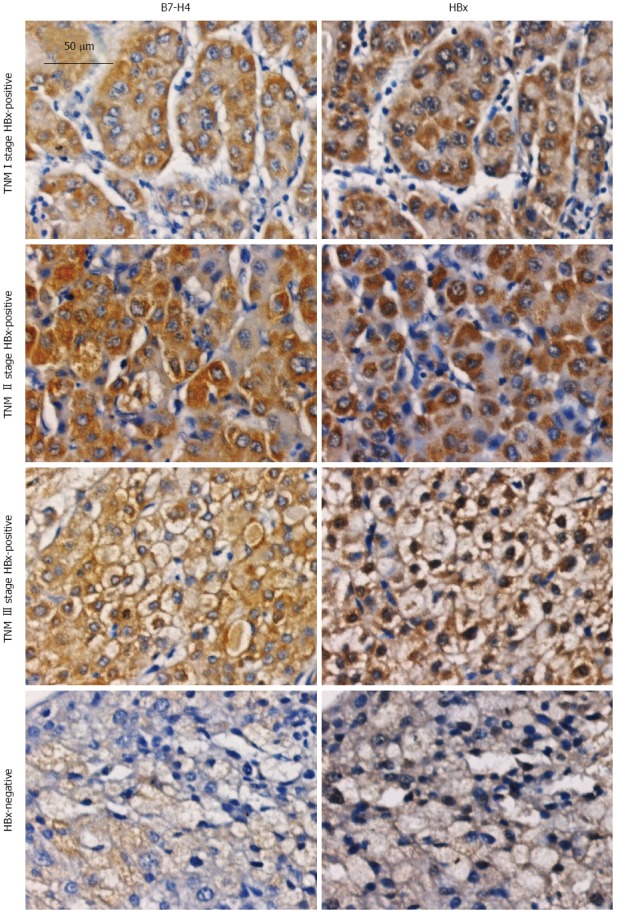
Expression of B7-H4 and hepatitis B virus X in hepatitis B virus related hepatocellular carcinoma tissues detected by immunohistochemical staining. Paraffin sections were generated from 83 hepatitis B virus related hepatocellular carcinoma (HBV-HCC) patients (22 females and 61 males) were enrolled in this study. The age of these patients ranged from 35 to 77 years, with an average of 52.5 ± 11.3 years. HBV-HCC tissues were subjected to IHC staining using antibodies specific for B7-H4 and HBx and recorded with a light microscope at 200 × magnification. HBx: Hepatitis B virus X; IHC: Immunohistochemical.
DISCUSSION
HBV infection is the primary cause of liver disease that has the potential to develop HCC[3,28]. Although the pathogenic mechanisms of HBV-HCC are not clear, it has been reported in many studies that immune-mediated hepatocyte malignant transformation might play a key role. HCC has poor immunogenicity, partly due to poor antigen expression, the lack of costimulatory molecules to positively regulate T cell immune responses[29], and an increased number of costimulatory molecules that negatively regulate T cell immune response[30].
There is accumulating evidence that immunotherapy may become a powerful therapeutic option for HCC patients[31]. Stimulating or eliminating inhibitory T cell signaling to enforce antitumor responses is central to immune-based therapies to eradicate human cancers. It is clear that costimulatory molecules play pivotal roles in regulating the immune response and are engaged in the pathogenesis of many diseases, especially chronic virus infection and tumor development[32]. B7-H4 is a member of the B7 family, and its mRNA is broadly expressed in many tissues[13,14]. In addition to the inhibition of T cell tolerance, our previous data showed that B7-H4 was also involved in the regulation of cellular tumor apoptosis[22]. More importantly, after performing a multivariate adjustment for conventional prognostic factors, elevation in B7-H4 expression was a significant predictor of tumor recurrence[33]. The expression and distribution of B7-H4 in HCC tissues from HBV patients has not been previously reported. In this study, we found that the expression of B7-H4 was significantly increased in HCC cells transfected with HBV compared with control HCC cells. A high percentage of B7-H4 positive staining was observed in a subset of HBx-positive HBV-HCC tissues and was significantly associated with the TNM stage of HCC and level of HBx expression. Thus, HBx and B7-H4 may jointly promote the development of HBV-HCC and prove useful for the clinical evaluation of HBV-HCC patients, especially to identify high-risk patients who are at increased risk of cancer progression.
Although several studies have examined the role of B7-H4 in tumor immunity, the pathophysiologic function of B7-H4 has yet to be fully elucidated. In the present study, we found a detrimental role for B7-H4 in HBV-HCC patients. Costimulatory molecules, including vascular cell adhesion molecule (VCAM)-1[34], cluster of differentiation (CD)40[35], CD28, and programmed death (PD)-1[36] as well as several members of the B7 superfamily (e.g., B7-1, B7-2[29], B7-H1[12,30], and B7-H3[37]), have been reported to be expressed in HCC tissues. These costimulatory molecules could provide positive (VCAM-1, CD28, CD40, B7-1, and B7-2) or negative (PD-1 and B7-H1) signals to local T cells response and regulate the pathogenesis of HCC. It has been reported that HBeAg suppresses the specific cellular immunity that clears the virus by upregulating B7-H1 expression, eventually leading to immune tolerance to HBV infection[38]. B7-H4 was described to be a membrane costimulatory ligand of the B7 superfamily, which is involved in the downregulation of T cell activation under certain circumstances. Ectopic B7-H4-Ig may protect animals from liver injury induced by concanavalin A (ConA), which could be associated with reduced serum levels of interleukin (IL)-2, interferon (IFN)-gamma, and IL-4 as well as enhanced IL-10 production[39]. Thus, B7-H4 may lead to immune tolerance to HBV infection and play an important role in immune suppression in chronic HBV-HCC patients. In addition to surface location, intracellular expression of B7-H4 has been reported in primary ovarian tumor cells[9], with similar expression levels to what we observed here in HBV-HCC (Figures 2 and 3). However, we also found that B7-H4 was an intracellular protein that was upregulated in HBV-HCC patients. This expression pattern suggests that B7-H4 might possess functions that are different from other cell surface molecules of the B7 family, which were previously reported to a play role in the pathogenesis of cancers by inhibiting the T cell-mediated immune response. Our previous results indicated that intracellular B7-H4 enhanced oncogenicity and inhibited apoptosis in pancreatic cancer cells[22]. In addition, Salceda et al[17] suggested that the expression of B7-H4 on breast cancer cell surface acts as an anti-apoptosis molecule that inhibits tumor apoptosis and ultimately protects tumors from cell-mediated immune surveillance. Furthermore, B7-H4 expression was enhanced in B cells infected with EBV, and the engagement of B7-H4 initially increased the levels of intracellular ROS, which induced the expression of FasL and subsequently aroused Fas-mediated and caspase-dependent apoptosis of EBV-transformed B cells[23]. These results together with our previous data indicate that B7-H4 signaling is involved in regulating cell apoptosis and that intracellular B7-H4 might possess an anti-apoptotic effect in HCC cells. Additional research is needed to determine if this effect is a function of B7-H4.
Interestingly, the expression of B7-H4 in HBx-positive HBV-HCC tissues was negatively correlated with the TNM stage. The epitope binding to the 3E8 mAb of B7-H4 may be altered. It has been reported that some cell surface adhesive proteins, such as mucin 4 (MUC4), can mask the human epidermal growth factor receptor (HER)2 receptor and inhibit Herceptin binding to HER2 through a stereospecific blockade, leading to Herceptin resistance[40]. Genetic mutations of epidermal growth factor receptor lead to gefitinib resistance[41]. Thus, we suspected that an epitope change of B7-H4 might be involved in immune-mediated tumor escape, thereby contributing to tumor development. Moreover, there was extensive necrosis in the advanced stage HBV-HCC tissues, leading to a decrease in the expression level of B7-H4. The mechanism by which this process occurs will be persued as an anvenue of future research.
In conclusion, our results indicate that B7-H4 is highly expressed in human HBx-positive HBV-HCC tissues and is associated with the TNM stage. Therefore, HBx and B7-H4 may play important roles in the development of HBV-HCC. In addition, B7-H4 may be involved in HBx-induced hepatocarcinogenesis, and an increased understanding of the functional roles of B7-H4 could aid in clarifying the mechanism of HBx in HBV-HCC. Furthermore, targeting B7-H4 may present a novel strategy by which disease diagnosis or immunotherapy against HBV infection can be achieved.
COMMENTS
Background
Hepatitis B virus-related hepatocellular carcinoma (HBV-HCC) has an extremely poor prognosis due to the lack of effective treatments. B7-H4 is a newly characterized member of the B7 superfamily of proteins, which are actively involved in regulating the pathogenesis of tumors. It is clear that both hepatitis B virus X (HBx) and B7-H4 are involved in the pathogenesis of HBV-HCC. However, the intrahepatic expression of B7-H4 in HBV-HCC patients has not been described.
Research frontiers
HBx originates from the HBV genome and is a multifunctional regulatory protein. Although HBx does not bind directly to DNA, it can trans-activate gene transcription through multiple cis-acting elements. HBx was thought to be associated with the development of human HCC. It has been reported that B7-H4 is expressed in human tumors and is implicated as coregulatory inhibitors that may inhibit T cell activation or induce T cell apoptosis upon antigen recognition. B7-H4 expression was enhanced in cells infected with a virus, such as Epstein-Barr virus (EBV) and HBV. B7-H4 signaling is most likely to affect the pathogenesis of a viral infection and a clear understanding of its functional role may further elucidate the disease process. Although the presence of B7-H4 in human tumors appears to be a general phenomenon, there is little available on the expression level of B7-H4 in human HBV-HCC.
Innovations and breakthroughs
In this article, the authors reached the conclusion that co-inhibitory molecules, such as B7-H4, are always involved in HBx-induced hepatocarcinogenesis. Targeting B7-H4 may represent a novel strategy by which disease diagnosis or immunotherapy against HBV infection can be achieved.
Applications
Based on these data, the authors concluded that B7-H4 is highly expressed in human HBx-positive HBV-HCC tissues and is associated with TNM stage. Therefore, HBx and B7-H4 may play an important role in the development of HBV-HCC. In addition, B7-H4 may be involved in HBx-induced hepatocarcinogenesis, and an increased understanding of the functional roles of B7-H4 could aid in clarifying the mechanism of HBx in HBV-HCC. This study provides new insight into the function of a coinhibitory signaling pathway during the clinical course of HBV-HCC.
Peer-review
The authors have presented a highly interesting study. The design, interpretation, and presentation of their study are well performed.
Footnotes
Supported by the National Natural Science Foundation of China, No. 81272679 and No. 81470851; Zhejiang Provincial Natural Science Foundation of China, No. LQ16H160005 and No. LY15H080002; the Medical Science and Technology Project of Zhejiang Province, No. 201337756; and the Educational Commission of Zhejiang Province of China, No. Y201328079. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was reviewed and approved by The Second Affiliated Hospital, Zhejiang University School of Medicine Institutional Review Board.
Conflict-of-interest statement: The authors declare that they have no conflict of interest related to the publication of this manuscript.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at yaohangping@zju.edu.cn. Participants gave informed consent for data sharing. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 16, 2016
First decision: February 18, 2016
Article in press: March 18, 2016
P- Reviewer: Bock CT, Chung YH S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma S
References
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol. 2005;34 Suppl 1:S75–S78. doi: 10.1016/s1386-6532(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 4.Lucito R, Schneider RJ. Hepatitis B virus X protein activates transcription factor NF-kappa B without a requirement for protein kinase C. J Virol. 1992;66:983–991. doi: 10.1128/jvi.66.2.983-991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen TS. Hepadnaviral X Protein: Review of Recent Progress. J Biomed Sci. 1996;3:20–30. doi: 10.1007/BF02253575. [DOI] [PubMed] [Google Scholar]
- 6.Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH, et al. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31:123–132. doi: 10.1016/s0168-8278(99)80172-x. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 9.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Yang C, Guo S, Fei L, Luo N, Fu X, Chen Y, Wu Y. Stimulation of B7-H1 in hepatocarcinoma cells by hepatitis B virus X antigen. Immunol Invest. 2010;39:754–769. doi: 10.3109/08820139.2010.494193. [DOI] [PubMed] [Google Scholar]
- 13.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 14.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 15.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyatake T, Tringler B, Liu W, Liu SH, Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A, Shroyer KR. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol. 2007;106:119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 19.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 20.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Qian Y, Hong B, Shen L, Wu Z, Yao H, Zhang L. B7-H4 enhances oncogenicity and inhibits apoptosis in pancreatic cancer cells. Cell Tissue Res. 2013;353:139–151. doi: 10.1007/s00441-013-1640-8. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Park G, Kim YS, Hur I, Kim H, Ryu JW, Lee HK, Cho DH, Choi IH, Lee WJ, et al. B7-H4 reverse signaling induces the apoptosis of EBV-transformed B cells through Fas ligand up-regulation. Cancer Lett. 2008;266:227–237. doi: 10.1016/j.canlet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 24.Guo G, Cao D, Xu H, Ruan Z, Fei L, Xie Z, Wu Y, Chen Y. The characteristic expression of B7-H3 and B7-H4 in liver biopsies from patients with HBV-related acute-on-chronic liver failure. Pathol Int. 2012;62:665–674. doi: 10.1111/j.1440-1827.2012.02856.x. [DOI] [PubMed] [Google Scholar]
- 25.Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, Shen L, Xu C, Wu Z, Brockmeyer NH, Altmeyer P, Wu N, Yao HP. Development of a novel monoclonal antibody to B7-H4: characterization and biological activity. Eur J Med Res. 2011;16:295–302. doi: 10.1186/2047-783X-16-7-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13:74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsumi T, Takehara T, Katayama K, Mochizuki K, Yamamoto M, Kanto T, Sasaki Y, Kasahara A, Hayashi N. Expression of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on human hepatocellular carcinoma. Hepatology. 1997;25:1108–1114. doi: 10.1002/hep.510250511. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61:101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 32.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 33.Jung SG, Choi KU, Lee SD, Lee ZZ, Chung MK. The Relationship between B7-H4 Expression and Clinicopathological Characteristics in Clinical Stage T1 Conventional Renal Cell Carcinoma. Korean J Urol. 2011;52:90–95. doi: 10.4111/kju.2011.52.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YI, Liu Z, Chen Y, Xu K, Dong J. PPARγ activation reduces ischemia/reperfusion-induced metastasis in a murine model of hepatocellular carcinoma. Exp Ther Med. 2016;11:387–396. doi: 10.3892/etm.2015.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, Chen Y, Ni B, Yang D, Guo S, Wu Y. Up-regulation of the expression of costimulatory molecule CD40 in hepatocytes by hepatitis B virus X antigen. Biochem Biophys Res Commun. 2009;384:12–17. doi: 10.1016/j.bbrc.2009.03.139. [DOI] [PubMed] [Google Scholar]
- 36.Hsu PN, Yang TC, Kao JT, Cheng KS, Lee YJ, Wang YM, Hsieh CT, Lin CW, Wu YY. Increased PD-1 and decreased CD28 expression in chronic hepatitis B patients with advanced hepatocellular carcinoma. Liver Int. 2010;30:1379–1386. doi: 10.1111/j.1478-3231.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, Shi JY, Xu YF, Shi YH, Song K, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Li J, Jiang L, Xu Q, Liu B, Jin K, Liu Y, Huang Z. Regulation of B7-H1 expression on peripheral monocytes and IFN-γ secretion in T lymphocytes by HBeAg. Cell Immunol. 2013;283:25–30. doi: 10.1016/j.cellimm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Xu JF, Xiao H, Hu GY, Zheng SH, Liu W, Yuan CL, Yang H, Lü J, Zheng F, Wang CY, et al. Ectopic B7-H4-Ig expression attenuates concanavalin A-induced hepatic injury. Clin Immunol. 2010;136:30–41. doi: 10.1016/j.clim.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011;102:1–8. doi: 10.1111/j.1349-7006.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]


