Abstract
AIM: To investigate the changes in clinical symptoms and gastric emptying and their association in functional dyspepsia (FD) patients.
METHODS: Seventy FD patients were enrolled and divided into 2 groups Helicobacter pylori (H. pylori)-negative group (28 patients), and H. pylori-positive group (42 patients). Patients in the H. pylori-positive group were further randomly divided into groups: H. pylori-treatment group (21 patients) and conventional treatment group (21 patients). Seventy two healthy subjects were selected as the control group. The proximal and distal stomach area was measured by ultrasound immediately after patients took the test meal, and at 20, 40, 60 and 90 min; then, gastric half-emptying time was calculated. The incidence of symptoms and gastric half-emptying time between the FD and control groups were compared. The H. pylori-negative and conventional treatment groups were given conventional treatment: domperidone 0.6 mg/(kg/d) for 1 mo. The H. pylori-treatment group was given H. pylori eradication treatment + conventional treatment: lansoprazole 30 mg once daily, clarithromycin 0.5 g twice daily and amoxicillin 1.0 g twice daily for 1 wk, then domperidone 0.6 mg/(kg/d) for 1 mo. The incidence of symptoms and gastric emptying were compared between the FD and control groups. The relationship between dyspeptic symptoms and gastric half-emptying time in the FD and control groups were analyzed. Then total symptom scores before and after treatment and gastric half-emptying time were compared among the 3 groups.
RESULTS: The incidence of abdominal pain, epigastric burning sensation, abdominal distension, nausea, belching, and early satiety symptoms in the FD group were significantly higher than in the control group (50.0% vs 20.8%; 37.1% vs 12.5%; 78.6% vs 44.4%; 45.7% vs 22.2%; 52.9% vs 15.3%; 57.1% vs 19.4%; all P < 0.05). The gastric half-emptying times of the proximal end, distal end, and the whole stomach in the FD group were slower than in the control group (93.7 ± 26.2 vs 72.0 ± 14.3; 102.2 ± 26.4 vs 87.5 ± 18.2; 102.1 ± 28.6 vs 78.3 ± 14.1; all P < 0.05). Abdominal distension, belching and early satiety had an effect on distal gastric half-emptying time (P < 0.05). Abdominal distension and abdominal pain had an effect on the gastric half-emptying time of the whole stomach (P < 0.05). All were risk factors (odds ratio > 1). The total symptom score of the 3 groups after treatment was lower than before treatment (P < 0.05). Total symptom scores after treatment in the H. pylori-treatment group and H. pylori-negative group were lower than in the conventional treatment group (5.15 ± 2.27 vs 7.02 ± 3.04, 4.93 ± 3.22 vs 7.02 ± 3.04, All P < 0.05). The gastric half-emptying times of the proximal end, distal end, and the whole stomach in the H. pylori-negative and H. pylori-treatment groups were shorter than in the conventional treatment group (P < 0.05).
CONCLUSION: FD patients have delayed gastric emptying. H. pylori infection treatment helps to improve symptoms of dyspepsia and is a reasonable choice for treatment in clinical practice.
Keywords: Functional dyspepsia, Gastric emptying, Ultrasound
Core tip: Stomach half-emptying time was determined in Helicobacter pylori (H. pylori) patients and healthy controls. The half-emptying times at the proximal end, distal end, and the whole stomach in the functional dyspepsia (FD) group were slower than in the control group. Total symptom scores in the H. pylori-treatment group and H. pylori-negative group were lower than in the conventional treatment group after treatment. Patients with FD have delayed gastric emptying. Treatment of H. pylori infection helps to improve symptoms of dyspepsia and is a reasonable choice for therapy in clinical practice.
INTRODUCTION
Functional dyspepsia (FD) is the most common functional gastrointestinal disorder, but its etiology remains unclear[1-3]. However, it is generally believed that an abnormality in gastric motility is an important factor[4]. Patients with FD often appear to have abdominal distension, belching, nausea, and other symptoms of dyspepsia. In severe cases, it has a major impact on daily life, and treatment is required to alleviate the symptoms[5-7]. FD is associated with a variety of factors that include gastrointestinal motility disorders, gastrointestinal hormone secretion abnormalities, or Helicobacter pylori (H. pylori) infection[8]. Among them, H. pylori infection probably induces symptoms by increasing the sensitivity to mechanical distension or increasing gastric acid secretion[9]. Clinically, gastric motility drug treatment is primarily given to these patients. However, whether there is a need for eradication therapy for H. pylori in FD patients remains controversial[10]. Furthermore, determination of gastric emptying by ultrasound is convenient for observation, has economic advantages, is easy for patients, and is suitable for widespread or repeated use[11,12]. Therefore, this study compared symptoms of dyspepsia, gastric emptying time, and other indicators before and after H. pylori treatment in patients with FD in our hospital, and in healthy volunteers. The relationship between the symptoms of dyspepsia and gastric half-emptying time were observed in FD patients, and whether H. pylori treatment could alleviate the symptoms was investigated, with the aim of providing a basis for the clinical treatment.
MATERIALS AND METHODS
Study population
A total of 70 adult FD patients admitted to our hospital from January 2013 to March 2015 were included in this study as the FD group, and were divided into 2 groups: H. pylori-negative group (n = 28) and H. pylori-positive group (n = 42). The H. pylori-positive group was further randomly divided into 2 groups: an H. pylori treatment group and a conventional treatment group (n = 21 each group). Patients with FD met the following criteria[13]: (1) the Rome III diagnostic criteria of FD; (2) duration of disease 1-3 years, with no gastrointestinal motility or H. pylori drug treatment in the previous month; (3) had not been treated with systematic FD or H. pylori drugs; and (4) underwent pathological examination by gastroscopy and a 13C-urea breath test. H. pylori- positive patients were required to have 2 positive checks, and excluded single-positive patients. In addition, 72 healthy adults were selected from the Medical center as the control group. None of the subjects had the following exclusion criteria[13]: (1) organic lesions in the stomach and duodenum revealed by endoscopic examinations; (2) history of gastrointestinal surgery; (3) diabetes or connective tissue diseases; and (4) long-term smoking or alcoholism. Age and other characteristics were similar between the FD group and control group (P > 0.05, Table 1).
Table 1.
Comparison of baseline characteristics between the functional dyspepsia and control groups
| Group | Age | Sex (male/female) | BMI (kg/m2) | Duration of disease (yr) |
| FD group (n = 70) | 44.82 ± 13.41 | 27/43 | 21.38 ± 3.87 | 2.01 ± 1.32 |
| Control group (n = 72) | 40.70 ± 6.39 | 22/48 | 21.20 ± 2.95 | 1.89 ± 1.73 |
| t-test value | t = 2.347 | χ2 = 0.785 | t = 0.312 | t = 0.464 |
| P value | 0.020 | 0.376 | 0.755 | 0.644 |
| H. pylori therapeutic group | 42.73 ± 11.97 | 10/18 | 20.98 ± 3.27 | 2.10 ± 1.12 |
| Conventional therapeutic group | 43.79 ± 14.28 | 9/12 | 22.08 ± 2.96 | 1.97 ± 1.28 |
| H. pylori-negative group | 45.82 ± 12.83 | 8/13 | 21.16 ± 3.64 | 1.95 ± 1.31 |
| t-test value | F = 2.191 | F = 2.315 | F = 1.923 | F = 1.872 |
| P value | 0.266 | 0.221 | 0.397 | 0.426 |
BMI: Body mass index; FD: Functional dyspepsia; H. pylori: Helicobacter pylori.
Methods
A GE Voluson E8 ultrasound with a C1-5 transducer was used for examination of all subjects by an experienced sonographer. Subjects were not allowed to drink and eat 12 h before the ultrasound examination, and the empty state of the stomach was confirmed before examination[14]. Patients were asked to finish a 500 mL standard test meal within 4-5 min (500 mL of 80 g black sesame paste in boiled water, cooled to about 25 °C; about 1960 kJ)[15]. With patients in a sitting position, the abdomen between the xiphoid and navel was first scanned by an ultrasound 4C1 convex array probe The “figure-of-eight-like” double ring sign (Figure 1) junction is the angulus, which is the boundary between the proximal end and distal ends of the stomach. The proximal and distal stomach areas were measured immediately after patients took the test meal and at 20, 40, 60, and 90 min thereafter, and gastric half-emptying time was obtained by computer analysis.
Figure 1.
Gastric “figure-of-eight-like” double ring sign in different patients.
FD patients in the H. pylori-negative group and conventional treatment group were given domperidone 0.6 mg/kg/d 30 min before a meal for 1 mo; while patients in the H. pylori treatment group took a combination of 3 drugs as eradication therapy for H. pylori, including lansoprazole tablets 30 mg once daily, clarithromycin tablets 0.5 g twice daily, and amoxicillin 1.0 g twice daily for 1 wk. Confirmation of H. pylori eradication was performed with a gastroscope and 13C-urea breath test. Then domperidone 0.6 mg/kg per day) was given 30 min before a meal for 1 mo. Dyspepsia symptoms pre-and-post treatment were scored in all these groups. Gastric half-emptying time was determined after the end of treatment.
Evaluation index
Dyspepsia symptoms of all subjects were statistically compared, and the correlation with gastric half-emptying time was analyzed. Total dyspepsia symptom scores before and after treatment were compared and analyzed among the 3 groups of patients with FD. Symptom scores for abdominal pain, epigastric burning sensation, abdominal distension, nausea, belching, vomiting, and early satiety were scored according to the severity of symptoms: 0, asymptomatic; 1, mild (between asymptomatic and moderate); 2, moderate (symptoms that can be tolerated); 3, severe (symptoms that are intolerable and have a serious impact on daily life)[16,17]. Then, gastric half-emptying time after treatment was compared among the 3 groups of patients with FD.
Statistical analysis
SPSS 20.0 was used to analyze all data (IBM Corp., Armonk, NY, United States). P < 0.05 was considered statistically significant. The incidence of epigastric abdominal pain, epigastric burning sensation, abdominal distension, nausea, belching, vomiting, and early satiety symptoms was compared between the FD group and control group using the χ2 test. Gastric half-emptying time: Gastric half-emptying time of the proximal end, distal end, and the whole gastric region was compared between the FD group and control group using the t-test. The relationship between dyspepsia symptoms and gastric half-emptying was determined using logistic regression analysis, with gastric half-emptying time as the dependent variable and the symptoms of dyspepsia as the independent variables. Total symptom scores before and after treatment in the 3 groups of FD patients were compared by one-way analysis of variance (ANOVA test). The SNK-Q test was then performed for paired comparisons when the difference between groups was statistically significant. The gastric half-emptying times at the proximal end, distal end, and the whole gastric region after treatment among the 3 groups of patients were compared by ANOVA, followed by the SNK-Q test for paired comparisons.
RESULTS
Comparison of dyspepsia symptoms
The incidence of abdominal pain, epigastric burning sensation, abdominal distension, nausea, belching, and early satiety symptoms was significantly higher in patients in the FD group compared with the control group (P < 0.05). However, there was no significant difference in the incidence of vomiting between the 2 groups (χ2 = 1.624, P = 0.203), as shown in Table 2.
Table 2.
Incidence of dyspeptic symptoms in functional dyspepsia and control groups n (%)
| Groups | Abdominal pain | Epigastric burning sensation | Abdominal distension | Nausea | Belching | Vomiting | Early satiety |
| FD group (n = 70) | 35 (50.0) | 26 (37.1) | 55 (78.6) | 32 (45.7) | 37 (52.9) | 11 (15.7) | 40 (57.1) |
| Control (n = 72) | 15 (20.8) | 9 (12.5) | 32 (44.4) | 16 (22.2) | 5 (15.3) | 7 (9.7) | 14 (19.4) |
| χ2 value | 18.642 | 16.224 | 24.699 | 12.314 | 31.456 | 1.624 | 30.087 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.203 | 0.000 |
FD: Functional dyspepsia.
Comparison of gastric half-emptying conditions
Gastric half-emptying times at the proximal end, distal end, and the whole gastric region were significantly slower in the FD group than in the control group (P < 0.05, Table 3). As observed from the gastric emptying curve for the 2 groups, gastric contents in the proximal end presented a gradual downward trend in the control group, which slowed down between 40 min and 60 min. However, the rate of decline was slower in the FD group compared with the control group, and the decline between 40 and 60 min time points was significantly slower, presenting a plateau phase. The control group continued to present a slow downward trend in the gastric emptying curve at the distal end. However, the curve presented an upward and a downward trend in the FD group within the first 20 min after eating, and was slower than that in the control group. In the gastric emptying curve of the whole gastric region, the control group continued to present a slow downward trend. However, the curve for the FD group declined at different rates (Figure 2).
Table 3.
Comparison of gastric half-emptying time between functional dyspepsia and control groups
| Groups | Gastric half-emptying time at the proximal end (min) | Gastric half-emptying time at the distal end (min) | Gastric half-emptying time at the whole gastric region (min) |
| FD group (n = 70) | 93.7 ± 26.2 | 102.2 ± 26.4 | 102.1 ± 28.6 |
| Control group (n = 72) | 72.0 ± 14.3 | 87.5 ± 18.2 | 78.3 ± 14.1 |
| t value | 6.149 | 3.782 | 6.316 |
| P value | 0.000 | 0.000 | 0.000 |
FD: Functional dyspepsia.
Figure 2.
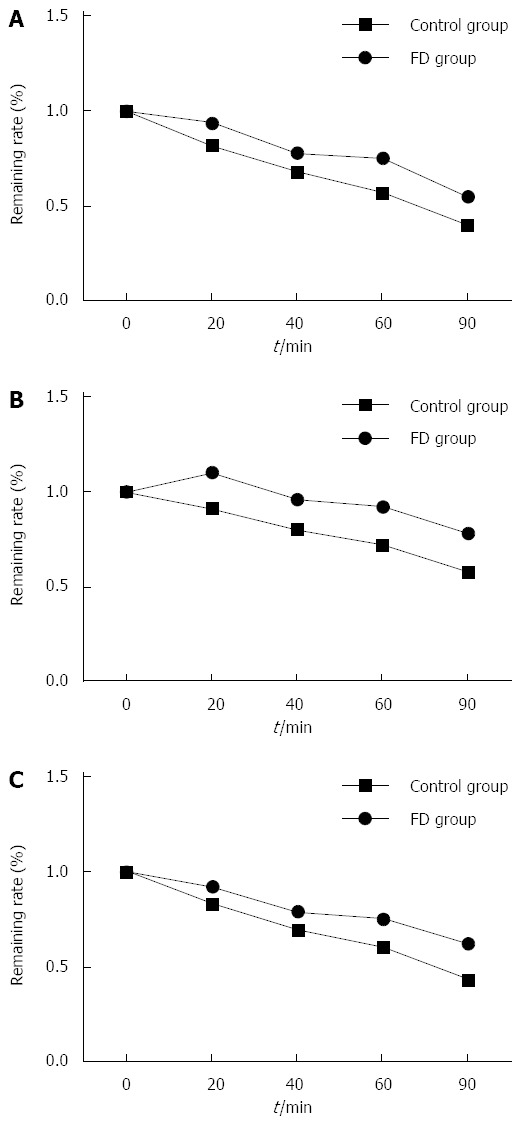
Gastric emptying curves. A: Proximal end; B: Distal end; C: Whole gastric region.
Relationship between symptoms of dyspepsia and gastric half-emptying time
There was no significant association of any of the symptoms with prolonged gastric half-emptying time at the proximal end (P > 0.05). However, abdominal distension, belching, and early satiety was associated with abdominal distension at the distal end (P < 0.05), and were risk factors for delayed gastric half-emptying time at the distal end (OR > 1). Abdominal distension and abdominal pain were associated with gastric half-emptying time of the whole gastric region (P < 0.05), and were risk factors for delayed gastric half-emptying of the whole gastric region (OR > 1, Table 4).
Table 4.
Logistic regression analysis of symptoms of dyspepsia and gastric half-emptying time
| Influential factors | Prolonged gastric half-emptying time | β | SE | Wald value | OR | 95%CI | P value |
| Abdominal pain | Proximal | 0.255 | 0.322 | 5.743 | 1.291 | 0.687-2.426 | 0.791 |
| Distal | 0.123 | 0.429 | 6.841 | 1.131 | 0.488-2.622 | 0.387 | |
| Whole | 0.780 | 0.117 | 15.935 | 2.182 | 1.735-2.744 | 0.008 | |
| Epigastric burning sensation | Proximal | 0.653 | 0.412 | 6.472 | 1.921 | 0.857-4.308 | 0.391 |
| Distal | -0.272 | 0.366 | 5.937 | 0.762 | 0.372-1.561 | 0.752 | |
| Whole | -0.183 | 0.621 | 6.935 | 0.833 | 0.247-2.814 | 0.326 | |
| Abdominal distension | Proximal | -0.451 | 0.429 | 7.299 | 0.637 | 0.275-1.477 | 0.261 |
| Distal | 0.600 | 0.128 | 15.643 | 1.823 | 1.419-2.343 | 0.016 | |
| Whole | 0.273 | 0.118 | 14.984 | 1.314 | 1.043-1.656 | 0.021 | |
| Nausea | Proximal | 0.545 | 0.529 | 8.327 | 1.725 | 0.612-4.865 | 0.134 |
| Distal | 0.205 | 0.326 | 7.565 | 1.227 | 0.648-2.325 | 0.142 | |
| Whole | 0.699 | 0.762 | 7.418 | 2.011 | 0.452-8.954 | 0.221 | |
| Belching | Proximal | 0.023 | 0.376 | 7.488 | 1.023 | 0.490-2.138 | 0.172 |
| Distal | 0.745 | 0.223 | 12.473 | 2.106 | 1.360-3.260 | 0.031 | |
| Whole | -0.583 | 0.515 | 9.304 | 0.558 | 0.203-1.531 | 0.086 | |
| Early satiety | Proximal | -0.467 | 0.718 | 8.471 | 0.627 | 0.153-2.561 | 0.096 |
| Distal | 0.461 | 0.202 | 13.845 | 1.585 | 1.067-2.355 | 0.026 | |
| Whole | -0.028 | 0.486 | 8.737 | 0.972 | 0.375-2.520 | 0.093 |
Total score of patients with symptoms of dyspepsia occurring before and after treatment
There was no statistically significant difference in total patient symptom scores before treatment among the 3 groups (F = 3.021, P = 0.291). However, the scores were lower after treatment than before treatment, and the difference was statistically significant (P < 0.05). There was a statistically significant difference in scores after treatment among the 3 groups (F = 3.162, P = 0.014). Pairwise comparisons showed total symptom scores after treatment were significantly lower in the H. pylori treatment group and H. pylori-negative group than in the conventional treatment group (H. pylori treatment group: Q = 2.259, P = 0.029; H. pylori-negative group: Q = 2.163, P = 0.037); however, there was no significant difference between the H. pylori treatment group and H. pylori-negative group (Q = 0.270, P = 0.791; Table 5).
Table 5.
Comparison of total symptom scores of patients before and after treatment
| Groups | Before treatment | After treatment | Q value | P value |
| H. pylori treatment group (n = 21) | 10.14 ± 4.02 | 5.15 ± 2.27 | 4.953 | 0.000 |
| Conventional treatment group (n = 21) | 11.01 ± 3.92 | 7.02 ± 3.04 | 3.686 | 0.001 |
| H. pylori-negative group (n = 28) | 11.61 ± 4.81 | 4.93 ± 3.22 | 6.107 | 0.000 |
H. pylori: Helicobacter pylori.
Gastric half-emptying time after treatment
There was a statistically significant difference in gastric half-emptying time at the proximal end, distal end, and whole gastric region after treatment among the 3 groups (P < 0.05). Pairwise comparisons showed that gastric half-emptying times at the proximal end, distal end, and whole gastric region were significantly shorter in the H. pylori-negative group and H. pylori treatment group compared with the conventional treatment group (P < 0.05); while there was no significant difference between the H. pylori treatment group and H. pylori-negative group (P > 0.05, Table 6).
Table 6.
Comparison of gastric half-emptying time (min) of patients after treatment
| Groups | Gastric half-emptying time at the proximal end | Gastric half-emptying time at the distal end | Gastric half-emptying time at whole gastric region |
| (1) H. pylori treatment group (n = 21) | 74.0 ± 12.4 | 87.7 ± 13.4 | 80.3 ± 14.4 |
| (2) Conventional treatment group (n = 21) | 83.1 ± 15.8 | 97.5 ± 15.1 | 91.9 ± 17.2 |
| (3) H. pylori-negative group (n = 28) | 73.6 ± 11.7 | 88.3 ± 15.4 | 79.8 ± 15.9 |
| F value | 3.211 | 3.143 | 3.188 |
| P value | 0.004 | 0.019 | 0.007 |
| (1):(2) Q value | 2.076 | 2.225 | 2.37 |
| P value | 0.044 | 0.032 | 0.023 |
| (1):(3) Q value | 0.115 | 0.143 | 0.107 |
| P value | 0.909 | 0.887 | 0.915 |
| (3):(2) Q value | 2.317 | 2.087 | 2.516 |
| P value | 0.025 | 0.042 | 0.015 |
H. pylori: Helicobacter pylori.
DISCUSSION
In patients with FD, although there is no organic disease, belching, nausea, abdominal pain, and other symptoms of dyspepsia can continue for more than 6 mo, and if symptoms persist for 3 mo or more, there is a greater impact on the quality of life[17-20]. The incidence of FD is about 20%, and is mostly caused by gastric motility disorders, including abnormal gastric emptying, stomach discomfort from reduced capacity, and gastric electrical rhythm abnormalities[21-24]. The stomach can be divided into 2 regions at the angulus, and the proximal and distal regions function differently to some extent. The proximal region mainly functions to receive and store food, and control liquid emptying. The distal region may conduct peristalsis to grind the food and mix it with the gastric juice[25-29]. Observation of gastric emptying by ultrasound is a simple method, does not cause injury, and is easily accepted by patients. Therefore, gastric emptying studies by ultrasound observation enable the comparison of the relationship between the symptoms of patients with FD. In this study, a comparative analysis was performed for gastric emptying and various common FD symptoms in the proximal end, distal end and full stomach. H. pylori infection affects the endocrine aspects of the smooth muscle in the human gastrointestinal tract, and this is generally considered to have a significant impact on the symptoms in patients with FD[30-32]. Therefore, in this study, H. pylori-positive and -negative symptoms and gastric emptying between healthy subjects and patients with FD were compared in order to observe the effects of H. pylori infection in FD patients.
The study revealed that the incidence of symptoms in the FD group, except vomiting, was high compared with the control group. Gastric half-emptying time at the proximal end, distal end, and the whole gastric region was slower in the FD group than in the control group. Abdominal distension, belching, and early satiety were associated with gastric half-emptying time at the distal end. Abdominal distension and abdominal pain were associated with gastric half-emptying time of the whole gastric region. Total symptom scores of patients in the 3 groups decreased after treatment. In the H. pylori-negative group and H. pylori treatment group, total symptom scores after treatment were lower compared with the conventional treatment group; and gastric half-emptying times at the proximal end, distal end, and the whole gastric region were shorter than in the conventional treatment group. However, there was no difference between the H. pylori treatment group and H. pylori-negative group. It can be observed that delayed gastric emptying and H. pylori infection have an important impact on the occurrence of symptoms in FD patients. As observed from the gastric emptying curve at the distal end after eating, the amount of food inside the stomach at the distal end increased and slowly declined. It is considered that this is because the proximal end of the stomach suffers from receptive dysfunction for food. When food increases, relaxation is delayed, resulting in regurgitation or the rapid discharge of food into the distal end, causing FD patients to have early satiety, abdominal distension, and other symptoms[33-36]. In addition, the abnormal distribution of food at the proximal and distal end of the stomach further affects the emptying of food, resulting in the occurrence of symptoms of dyspepsia[37].
After administration of drugs promoting gastric motility to patients, the clinical symptoms of FD patients were alleviated regardless of whether H. pylori infection was present or not. As observed, abnormal gastric motility is an important reason for the occurrence of FD symptoms. H. pylori eradication treatment to improve symptoms and gastric emptying of H. pylori-positive patients is better than no H. pylori eradication treatment. It has been considered that H. pylori participate in the occurrence of FD symptoms through a variety of mechanisms[9]. This includes H. pylori infections in the gastrointestinal tract causing an increase in mechanical expansion sensitivity[38,39]. H. pylori infection can directly lead to increased gastric acid secretion or promote gastric acid secretion by increasing gastrin, leading to abdominal pain, epigastric burning sensations, and other symptoms[40]. H. pylori infection can cause gastrointestinal hormone secretion disorders, such as increased somatostatin and cholecystokinin, leading to an increase in the incidence of symptoms in patients with FD[41-44]. H. pylori infection affects gastric emptying, and influencing factors include increased release of leukotrienes[45,46], or nitric oxide and other substances. This leads to gastrointestinal smooth muscle relaxation and delayed gastric emptying, or an increase in 5-HT and other substances affecting gastrointestinal smooth muscle contraction, resulting in gastrointestinal tract motility disorders[47-50].
As the amount of samples collected in this study was small and it was a single center study, the relationship between symptoms of dyspepsia in patients with FD, gastrointestinal tract motility disorders, and H. pylori infections requires further larger scale investigations to further determine the pathophysiological mechanisms in order to provide good guidance for clinical diagnosis and treatment of patients with FD.
In summary, gastric emptying is delayed to some extent in patients with FD. For patients infected with H. pylori, H. pylori eradication treatment helps to improve dyspepsia symptoms. This may be a reasonable choice for therapy in clinical practice.
COMMENTS
Background
Functional dyspepsia (FD) is the most common functional gastrointestinal disorder, but the etiology remains unclear. Patients with FD often appear to have abdominal distension, belching, nausea, and other symptoms of dyspepsia. In severe cases, it seriously impacts their daily life. FD is related to a variety of causes that include gastrointestinal motility disorders, gastrointestinal hormone secretion abnormalities, or Helicobacter pylori (H. pylori) infection. However whether there is a need for eradication therapy for H. pylori in FD patients remains controversial. Therefore, the study investigated the relationship between symptoms of dyspepsia and gastric half-emptying time in FD patients, and whether H. pylori treatment could alleviate the symptoms, to provide a basis for clinical treatment.
Research frontiers
In recent years, FD incidence has gradually increased and its cause is unknown. Studies have reported that H. pylori infection can cause gastrointestinal hormone secretion disorders such as increased somatostatin and cholecystokinin secretion, leading to a corresponding increase in the incidence of symptoms in patients with FD. The symptoms of FD are mostly caused by gastric motility disorders, including abnormal gastric emptying, stomach discomfort from reduced capacity, and gastric electrical rhythm abnormalities. The use of ultrasound to observe gastric emptying, and compare symptoms score, is a simple and effective method.
Innovations and breakthroughs
FD is mostly caused by gastric motility disorders including abnormal gastric emptying. Gastric emptying observation by ultrasound is a simple and effective method, does not cause injury, and is easily accepted by patients. Using symptom scoring for a variety of FD symptoms, a more comprehensive evaluation of symptoms can be made.
Applications
This study demonstrated that patients with FD have delayed gastric emptying. H. pylori infection treatment helps to improve symptoms of dyspepsia. FD is closely associated with abnormal gastric emptying and H. pylori infection. This provides a basis for clinical treatment with gastrointestinal motility drugs and H. pylori eradication therapy in FD patients.
Peer-review
This study compared and analyzed the gastric emptying and symptoms between FD patients and healthy people; H. pylori positive group, H. pylori-negative group and the conventional treatment. It demonstrates that patients with FD have delayed gastric emptying. H. pylori infection treatment helps to improve symptoms of dyspepsia. Provide a reliable basis to applications gastrointestinal drugs and drugs to cure H. pylori for the treatment of patients with H. pylori.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Daqing oilfield general hospital.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: We declare that no conflict of interests in our study is going to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 25, 2016
First decision: February 18, 2016
Article in press: March 18, 2016
P- Reviewer: Handan OB, Thornton GD S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Ma S
References
- 1.Dibaise JK, Islam RS, Dueck AC, Roarke MC, Crowell MD. Psychological distress in Rome III functional dyspepsia patients presenting for testing of gastric emptying. Neurogastroenterol Motil. 2016;28:196–205. doi: 10.1111/nmo.12709. [DOI] [PubMed] [Google Scholar]
- 2.Zybach K, Friesen CA, Schurman JV. Therapeutic effect of melatonin on pediatric functional dyspepsia: A pilot study. World J Gastrointest Pharmacol Ther. 2016;7:156–161. doi: 10.4292/wjgpt.v7.i1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimura S, Ishimura N, Mikami H, Okimoto E, Uno G, Tamagawa Y, Aimi M, Oshima N, Sato S, Ishihara S, et al. Small Intestinal Bacterial Overgrowth in Patients with Refractory Functional Gastrointestinal Disorders. J Neurogastroenterol Motil. 2016;22:60–68. doi: 10.5056/jnm15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolino MC, Furia M, Facio L, Delli Quadri I, Lien Y, Espinosa F, Vera F, Corti R, Vázquez H, Iantorno G. [Functional dyspepsia and the satiety test: its usefulness in clinical practice] Rev Gastroenterol Mex. 2013;78:127–134. doi: 10.1016/j.rgmx.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano M, Miceli E, Tana P, Mengoli C, Bergonzi M, Pagani E, Corazza GR. Fasting and postprandial gastric sensorimotor activity in functional dyspepsia: postprandial distress vs. epigastric pain syndrome. Am J Gastroenterol. 2014;109:1631–1639. doi: 10.1038/ajg.2014.231. [DOI] [PubMed] [Google Scholar]
- 6.Keely S, Walker MM, Marks E, Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest. 2015;45:1350–1359. doi: 10.1111/eci.12548. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Li F, Tang XD, Ma J, Ma X, Ge DY, Li GM, Wang Y. XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement Altern Med. 2015;15:387. doi: 10.1186/s12906-015-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naumann J. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia. Gastroenterology. 2016;150:532. doi: 10.1053/j.gastro.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 9.Peng YC, Huang LR, Shyu CL, Cheng CC, Ho SP. Interaction of omeprazole and Helicobacter pylori-induced nuclear factor-κB activation and mediators in gastric epithelial cells. J Chin Med Assoc. 2014;77:567–572. doi: 10.1016/j.jcma.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Masjedizadeh A, Zaeemzadeh N, Mard SA, Vanani GS. Comparing the efficacy of four different protocols for eradicating of Helicobacter pylori infection in Ahvaz, southwest Iran. Prz Gastroenterol. 2015;10:94–99. doi: 10.5114/pg.2015.49001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielefeldt K. From ischochymia to gastroparesis: proposed mechanisms and preferred management of dyspepsia over the centuries. Dig Dis Sci. 2014;59:1088–1098. doi: 10.1007/s10620-014-3144-0. [DOI] [PubMed] [Google Scholar]
- 12.Kushch I, Korenev N, Kamarchuk L, Pospelov A, Kravchenko A, Bajenov L, Kabulov M, Amann A, Kamarchuk G. On the importance of developing a new generation of breath tests for Helicobacter pylori detection. J Breath Res. 2015;9:047111. doi: 10.1088/1752-7155/9/4/047111. [DOI] [PubMed] [Google Scholar]
- 13.Korkmaz H, Kesli R, Karabagli P, Terzi Y. Comparison of the diagnostic accuracy of five different stool antigen tests for the diagnosis of Helicobacter pylori infection. Helicobacter. 2013;18:384–391. doi: 10.1111/hel.12053. [DOI] [PubMed] [Google Scholar]
- 14.Stein B, Everhart KK, Lacy BE. Treatment of functional dyspepsia and gastroparesis. Curr Treat Options Gastroenterol. 2014;12:385–397. doi: 10.1007/s11938-014-0028-5. [DOI] [PubMed] [Google Scholar]
- 15.Singh H, Bala R, Kaur K. Efficacy and tolerability of levosulipride, domperidone and metoclopramide in patients with non-ulcer functional dyspepsia: a comparative analysis. J Clin Diagn Res. 2015;9:FC09–FC12. doi: 10.7860/JCDR/2015/11613.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma T, Zeng F, Li Y, Wang CM, Tian X, Yu S, Zhao L, Wu X, Yang M, Wang D, et al. Which subtype of functional dyspepsia patients responses better to acupuncture? A retrospective analysis of a randomized controlled trial. Forsch Komplementmed. 2015;22:94–100. doi: 10.1159/000380983. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Holtmann G, Walker MM. Therapeutic strategies for functional dyspepsia and irritable bowel syndrome based on pathophysiology. J Gastroenterol. 2015;50:601–613. doi: 10.1007/s00535-015-1076-x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Matsuzaki J, Fukushima Y, Suzaki F, Kasugai K, Nishizawa T, Naito Y, Hayakawa T, Kamiya T, Andoh T, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia--a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. 2014;26:950–961. doi: 10.1111/nmo.12348. [DOI] [PubMed] [Google Scholar]
- 19.Du Y, Su T, Song X, Gao J, Zou D, Zuo C, Xie W, Wang B, Zhang Z, Xu J, et al. Efficacy and safety of cinitapride in the treatment of mild to moderate postprandial distress syndrome-predominant functional dyspepsia. J Clin Gastroenterol. 2014;48:328–335. doi: 10.1097/MCG.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 20.Kessing BF, Smout AJ, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. 2014;26:1079–1086. doi: 10.1111/nmo.12359. [DOI] [PubMed] [Google Scholar]
- 21.Tseng PH, Wu YW, Lee YC, Cheng MF, Tzen KY, Wang HP, Lin JT, Hsieh ST, Yang WS, Wu MS. Normal values and symptom correlation of a simplified oatmeal-based gastric emptying study in the Chinese population. J Gastroenterol Hepatol. 2014;29:1873–1882. doi: 10.1111/jgh.12640. [DOI] [PubMed] [Google Scholar]
- 22.Valeur J, Berstad A, Hausken T. The effect of body position on postprandial perceptions, gastric emptying, and intragastric meal distribution: an ultrasonographic study in reclining healthy subjects. Scand J Gastroenterol. 2015;50:170–173. doi: 10.3109/00365521.2014.990506. [DOI] [PubMed] [Google Scholar]
- 23.Chirila I, Morariu ID, Barboi OB, Drug VL. The role of diet in the overlap between gastroesophageal reflux disease and functional dyspepsia. Turk J Gastroenterol. 2016;27:73–80. doi: 10.5152/tjg.2015.150238. [DOI] [PubMed] [Google Scholar]
- 24.DiBaise JK, Patel N, Noelting J, Dueck AC, Roarke M, Crowell MD. The relationship among gastroparetic symptoms, quality of life, and gastric emptying in patients referred for gastric emptying testing. Neurogastroenterol Motil. 2016;28:234–242. doi: 10.1111/nmo.12718. [DOI] [PubMed] [Google Scholar]
- 25.Vanheel H, Vanuytsel T, Van Oudenhove L, Farré R, Verbeke K, Tack J. Postprandial symptoms originating from the stomach in functional dyspepsia. Neurogastroenterol Motil. 2013;25:911–e703. doi: 10.1111/nmo.12227. [DOI] [PubMed] [Google Scholar]
- 26.Yamawaki H, Futagami S, Shimpuku M, Shindo T, Maruki Y, Nagoya H, Kodaka Y, Sato H, Gudis K, Kawagoe T, et al. Leu72Met408 Polymorphism of the Ghrelin Gene Is Associated With Early Phase of Gastric Emptying in the Patients With Functional Dyspepsia in Japan. J Neurogastroenterol Motil. 2015;21:93–102. doi: 10.5056/jnm14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zai H, Matsueda K, Kusano M, Urita Y, Saito Y, Kato H. Effect of acotiamide on gastric emptying in healthy adult humans. Eur J Clin Invest. 2014;44:1215–1221. doi: 10.1111/eci.12367. [DOI] [PubMed] [Google Scholar]
- 28.Kanda M, Oya H, Nomoto S, Takami H, Shimizu D, Hashimoto R, Sueoka S, Kobayashi D, Tanaka C, Yamada S, et al. Diversity of clinical implication of B-cell translocation gene 1 expression by histopathologic and anatomic subtypes of gastric cancer. Dig Dis Sci. 2015;60:1256–1264. doi: 10.1007/s10620-014-3477-8. [DOI] [PubMed] [Google Scholar]
- 29.Witte AB, Hilsted L, Holst JJ, Schmidt PT. Peptide YY3-36 and glucagon-like peptide-1 in functional dyspepsia. Secretion and role in symptom generation. Scand J Gastroenterol. 2016;51:400–409. doi: 10.3109/00365521.2015.1101780. [DOI] [PubMed] [Google Scholar]
- 30.Targosz A, Brzozowski T, Pierzchalski P, Szczyrk U, Ptak-Belowska A, Konturek SJ, Pawlik W. Helicobacter pylori promotes apoptosis, activates cyclooxygenase (COX)-2 and inhibits heat shock protein HSP70 in gastric cancer epithelial cells. Inflamm Res. 2012;61:955–966. doi: 10.1007/s00011-012-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakshi SS. Outcome evaluation of clarithromycin, metronidazole and lansoprazole regimens in Helicobacter pylori positive or negative children with resistant otitis media with effusion. J Laryngol Otol. 2016;130:318. doi: 10.1017/S0022215116000086. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Dang Y, Zhou X, Liu B, Liu S, Zhang G. Tailored Therapy Versus Empiric Chosen Treatment for Helicobacter pylori Eradication: A Meta-Analysis. Medicine (Baltimore) 2016;95:e2750. doi: 10.1097/MD.0000000000002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdel-Aziz H, Wadie W, Zaki HF, Müller J, Kelber O, Efferth T, Khayyal MT. Novel sequential stress model for functional dyspepsia: Efficacy of the herbal preparation STW5. Phytomedicine. 2015;22:588–595. doi: 10.1016/j.phymed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Dixon BR, Radin JN, Piazuelo MB, Contreras DC, Algood HM. IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori. PLoS One. 2016;11:e0148514. doi: 10.1371/journal.pone.0148514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng X, Liu GF, Wu J, Kong CC, Zhao LW, Zhu XY, Ji CG, Yang L. [Antibiotic resistance of Helicobacter pylori clinical isolates in Hebei Province] Zhonghua Yi Xue Zazhi. 2016;96:270–272. doi: 10.3760/cma.j.issn.0376-2491.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Turanli S, Bozdogan N, Mersin H, Berberoglu U. The Effect of Helicobacter pylori on Gastric Cancer Treated with Adjuvant Chemotherapy After Curative Resection. Indian J Surg. 2015;77:489–494. doi: 10.1007/s12262-015-1305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY, Rew JS. Is Lipase Supplementation before a High Fat Meal Helpful to Patients with Functional Dyspepsia? Gut Liver. 2015;9:433–434. doi: 10.5009/gnl15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazdanbod A, Salimian S, Habibzadeh S, Hooshyar A, Maleki N, Norouzvand M. Effect of Helicobacter pylori eradication in Iranian patients with functional dyspepsia: a prospective, randomized, placebo-controlled trial. Arch Med Sci. 2015;11:964–969. doi: 10.5114/aoms.2015.54851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinova Z, Leng Y, Leeds P, Chuang DM. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology. 2011;60:1109–1115. doi: 10.1016/j.neuropharm.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janjetic MA, Mantero P, Cueto Rua E, Balcarce N, Zerbetto de Palma G, Catalano M, Zubillaga MB, Boccio JR, Goldman CG. Dietary and anthropometric indicators of nutritional status in relation to Helicobacter pylori infection in a paediatric population. Br J Nutr. 2015;113:1113–1119. doi: 10.1017/S0007114515000483. [DOI] [PubMed] [Google Scholar]
- 41.Yu K, Ke MY, Li WH, Zhang SQ, Fang XC. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac J Clin Nutr. 2014;23:210–218. doi: 10.6133/apjcn.2014.23.2.01. [DOI] [PubMed] [Google Scholar]
- 42.Welsh C, Jarrin J, Daneman A, Belik J. In vivo ultrasound assessment of gastric emptying in newborn mice. J Pediatr Gastroenterol Nutr. 2015;60:322–326. doi: 10.1097/MPG.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 43.Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Influences of breast milk composition on gastric emptying in preterm infants. J Pediatr Gastroenterol Nutr. 2015;60:264–271. doi: 10.1097/MPG.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 44.Kubo TT, Moraes ER, Secaf M, Troncon LE. A novel dynamic scintigraphic technique for assessing duodenal contractions during gastric emptying in humans: a feasibility study. Nucl Med Commun. 2015;36:95–101. doi: 10.1097/MNM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 45.Benhamou D. Ultrasound assessment of gastric contents in the perioperative period: why is this not part of our daily practice? Br J Anaesth. 2015;114:545–548. doi: 10.1093/bja/aeu369. [DOI] [PubMed] [Google Scholar]
- 46.Faria M, Pavin EJ, Parisi MC, Nagasako CK, Mesquita MA. Dyspeptic symptoms in patients with type 1 diabetes: endoscopic findings, Helicobacter pylori infection, and associations with metabolic control, mood disorders and nutritional factors. Arch Endocrinol Metab. 2015;59:129–136. doi: 10.1590/2359-3997000000025. [DOI] [PubMed] [Google Scholar]
- 47.El-Salhy M, Gilja OH, Gundersen D, Hausken T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J Gastrointest Endosc. 2014;6:176–185. doi: 10.4253/wjge.v6.i5.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767–12780. doi: 10.3748/wjg.v20.i36.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adler I, Muiño A, Aguas S, Harada L, Diaz M, Lence A, Labbrozzi M, Muiño JM, Elsner B, Avagnina A, et al. Helicobacter pylori and oral pathology: relationship with the gastric infection. World J Gastroenterol. 2014;20:9922–9935. doi: 10.3748/wjg.v20.i29.9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wouters MM, Boeckxstaens GE. Is there a causal link between psychological disorders and functional gastrointestinal disorders? Expert Rev Gastroenterol Hepatol. 2016;10:5–8. doi: 10.1586/17474124.2016.1109446. [DOI] [PubMed] [Google Scholar]


