Abstract
People with Parkinson disease (PD) who show freezing of gait also have dysfunction in cognitive domains that interact with mobility. Specifically, freezing of gait is associated with executive dysfunction involving response inhibition, divided attention or switching attention, and visuospatial function. The neural control impairments leading to freezing of gait have recently been attributed to higher-level, executive and attentional cortical processes involved in coordinating posture and gait rather than to lower-level, sensorimotor impairments. To date, rehabilitation for freezing of gait primarily has focused on compensatory mobility training to overcome freezing events, such as sensory cueing and voluntary step planning. Recently, a few interventions have focused on restitutive, rather than compensatory, therapy. Given the documented impairments in executive function specific to patients with PD who freeze and increasing evidence of overlap between cognitive and motor function, incorporating cognitive challenges with mobility training may have important benefits for patients with freezing of gait. Thus, a novel theoretical framework is proposed for exercise interventions that jointly address both the specific cognitive and mobility challenges of people with PD who freeze.
Freezing of gait (FoG) is defined as a “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk.”1(p734) Approximately 26% of people with mild Parkinson disease (PD) and 80% of those with severe PD are affected by FoG, and it is one of the most common reasons for falls and dependency.2,3 Thus, although it can occur throughout the course of PD, FoG is more common in the later stages of the disease. Identifying whether an individual experiences FoG can be done by: (1) observation during tasks that commonly elicit FoG (eg, turning in place, short rapid steps4,5), (2) self-report questionnaire,6 or (3) frequency analysis of lower leg trembling with inertial sensors.7–9 Current therapies for PD, including deep brain stimulation and levodopa, are inadequate for treating FoG.10 Currently, the most common rehabilitative approach for helping patients overcome FoG episodes is to teach compensatory mechanisms, such as cueing (for a review, see Heremans et al11). Even though using external cues can be beneficial, success relies on patients having sufficiently preserved cognitive abilities to consolidate and retrieve these compensatory strategies. Furthermore, the benefits of cues may be transient, as FoG episodes often return after withdrawal of cues.12 Therefore, approaches (eg, restitutive rehabilitation) that also target the underlying dysfunction may be more effective than compensatory strategies alone for retention of improvements.
Cognitive function—specifically, executive function and attention—is critical for mobility, and rehabilitation interventions aimed at improving cognitive function and movement may be particularly beneficial for improving mobility. Indeed, recent work in healthy adults has shown interventions that incorporate cognitive and motor tasks to improve physical and cognitive fall risk factors.13 Integrating cognitive and motor rehabilitation may be especially important for individuals with PD who experience FoG, as freezing itself may be due to impaired executive function and attention.14–16 However, although cognitive-motor training has begun to be used in healthy older adults and people with PD,17–19 no studies have incorporated FoG-specific cognitive remediation into mobility training. Given the specific and pronounced cognitive and mobility profiles of individuals with FoG, it may be useful to develop targeted rehabilitation strategies to improve functional mobility in this population.
In this article, we first summarize how mobility relies on executive, attentional, and visuospatial function. Then, we discuss common models of executive dysfunction and how they are impaired in people with FoG. Finally, we propose a theoretical framework to incorporate focused and specific cognitive challenges into exercise progressions.
Mobility Requires Cognitive Function
Early investigations suggested that locomotion was controlled primarily by central pattern generators in the spinal cord and brain stem. Although these structures play a critical role in locomotion, converging evidence from behavioral and imaging studies shows that higher-level, cortical structures also are essential for functional gait (for reviews, see Yogev-Seligmann et al20 and Takakusaki21). For example, intracortical recording in cats demonstrates the critical function of the frontal cortex during gait, particularly during precise stepping.22,23 In humans, brain imaging studies have shown that reduced volume of prefrontal brain regions (areas that play a critical role in cognitive function) is related to reduced gait performance.24 Similarly, transcranial magnetic stimulation over cortical regions, including the supplementary motor area, can alter stepping and step initiation.25,26 Furthermore, a number of recent investigations using mobile brain imaging have shown considerable activity in prefrontal cortical regions related to executive function during gait.27,28
The role of cognition in functional mobility is illustrated by real-world scenarios. Take, for example, the mental operations required to successfully cross a busy intersection. A person must attend to a number of different, often conflicting, stimuli, including walk signs and stop signs, traffic lights, other pedestrians, and velocities of approaching vehicles. Success requires not only an ability to divide attention but also the ability to effectively focus attention on particular stimuli while ignoring others. For example, a person may have to inhibit a response, such as obeying the walk signal, if other important information (eg, an oncoming car) is present. Attention needs to be divided or switched between, on the one hand, the coordination of balance and gait to step down the curb, and, on the other hand, the dangers of traffic. Visuospatial function also is needed to judge the height of the curb and to estimate time to potential contact of oncoming vehicles. Cognitive dysfunction may lead to an inability to appropriately respond to such complex situations, resulting in decrements in gait coordination and functional mobility.
Navigating such a scenario is especially challenging to individuals with PD who experience FoG. As will be discussed in the following sections, we believe this challange is due, in part, to the fact that people with PD+FoG often have impaired cognitive function compared with people with PD but without FoG (PD−FoG).11 These cognitive deficits are interrelated with motor deficits and can lead to reduced functional mobility and increased freezing events during complex scenarios such as that described above.
Models of Executive Function and Attention
In the following sections, we will outline changes in cognition in people with PD+FoG and describe a framework for the incorporation of relevant cognitive challenges into exercise. For clarity, it may be helpful to briefly review some prominent models of executive function and attention.
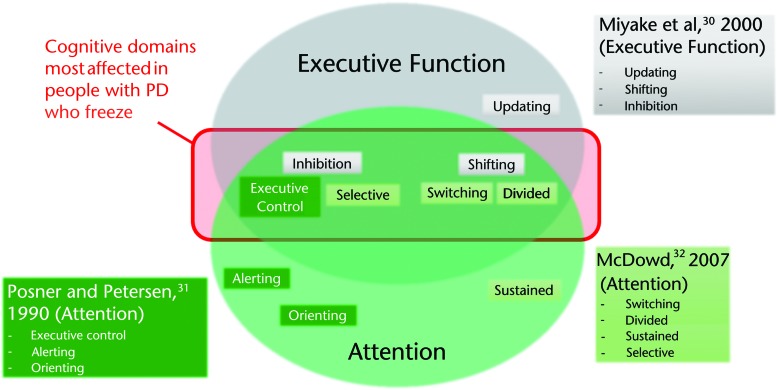
Although there are many models of executive function and attention (for a review, see Chan et al29), for the purposes of this article, we will summarize Miyake and colleagues' model of executive function,30 Posner and Petersen's model of attention,31 and McDowd's model of attention.32 Miyake and colleagues defined executive function as “general purpose control mechanisms that modulate the operations of various subprocesses and thereby regulate the dynamics of human cognition.”30(p50) Miyake's model describes 3 primary domains of executive function: shifting (shifting back and forth among multiple tasks, operations, or mental sets), inhibition (deliberately inhibiting dominant, automatic, or prepotent responses when appropriate), and updating (updating and monitoring working memory representations). With respect to attention, we draw upon 2 prominent models described by McDowd32 and Posner and Petersen.31 McDowd suggested that attention can be parsed into 4 partially overlapping components: divided, switching, sustained, and selective.32 These components, described in detail below, overlap with some components of Miyake and colleagues' model of executive function (Fig. 1). They also overlap with Posner and Petersen's model of attention,31 which suggests that attention is related to 3 “attentional networks” (executive control, orienting, and alerting). These networks can be assessed by the Attention Network Test (ANT).33 The executive control network, defined as resolving conflict among responses, is assessed by the Eriksen flanker test embedded in the ANT. Alerting, defined as achieving and maintaining an alert state, and orienting, defined as selection of information from sensory input, are assessed by changes in reaction times in the presence or absence of cues placed above and below the flankers stimuli.33
Figure 1.
Overlap across models of executive function30 and attention.31,32 Domains within each model are grouped to show similarity among models (eg, inhibition, executive control, and selective attention). The domains in the shaded red box (broadly: inhibition and divided/switching attention) are most commonly dysfunctional in people with Parkinson disease (PD) who freeze. Dysfunction of these domains can lead to changes in functional mobility and falls in this population.
Although there is no consensus regarding the independence or overlap of the cognitive domains described above, it is clear that these models are not fully distinct from one another. Indeed, the same cognitive test is often used to assess domains described by different models. For example, the flankers task can be used to assess domains of all 3 models: the executive control component of attention, defined by Posner and Petersen31; selective attention, defined by McDowd32; and inhibition, defined by Miyake and colleagues.30 Similarly, cognitive tasks that measure the ability to shift focus (eg, the Trail Making Test, which involves drawing a path between alternating letters and numbers) have been used to measure the shifting component of executive function, as defined by Miyake and colleagues, as well as attentional switching, as defined by McDowd. Such overlap demonstrates the commonality of some domains described by these models. In Figure 1, we lay out the domains of each model, with similar domains from each model grouped together to illustrate the similar and distinct components of these common models.
Also discussed in our framework, although not included in Figure 1, is visuospatial function. Visuospatial function consists of several components, including, but not limited to, visuoperceptual abilities (ie, identification of a stimulus, its orientation, and its location) and visuoconstructional abilities (ie, organization and manipulation of spatial information to make a design).34–36 Visuospatial function is often considered somewhat distinct from executive function and attention. However, as described below, some tests designed to assess visuospatial functions do tap into domains of both executive function and attention.
Altered Cognition Affects Mobility in Patients With PD Who Freeze
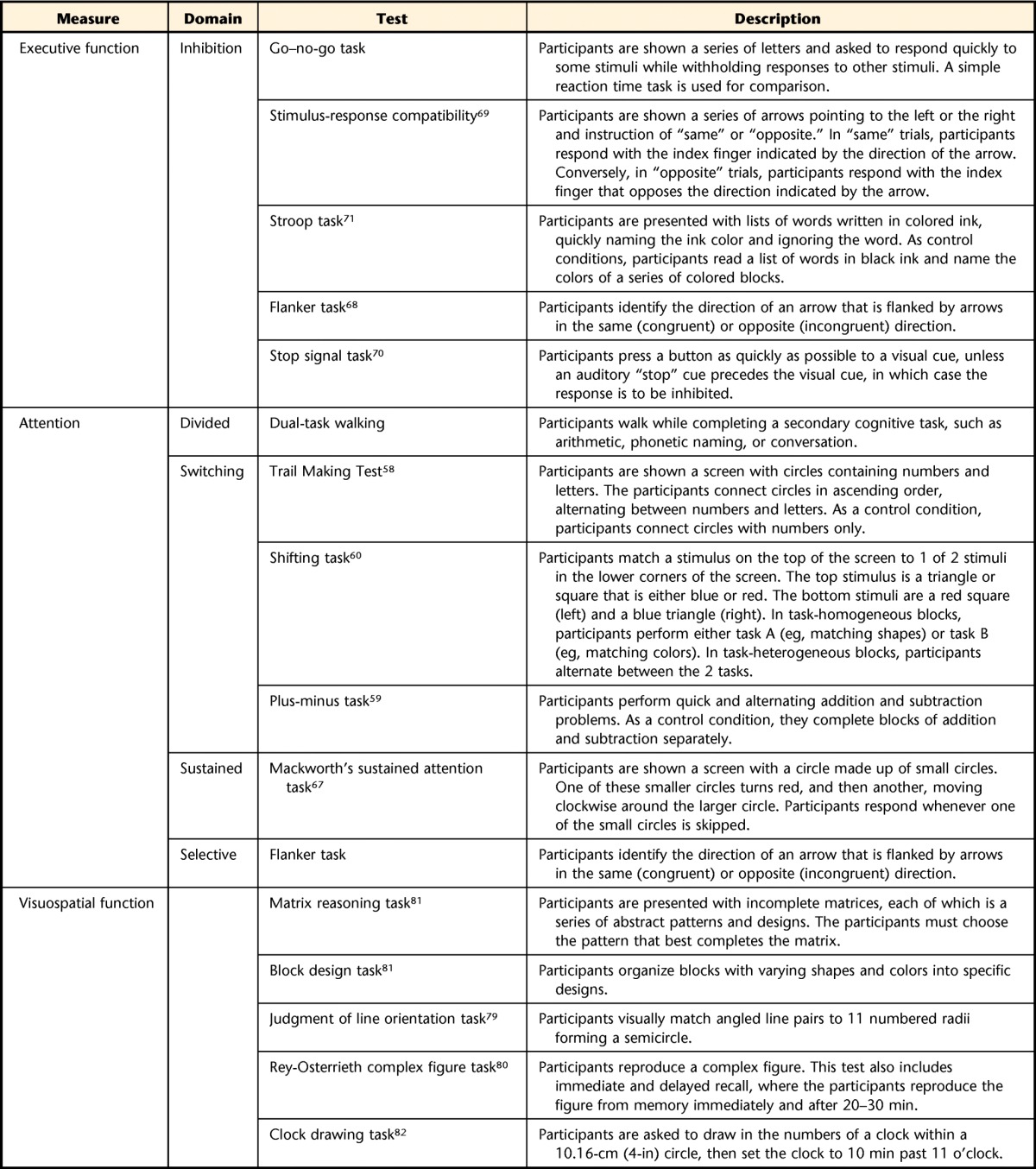
Individuals with PD often exhibit altered cognition compared with healthy adults.37,38 However, people with PD+FoG often exhibit even more pronounced cognitive dysfunction than those who do not experience FoG. In particular, FoG is associated with deficits in attention, especially divided attention and attentional switching39–44; executive function, especially shifting and inhibition15,45–48; and visuospatial function.34,46,49–51 In the following section, we provide a brief review describing evidence of cognitive deficits in people with PD+FoG and discuss how these deficits may lead to FoG events. Then, we suggest ways in which executive and attention function deficits may be related to motor dysfunction. Table 1 outlines and describes some neuropsychological tests commonly used to probe these cognitive domains.
Table 1.
Common Examinations to Assess Domains of Executive Function, Attention, and Visuospatial Function
Attention
Divided attention is the ability to complete 2 different attention-demanding tasks at the same time. In physical therapy, this ability is commonly tested by having patients complete a secondary cognitive task (ie, dual task [DT]) during stance or gait and measuring decrements in performance of each task during DT performance compared with when it is performed alone. This DT analysis allows clinicians to quantify the cost to mobility of adding a secondary task and to determine whether a person prioritizes mobility over the cognitive task. Analysis of prioritization of tasks is particularly important for people with PD. Previous studies have shown that people with PD may prioritize the secondary cognitive task over mobility, a “posture-second” strategy,52 although recent work suggests this strategy may not be a consistent feature of DT performance in people with PD.53 If utilized, a posture-second strategy could result in disproportionate posture and gait dysfunction in DT situations. Interestingly, individuals who experience freezing may exhibit even more pronounced “posture-second” prioritization than people with PD−FoG.39,42,44
Gait characteristics during DT walking are more affected in people with PD+FoG than in people with PD−FoG,39,42,44 and the changes in gait while dual tasking may have a causal role in FoG. Indeed, gait variables affected by DT walking (eg, smaller step length, increased variability) have been linked to FoG,54,55 and recent work suggests that there may be a threshold of gait dysfunction beyond which freezing occurs.55–57 Thus, the reduced gait function during DT walking may bring people with PD+FoG closer to this hypothetical threshold, increasing the chances of a freezing event.54,57 In addition, prioritization of cognitive tasks over gait tasks in conjunction with already reduced cognitive resources may further increase the risk of freezing. Importantly, previous reports have shown that people with PD show benefits in DT walking with practice.41,42 However, DT training has not been carried out explicitly on people PD+FoG; therefore, the degree to which freezing is reduced from these exercises is not known.
Attention switching refers to alternation of the focus of attention between 2 different tasks or sources of information.32 As noted above, “attention switching” is similar to the “shifting” domain described in Miyake and colleagues' model of executive function.30 These domains are commonly assessed with the Trail Making Test,58 the plus-minus task,59 and the shifting task.60 The ability to switch or shift attention has been shown to be associated with clinical severity of freezing and is worse in people with PD+FoG,40,41,43,61 although a recent report showed no differences in switching ability between people with PD+FoG and those with PD−FoG.45 Smulders and colleagues62 showed that shifting between lower extremity motor tasks (stepping forward and backward) resulted in larger delays in people who freeze than in people with PD−FoG, suggesting that switching deficits may contribute to the occurrence of FoG. Mobility in complex environments requires constantly switching attention among posture, locomotion, and surrounding sensory input. In people with PD+FoG, the inability to quickly and effectively switch attention during walking, turning, or initiating gait, particularly when completing secondary tasks, such as conversing with a friend, may contribute to freezing episodes and falls. Training that incorporates switching attentional focus improves retention of DT training benefits in healthy elderly people63 and may lead to improvements in DT gait in people with PD64,65 (for a review, see Kelly et al66). To our knowledge, no reports have investigated the effects of training attention switching on postural or locomotor control in people with PD+FoG. However, given the promising prior results and the attention switching dysfunction observed with FoG, incorporating this type of training into exercise for people with PD+FoG may improve their mobility during complex gait and posture tasks.
Sustained attention refers to the ability to maintain attention to a task over prolonged periods. This domain of attention has not been thoroughly investigated with respect to FoG. However, one recent study45 showed that performance on Mackworth's sustained attention task67 was similar between people with PD+FoG and those with PD−FoG.
Selective attention is the ability to intentionally focus attention on one source of information while excluding irrelevant information. As noted above, this domain shares some similarity to the inhibition domain of executive function (described by Miyake et al30) and the inhibition domain of executive control (described by Posner and Petersen31). These domains are commonly assessed with the flankers task,68 in which an individual must discern the direction an arrow is pointing while ignoring the directions of the flanking arrows. Two recent investigations compared selective attention in people with PD+FoG and people with PD−FoG using the ANT,33 which has a flankers task embedded in it.47,48 The results showed that people with PD+FoG performed worse on the flankers portion of the ANT, suggesting that selective attention may be worse in people with PD+FoG than in those with PD−FoG. Other components of attention defined by Posner and Petersen, namely the orienting and alerting networks of attention, were not affected by freezing status. Clearly, the flankers task may call on a number of cognitive functions other than selective attention, including inhibition of unwanted responses. Furthermore, a study that examined performance on the flankers task outside of the context of the ANT did not demonstrate a difference in performance between people with PD+FoG and those with PD−FoG.45 Although these results suggest altered attention in people with PD+FoG, additional work is necessary to understand the selective or sustained attention deficits in this population.
Executive Function
Of the 3 domains of executive function described by Miyake et al30 (ie, shifting, inhibition, and updating), shifting and inhibition are most consistently altered in people with PD+FoG.
Shifting (shifting back and forth between multiple tasks, operations, or mental sets) shares considerable overlap with the attention domain described in detail in the Attention section.
Inhibition (commonly assessed via the go–no-go task, various stimulus-response compatibility tasks,69 stop signal task,70 flanker task,68 and Stroop task71) is consistently affected in people with PD+FoG.15,45,47,48,51 For example, Cohen and colleagues45 showed that performance on a go–no-go task was worse in people with PD+FoG than in those with PD−FoG. Furthermore, both false alarms and misses in the go–no-go task were associated with severity of physician-rated FoG, illustrating difficulty both with inhibiting responses and with allowing responses to proceed after inhibition.45 Performance on the Stroop task has also been related to FoG, as people with PD+FoG performed worse than those with PD−FoG.15,45 Furthermore, people with PD+FoG demonstrate faster decline in Stroop performance over time than those with PD−FoG.14 Fling and colleagues72 also showed that performance deficits on the Stroop task in people with PD+FoG were correlated with asymmetry of white matter tracts between deep brain (pedunculopontine nucleus) and cortical (supplementary motor area) locomotor regions. A recent report by Matar and colleagues46 provides an additional link between performance in Stroop-like tasks and freezing events. Using virtual reality, researchers assessed step latency, a measure directly related to FoG, in people with PD+FoG and people with PD−FoG after conflicting stimuli in a Stroop-like task. The results showed that conflicting Stroop-like stimuli increased step latency more in people with FoG than in those without FoG.46 Applied to real-world environments, a reduced ability to interpret conflicting sensory input, inhibit inappropriate responses, and allow appropriate responses during gait could delay stepping, leading to freezing and falls.
Previous work from our laboratory suggests that freezing may be directly related to alterations in inhibition and release of motor programs. Jacobs and colleagues73 found that the coupling of a weight shift (or anticipatory postural response [APA]) with a step was altered in individuals with PD+FoG. Furthermore, the 4- to 6-Hz trembling of the knees often observed during a freezing event prior to step initiation may represent multiple unwanted APAs. This potential inability to couple the APA to the stepping motor program may be related to cognitive dysfunction, as noted above. For example, dysfunctional response inhibition could be related to an inability to effectively inhibit unwanted movements (extra APAs) or to release wanted (stepping) movements. Recent neuroimaging results provide further evidence suggesting a link between freezing and inhibition. Fling and colleagues showed that freezers exhibit altered functional connectivity in the hyperdirect pathway between the subthalamic nucleus and supplementary motor area,74 regions known to play important roles in inhibition.75 Together, these results suggest that the same neural circuits may be involved in both motor and cognitive inhibition and that they may be altered in people with PD+FoG. These results also are partially consistent with the suggestion by Vandenbossche and colleagues that alterations in inhibition and release of movements may reflect, in part, impaired movement automaticity in people with PD+FoG.48,76 This idea is consistent with previous observations that suggest automatic movement is altered in people with PD+FoG (for a review, see Nutt et al10). Importantly, recent research shows that inhibitory control can be improved with training in healthy adults.77,78 These improvements have not yet been replicated in people with PD; however, given the dysfunction observed in this domain, research investigating improvement in inhibition with training in PD is warranted.
Visuospatial Function
Visuospatial function can be assessed with a number of tests, including the judgment of line orientation task,79 the Rey-Osterrieth Complex Figure Test,80 the matrix reasoning task,81 the block design task,81 or the clock drawing test82 (Tab. 1). Previous reports suggest that individuals with PD exhibit visuospatial deficits and that these deficits relate to difficulties in everyday life.83 Two recent investigations have directly compared visuospatial function specifically in people with PD+FoG. Nantel and colleagues51 showed that people with PD+FoG scored worse than those with PD−FoG on matrix reasoning and block design tasks, and scores on these tasks were correlated to FoG severity. Lord and colleagues34 also showed dysfunction of visuospatial function in people with PD+FoG, as this population took longer to match angles presented on a screen (a task similar to the judgment of line orientation task) compared with people with PD−FoG. In partial support of these findings, a recent resting state functional connectivity assessment demonstrated that visual networks may be altered in people with PD+FoG in comparison with people with PD−FoG.84 It should be noted, however, that as with many executive function tests, complex visuospatial tasks such as the matrix reasoning and block design tasks incorporate a number of cognitive functions, including visuoconstructional abilities and nonverbal problem solving,36 which could confound conclusions specific to visuospatial function.
Adding to these findings, 2 recent investigations showed that walking through doorways is particularly problematic for people with PD+FoG,49,50 suggesting that altered visuospatial function may contribute to freezing in this scenario. Similarly, Matar and colleagues showed that, while moving through a virtual reality environment with wide, narrow, and sliding doorways, individuals with PD+FoG exhibited larger delays in stepping during narrow and sliding door conditions.46 Interestingly, follow-up studies showed that people with PD+FoG are able to effectively predict door size.50,85 However, they do not correctly predict how their gait will be influenced by the narrowness.85 Thus, it is possible that freezing while approaching or moving through doorways could be related to the integration of visuospatial information. Alternatively, moving through doorways adds an additional distracting task that could contribute to freezing, as noted above. Clearly, additional research is necessary to better understand the specific visuospatial dysfunction associated with FoG and whether this dysfunction plays a causal role in FoG.
Overall, the data summarized above suggest that the most prominent cognitive dysfunctions exhibited by people with PD+FoG are divided attention, attention switching or shifting, inhibitory control, and visuospatial function. Given the emerging evidence of the relationship between cognition and mobility, improving cognition could reduce freezing and improving mobility in people with PD+FoG. Indeed, recent evidence suggests that improving cognition with training may improve mobility in healthy older adults86 and individuals with PD.87 Additional studies have shown that training incorporating both cognitive and motor components also may be effective at improving cognition in healthy individuals88 (for a review, see Schoene et al13) and people with PD.19 However, considerably less research has focused on the effects of cognitive or motor programs on people with PD+FoG,89 and it is unknown whether this population will benefit from such an intervention. Given the fact that people with PD+FoG typically exhibit more pronounced cognitive dysfunction than those with PD−FoG, this cognitive dysfunction could impede mobility and cognitive benefits to training people with PD−FoG. A recent Cochrane review,90 however, suggests that even individuals with nonparkinsonian dementia may improve cognitive function through exercise. Thus, it is unlikely that cognitive dysfunction alone in people with PD+FoG would abolish the ability to improve cognitive ability through cognitive or mobility training.
Combining Cognitive and Exercise Training for People With PD Who Freeze
In clinical practice, cognitive training is typically carried out separately from mobility training. This model can clearly be effective for people with PD87,91 (for reviews, see Heremans et al,11 Walton et al,89 Calleo et al,92 and Segev-Jacubovski et al93). The improvements in function include reductions in FoG severity, as recent investigations have demonstrated improvements in FoG after mobility or cueing interventions.11,94 However, given the evidence of overlap between cognition and mobility, training cognitive and mobility together (rather than separately) may enhance gains of each area, increasing global function in people with FoG. Although people with PD+FoG exhibit clear overlap between cognitive and motor deficits (eg, increased FoG episodes with stress), research to date has not focused on the integration of freezing-specific motor and cognitive therapies for this population. Given previous literature demonstrating how interventions that incorporate cognition and motor tasks can improve function in healthy adults and people with PD−FoG13,17–19,64,86,88,95 (for a review, see Wollesen and Voelcker-Rehage96), research investigating the effects of targeted cognitive and motor interventions on FoG severity is warranted. Thus, in the following section, we provide examples of exercises that integrate cognitive and motor components. As the previous sections have provided a rationale for the integration of cognition and motor training, the following examples are meant to provide a starting point for evaluation of such targeted cognition-mobility training in people with PD+FoG and have not yet been tested.
Integrating FoG-Specific Cognitive Training With Mobility Training
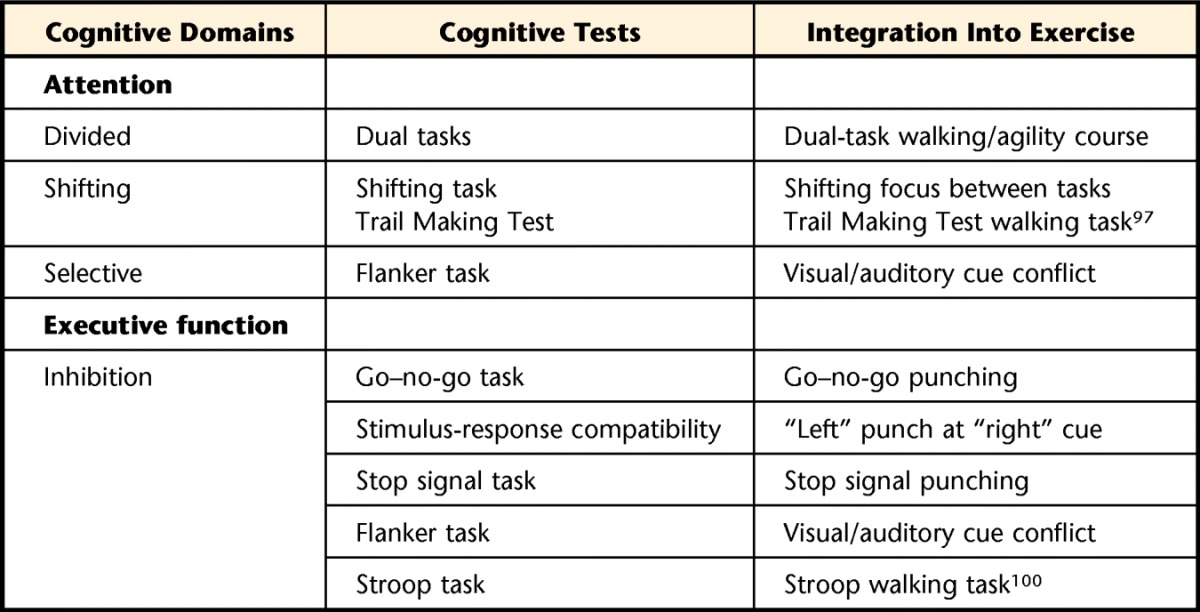
Table 2 lists some of the cognitive domains we suggest as targets for rehabilitation for FoG, as well as examples of tasks that integrate these domains into exercise.
Table 2.
Examples of Cognitive Domains Associated With Freezing and Exercises That Challenge These Deficits
Attention (Divided Attention and Attention Switching)
Attentional control can be integrated into exercise by instructing patients to carry out a secondary cognitive task (eg, arithmetic, phonetic/categorical naming, conversations) while exercising. Many physical therapists already incorporate DT elements into gait training to assess attention or to increase difficulty of gait tasks, and recent reports have demonstrated that DT practice may improve DT ability.64,86,95 However, despite the fact that the ability to divide attention is particularly altered in people with PD+FoG, the effect of DT practice on this population is not well characterized.
The ability to switch attention also is likely altered in people with PD+FoG, and recent work has demonstrated the feasibility of integrating attentional switching tasks into walking (Fig. 2A). For example, Perrochon and Kemoun97 used a Trail Making Test walking task to differentiate healthy older adults from those with mild cognitive impairment. In this task, participants walked along a mat with numbers and letters. Similarly to the traditional paper-and-pencil Trail Making Test, participants stepped on alternating and ascending letters and numbers (eg, 1-A-2-B…).97 Shifting also can be integrated into nongait exercises. Well-established tests to challenge shifting ability, such as the shifting task,60 can be integrated into upper or lower limb movements.
Figure 2.
(A) Example of task prioritization during agility training. The patient completes a secondary cognitive task during agility training and is instructed to switch prioritization between the mobility/stepping component (left) and the cognitive component (right). (B) Example of visual-auditory cue conflict during boxing. Simultaneously, the instructor visually cues for a left punch and verbally cues for a right punch. For this trial, the patient is instructed to respond to the visual cue only and ignore the auditory cue.
Boxing and agility courses also provide opportunities to incorporate dual tasking into exercise. During boxing, the trainer can cue punches verbally (saying “left arm” or “right arm”) and visually (moving the target to the left or right). With multiple cueing modalities, the patient is forced to switch attention between visual and auditory cues, prioritizing one over the other as instructed. Thus, through the application of multiple cues during mobility tasks, patients can practice divided attention and switching attention between cues (Fig. 2A). As will be discussed later, this paradigm also allows practice responding to conflicting cues and practice inhibiting prepotent responses (Fig. 2B). Agility courses also can integrate DT practice. Such courses integrate obstacles associated with FoG (eg, doorways, turning, tight spaces, backward walking, stepping over obstacles, change in surface)98,99 or secondary cognitive tasks, forcing practice with divided attention. Furthermore, the patient can be instructed to switch focus between primary (locomotion) and secondary (cognitive or motor secondary) tasks to practice switching task and attention priorities. In this way, patients receive practice with divided attention, switching attention, and integration of internal and external cues.
Inhibition
Inhibition tasks also can be integrated into motor training (see Tab. 2 for examples). Exercises such as boxing and lunges are particularly well suited to incorporate these challenges while maintaining a level of aerobic challenge. Aspects of go–no-go, stimulus-response compatibility, stop signal, flankers, and Stroop tasks can all be incorporated into these mobility exercises. For example, during a partnered boxing station, participants can be instructed to punch or step as quickly as possible in response to certain stimuli (given by instructor) while ignoring other stimuli (analogous to the go–no-go task). Participants also can be instructed to punch with the opposite arm as what is cued (ie, “right”=punch with left arm), thereby forcing participants to inhibit prepotent responses and resolve stimulus-response compatibility. As noted above, partnered boxing can integrate multiple cueing modalities (eg, visual, auditory). In addition to allowing the patient to practice switching attention between cues, these cues provide practice with inhibition tasks. For example, the trainer can provide conflicting information by verbally cueing the participant to punch with the left hand while visually cueing a punch with the right hand by moving the right target (Fig. 2B). This approach provides conflicting stimuli that the patient must decipher, as in a Simon, flankers, or Stroop task, requiring inhibition of inappropriate responses. Similarly, stop signal tasks can easily be incorporated: after the instructor cues a movement, he or she may occasionally give a stop signal, forcing inhibition of movement.
Finally, tasks related to response inhibition can be integrated into walking, such as a Stroop walking task.100 In this task, participants walk on a mat with different words (eg, “RED,” “BLUE,” “YELLOW”) printed in different colors. Participants hear color word cues, and, depending on the condition, they step either on a printed version of the word they heard or on a word that is printed in the same color ink as the word they heard. Participants can also practice doing the Stroop task mounted on a large board while they practice lunging in various directions.
Visuospatial Function
A common functional outcome of visuospatial dysfunction in people with PD+FoG is a change in gait when approaching doorways or walking surface transitions. Thus, incorporating such obstacles (eg, doorways of varying widths, obstacles) into training courses can provide individuals practice with these challenges. Previous investigations provide support for such an approach. Plotnik et al98 showed that obstacle-based training that incorporated narrow passages led to improvements in FoG. Although this intervention incorporates a number of approaches, including cueing before and after FoG-provoking obstacles, it provides some evidence that practicing gait through obstacles, including doorways, may be beneficial in reducing FoG. A recent study also showed that gait in patients with PD can be improved by treadmill training walking over a virtual obstacle with visual feedback of foot trajectories.101 Providing additional visual information about body motion in relation to environmental obstacles may allow compensatory mechanisms to control locomotion or may be restitutive.
Challenges in FoG Rehabilitation
Cognitive dysfunction in people with PD in general, and in people with PD+FoG specifically, can create challenges to rehabilitation. Some previous interventions aimed to improve FoG use compensatory strategies, such as providing external cues to trigger and guide movement and encouraging altered allocation of attention (ie, task prioritization).102 Although this type of compensatory training often improves FoG11,98 the cognitive dysfunction often observed in individuals with PD+FoG may limit their ability to deploy such strategies in daily life. Indeed, recent results suggest that, when cues are removed, people with PD+FoG revert to dysfunctional movement more than people with PD−FoG.12,103 Alternatively, attempts to improve the underlying dysfunction (ie, restitutive rehabilitation) may be able to reduce the cognitive limitations of this population. For example, training individuals to take larger, more consistent steps (eg, through treadmill walking) may partially circumvent attentional cues such as lines (visual) or tones (auditory). However, this approach relies, in part, on implicit motor learning, which has been shown to be deficient in people with PD+FoG.104 Due to the drawbacks of both treatment approaches, we believe that incorporating both restitutive and compensatory approaches will provide the greatest chance of cognitive-motor improvements. Therefore, we have incorporated each of these approaches into the current framework.
Cognitive dysfunction can create specific challenges for application of therapeutic approaches. For example, DT walking is challenging for people with PD, and too much cognitive challenge may lead to breakdown of gait and FoG. Thus, therapy must be tailored to the individual to find the level of dual tasking that challenges the system but does not fully overload it. Indeed, further research on ways to quickly indicate or contraindicate different cognitive approaches is necessary. Dual-task training also may increase risk of falls during training. However, despite these concerns, the use of DT training appears to be beneficial in people with PD and, given appropriate assessment and safety assessments, can be used effectively.105
A third challenge to rehabilitation is the possible effect of levodopa on cognition. A number of recent studies suggest that levodopa, the most common pharmacological therapy for PD, may have negative effects on specific cognitive tasks. These effects are thought to be most pronounced in the early stages of PD and may result from “overdosing” the ventral striatum with dopamine.106–108 This hypothesized overdosing of the ventral striatum may impede certain types of probabilistic reversal learning and, particularly important for rehabilitation, motor learning. Some investigations suggest that upper extremity motor learning may be subtly inhibited by levodopa.109,110 Although recent studies have not confirmed these findings in postural motor learning,111,112 additional research will be necessary to identify the effect of levodopa on neurorehabilitation.
Although a number of barriers exist for efficient treatment of FoG, it is important to keep in mind that despite the cognitive deficits and incomplete recovery noted above, individuals with PD+FoG can improve FoG symptoms through training.11,94 Thus, improvements can be made to rehabilitation for people with PD+FoG, and there is strong evidence that such efforts can have important positive effects on mobility and quality of life in this population.
Summary and Conclusions
Given the immense burden of FoG and cognitive deficits on quality of life, rehabilitation strategies should be designed based on current evidence to provide maximum benefit to both domains to patients with PD. Previous investigations have provided evidence that cognitive and mobility training are each separately beneficial in PD. We propose that FoG-specific cognitive training integrated with mobility training may enhance the benefits of both types of training in people with PD+FoG. However, this approach has not yet been investigated as an intervention targeting FoG. Thus, we have developed a framework for integrating cognitive-motor training for people who experience FoG and provided specific examples of exercises that integrate cognitive and motor challenges. Future research should assess the effectiveness of such a program on cognitive and mobility function in people with PD who freeze.
Footnotes
All authors provided concept/idea/project design and writing. Dr Horak provided project management, facilities/equipment, and institutional liaisons. Dr Peterson and Dr Horak provided fund procurement. Dr King, Dr Cohen, and Dr Horak provided consultation (including review of manuscript before submission).
This work was supported by grants from the US Department of Veterans Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080, Principal Investigator: Dr Peterson; VA Merit Award: E1075-R, Principal Investigator: Dr Horak); the National Institutes of Health (R01 AG006457 29, Principal Investigator: Dr Horak); NIGMS 5 U54 GM104944 Pilot Award, Principal Investigator: Dr Cohen; and the Medical Research Foundation of Oregon (Early Investigator Award; Principal Investigator: Dr Peterson). The contents of this manuscript do not represent the views of the US Department of Veterans Affairs or the US Government.
Dr Horak and OHSU have significant financial interests in APDM, a company that might have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Oversight Council. No other authors declare any conflict of interest.
References
- 1. Nutt JG, Bloem BR, Giladi N, et al. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neuron. 2011;10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giladi N, Kao R, Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. Mov Disord. 1997;12:302–305. [DOI] [PubMed] [Google Scholar]
- 3. Macht M, Kaussner Y, Möller JC, et al. Predictors of freezing in Parkinson's disease: a survey of 6,620 patients. Mov Disord. 2007;22:953–956. [DOI] [PubMed] [Google Scholar]
- 4. Nonnekes J, Janssen AM, Mensink SH, et al. Short rapid steps to provoke freezing of gait in Parkinson's disease. J Neurol. 2014;261:1763–1767. [DOI] [PubMed] [Google Scholar]
- 5. Snijders AH, Haaxma CA, Hagen YJ, et al. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18:149–154. [DOI] [PubMed] [Google Scholar]
- 6. Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. [DOI] [PubMed] [Google Scholar]
- 7. Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods. 2008;167:340–348. [DOI] [PubMed] [Google Scholar]
- 8. Moore ST, Yungher DA, Morris TR, et al. Autonomous identification of freezing of gait in Parkinson's disease from lower-body segmental accelerometry. J Neuroeng Rehabil. 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson's disease during the Instrumented Timed Up and Go test. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1198–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nutt JG, Horak FB, Bloem BR. Milestones in gait, balance, and falling. Mov Disord. 2011;26:1166–1174. [DOI] [PubMed] [Google Scholar]
- 11. Heremans E, Nieuwboer A, Spildooren J, et al. Cognitive aspects of freezing of gait in Parkinson's disease: a challenge for rehabilitation. J Neural Transm. 2013;120:543–557. [DOI] [PubMed] [Google Scholar]
- 12. Spildooren J, Vercruysse S, Meyns P, et al. Turning and unilateral cueing in Parkinson's disease patients with and without freezing of gait. Neuroscience. 2012;207:298–306. [DOI] [PubMed] [Google Scholar]
- 13. Schoene D, Valenzuela T, Lord SR, de Bruin ED. The effect of interactive cognitive-motor training in reducing fall risk in older people: a systematic review. BMC Geriatr. 2014;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amboni M, Barone P, Picillo M, et al. A two-year follow-up study of executive dysfunctions in parkinsonian patients with freezing of gait at on-state. Mov Disord. 2010;25:800–802. [DOI] [PubMed] [Google Scholar]
- 15. Amboni M, Cozzolino A, Longo K, et al. Freezing of gait and executive functions in patients with Parkinson's disease. Mov Disord. 2008;23:395–400. [DOI] [PubMed] [Google Scholar]
- 16. Vercruysse S, Spildooren J, Heremans E, et al. Freezing in Parkinson's disease: a spatiotemporal motor disorder beyond gait. Mov Disord. 2012;27:254–263. [DOI] [PubMed] [Google Scholar]
- 17. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson's disease—effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98:217–223. [DOI] [PubMed] [Google Scholar]
- 18. King LA, Salarian A, Mancini M, et al. Exploring outcome measures for exercise intervention in people with Parkinson's disease. Parkinsons Dis. 2013;2013:572134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reuter I, Mehnert S, Sammer G, et al. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson's disease. J Aging Res. 2012;2012:235765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28:1483–1491. [DOI] [PubMed] [Google Scholar]
- 22. Criado JM, de la Fuente A, Heredia M, et al. Electrophysiological study of prefrontal neurones of cats during a motor task. Pflugers Arch. 1997;434:91–96. [DOI] [PubMed] [Google Scholar]
- 23. Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. [DOI] [PubMed] [Google Scholar]
- 24. Rosenberg-Katz K, Herman T, Jacob Y, et al. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. 2013;80:1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 2009;164:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nonnekes J, Arrogi A, Munneke MA, et al. Subcortical structures in humans can be facilitated by transcranial direct current stimulation. PLoS One. 2014;9:e107731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gramann K, Jung TP, Ferris DP, et al. Toward a new cognitive neuroscience: modeling natural brain dynamics. Front Hum Neurosci. 2014;8:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. [DOI] [PubMed] [Google Scholar]
- 30. Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
- 31. Posner MI, Petersen SE. The attention system of the human brain. Ann Rev Neurosci. 1990;13:25–42. [DOI] [PubMed] [Google Scholar]
- 32. McDowd JM. An overview of attention: behavior and brain. J Neurol Phys Ther. 2007;31:98–103. [DOI] [PubMed] [Google Scholar]
- 33. Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. [DOI] [PubMed] [Google Scholar]
- 34. Lord S, Archibald N, Mosimann U, et al. Dorsal rather than ventral visual pathways discriminate freezing status in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:1094–1096. [DOI] [PubMed] [Google Scholar]
- 35. Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010;16:466–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Ann Rev Psychol. 2009;60:257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7:193–224. [DOI] [PubMed] [Google Scholar]
- 38. Reid WG, Hely MA, Morris JG, et al. Dementia in Parkinson's disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry. 2011;82:1033–1037. [DOI] [PubMed] [Google Scholar]
- 39. Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48:955–958. [DOI] [PubMed] [Google Scholar]
- 40. Hall JM, Shine JM, Walton CC, et al. Early phenotypic differences between Parkinson's disease patients with and without freezing of gait. Parkinsonism Relat Disord. 2014;20:604–607. [DOI] [PubMed] [Google Scholar]
- 41. Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson's disease. Mov Disord. 2010;25:1000–1004. [DOI] [PubMed] [Google Scholar]
- 42. Peterson DS, Fling BW, Mancini M, et al. Dual-task interference and brain structural connectivity in people with Parkinson's disease who freeze. J Neurol Neurosurg Psychiatry. 2015;86:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shine JM, Naismith SL, Palavra NC, et al. Attentional set-shifting deficits correlate with the severity of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:388–390. [DOI] [PubMed] [Google Scholar]
- 44. Spildooren J, Vercruysse S, Desloovere K, et al. Freezing of gait in Parkinson's disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–2570. [DOI] [PubMed] [Google Scholar]
- 45. Cohen RG, Klein KA, Nomura M, et al. Inhibition, executive function, and freezing of gait. J Parkinsons Dis. 2014;4:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matar E, Shine JM, Naismith SL, Lewis SJ. Using virtual reality to explore the role of conflict resolution and environmental salience in freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:937–942. [DOI] [PubMed] [Google Scholar]
- 47. Vandenbossche J, Deroost N, Soetens E, et al. Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabil Neural Repair. 2011;25:765–773. [DOI] [PubMed] [Google Scholar]
- 48. Vandenbossche J, Deroost N, Soetens E, et al. Conflict and freezing of gait in Parkinson's disease: support for a response control deficit. Neuroscience. 2012;206:144–154. [DOI] [PubMed] [Google Scholar]
- 49. Almeida QJ, Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010;81:513–518. [DOI] [PubMed] [Google Scholar]
- 50. Cowie D, Limousin P, Peters A, Day BL. Insights into the neural control of locomotion from walking through doorways in Parkinson's disease. Neuropsychologia. 2010;48:2750–2757. [DOI] [PubMed] [Google Scholar]
- 51. Nantel J, McDonald JC, Tan S, Bronte-Stewart H. Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson's disease. Neuroscience. 2012;221:151–156. [DOI] [PubMed] [Google Scholar]
- 52. Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson's disease. J Neurol Sci. 2006;248:196–204. [DOI] [PubMed] [Google Scholar]
- 53. Rochester L, Galna B, Lord S, Burn D. The nature of dual-task interference during gait in incident Parkinson's disease. Neuroscience. 2014;265:83–94. [DOI] [PubMed] [Google Scholar]
- 54. Chee R, Murphy A, Danoudis M, et al. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132(pt 8):2151–2160. [DOI] [PubMed] [Google Scholar]
- 55. Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson's disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80:347–350. [DOI] [PubMed] [Google Scholar]
- 56. Peterson DS, Plotnik M, Hausdorff JM, Earhart GM. Evidence for a relationship between bilateral coordination during complex gait tasks and freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Plotnik M, Giladi N, Hausdorff JM. Is freezing of gait in Parkinson's disease a result of multiple gait impairments? Implications for treatment. Parkinsons Dis. 2012;2012:459321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 59. Jersild A. Mental set and shift. Arch Psychol. 1927;89(whole issue). [Google Scholar]
- 60. Kramer JH, Mungas D, Possin KL, et al. NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc. 2014;20:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walton CC, Shine JM, Mowszowski L, et al. Impaired cognitive control in Parkinson's disease patients with freezing of gait in response to cognitive load. J Neural Transm. 2015;122:653–660. [DOI] [PubMed] [Google Scholar]
- 62. Smulders K, Esselink RA, Bloem BR, Cools R. Freezing of gait in Parkinson's disease is related to impaired motor switching during stepping. Mov Disord. 2015;30:1090–1097. [DOI] [PubMed] [Google Scholar]
- 63. Silsupadol P, Shumway-Cook A, Lugade V, et al. Effects of single-task versus dual-task training on balance performance in older adults: a double-blind, randomized controlled trial. Arch Phys Med Rehabil. 2009;90:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brauer SG, Morris ME. Can people with Parkinson's disease improve dual tasking when walking? Gait Posture. 2010;31:229–233. [DOI] [PubMed] [Google Scholar]
- 65. Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructions on dual-task walking and cognitive task performance in people with Parkinson's disease. Parkinsons Dis. 2012;2012:671261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mackworth N. Researches on the Measurement of Human Performance. Medical Research Council Special Report Series No. 268 London, United Kingdom: His Majesty's Stationery Office; 1950. [Google Scholar]
- 68. Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- 69. Wager TD, Sylvester CY, Lacey SC, et al. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. [DOI] [PubMed] [Google Scholar]
- 70. Verbruggen F, Logan GD, Stevens MA. STOP-IT: Windows executable software for the stop-signal paradigm. Behav Res Methods. 2008;40:479–483. [DOI] [PubMed] [Google Scholar]
- 71. Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 72. Fling BW, Cohen RG, Mancini M, et al. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136(pt 8):2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jacobs JV, Nutt JG, Carlson-Kuhta P, et al. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fling BW, Cohen RG, Mancini M, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9:e100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vandenbossche J, Deroost N, Soetens E, et al. Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manuel AL, Bernasconi F, Spierer L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: an electrical neuroimaging study. Cortex. 2013;49:1141–1417. [DOI] [PubMed] [Google Scholar]
- 78. Spierer L, Chavan CF, Manuel AL. Training-induced behavioral and brain plasticity in inhibitory control. Front Hum Neurosci. 2013;7:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol. 1978;35:364–367. [DOI] [PubMed] [Google Scholar]
- 80. Shin MS, Park SY, Park SR, et al. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 2006;1:892–899. [DOI] [PubMed] [Google Scholar]
- 81. Groth-Marnat G. Handbook of Psychological Assessment. 5th ed Hoboken, NJ: John Wiley & Sons Inc; 2009. [Google Scholar]
- 82. Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatry Med. 1994;24:229–244. [DOI] [PubMed] [Google Scholar]
- 83. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res. 2005;45:1285–1296. [DOI] [PubMed] [Google Scholar]
- 84. Tessitore A, Amboni M, Esposito F, et al. Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord. 2012;18:781–787. [DOI] [PubMed] [Google Scholar]
- 85. Cohen RG, Chao A, Nutt JG, Horak FB. Freezing of gait is associated with a mismatch between motor imagery and motor execution in narrow doorways, not with failure to judge doorway passability. Neuropsychologia. 2011;49:3981–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verghese J, Mahoney J, Ambrose AF, et al. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010;65:1338–1343. [DOI] [PubMed] [Google Scholar]
- 87. Milman U, Atias H, Weiss A, et al. Can cognitive remediation improve mobility in patients with Parkinson's disease? Findings from a 12-week pilot study. J Parkinsons Dis. 2014;4:37–44. [DOI] [PubMed] [Google Scholar]
- 88. Theill N, Schumacher V, Adelsberger R, et al. Effects of simultaneously performed cognitive and physical training in older adults. BMC Neurosci. 2013;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walton CC, Shine JM, Mowszowski L, et al. Freezing of gait in Parkinson's disease: current treatments and the potential role for cognitive training. Restor Neurol Neurosci. 2014;32:411–422. [DOI] [PubMed] [Google Scholar]
- 90. Forbes D, Thiessen EJ, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;12:CD006489. [DOI] [PubMed] [Google Scholar]
- 91. Petrelli A, Kaesberg S, Barbe MT, et al. Effects of cognitive training in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. 2014;20:1196–1202. [DOI] [PubMed] [Google Scholar]
- 92. Calleo J, Burrows C, Levin H, et al. Cognitive rehabilitation for executive dysfunction in Parkinson's disease: application and current directions. Parkinsons Dis. 2012;2012:512892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Segev-Jacubovski O, Herman T, Yogev-Seligmann G, et al. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother. 2011;11:1057–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database Syst Rev. 2013;9:CD002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yogev-Seligmann G, Giladi N, Brozgol M, Hausdorff JM. A training program to improve gait while dual tasking in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil. 2012;93:176–181. [DOI] [PubMed] [Google Scholar]
- 96. Wollesen B, Voelcker-Rehage C. Training effects on motor-cognitive dual-task performacne in older adults. Eur Rev Aging Phys Act. 2014;11:5–24. [Google Scholar]
- 97. Perrochon A, Kemoun G. The Walking Trail-Making Test is an early detection tool for mild cognitive impairment. Clin Interv Aging. 2014;9:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Plotnik M, Shema S, Dorfman M, et al. A motor learning-based intervention to ameliorate freezing of gait in subjects with Parkinson's disease. J Neurol. 2014;261:1329–1339. [DOI] [PubMed] [Google Scholar]
- 99. King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009;89:3843–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perrochon A, Kemoun G, Watelain E, Berthoz A. Walking Stroop carpet: an innovative dual-task concept for detecting cognitive impairment. Clin Interv Aging. 2013;8:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mirelman A, Maidan I, Herman T, et al. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson's disease? J Gerontol A Biol Sci Med Sci. 2011;66:234–240. [DOI] [PubMed] [Google Scholar]
- 102. Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson's disease. Mov Disord. 2009;24:64–71. [DOI] [PubMed] [Google Scholar]
- 103. Vercruysse S, Spildooren J, Heremans E, et al. Abnormalities and cue dependence of rhythmical upper-limb movements in Parkinson patients with freezing of gait. Neurorehabil Neural Repair. 2012;26:636–645. [DOI] [PubMed] [Google Scholar]
- 104. Vandenbossche J, Deroost N, Soetens E, et al. Impaired implicit sequence learning in Parkinson's disease patients with freezing of gait. Neuropsychology. 2013;27:28–36. [DOI] [PubMed] [Google Scholar]
- 105. Strouwen C, Molenaar EA, Munks L, et al. Dual tasking in Parkinson's disease: should we train hazardous behavior? Expert Rev Neurother. 2015:1–9. [DOI] [PubMed] [Google Scholar]
- 106. Cools R, Lewis SJ, Clark L, et al. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology. 2007;32:180–189. [DOI] [PubMed] [Google Scholar]
- 107. Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. [DOI] [PubMed] [Google Scholar]
- 108. Vaillancourt DE, Schonfeld D, Kwak Y, et al. Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord. 2013;28:1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kwak Y, Muller ML, Bohnen NI, et al. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. J Neurophysiol. 2010;103:942–949. [DOI] [PubMed] [Google Scholar]
- 110. Kwak Y, Muller ML, Bohnen NI, et al. L-DOPA changes ventral striatum recruitment during motor sequence learning in Parkinson's disease. Behav Brain Res. 2012;230:116–124. [DOI] [PubMed] [Google Scholar]
- 111. Roemmich RT, Hack N, Akbar U, Hass CJ. Effects of dopaminergic therapy on locomotor adaptation and adaptive learning in persons with Parkinson's disease. Behav Brain Res. 2014;268:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Anderson ED, Horak FB, Lasarev MR, Nutt JG. Performance of a motor task learned on levodopa deteriorates when subsequently practiced off. Mov Disord. 2014;29:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]