Abstract
Background and Purpose
Pompe disease is an inherited disorder notable for severe, progressive ventilatory compromise. Although ventilatory failure has been attributed to myofiber dysfunction secondary to diaphragmatic glycogen accumulation, neural involvement of the phrenic motor system is also a prominent feature. Direct diaphragm pacing supplements respiratory function in other disorders of the phrenic motor system. Accordingly, it is hypothesized that augmented neuromuscular activity via diaphragm pacing would promote weaning from mechanical ventilation in patients with Pompe disease who are unresponsive to conventional, muscle-directed treatments.
Case Description
Three patients with Pompe disease developed diaphragm paresis that resulted in chronic mechanical ventilation dependence. After preoperative inspiratory muscle strengthening exercises failed to improve function, fine-wire pacing electrodes were laparoscopically implanted into the diaphragm. Diaphragm conditioning was initiated the first postoperative week and consisted of gradual increases in stimulation parameters, lengthening of stimulation sessions, and ventilator weaning. Ventilation and intramuscular electromyographic activity were recorded periodically during conditioning to quantify diaphragm neuromuscular function.
Outcomes
During paced breathing without mechanical ventilation, tidal volumes increased, and 2 patients were weaned from daytime ventilator dependence within the first 3 months of pacing, which has been sustained over the long-term. A third patient reduced reliance on daytime ventilation, but weaning was delayed by malacia of the large airways. In all patients, pacing appeared to facilitate spontaneous phrenic motor unit activity during independent breathing without ventilator or pacer support.
Discussion
The findings are consistent with the view that diaphragm pacing has potential rehabilitative value to reduce reliance on mechanical ventilation in people with Pompe disease, but further study is needed. Diaphragm pacing represents a paradigm shift in the management of respiratory insufficiency for Pompe disease that warrants further controlled examination.
Pompe disease is an inherited neuromuscular disorder characterized by a deficiency or absence of acid α-glucosidase (GAA), resulting in glycogen aggregations in various tissues, including striated muscle and neural tissue.1 The age of onset and progression are variable and reflect residual GAA activity. In all presentations, progressive diaphragm dysfunction is a dominant feature, and the resulting ventilatory insufficiency represents a major source of morbidity and mortality.2 Motor deficits in people with Pompe disease have traditionally been attributed to myopathy,3,4 but accumulating evidence also has revealed glycogen accumulation in motoneurons and neural dysfunction (reviewed by Fuller et al5). These findings are important because the current therapy for Pompe disease—intravenous enzyme replacement therapy (ERT) using recombinant GAA6—does not affect the central nervous system.7 Although ERT has enhanced survival in patients with infantile-onset Pompe disease, most children still eventually require assisted ventilation.8 Furthermore, the respiratory benefits are limited for ERT-treated adults,6,9 and they may require mechanical ventilation (MV) over the long-term. The modest impact of muscle-directed ERT on limb and ventilatory motor function6,10 is consistent with a mixed neuromuscular etiology of disease progression.11,12 Thus, additional rehabilitative strategies may be indicated to target neuromuscular sources of ventilatory dysfunction.
Phrenic stimulation via intramuscular pacing of the diaphragm (“diaphragm pacing”) has facilitated ventilator weaning in other neuromuscular disorders with impaired phrenic motor function, most notably in spinal cord injury,13,14 and it may offer rehabilitative benefits to restore spontaneous activity of the phrenic motor system.15 We reasoned that if respiratory failure in people with Pompe disease is partially due to impaired efferent signals to the diaphragm,5,10,11,16 diaphragm pacing should promote ventilator weaning to a greater degree than using ERT alone. Here, we report the first implantations of diaphragm pacemakers to treat chronic ventilator dependence in 3 patients with Pompe disease. In each case, other clinical approaches to reduce reliance on MV were unsuccessful.
Case Descriptions: Patient History and Systems Review
Patient 1 was a 53-year-old man referred to our clinic after 4 years of continual invasive ventilator dependence (Tab. S1 of the eAppendix). A 15-year history of undiagnosed proximal weakness and progressive respiratory insufficiency escalated to an episode of acute respiratory failure, and full-time invasive MV was instituted. He then was diagnosed with Pompe disease and began ERT (Lumizyme, Genzyme, Cambridge, Massachusetts). After the patient was stabilized, repeated efforts to wean him from MV failed during the subsequent 4 years, and he remained confined to a medical care facility due to the MV dependence. At the preoperative evaluation, maximal inspiratory pressure (MIP) measured −38 cm H2O, reflecting inspiratory weakness.17 However, a maximal twitch transdiaphragmatic pressure of <3 cm H2O indicated severe, preferential diaphragm dysfunction,17 and the diaphragm descended <0.5 cm during a “maximal sniff” videofluorography test. These clinical findings indicated diaphragm paresis.14 Off-ventilator tolerance was within 2 minutes due to hypoventilation (tidal volume approximately 4 mL/kg).
Patient 2 was a 3-year-old girl with infantile presentation of hypotonia, developmental delay, feeding difficulties, and severe dilated cardiomyopathy with cardiac hypertrophy who was diagnosed with Pompe disease at 8 months of age (Tab. S2 of the eAppendix). Despite ERT (Myozyme, Genzyme) and immune modulation to block anti-GAA antibodies, she developed progressive sleep-disordered breathing and daytime respiratory insufficiency. Dose escalation of ERT (40 mg/kg weekly) and noninvasive ventilation (NIV) failed to stabilize cardiopulmonary dysfunction. Tracheostomy and full-time MV reduced the chronic cardiac failure, but respiratory muscle function remained severely compromised. Her MIP was −35 cm H2O with reduced diaphragmatic descent during sniff maneuvers (right: one costal space; left: one-half costal space). Soon after tracheostomy, she tolerated reduced ventilator support trials for 10-minute bouts.
Patient 3 was a 48-year-old man with Pompe disease who was unresponsive to ERT. He experienced nearly a decade of progressive quadriparesis with critical hypoventilation that required high rate and pressure settings on continuous NIV (daytime arterial pressure of carbon dioxide obtained overnight=44 mm Hg). Due to a chronic, full-time requirement for NIV, the patient elected to withdraw from ERT 2 years prior to our initial consultation. On examination, MIP was −13.5 cm H2O, with a maximal twitch transdiaphragmatic pressure of 1.26 cm H2O. Fluoroscopic sniff revealed no inspiratory diaphragmatic excursion, and removal of the ventilator resulted in desaturation within 40 seconds with tidal volume approximately 2 mL/kg (Tab. S3 of the eAppendix).
The preoperative clinical evaluation included tests for MIP, evoked phrenic motor potentials, breathing pattern assessment, and off-ventilator endurance time. Each test procedure is detailed in the eAppendix.
Clinical Impression 1
Each patient was classified with severe chronic hypoventilation resulting from respiratory muscle weakness and preferential diaphragm dysfunction. Each patient and his or her family reported that MV resulted in profound restrictions on activities of daily living and mobility, and the primary functional goal was to regain the ability to breathe without positive pressure ventilation.
Preoperative Intervention: Inspiratory Muscle Strength Training
Patients received respiratory muscle strength training 3 to 5 days per week for at least 4 weeks prior to implantation.18 Training sessions consisted of 4 sets of 8 to 10 “best-effort” breaths, using a pressure-threshold trainer set at the highest load that permitted valve opening. Additional details of the training device and regimen are described in the eAppendix.
Clinical Impression 2: Eligibility for Diaphragm Pacing
Each patient tolerated inspiratory muscle strength training (IMST) exercises well and did not experience any adverse events related to IMST. Patient 2 experienced an increase in MIP from −35 cm H2O to −65 cm H2O and could tolerate up to 60-minute spontaneous breathing trials, with a paradoxical breathing pattern. However, the exercise intervention did not relieve dyspnea or reliably increase tolerance to breathing without MV (Tabs. S1, S2, and S3 of the eAppendix). Based on the evaluation, results of training, and the judgment of the surgeon, it was determined that the patients were eligible for diaphragm pacing.
Approach
As preoperative assessments were consistent with impaired phrenic motor unit recruitment, an off-label compassionate use diaphragm pacing implantation was performed under guidelines of a US Food and Drug Administration (FDA) humanitarian device exemption for a humanitarian use device, following institutional review board (IRB) approval. The adult patients and the parents of the child consented to the implantation, postoperative respiratory monitoring through an existing IRB-approved natural history study, and this report.
Placement of the Diaphragm Pacing System
The NeuRx RA/4 (Synapse Biomedical Inc, Oberlin, Ohio) diaphragm pacing system was placed laparoscopically, as described in detail elsewhere.13 Briefly, the surgeon identified the motor point in each costal hemidiaphragm by a systematic mapping technique using a stimulating probe.19 When the motor point was identified, 2 customized insulated wire electrodes were secured in each hemidiaphragm. A fifth electrode (anode) was placed subcutaneously. Electrodes were secured to the abdominal wall, externalized at the right upper quadrant of the abdomen, and mounted onto an electrode connector block. The connector block connected to the pacemaker's external stimulator, which was programmed to deliver timed stimulus pulses. Further information on the external stimulator and pacing settings is presented in the eAppendix. The initial settings were set in the operating department by the pacemaker manufacturer's representative.
Diaphragm Conditioning
Daily diaphragm conditioning commenced after a minimum 3-day postoperative recovery period (POD4 for patients 1 and 3 and POD5 for patient 2), when postoperative pain and inflammation were controlled for 24 hours. Diaphragm atrophy was anticipated after prolonged periods of MV support20; therefore, we aimed to postoperatively recondition the diaphragm by gradually increasing:
External stimulator settings. The manufacturer's representative and the surgical care team set the initial external stimulator amplitude, frequency, and pulse width to below the patient's pain threshold. The settings were re-evaluated at least weekly for the first 30 days and then at least monthly for the first 90 days after implantation. Progression of settings typically included increase in the stimulation amplitude or pulse width, with minimal changes to the stimulation frequency.
Duration of diaphragm stimulation. The goal for duration of the initial pacing conditioning session was 30 minutes. The duration of diaphragm stimulation was systematically increased by 30 to 60 minutes, every 1 to 2 days as tolerated, with the goal to pace continuously by POD30.
Off-ventilator spontaneous breathing. On POD4 or POD5, the patients underwent an initial trial of breathing with reduced or no MV support while the pacer was powered on. For patients who could not initially complete more than a few minutes of off-ventilator breathing, even with the diaphragm pacer turned on, the focus was to breathe for prolonged periods with progressively lower levels of MV. The duration of reduced or off-ventilator breathing with diaphragm pacing was increased at least twice weekly for the first month. Clinical laboratory tests were done following major increases in off-ventilator spontaneous breathing trials.
Postoperative Electrophysiological Assessments
Postoperatively, we recorded minute ventilation and diaphragm electrical activity simultaneously using the implanted pacing electrodes during spontaneous (unpaced) breathing. The right and left electromyographic (EMG) signals were recorded every 1 to 2 weeks for the first postoperative month and periodically thereafter. The purposes of the recordings were: (1) to identify the baseline postoperative activity of the diaphragm with various spontaneous breathing maneuvers and (2) to determine whether diaphragm activation was increased in patients following reconditioning by pacing. Electromyographic activity was recorded with each patient seated fully upright during resting MV support, during breathing without MV support, and with maximal-effort maneuvers, the latter being used to normalize the EMG signals.
A customized adaptor was connected to the pacemaker's external connector block in order to record from the implanted diaphragmatic electrodes. Signals were band-pass filtered (1–5,000 Hz), sampled at 20 kHz (PowerLab 16/30, AD Instruments Inc, Colorado Springs, Colorado), and rectified, integrated, and averaged together for 1-minute intervals during each condition. The EMG amplitude was normalized to the within-session MIP. Ventilatory flow, pressure, and timing parameters were captured simultaneously with a pneumotachograph/capnograph.
Outcome
Diaphragm Conditioning
In the 2 adult patients, diaphragm pacing was initiated at POD4 for 30 minutes at intensities that were detectable but did not elicit pain. For patient 2, diaphragm conditioning began on POD5 with 1 hour of pacing at the intensity the stimulation was first detected by the patient. The immediate goal was to achieve 24 hours of continuous pacing comfortably and without adverse side effects. Patient 1 achieved this goal by POD30, and patients 2 and 3 achieved 24-hour pacing by POD21. The stimulation intensity also was gradually increased individually. Over the next several months of paced breathing, patients completed progressively longer sessions of breathing with reduced or no MV support, with the long-term goal of eliminating MV support. Clinical laboratory tests and ventilation were monitored to evaluate the patients' tolerance to conditioning and weaning (Tabs. S1, S2, and S3 of the eAppendix).
Diaphragm EMG Activity
Initially, the spontaneous EMG activity was marginal (patient 2) or absent (patients 1 and 3) during periods of full MV support. With lowered support, diaphragm inspiratory bursting emerged, but the patients' accessory respiratory muscles were disproportionately recruited, evident by observations of an abdominal inspiratory paradox and negative gastric pressure waveform (Fig. 1). When pacing acutely activated the diaphragm, the resulting contractions restored the gastric pressure to positive inspiratory deflections. After weeks to months of daily diaphragm reconditioning, each patient's relative (ie, normalized) and absolute (ie, mV) unpaced diaphragm EMG signal intensified during MV and spontaneous breathing (Figs. 2 and 3).
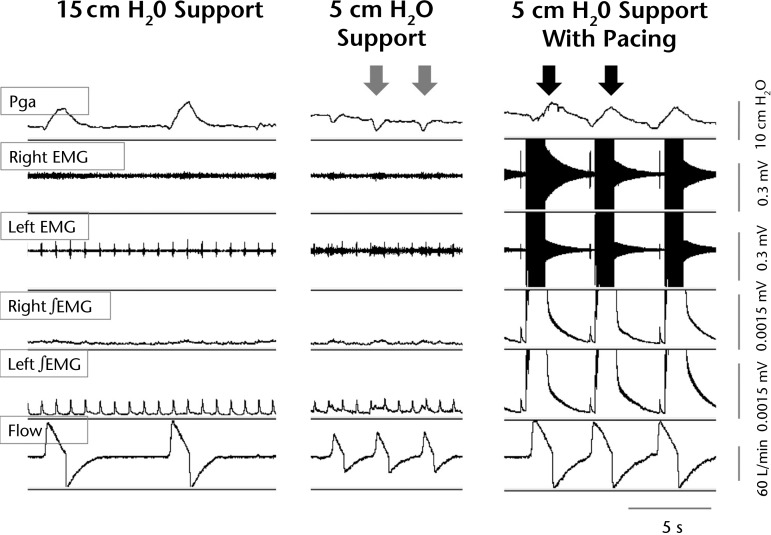
Figure 1.
Acute reversal of abdominal inspiratory paradox with pacing. A sample recording from patient 1 captures gastric pressure (Pga), raw and integrated electromyographic (EMG) signals, and flow waveforms during resting mechanical ventilation (MV) settings, lowest tolerated MV support, and lowest MV support plus pacing. Reduced MV settings resulted in slower peak flow, and a rapid, shallow breathing pattern was accompanied by negative inflections of the gastric pressure waveform (gray arrows), reflecting an abdominal paradox. At the same reduced MV settings, the addition of diaphragm pacing improved flow and restored positive inspiratory gastric pressures (black arrows).
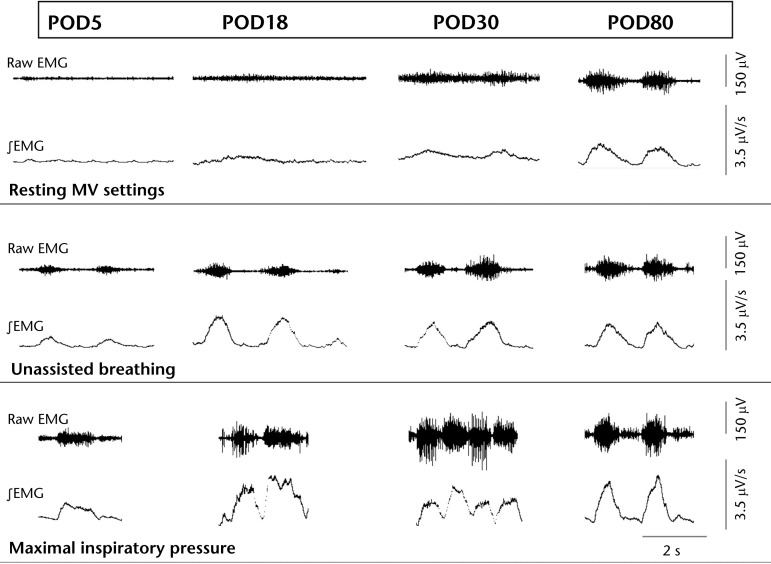
Figure 2.
Improved diaphragm activation after conditioning. Representative right hemidiaphragm electromyographic (EMG) activity from patient 1 captured during resting mechanical ventilation (MV) settings, unassisted breathing (no assistance by MV or diaphragm pacing), and maximal inspiratory pressure maneuvers. Electromyographic activity from POD5 (left) to POD80 (right) reveals that adjacent diaphragm motor units became progressively more active under all conditions. In conjunction, the patient tolerated both a progressively longer time without MV support and greater minute ventilation with pacing. Similar increases in diaphragm EMG activity were observed in the other patients. POD=postoperative day.
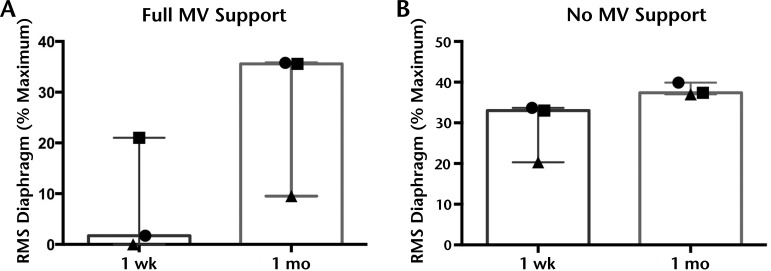
Figure 3.
Normalized activity of the diaphragm increased after 1 month of pacing. Spontaneous electromyographic activity of the diaphragm was recorded during full mechanical ventilation (MV) support (A) and unsupported spontaneous breathing (B) and normalized to the within-session maximal voluntary effort. Within 1 month, the averaged normalized root mean square (RMS) of the diaphragm was higher, suggesting the muscle was more active during these behaviors. Graphs depict median and range.
Respiratory Outcomes
Within 1 month, gains in tidal volume were evident, with ventilator-free time increasing by 3 months (Fig. 4). Patient 1 began conditioning on reduced MV support and transitioned to paced breathing with no MV support by POD35. By POD62, he could comfortably breathe without MV for 8 to 10 continuous hours while pacing. In conjunction, the average paced tidal volume increased 131% by POD80. This gradual introduction of diaphragm pacing and ventilator weaning culminated in patient 1's discharge to home on POD65, after a 4.5-year hospitalization for full-time MV. He continues diaphragm pacing on most days for more than 12 hours a day and remains liberated from daytime MV after more than 3 years.
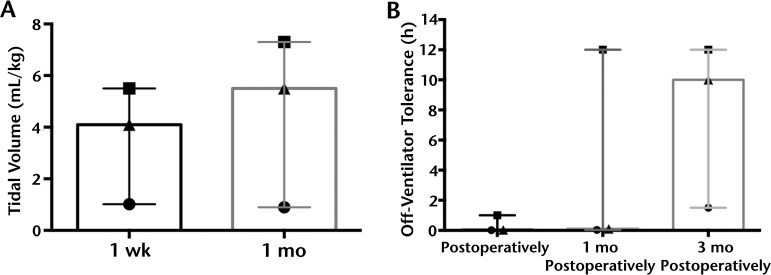
Figure 4.
Tidal volume and ventilator-free time increased after diaphragm conditioning with pacing. After 1 month of conditioning, modest gains in tidal volume (A) were detected, but the effect was variable among patients. Ventilator-free time (B) progressively increased in the first 3 months of diaphragm conditioning, which is consistent with published reports of pacing in spinal cord injury. Graphs depict median and range.
For patient 2, diaphragm conditioning was initiated without MV support on POD7. By POD30, she could sustain paced breathing for 12 hours without MV and increased to 16-hour bouts by POD50. At POD50, the breathing pattern was notable for 33% reduced respiratory rate, with a concurrent 45% increase in tidal volume during pacing. More than 2 years later, patient 2 also regained many trunk and upper extremity developmental skills and attends school full-time. The tracheostomy remains capped during waking hours, and the patient uses the pacer and ventilator only during sleep.
Because his preoperative diaphragm function was severely compromised, patient 3 could not clear secretions effectively after intubation, and he underwent elective tracheostomy on POD8. Severe malacia of the trachea and left main stem bronchus significantly slowed his progress with spontaneous breathing trials. Furthermore, electrophysiological tests identified denervated motor units in the left hemidiaphragm (Fig. 5). Patient 3 was discharged home from inpatient rehabilitation on POD75, and he tolerated up to 60-minute periods of pacing without assistance by POD90, which had not been possible for more than 3 years preoperatively. In an effort to stabilize the malacia, weaning efforts shifted from daily spontaneous breathing trials without MV to all-day tolerance of progressively reduced positive pressure. His MV requirement progressively decreased over the first year (intermittent mandatory ventilation from 14 to 3 breaths per minute, positive end-expiratory pressure from 6 to 5 cm H2O, peak inspiratory pressure support from 29 to 16 cm H2O), and he regained the ability to clear secretions independently. Progress remains ongoing, toward the goal of independent spontaneous breathing.
Figure 5.
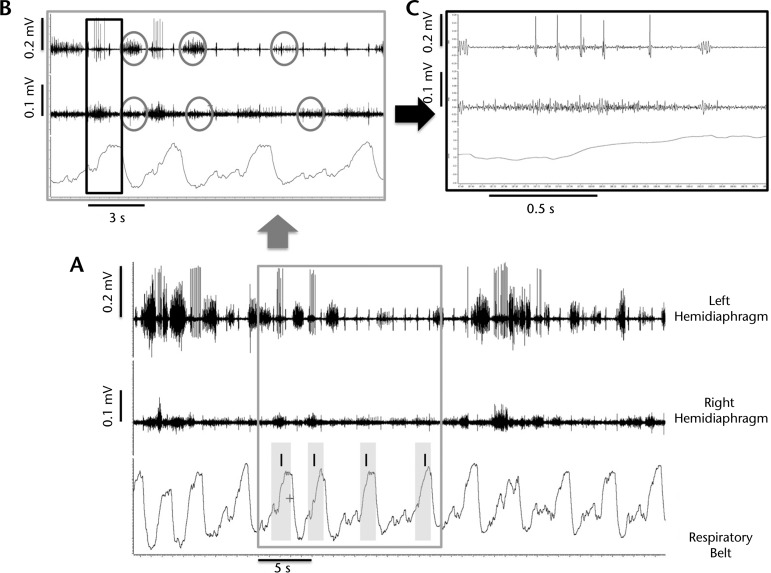
Right and left hemidiaphragm asynchrony in patient 3. Evidence of asynchrony and loss of phasic inspiratory activity of the left hemidiaphragm reflects denervation of phrenic motor units and altered control of breathing, which may have influenced the lower functional recovery of breathing in patient 3. (A) Respiratory plethysmography and diaphragm electromyographic (EMG) tracings, recorded spontaneous diaphragm activity during full mechanical ventilation, 1 month after implantation of the diaphragm pacemaker. Shaded areas show inspiration. (B) Gray cutout box from graph A illustrates expiratory phase activity (circles), which could be co-contraction to generate positive end-expiratory pressure and offset malacia. In addition, intermittent periods of inspiratory quiescence and large amplitude inspiratory bursts are apparent on the left hemidiaphragm (top) EMG tracing. (C) Black cutout box from graph B shows bursting pattern from a single motor unit. The large-amplitude complex bursts and repetitive discharges suggest partial denervation of this motor unit.
Discussion
A novel and clinically important finding emerges from this report: direct diaphragm muscle stimulation by pacing replaced the use of daytime MV in 2 of the 3 patients with chronic ventilator dependence, and it reduced MV dependence in the third patient. Gains in independent breathing were accompanied by changes in the spontaneous activity of the unpaced diaphragm. Thus, in at least a subset of patients with Pompe disease who are ventilator-dependent, the diaphragm can be “trained,” via electrical stimulation, to counteract chronic hypoventilation. This result has important implications for the underlying pathology and treatment of respiratory insufficiency in ventilator-dependent Pompe disease, where conventional thinking is that glycogen accumulation in the diaphragm muscle causes hypoventilation.4,21
Potential mechanisms for the critical hypoventilation observed in the cases include an insufficient phrenic motor drive and diaphragm contractile dysfunction secondary to chronic MV support. Muscle-directed ERT is the only FDA-approved therapy for Pompe disease, but it has limited, if any, impact on the central nervous system.22 In the pivotal studies for ERT approval, respiratory endpoints included forced vital capacity and ventilator-free survival.6,23 However, studies of ERT in adults with Pompe disease showed no long-term improvements in respiratory function.9 Moreover, the majority of pediatric ERT-treated patients eventually required assistance.8 The limited effectiveness of ERT on ventilation indicates that adjunctive therapies may need to address the neuromuscular manifestations of Pompe disease.
The sustained improvement in ventilation reported here has not, to our knowledge, been documented previously with standard clinical care in people with severe Pompe disease who are chronically ventilator-dependent. Accumulating data (including the present report) indicate the importance of targeting pathology of the respiratory-related neurons and neural networks.10,12,16,24 If respiratory insufficiency was exclusively due to muscular contractile dysfunction secondary to GAA deficiency, direct muscle pacing would not acutely or chronically improve diaphragm activity or MV. Accordingly, the current findings are consistent with a role for a mixed neuromuscular pathology, versus restricted respiratory muscle dysfunction, in the etiology of ventilator-dependent Pompe disease.
An unexpected and novel finding is that chronic diaphragm stimulation, which was implemented to maintain alveolar ventilation, also may have elicited rehabilitative effects on the spontaneous, unpaced phrenic motor output. Specifically, EMG recordings suggest the patients' ability to voluntarily activate the diaphragm improved following pacemaker implantation. The progressively more robust diaphragm motor activation was associated with a greater ability to breathe independently. Similar findings have been reported in the literature on spinal cord injury, where anecdotal reports show that some patients with an initially paralyzed diaphragm can be weaned from diaphragm pacing to fully independent breathing after weeks to months.15,25 The EMG data are supported by observations that patients 1 and 2 do not need full-time pacing to sustain independent daytime ventilation. Although the EMG data must be interpreted with caution, we speculate that phrenic stimulation via diaphragm pacing could provide targeted rehabilitation of the neurons and networks that regulate breathing.
A few caveats should be noted. Our methodology cannot fully distinguish the effects of prolonged MV support from those of Pompe disease at baseline. High ventilator settings and control-mode MV inhibit respiratory drive26 and precipitate ventilator-induced diaphragm dysfunction, which is marked by diaphragm atrophy and contractile dysfunction20 with relatively spared neural function and synaptic transmission.27 Therefore, at least some of the neuromuscular and functional observations may have resulted from a reversal of MV-induced diaphragm fiber atrophy. Respiratory strengthening exercises may counter some manifestations of ventilator-induced diaphragm dysfunction in patients without neuromuscular diseases,18 but the existence of ventilator-induced diaphragm dysfunction and effects of preventative measures are not well understood in Pompe disease. Although respiratory exercises have been reported to improve maximal respiratory pressures in some patients with milder symptoms of Pompe disease,28 they did not alter strength or function in a sample of tracheostomized, chronically ventilator-dependent children.29 Likewise, our patients did not respond to the conventional weaning strategies and preoperative exercises typically used to facilitate weaning in the intensive care unit,18 suggesting that neuromuscular effects of Pompe disease at least partially contributed to the baseline diaphragm dysfunction.
As ventilatory function improved but was not fully corrected, we speculate that existing neurological impairments in respiratory motor control5,11,16 were relevant to the clinical outcomes in our case series. Denervation of some phrenic motor units, in conjunction with airway disease, likely contributed to patient 3's lower rate and extent of weaning. Also, patients 1 and 2 achieved long-term independence from daytime MV support following rehabilitation with pacing but still used nighttime MV. The central control of breathing in people with Pompe disease remains under active investigation and may play a role in the need for some nighttime ventilatory support.16,24 Further mechanistic study is recommended in this area. Consistent with preclinical data, a logical interpretation of these observations is that during spontaneous breathing, pacing improved activation and function of the remaining phrenic motor units (which have been shown to exhibit considerable pathology in Pompe disease, as reviewed by Fuller et al5). Earlier therapies targeted to neurons may be needed to prevent extensive glycogen accumulation and autophagy of the phrenic motor system.10
There were limitations to the approach. As the data were generated from 3 patients, we caution against generalizing these findings to the entire ventilator-dependent population with Pompe disease. In addition, the proximity of the intramuscular electrodes to the phrenic nerve insertions prevents us from distinguishing neural from muscular plasticity by pacing. Furthermore, the long-term safety and expected duration of the pacing benefit are undefined and warrant further controlled study.
In conclusion, using diaphragm pacing as an essential part of respiratory management, each patient achieved meaningful gains in ventilatory function. Diaphragm pacing may have potential in some cases to serve as a surrogate for MV and potentially promote rehabilitation of neural circuits and the diaphragm itself.25 Treatment strategies that eliminate glycogen storage in neural tissue will be needed for long-term improvement of ventilation.5,10
Footnotes
All authors contributed to data acquisition and interpretation, manuscript draft and revision, and approval of the submitted work. The corresponding author had full access to all data and accepts final responsibility for the article.
Dr Onders is cofounder of Synapse Biomedical, which manufactured the diaphragm pacing technology used in this report.
Training support to Dr Smith was provided by the National Institutes of Health (NICHD: K12HD055929). The authors are grateful to the Acid Maltase Deficiency Association (AMDA) for supporting this work.
References
- 1. Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease). Curr Mol Med. 2002;2:145–166. [DOI] [PubMed] [Google Scholar]
- 2. Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011;103:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Muller-Felber W, Horvath R, Gempel K, et al. Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord. 2007;17:698–706. [DOI] [PubMed] [Google Scholar]
- 4. American Association of Neuromuscular & Electrodiagnostic Medicine. Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve. 2009;40:149–160. [DOI] [PubMed] [Google Scholar]
- 5. Fuller DD, ElMallah MK, Smith BK, et al. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol. 2013;189:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. 2010;362:1396–1406. [DOI] [PubMed] [Google Scholar]
- 7. Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. [DOI] [PubMed] [Google Scholar]
- 8. Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. [DOI] [PubMed] [Google Scholar]
- 9. van der Ploeg AT, Barohn R, Carlson L, et al. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol Genet Metab. 2012;107:456–461. [DOI] [PubMed] [Google Scholar]
- 10. Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet. 2011;20:R61–R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeRuisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA. 2009;106:9419–9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hobson-Webb LD, Proia AD, Thurberg BL, et al. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab. 2012;106:462–469. [DOI] [PubMed] [Google Scholar]
- 13. Onders RP, Elmo M, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23:1433–1440. [DOI] [PubMed] [Google Scholar]
- 14. DiMarco AF. Restoration of respiratory muscle function following spinal cord injury: review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147:273–287. [DOI] [PubMed] [Google Scholar]
- 15. Posluszny JA, Jr, Onders R, Kerwin AJ, et al. Multicenter review of diaphragm pacing in spinal cord injury: successful not only in weaning from ventilators but also in bridging to independent respiration. J Trauma Acute Care Surg. 2014;76:303–309, discussion 309–310. [DOI] [PubMed] [Google Scholar]
- 16. ElMallah MK, Pagliardini S, Turner SM, et al. Ampakines stimulate respiratory motor output and ventilation in a murine model of Pompe disease. Am J Respir Cell Mol Biol. 2015;53:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- 18. Martin AD, Smith BK, Davenport P, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Onders RP, Dimarco AF, Ignagni AR, et al. Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery. 2004;136:819–826. [DOI] [PubMed] [Google Scholar]
- 20. Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37(10 suppl):S347–S353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Case LE, Beckemeyer AA, Kishnani PS. Infantile Pompe disease on ERT: update on clinical presentation, musculoskeletal management, and exercise considerations. Am J Med Genet C Semin Med Genet. 2012;160:69–79. [DOI] [PubMed] [Google Scholar]
- 22. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid α-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. [DOI] [PubMed] [Google Scholar]
- 24. Lee KZ, Qiu K, Sandhu MS, et al. Hypoglossal neuropathology and respiratory activity in Pompe mice. Front Physiol. 2011;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onders RP, Ponsky TA, Elmo M, et al. First reported experience with intramuscular diaphragm pacing in replacing positive pressure mechanical ventilators in children. J Pediatr Surg. 2011;46:72–76. [DOI] [PubMed] [Google Scholar]
- 26. Colombo D, Cammarota G, Bergamaschi V, et al. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 2008;34:2010–2018. [DOI] [PubMed] [Google Scholar]
- 27. Radell PJ, Remahl S, Nichols DG, Eriksson LI. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med. 2002;28:358–364. [DOI] [PubMed] [Google Scholar]
- 28. Jones HN, Crisp KD, Robey RR, et al. Respiratory muscle training (RMT) in late-onset Pompe disease (LOPD): effects of training and detraining. Mol Genet Metab. 2016;117:120–128. [DOI] [PubMed] [Google Scholar]
- 29. Smith BK, Collins S, Conlon T, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013;24:630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]