Abstract
The lack of cell retention clearly represents a potentially serious limitation for therapeutic efficacy of stem cells. To enhance the efficacy, we developed a novel hydrogel that is thermosensitive and biodegradable and possesses desirable stiffness in a solid form. Immediately after induction of myocardial infarction of male rat, cardiac outgrowth cells embedded in hydrogel (HG) or saline (CO) were injected directly into the peri-infarct area. Left ventricular ejection fraction, cell retention rate, and a spectrum of biochemical markers were measured to evaluate the effect of the treatment. Left ventricular ejection fraction was significantly higher in the cell-injected groups (HG and CO) than in the control group at 1 week after treatment. This functional benefit was continued only in the HG group, accompanied with more retained cells. Furthermore, the expression of insulin-like growth factor-1 was significantly higher in the HG group with less progression of cell apoptosis.
Keywords: Insulin-like growth factor-1, retention rate, micro-computed tomography, paracrine effect, stiffness
Introduction
Among the many cell types used to treat patients with heart disease, cardiac stem cells are attractive candidates because they are of cardiac origin,1 can be cultured from autologous cardiac tissue without special techniques,2 and have been reported to differentiate into heart cells.3,4 In addition, recent phase-1 clinical trials have demonstrated their safety and modest efficacy.5,6 However, a major limitation of cardiac stem cell therapy has been the low number of retained cells after administration. Direct intramyocardial injection was reported to be the best route to facilitate early retention of the administrated cells.7 However, when labeled stem cells are injected into the myocardium, as many as 90% disappear by 24 h after injection, and frequently, none can be found at 3 weeks. In addition, the inflammatory environment of the acute infarct area is often too intense for cell survival and proliferation. Recently, many researchers have attempted administration of cells embedded in a hydrogel to improve functional recovery of the heart.8–12 Importantly, one-fifth of the injected cells are rapidly lost from the needle hole at the injection site or washed out by blood flow.13 Therefore, an intra-muscular injectable gel that can solidify immediately after injection is required.
Recently, we developed a thermosensitive and biodegradable hydrogel that can last for 4–8 weeks.14,15 It exists in liquid form at cold temperatures and immediately turns to a solid at temperatures >22°C. We designed the stiffness of the solid form to be 30–80 kPa, which is similar to that of the left ventricle (LV) of a normal heart. To promote cell survival, we conjugated catalase, an oxygen-producing enzyme, into the hydrogel. In our previous study of cardiac outgrowth cell cultures in hydrogels, we found that our hydrogel was capable of loading a cytokine, insulin-like growth factor-1 (IGF-1),15 and its formulation upregulated the messenger RNA (mRNA) levels of the mature cardiac markers, cardiac troponin-T, and cardiac myosin heavy chain, in vitro.16
Here, we sought to determine if cardiac outgrowth cells embedded in our hydrogel were able to enhance functional recovery following acute myocardial infarction in a rat model.
Materials and methods
Animal experimentation protocol
Experimental animals were treated in compliance with the institutional guidelines for animal experimentation of the Institutional Animal Care and Usage Committee (IACUC) of Juntendo University, School of Medicine. All experimental procedures were approved by IACUC of Juntendo University.
Cell culture
The green fluorescent protein (GFP)-induced male Sprague-Dawley rats (SD-Tg(CAG-EGFP); Sankyo Lab Inc., Tokyo, Japan) were used. The heart was isolated under anesthesia and rinsed with phosphate-buffered saline (PBS; Wako, Tokyo, Japan) containing heparin sodium to wash out the blood. The atrium of the heart was diced into small pieces and was subjected to 10-min digestion with 0.05% trypsin-EDTA (Sigma-Aldrich, Tokyo, Japan). These explants were plated onto fibronectin-coated dishes (BD Biosciences, Tokyo, Japan) in Iscove’s modified Eagle medium (IMDM; Life Technologies, Tokyo, Japan) supplemented with 20% fetal bovine serum (FBS; Thermo Scientific, Yokohama, Japan), 1% penicillin-streptomycin (Life Technologies), and 2 mM l-glutamine (Life Technologies). Two weeks later, a primary outgrowth of adherent cells grew out radially in monolayer from the cardiac tissue. The outgrowth cells were then harvested to continue to culture to second passage. The cells were stored at −80°C and transferred to liquid nitrogen until thawed 1 week before injection.
Hydrogel
The hydrogels were based on polycaprolactone, N-isopropylacrylamide, 2-hydroxyethyl methacrylate, and dimethyl-g-butyrolactone acrylate. The detailed characteristics of the hydrogel polymer have been described previously.16 The powder form of the hydrogel polymer was dissolved in PBS at 4°C to form a 20% solution, which was then mixed with type I collagen powder (Sigma-Aldrich) to yield a final polymer concentration of 8%. The hydrogel and collagen mixture was stirred at 4°C overnight. The hydrogel is thermosensitive; it is liquid at 4°C, can pass through 26-gauge needles, and turns into a gel immediately at 37°C. The stiffness of the gel is 31.4 kPa.
Cell preparation for injection
For injection of cells within the hydrogel, 1.0 × 107 cells from the outgrowth culture were mixed with 2 mL of hydrogel on ice. For injection of cells alone, the same number of cells was mixed with 2 mL of PBS instead of hydrogel.
Myocardial infarction
Male Sprague-Dawley rats at 8 weeks of age (weight: 240–280 g) were anesthetized by intraperitoneal injection of sodium pentobarbital (Somnopentyl; Kyoritsu Seiyaku, Tokyo, Japan; 30 mg/kg). After anesthesia, each rat was intubated with a 14-gauge angiocatheter (Terumo, Tokyo, Japan). Artificial ventilation was then started with a tidal volume of 4.5 mL and a respiration rate of 70 breaths/min. A lateral incision was made to approach the heart, followed by ligation of the coronary artery with a 7-0 monofilament suture (Prolene; Johnson and Johnson Japan, Tokyo, Japan). Immediately after ligation, 70 µL of cells only (CO) or cells suspended in hydrogel (HG) were injected into the peri-infarct area with a 26-gauge needle. As a control (CTL), an equivalent volume of PBS was injected.
Micro-computed tomography
Cardiac function was measured by micro-computed tomography (mCT) on the day of, 1 week, and 4 weeks after surgery. The rat was anesthetized with the same method described above, and body weight was measured to determine the volume of the contrast agent. Detailed procedure for the measurement of heart function was described previously.17 Briefly, the rat was set in the mCT equipment (Latheta LCT-200; Hitachi-Aloka Medical, Tokyo, Japan) with a 120-mm holder. The iodized contrast agent (Bystage 370; Teva Pharma, Tokyo, Japan) was intravenously administrated (150 µL/min) through a 24-gauge angiocatheter. When the dose of the contrast agent reached 20 mL/kg, image acquisition was started. The image acquisition of mCT was set to “cardiac synchronized” mode with 480 µm of disk wideness and thickness.
Histological analysis
Under anesthesia, the heart was perfused with 50 mL of PBS containing 1000 U of heparin sodium (Mochida Pharma, Tokyo, Japan) followed by 4% paraformaldehyde (PFA; Sigma-Aldrich). Cross sections of the heart, including the ligation site (base), apex, and the areas in between, were obtained and immersed in 4% PFA for 30 min. The sections were dehydrated with 10%, 15%, and 20% sucrose solutions in succession, embedded into the optimum cutting temperature (O.C.T) compound (Sakura Finetek, Tokyo, Japan), and stored at −80°C. Each section was cut into 5-µm slices using a cryostat and mounted on a glass slide.
To determine the size of the infarction, Masson’s trichrome staining was performed on hearts excised at 4 weeks after surgery. After being immersed in Bouin’s solution (Muto Pure Chemicals, Tokyo, Japan) overnight at room temperature, the slides were processed using a staining kit (Sigma-Aldrich) according to manufacturer’s instructions. The infarct area was determined using the NIH ImageJ software. The blue area (collagen) was divided by whole LV area excluding the right ventricular wall. To normalize the infarct size, three sections from different parts of the heart (see above) were analyzed, and the average was considered as the percentage of infarction for each heart.
To detect the apoptotic cells, TdT-mediated dUTP nick end labeling (TUNEL) staining was performed using heart sections dissected at 1 week after surgery. The In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN, USA) was used.
To determine the retention rate of the injected cells, the section at 1 week after surgery was double stained using rhodamine-phalloidin (Sigma-Aldrich) for filamentous actin and 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies) for nuclei. A whole image of the cross-sectioned LV was captured using “image stitching” software (Keyence, Tokyo, Japan) at 400× magnification, which allowed for high-resolution imaging of the cells. The area of GFP-positive cells in the whole LV was calculated using NIH ImageJ software.
Reverse transcription polymerase chain reaction
The heart was harvested at 1 week and 4 weeks after surgery, for RNA analysis. The peri-infarct area including the infarct area was excised and immersed in RNAlater solution (Life Technologies). The tissue was reverse-transcribed (iScript cDNA Synthesis Kit; Bio-Rad, Tokyo, Japan) to form complementary DNA (cDNA). The polymerase chain reaction (PCR) and analysis were carried out using HT-7700 (Life Technologies) with SYBR-green kit (Life Technologies). Primers were designed to amplify and quantify vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-2 (VEGF-R2), angiopoietin-like 2 (ANGPTL-2), beta-fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), IGF-1, and platelet-derived growth factor beta (PDGF-β). The relative abundance of target genes was obtained by calculating gene-specific mRNA abundance relative to a time zero heart, and normalized to an internal control (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression).
Statistical analysis
All data are shown as the mean ± standard deviation (SD). Comparisons of heart function, infarction area analysis on Masson’s staining, and expression of mRNA were first analyzed using the Kruskal–Wallis test then post hoc analyzed using the Steel–Dwass test. Densitometry analysis of Western blotting was performed by Welch’s t-test using commercially available software (Statcel 3; O.M.S. publisher, Saitama, Japan). A value of p < 0.05 is considered as statistically significant.
Results
Cardiac function
Figure 1 shows the time course change in left ventricular ejection fraction (LVEF) at baseline (immediate after surgery), 1 week, and 4 weeks after surgery. There were eight animals in each group. The baseline LVEF was not significantly different among the control (CTL), CO, and the cells injected with hydrogel (HG) groups (16.9% ± 4.2%, 18.4% ± 6.6%, 19.1% ± 4.5%; CTL, CO, and HG; p = not significant (NS)). While LVEF in the CTL group deteriorated over the 4 weeks, the other two groups showed improvement 1 week after cell treatment (14.8% ± 1.4%, 25.9% ± 7.3%, 25.1% ± 6.1%; CTL, CO, and HG; p < 0.01 in CTL vs CO and in CTL vs HG). At 4 weeks after surgery, the HG group showed further improvement in LVEF compared to 1 week (28.9% ± 5.0%). On the other hand, the functional benefit in the CO group observed at 1 week diminished during this period (21.6% ± 7.7%). In addition, the CTL heart showed further reduction in ejection fraction at 4 weeks (13.6% ± 3.5%).
Figure 1.
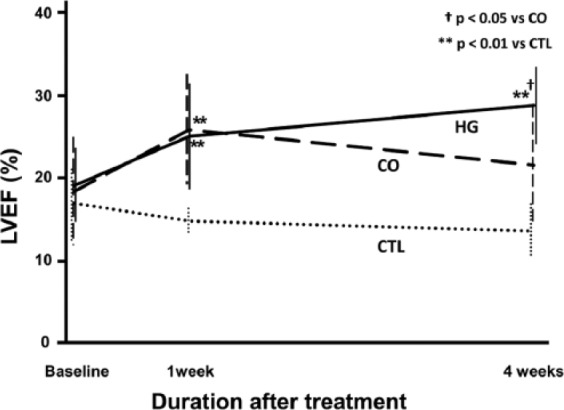
Cardiac function. A time course of changes in left ventricular ejection fraction (LVEF) following injection of cardiac cells into infarcted rat hearts. The baseline (immediately after surgery) values among treatment groups were not significantly different. At 1 week after treatment, although the LVEF of the control (CTL; PBS only) hearts decreased, the cell-injected hearts showed similar LVEF improvements. At 4 weeks after treatment, although the LVEF of cells in hydrogel (HG)-injected hearts increased, the LVEF of cells only (CO)-injected hearts decreased. Furthermore, only HG showed significant increase of LVEF between baseline and 4 weeks (+11.8% ± 8.0%, p < 0.01). In addition, the CTL hearts showed a further decrease in LVEF after 1 week (n = 8).
Infarction size
The heart was sectioned to semi-quantify infarct area by Masson’s trichrome staining at 4 weeks after surgery (Figure 2). The infarct area was significantly smaller in HG (13.8% ± 3.3%) than in CTL (28.8% ± 7.1%; p < 0.05). Infarct area in CO (20.6% ± 7.0%) was not significantly different, although the trend indicates that it was smaller than that of CTL.
Figure 2.
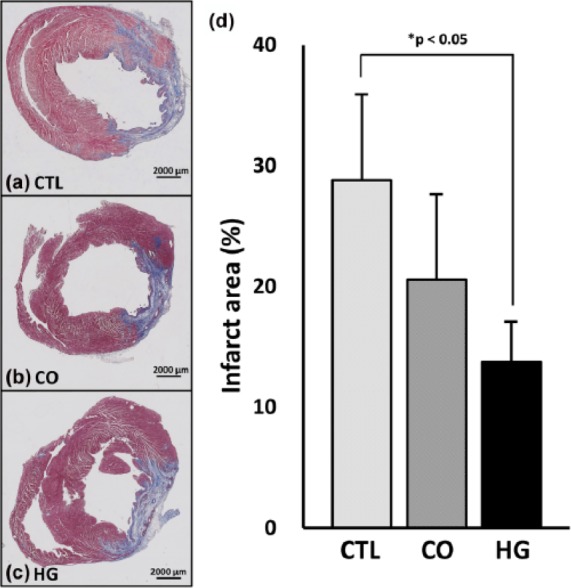
Infarct area. (a–c) Representative images of Masson’s trichrome–stained sections at 4 weeks after treatment (bar = 2000 µm). (d) Area analysis of infarction size (n = 5, each) demonstrated that there was no significant difference between the control (CTL) and cells only (CO) groups. In contrast, the cells in hydrogel (HG) group showed a significantly smaller infarct size than the CTL group (p < 0.05), although there was no significant difference compared to the CO group (p = 0.09).
Cell retention
The heart sections of CO and HG at 1 week were co-immunostained with F-actin and DAPI (Figure 3(a) and (b)). Since the injected cells were positive for GFP, the ratio of GFP-positive cells in the whole section was calculated. The ratio of GFP-positive cells per unit area was 3.5-fold more in HG compared to CO (0.8% ± 0.8% in CO vs 2.9% ± 0.5% in HG; p < 0.05, Figure 3(c)).
Figure 3.
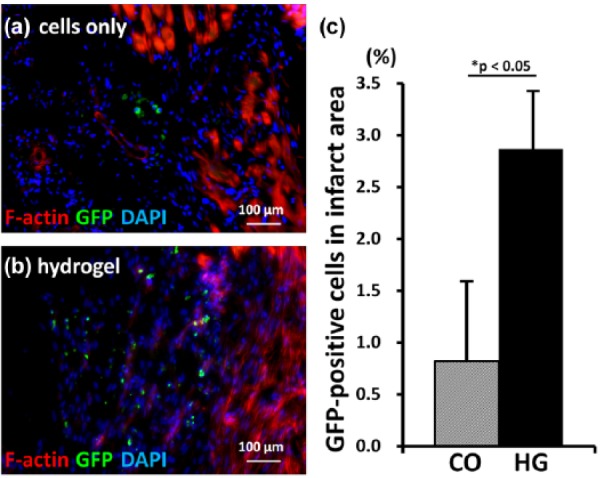
Injected cell retention. (a and b) Images of heart sections at 1 week after cell injection are shown (400×). In the peri-infarct area, actin fibers were deformed due to myocardial infarction (left side). More injected (GFP-positive) cells were observed in the hearts of the cells in hydrogel (HG) group rats than in those of the cells only (CO) group rats (bar = 100 µm). (c) Semi-quantitative analysis demonstrated that the number of GFP-positive cells in the infarct area was 2.8-fold higher in the HG group than in the CO group (n = 5).
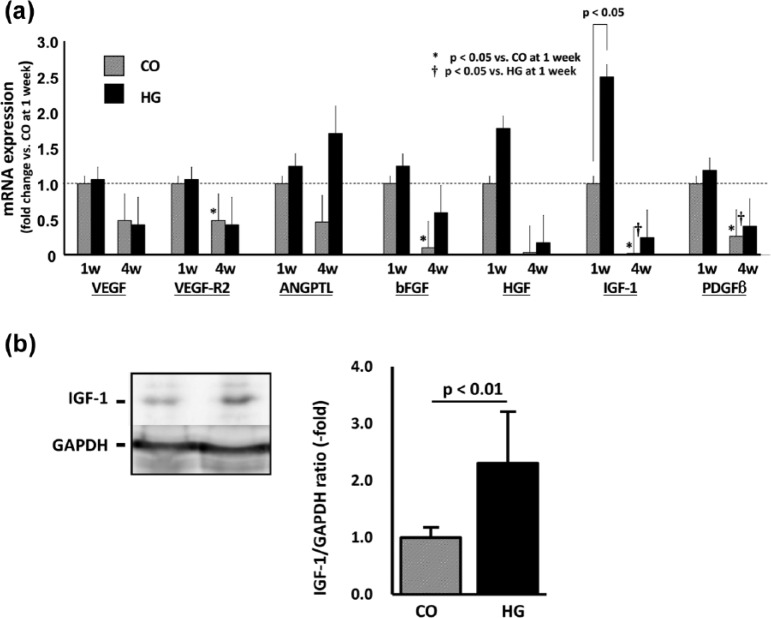
Cytokine expression in infarcted area
To evaluate the causation of functional benefit, the expression of tissue cytokines was measured using reverse transcription polymerase chain reaction (RT-PCR) (Figure 4). Although all the cytokines showed increasing trends in the HG group when compared to their expression in the CO group at 1 week, only IGF-1 showed a significant increase (2.5 ± 0.6-fold; p < 0.05). At the 4-week time-point, expression tended to be reduced in all genes except for ANGPTL-2 of the HG group. In addition, the expression of VEGF-R2 and bFGF in the CO group and IGF-1 and PDGF-β2 in both the CO and HG groups were significantly less at 4 weeks compared to their respective counterparts at the 1-week time-point (p < 0.05, n = 5 each).
Figure 4.
Cytokine expression. (a) The expression of cytokines mRNA in the peri-infarct area was measured at 1 and 4 weeks after treatment and is shown as the fold change based on the expression in the cells only (CO) at 1 week. Only IGF-1 showed a significant increase (2.5-fold; p < 0.05, n = 5). At 4 week, the expressions of VEGF-R2 and bFGF in CO and IGF-1 and PDGF-β2 in both CO and HG were significantly less compared to that at 1 week (p < 0.05, respectively, n = 5 each). (b) The expression of IGF-1 protein at 1 week was also significantly higher in HG group compared to that in CO group (2.3-fold; p < 0.01, n = 6 each).
In RT-PCR analysis, only IGF-1 mRNA was increased significantly in the HG group compared to that in the CO group at 1 week. We further evaluated the expression of IGF-1 at the protein level (Figure 4(b)). Western blotting results demonstrated that the expression of IGF-1 was significantly higher in the HG group at the 1-week time-point (2.3 ± 0.9-fold vs CO, p < 0.01).
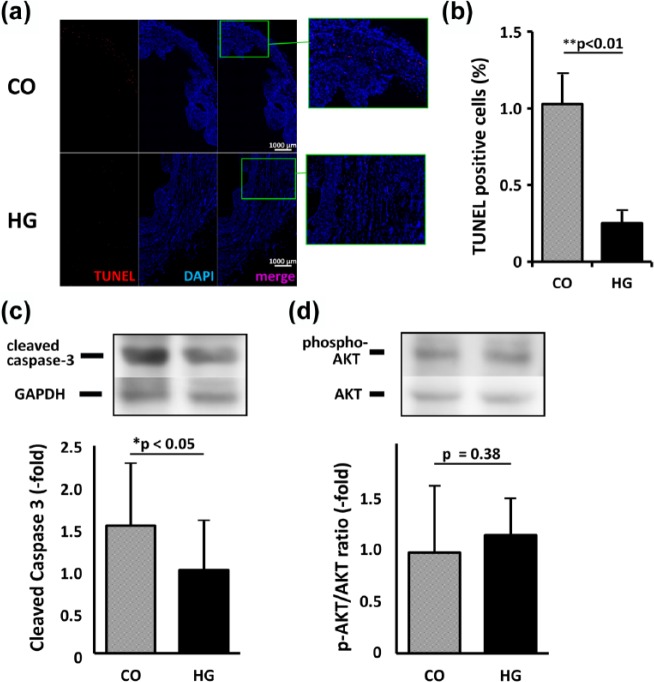
Attenuated cell apoptosis in the peri-infarct area
The HG-injected heart contained fewer TUNEL-positive cells than the CO heart at 1 week after treatment (Figure 5(a) and (b)). Densitometric analysis of the bands on Western blotting showed that the expression of cleaved caspase-3, a marker of cell apoptosis, was 1.54-fold higher in the CO group than in the HG group (p < 0.05, Figure 5(c)). In addition, the ratio of phospho-AKT to total AKT was higher in the HG group (1.17-fold); however, the difference did not reach statistical significance (p = 0.38, Figure 5(d)).
Figure 5.
Apoptosis in heart tissue. (a) The composite images of the heart stained with TUNEL (red) and DAPI (blue) were shown. When compared to the heart injected with cells only (CO), fewer TUNEL-positive cells were observed in the heart injected with cells in hydrogel (HG) (bar = 1000 µm). (b) The ratio of TUNEL-positive cells per nuclei (DAPI-positive) was 1.03% ± 0.56% versus 0.25% ± 0.19% for CO versus HG, respectively (p < 0.01). (c and d) Quantitative analysis of the bands in the Western blot revealed that the expression of cleaved caspase-3 was significantly higher in the CO group than in the HG group (1.54-fold, p < 0.05). (c) Although the ratio of phosphorylated AKT to total AKT tended to be higher in the HG heart, the difference was not statistically significant (1.17-fold, p = 0.38).
Discussion
The hydrogel was originally developed in a previous study, wherein it yielded promising results in in vitro experiments.16 In this study, we tested the efficacy of the gel for cell therapy of myocardial infarction in a rat model. The most important finding of our study is that compared to the control, hearts implanted with cells, in both saline buffer and hydrogel, showed a functional benefit at 1 week after treatment. However, this benefit in cardiac function only persisted in the group treated with cells suspended in hydrogel. In the cells in hydrogel-injected group, we found increased cell persistence and enhanced expression of protective cardiac cytokines relative to the group injected with cells in PBS, suggesting that the cardiac function enhancement was related to improved cell retention. An additional potential benefit of the hydrogel, with regard to its comparable stiffness to normal myocardium, may be its splinting effect on injured myocardium, which would otherwise be stretched by the force of the adjacent normal myocardium.
One of the benefits of stem cell therapy is its preventative effect on cardiac remodeling after myocardial injury. Since cardiac function continues to deteriorate 1 week after myocardial injury,18 sustained secretion of cytokines is thought to be an important factor in preventing further remodeling of the injured heart. Among the methods for administering cardiac stem cells, direct injection into the cardiac muscle, although comparatively invasive, has proven beneficial. Nonetheless, the cell retention rates following injection have been strikingly low, with reported retention rates of less than 20% at 1 h and 5.3% at 3 weeks.13 Further studies suggest that 20% of the injected cells would be lost from the needle hole, 70% of the remaining cells would be washed out by blood flow, and another 5% would die at the injection site, probably due to severe local ischemia or an intense inflammatory response. Many attempts have been made to improve cell retention, using fibrin glue,13 hydrogel,8–10 magnets,19,20 or cell sheets.21,22 In this study, we used an injectable, thermosensitive hydrogel. The hydrogel is in a liquid form on ice and is immediately solidified within 5 s at 37°C. This distinctive feature allowed the cells to be injected with a narrow (26-gauge) needle and prevented the loss of cells at the injection site and into the blood stream. Furthermore, in its solid state, the stiffness of our hydrogel is designed to be approximately 31 kPa, which is more favorable for mature cardiac genes (myosin heavy chain-6 and troponin-T) compared to softer (5 kPa) or harder (63 kPa) environments.16 IGF-1 is known to play an important role in repairing the damaged tissue by inhibiting apoptosis and enhancing proliferation. Our results demonstrate that IGF-1 was significantly upregulated in the HG group at 1 week after treatment compared to its expression in the controls. Although the expression of the other tested genes did not increase, which is consistent with our previous results,23 apoptosis was significant lower in the hearts injected with cells in hydrogel than in the hearts injected with CO. In addition, this cascade might not depend on the AKT cascade. According to our previous in vitro studies, our biodegradable hydrogel persists for 4–8 weeks. In this in vivo study, the gel was present in the rat heart when it was excised at 1 week after treatment, but it was not detectable at 4 weeks after treatment, suggesting that enhanced degradation occurred in the current in vivo conditions.
Previous studies demonstrated that c-Kit-positive cells cultured from cardiac specimen even with1,4,18,19 or without3 cardiosphere-forming method has an ability to differentiate into cardiac components cells. Two recent clinical studies using c-Kit-positive cells5 and cardiosphere-derived cells6 showed that both type of the cells had beneficial effects on cell therapy. Furthermore, a comparison of various cell therapeutic approaches for myocardial injury showed that the functional benefit was greater when the whole portion of cardiac outgrowth cells was used than when more processed cells were used.24 In this study, we used cardiac outgrowth cells containing 5%–15% c-Kit-positive cells as the source of the cells. In contrast, in other clinical trials using non-cardiac stem cells, myoblast cells,20 or endothelial progenitor cells co-cultured with fibroblasts,25 a modest recovery of cardiac function was also shown. In addition, recent study suggested that the c-Kit-positive population barely contributed to cardiac regeneration.26 These results, combined with the rapid disappearance of cells in animal studies, suggest that a paracrine effect may be the main factor in cardiac recovery. In our study, we found that double-positive cells (GFP and F-actin) rarely existed at 1 week after infarction. Although it may be argued that the cells might not have had enough time to differentiate into mature cardiomyocytes, our results seem to be more consistent with the hypothesis that recovery of function is mainly due to a paracrine effect.
Our findings raised a question for future study. First, when we measured cardiac function at 8 weeks after treatment, in the cells in hydrogel-injected heart, we observed that the LVEF effect was sustainable; however, it did not increase further. Since our hydrogel had disappeared by 4 weeks after treatment, these data raise the question of whether a long-lasting hydrogel might provide a further recovery of heart function. Second, since our hydrogel was easy to inject, it would seem to be potentially relevant for clinical applications with current imaging technologies. However, we have not yet evaluated any possible long-term adverse effects, including toxicity, resulting from the injection of a cold material into the heart.
Conclusion
Our thermosensitive, stiffness-controlled hydrogel was suitable for use in cell therapy for the treatment of ischemic myopathy in the rat heart due to the enhancement of cell retention and increased IGF-1 release.
Acknowledgments
The authors thank Mrs Yuko Kojima for correcting English in this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Young Scientists (Grant number: 23700485).
References
- 1. White AJ, Smith RR, Matsushita S, et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur Heart J 2013; 34: 68–75. [DOI] [PubMed] [Google Scholar]
- 2. Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004; 95: 911–921. [DOI] [PubMed] [Google Scholar]
- 3. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114: 763–776. [DOI] [PubMed] [Google Scholar]
- 4. Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007; 115: 896–908. [DOI] [PubMed] [Google Scholar]
- 5. Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011; 378: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012; 379: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li SH, Lai TY, Sun Z, et al. Tracking cardiac engraftment and distribution of implanted bone marrow cells: comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg 2009; 137: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 8. Hashizume R, Fujimoto KL, Hong Y, et al. Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia-reperfusion injury: a preclinical study of a porous polyurethane material in a porcine model. J Thorac Cardiovasc Surg 2013; 146: 391.e1–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu WN, Lü SH, Wang HB, et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Part A 2009; 15: 1437–1447. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Sakakibara Y, Nishimura K, et al. Improved therapeutic efficacy in cardiomyocyte transplantation for myocardial infarction with release system of basic fibroblast growth factor. Artif Organs 2003; 27: 181–184. [DOI] [PubMed] [Google Scholar]
- 11. Yao X, Liu Y, Gao J, et al. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 2015; 60: 130–140. [DOI] [PubMed] [Google Scholar]
- 12. Nakajima K, Fujita J, Matsui M, et al. Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and angiogenesis to ameliorate cardiac function after myocardial infarction. PLoS One 2015; 10: e0133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terrovitis J, Kwok KF, Lautamäki R, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol 2008; 52: 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan J, Stankus JJ, Wagner WR. Development of composite porous scaffolds based on collagen and biodegradable poly(ester urethane)urea. Cell Transplant 2006; 15(Suppl. 1): S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang F, Li Z, Tamama K, et al. Fabrication and characterization of prosurvival growth factor releasing, anisotropic scaffolds for enhanced mesenchymal stem cell survival/growth and orientation. Biomacromolecules 2009; 10: 2609–2618. [DOI] [PubMed] [Google Scholar]
- 16. Li Z, Guo X, Matsushita S, et al. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 2011; 32: 3220–3232. [DOI] [PubMed] [Google Scholar]
- 17. Matsushita S, Naito M, Amano A. Micro-computed tomography provides accurate measurement for cardiac function in infarcted rat heart. Open J Med Imag 2014; 4: 72–79. [Google Scholar]
- 18. Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol 2011; 57: 455–465. [DOI] [PubMed] [Google Scholar]
- 19. Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res 2010; 106: 1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugioka T, Ochi M, Yasunaga Y, et al. Accumulation of magnetically labeled rat mesenchymal stem cells using an external magnetic force, and their potential for bone regeneration. J Biomed Mater Res A 2008; 85: 597–604. [DOI] [PubMed] [Google Scholar]
- 21. Memon IA, Sawa Y, Fukushima N, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg 2005; 130: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 22. Shimizu T, Yamato M, Kikuchi A, et al. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng 2001; 7: 141–151. [DOI] [PubMed] [Google Scholar]
- 23. Wang F, Li Z, Khan M, et al. Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomater 2010; 6: 1978–1991. [DOI] [PubMed] [Google Scholar]
- 24. Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One 2009; 4: e7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi H, Shimizu T, Yamato M, et al. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J Artif Organs 2008; 11: 141–147. [DOI] [PubMed] [Google Scholar]
- 26. Van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014; 509: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]