Abstract
It remains hotly debated whether latitudinal diversity gradients are common across taxonomic groups and whether a single mechanism can explain such gradients. Investigating species richness (SR) patterns of European land plants, we determine whether SR increases with decreasing latitude, as predicted by theory, and whether the assembly mechanisms differ among taxonomic groups. SR increases towards the south in spermatophytes, but towards the north in ferns and bryophytes. SR patterns in spermatophytes are consistent with their patterns of beta diversity, with high levels of nestedness and turnover in the north and in the south, respectively, indicating species exclusion towards the north and increased opportunities for speciation in the south. Liverworts exhibit the highest levels of nestedness, suggesting that they represent the most sensitive group to the impact of past climate change. Nevertheless, although the extent of liverwort species turnover in the south is substantially and significantly lower than in spermatophytes, liverworts share with the latter a higher nestedness in the north and a higher turn-over in the south, in contrast to mosses and ferns. The extent to which the similarity in the patterns displayed by spermatophytes and liverworts reflects a similar assembly mechanism remains, however, to be demonstrated.
The existence of a latitudinal diversity gradient (LDG) peaking near the equator and decreasing towards the poles has been a persistent feature during the history of life on earth1 (but see ref. 2). This gradient has been quoted as one of the few laws in ecology3, and it demonstrates remarkable consistency across geographic areas, scales, habitats, and taxonomic groups4,5,6,7. Mounting evidence suggests that this convergence of distribution patterns across taxonomic groups is due to environmental forcing8.
On the one hand, macroclimate (primarily energy and water) is postulated to control species richness through the ecological sorting of regional and global species pools according to species climatic tolerances or by affecting rates of speciation9. In particular, dry or cold environments are specifically challenging for plants because adaptations must evolve to enable the tolerance or avoidance of extremely low water potentials10. In lineages that have successfully adapted to high-latitude environments, increased seasonal variability at higher latitudes is assumed to have resulted in broader thermal tolerances and consequently larger ranges: i.e., Rapoport’s rule11.
In addition, habitat heterogeneity promotes species richness because diverse habitats allow greater niche separation and therefore promote species coexistence12,13,14. Habitat heterogeneity, when represented by topographic heterogeneity, is also related to historical factors because mountains facilitate the long-term survival of species by allowing climate tracking via short-distance altitudinal migration15.
Investigating the factors determining the distribution of 1016 European plant species, Normand et al.16 confirmed the key role of extant climate and also demonstrated the crucial role of historical factors. Decreased extinction and increased speciation in climatically stable areas is expected to contribute to shaping extant distribution patterns17,18. For example, the increase in dung beetle species richness towards lower latitudes in Europe results from both the orderly exclusion of species towards the north, resulting in a higher nestedness of northern assemblages compared with southern assemblages and from the high species turnover caused by steep ecological gradients in southern areas19. In addition to climate stability, the time required for colonisation can also contribute to the observed latitudinal species richness gradient13,15. In particular, latitudinal species richness gradients might result from the incomplete post-glacial recolonisation of high-latitude regions. In this context, less mobile organisms are expected to exhibit steeper latitudinal species richness gradients than vagile organisms20.
There are, however, some notable exceptions to these patterns, as groups originating during warmer periods of earth’s history display a steeper latitudinal gradient, whereas groups originating during colder periods display a shallower diversity gradient due to a weak affinity or no affinity for lower latitudes21. For example, grasses are among the relatively few higher-order lineages that exhibit a shallow, atypical latitudinal gradient due to the climatic specialisation of particular lineages to cold and arid environments22. Similarly, although approximately 50% of extant gymnosperm species occur primarily between the tropics, the diversity of gymnosperms decreases at equatorial latitudes23.
A latitudinal species richness gradient was observed in spermatophytes24 and in ferns25 wherein, however, regional species richness patterns do not always correlate with latitude26,27, and even challenged in bryophytes, a group with approximately 20,000 species of mosses, liverworts, and hornworts, which represents the second most speciose lineage of land plants after the angiosperms. Similar levels of bryophyte species richness have been repeatedly reported from tropical and extra-tropical areas28,29,30,31, and Rozzi et al.32 even documented an inverted bryophyte species richness gradient that increased towards the pole in southern south America. Such weak, if not inverted, gradients of species richness towards high latitudes appear consistent with three major features of bryophyte biology. First, bryophytes typically fail to radiate in contrasted environments33, reducing their opportunities to diversify along the steep ecological gradients found at low latitudes. Second, bryophyte species, including tropical species, are inherently better adapted to cold conditions than are angiosperms34. They are universally able to grow at low temperatures, showing a growth reduction of less than 50% at 5 °C compared with growth at their optimal temperatures35. Simultaneously, because they are poikilohydric, they are much less well equipped to face drought and warm conditions. Bryophytes therefore exhibit lower temperature optima than higher plants35; all temperate and boreal species investigated by Furness & Grime35 died when kept continuously at 35 °C, and most shoots died at > 30 °C. Third, bryophyte species display larger geographic ranges than angiosperm species and display a high dispersal capacity, resulting in a much lower global rate of species turn-over than angiosperms36, suggesting that rapid post-glacial recolonisation prevents the formation of the richness gradients that result from limited post-glacial dispersal processes, as has been observed in vascular plants15.
Here, we examined the spatial variation in species richness and beta-diversity (disentangling its two components: species replacement or turnover, and species loss or nestedness) in European bryophytes and compared them with those observed in ferns and spermatophytes. Specifically, we tested three hypotheses: 1) as a result of differences in temperature optima and drought tolerance between bryophytes and spermatophytes, bryophyte species richness should decrease towards lower latitudes, whereas spermatophytes should display the opposite pattern; 2) given the failure of bryophytes to radiate in ecologically contrasted areas with a long history of climate stability such as the Mediterranean, and given their large range sizes, the spatial turnover of bryophyte richness across latitudinal gradients should be less pronounced than in spermatophytes; 3) in spermatophytes, nestedness should increase in the north as a result of limitations in the ability to evolve adaptations to cold conditions and the consequent ordered loss of species towards the north, whereas species turn-over should increase in the south. In bryophytes, we expect to observe a reverse pattern as a result of the inherent cold tolerance in the north and the filtering-out of species towards the south.
Results
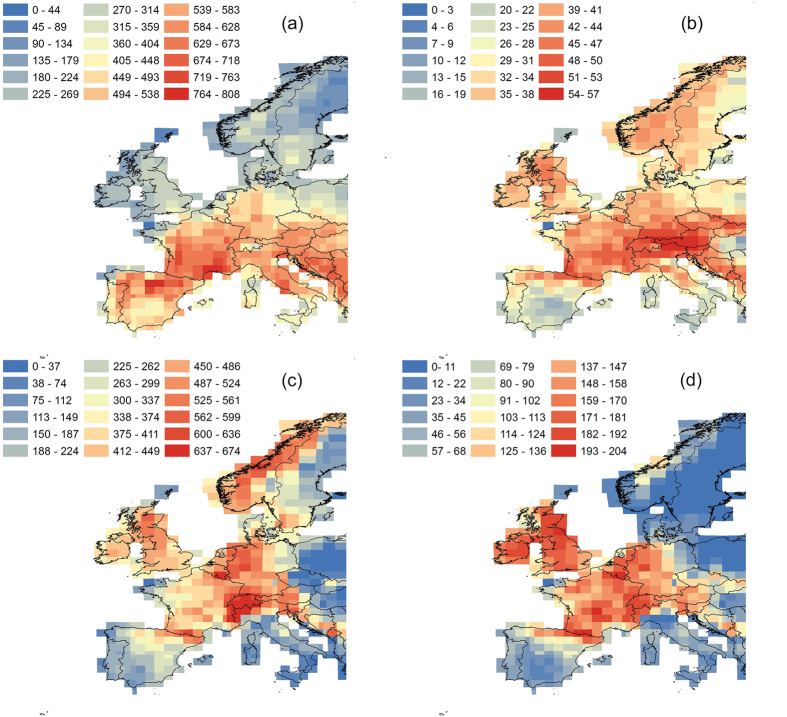
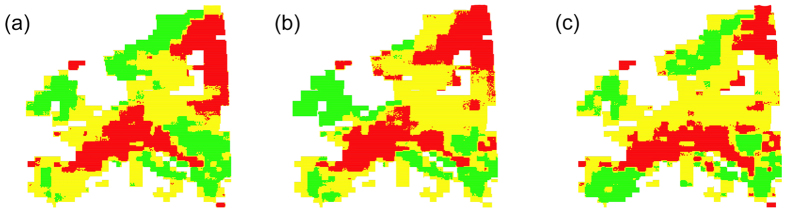
Consistent with our first hypothesis, European ferns, mosses, liverworts and spermatophytes exhibited contrasting patterns of species richness around a central axis running approximately through the chains of the Pyrenees and the Alps, close to the 46th parallel (Figs 1 and 2). Ferns, mosses and liverworts exhibited a similar impoverishment of species richness towards the Mediterranean and a peak of richness at mid-latitudes that reaches Scandinavia in ferns and mosses, but not in liverworts (Figs 1 and 2). Thus, regions of significant spatial association in the distribution of the four taxonomic groups are distributed along this central axis, whereas negative associations are found southwards and northwards of it (Fig. 3).
Figure 1. Potential species richness of spermatophytes (a), ferns (b), mosses (c) and liverworts (d) across Europe.
Maps based on ensemble stacked species distribution models (S-SDMs) of 1359, 79, 810 and 224 species of spermatophytes, ferns, mosses and liverworts, respectively. Maps generated by R.G. Mateo using the ArcMap extension in ArcGIS 10.2 (ESRI Inc., Redlands, CA, USA, http://www.esri.com).
Figure 2. Predicted numbers of species of spermatophytes (green), ferns (black), mosses (red) and liverworts (blue) in 100 km latitudinal bands across Europe.
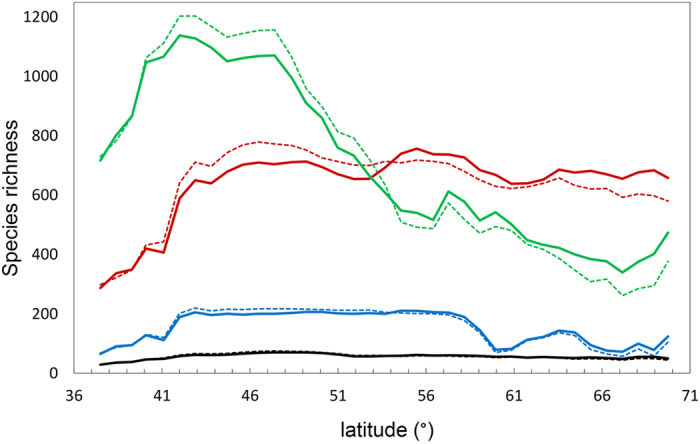
Dashed lines indicate crude SR values predicted by S-SDMs, solid lines correspond to SR values normalized according to species-area relationships (see Supplementary Methods 5).
Figure 3. Correlation between the species richness of taxonomical groups across Europe corrected for spatial autocorrelation, as measured by re-scaled Lee’s L bivariate spatial association.
Regions of significant spatial association using a Monte Carlo test on Lee’s statistic at the 95% level. ‘Positive’ indicates values of the Lee’s statistic ranked in the top 97'5% of Monte Carlo values, whilst ‘Negative’ indicates a statistic ranked among the bottom 2'5% Monte Carlo values. Maps generated by V. Gómez-Rubio using R 3.2.2 (R Core Team, https://www.r-project.org). (a) Correlation between mosses and spermatophytes. (b) Correlation between liverworts and spermatophytes. (c) Correlation between ferns and spermatophytes.
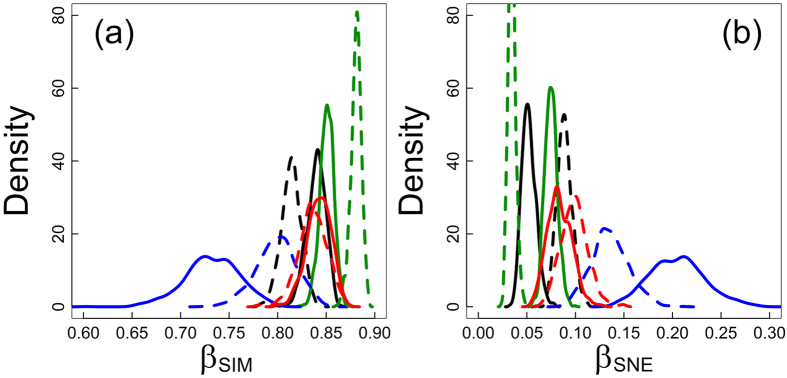
These differences in species richness patterns among taxonomic groups were paralleled by substantial differences in their patterns of species turnover (β SIM) and nestedness-resultant dissimilarity (β SNE) in northern (> 46° N) and southern areas (< 46° N) (Fig. 4, Tables 1 and 2). In spermatophytes, β SIM was significantly higher in the south than in the north (p < 0.01). Spermatophytes thus exhibited significantly and substantially higher β SIM than ferns, mosses and liverworts in the south (p = 0.001), but not in the north. Liverworts exhibited a similar trend as spermatophytes, with β SIM in the south being significantly higher than in the north (p = 0.026). Mosses and ferns exhibited the reverse pattern, with significantly higher (p = 0.036 in ferns) or similar (p = 0.37 in mosses) β SIM in the north than in the south. Thus, there was no difference of β SIM between mosses and ferns in the north (p = 0.429) or in the south (p = 0.111), whereas both groups exhibited significantly higher β SIM than liverworts in the north (p < 0.001 in both mosses and ferns), but not in the south (p = 0.067 in mosses and 0.266 in ferns).
Figure 4. Density plots representing (a) the distribution of the turnover (β SIM) and (b) nestedness-resultant multiple-site dissimilarity (β SNE) across 1000 samples of 50 pixels.
Components of multiple-site dissimilarity were computed for potential species composition in mosses (red), liverworts (blue), ferns (black) and spermatophytes (green) in northern (latitude above 46th parallel, solid line) and southern Europe (latitude below 46th parallel, dashed line).
Table 1. Species turnover (βSIM) and nestedness (βSNE) in European spermatophytes (Sp), ferns (Fe), mosses (Mo) and liverworts (Li): significance level (p-value) of the difference between northern (N, latitude above 46th parallel) and southern Europe (S, latitude below 46th parallel) within taxonomic groups (Fig. 4).
| βSIM | βSNE | |
|---|---|---|
| Spermatophytes | N < S | N > S |
| p < 0.001 | p = 0.001 | |
| Ferns | N > S | N < S |
| p = 0.036 | p = 0.001 | |
| Mosses | S= N | S = N |
| p = 0.370 | p = 0.220 | |
| Liverworts | N < S | N > S |
| p = 0.026 | p = 0.019 |
Table 2. Species turnover (βSIM) and nestedness (βSNE) in European spermatophytes (Sp), ferns (Fe), mosses (Mo) and liverworts (Li): significance level (p-value) of the difference between groups within northern (N, latitude above 46th parallel) and southern Europe (S, latitude below 46th parallel). (Fig. 4).
| Taxonomic groups | north |
south |
||
|---|---|---|---|---|
| βSIM | βSNE | βSIM | βSNE | |
| Mo vs. Li | Mo > Li | Mo < Li | Mo = Li | Mo = Li |
| p < 0.001 | p < 0.001 | p = 0.067 | p = 0.052 | |
| Mo vs. Fe | Mo = Fe | Mo > Fe | Mo = Fe | Mo = Fe |
| p = 0.429 | p = 0.009 | p = 0.111 | p = 0.253 | |
| Mo vs. Sp | Mo = Sp | Mo = Sp | Sp > Mo | Mo > Sp |
| p = 0.292 | p = 0.278 | p = 0.002 | p < 0.001 | |
| Li vs. Fe | Fe > Li | Li > Fe | Fe = Li | Li > Fe |
| p < 0.001 | p < 0.001 | p = 0.266 | p = 0.009 | |
| Li vs. Sp | Sp > Li | Li > Sp | Sp > Li | Li > Sp |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Fe vs. Sp | Fe = Sp | Sp > Fe | Sp > Fe | Fe > Sp |
| p = 0.178 | p = 0.011 | p < 0.001 | p < 0.001 | |
In turn, β SNE was significantly higher in the north than in the south (p < 0.001) in spermatophytes. Again, although β SNE was significantly higher for ferns, mosses and liverworts than for spermatophytes in the south (p < 0.001), whereas the opposite trend was observed in the north (p < 0.001 in mosses and liverworts and p = 0.011 in ferns), liverworts exhibited a similar trend to spermatophytes as β SNE in the north was significantly higher than in the south (0.019). The opposite trend was observed in mosses and ferns, wherein β SNE was significantly lower in the north than in the south (p < 0.001) in the latter, and similar in the north and the south (p = 0.220) in the former. Thus, β SNE was significantly higher for liverworts than for mosses and ferns in the north (p < 0.001).
Discussion
Although some previous studies report shallow increases in species richness towards low latitudes21 and even of inverted patterns in specific taxonomic groups37, we report here a marked increase in species richness towards high latitudes for an entire phylum of land plants at the continental scale. The investigated area in the present study did not encompass the tropics, so that the hypothesis of a global LDG in bryophytes cannot be rejected (but see ref. 29). Nonetheless, our results contrast with the suggestion of a consistent LDG pattern across geographic areas, scales, habitats and taxonomic groups4,5,6,7. This pattern was revealed by the analysis of both raw data and stacked species distribution models (see Methods), reducing the potential effect of dispersal limitations on extant species richness patterns. Thus, in contrast with less-mobile organisms, for which historical factors can account for an inverted LDG37, major ecological factors also contribute to the observed patterns of increased species richness towards high latitudes in European bryophytes, consistent with the suggestion that habitat suitability and diversity prevail over historical factors (time, speciation, dispersal) in explaining patterns of biodiversity for bryophytes38.
In spermatophytes, the marked increase in SR towards the south is paralleled by an increase in species turnover and a reduced nestedness compared with northern areas. The higher spatial species turnover in southern vs. northern areas of Europe is consistent with the strong topographical variation in the Mediterranean basin and with the long-term isolation of specialised populations along ecological gradients, which have accumulated mutations within a relatively stable environment resulting in high rates of local endemism39. The markedly lower levels of species turnover in southern mosses, liverworts and ferns compared with southern vascular plants is consistent with our second hypothesis that, unlike spermatophytes, ferns and bryophytes have failed to radiate in situ along the strong ecological gradients of the Mediterranean. The significantly higher species turnover observed in southern liverworts as compared to northern ones points, however, to the large difference between assemblages dominated by leafy species in the most humid areas as compared to the assemblages dominated by highly specialized thalloid species such as Riccia, characterized by an annual life-cycle and very large spores able to persist underground during the drought season in the most xeric areas40.
The higher levels of nestedness-resultant dissimilarity observed in northern spermatophytes compared with southern ones point, in turn, to the exclusion of species from northern areas, in support of our third hypothesis that the failure to evolve adaptations to cold climates is a key mechanism of the LDG in this group22. Ferns and mosses exhibited the reverse trend. Fern assemblages, whose distribution and richness patterns are indeed mainly controlled by precipitation levels41,42,43, were significantly more dissimilar due to nested patterns in the south than in the north, pointing to the exclusion of drought-intolerant species from the south. Moss assemblages, however, did not exhibit significantly higher levels of nestedness-resultant dissimilarity in the south than in the north. Since beta diversity, which represents the slope in species-area relationships44, does not significantly vary across latitudes in mosses, low levels of moss species richness in the south must be interpreted in terms of the lower carrying capacity (i.e., the intercept of the species-area relationship) of southern areas due to the severe constraint of the poikilohydric condition.
The significant difference in beta diversity along a latitudinal gradient among land plants sheds light on the question of whether the turn-over in community composition is progressively slower from spermatophytes, ferns, and bryophytes, in relationship with the difference in dispersal capacities between these groups36, and suggests different mechanisms of assembly in these groups. Liverworts, in particular, strikingly differed from all groups by exhibiting the highest levels of nestedness both in the north and in the south. Such a pattern suggests that liverworts are the most sensitive group to the impact of past climate change, and in particular, to the higher levels of drought that characterized the glacial periods of the Quaternary and particularly affected frost- and drought-sensitive taxa45. Nevertheless, although the extent of species turnover in the south was substantially and significantly lower than in spermatophytes, liverworts exhibit a similar pattern towards higher species turnover in the south than in the north, strikingly differing from mosses and ferns in this respect. The patterns of beta diversity displayed by ferns and mosses as compared to liverworts observed here is reminiscent of the differences in the slope of the species-area relationships in liverworts as compared to mosses and ferns36. In turn, the similarity in the patterns of beta diversity between liverworts and spermatophytes is puzzling. The extent to which the similarity in the patterns displayed by spermatophytes and liverworts along the latitudinal gradient reflects a similar mechanism of assembly remains, however, to be demonstrated. In the mid-western islands of the Canaries, Madeira and Azores for example, congeneric endemic species generally result from the diversification of a single common ancestor in angiosperms (cladogenetic speciation), but from several independent colonization events in bryophytes (anagenetic speciation)46. This suggests that, in the Mediterranean, the diversity of genera such as Riccia may not necessarily result, like in angiosperms, from a local radiation, but from the recurrent recruitment of pre-adapted species from south-west Asia, where these genera are highly diversified47. While the evolutionary processes underlying the unique diversity of Mediterranean angiosperms have been thoroughly studied39, the evolutionary history of the highly specialized Mediterranean liverwort flora remains a large avenue of research to better understand how poikilohydric organisms may thrive and diversify in dry environments.
Methods
Implementation of species distribution models to circumvent sampling bias
Although Europe is arguably the continent for which the information on species distributions is most detailed, the corresponding databases and richness patterns are, even in the best-known groups such as vascular plants, globally biased because some areas have been more intensively investigated than others48. This problem is exacerbated in less-studied organisms such as bryophytes49. To circumvent this issue, we employed species distribution models (SDMs)50, which have become a powerful tool for generating maps of potential distribution, or ecological suitability, in areas where distribution information is scarce or lacking.
Available bryophyte distributions include 113,321 records for 1726 species (see Supplementary Methods 1). After removal of the species with less than 15 presences, the data contained 1040 species (including 224 of the 453 liverwort (49%), 810 of the 1,292 moss (63%) and 6 of the 8 hornwort species (73%) of Europe) at a 100 km pixel resolution. Bryophyte species richness was split into mosses and liverworts, two lineages of about 12,000 and 5,000 species. Hornworts should, for consistency, have also been analysed separately. Hornworts are, however, a small group of only about 250 species worldwide whose diversity pales in comparison to the much more diverse liverworts and mosses. The number of hornwort species in our data set did not warrant separate analyses and, because hornworts exhibit a suite of functional vegetative traits and ecological features that are similar to those of thalloid liverworts, the data from the two groups were merged (hereafter referred to as liverworts). Species that were present in fewer than 15 pixels were removed, leaving a total of 1040 species of bryophytes, representing 58% of the total number of species in Europe.
We ran an ensemble model using three different techniques: Generalised Linear Models, Maxent, and Random Forests, as implemented in the R (R Core Team60) package BIOMOD 2.051 (see Supplementary Methods 1). We used the 35 macroclimatic variables of CliMond52 as environmental predictors, as well as monthly and annual potential evapotranspiration53. To avoid multicollinearity, we ran a Pearson correlation analysis eliminating one of the variables in each pair with a correlation value greater than 0.8, as advised by Dormann et al.54. A final set of six variables was used to run the models (see Supplementary Methods 1). For proper evaluation, the models were trained on 70% of the data and evaluated on the remaining 30%. This split-sampling was replicated 10 times. For each species, the potential distribution was considered as a consensus across statistical techniques, evaluation indices and thresholds used to binarise continuous predictions. These individual potential species distributions were stacked (S-SDMs, stacked species distributions models55) to depict the potential SR across Europe (see Supplementary Methods 1).
To validate the resulting potential SR (see Supplementary Methods 2), we compared the maps generated by S-SDMs with 1) the observed bryophyte richness values from a literature review (Supplementary Methods 3), 2) macroecological models (MEMs) of SR for the same study area56 and 3) a sampling effort map for bryophytes in Europe (see Supplementary Methods 3).
To obtain comparable results for bryophytes and vascular plants, we further generated a potential richness S-SDM for ferns and spermatophytes (see Supplementary Methods 1). Data for 2,728 native spermatophyte and fern species from the Atlas Florae Europaeae at 50 km pixel resolution16 were upscaled to a 100 km pixel resolution for consistency with the bryophyte data. Gymnosperms should have been analysed separately but, as in the case of hornworts (see above), the low number of species (20) did not warrant a specific analysis, so that spermatophytes were analysed globally. After removal of the species with less than 15 presences, the data included 1,359 and 79 spermatophyte and fern species, representing 12% and 49% of their total diversity in Europe, respectively. The potential richness generated by the S-SDM was then compared with the observed richness values for all the species available in this study (see Supplementary Methods 2)57.
The predictions of the S-SDMs were highly correlated with the observed richness values and the potential richness values of the macroecological models (MEMs, Supplementary Methods 2), supporting the notion that S-SDMs appear as a very promising tool for modelling species assemblages and providing reliable predictions of the geographical variation in species richness55,58. Moreover, these predictions showed only a low correlation with the map of sampling effort, indicating that the effects of sampling bias were adequately removed (Supplementary Methods 2).
Comparison of SR patterns between bryophytes and vascular plants
The potential richness predicted by S-SDMs was employed to compare the spatial patterns of SR between bryophytes and vascular plants, using three approaches: 1) comparison of potential richness maps, 2) spatial turnover and nestedness, and 3) a latitudinal band analysis of SR.
Comparison of potential richness maps of bryophytes and vascular plants
We began with a comparison of potential richness maps using two different techniques. First, we calculated and mapped the local Lee’s L bivariate spatial association59 using our own implementation of this statistic with the R language60, which is now included in the ‘spdep’ package (Supplementary Methods 4). In contrast to bivariate association measures such as Pearson’s correlation, Lee’s L captures spatial associations among observations in terms of their point-to-point relationships across two spatial patterns.
Spatial turnover and nestedness in species composition
In the second approach, assemblage multiple-site dissimilarity was measured using the Sørensen index (β SOR) and was partitioned into its turnover (β SIM) and nestedness-resultant (β SNE) components to distinguish between the contribution of spatial species replacement and species loss, respectively61, along the environmental gradients. Potential values for β SIM and β SNE were computed independently for mosses, liverworts, ferns, and spermatophytes in northern and southern Europe (defined by the limit of the 46th parallel). Multiple-site dissimilarity was computed 1000 times for randomly sampled subsets of 50 pixels (command beta.sample in R package betapart62), and the resulting distributions of β SIM and β SNE values across the 1000 samples were used to empirically assess whether there were significant differences between northern and southern Europe and between mosses, liverworts, ferns and spermatophytes.
Latitudinal band analysis of species richness
Lastly, we plotted a set of the environmental variables indicated in Table 3 and the potential species richness values for mosses, liverworts, ferns, and spermatophytes for each 100 km latitudinal band across Europe (Supplementary Methods 5).
Table 3. Variables used in the canonical analyses and macroecological models.
| Environmental variable | Driver |
|---|---|
| Mean annual temperature | Kinetic energy |
| Standard deviation of mean annual temperature | Environmental heterogeneity |
| Mean annual precipitation in each pixel | Water balance |
| Standard deviation of mean annual precipitation | Environmental heterogeneity |
| Standard deviation of altitude | Environmental heterogeneity |
| Mean potential evapotranspiration in each pixel | Potential energy |
| Standard deviation of potential evapotranspiration | Environmental heterogeneity |
| Mean distance to the coast (continentality) | Spatial and climatic |
| Mean distance to refugia16 | Spatial and historical |
Each variable define a possible driver for species richness patterns.
Additional Information
How to cite this article: Mateo, R. G. et al. The mossy north: an inverse latitudinal diversity gradient in European bryophytes. Sci. Rep. 6, 25546; doi: 10.1038/srep25546 (2016).
Supplementary Material
Acknowledgments
RGM was funded by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (ACONITE, PIEF-GA-2013-622620). Many thanks are due to two referees for their constructive comments on the manuscript.
Footnotes
Author Contributions R.G.M., S.N., J.M. and A.V. collected the data. R.G.M., O.B., B.P., V.G.R. and A.B. analysed the data. All the authors contributed to the writing of the manuscript.
References
- Crane P. R. & Lidgard S. Angiosperm diversification and paleolatitudinal gradients in cretaceous floristic diversity. Science 246, 675–678 (1989). [DOI] [PubMed] [Google Scholar]
- Mannion P. D., Upchurch P., Benson R. B. J. & Goswami A. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 29, 42–50 (2013). [DOI] [PubMed] [Google Scholar]
- Lomolino M. V., Riddle B. R., Whittaker R. J. & Brown J. H. Biogeography (Sinauer Associates Inc., Sunderland, MA, 2010). [Google Scholar]
- Gaston K. J. Global patterns in biodiversity. Nature 405, 220–227 (2000). [DOI] [PubMed] [Google Scholar]
- Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004). [DOI] [PubMed] [Google Scholar]
- Beaugrand G., Rombouts I. & Kirby R. R. Towards an understanding of the pattern of biodiversity in the oceans. Glob. Ecol. Biogeogr. 22, 440–449 (2013). [Google Scholar]
- Jablonski D. et al. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA 110, 10487–94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen R. K., Marmion M. & Luoto M. Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography 35, 276–288 (2012). [Google Scholar]
- Currie D. J. et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (2004). [Google Scholar]
- Chaves M. M., Maroco J. P. & Pereira J. S. Understanding plant responses to drought – from genes to the whole plant. Funct. Plant Biol. 30, 239–264 (2003). [DOI] [PubMed] [Google Scholar]
- Stevens G. C. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256 (1989). [Google Scholar]
- Simpson G. G. Species density of north American recent mammals. Syst. Zool. 13, 57 (1964). [Google Scholar]
- Currie D. J. Energy and large-scale patterns of animal- and plant-species richness. Am. Nat. 137, 27–49 (1991). [Google Scholar]
- Tognelli M. F. & Kelt D. A. Analysis of determinants of mammalian species richness in south America using spatial autoregressive models. Ecography 27, 427–436 (2004). [Google Scholar]
- Svenning J. C. & Skov F. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460 (2007). [DOI] [PubMed] [Google Scholar]
- Normand S. et al. Postglacial migration supplements climate in determining plant species ranges in Europe. Proc. Biol. Sci. 278, 3644–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynesius M. & Jansson R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl. Acad. Sci. USA 97, 9115–20 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo M. B. et al. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 31, 8–15 (2008). [Google Scholar]
- Hortal J. et al. Ice age climate, evolutionary constraints and diversity patterns of European dung beetles. Ecol. Lett. 14, 741–8 (2011). [DOI] [PubMed] [Google Scholar]
- Baselga A., Lobo J. M., Svenning J.-C., Aragón P. & Araújo M. B. Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Glob. Ecol. Biogeogr. 21, 1106–1113 (2012). [Google Scholar]
- Romdal T. S., Araújo M. B. & Rahbek C. Life on a tropical planet: niche conservatism and the global diversity gradient. Glob. Ecol. Biogeogr. 22, 344–350 (2013). [Google Scholar]
- Visser V., Clayton W. D., Simpson D. A., Freckleton R. P. & Osborne C. P. Mechanisms driving an unusual latitudinal diversity gradient for grasses. Glob. Ecol. Biogeogr. 23, 61–75 (2014). [Google Scholar]
- Fragnière Y., Bétrisey S., Cardinaux L., Stoffel M. & Kozlowski G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 42, 809–820 (2015). [Google Scholar]
- Kreft H. & Jetz W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 104, 5925–5930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. Biogeography of ferns. In Fern Ecology (eds Mehltreter K., Walker L. R., Sharpe J. M.). Cambridge University Press (2010). [Google Scholar]
- Salazar L. et al. Diversity patterns of ferns along elevational gradients in Andean tropical forests. Plant Ecol Divers 8, 13–24 (2015). [Google Scholar]
- Parra M. J., Rodríguez R., Cavieres L., Muñoz-Tapia L. & Atala C. Latitudinal patterns in pteridophyte distribution of continental Chile. Gayana - Botanica 72, 58–69 (2015). [Google Scholar]
- Shaw A. J., Cox C. J. & Goffinet B. Global patterns of moss diversity: taxonomic and molecular inference. Taxon 54, 337–352 (2005). [Google Scholar]
- Geffert J. L., Frahm J. P., Barthlott W. & Mutke J. Global moss diversity: spatial and taxonomic patterns of species richness. J. Bryol. 35, 1–11 (2013). [Google Scholar]
- Tan B. C. & Pocs T. Bryogeography and conservation of bryophytes. In Bryophyte Biology (eds Shaw A. J. and Goffinet B.) 403–448, Cambridge (Cambridge University Press, 2000). [Google Scholar]
- Chen S.-B. et al. Latitudinal diversity gradients in bryophytes and woody plants: Roles of temperature and water availability. J. Syst. Evol. 53, 535–545 (2015). [Google Scholar]
- Rozzi R. et al. Changing lenses to assess biodiversity: patterns of species richness in sub-Antarctic plants and implications for global conservation. Front. Ecol. Environ. 6, 131–137 (2008). [Google Scholar]
- Patiño J., Carine M., Fernández-Palacios J. M., Otto R., Schaefer H. & Vanderpoorten A. The anagenetic world of spore-producing land plants. New Phytol. 201, 305–311 (2014). [DOI] [PubMed] [Google Scholar]
- Glime J. M. Bryophyte ecology (Michigan Technological University, Botanical Society of America & International Association of Bryologists, 2007).
- Furness S. B. & Grime J. P. Growth rate and temperature responses in bryophytes: II. A comparative study of species of contrasted ecology. J. Ecol. 70, 525–536 (1982). [Google Scholar]
- Patiño J. et al. Differences in species–area relationships among the major lineages of land plants: a macroecological perspective. Glob. Ecol. Biogeogr. 23, 1275–1283 (2014). [Google Scholar]
- Pyron R. A. & Burbrink F. T. Can the tropical conservatism hypothesis explain temperate species richness patterns? An inverse latitudinal biodiversity gradient in the New World snake tribe Lampropeltini. Glob. Ecol. Biogeogr. 18, 406–415 (2009). [Google Scholar]
- Patiño J. et al. Accounting for data heterogeneity in patterns of biodiversity: an application of linear mixed effect models to the oceanic island biogeography of spore-producing plants. Ecography 36, 904–913 (2013). [Google Scholar]
- Thompson J. D. Plant Evolution in the Mediterranean (Oxford University Press, Oxford, 2005). [Google Scholar]
- Bishler H. Liverworts of the Mediterranean: Ecology, diversity and distribution (J. Cramer, Berlin, 2004). [Google Scholar]
- Ferrer-Castán D. & Vetaas O. R. Pteridophyte richness, climate and topography in the Iberian Peninsula: Comparing spatial and nonspatial models of richness patterns. Glob. Ecol. Biogeogr. 14, 155–165 (2005). [Google Scholar]
- Kessler M. Patterns of diversity and range size of selected plant groups along an elevational transect in the Bolivian Andes. Biodiver. Conser. 10, 1897–1921 (2001). [Google Scholar]
- Bickford S. A. & Laffan S. W. Multi-extent analysis of the relationship between pteridophyte species richness and climate. Glob. Ecol. Biogeogr. 15, 588–601 (2006). [Google Scholar]
- Triantis K. A., Guilhaumon F. & Whittaker R. J. The island species–area relationship: biology and statistics. J. of Biogeogr. 39, 215–231 (2012). [Google Scholar]
- Svenning J.-C. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol. Lett. 6, 646–653 (2003). [Google Scholar]
- Patiño J. & Vanderpoorten A. Macaronesia is a departure gate of anagenetic speciation in the moss genus Rhynchostegiella. J. Biogeogr. 42, 2122–2130 (2015). [Google Scholar]
- Kürschner H. & Frey W. Liverworts, Mosses and Hornworts of southwest Asia (Marchantiophyta, Bryophyta, Anthocerotophyta). Nova Hedwigia, Beihefte 139, 1–240 (2011). [Google Scholar]
- Petřík P., Pergl J. & Wild J. Recording effort biases the species richness cited in plant distribution atlases. Perspect. Plant Ecol. Evol. Syst. 12, 57–65 (2010). [Google Scholar]
- Mateo R. G., Vanderpoorten A., Munoz J., Laenen B. & Desamore A. Modeling species distributions from heterogeneous data for the biogeographic regionalization of the European bryophyte flora. Plos One 8, e55648 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A. & Zimmermann N. E. Predictive habitat distribution models in ecology. Ecol. Model. 135, 147–186 (2000). [Google Scholar]
- Thuiller W., Lafourcade B., Engler R. & Araújo M. B. BIOMOD - a platform for ensemble forecasting of species distributions. Ecography 32, 369–373 (2009). [Google Scholar]
- Kriticos D. J. et al. CliMond: global high resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 3, 53–64 (2012). [Google Scholar]
- Zomer R. J., Trabucco A., Bossio D. A. & Verchot L. V. Climate change mitigation: a spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agric. Ecosyst. Environ. 126, 67–80 (2008). [Google Scholar]
- Dormann C. F. et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 027–046 (2013). [Google Scholar]
- Mateo R. G., Felicísimo Á. M., Pottier J., Guisan A. & Muñoz J. Do stacked species distribution models reflect altitudinal diversity patterns? Plos ONE 7, e32586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli N. J. et al. Patterns and causes of species richness: a general simulation model for macroecology. Ecol. Lett. 12, 873–886 (2009). [DOI] [PubMed] [Google Scholar]
- Humphries C., Araújo M. B., Williams P., Lampinen R., Lahti T. & Uotila P. Plant diversity in Europe: Atlas Florae Europaeae and WORLDMAP. Acta Botanica Fennica 162, 11–21 (1999). [Google Scholar]
- Aranda S. C. & Lobo J. M. How well does presence-only-based species distribution modelling predict assemblage diversity? A case study of the Tenerife flora. Ecography 34, 31–38 (2010). [Google Scholar]
- Lee S. I. Developing a bivariate spatial association measure: An integration of Pearson’s r and Moran’s I. J. Geogr. Syst. 3, 369–385 (2001). [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- Baselga A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010). [Google Scholar]
- Baselga A. & Orme C. D. L. betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.