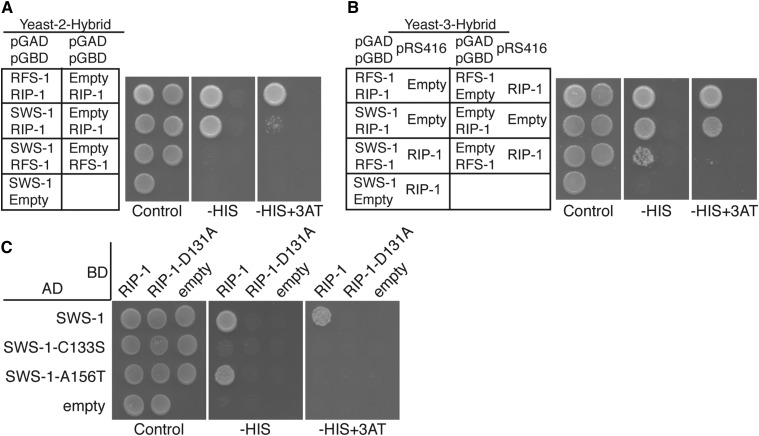
Figure 7.
RIP-1 interacts with SWS-1 and bridges an interaction between SWS-1 with RFS-1. Y2H (A and C) and Y3H (B) from left to right show plating controls on SC−LEU−TRP or SC−LEU−TRP−URA, respectively, with the additional dropout of histidine (−HIS) and histidine with 3-amino-1,2,4-triazole (−HIS+3AT), indicating a Y2H or Y3H interaction. Within each panel, the left column shows potential interactions between two proteins and the right column shows an empty vector control. RIP-1 interacts with both SWS-1 and RFS-1. SWS-1 and RFS-1 do not interact (A). With constitutive expression of RIP-1, SWS-1 and RFS-1 promote growth on SC−LEU−TRP− URA−HIS, indicating a Y3H interaction (row 3 in B). Two SWIM domain mutations were created in SWS-1, C133S, and A156T. SWS-1-C133S disrupts interaction with RIP-1 (row 2 in C). SWS-1-A156T decreases interaction with RIP-1 on –HIS+3AT (row 3 in C). A Walker B motif mutation that disrupts interaction with SWS-1, SWS-1-C133S, and SWS-1-A146T (column 2 in C) was introduced into RIP-1.