Abstract
Double-strand DNA breaks occur upon exposure of cells to ionizing radiation and certain chemical agents or indirectly through replication fork collapse at DNA damage sites. If left unrepaired, double-strand breaks can cause genome instability and cell death, and their repair can result in loss of heterozygosity. In response to DNA damage, proteins involved in double-strand break repair by homologous recombination relocalize into discrete nuclear foci. We identified 29 proteins that colocalize with recombination repair protein Rad52 in response to DNA damage. Of particular interest, Ygr042w/Mte1, a protein of unknown function, showed robust colocalization with Rad52. Mte1 foci fail to form when the DNA helicase gene MPH1 is absent. Mte1 and Mph1 form a complex and are recruited to double-strand breaks in vivo in a mutually dependent manner. MTE1 is important for resolution of Rad52 foci during double-strand break repair and for suppressing break-induced replication. Together our data indicate that Mte1 functions with Mph1 in double-strand break repair.
Keywords: DNA repair, recombination, double-strand breaks, break-induced replication, loss of heterozygosity, nuclear foci
EFFECTIVE repair of double-strand DNA breaks (DSBs) is critical to the preservation of genome stability, yet most modes of DSB repair have significant potential to generate sequence alterations or sequence loss. Repair of DSBs by homologous recombination can result in loss of heterozygosity when resolution of recombination intermediates between homologous chromosomes results in a crossover. As such, cells possess several mechanisms by which crossing over can be suppressed in favor of noncrossover recombination products. Double Holliday junction (dHJ) intermediates that result from invasion of a homologous chromosome by both ends of a resected DSB (Szostak et al. 1983) can be resolved nucleolytically by the action of the Yen1 and Mus81/Mms4 endonucleases (Blanco et al. 2010; Ho et al. 2010) to produce a random distribution of crossover and noncrossover products. By contrast, the same dHJ intermediates can be dissolved by the combined helicase and ssDNA decatenase action of the Bloom/TopIIIα/Rmi1 complex (Sgs1/Top3/Rmi1 in yeast) (Wu et al. 2006; Yang et al. 2010) to yield exclusively noncrossover products (Wu and Hickson 2003). Crossovers can also be prevented if the D-loop structure that results from the first strand invasion by one end of a resected DSB into the homologous chromosome is unwound before capture of the second end to form the dHJ. Unwinding of D-loops is catalyzed in vitro and in vivo by the 3′-to-5′ DNA helicase Mph1 (Sun et al. 2008; Prakash et al. 2009) to prevent loss of heterozygosity due to crossovers and break-induced replication (BIR) (Luke-Glaser and Luke 2012; Mazon and Symington 2013; Stafa et al. 2014).
The Mph1 DNA helicase was first identified as a deletion mutant with an increased mutation frequency (Entian et al. 1999). Subsequent characterization revealed that mph1 mutants are sensitive to the alkylating agent MMS and to a lesser degree to ionizing radiation (Scheller et al. 2000), and that mph1 mutants are proficient for mitotic recombination (Schurer et al. 2004). Molecular insight into Mph1 function in recombination reactions comes from evidence that Mph1 is a DNA helicase (Prakash et al. 2005), and that Mph1 can unwind Rad51 D-loops (Sun et al. 2008; Prakash et al. 2009) and extended D-loops (Sebesta et al. 2011). Consistent with an antirecombination role for Mph1, overexpression of MPH1 reduces recombination rate and reduces loading of Rad51 at an induced DSB (Banerjee et al. 2008). Indeed, Mph1 suppresses crossing over during mitotic recombination, likely by unwinding D-loop recombination intermediates formed by Rad51 (Prakash et al. 2009) and preventing ectopic resolution of early strand exchange intermediates by the Mus81–Mms4 nuclease (Mazon and Symington 2013). Mph1 inhibits BIR repair of double-strand breaks (Luke-Glaser and Luke 2012) and promotes template switching during BIR (Stafa et al. 2014), both consistent with the ability of Mph1 to unwind recombination intermediates in vitro. In addition to functioning in crossover suppression, Mph1 plays a prorecombinogenic role in repair of stressed DNA replication forks (Sun et al. 2008; Chen et al. 2009, 2013; Choi et al. 2010; Chavez et al. 2011; Zheng et al. 2011; Xue et al. 2014) and inhibits nonhomologous end-joining repair at telomeres (Luke-Glaser and Luke 2012). Mph1 is thought to be the functional homolog of the human FANCM protein (Kee and D’Andrea 2010; Whitby 2010; Xue et al. 2015). Thus, available evidence points to diverse functions for Mph1, and these functions are likely connected to the ability of Mph1 to unwind and remodel DNA structures.
Here we leverage intracellular protein location data to identify the complement of proteins that colocalize with the recombination repair protein Rad52 in nuclear foci during the response to DNA double-strand breaks. In addition to defining the membership of Rad52 foci, we identify an uncharacterized protein, Ygr042w/Mte1, that functions in double-strand break repair. Mte1 acts in complex with Mph1 at double-strand breaks in vivo, is important for DSB repair as assessed by resolution of Rad52 foci, and functions, as is the case for Mph1, in suppressing BIR repair of double-strand DNA breaks.
Materials and Methods
Yeast strains and media
All yeast strains used in this study are derivatives of BY4741 (Brachmann et al. 1998), CL11-7, or W303, and are listed in Supplemental Material, Table S1. Strains were constructed using genetic crosses and standard PCR-based gene disruption techniques. Standard yeast media and growth conditions were used.
Chromatin immunoprecipitation and deep sequencing
Chromatin immunoprecipitation (IP) was performed using Flag-epitope-tagged versions of each indicated protein, as previously described (Roberts et al. 2008; Balint et al. 2015), with modifications. Cells were grown to midlogarithmic phase in YPR (1% yeast extract, 2% peptone, 3% raffinose) at 28° and then arrested in G2/M with 20 μg/ml nocodazole for 4 hr. Galactose was added to 2% final concentration to induce expression of the HO endonuclease gene. Cells were sampled before galactose addition and after 4 hr of induction and cross-linked with formaldehyde overnight. Cells were harvested and washed twice with cold TBS (20 mM Tris-HCl pH 7.5, 150 mM NaCl), resuspended in FA-lysis buffer (50 mM HEPES pH 7.5, 2 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 150 mM NaCl) containing 0.05% SDS, lysed, and sonicated. Immunoprecipitates were washed sequentially with 1 ml of FA-lysis buffer, FA-lysis buffer containing 1 M NaCl, FA-lysis buffer containing 0.5 M NaCl, wash buffer (50 mM HEPES pH 7.5, 0.25 M LiCl, 2 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 1% NP-40, 10 mM Tris-HCl pH 8.0), and TE (10 mM Tris-HCl pH 8.0, 1 mM EDTA). Protein–DNA complexes were eluted, cross-links were reversed, protein and RNA was digested, and DNA was isolated by phenol/chloroform extraction and ethanol precipitation. Sequencing libraries were generated using the Nextera XT DNA Sample Preparation Kit (Illumina) with custom index primers for the PCR amplification step. Libraries were quantified using a 2100 Bioanalyzer (Agilent) and the KAPA SYBR FAST Universal qPCR Kit (KAPA Biosystems).
Sequencing data analysis
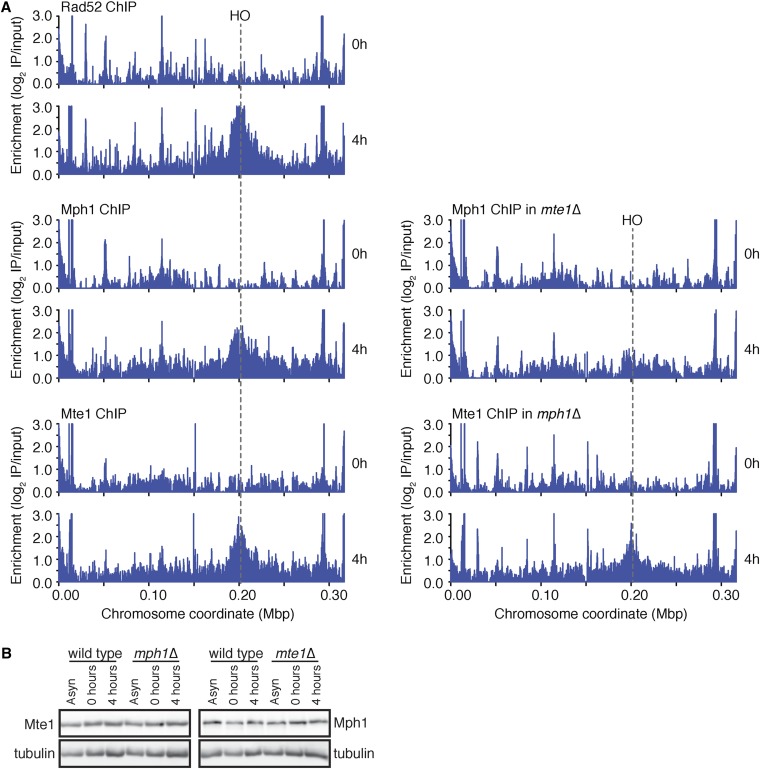
Input and IP samples from each experiment were sequenced on an Illumina HiSeq 2500 (50 nucleotide single-end reads). All sequencing data are deposited in the Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra, study accession SRP064493). The number of reads for each sample ranges from 12.8 M to 25.7 M. The quality of sequencing reads was first assessed using FastQC (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc). All samples have a median PHRED score of ≥30 for all positions. Sequenced reads were mapped to the Saccharomyces cerevisiae reference genome version WS220 (downloaded from the Saccharomyces Genome Database) (Cherry et al. 2012; Engel et al. 2014) using Bowtie2 (version 2.0.0) (Langmead and Salzberg 2012) with default settings, except for forcing end-to-end alignment. Greater than 96% mapping frequencies were achieved for all samples, yielding a minimum 50× coverage for all samples (Table S2). To reduce any bias from DNA sequencing, the data were normalized by the ratio of coverage for each IP and input pair prior to each comparison. We used a 100-bp sliding window with a step size of 50 bp to calculate enrichment scores as a log2 ratio of normalized read counts for each IP:input pair. The enrichment scores for all of IP:input pairs, plotted across chromosome III, are shown in Figure 5.
Figure 5.
Mte1 and Mph1 are recruited to double-strand DNA breaks. (A) ChIP-seq analysis was performed on RAD52–FLAG, MTE1–FLAG, MTE1–FLAG mph1∆, MPH1–FLAG, and MPH1–FLAG mte1∆ cells at 0 hr and 4 hr following the induction of a specific irreparable double-strand break at the MAT locus by the HO endonuclease. ChIP enrichment scores representing the log2 immunoprecipitate to input ratio are plotted across chromosome III for each time point. The position of the HO cut site is indicated by a dashed line. (B) Extracts from cells used in A were subjected to immunoblot analysis and probed with an antiflag antibody and an antitubulin antibody (as a loading control).
Whole cell extracts, immunoblotting, and immunoprecipitation
Logarithmically growing cells at 30° were treated with or without 5 µg/ml phleomycin (BioShop PEO422.25) for 2 hr before cells were collected, fixed with 10% trichloroacetic acid, and whole cell extracts were prepared (Pellicioli et al. 1999). Proteins were resolved by SDS-PAGE and subjected to immunoblotting with α-Flag M2 (F3165, Sigma-Aldrich), α-HA (ab16918, Abcam), or α-tubulin (YOL1/34, Serotec) antibodies. Native extracts for immunoprecipitation were prepared from 5 × 108 cells as previously described (Shimomura et al. 1998), with some modifications. Cell pellets were resuspended in FA-lysis buffer containing 1 mM DTT, 2 mM sodium fluoride, 1 mM sodium orthovanadate, 1× Complete Mini EDTA-free protease inhibitor cocktail (Roche 11836170001), 2.5 μg/ml aprotinin, 10 mM β-glycerophosphate, 5 μg/mL leupeptin, 2 μg/mL pepstatin A, 1 mM PMSF, and 5 μg/ml tosyl-L-lysyl-chloromethane hydrochloride, and then lysed with glass beads. Cleared extracts were immunoprecipitated with α-Flag M2 antibody. Beads were washed twice with 0.5 ml FA-lysis buffer as above and eluted in 5× SDS loading buffer.
DNA damage sensitivity
Yeast strains were grown overnight in YPD, diluted serially, and spotted onto YPD plates containing the indicated concentrations of phleomycin. Plates were incubated at 30° for 2–3 days before imaging. The experiment was repeated twice, and a representative example is shown.
Fluorescence microscopy
For analysis of GFP fusion protein nuclear foci, strains were grown to midlog phase in YPD, diluted into fresh YPD and cultured overnight to OD600 = 0.3. Cells were treated for 120 min with 5 µg/ml phleomycin, or cultured without phleomycin, harvested, and washed once in low fluorescence medium with or without phleomycin before imaging. Eleven z slices with a 0.4-µm step size were acquired using Volocity imaging software (PerkinElmer) controlling a Leica DMI6000 confocal fluorescence microscope with fluorescein isothiocyanate, Texas Red, and differential interference contrast filter sets (Quorum Technologies). Images were scored by visual inspection for GFP fusion protein foci. Samples were compared using the t-test or the Wilcoxon rank sum test, as appropriate, in R (www.r-project.org). Data were plotted using ggplot2 in R. For Rad52–GFP foci, the same procedure was used except that cells were blocked in G2/M phase by treatment with 20 µg/ml nocodazole for 3 hr and exposed to 50 µg/ml phleomycin for 30 min. To screen Mte1–GFP foci in different mutant backgrounds, we imaged a single z-slice on an EVOTEC Opera confocal microscope system (PerkinElmer), as described (Tkach et al. 2012).
Recombination assays
Recombination rates (events/cell/generation) were calculated using a direct repeat recombination assay (Smith and Rothstein 1999) and quantifying recombination from the number of Leu+ recombinant colonies using the method of the median (Lea and Coulson 1949). Each fluctuation test comprised nine independent cultures, and the results from 10 fluctuation tests were plotted in R. Rates were compared using a Welch two-sample t-test in R.
BIR efficiencies were calculated as described previously (Anand et al. 2014). Briefly, cells were plated for individual colonies on YEPD + clonNat to retain the HOcs (which is marked with natMX). Approximately 1 million cells from individual colonies were appropriately diluted and plated on YEPD plates to get the total cell count and on YEP-Gal plates for HO induction. Cells that grew on YEP-Gal plates (DNA break survivors) were counted and replica plated to plates lacking uracil to determine BIR frequencies. For each replicate, Ura+ frequencies were calculated as total Ura+ cells that grew on plates lacking uracil over total cells that grew on YEPD. Experiments were repeated at least three times, plotted in R, and compared using a Welch two-sample t-test in R.
Data availability
Strains are available upon request. Table S1 contains the genotypes of all strains used. Table S2 contains statistics for all deep sequencing, including NCBI Sequence Read Archive (SRA) accession numbers.
Results
Twenty-nine proteins form nuclear foci that detectably colocalize with Rad52 foci
A number of DNA repair proteins change their intracellular localization from pannuclear to nuclear foci in response to DNA damage. Proteins that localize in nuclear foci have been identified in candidate approaches (Lisby et al. 2001, 2004; Melo et al. 2001; Zhu et al. 2008; Burgess et al. 2009; Germann et al. 2011) and in genome-scale screens (Tkach et al. 2012; Denervaud et al. 2013; Mazumder et al. 2013; Yu et al. 2013). Nuclear foci are commonly thought of as centers of DNA repair, in part because foci formed by recombination repair proteins colocalize with double-strand DNA breaks (Lisby et al. 2003). However, not all nuclear foci are identical to the canonical DNA repair centers that are marked by the recombination protein Rad52. For example, Cmr1 forms foci that do not colocalize detectably with Rad52 (Tkach et al. 2012), but rather colocalize with a distinct set of proteins in an intranuclear quality control compartment (Gallina et al. 2015).
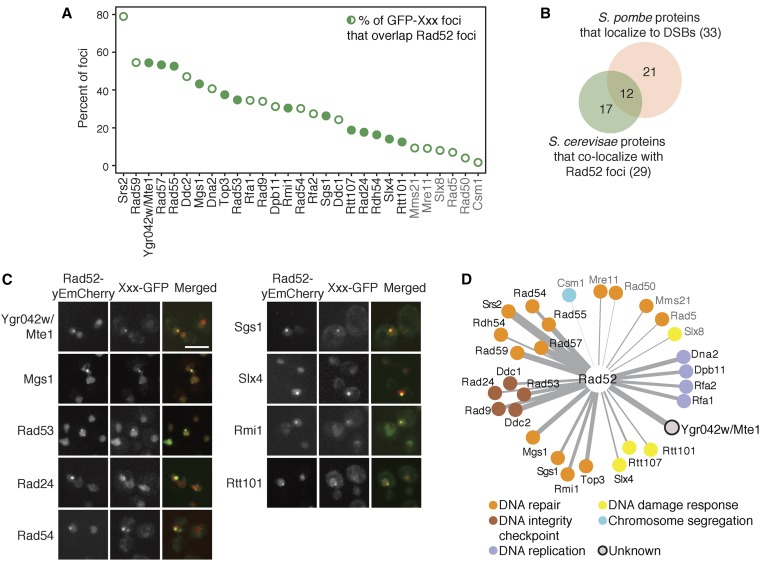
We tested 61 budding yeast proteins that form nuclear foci in response to DNA damage to identify those that colocalize detectably with Rad52. Nuclear foci proteins were tagged with GFP (Huh et al. 2003), Rad52 was tagged with mCherry, and cells were examined by fluorescence microscopy after treatment with the double-strand DNA break-inducing agent phleomycin (Figure 1). Twenty-nine proteins colocalized detectably with Rad52 (Figure 1A, Table S3, Table S4, and Table S5). The extent of colocalization ranged from 79% of foci for Srs2 to 2% of foci for Csm1 (Table S3). Six colocalizations we regard with caution, as the extent of colocalization was equal or less than that seen with Mre11 (indicated in gray in Figure 1). A low extent of Mre11 colocalization with Rad52 has been observed previously and attributed to colocalization of DSBs that are at different stages of repair (Lisby et al. 2004). Fourteen proteins had not previously been described as components of Rad52 foci (Figure 1, A and C), although most are known DNA repair, DNA replication, or checkpoint signaling proteins (Figure 1D). We identified one protein, Ygr042w, with no known role in recombination repair. Mutants in YGR042W affect telomere length (Askree et al. 2004), and the fission yeast homolog of Ygr042w, Dbl2, forms foci that colocalize with an induced double-strand DNA break (Yu et al. 2013). The extensive colocalization of Ygr042w with Rad52 foci, similar to the extent of colocalization observed for members of the Rad52 epistasis group (Symington 2002) Rad55, Rad57, and Rad59, suggests that Ygr042w could function in repair of double-strand DNA breaks. While this work was in progress, a name for YGR042W was reserved in the Saccharomyces Genome Database, MTE1 (Mph1-associated telomere maintenance protein). Thus, we now refer to YGR042W as MTE1.
Figure 1.
Twenty-nine proteins form nuclear foci that detectably colocalize with Rad52 foci. (A) The percent of nuclear foci formed by each GFP fusion protein that overlaps with Rad52–yEmCherry foci after 2 hr in 5 µg/ml phleomycin is plotted. Open circles indicate colocalizations that were previously identified. Closed circles indicate Rad52 colocalizations that have not been previously described. Protein names in gray indicate those with a percent colocalization at or below that seen with Mre11. (B) The overlap between the proteins that colocalize with Rad52 foci and those that colocalize with an induced double-strand DNA break in fission yeast is shown. (C) Representative fluorescence micrographs showing colocalization of the indicated GFP fusion proteins with Rad52 foci. The mCherry, GFP, and merged images are shown. Bar, 5 µm. (D) A network of the proteins that colocalize detectably with Rad52. Protein function is indicated by color and edge thickness is proportional to the extent of protein colocalization with Rad52 foci.
Mte1 foci form in S/G2 phase and in response to double-strand breaks
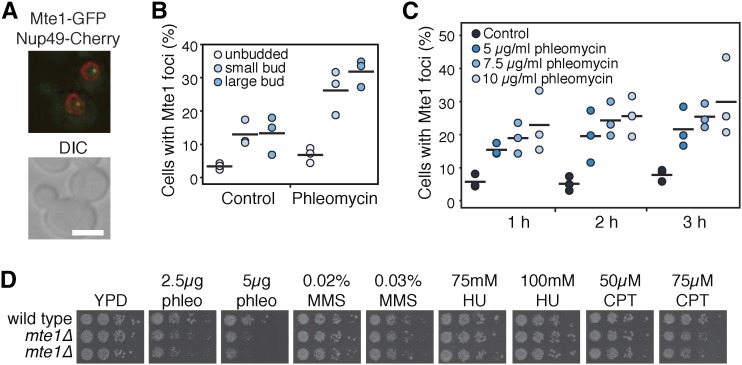
The foci formed by Mte1 in response to phleomycin localize to the nucleus (Figure 2A) and form more frequently in cells in S and G2 phases than in G1 cells (Figure 2B). Mte1 foci also form in the absence of DNA damaging agents, in 13% of cells during S or G2 phase, but in only 3% of cells during G1 phase (Figure 2B), similar to Rad52 foci (Lisby et al. 2001). As expected, Mte1 foci levels increase with increasing phleomycin concentration and with increasing time of phleomycin exposure (Figure 2C). Deletion of MTE1 confers modest sensitivity to phleomycin, but not to other DNA damaging and replication stress agents, methyl methanesulfonate, hydroxyurea, and camptothecin (Figure 2D).
Figure 2.
Mte1 foci form in S/G2 phase and in response to double-strand breaks. (A) Mte1–GFP nuclear foci are shown in a merged fluorescence micrograph. The lower panel is the DIC image of the same cells. Bar, 5 µm. (B) The percent of cells in each morphology category (unbudded, small-budded, and large-budded) with Mte1 nuclear foci after 2 hr in 5 µg/ml phleomycin, or untreated, is plotted. The solid bars show the means of the three replicates. N ranged from 46 to 188 cells per morphology category per replicate. (C) The percent of cells with Mte1 nuclear foci after 1, 2, or 3 hr in the indicated concentrations of phleomycin is plotted. The solid bars show the means of the three replicates. N ranged from 60 to 166 cells per drug concentration per replicate. (D) Serial 10-fold dilutions of the indicated strains were spotted on the indicated concentrations of phleomycin (phleo), methyl methanesulfonate (MMS), hudroxyurea (HU), or camptothecan (CPT). Plates were photographed after 2–3 days.
Mte1 foci are increased when end resection is defective and depend on MPH1
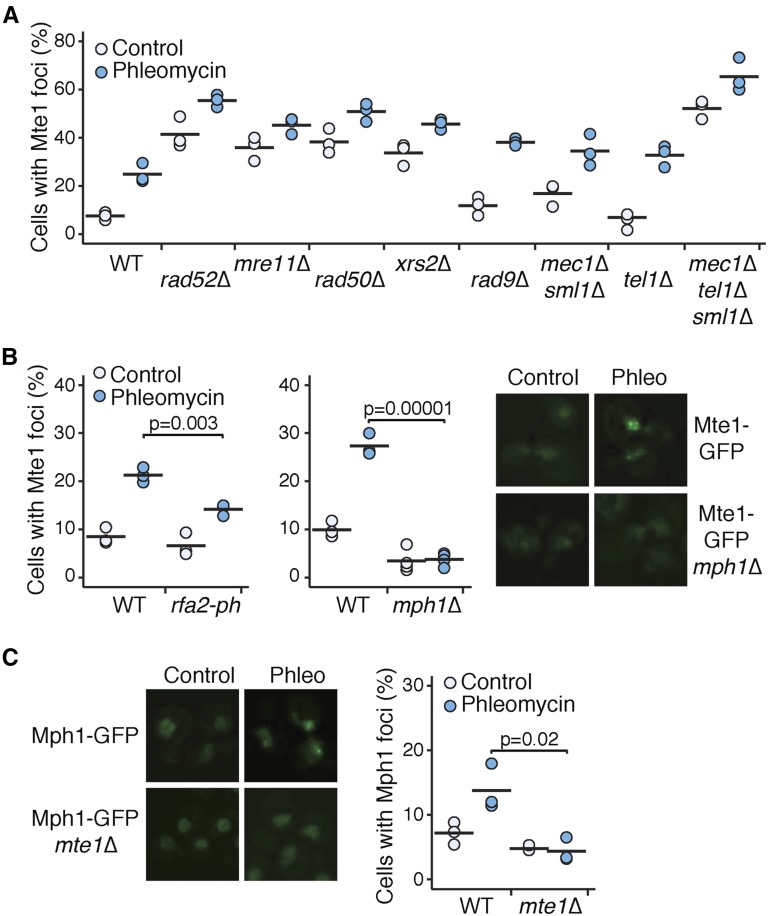
We tested whether Mte1 focus formation was altered in mutants of genes encoding other proteins that form nuclear foci. Of 52 mutants tested, 5 led to increased Mte1 focus formation (Figure 3A and Table S6). Three of the mutants, in MRE11, RAD50, and XRS2, would eliminate the DSB end-resection function of the MRX complex (Ivanov et al. 1994), and RAD52 is critical for formation of the Rad51 filament at resected DSBs (Sugawara et al. 2003), among other functions. Mte1 foci increase in both the presence and absence of phleomycin in mre11∆, rad50∆, xrs2∆, and rad52∆, indicating that spontaneous DSBs are either more prevalent in these mutants or are repaired less effectively. By contrast, the rad9∆ mutant, which is defective in DNA damage checkpoint signaling and results in faster end resection at a double-strand break (Lazzaro et al. 2008; Ferrari et al. 2015), displays increased Mte1 foci only in the presence of phleomycin. We tested whether other checkpoint mutants result in increased Mte1 foci (Figure 3A). We disrupted checkpoint signaling upstream of Rad9 by deleting MEC1, TEL1, or both, and found that only the mec1∆ tel1∆ double mutant had a statistically evident increase in Mte1 foci, in both the absence and presence of phleomycin (P = 5.2 × 10−5 and P = 0.00095, one-sided t-test). Interestingly, mec1∆ tel1∆ cells, like rad9∆, have a higher rate of resection (Tsabar et al. 2015), and so increased Mte1 foci in these mutants could reflect increased resection of the DSB.
Figure 3.
Mte1 foci are increased in MRX mutants and depend on MPH1. (A) The percent of cells with Mte1 foci is plotted for mutants with increased numbers of foci. Three replicates for each strain, in the absence and presence of 5 µg/ml phleomycin, are plotted. The black bars show the means of the three replicates. N ranged from 41 to 211 cells per strain per replicate. (B) The percent of cells with Mte1 foci is plotted for mutants with decreased numbers of foci (left) for untreated cells and cells grown in the presence of 5 µg/ml phleomycin for 2 hr. The black bars show the means of the replicates. The indicated samples were compared using a one-sided t-test. N ranged from 65 to 174 cells per strain per replicate. Representative images (right) of cells with Mte1 foci are shown for untreated cells and cells grown in the presence of 5 µg/ml phleomycin for 2 hr, for wild-type cells, and mph1∆ cells. (C) Representative images (left) and the percent of cells with Mph1 foci (right) are shown for untreated cells and cells grown in the presence of 5 µg/ml phleomycin for 2 hr, for wild-type cells and mte1∆ cells. The black bars show the means of the replicates. The indicated samples were compared using a one-sided t-test. N ranged from 89 to 276 cells per strain per replicate.
Two mutants, mph1∆ and rpa2-ph (a temperature-sensitive allele of RPA2), caused decreased Mte1 focus formation (Figure 3B). Interestingly, Mph1 and RPA are proposed to function together to suppress recombination (Banerjee et al. 2008), and so perhaps the effect of rpa2-ph is indirect via Mph1 recruitment. Mph1 forms nuclear foci in unperturbed cells and in MMS (Chen et al. 2009), and we find that Mph1 foci increase in the presence of phleomycin (Figure 3C). Deletion of MTE1 reduces Mph1 foci to background levels (Figure 3C), suggesting that Mph1 and Mte1 might function in concert.
Mte1 and Mph1 interact physically and are in the same genetic pathway
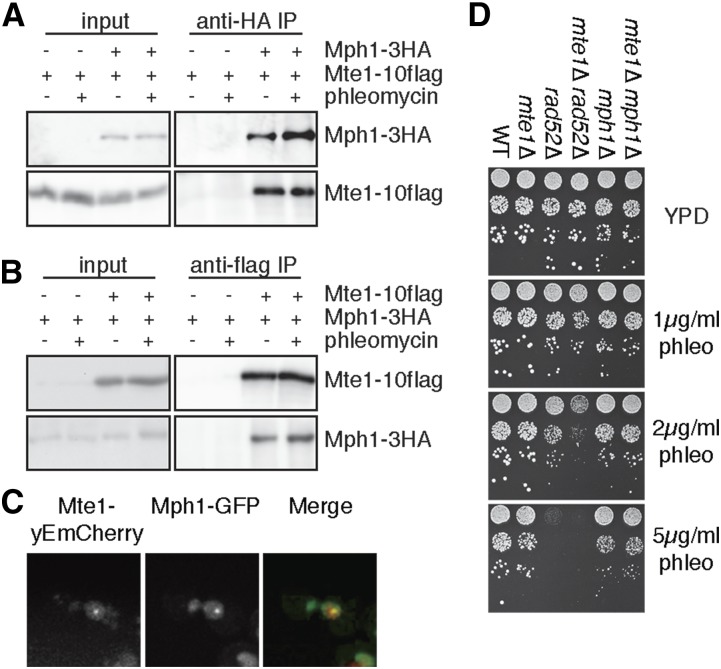
We tested whether Mte1 interacts with Mph1 in coimmunoprecipitation experiments (Figure 4). We found that Mte1 immunoprecipitates contain Mph1 (Figure 4A), and that Mph1 immunoprecipitates contain Mte1 (Figure 4B). Mte1 and Mph1 appear to interact constitutively, as the extent of coimmunoprecipitation is unaffected by the presence of phleomycin. Consistent with Mte1 and Mph1 forming a complex, 38% of Mte1 foci colocalize with Mph1 after 3 hr in phleomycin (Figure 4C). Both mte1∆ and mph1∆ confer modest sensitivity to phleomycin, and the double mutant mte1∆ mph1∆ is no more sensitive than either of the single mutants, suggesting the MTE1 and MPH1 function in the same genetic DSB response pathway. By contrast, mte1∆ and rad52∆ show additive phleomycin sensitivity (Figure 4D), indicating that MTE1 and RAD52 play nonredundant roles in DSB repair.
Figure 4.
Mte1 and Mph1 interact physically and are in the same genetic pathway. (A) Extracts of cells expressing Mte1–10flag and Mph1–3HA proteins as indicated were subjected to immunoprecipitation with an anti-HA antibody. Input and immunoprecipitate (IP) fractions were immunoblotted to detect Mte1–10flag or Mph1–3HA. (B) In the reciprocal of A, extracts of cells expressing Mte1–10flag and Mph1–3HA proteins as indicated were subjected to immunoprecipitation with an antiflag antibody. Input and IP fractions were immunoblotted to detect Mte1–10flag or Mph1–3HA. (C) Representative fluorescence micrographs showing colocalization of Mte1 with Mph1 following phleomycin treatment. The mCherry, GFP, and merged images are shown. (D) Serial 10-fold dilutions of the indicated strains were spotted on media containing the indicated concentrations of phleomycin. Plates were photographed after 2–3 days.
Mte1 and Mph1 localize to double-strand DNA breaks
Many proteins involved in double-strand DNA break repair are physically associated with chromatin adjacent to strand breaks in vivo, including Mph1 (Prakash et al. 2009). We used chromatin immunoprecipitation followed by deep sequencing to assess binding of Mte1 and Mph1 to the region flanking an induced HO double-strand break (Figure 5). The HO double-strand break was induced by growth in galactose to induce expression of the HO endonuclease gene. Cultures were sampled before HO induction, and after 4 hr in galactose, cross-linked with formaldehyde, and subjected to chromatin immunoprecipitation. Enrichment of DNA sequences in the immunoprecipitate relative to the input sample indicates regions of protein binding. We first tested Rad52, which is known to localize robustly to DSBs in vivo (Wolner et al. 2003), and found a peak of enrichment on chromosome III following HO induction, centered on the HO endonuclease site (Figure 5A). Similar peaks were detected at the induced DSB for both Mte1 and Mph1, indicating that the Mte1–Mph1 protein complex is recruited to DNA double-strand breaks in vivo (Figure 5A). Of particular interest, Mph1 enrichment at the DSB was reduced in an mte1∆ mutant, and Mte1 enrichment at the DSB was reduced (although to a lesser extent) in an mph1∆ mutant (Figure 5A). Mte1 and Mph1 protein levels were unchanged in the mutant backgrounds (Figure 5B), suggesting that the functional unit recruited to DSBs is an Mte1–Mph1 complex.
Increased phleomycin-induced DSBs in the absence of MTE1
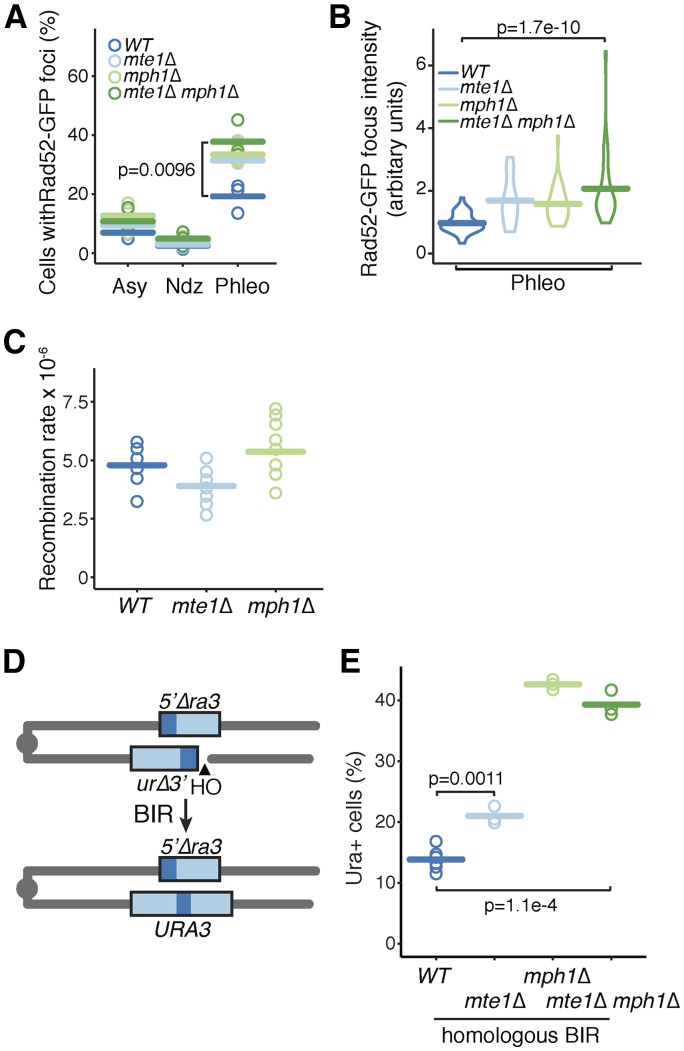
The presence of Mte1 at an induced DSB, and the sensitivity of mte1∆ strains to DSBs, suggested that Mte1 could play a role in DSB repair. We measured Rad52 focus formation as a proxy for the presence of DNA damage. Cells were blocked in G2 phase with nocodazole and treated with 50 µg/ml phleomycin for 30 min. Phleomycin caused an increase in the fraction of cells with Rad52 foci in mph1∆, mte1∆, and the mph1∆ mte1∆ double mutant compared to the wild type (Figure 6A) and an increase in Rad52 focus intensity (Figure 6B). The mph1∆ and mte1∆ single mutants and the mph1∆ mte1∆ double mutant had similar effects in both assays, suggesting that MTE1 and MPH1 function together in DSB repair. We measured recombination directly in mte1∆ mutants (Figure 6C). In the absence of DNA damage, mte1∆, like mph1∆ (Schurer et al. 2004), is proficient in mitotic recombination, displaying a recombination rate that is highly similar to the wild type.
Figure 6.
MTE1 contributes to double-strand break repair. (A) The percent of cells with Rad52 foci is plotted for the indicated strains. Samples were from logarithmic phase (Asy), G2/M (Ndz), and after treatment with 50 µg/ml phleomycin for 30 min (Phleo). Three replicates for each strain, for each condition, are plotted. The bars show the mean of the three replicates. N ranged from 42 to 160 cells per strain per replicate. The indicated samples were compared using a one-sided t-test. (B) The distribution of Rad52 focus intensity is plotted for the indicated strains, after treatment with 50 µg/ml phleomycin for 30 min (Phleo). The width of the box indicates the number of foci with a given intensity, and the bar indicates the mean. N = 30 for all samples. The distributions were compared using the Wilcoxon rank sum test. (C) The direct repeat recombination rate (events/cell/generation) was measured for the indicated strains. Each assay was a fluctuation test of nine cultures. The bars show the means of 10 replicates. (D) Schematic of the strain used to measure BIR. A DSB is induced by expression of the HO endonuclease gene. The right arm of chromosome III invades the left arm, and replication restores a functional URA3 gene. (E) BIR was quantified for wild-type, mte1∆, mph1∆, and mte1∆ mph1∆ strains. N ranged from 3 to 6. The bars show the means of the replicates, and strains were compared using a t-test.
MTE1 suppresses BIR
MPH1 suppresses BIR during double-strand break repair (Luke-Glaser and Luke 2012; Stafa et al. 2014). Given the physical and genetic interactions between Mte1 and Mph1 that our work has revealed, we tested whether MTE1 also plays a role in suppressing BIR. We induced a DSB in strains carrying a modified chromosome V with a truncated ura3 allele adjacent to an HO endonuclease site. Upon induction of the double-strand break, the truncated allele is repaired using donor sequences located on the other arm of chromosome V to yield Ura+ colonies (Figure 6D). In homologous BIR, where the sequences that recombine share 108 bp of homology, deletion of mte1 results in increased BIR (Figure 6E). Deletion of mph1 also results in increased BIR (Figure 6E), as previously reported (Stafa et al. 2014). The double mutant mph1∆ mte1∆ displays increased BIR, much like mph1∆ (Figure 6E), indicating that MPH1 and MTE1 function in the same genetic pathway, and that mph1∆ is epistatic to mte1∆. Since the magnitude of effect is greater in the mph1∆, we infer that loss of MTE1 only partially compromises MPH1 function, consistent with reduced (but not eliminated) recruitment of Mph1 to DSBs in mte1∆ (Figure 5A). Together the data indicate that MTE1, like MPH1, is an important suppressor of BIR and therefore a suppressor of loss of heterozygosity.
Discussion
In response to DNA damage, most homologous recombination proteins are recruited to sites of double-strand DNA breaks. Among them, Rad52 is a key recombination protein and the Rad52 focus is considered to be a sensitive indicator of DNA repair (Lisby et al. 2001, 2003; Alvaro et al. 2007). We identified 29 proteins that localize to Rad52 foci in response to DNA damage. Among them, we identified a role for YGR042W/MTE1 in DNA double-strand break repair. Similar to many DNA repair proteins, Mte1 forms nuclear foci in response to double-strand breaks, and Mte1 foci only form when the DNA helicase Mph1 is present. Mte1 forms protein complexes with Mph1, and both proteins are recruited to the chromatin flanking double-strand DNA breaks in vivo. In the absence of MTE1 the Rad52 repair centers accumulate, and MTE1 is important for suppressing break-induced replication. Together our data indicate that Mph1 function in recombination repair of double-strand breaks requires Mte1.
How does Mte1 impact Mph1 function?
Mte1 and Mph1 appear to be members of a constitutive complex. The interaction between these two proteins, whether direct or indirect, was readily detected by coimmunoprecipitation of either protein even in the absence of DNA damage. Mte1 is important for Mph1 nuclear focus formation, and more importantly, for recruitment of Mph1 to double-strand breaks in vivo. These data suggest that Mph1 functions as part of a protein complex containing Mte1. Consistent with this notion, deletion of MTE1 conferred sensitivity to phleomycin that was similar to that conferred by deletion of MPH1, and the mte1∆ mph1∆ double mutant was no more sensitive, indicating that these genes function in the same genetic pathway for phleomycin resistance.
Our data suggest that Mte1 is not simply a structural component of Mph1 complexes, as Mte1 appears to have little effect on Mph1 stability in vivo. Mte1 could presumably play a role in targeting Mph1 to specific substrates in vivo. Such a role would be consistent with our findings that Mph1 nuclear foci and recruitment or retention of Mph1 at double-strand breaks is compromised when MTE1 is absent. MPH1 suppresses crossovers and BIR by unwinding D-loop recombination intermediates (Prakash et al. 2009; Mazon and Symington 2013; Stafa et al. 2014). We find that MTE1 suppresses BIR much like MPH1, thus it is also possible that Mte1 facilitates some aspect of Mph1 catalysis. Mte1 lacks obvious catalytic domains, and purified Mph1 is capable of unwinding D-loops and extended D-loops in vitro in the absence of Mte1 (Sun et al. 2008; Prakash et al. 2009; Sebesta et al. 2011). Nonetheless, it will be of great interest to determine whether Mte1 modulates Mph1 activity in vitro, as it appears that in vivo Mph1 is normally assembled into complexes that contain Mte1.
Orthologs of MTE1
MTE1 has readily identifiable orthologs in other yeasts, including Kluyveromyces, Candida, Pichia, and Ashbya species. MTE1 appears to be an ortholog of the Schizosaccharomyces pombe dbl2+ gene (Yu et al. 2013). Dbl2 colocalizes with the fission yeast Rad52, and with double-strand breaks, and is important for nuclear focus formation by Fml1, the fission yeast ortholog of Mph1 (Yu et al. 2013). dbl2+ does not have a clear role in fml1+ inhibition of crossovers or inhibition of BIR as of yet, so it is not known if dbl2+ plays a functional role similar to MTE1. Mte1 contains a domain of unknown function, DUF2439, which is found in the human ZGRF1 protein. The DUF2439 domain is also found in Dbl2 (Yu et al. 2013), but does not appear to be important for DNA damage resistance or for nuclear focus formation. Further, ZGRF1 is likely membrane anchored and so might not be a true ortholog of Mte1. Nonetheless, as several lines of evidence suggest that Mph1 is an ortholog of the human FANCM protein (Whitby 2010; Xue et al. 2015), our evidence that Mph1 functions in concert with an important cofactor is consistent with modulation of diverse FANCM activities by different binding partners in metazoans (Ciccia et al. 2007; Deans and West 2009; Singh et al. 2010; Yan et al. 2010; Leung et al. 2012; Yan et al. 2012). It will be of interest to determine how Mte1 modulates Mph1 function, and whether FANCM is similarly regulated.
Acknowledgments
We thank members of the laboratories of Brenda Andrews and Charlie Boone for assistance with microscopy and Tobit Glenhaber and Linus Glenhaber for assistance in constructing mte1 deletion strains to measure BIR efficiency. We also thank Lorraine Symington and Daniel Durocher for providing strains and Patrick Sung, Michael Lisby, and Lorraine Symington for sharing data prior to publication. This work was supported by grants from the Canadian Cancer Society Research Institute (impact grant 702310 to G.W.B.), the Cancer Research Society (G.W.B.), the Natural Sciences and Engineering Research Council of Canada (discovery grant 327612 to Z.Z.), and the National Institutes of Health (GM76020 to J.E.H.).
Author contributions: A.Y. designed and carried out the experiments, wrote the paper, and edited the paper; T.K. analyzed ChIP-seq data and edited the paper; R.P.A. performed and analyzed BIR assays; S.M. performed recombination assays and edited the paper; J.O. performed recombination analysis and constructed strains; J.E.H. edited the paper; Z.Z. analyzed ChIP-seq data and edited the paper; and G.W.B. designed the experiments, wrote the paper, and edited the paper. The authors declare that they have no conflict of interest.
Footnotes
Communicating editor: N. M. Hollingsworth
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.185454/-/DC1.
Literature Cited
- Alvaro D., Lisby M., Rothstein R., 2007. Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination. PLoS Genet. 3: e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R. P., Tsaponina O., Greenwell P. W., Lee C. S., Du W., et al. , 2014. Chromosome rearrangements via template switching between diverged repeated sequences. Genes Dev. 28: 2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askree S. H., Yehuda T., Smolikov S., Gurevich R., Hawk J., et al. , 2004. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 101: 8658–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint A., Kim T., Gallo D., Cussiol J. R., Bastos de Oliveira F. M., et al. , 2015. Assembly of Slx4 signaling complexes behind DNA replication forks. EMBO J. 34: 2182–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Smith S., Oum J. H., Liaw H. J., Hwang J. Y., et al. , 2008. Mph1p promotes gross chromosomal rearrangement through partial inhibition of homologous recombination. J. Cell Biol. 181: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M. G., Matos J., Rass U., Ip S. C., West S. C., 2010. Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae. DNA Repair (Amst.) 9: 394–402. [DOI] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Burgess R. C., Lisby M., Altmannova V., Krejci L., Sung P., et al. , 2009. Localization of recombination proteins and Srs2 reveals anti-recombinase function in vivo. J. Cell Biol. 185: 969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez A., Agrawal V., Johnson F. B., 2011. Homologous recombination-dependent rescue of deficiency in the structural maintenance of chromosomes (Smc) 5/6 complex. J. Biol. Chem. 286: 5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Choi K., Szakal B., Arenz J., Duan X., et al. , 2009. Interplay between the Smc5/6 complex and the Mph1 helicase in recombinational repair. Proc. Natl. Acad. Sci. USA 106: 21252–21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Szakal B., Castellucci F., Branzei D., Zhao X., 2013. DNA damage checkpoint and recombinational repair differentially affect the replication stress tolerance of Smc6 mutants. Mol. Biol. Cell 24: 2431–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Hong E. L., Amundsen C., Balakrishnan R., Binkley G., et al. , 2012. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res. 40: D700–D705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Szakal B., Chen Y. H., Branzei D., Zhao X., 2010. The Smc5/6 complex and Esc2 influence multiple replication-associated recombination processes in Saccharomyces cerevisiae. Mol. Biol. Cell 21: 2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A., Ling C., Coulthard R., Yan Z., Xue Y. et al, 2007. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol. Cell 25: 331–343. [DOI] [PubMed] [Google Scholar]
- Deans A. J., West S. C., 2009. FANCM connects the genome instability disorders Bloom’s Syndrome and Fanconi Anemia. Mol. Cell 36: 943–953. [DOI] [PubMed] [Google Scholar]
- Denervaud N., Becker J., Delgado-Gonzalo R., Damay P., Rajkumar A. S., et al. , 2013. A chemostat array enables the spatio-temporal analysis of the yeast proteome. Proc. Natl. Acad. Sci. USA 110: 15842–15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S. R., Dietrich F. S., Fisk D. G., Binkley G., Balakrishnan R., et al. , 2014. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 (Bethesda) 4: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D., Schuster T., Hegemann J. H., Becher D., Feldmann H., et al. , 1999. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol. Gen. Genet. 262: 683–702. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Dibitetto D., De Gregorio G., Eapen V. V., Rawal C. C., et al. , 2015. Functional interplay between the 53BP1-ortholog Rad9 and the Mre11 complex regulates resection, end-tethering and repair of a double-strand break. PLoS Genet. 11: e1004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina I., Colding C., Henriksen P., Beli P., Nakamura K., et al. , 2015. Cmr1/WDR76 defines a nuclear genotoxic stress body linking genome integrity and protein quality control. Nat. Commun. 6: 6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann S. M., Oestergaard V. H., Haas C., Salis P., Motegi A., et al. , 2011. Dpb11/TopBP1 plays distinct roles in DNA replication, checkpoint response and homologous recombination. DNA Repair (Amst.) 10: 210–224. [DOI] [PubMed] [Google Scholar]
- Ho C. K., Mazon G., Lam A. F., Symington L. S., 2010. Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Mol. Cell 40: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., et al. , 2003. Global analysis of protein localization in budding yeast. Nature 425: 686–691. [DOI] [PubMed] [Google Scholar]
- Ivanov E. L., Sugawara N., White C. I., Fabre F., Haber J. E., 1994. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 3414–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y., D’Andrea A. D., 2010. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 24: 1680–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro F., Sapountzi V., Granata M., Pellicioli A., Vaze M., et al. , 2008. Histone methyltransferase Dot1 and Rad9 inhibit single-stranded DNA accumulation at DSBs and uncapped telomeres. EMBO J. 27: 1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea D. E., Coulson C. A., 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49: 264–285. [DOI] [PubMed] [Google Scholar]
- Leung J. W., Wang Y., Fong K. W., Huen M. S., Li L., et al. , 2012. Fanconi anemia (FA) binding protein FAAP20 stabilizes FA complementation group A (FANCA) and participates in interstrand cross-link repair. Proc. Natl. Acad. Sci. USA 109: 4491–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M., Rothstein R., Mortensen U. H., 2001. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. USA 98: 8276–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M., Mortensen U. H., Rothstein R., 2003. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat. Cell Biol. 5: 572–577. [DOI] [PubMed] [Google Scholar]
- Lisby M., Barlow J. H., Burgess R. C., Rothstein R., 2004. Choreography of the DNA damage response; spatiotemporal relationships among checkpoint and repair proteins. Cell 118: 699–713. [DOI] [PubMed] [Google Scholar]
- Luke-Glaser S., Luke B., 2012. The Mph1 helicase can promote telomere uncapping and premature senescence in budding yeast. PLoS One 7: e42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon G., Symington L. S., 2013. Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates. Mol. Cell 52: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Pesudo L. Q., McRee S., Bathe M., Samson L. D., 2013. Genome-wide single-cell-level screen for protein abundance and localization changes in response to DNA damage in S. cerevisiae. Nucleic Acids Res. 41: 9310–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo J. A., Cohen J., Toczyski D. P., 2001. Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev. 15: 2809–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicioli A., Lucca C., Liberi G., Marini F., Lopes M., et al. , 1999. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 18: 6561–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R., Krejci L., Van Komen S., Anke Schurer K., Kramer W., et al. , 2005. Saccharomyces cerevisiae MPH1 gene, required for homologous recombination-mediated mutation avoidance, encodes a 3′ to 5′ DNA helicase. J. Biol. Chem. 280: 7854–7860. [DOI] [PubMed] [Google Scholar]
- Prakash R., Satory D., Dray E., Papusha A., Scheller J., et al. , 2009. Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes Dev. 23: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Zaidi I. W., Vaisica J. A., Peter M., Brown G. W., 2008. Regulation of Rtt107 recruitment to stalled DNA replication forks by the cullin Rtt101 and the Rtt109 acetyltransferase. Mol. Biol. Cell 19: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., Schurer A., Rudolph C., Hettwer S., Kramer W., 2000. MPH1, a yeast gene encoding a DEAH protein, plays a role in protection of the genome from spontaneous and chemically induced damage. Genetics 155: 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer K. A., Rudolph C., Ulrich H. D., Kramer W., 2004. Yeast MPH1 gene functions in an error-free DNA damage bypass pathway that requires genes from Homologous recombination, but not from postreplicative repair. Genetics 166: 1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebesta M., Burkovics P., Haracska L., Krejci L., 2011. Reconstitution of DNA repair synthesis in vitro and the role of polymerase and helicase activities. DNA Repair (Amst.) 10: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura T., Ando S., Matsumoto K., Sugimoto K., 1998. Functional and physical interaction between Rad24 and Rfc5 in the yeast checkpoint pathways. Mol. Cell. Biol. 18: 5485–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. R., Saro D., Ali A. M., Zheng X. F., Du C. H., et al. , 2010. MHF1–MHF2, a histone-fold-containing protein complex, participates in the Fanconi anemia pathway via FANCM. Mol. Cell 37: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Rothstein R., 1999. An allele of RFA1 suppresses RAD52-dependent double-strand break repair in Saccharomyces cerevisiae. Genetics 151: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafa A., Donnianni R. A., Timashev L. A., Lam A. F., Symington L. S., 2014. Template switching during break-induced replication is promoted by the Mph1 helicase in Saccharomyces cerevisiae. Genetics 196: 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N., Wang X., Haber J. E., 2003. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell 12: 209–219. [DOI] [PubMed] [Google Scholar]
- Sun W., Nandi S., Osman F., Ahn J. S., Jakovleska J., et al. , 2008. The FANCM ortholog Fml1 promotes recombination at stalled replication forks and limits crossing over during DNA double-strand break repair. Mol. Cell 32: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., 2002. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66: 630–670, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W., 1983. The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Tkach J. M., Yimit A., Lee A. Y., Riffle M., Costanzo M., et al. , 2012. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol. 14: 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsabar M., Eapen V. V., Mason J. M., Memisoglu G., Waterman D. P., et al. , 2015. Caffeine impairs resection during DNA break repair by reducing the levels of nucleases Sae2 and Dna2. Nucleic Acids Res. 43: 6889–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby M. C., 2010. The FANCM family of DNA helicases/translocases. DNA Repair (Amst.) 9: 224–236. [DOI] [PubMed] [Google Scholar]
- Wolner B., van Komen S., Sung P., Peterson C. L., 2003. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol. Cell 12: 221–232. [DOI] [PubMed] [Google Scholar]
- Wu L., Hickson I. D., 2003. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874. [DOI] [PubMed] [Google Scholar]
- Wu L., Bachrati C. Z., Ou J., Xu C., Yin J., et al. , 2006. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc. Natl. Acad. Sci. USA 103: 4068–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Choi K., Bonner J., Chiba T., Kwon Y., et al. , 2014. Restriction of replication fork regression activities by a conserved SMC complex. Mol. Cell 56: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Sung P., Zhao X., 2015. Functions and regulation of the multitasking FANCM family of DNA motor proteins. Genes Dev. 29: 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Delannoy M., Ling C., Daee D., Osman F., et al. , 2010. A histone-fold complex and FANCM form a conserved DNA-remodeling complex to maintain genome stability. Mol. Cell 37: 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Guo R., Paramasivam M., Shen W., Ling C., et al. , 2012. A ubiquitin-binding protein, FAAP20, links RNF8-mediated ubiquitination to the Fanconi anemia DNA repair network. Mol. Cell 47: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Bachrati C. Z., Ou J., Hickson I. D., Brown G. W., 2010. Human topoisomerase IIIalpha is a single-stranded DNA decatenase that is stimulated by BLM and RMI1. J. Biol. Chem. 285: 21426–21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Ren J. Y., Zhang J. M., Suo F., Fang X. F., et al. , 2013. A proteome-wide visual screen identifies fission yeast proteins localizing to DNA double-strand breaks. DNA Repair (Amst.) 12: 433–443. [DOI] [PubMed] [Google Scholar]
- Zheng X. F., Prakash R., Saro D., Longerich S., Niu H., et al. , 2011. Processing of DNA structures via DNA unwinding and branch migration by the S. cerevisiae Mph1 protein. DNA Repair (Amst.) 10: 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chung W. H., Shim E. Y., Lee S. E., Ira G., 2008. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains are available upon request. Table S1 contains the genotypes of all strains used. Table S2 contains statistics for all deep sequencing, including NCBI Sequence Read Archive (SRA) accession numbers.