Abstract
Accurate segregation of homologous chromosomes during meiosis depends on their ability to remain physically connected throughout prophase I. For homologs that achieve a crossover, sister chromatid cohesion distal to the chiasma keeps them attached until anaphase I. However, in Drosophila melanogaster wild-type oocytes, chromosome 4 never recombines, and the X chromosome fails to cross over in 6–10% of oocytes. Proper segregation of these achiasmate homologs relies on their pericentric heterochromatin-mediated association, but the mechanism(s) underlying this attachment remains poorly understood. Using an inducible RNA interference (RNAi) strategy combined with fluorescence in situ hybridization (FISH) to monitor centromere proximal association of the achiasmate FM7a/X homolog pair, we asked whether specific heterochromatin-associated proteins are required for the association and proper segregation of achiasmate homologs in Drosophila oocytes. When we knock down HP1a, H3K9 methytransferases, or the HP1a binding partner Piwi during mid-prophase, we observe significant disruption of pericentric heterochromatin-mediated association of FM7a/X homologs. Furthermore, for both HP1a and Piwi knockdown oocytes, transgenic coexpression of the corresponding wild-type protein is able to rescue RNAi-induced defects, but expression of a mutant protein with a single amino acid change that disrupts the HP1a-Piwi interaction is unable to do so. We show that Piwi is stably bound to numerous sites along the meiotic chromosomes, including centromere proximal regions. In addition, reduction of HP1a or Piwi during meiotic prophase induces a significant increase in FM7a/X segregation errors. We present a speculative model outlining how HP1a and Piwi could collaborate to keep achiasmate chromosomes associated in a homology-dependent manner.
Keywords: meiosis, chromosome segregation, H3K9 methyltransferases, pericentric heterochromatin, nondisjunction
ONE unique feature of meiosis is segregation of homologous chromosomes during the first division followed by segregation of sisters during meiosis II. For their proper segregation, homologs need to find each other and physically associate during meiotic prophase I. Prolonged association of homologous chromosomes is essential for establishing biorientation of their centromeres and proper microtubule attachments on the meiosis I spindle. If their association is not maintained, homologs are free to attach to microtubules emanating from the same pole, which can lead to nondisjunction and the production of aneuploid gametes. A crossover between homologous chromosomes is normally sufficient to ensure their accurate segregation because sister chromatid cohesion distal to the chiasma keeps the pair of recombinant homologs physically attached until anaphase I (Buonomo et al. 2000; Bickel et al. 2002; Hodges et al. 2005).
In the Drosophila oocyte, the X chromosome fails to cross over 6–10% of the time, and chromosome 4 never recombines (Ashburner et al. 2005). Still, these achiasmate chromosomes segregate faithfully in wild-type oocytes (Grell 1976). Elegant genetic experiments have uncovered the existence of a backup system in Drosophila oocytes that ensures proper segregation of homologs that lack a crossover and that is distinct from the achiasmate pathway that operates in the male germline (Theurkauf and Hawley 1992; Hawley et al. 1993; Whyte et al. 1993; Moore et al. 1994; Karpen et al. 1996). Accurate segregation of achiasmate homologs in Drosophila oocytes requires homology-dependent interactions within their pericentric heterochromatin (Hawley et al. 1993; Karpen et al. 1996). Pericentric heterochromatin-mediated association of achiasmate homologs (for which we have adopted the acronym PHeMAAH) persists throughout prophase I (Dernburg et al. 1996), and heterochromatic threads connecting homologs have been visualized in oocytes during prometaphase I congression (Hughes et al. 2009). Models to explain how achiasmate homologs remain physically associated include the possibility of heterochromatin entanglements (Hughes et al. 2009) as well as heterochromatin-bound proteins acting to connect the two homologs (Karpen et al. 1996). Although a role for pericentric heterochromatin in PHeMAAH is well established, the function of heterochromatin proteins in this process remains largely unexplored.
HP1a, an essential heterochromatin protein encoded by the Drosophila Su(var)205 gene, is enriched in pericentric heterochromatin (James et al. 1989), making it an attractive candidate for participation in PHeMAAH. HP1a is highly conserved, and functional orthologs in other species are also required for proper heterochromatin structure and function (Vermaak and Malik 2009; Zeng et al. 2010a; Canzio et al. 2014). The N-terminal chromodomain of HP1a binds to the canonical H3K9me2/3 heterochromatin mark, while its chromo shadow domain (CSD) at the C-terminus mediates the formation of homodimers (Mendez et al. 2011). In vitro experiments have demonstrated that HP1 dimers can interact with two nonadjacent nucleosomes within a multinucleosomal array (Canzio et al. 2011) or serve as a bridge between two independent nucleosomal arrays (Azzaz et al. 2014). In addition, when HP1a is tethered to a specific chromosomal site within Drosophila polytene chromosomes, its interaction with a distant location on the chromosome promotes looping (Azzaz et al. 2014). HP1a-mediated nucleosome-nucleosome interactions are thought to be critical in establishing and maintaining the compact structure of heterochromatin. Another key feature of the HP1a dimer is the binding surface created when its two CSDs come together, and this interaction platform is able to recruit additional proteins to the heterochromatin, including the nuclear protein Piwi (Mendez et al. 2013).
Piwi, a member of the Argonaute superfamily of proteins, is best known for its ability to bind Piwi-interacting RNAs (piRNAs) and prevent transposon mobilization in the germline (Mani and Juliano 2013; Ku and Lin 2014). Most piRNA sequences in the Drosophila genome map to heterochromatic regions (Saito et al. 2006), and several lines of investigation have demonstrated that Piwi and HP1a can physically interact (Brower-Toland et al. 2007; Mendez et al. 2011). Moreover, transcriptional silencing of retrotransposons by the Piwi-piRNA system involves recruitment of HP1a to transposable elements (Wang and Elgin 2011; Sentmanat and Elgin 2012; Huang et al. 2013). Piwi also participates in activities that are not directly related to transposon silencing. In the early Drosophila embryo, maternally inherited Piwi protein is required for localization of HP1a to specific genomic regions and subsequent establishment of heterochromatin at those locations (Gu and Elgin 2013). Piwi also has been implicated in a phenomenon termed pairing-sensitive silencing, in which “crosstalk” between homologs results in transcriptional repression of two transgenes on homologous chromosomes, but only when the transgenes are paired (Grimaud et al. 2006). The involvement of Piwi in heterochromatin formation and its role in facilitating communication between paired homologs raise the possibility that Piwi also may promote PHeMAAH in the Drosophila oocyte.
Here we explore a potential role for HP1a and Piwi in maintaining the association of achiasmate homologs during meiotic prophase I. We find that reduction of either protein in the oocyte disrupts PHeMAAH and causes achiasmate chromosomes to missegregate. Significantly, we find that the ability of HP1a and Piwi to promote PHeMAAH depends on their ability to interact with each other. We provide a speculative model for how these two heterochromatin localized proteins could collaborate to keep the pericentric heterochromatin of achiasmate homologs physically associated in a homology-dependent manner.
Materials and Methods
Fly stocks
Flies were raised on standard cornmeal/molasses food at 25°. Stocks containing a wild-type or mutant V30A Piwi transgene were provided by S. Elgin (Brower-Toland et al. 2007). All other fly stocks were obtained from Bloomington Drosophila Stock Center at Indiana University, the Vienna Drosophila RNAi Center, and the Harvard Transgenic RNAi Project or generated for this study (Supplemental Material, Table S1 provides a complete list of stocks and their sources). The Su(var)2055 allele has been characterized previously (Eissenberg et al. 1992), and the GFP-HP1a transgenic construct containing the native Su(var)205 promoter is derived from the previously described RFP-HP1a transgene (Wen et al. 2008; Lipsick 2010).
Nondisjunction tests
Because Drosophila tolerate certain sex chromosome aneuploidies (XXY and XO), we can assay meiotic nondisjunction (NDJ) in females by using X chromosome markers that allow us to visually distinguish progeny arising from normal X chromosome segregation and exceptional progeny for which X chromosome missegregation resulted in diplo-X or nullo-X oocytes. To accommodate the visible markers carried on specific transgenes, we used two slightly different cross schemes to measure nondisjunction (Figure S1). For HP1a mutant and HP1a RNAi NDJ tests, FM7a/y females were crossed to attached X^Y, v f B males, and progeny were scored based on sex and body color. For Piwi RNAi NDJ tests, FM7a/w B females were crossed to males with attached X^Y chromosomes that carried a wild-type Bar allele, and progeny were scored based on sex and eye morphology. In both cross schemes, all the normal progeny but only half the exceptional progeny are expected to survive (XXX^Y and 00 are lethal), so total %NDJ was calculated as {[2 × (diplos + nullos)]/(n + diplos + nullos)} × 100, where n is the total number of progeny scored. P-values were calculated using the method described in Zeng et al. (2010b).
Creation of GFP-HP1a constructs
Site-directed mutagenesis was used (Agilent QuikChange II Kit) to introduce silent mutations into the enhanced green fluorescent protein (EGFP) complementary DNA (cDNA) sequence in pEGFP-C1 (Clontech Laboratories) in order to remove KpnI, BamHI, and XbaI sites from the coding sequence and facilitate downstream cloning. During PCR amplification of EGFP, a short linker sequence encoding four amino acids was introduced at the 3′ end of the open reading frame (ORF), as well as EagI and SpeI restriction sites flanking the ORF, which were used to clone the PCR product into the pUASP-attB vector (Takeo et al. 2012). Full-length HP1a cDNA (LD10408) in pBluescript SK(−) was obtained from the Drosophila Genome Resource Center and used as a template for site-directed mutagenesis to create a W200A mutant HP1a construct in the SK vector. The wild-type and W200A HP1a clones were used as PCR templates to generate HP1a ORF amplicons lacking the 3′ UTR (thereby conferring HP1a RNAiV20 resistance), and each was inserted downstream of EGFP in pUASP. Table S2 contains the sequences of all primers used for site-directed mutagenesis and cloning. The EGFP-HP1a inserts were excised from pUASP-attB and inserted into the pUAST-attB vector (Bischof et al. 2007) using EagI and XbaI. All clones were verified by sequencing, and transgenic flies with insertions at the attP40 site on chromosome 2 were generated by Genetic Services, Inc. (Cambridge, MA).
For each insertion, a series of crosses was performed to generate a stock carrying both the GFP-HP1a transgene (chromosome 2) and the HP1a RNAiV20 transgene (chromosome 3). Expression of pUASP and pUAST versions of wild-type HP1a insertions was tested in fluorescence in situ hybridization (FISH) experiments, but preliminary results indicated that the pUAST resulted in more robust rescue of the HP1a knockdown (KD) phenotype; therefore, pUAST insertions were used for subsequent experiments.
Immunoblotting
Flies were fattened for 2–3 days on yeast, and ovaries from 20 females were dissected in 1× PBS, frozen in liquid nitrogen, and stored at −80° until ready to use. Frozen ovaries were homogenized in a solution of 8 M urea, 2% SDS, 100 mM Tris HCl (pH 6.8), 5% Ficoll, and 2 mM Pefabloc (Sigma-Aldrich), heated at 95° for 5 min, and centrifuged at 13,000 rpm for 5 min to pellet debris. The supernatant was transferred to a new tube, and protein concentration was quantified using the Bio-Rad DC protein assay. Then 15 µg of protein per lane (for HP1a blots) or 25 µg protein per lane (for Piwi blots) was separated on a Bio-Rad Mini-PROTEAN TGX Stain-Free 4–20% Gradient Gel and transferred to a Millipore PVDF membrane using a Bio-Rad Trans-Blot SD Semi-Dry apparatus. HP1a blots were cut at 37 kDa to allow separate processing of HP1a and tubulin bands. Each blot was stained with rabbit α-Piwi 1:5000 (Klattenhoff et al. 2009), rabbit α-HP1a 1:5000 (Covance PRC-291C), or monoclonal mouse α-tubulin 1:1000 (DM1A, Sigma T9206). After incubation with Promega AP-conjugated secondary antibodies diluted 1:7500, Lumi-Phos reagent (Thermo Scientific) or Immuno-Star AP substrate (Bio-Rad) was applied to the blots, and they were imaged on a Bio-Rad ChemiDoc Touch System. Signal intensity was quantified with Bio-Rad Image Lab 5.2.1 software. HP1a bands were normalized to the tubulin signal, and the normalized KD signal was divided by the normalized control signal to determine relative protein amount. Because Piwi KD ovaries were consistently smaller than control ovaries, we were concerned that normalization using tubulin might not be accurate. Therefore, for Piwi blots, we used Bio-Rad Stain-Free Gel Technology to quantify the total protein signal in each lane of the blot and then normalized the Piwi band intensity to the total protein in each lane. Normalized KD signal was divided by the normalized control signal to quantify Piwi knockdown.
Fluorescence in situ hybridization (FISH)
Ovaries were probed using a Cy3-labeled oligo (5′-Cy3-AGGGATCGTTAGCACTCGTAAT; Integrated DNA Technologies) that corresponds to a portion of the 359-bp sequence of the 1.688 satellite DNA, repeats of which comprise approximately 11 Mb of the heterochromatin proximal to the X chromosome centromere (Dernburg et al. 1996). Females of each genotype were fattened for 1–2 days on yeast with males. Ovaries from 12 females were dissected in 1× modified Robb’s solution (Theurkauf and Hawley 1992), gently splayed open with a tungsten needle, and transferred to a microfuge tube. Preheated (37°) fix solution (4% formaldehyde, 0.1 M sodium cacodylate, 0.1 M sucrose, 0.04 M sodium acetate, and 0.01 M EGTA) was added to the ovaries, the tube was nutated for 3 min at room temperature, and the ovaries were allowed to settle for 1 min. Ovaries were rinsed three times and washed three times for 10 min in 2× SSCT (0.3 M sodium chloride, 0.03 M sodium citrate, and 0.1% Tween-20) and then incubated for 10 min each in 20, 40, and 50% formamide in 2× SSCT at room temperature. Samples were incubated for 2 hr at 37° in prewarmed 50% formamide in 2× SSCT and then transferred to a 0.2-ml PCR tube. Then 40 µl of hybridization buffer (3× SSC, 50% formamide, 10% dextran sulfate, and 2.5 ng/µl 359 probe) was added, and the samples were incubated in a PCR machine at 37° for 5 min, 92° for 3 min, and 37° overnight. Following hybridization, ovaries were transferred to a 0.5-ml microfuge tube and washed three times for 20 min and three times for 10 min at 37° in prewarmed 50% formamide in 2× SSCT, moved to room temperature, and washed for 10 min each in 50, 40, and 20% formamide in 2× SSCT and for 10 min in 2× SSCT. A 20-min incubation in DAPI (1.0 µg/ml in 2× SSCT) was followed by three rinses and two times 10-min washes in 2× SSCT. Stained ovaries were separated into individual ovarioles with tungsten needles, spread on poly-l-lysine-coated coverslips, and mounted in Prolong Gold (Molecular Probes).
FISH scoring
Each slide was scanned systematically, and ovarioles were scored as they were encountered. Ovarioles were scored only if they contained four or more egg chambers with clearly discernible oocyte nuclei. The developmental stage of each egg chamber was determined based on size and morphology (Ashburner et al. 2005). The “two-spot” phenotype was assigned to oocytes in which the DAPI signal colocalized with two distinct FISH spots and in which a small spot was clearly visible, distinct from the larger spot, and in which the two spots were separated by at least the diameter of the small spot. In addition to the phenotypes depicted in Figure 1C, other configurations were observed occasionally. Two spots of equal size were interpreted as representing the satellite region of the normal X chromosome and the distal end of FM7a, with the small FM7a pericentric region associated with one or the other. Because we could not be certain in this case whether the pericentric regions were associated or apart, these oocytes were conservatively scored as exhibiting “normal” PHeMAAH. When two large spots and a single small spot were observed, this was interpreted as indicating separation of all three regions of satellite sequence and scored as “defective” PHeMAAH because the pericentric regions of the two chromosomes were unambiguously separated. KD and control genotypes were compared using a two-tailed Fisher’s exact test to obtain P-values. Thirty ovarioles were scored per slide. For each genotype, because we observed negligible variation between slides, we pooled our results when more than one slide was scored. Then 95% confidence intervals were calculated using the modified Wald method (Agresti and Coull 1998) available on GraphPad.
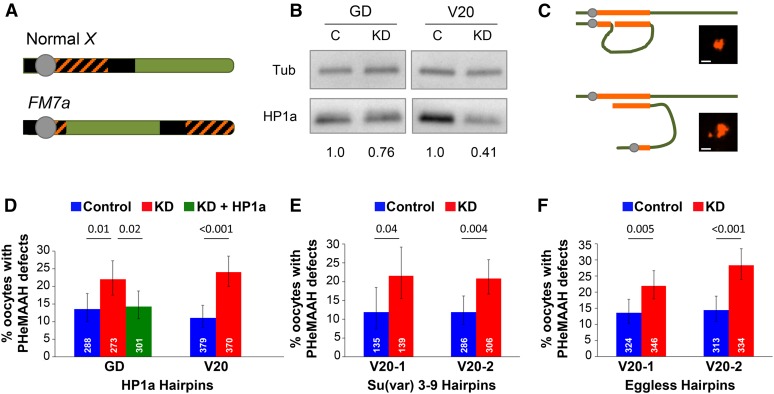
Figure 1.
Reduction of HP1a protein or H3K9 methyltransferases in FM7a/X oocytes disrupts PHeMAAH. (A) Diagram of wild-type Drosophila X chromosome and its derivative, the FM7a balancer. Heterochromatin is shown as black. Orange cross-hatching denotes the 359-bp satellite DNA repeats recognized by the FISH probe. The centromere is the gray circle. Rearrangements within the FM7a euchromatin (green) suppress recombination with a normal X chromosome. (B) Immunoblots of ovary extracts from HP1a knockdown (KD) and control (C) flies. The relative intensity of HP1a signal for each set of knockdown and control extracts is shown below the blot. For each lane, HP1a signals were normalized to tubulin. GD and V20 correspond to the HP1a hairpins used for knockdown. (C) Schematic diagram of heterochromatic pairing between FM7a and a normal X chromosome. Pairing between euchromatic regions has been omitted for clarity. The 359-bp repeats are shown in orange. When PHeMAAH is normal (top), association between heterochromatic regions results in one large, visible FISH signal. When PHeMAAH is disrupted (bottom), the pericentric regions separate, resulting in one small dot corresponding to the FM7a pericentric region and one large dot representing the remaining 359-bp repeats of the FM7a and normal X chromosomes. Inset images show representative oocyte FISH signals. Scale bars, 1.0 µm. See Materials and Methods for other heterochromatin pairing configurations not shown. (D–F) Levels of FM7a/X PHeMAAH defects measured by FISH. P-values are shown above the bars, and 95% confidence intervals are presented as error bars. Number of oocytes assayed for each genotype is indicated in white. Control flies carry the UAS hairpin transgene but lack the matα driver, so the hairpin is not expressed. KD flies express the hairpin under the control of the matα driver (see Materials and Methods). Complete data for these experiments are provided in Table S3. (D) In HP1a RNAi experiments, 60 ovarioles were scored for each genotype using the GD hairpin, and 90 ovarioles were scored for each V20 hairpin genotype. (E) In Su(var)3–9 RNAi experiments, 30 and 60 ovarioles were scored for each genotype containing the V20-1 and V20-2 hairpins, respectively. (F) For both Eggless hairpins, 60 ovarioles were scored for each genotype.
Chromosome spreads
Preparation of chromosome spreads was performed as described previously (Khetani and Bickel 2007), with the exception that incubation in primary antibodies extended overnight, and slides were mounted in Prolong Gold (Molecular Probes). Primary antibodies and dilutions used were chicken α-CID at 1:250 (Blower and Karpen 2001), mouse α-C(3)G at 1:2000 (Page and Hawley 2001), and rabbit α-Piwi at 1:1000 (Klattenhoff et al. 2009). Secondary antibodies, all used at a final dilution of 1:400, were Alexa 488–conjugated donkey α-mouse (Invitrogen) and Cy3-conjugated donkey α-rabbit and Cy5-conjugated donkey α-chicken (both from Jackson Immunoresearch Laboratories).
Visualization of ovary GFP-HP1a expression
Ovaries from eight or nine fattened females were dissected in 1× PBS, fixed in 4% formaldehyde in 1× PBS for 5 min, and rinsed three times in 1× PBS. Samples were incubated for 30 min in 2.0 µg/ml Hoechst 33342 in 1× PBS in the dark and then rinsed and washed in 1× PBS. Individual ovarioles were separated and mounted on poly-l-lysine-coated coverslips in Prolong Diamond (Molecular Probes).
Microscopy, imaging, and image processing
FISH analysis was performed using a 1.4-NA 100× Plan APOCHROMAT Objective on a Zeiss Axioimager M1 Microscope with a Hamamatsu C10600-10B Orca-R2 camera controlled by Volocity 6.3 software (Perkin-Elmer). FISH spots were analyzed by focusing up and down and while viewing an enlarged image on the computer screen. Images of FISH spots and chromosome spreads were captured using a 1.4-NA 63× Plan APOCHROMAT Objective on the same microscope and represent maximum-intensity projections of Z series (0.1-μm steps) that were deconvolved using Volocity 6.3 (20 iterations) and then cropped in the Z to 0.7 μm (spreads) or 0.5 μm (FISH). For chromosome spreads, Cy3 (Piwi) images corresponding to “plus” or “minus” Piwi primary antibody were acquired, processed, and contrast enhanced identically. However, different settings were used for 16-cell cysts and individual nuclei. Images of GFP-HP1a expression in ovaries were acquired using a 40× Oil Plan Fluor DIC objective (NA 1.3) on a Nikon A1RSi Laser Scanning Confocal Microscope controlled by NIS Elements software v4.30.02. Single optical sections were captured using 407- (DAPI) and 488-nm (GFP) lasers. Ovariole images were captured without digital zoom, while magnified images of oocyte nuclei were captured using 4× digital zoom; otherwise, all images were captured and processed identically.
Quantification of ovary GFP-HP1a expression
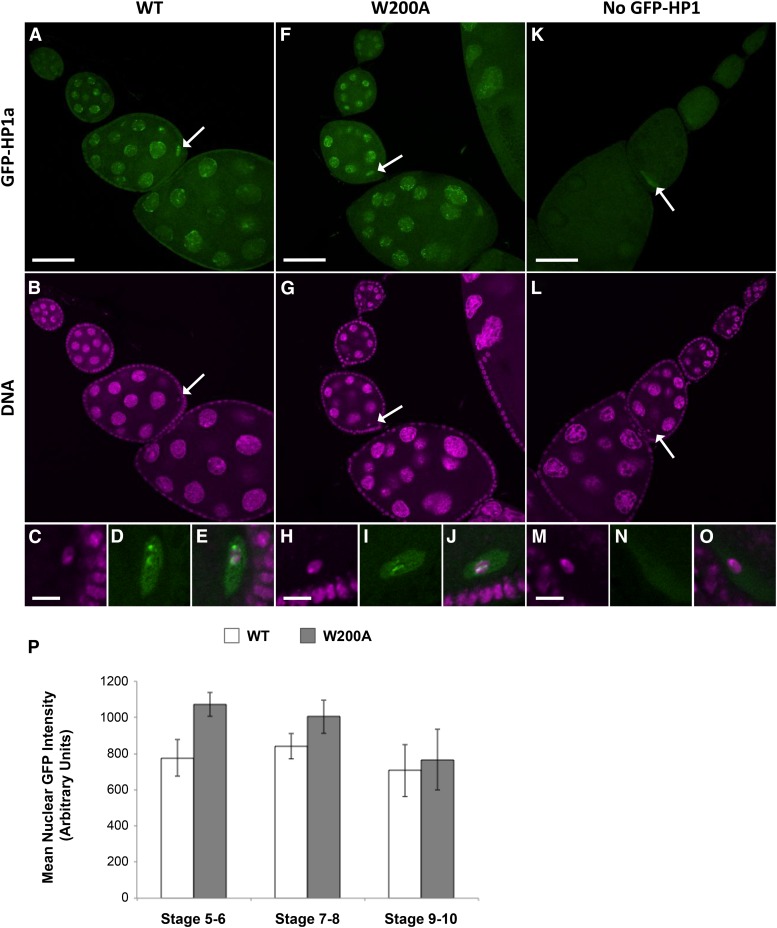
Confocal images of ovarioles expressing the UAS-driven wild-type or W200A GFP-HP1a transgene were used to quantify GFP-HP1a expression and compare relative expression levels at different stages of oogenesis. For each genotype, three representative ovarioles were selected, and egg chambers were assigned a developmental stage based on size and morphology. Within each egg chamber, three nurse cell nuclei with clearly defined boundaries were selected. The GFP signal within each nucleus was quantified using Volocity 6.3 software, and the mean GFP nuclear intensity was calculated for that egg chamber. For each developmental stage, the average nuclear GFP signal intensity for three egg chambers was graphed. An unpaired t-test was used to compare the nuclear GFP intensity from the three wild-type GFP-HP1a and three W200A GFP-HP1a egg chambers for each stage.
Data availability
The authors state that all data necessary for confirming the conclusions presented in this article are represented fully within the article. Constructs and strains are available on request.
Results
Reducing HP1a levels causes FM7a/X segregation errors in oocytes
Drosophila FM7a/X females are a useful model system for studying the association and segregation of achiasmate chromosomes. The FM7a balancer is a derivative of the X chromosome for which much of the pericentric heterochromatin has been translocated to the distal end of the arm (Figure 1A). The euchromatin of FM7a also contains several inversions that suppress recombination with a normal X even though pairing and synapsis occur normally in the majority of FM7a/X oocytes (Hawley et al. 1993; Gong et al. 2005). Therefore, FM7a/X homologs rely entirely on the achiasmate system for accurate segregation. We have previously shown that because centromere proximal heterochromatin on FM7a is greatly reduced, the FM7a/X pair provides a sensitized system to investigate the mechanisms underlying PHeMAAH (Subramanian and Bickel 2009).
We predicted that reducing the amount of any protein required for PHeMAAH would lead to weakened FM7a/X association, which should, in turn, cause an increase in X chromosome segregation errors. Therefore, to begin to investigate the role of HP1a in achiasmate homolog association, we measured X chromosome NDJ during meiosis in FM7a/X females that also were heterozygous for the Su(var)2055 null allele (Eissenberg et al. 1992). In a genetic test that provides a quantitative measure of the fidelity of chromosome segregation during meiosis (Figure S1), we observed a small but statistically significant increase in FM7a/X chromosome NDJ in Su(var)2055 heterozygotes compared to control FM7a/X flies lacking the mutation (1.69% in mutant vs. 0.49% in control, P < 0.0001; Table 1). This result suggests that the heterochromatin protein HP1a promotes accurate segregation of achiasmate homologs in Drosophila oocytes.
Table 1. Reduction of HP1a induces increased FM7A/X nondisjunction.
| Genotype | % NDJ | % Diplo (n) | % Nullo (n) | Total n | P-value |
|---|---|---|---|---|---|
| FM7a/y; Su(var)2055/+ | 1.69% | 0.83% (23) | 0.86% (24) | 5513 | <0.0001 |
| FM7a/y; cn bw sp/+ | 0.49% | 0.27% (5) | 0.22% (4) | 3680 | |
| FM7a/y w; HP1a RNAiGD/+; matα/+ | 1.69% | 0.92% (12) | 0.77% (10) | 2610 | 0.001 |
| FM7a/y w; HP1a RNAiGD/+; +/+ | 0.33% | 0.25% (3) | 0.08% (1) | 2399 |
Because HP1a is an essential gene product, we cannot assay for meiotic chromosome segregation defects in homozygotes (which are nonviable), and heterozygotes have reduced levels of HP1a in all somatic cells as well as in the germline. In order to focus more narrowly on the role of HP1a in oocytes, we turned to a UAS/Gal4-inducible RNAi strategy. We used the female germline-specific driver matα Gal4:VP16 (matα driver) (Januschke et al. 2002), which is first expressed in region three of the germarium and remains active as oogenesis progresses (Weng et al. 2014). The matα driver allowed us to induce expression of an HP1a RNAi hairpin starting in mid-prophase, well after homologs synapse and meiotic recombination begins. Therefore, this approach allowed us to specifically ask whether normal levels of HP1a in the oocyte are required to maintain pericentric association and for proper segregation of achiasmate homologs. For all the RNAi experiments we describe, “knockdown” (KD) refers to flies that express an RNAi hairpin driven by the matα driver, while “control” refers to flies that carry the RNAi transgene but lack the driver and therefore do not express the hairpin. We measured meiotic NDJ in FM7a/X flies expressing the HP1a RNAiGD hairpin (Figure S2) in the germline and observed a result very similar to that seen in Su(var)2055 heterozygotes: knockdown of HP1a caused a small but statistically significant increase in X chromosome NDJ during meiosis (1.69% in KD vs. 0.33% in control, P = 0.001; Table 1). Immunoblotting confirmed that matα-driven expression of the HP1a RNAiGD hairpin reduced HP1a protein in the ovary (Figure 1B) to a level that was comparable to that observed in Su(var)2055 heterozygotes (Figure S3). Our finding that reduction of HP1a by two different methods causes similar levels of achiasmate homolog missegregation confirms that the NDJ phenotype we observe is not an artifact of genetic background. We conclude that reduced levels of HP1a protein in the oocyte compromises segregation of the achiasmate FM7a/X chromosome pair.
Knockdown of HP1a in oocytes weakens pericentric heterochromatin-mediated association of achiasmate homologs
Because accurate segregation of achiasmate homologs depends on their stable association during meiotic prophase, our finding that HP1a knockdown in oocytes causes chromosome segregation errors suggests that the physical association of achiasmate homologs may be disrupted when HP1a levels are reduced. To directly visualize PHeMAAH in FM7a/X oocytes, we performed FISH using a probe that hybridizes to the 359-bp satellite repeat sequence that comprises a large percentage of the heterochromatin on the X chromosome (Hsieh and Brutlag 1979) (Figure 1A). As illustrated in Figure 1C, one FISH signal within the oocyte nucleus indicates that the centromere proximal heterochromatin of FM7a is physically associated with that of the normal X chromosome, and this represents “normal” PHeMAAH (Dernburg et al. 1996). However, defects in PHeMAAH are manifest as visible separation of the small region of pericentric heterochromatin on FM7a (small dot) from the large heterochromatin signal corresponding to the normal X chromosome and distal heterochromatin on FM7a (large dot) (Figure 1C). We used the presence of a small FISH dot in combination with one or two large FISH signals to quantify the number of FM7a/X oocytes exhibiting “defective” PHeMAAH.
When we probed ovaries from FM7a/X females expressing the HP1a RNAiGD hairpin, we observed a significant increase in PHeMAAH defects in KD oocytes compared to control (22.0% in KD vs. 13.5% in the control, P = 0.01), indicating that the pericentric heterochomatin-mediated association of FM7a and a normal X chromosome was weaker and/or disrupted in HP1a KD oocytes (Figure 1D). We also obtained a very similar result (24.0% in KD vs. 11.1% in control, P < 0.001; Figure 1D) when using the HP1a RNAiV20 hairpin, which targets a different region of the HP1a mRNA (Figure S2). Because two different hairpins that reduce HP1a protein levels in ovaries (Figure 1B) both result in disruption of PHeMAAH, we can conclude that the defects that we observe are not the result of RNAi off-target effects. Interestingly, when we pooled data from multiple experiments and tabulated FISH results for different stages of oogenesis (Figure S4 and Table S4), we found that PHeMAAH defects were significantly increased in HP1a KD oocytes primarily during middle to late oogenesis (stages 7–10), after disassembly of the synaptonemal complex. However, knockdown of HP1a also caused a significant increase in PHeMAAH defects at earlier stages (stages 2–3) when the synaptonemal complex is still intact.
We reasoned that if the PHeMAAH defects we observe in FM7a/X HP1a KD oocytes are due solely to reduction of HP1a protein, increasing HP1a levels in HP1a KD oocytes should rescue the phenotype. To test this hypothesis, we used the matα driver to induce germline expression of the HP1a RNAiGD transgene in FM7a/X females that also harbored one copy of a GFP-HP1a transgene containing Su(var)205 regulatory sequences that control its expression (Wen et al. 2008; Lipsick 2010). When we performed FISH, the number of oocytes exhibiting PHeMAAH defects was significantly lower (P = 0.02) for HP1a RNAiGD KD oocytes expressing the GFP-HP1a transgene (14.3%) than for HP1a RNAiGD KD oocytes that lacked the GFP-HP1a transgene (22.0%) (Figure 1D). Notably, expression of GFP-HP1a resulted in pericentric heterochromatin-mediated association between FM7a and a normal X chromosome that was comparable to that observed in control oocytes (P = 0.91) (Figure 1D). Together these data support the conclusion that reduction of HP1a levels in the oocyte leads to weakening and/or loss of pericentric heterochromatin-mediated association of achiasmate chromosomes.
Knockdown of two different H3K9 methyltransferases disrupts PHeMAAH
Because PHeMAAH specifically depends on pericentric heterochromatin, and because proper localization of HP1a to pericentric heterochromatin depends on deposition of the H3K9me2/3 mark by the methyltransferase Su(var)3–9 (Schotta et al. 2002), we asked whether PHeMAAH is affected by reduction of Su(var)3–9 in the oocyte. Using two different RNAi hairpins that target different regions of the mRNA (Figure S2), we knocked down Su(var)3–9 in FM7a/X oocytes and assayed for PHeMAAH defects using FISH. With both hairpins, we observed that Su(var)3–9 knockdown induced defects at levels similar to those in HP1a knockdown oocytes (V20-1: 21.6% in KD vs. 12.4% in control, P = 0.04; V20-2: 20.9% in KD vs. 11.9% in control, P = 0.004; Figure 1E). These results are consistent with the hypothesis that proper localization of HP1a to pericentric heterochromatin is required to support PHeMAAH.
Interpretation of the Su(var)3–9 result is somewhat complicated by the fact that owing to a quirk of Drosophila evolutionary history, the Su(var)3–9 gene shares an exon with that of the translation initiation factor eIF-γ (Krauss and Reuter 2000). Unfortunately, all publically available Su(var)3–9 hairpins target sequences in this common exon, raising the possibility that the PHeMAAH defects that we observe in Su(var)3–9 KD oocytes result from disruption of eIF-γ function rather than reduced H3K9 methyltransferase activity. To further investigate the requirement of the H3K9me2/3 mark for promoting centromere proximal association of achiasmate homologs, we knocked down Eggless (also known as dSETDB1), a second methyltransferase that is also required to maintain the H3K9me2/3 mark in the pericentric heterochromatin (Brower-Toland et al. 2009). With two different hairpins targeting different sequences within the Eggless mRNA (Figure S2), FM7a/X KD oocytes exhibited a significant increase in PHeMAAH defects compared to control oocytes (V20-1: 22.0% in KD vs. 13.6% in control, P = 0.005; V20-2: 28.4% in KD vs. 14.4% in control, P < 0.001; Figure 1F). Although we cannot rule out the possibility that knockdown of eIF-γ may contribute to PHeMAAH defects, the striking similarity in the results we observe for Su(var)3–9 and Eggless KD argues that maintenance of the H3K9 methyl mark and recruitment of HP1a to pericentric heterochromatin are required to promote the centromere proximal association of achiasmate homologs in Drosophila oocytes.
Given that the knockdown of HP1a, Su(var)3–9, and Eggless all result in a similar elevation of PHeMAAH defects compared to control oocytes, we performed an additional control to rule out the possibility that matα-driven expression of any RNAi hairpin in the oocyte will cause disruption of PHeMAAH. For this experiment, we used the matα driver to induce expression of a GFP RNAi hairpin in FM7a/X females that lacked a GFP target (no transgene encoding GFP or a GFP fusion protein). Expression of the GFP RNAi hairpin did not cause a significant increase in PHeMAAH defects compared to control oocytes lacking the driver (16.6% in KD vs. 13.3% in control, P = 0.4), demonstrating that matα-driven hairpin expression alone does not cause weakening of centromere proximal heterochromatin-mediated pairing of the FM7a/X homologs (Figure S5).
Piwi protein stably associates with oocyte chromosomes
A member of the Argonaute protein family, Piwi is able to bind piRNAs and repress the mobilization of retrotransposons within the germline (Mani and Juliano 2013; Ku and Lin 2014). However, Piwi is also a recognized binding partner of HP1a (Brower-Toland et al. 2007), plays a role in the establishment of heterochromatin during early embryogenesis (Gu and Elgin 2013), and is required for pairing-sensitive silencing (Grimaud et al. 2006), a phenomenon that involves crosstalk between homologous chromosomes (Kassis 1994; Americo et al. 2002). Therefore, we set out to test the hypothesis that Piwi, possibly in collaboration with HP1a, promotes the pericentric heterochromatin-mediated association of achiasmate homologs that is required for their accurate segregation in the Drosophila oocyte.
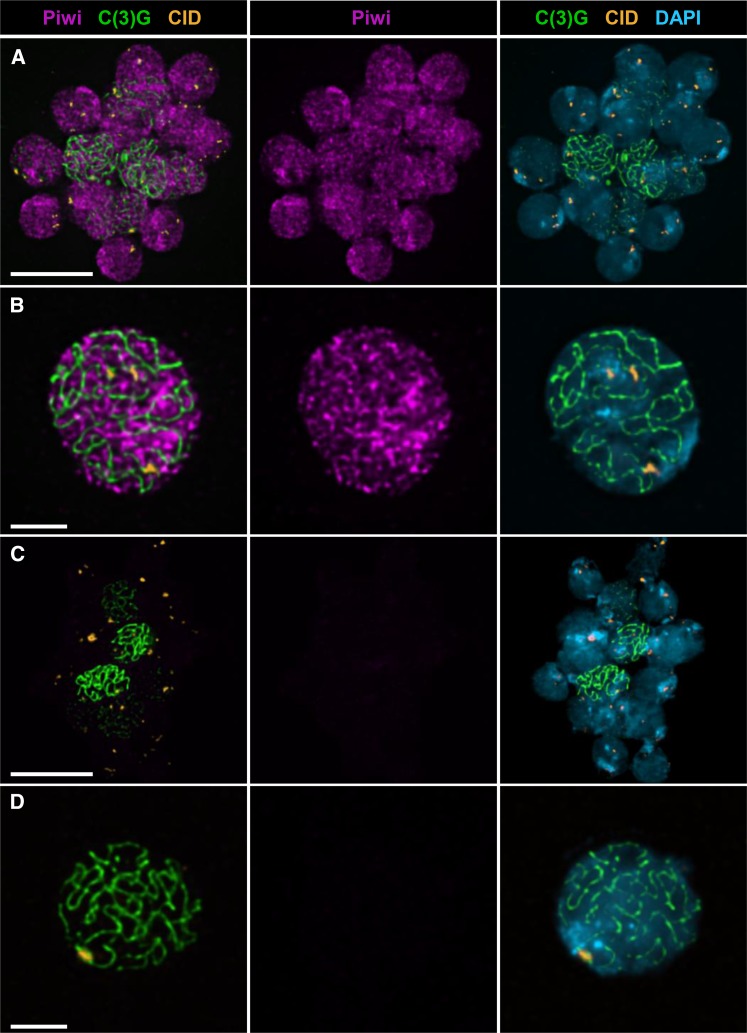
Localization of Piwi within the nuclei of nurse cells and the oocyte has been well established using whole-mount ovary preparations (Brennecke et al. 2007; Klattenhoff et al. 2009; Wang and Elgin 2011; Darricarrère et al. 2013; Dufourt et al. 2014); however, these data have not yielded conclusive evidence that Piwi physically associates with oocyte chromatin. Therefore, we used ovaries from wild-type females to prepare chromosome spreads, a technique in which cytosolic proteins are removed as well as nuclear proteins that are not bound to chromatin (Peters et al. 1997; Khetani and Bickel 2007). This approach, coupled with standard immunostaining, facilitates visualization of proteins that are stably associated with the oocyte chromosomes (Webber et al. 2004; Khetani and Bickel 2007; Page et al. 2008). We used the synaptonemal complex protein C(3)G (Page and Hawley 2001) as a marker to identify oocyte chromosomes and the centromere-specific histone variant CID (Blower and Karpen 2001) to mark the centromeres. Using antiserum specific for Piwi (Klattenhoff et al. 2009), we observed binding of Piwi protein to multiple sites along the meiotic chromosomes. In some cases, the spread chromosomes from all 16 nuclei of a germarial cyst remain in close proximity on the slide (Figure 2A). Note that Piwi is localized to the chromatin of all 16 nuclei and that the signal intensity in the two pro-oocytes [long C(3)G threads] is similar to that of the adjacent pro-nurse cells. Interestingly, Piwi binding to the chromatin does not appear uniform; instead, numerous patches of enrichment are visible, contrasting sharply with the homogeneous signal we have reported for spread preparations stained with histone antibodies (Khetani and Bickel 2007). This nonuniform Piwi staining pattern is especially apparent in Figure 2B, which shows an isolated spread from a pachytene oocyte, most likely at a later stage of oogenesis. Regions of Piwi enrichment often lie adjacent to the C(3)G threads but do not colocalize extensively with the synaptonemal complex. Moreover, Piwi signal is not restricted to the pericentric heterochromatin, but it is clearly visible in the vicinity of the centromeres. Because piwi mutations disrupt germline stem cell maintenance, we could not prepare spreads from ovaries lacking Piwi protein. However, Figure 2, C and D, shows negative controls in which spread preparations were stained identically except that the Piwi antibody was omitted. From these experiments, we conclude that Piwi is able to associate with the oocyte chromosomes and that its localization within pericentric heterochromatin is consistent with a possible role in PHeMAAH.
Figure 2.
Piwi localizes to oocyte chromatin throughout the genome. Chromosome spread preparations from wild-type ovaries were stained with antibodies that recognize Piwi (magenta), the synaptonemal complex protein C(3)G (green), and the centromere marker CID (orange) and counterstained with DAPI (blue). Images are maximum-intensity projections of a deconvolved Z series (see Materials and Methods). (A) Chromatin from 16 interconnected cells of an early meiotic cyst. C(3)G staining indicates that full-length synaptonemal complex is present in two adjacent cells, corresponding to region 2B of the germarium. Piwi localizes to the chromatin in a nonuniform pattern in both pro-oocytes and pro-nurse cells. (B) Chromosome spread from a single pachytene nucleus, most likely from a postgermarial egg chamber. Piwi localizes to discrete regions along the meiotic chromosomes. Regions of Piwi enrichment are visible, including heterochromatin near the centromeres (marked with CID). (C and D) Chromatin from semi-intact 16-cell cyst (C) and single nucleus (D) stained for C(3)G and CID and counterstained with DAPI. Primary antibody against Piwi was omitted, but secondary antibody was included. Piwi images were captured and processed identically for A and C and for B and D pairs. Scale bars correspond to 10 µm in A and C and 3 µm in B and D.
Reduction of Piwi during mid-prophase causes PHeMAAH defects and missegregation of achiasmate homologs
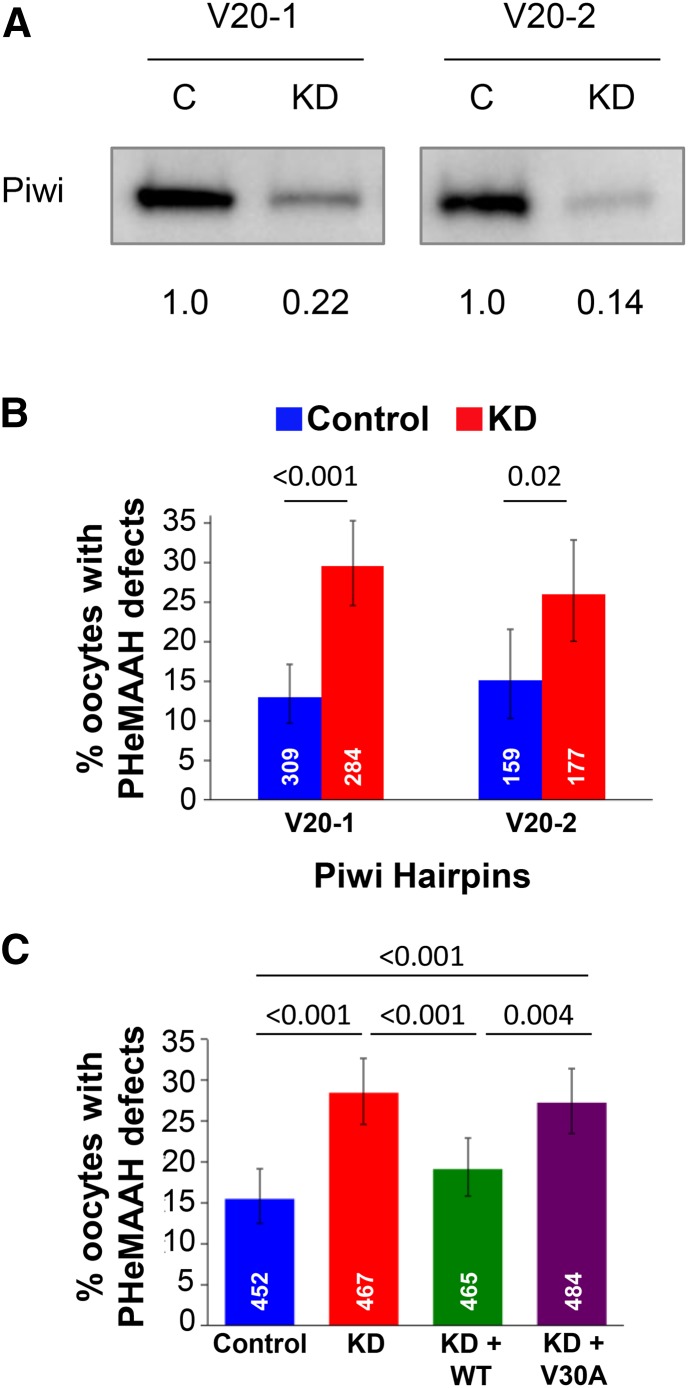
If Piwi promotes the centromere proximal association of the FM7a/X homologs, then reduction of Piwi levels by RNAi may affect their segregation. Immunoblotting confirmed that matα-driven RNAi knockdown of Piwi lowered Piwi protein levels in ovary extracts (Figure 3A). Although induction of Piwi knockdown with the matα driver does not begin until mid-prophase, fertility of Piwi KD females was still strongly reduced. However, we were able to recover sufficient progeny from FM7a/X females expressing the Piwi RNAiV20-1 hairpin (Figure S2) to measure meiotic NDJ levels (Table 2). We observed a significant increase in FM7a/X NDJ in Piwi KD oocytes compared to control oocytes (16.4% NDJ in KD vs. 1.4% in control, P = 0.004), indicating that normal levels of Piwi are required for the accurate segregation of achiasmate chromosomes.
Figure 3.
Knockdown of Piwi in the germline causes PHeMAAH defects, which can be rescued by wild-type, but not mutant, Piwi expression. (A) Western blot comparing Piwi levels in knockdown (KD) and control (C) ovary extracts from flies harboring the Piwi V20-1 or the V20-2 hairpin. For each lane, the Piwi signal intensity was normalized to the total protein in the corresponding lane of the blot, and the relative levels of Piwi protein in knockdown and control extracts are shown below the blots. (B) Matα-driven expression of two hairpins targeting different regions of the Piwi mRNA both result in PHeMAAH defects for FM7a/X chromosomes. Sixty ovarioles per genotype were scored for the V20-1 hairpin, and 30 ovarioles were scored for each V20-2 genotype. (C) FM7a/X PHeMAAH defects caused by Piwi knockdown can be rescued by expression of a wild-type (WT) Piwi transgene but not a transgene encoding the V30A Piwi mutant protein. “Control” oocytes carry the Piwi RNAiV20-1 hairpin transgene but lack the Gal4 driver, so the hairpin is not expressed. “KD” oocytes express the RNAiV20-1 hairpin under control of the matα driver. “KD + WT” oocytes express the Piwi UAS-RNAiV20-1 hairpin and also contain a wild-type Piwi transgene in which Piwi expression is controlled by native Piwi regulatory sequences. “KD + V30A” oocytes express the RNAiV20-1 Piwi hairpin and also harbor a transgene in which native Piwi regulatory sequences control expression of Piwi protein containing a V30A mutation. Ninety ovarioles of each genotype were scored. For B and C, the number of individual oocytes scored for each genotype is indicated in white, and P-values are shown above the bars. Error bars represent 95% confidence intervals. Complete data for FISH experiments are provided in Table S3.
Table 2. Piwi RNAi KD increases nondisjunction of FM7A/X chromosomes.
| Genotype | % NDJ | % Diplo (n) | % Nullo (n) | Total n | P-value |
|---|---|---|---|---|---|
| w B/FM7a;+;Piwi RNAiV20-1/matα | 16.39% | 9.84% (6) | 6.56% (4) | 122 | 0.004 |
| w B/FM7a:+;Piwi RNAiV20-1/+ | 1.44% | 1.03% (5) | 0.41% (2) | 969 |
If chromosome segregation errors arise in Piwi KD oocytes because Piwi is required to promote pericentric heterochromatin-mediated association of achiasmate homologs, we reasoned that a significant increase in PHeMAAH defects should be evident in FM7a/X Piwi KD oocytes analyzed by FISH. Using two independent Piwi hairpins (denoted V20-1 and V20-2; Figure S2) that both reduce Piwi protein levels in the ovary (Figure 3A), we observed a significantly higher percentage of PHeMAAH defects in KD oocytes than in control oocytes (V20-1: 29.6% in KD vs. 12.9% in control, P < 0.001; V20-2: 26.0% in KD vs. 15.1% in control, P = 0.02; Figure 3B). In addition, when we analyzed FISH data from multiple experiments and quantified PHeMAAH defects at different oocyte stages, we found that the temporal profile for Piwi KD-induced defects was strikingly similar to what we observed for HP1a KD oocytes (Figure S4 and Table S4). Notably, it was during middle to late oogenesis that the most dramatic differences were observed between Piwi KD and control oocytes, specifically the stages that follow disassembly of the synaptonemal complex. However, like HP1a KD, knockdown of Piwi caused a significant increase in PHeMAAH defects as oocytes exited the germarium (stage 2). These data, combined with the published evidence that HP1a and Piwi physically interact (Brower-Toland et al. 2007), suggest that the two proteins may indeed be collaborating to promote the association and segregation of achiasmate homologs in Drosophila oocytes.
Wild-type Piwi protein but not the V30A Piwi mutant protein promotes PHeMAAH
In order to demonstrate that the elevation of PHeMAAH defects we observed in Piwi KD oocytes is genuinely due to reduced levels of Piwi protein, we performed a rescue experiment similar to that described earlier for HP1a. For this experiment, we used a Piwi transgene in which the native Piwi promoter controls expression of wild-type Piwi protein (Brower-Toland et al. 2007). For FM7a/X flies that expressed both the Piwi RNAiV20-1 hairpin and a single copy of the wild-type Piwi transgene (KD + WT, Figure 3C), PHeMAAH defects were significantly lower than for Piwi RNAiV20-1 KD oocytes that lacked the Piwi transgene (19.1% in KD + WT vs. 28.5% in KD, P < 0.001) and not significantly different from control females that contained the Piwi RNAiV20-1 hairpin but no matα driver (15.5% in control; for control vs. KD + WT, P = 0.16; Figure 3C). We also tested an additional Piwi transgene encoding a mutant version of Piwi protein (V30A) that is unable to interact with HP1a (Brower-Toland et al. 2007). Unlike the wild-type Piwi transgene, V30A Piwi failed to rescue the PHeMAAH defects caused by expression of the Piwi RNAiV20-1 hairpin in FM7a/X oocytes (27.3% in KD + V30A vs. 28.5% in KD, P = 0.7; vs. 15.5% in control, P < 0.001; Figure 3C). We verified that Piwi protein levels were increased in both KD + WT and KD + V30A extracts relative to KD only (Figure S6). From these data, we conclude that Piwi is required for accurate chromosome segregation of achiasmate homologs because it helps to promote their pericentric heterochromatin-mediated association during prophase I. Importantly, our finding that the V30A point mutation in the Piwi transgene abolishes its ability to rescue PHeMAAH defects in Piwi KD oocytes supports the model that centromere proximal association of achiasmate chromosomes in Drosophila oocytes requires physical interaction of Piwi with the HP1a protein.
Mutant HP1a protein that is unable to bind Piwi fails to promote PHeMAAH
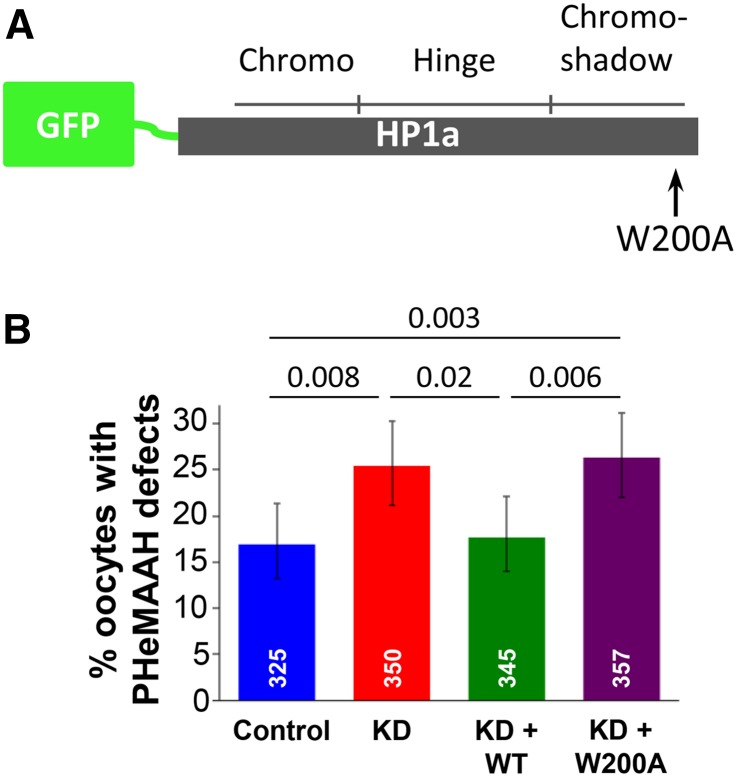
We used an alternative approach to confirm that interaction of Piwi with HP1a is required for pericentric heterochromatin-mediated association of achiasmate homologs. The W200A mutation in HP1a lies within the CSD and has been shown to abolish the ability of HP1a to interact with Piwi (Brower-Toland et al. 2007; Mendez et al. 2011). W200A HP1a dimers still form, but the binding surface formed by HP1a dimerization does not support association with Piwi (Brower-Toland et al. 2007; Mendez et al. 2011). Therefore, we generated UAS/Gal4-inducible transgenes that encoded either wild-type or W200A mutant HP1a tagged with GFP (Figure 4A). Both transgenes lack the 3′ UTR region of the endogenous HP1a mRNA and are therefore resistant to the HP1a RNAiV20 hairpin (Figure S2). In FM7a/X oocytes in which the matα driver simultaneously induced expression of both the wild-type GFP-HP1a transgene and the HP1a RNAiV20 hairpin (KD + WT), PHeMAAH defects were significantly lower than in KD oocytes expressing only the HP1a RNAiV20 hairpin (KD + WT: 17.7%, KD: 25.4%, P = 0.02; Figure 4B).
Figure 4.
PHeMAAH defects induced by expression of the HP1a RNAiGD hairpin can be rescued by wild-type HP1a expression but not by the W200A mutant HP1a protein. (A) UAS/Gal4-inducible transgenes were generated that encode wild-type (WT) or mutant (W200A) HP1a protein tagged with GFP at its N-terminus. The three HP1a domains are indicated, as is the site of the W200A mutation within the shadow domain. (B) “Control” oocytes carry the HP1a RNAiGD transgene, but it is not expressed due to the lack of a Gal4 driver. “KD” oocytes express the hairpin under control of the matα driver. In “KD + WT” ovaries, the matα driver induces expression of the HP1a RNAiV20 hairpin as well as expression of GFP-tagged wild-type HP1a protein. “KD + W200A” indicates HP1a RNAiV20 knockdown as well as simultaneous expression of GFP-tagged HP1a protein containing the W200A mutation, which disrupts HP1a interaction with Piwi. Numbers of oocytes scored for each genotype are shown in white, and P-values are shown above the bars. Sixty ovarioles were scored for each genotype, and 95% confidence intervals are shown as error bars. For complete FISH data, see Table S3.
Importantly, expression of the wild-type GFP-HP1a transgene in HP1a KD oocytes (KD + WT) resulted in PHeMAAH defects that were comparable to control oocytes harboring both HP1a transgenes but lacking the matα driver (KD + WT: 17.7%, control: 16.9%, P = 0.84; Figure 4B). These data indicate that matα-driven expression of our UAS/Gal4-inducible wild-type GFP-HP1a construct can rescue the KD phenotype. In contrast, simultaneous expression of the W200A GFP-HP1a transgene with the HP1a RNAiV20 hairpin in FM7a/X oocytes fails to rescue PHeMAAH defects caused by HP1a KD (KD + W200A: 26.3%; P = 0.80 when compared to KD alone and P = 0.003 when compared to control; Figure 4B). Therefore, results from two independent experimental approaches (Figure 3C and Figure 4B) support the hypothesis that physical interaction of HP1a with Piwi is required to maintain the pericentric heterochromatin-mediated association of achiasmate homologs during meiotic prophase.
We performed an additional control to verify that the lack of rescue observed with the W200A mutant HP1a protein was not due to low expression or mislocalization. Using confocal imaging, we compared GFP-HP1a signal intensity and localization in the ovarioles of three genotypes: females with matα-driven expression of wild-type GFP-HP1a and HP1a RNAiV20 (WT), females in which matα is driving expression of the W200A GFP-HP1a transgene and the HP1a RNAiV20 hairpin (W200A), and females that harbored both the WT GFP-HP1a and the HP1a RNAiV20 transgenes but lacked a driver (no GFP-HP1a expression). Figure 5 shows representative ovariole images that were captured and processed identically for each genotype. The GFP fluorescence intensity is comparable for WT GFP-HP1a and W200A GFP-HP1a ovarioles, and both WT and W200A GFP HP1a proteins localize to the nurse cell and oocyte nuclei (compare Figure 5, A and F). No nuclear GFP signal is detectable in the control ovarioles (no GFP-HP1) (Figure 5K). In nurse cell nuclei, bright foci for both WT and W200A GFP-HP1a are visible and most likely correspond to HP1a association with specific genomic regions. In the oocyte nuclei, bright regions of GFP-HP1a signal are restricted to the area occupied by the karyosome (oocyte DNA), indicating that both WT and W200A GFP-HP1a can localize to the oocyte chromosomes (Figure 5, C–E and H–J). Quantification of GFP-HP1a signal intensity in nurse cell nuclei at different stages of oogenesis confirms that the level of nuclear W200A is comparable or slightly higher than that of WT GFP-HP1a (Figure 5P). Therefore, failure of W200A GFP-HP1a to rescue the PHeMAAH defects caused by HP1a KD cannot be explained by poor expression or mislocalization of the mutant protein.
Figure 5.
Wild-type and W200A GFP-HP1a protein expression levels and localization in the female germline are similar. Representative confocal images of ovarioles in which the indicated UAS-driven GFP-HP1a protein (WT or W200A) as well as the HP1a RNAiV20 hairpin was induced by the matα Gal4 driver. Each image represents a single confocal optical section, and image acquisition and processing were identical for all three genotypes presented. GFP fluorescence is green, and Hoechst signal (DNA) is magenta. Arrows indicate oocyte nuclei for which enlarged insets are shown in the bottom row. (A–E) Ovariole from female expressing wild-type (WT) GFP-HP1a protein. GFP signal is visible in nurse cell and oocyte nuclei. (C–E) Distinct patches of wild-type HP1a signal colocalize with the oocyte chromatin. Note that GFP signal is absent in follicle cells in which the matα driver is not expressed. (F–J) Expression of GFP-HP1a W200A mutant protein in the ovariole. GFP signal intensity in germline nuclei is comparable to that seen in wild type (compare A to F and see below). (H–J) Mutant W200A HP1a protein also localizes in distinct patches to the oocyte DNA. (K–O) Ovariole from control female lacking GFP-HP1a expression because the matα driver is absent. In this genotype, weak signal is often visible in the oocyte cytosol, most likely due to autofluorescence. However, note the absence of GFP signal associated with nurse cell or oocyte chromatin. Scale bars correspond to 50 µm in A, F, and K and 5 µm in C, H, and M. (P) Quantification of nuclear GFP signal demonstrates that the lack of rescue observed in W200A GFP-HP1a-expressing flies is not due to insufficient transgene expression. Each bar represents the average GFP intensity for nurse cell nuclei within three representative egg chambers for each genotype at the specified stage of oogenesis (see Materials and Methods). Error bars represent SD. P-values comparing wild type and W200A were calculated using an unpaired t-test (P = 0.01 for stage 5–6, P = 0.15 for stage 7–8, and P = 0.62 for stage 9–10).
We also carried out an additional set of experiments to rule out the possibility that titration of the Gal4:VP16 produced by the matα driver was limiting in the presence of two UAS-controlled transgenes (Figure S7). If this were the case, a reduction of HP1a KD PHeMAAH defects could arise when the UAS/Gal4-inducible WT GFP-HP1a transgene is present not because of “true rescue” but because expression of the UAS-RNAi hairpin is lower in the presence of another UAS transgene. However, when we measured PHeMAAH defects in FM7a/X females that harbored the UAS-HP1a RNAiV20 hairpin as well as a UAS-GFP RNAi hairpin (but no transgene encoding a GFP protein), we found that the levels of PHeMAAH defects were comparable to those observed when matα was driving only the single UAS-HP1a RNAiV20 hairpin (Figure S7; compare HP1a KD with HP1a KD + GFP RNAi, P = 0.86). Therefore, we conclude that the level of Gal4:VP16 protein produced by the matα driver is not limiting in the presence of two UAS-controlled transgenes and that failure of the W200A GFP-HP1a transgene to suppress the HP1a RNAi-induced PHeMAAH defects indicates that physical interaction between HP1a and Piwi is required to promote centromere proximal association of achiasmate homologs.
Discussion
HP1a and Piwi collaborate to promote the association of achiasmate homologs
In this study, we reveal a previously unknown role for heterochromatin-associated proteins in maintaining pericentric heterochromatin-mediated association of achiasmate homologs (PHeMAAH) during meiotic prophase. Reducing either HP1a or Piwi in the Drosophila female germline induces a significant increase in the frequency of meiotic nondisjunction of FM7a/X homologs, demonstrating that both proteins are required to ensure proper biorientation and segregation of achiasmate chromosomes. To test the hypothesis that increased NDJ is caused by weakening or failure of PHeMAAH, we used a FISH assay to quantitatively assess the centromere proximal association of the achiasmate FM7a/X homolog pair in KD and control oocytes. RNAi knockdown of either HP1a or Piwi resulted in a significant increase in the percentage of oocytes in which the pericentric region of FM7a was separated from the normal X chromosome. In FM7a/X flies that expressed an HP1a or Piwi RNAi hairpin in combination with an additional transgenic copy of the corresponding wild-type gene, the RNAi-induced PHeMAAH defects were reduced to levels comparable to those of controls. These data confirm that pericentric heterochromatin-mediated association of achiasmate homologs depends on a threshold level of HP1a and Piwi protein in oocytes. Because matα driver expression does not begin until mid-prophase (Weng et al. 2014), when homologs are already fully synapsed, our experimental strategy has allowed us to specifically monitor how reduction of HP1a or Piwi affects the maintenance of PHeMAAH. Our approach differs from that of a previous study that monitored homolog pairing at earlier stages of meiosis by examining mutant germaria (Blumenstiel et al. 2008). Because germarial morphology is severely disrupted in piwi1 homozygotes, analysis of pairing was not possible in this mutant background. However, mutations in other components of the piRNA pathway did not cause synapsed homologs to exhibit pairing defects during early prophase (Blumenstiel et al. 2008). We suspect that our knockdown strategy combined with targeted analysis of the FM7a/X achiasmate homolog pair during later stages of oogenesis accounts for our different findings.
Our results argue that association of achiasmate homologs also depends on H3K9 methyl marks that recruit HP1a to the pericentric heterochromatin. Previous work has demonstrated that Su(var)3–9 mutants display defects in H3K9 methylation patterns and reduced pericentric HP1a localization in both the ovary (Schotta et al. 2002; Yoon et al. 2008) and salivary glands (Brower-Toland et al. 2009), and similar phenotypes have been observed in Eggless RNAi KD salivary glands (Brower-Toland et al. 2009). When we use the matα driver to knock down either Su(var)3–9 or Eggless protein during meiotic prophase, we observe PHeMAAH defects at levels similar to those observed in HP1a and Piwi knockdown ovaries. Therefore, our data are consistent with a model in which centromere proximal association of achiasmate homologs depends on recruitment of HP1a to the pericentric heterochromatin by H3K9 methyl marks laid down by histone methyltransferase proteins.
Because we found that both HP1a and Piwi are required for PHeMAAH, and several lines of evidence have demonstrated that Piwi binds to the HP1a dimer, we tested the hypothesis that interaction of HP1a with Piwi is required to maintain the association of achiasmate homologs. The HP1a W200A mutation prevents Piwi binding by altering the surface formed by HP1a dimerization (Brower-Toland et al. 2007; Mendez et al. 2011), while the Piwi V30A mutation is located in a pentapeptide motif (PXVXL) through which Piwi interacts with HP1a (Brower-Toland et al. 2007). When a transgene encoding either of these mutant proteins was expressed in conjunction with an RNAi hairpin targeting the respective wild-type endogenous protein, the mutant protein was unable to suppress the PHeMAAH defects caused by knockdown. This contrasts sharply with the rescue that we observed when a transgene encoding the corresponding wild-type protein was expressed in KD oocytes. Therefore, in two independent approaches, rescue of RNAi-induced PHeMAAH defects was eliminated by mutation of a single amino acid known to abolish the interaction of HP1a with Piwi. These data provide strong evidence that HP1a and Piwi must interact in order to keep achiasmate homologs physically together.
Weakened PHeMAAH predisposes achiasmate homologs to segregation errors
For Piwi KD oocytes, NDJ values (∼16%) and PHeMAAH defects (∼28%, as quantified by FISH) were reasonably well matched. However, we observed a much more pronounced difference between the severity of NDJ and FISH phenotypes in HP1a KD oocytes, with approximately 1.7% chromosome missegregation in NDJ tests compared to roughly 23% separated FISH signals in oocytes. In addition, the baseline value for separated FM7a/X pericentric heterochromatin in both HP1a and Piwi control oocytes hovered between 10 and 15% in most of our FISH experiments, but NDJ was considerably lower (0.33% for the HP1a control and 1.44% for the Piwi control). This means that weakened PHeMAAH between a pair of homologs during prophase I does not always result in missegregation during anaphase I. Our previous work has shown that a reduction of pericentric heterochromatin on one of the chromosomes of an achiasmate X chromosome pair sensitizes it to missegregation under conditions that perturb PHeMAAH (Subramanian and Bickel 2009). This is the case for the FM7a/X achiasmate chromosome pair that we have used in our analyses. However, we cannot rule out the possibility that the large distal block of heterochromatin on FM7a, even when removed from a centromere proximal location, still may contribute to accurate segregation of the achiasmate FM7a/X pair. Another possibility is that the physical separation of pericentric regions that we observe when HP1a levels are reduced does not reflect complete disengagement of the achiasmate homologs. This idea is reinforced by the observation that during prometaphase, achiasmate chromosomes move toward the poles but rejoin the chiasmate bivalents at the spindle equator by metaphase (Gilliland et al. 2009). Hawley and colleagues have observed heterochromatin threads that stretch between separated achiasmate homologs during prometaphase and have proposed that congression back to the metaphase plate is mediated by contraction of these threads (Hughes et al. 2009). Reducing topoisomerase II activity in mature oocytes disrupts the normal morphology of heterochromatin threads and separation of achiasmate homologs during prometaphase, suggesting that DNA entanglements also play a role in heterochromatin-mediated association (Hughes and Hawley 2014).
We suggest that the “two-spot” FISH phenotype that we observe in control oocytes represents an elastic state in which the bulk of pericentric heterochromatin for the two homologs has separated but that homologs remain physically connected by stretched heterochromatin threads that are not readily apparent in our FISH assay. The increased frequency of the “two-spot” phenotype observed in KD oocytes reflects weakened PHeMAAH and looser association between homologs. Indeed, HP1a knockdown most likely reduces the compaction of heterochromatin resulting in a less rigid state. However, even when levels of HP1a are reduced, most homologs remain connected by heterochromatin threads and are able to segregate correctly. Our finding that reduction of HP1a induces a significant elevation in chromosome segregation errors argues that increased stretching of pericentric heterochromatin can cause missegregation for a subset of chromosome pairs. Therefore, we conclude that weakened centromere proximal association predisposes the FM7a/X homolog pair to missegregation but does not guarantee it.
Interestingly, HP1a and Piwi KD oocytes that have disassembled their synaptonemal complex exhibit the most dramatic increase in PHeMAAH defects, but we do observe separation of FM7a/X pericentric heterochromatin at stages where the synaptonemal complex is still intact. Moreover, clustering of oocyte centromeres persists even after synaptonemal complex disassembly in wild-type oocytes (Takeo et al. 2011). These results suggest that even within the context of the synaptonemal complex or centromeric clustering, the elasticity of pericentric heterochromatin can allow this region of the FM7a homolog to stretch away from its partner. This most likely occurs because the FM7a/X homolog pair provides a sensitized system to detect PHeMAAH defects given that pericentric heterochromatin is substantially reduced on the FM7a chromosome. Supporting this idea, HP1a KD did not cause detectable PHeMAAH defects between a normal X chromosome and In(1)dl-49, an X chromosome derivative that carries euchromatic inversions that suppress recombination but contains un-rearranged full-length pericentric heterochromatin (data not shown).
A model for HP1a-Piwi interaction in PHeMAAH
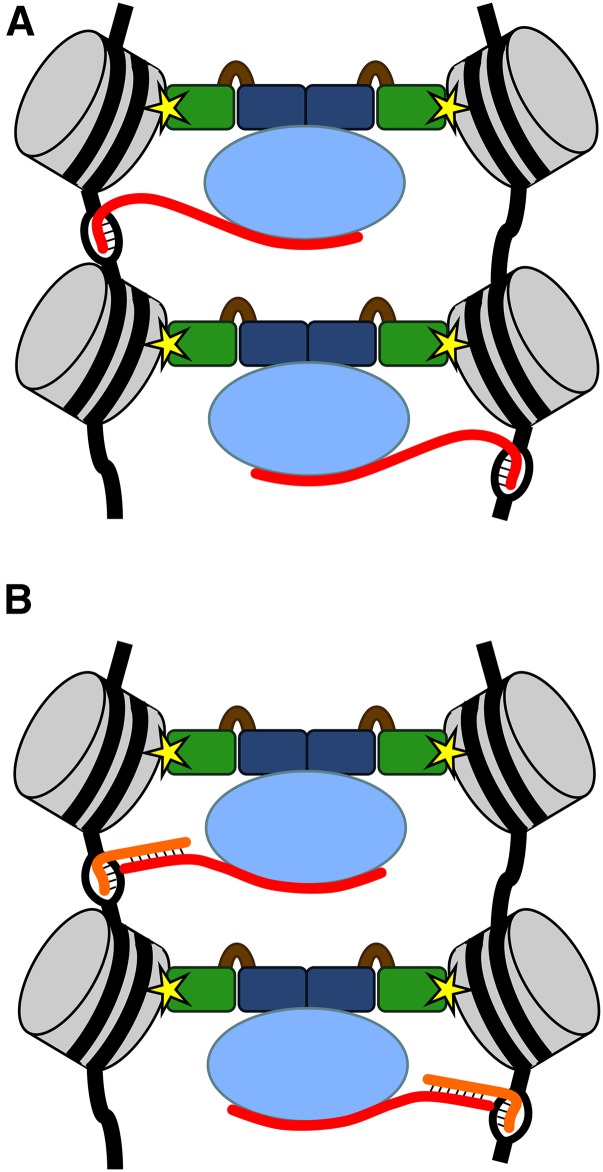
Previously published work has demonstrated that the yeast HP1a ortholog Swi6 forms dimers that can bridge two nucleosomes, with each chromodomain binding to a different histone H3 tail (Canzio et al. 2011). Interaction of human HP1α dimers with a single molecule of nucleosome-associated DNA can cause its compaction in vitro, and dimerization of Drosophila HP1a can facilitate looping within chromosomes in vivo, bringing distantly spaced regions of the chromosome into close proximity (Azzaz et al. 2014). Moreover, in vitro experiments have demonstrated that two separate nucleosomal DNA arrays are held together when HP1α dimers are present but remain separate when incubated with mutant HP1α proteins that cannot dimerize (Azzaz et al. 2014). With these observations in mind, we propose a speculative model suggesting how HP1a, H3K9 methylation, and Piwi may be working together to bring about the stable association of achiasmate homologous chromosomes during meiotic prophase (Figure 6). In this model, the chromodomains of an HP1a dimer bind to nucleosomes at the H3K9 methyl mark, with dimers physically tethering homologous chromosomes as they do with in vitro nucleosome arrays. HP1a bridging of homologs must be restricted to regions of homologous heterochromatin, and we suggest that Piwi provides this function, with Piwi-associated RNA molecules interacting with DNA and thereby providing sequence recognition. In addition, the inability of W200A HP1a dimers to mediate PHeMAAH independently of Piwi interaction suggests that Piwi also may be required to initiate or stabilize HP1a bridging.
Figure 6.
A model illustrating possible roles for HP1a and Piwi in PHeMAAH. In this model, nucleosomes (gray) from two homologs are physically connected by dimers of HP1a. HP1a chromodomains (green) bind to the chromatin at the H3K9 methyl mark (yellow star) and dimerize via interactions at their CSDs (dark blue). Piwi (light blue), along with an associated RNA (red), binds to the HP1a dimer at the CSD-CSD surface. The Piwi-associated RNA provides sequence specificity by interacting with the DNA sequence via (A) RNA-DNA hybridzation or (B) hybridization to a second RNA (orange), such as a nascent transcript, emerging from the DNA (black).
Figure 6 illustrates two different scenarios that rely on recruitment of HP1a to H3K9 methyl marks, HP1a dimerization and interaction with Piwi, as well as interaction of Piwi-bound RNA with the pericentric heterochromatin of homologous chromosomes. One possibility (Figure 6A) is that Piwi binds an RNA that directly hybridizes with the DNA, with RNA-DNA interactions occurring on both homologs such that stable HP1a bridging occurs only if both chromosomes are able to hybridize with the Piwi-associated RNA. Because pericentric satellite sequences are highly repetitive, a small number of RNA sequences may be sufficient to direct the HP1a-Piwi complex to a relatively large region of heterochromatin. Alternatively (Figure 6B), the Piwi-associated RNA could be interacting with DNA sequences via another RNA intermediate, such as a nascent satellite sequence transcript. Though not included in this model, it is interesting to note that like Drosophila HP1a, mammalian HP1α and Schizosaccharomyces pombe Swi6 also bind RNA (Muchardt et al. 2002; Keller et al. 2012). This activity is required in mammals for the targeting of HP1α to the pericentric heterochromatin, raising the possibility that in the context of PHeMAAH, HP1a-RNA interactions also could contribute to targeting or stabilizing the HP1a-Piwi complex.
Both versions of the model presented in Figure 6 rely on interaction between Piwi and RNA to provide the homology recognition that is essential for PHeMAAH. At this time, our suggestion that Piwi-bound RNA is required for PHeMAAH is based solely on extrapolation of published evidence detailing Piwi-piRNA interactions (Le Thomas et al. 2013; Mani and Juliano 2013; Ku and Lin 2014). We can only speculate on the identity of Piwi-associated RNAs that might be involved in PHeMAAH and whether these might be mature piRNAs or unprocessed nuclear transcripts. piRNAs largely originate from transcription of piRNA clusters enriched in heterochromatic regions, and long piRNA precursor molecules in excess of 200 nucleotides have been detected by RT-PCR (Pal-Bhadra et al. 2004; Saito et al. 2006; Brennecke et al. 2007; Usakin et al. 2007). After processing in the cytosol, mature piRNAs are loaded onto Piwi and transported back into the nucleus, where they are thought to guide Piwi to retrotransposon sequences throughout the genome (Brennecke et al. 2007; Huang et al. 2013; Weick and Miska 2014). In addition, RNA transcripts from both strands of the pericentric satellite DNA of all Drosophila chromosomes have been detected, including the 359-bp satellite sequence on the X chromosome (Usakin et al. 2007). Furthermore, pericentric repeat sequences have been detected among small RNAs bound to Piwi (Brennecke et al. 2007), raising the possibility that RNA molecules from these regions may contribute to homology recognition by the HP1a-Piwi complex. Further work will be required to establish whether RNA binding is required for Piwi to promote PHeMAAH and, if so, how those RNAs are generated and processed.
The proposal that Piwi facilitates the association of homologous sequences on different chromosomes is not without precedent. Piwi has been implicated previously in a phenomenon known as pairing-sensitive silencing in which transcriptional repression of transgenes on homologous chromosomes occurs only when they are able to pair (Kassis et al. 1991). In their analysis of transgenes carrying the Fab-7 silencing element upstream of a miniwhite reporter, Grimaud et al. (2006) found that pairing-sensitive silencing was disrupted in piwi1 and piwi2 mutant adults, resulting in an increase in eye pigmentation. In addition, production of short RNAs from the Fab-7 region of the paired transgenes was strongly reduced by piwi mutations. Although the mechanism of pairing-sensitive silencing is not completely understood, these results suggest that Piwi, with the guidance of small RNAs, can promote pairing between homologous regions of different chromosomes in a way that is similar to the mechanism that we envision for Piwi in our model of PHeMAAH (Figure 6). Our chromosome spread analysis indicates that Piwi stably localizes at many locations along the meiotic chromosomes, including the centromere proximal regions. Therefore, Piwi protein is in the right place at the right time to collaborate with HP1a and promote the sustained association of pericentric regions of achiasmate homologs during meiotic prophase.
Possible relevance to human biology
In humans, chromosome segregation errors lead to trisomy disorders, including Down syndrome, and are the leading cause of miscarriages (Nagaoka et al. 2012). Although direct evidence for the existence of an achiasmate chromosome association system in humans is lacking, multiple lines of inquiry indicate that human chromosomes frequently fail to recombine but are still able to segregate correctly (Oliver et al. 2008; Cheng et al. 2009; Fledel-Alon et al. 2009; Nagaoka et al. 2012). HP1a, Su(var)3–9, and Piwi are highly conserved (Saunders et al. 1993; Cox et al. 1998; Aagaard et al. 1999), and human Su(var)3–9 and HP1 proteins are able to functionally replace their Drosophila counterparts (Ma et al. 2001; Schotta et al. 2002). Therefore, if a human achiasmate segregation system does indeed exist and it relies on pericentric heterochromatin-mediated association of homologs, these three proteins may function as conserved components of this pathway. Our findings that HP1a and Piwi must collaborate to promote PHeMAAH in Drosophila oocytes opens the door to further investigation of the mechanisms that govern achiasmate chromosome segregation in flies and possibly even in humans.
Acknowlegments
We thank S. Elgin for providing the wild-type and mutant Piwi transgene stocks and W. Theurkauf for providing anti-Piwi antibodies. We are grateful to the Bloomington Drosophila Stock Center (NIH P40-OD018537), the Vienna Drosophila RNAi Center, and the Harvard Transgenic RNAi Project (NIH/NIGMS R01-GM084947) for providing the stocks necessary for this study and the Drosophila Genome Resource Center (supported by NIH grant 2P40OD010949-10A1) for supplying the HP1a cDNA. We acknowledge Brian Seitz for generating the w1118 B1 chromosome used for the Piwi NDJ tests and Charlotte Jeffreys for help with the Piwi NDJ tests. We thank Viji Subramanian and members of the Bickel laboratory for helpful discussions and critical reading of the manuscript. The confocal microscope used in this study was purchased with an MRI award from the National Science Foundation (DBI-1039423). This work was funded by the National Science Foundation (grant MCB-0919893) and the National Institutes of Health (grant R01-GM59354 awarded to S.E.B.).
Footnotes
Communicating editor: J. Sekelsky
Supplemental material is available online at www.genetics.org/cgi/data/genetics.115.186460/DC1/.
Literature Cited
- Aagaard L., Laible G., Selenko P., Schmid M., Dorn R., et al. , 1999. Functional mammalian homologues of the Drosophila PEV-modifier Su(var)3–9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J. 18: 1923–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A., Coull B. A., 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52: 119–126. [Google Scholar]
- Americo J., Whiteley M., Brown J. L., Fujioka M., Jaynes J. B., et al. , 2002. A complex array of DNA-binding proteins required for pairing-sensitive silencing by a polycomb group response element from the Drosophila engrailed gene. Genetics 160: 1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Golic K., Hawley R., 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- Azzaz A. M., Vitalini M. W., Thomas A. S., Price J. P., Blacketer M. J., et al. , 2014. Human heterochromatin protein 1α promotes nucleosome associations that drive chromatin condensation. J. Biol. Chem. 289: 6850–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S. E., Orr-Weaver T., Balicky E. M., 2002. The sister-chromatid cohesion protein ORD is required for chiasma maintenance in Drosophila oocytes. Curr. Biol. 12: 925–929. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower M. D., Karpen G. H., 2001. The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat. Cell Biol. 3: 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstiel J. P., Fu R., Theurkauf W. E., Hawley R. S., 2008. Components of the RNAi machinery that mediate long-distance chromosomal associations are dispensable for meiotic and early somatic homolog pairing in Drosophila melanogaster. Genetics 180: 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Aravin A. A., Stark A., Dus M., Kellis M., et al. , 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103. [DOI] [PubMed] [Google Scholar]
- Brower-Toland B., Findley S. D., Jiang L., Liu L., Yin H., et al. , 2007. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 21: 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower-Toland B., Riddle N. C., Jiang H., Huisinga K. L., Elgin S. C., 2009. Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of Drosophila melanogaster. Genetics 181: 1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo S. B., Clyne R. K., Fuchs J., Loidl J., Uhlmann F., et al. , 2000. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103: 387–398. [DOI] [PubMed] [Google Scholar]
- Canzio D., Chang E. Y., Shankar S., Kuchenbecker K. M., Simon M. D., et al. , 2011. Chromodomain-mediated oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol. Cell 41: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzio D., Larson A., Narlikar G. J., 2014. Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol. 24: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng E. Y., Hunt P. A., Naluai-Cecchini T. A., Fligner C. L., Fujimoto V. Y., et al. , 2009. Meiotic recombination in human oocytes. PLoS Genet. 5: e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. N., Chao A., Baker J., Chang L., Qiao D., et al. , 1998. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 12: 3715–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darricarrère N., Liu N., Watanabe T., Lin H., 2013. Function of Piwi, a nuclear Piwi/Argonaute protein, is independent of its slicer activity. Proc. Natl. Acad. Sci. USA 110: 1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A. F., Sedat J. W., Hawley R. S., 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Dufourt J., Dennis C., Boivin A., Gueguen N., Théron E., et al. , 2014. Spatio-temporal requirements for transposable element piRNA-mediated silencing during Drosophila oogenesis. Nucleic Acids Res. 42: 2512–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Morris G. D., Reuter G., Hartnett T., 1992. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledel-Alon A., Wilson D. J., Broman K., Wen X., Ober C., et al. , 2009. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 5: e1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland W. D., Hughes S. F., Vietti D. R., Hawley R. S., 2009. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Dev. Biol. 325: 122–128. [DOI] [PubMed] [Google Scholar]
- Gong W. J., McKim K. S., Hawley R. S., 2005. All paired up with no place to go: pairing, synapsis, and DSB formation in a balancer heterozygote. PLoS Genet. 1: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R., 1976. Distributive pairing, pp. 436–486 in The Genetics and Biology of Drosophila, edited by Ashburner M., Novitski E. Academic Press, New York. [Google Scholar]
- Grimaud C., Bantignies F., Pal-Bhadra M., Ghana P., Bhadra U., et al. , 2006. RNAi components are required for nuclear clustering of Polycomb group response elements. Cell 124: 957–971. [DOI] [PubMed] [Google Scholar]
- Gu T., Elgin S. C., 2013. Maternal depletion of Piwi, a component of the RNAi system, impacts heterochromatin formation in Drosophila. PLoS Genet. 9: e1003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., Irick H., Haddox D. A., Whiteley M. D., Arbel T., et al. , 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Hodges C. A., Revenkova E., Jessberger R., Hassold T. J., Hunt P. A., 2005. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 37: 1351–1355. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D., 1979. Sequence and sequence variation within the 1.688 g/cm3 satellite DNA of Drosophila melanogaster. J. Mol. Biol. 135: 465–481. [DOI] [PubMed] [Google Scholar]
- Huang X. A., Yin H., Sweeney S., Raha D., Snyder M., et al. , 2013. A major epigenetic programming mechanism guided by piRNAs. Dev. Cell 24: 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Hawley R. S., 2014. Topoisomerase II is required for the proper separation of heterochromatic regions during Drosophila melanogaster female meiosis. PLoS Genet. 10: e1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Gilliland W. D., Cotitta J. L., Takeo S., Collins K. A., et al. , 2009. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 5: e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. C., Eissenberg J. C., Craig C., Dietrich V., Hobson A., et al. , 1989. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol. 50: 170–180. [PubMed] [Google Scholar]
- Januschke J., Gervais L., Dass S., Kaltschmidt J. A., Lopez-Schier H., et al. , 2002. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 12: 1971–1981. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Le M. H., Le H., 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273: 118–122. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., 1994. Unusual properties of regulatory DNA from the Drosophila engrailed gene: three “pairing-sensitive” sites within a 1.6-kb region. Genetics 136: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A., VanSickle E. P., Sensabaugh S. M., 1991. A fragment of engrailed regulatory DNA can mediate transvection of the white gene in Drosophila. Genetics 128: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C., Adaixo R., Stunnenberg R., Woolcock K. J., Hiller S., et al. , 2012. HP1(Swi6) mediates the recognition and destruction of heterochromatic RNA transcripts. Mol. Cell 47: 215–227. [DOI] [PubMed] [Google Scholar]
- Khetani R. S., Bickel S. E., 2007. Regulation of meiotic cohesion and chromosome core morphogenesis during pachytene in Drosophila oocytes. J. Cell Sci. 120: 3123–3137. [DOI] [PubMed] [Google Scholar]
- Klattenhoff C., Xi H., Li C., Lee S., Xu J., et al. , 2009. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell 138: 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss V., Reuter G., 2000. Two genes become one: the genes encoding heterochromatin protein Su(var)3–9 and translation initiation factor subunit eIF-2γ are joined to a dicistronic unit in holometabolic insects. Genetics 156: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H. Y., Lin H., 2014. PIWI proteins and their interactors in piRNA biogenesis, germline development and gene expression. Natl. Sci. Rev. 1: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thomas A., Rogers A. K., Webster A., Marinov G. K., Liao S. E., et al. , 2013. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 27: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick, J., 2010 P{GFP-HP1} construct and insertion. Available at: http://flybase.org/reports/FBrf0210709.html.
- Ma J., Hwang K. K., Worman H. J., Courvalin J. C., Eissenberg J. C., 2001. Expression and functional analysis of three isoforms of human heterochromatin-associated protein HP1 in Drosophila. Chromosoma 109: 536–544. [DOI] [PubMed] [Google Scholar]
- Mani S. R., Juliano C. E., 2013. Untangling the web: the diverse functions of the PIWI/piRNA pathway. Mol. Reprod. Dev. 80: 632–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez D. L., Kim D., Chruszcz M., Stephens G. E., Minor W., et al. , 2011. The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. Chembiochem 12: 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez D. L., Mandt R. E., Elgin S. C., 2013. Heterochromatin protein 1a (HP1a) partner specificity is determined by critical amino acids in the chromo shadow domain and C-terminal extension. J. Biol. Chem. 288: 22315–22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. P., Miyazaki W. Y., Tomkiel J. E., Orr-Weaver T. L., 1994. Double or nothing: a Drosophila mutation affecting meiotic chromosome segregation in both females and males. Genetics 136: 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C., Guilleme M., Seeler J. S., Trouche D., Dejean A., et al. , 2002. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1α. EMBO Rep. 3: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T. R., Feingold E., Yu K., Cheung V., Tinker S., et al. , 2008. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet. 4: e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. L., Hawley R. S., 2001. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 15: 3130–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. L., Khetani R. S., Lake C. M., Nielsen R. J., Jeffress J. K., et al. , 2008. corona is required for higher-order assembly of transverse filaments into full-length synaptonemal complex in Drosophila oocytes. PLoS Genet. 4: e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M., Leibovitch B. A., Gandhi S. G., Chikka M. R., Rao M., et al. , 2004. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303: 669–672. [DOI] [PubMed] [Google Scholar]
- Peters A. H., Plug A. W., van Vugt M. J., de Boer P., 1997. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5: 66–68. [DOI] [PubMed] [Google Scholar]
- Saito K., Nishida K. M., Mori T., Kawamura Y., Miyoshi K., et al. , 2006. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 20: 2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W. S., Chue C., Goebl M., Craig C., Clark R. F., et al. , 1993. Molecular cloning of a human homologue of Drosophila heterochromatin protein HP1 using anti-centromere autoantibodies with anti-chromo specificity. J. Cell Sci. 104: 573–582. [DOI] [PubMed] [Google Scholar]
- Schotta G., Ebert A., Krauss V., Fischer A., Hoffmann J., et al. , 2002. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 21: 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentmanat M. F., Elgin S. C., 2012. Ectopic assembly of heterochromatin in Drosophila melanogaster triggered by transposable elements. Proc. Natl. Acad. Sci. USA 109: 14104–14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V. V., Bickel S. E., 2009. Heterochromatin-mediated association of achiasmate homologues declines with age when cohesion is compromised. Genetics 181: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Lake C. M., Morais-de-Sá E., Sunkel C. E., Hawley R. S., 2011. Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Curr. Biol. 21: 1845–1851. [DOI] [PubMed] [Google Scholar]
- Takeo S., Swanson S. K., Nandanan K., Nakai Y., Aigaki T., et al. , 2012. Shaggy/glycogen synthase kinase 3β and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc. Natl. Acad. Sci. USA 109: 6382–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Hawley R. S., 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usakin L., Abad J., Vagin V. V., de Pablos B., Villasante A., et al. , 2007. Transcription of the 1.688 satellite DNA family is under the control of RNA interference machinery in Drosophila melanogaster ovaries. Genetics 176: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaak D., Malik H. S., 2009. Multiple roles for heterochromatin protein 1 genes in Drosophila. Annu. Rev. Genet. 43: 467–492. [DOI] [PubMed] [Google Scholar]
- Wang S. H., Elgin S. C., 2011. Drosophila Piwi functions downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line. Proc. Natl. Acad. Sci. USA 108: 21164–21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber H. A., Howard L., Bickel S. E., 2004. The cohesion protein ORD is required for homologue bias during meiotic recombination. J. Cell Biol. 164: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick E. M., Miska E. A., 2014. piRNAs: from biogenesis to function. Development 141: 3458–3471. [DOI] [PubMed] [Google Scholar]
- Wen H., Andrejka L., Ashton J., Karess R., Lipsick J. S., 2008. Epigenetic regulation of gene expression by Drosophila Myb and E2F2-RBF via the Myb-MuvB/dREAM complex. Genes Dev. 22: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng K. A., Jeffreys C. A., Bickel S. E., 2014. Rejuvenation of meiotic cohesion in oocytes during prophase I is required for chiasma maintenance and accurate chromosome segregation. PLoS Genet. 10: e1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte W. L., Irick H., Abel T., Yasuda G., French R. L., et al. , 1993. The genetic analysis of achiasmate segregation in Drosophila melanogaster. III. The wild-type product of the Axs gene is required for the meiotic segregation of achiasmate homologs. Genetics 134: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Lee K. S., Park J. S., Yu K., Paik S. G., et al. , 2008. dSETDB1 and SU(VAR)3–9 sequentially function during germline-stem cell differentiation in Drosophila melanogaster. PLoS One 3: e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Ball A. R., Jr, Yokomori K., 2010a HP1: heterochromatin binding proteins working the genome. Epigenetics 5: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Li H., Schweppe N. M., Hawley R. S., Gilliland W. D., 2010b Statistical analysis of nondisjunction assays in Drosophila. Genetics 186: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in this article are represented fully within the article. Constructs and strains are available on request.