Abstract
The functional requirement of adapter protein 2 (AP2) complex in synaptic membrane retrieval by clathrin-mediated endocytosis is not fully understood. Here we isolated and functionally characterized a mutation that dramatically altered synaptic development. Based on the aberrant neuromuscular junction (NMJ) synapse, we named this mutation angur (a Hindi word meaning “grapes”). Loss-of-function alleles of angur show more than twofold overgrowth in bouton numbers and a dramatic decrease in bouton size. We mapped the angur mutation to σ2-adaptin, the smallest subunit of the AP2 complex. Reducing the neuronal level of any of the subunits of the AP2 complex or disrupting AP2 complex assembly in neurons phenocopied the σ2-adaptin mutation. Genetic perturbation of σ2-adaptin in neurons leads to a reversible temperature-sensitive paralysis at 38°. Electrophysiological analysis of the mutants revealed reduced evoked junction potentials and quantal content. Interestingly, high-frequency nerve stimulation caused prolonged synaptic fatigue at the NMJs. The synaptic levels of subunits of the AP2 complex and clathrin, but not other endocytic proteins, were reduced in the mutants. Moreover, bone morphogenetic protein (BMP)/transforming growth factor β (TGFβ) signaling was altered in these mutants and was restored by normalizing σ2-adaptin in neurons. Thus, our data suggest that (1) while σ2-adaptin facilitates synaptic vesicle (SV) recycling for basal synaptic transmission, its activity is also required for regenerating SVs during high-frequency nerve stimulation, and (2) σ2-adaptin regulates NMJ morphology by attenuating TGFβ signaling.
Keywords: Drosophila, angur, synapse, physiology, pMAD
SYNAPTIC transmission requires fusion of synaptic vesicles (SVs) at the active zones followed by their efficient retrieval and recycling through endocytic mechanisms (Heuser and Reese 1973; Jahn and Sudhof 1994). Retrieval and sorting of membrane lipids and vesicular proteins at the synapse are mediated by a well-orchestrated and coordinated action of several adapter and endocytic proteins (Stimson et al. 2001; Rikhy et al. 2002; Verstreken et al. 2002; Koh et al. 2004; Marie et al. 2004). Clathrin-mediated endocytosis (CME) is the primary pathway operative at the synapses for membrane retrieval (Granseth et al. 2006, 2007; Heerssen et al. 2008; Dittman and Ryan 2009; McMahon and Boucrot 2011; Saheki and De Camilli 2012). Genetic analysis of the components of the CME pathway in Caenorhabditis elegans and Drosophila has revealed that this pathway is required for SV re-formation, and in many cases, blocking CME at synapses results in temperature-sensitive paralysis (Gonzalez-Gaitan and Jackle 1997; Zhang et al. 1998; Stimson et al. 2001; Koh et al. 2004, 2007; Sato et al. 2009). Additionally, CME plays a crucial role in regulating synaptic morphology (Rikhy et al. 2002; Koh et al. 2004, 2007; Dickman et al. 2006). At Drosophila NMJs, blocking CME results in enhanced bone morphogenetic protein (BMP) signaling and affects synaptic growth (Coyle et al. 2004; O’Connor-Giles et al. 2008).
The heterotetrameric adapter protein 2 (AP2) complex is a major effector of the CME pathway. AP2 serves as a major hub for a large number of molecular interactions and links plasma membrane, cargo/signaling molecules, clathrin, and accessory proteins in the CME pathway (Traub 2003; Schmid and McMahon 2007) and hence can directly influence synaptic signaling. The AP2 complex is pseudo-asymmetric and contains four subunits—one each of large α and β2 subunits, one medium µ2 subunit, and a small σ2 subunit (Matsui and Kirchhausen 1990; Collins et al. 2002; Traub 2003). Depletion of clathrin or its major adapter, AP2, in either Drosophila or mammalian central synapses results in accumulation of endosome-like vacuoles and reduction of SVs, suggesting that CME may not be essential for membrane retrieval (Heerssen et al. 2008; Gu et al. 2013; Kononenko et al. 2014). Similarly, genetic perturbation of μ2-adaptin or α-adaptin shows only mild defects in vesicle biogenesis at C. elegans synapses, but simultaneous loss of both adaptins leads to severely compromised SV biogenesis and accumulation of large vacuoles at nerve terminals (Kim and Ryan 2009; Gu et al. 2013). While Drosophila loss-of-function mutations in α-adaptin are embryonic lethal, hypomorphic mutants exhibit reduced FM1-43 uptake, suggesting a compromised endocytosis in these mutants (Gonzalez-Gaitan and Jackle 1997). Whether reduced endocytosis reflects a defect in membrane retrieval or a defect in SV biogenesis remains unclear. Moreover, the consequences of AP2 reduction on synaptic morphology and physiology remain unknown.
Here we present an analysis of Drosophila σ2-adaptin in the context of regulating NMJ morphological plasticity and physiology. We first identified a mutation that dramatically altered NMJ morphology. Next, we mapped this mutation to σ2-adaptin by deficiency mapping. We show that AP2-dependent vesicle endocytosis regulates both synaptic growth and transmitter release. The AP2 complex is a heterotetramer, and our studies in Drosophila show that the four subunits are obligate partners of each other and are required for a functional AP2 complex (Collins et al. 2002). This finding is in contrast to the hemicomplex model in C. elegans, in which α/σ2 and β2/μ2 can sustain the function, if any one of the subunits is mutated (Gu et al. 2013). We find that loss of AP2 disrupts stable microtubule loops of the presynaptic cytoskeleton and exacerbates growth signaling through the phosphorylated Mothers Against Decapentaplegic (pMAD) pathway, suggesting that normal AP2 constrains the TGFβ signaling module. Reducing σ2-adaptin level results in synaptic fatigue at the larval NMJ synapses during high-frequency stimulation and causes temperature-sensitive paralysis in adults. Based on these results, we suggest that AP2 is essential for attenuating synaptic growth signaling mediated by the TGFβ pathway in addition to its requirement in regenerating SVs under high-frequency nerve firing.
Materials and Methods
Fly genetics
All the flies were maintained at 25° in standard corn meal medium containing sucrose, agar, and yeast granules. Flies for RNA interference (RNAi) experiments were reared at 28°. angur alleles were obtained as a second mutation through P-element mobilization of syndEP877/TM6B, Tb. All deficiency lines and mutants including the P-element insertion line AP2σKG02457 (BL13478) were obtained from the Bloomington Drosophila Stock Center at Indiana University. Mutant σ2-adaptin and control and rescue larvae were grown in noncrowded conditions on apple agar plates with a yeast paste dollop. All controls used in this study were w1118 unless stated otherwise.
Mutant eye clones
We generated angur7, FRT82B/TM6, Tb stock by recombining angur7 with FRT82B (BL2035). This recombinant was crossed to yw; ey GAL4 UAS-FLP; FRT82BGMR-hid 3R CL3R/TM6, Tb to generate flies with eyes homozygous for angur7. These mutant eye clones thus were generated using the EGUF-hid technique (Stowers and Schwarz 1999). The eye rescue construct angur7, FRT82B, UAS-AP2σ/TM6, Tb was similarly generated using standard fly genetics.
Electroretinograms (ERGs)
Flies were anesthetized and immobilized at the end of a disposable pipette tip using a drop of clear nail varnish. Recordings were done using glass microelectrodes filled with 0.8% w/v NaCl solution. Voltage changes were recorded between the surface of the eye and an electrode placed on the thorax. Following fixing and positioning, flies were dark adapted for 5 min. ERGs were recorded with 2-sec flashes of green light as a stimulus. Stimulating light was delivered from a light-emitting diode (LED) light source to within 5 mm of the fly’s eye through a fiber-optic guide. Calibrated neutral-density filters were used to vary the intensity of the light source. Voltage changes were amplified using a DAM50 amplifier (WPI) and recorded using pCLAMP 10.4. Analysis of traces was performed using Clampfit 10.4 (Axon Laboratories).
Transgene rescue
The σ2-adaptin ORF was amplified and cloned in pUAST vector. The transgenic flies were generated by the Fly Facility of the Centre for Cellular and Molecular Platforms–National Centre for Biological Sciences (CCAMP-NCBS), Bangalore, India. The transgene was expressed in a tissue-specific manner using the bipartite UAS-Gal4 system (Brand and Perrimon 1993). The Gal4 drivers used were the ubiquitous actin5C-Gal4 and the pan-neuronal elavC155-Gal4. All genetic combinations and recombinants were made using standard Drosophila genetics. The rescue experiments were performed at 25°.
RNAi knockdown experiments
The following RNAi lines were obtained from the Bloomington Drosophila Stock Center at Indiana University: RNAi α-adaptin (BL32866), RNAi β2-adaptin (BL28328), RNAi µ2-adaptin (BL28040), RNAi σ2-adaptin (BL27322), and RNAi PI4KIIIα (BL35256). All these RNAi lines were crossed to appropriate Gal4 drivers, and progeny were reared at 28°. Where lethality was observed at 28 or 25°, as in the case of α-adaptin and PI4KIIIα RNAi, the animals were raised at 18°.
Reverse transcription PCR (RT-PCR) and transcript quantification by real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from larval fillets of the mentioned genotypes using Qiagen’s RNA extraction kit as per the manufacturer’s instructions. The total amount of RNA from each genotype was quantified using a Nanodrop (Thermo Scientific), and 50 ng of RNA was taken to synthesize the first-strand complementary DNA (cDNA) using SuperScipt II (Life Technologies). One-tenth volume of the reaction mix was subjected to RT-qPCR with the 7500 Cycler (Applied Biosystems). The sequences of primers used in this study are listed in Supplemental Material, Table S2.
The primers used for the analysis were designed using the Primer-BLAST tool from the National Center for Biotechnology Information (NCBI). Cycling conditions during RT-qPCR were as follows: 50° for 2 min, 95° for 10 min, and 40 cycles at 95° for 15 sec, followed by 60° for 1 min. SYBR Green was used for the detection of amplicons with a final primer concentration of 200 nM. Individual RT-qPCR runs were performed in triplicate. The fold change was calculated using 2−Δ(ΔCt).
Brightfield imaging
Three-day-old flies were anesthetized with ether, and images were captured using a Leica M205FA Stereo Zoom Microscope.
Scanning electron microscopy
Three-day-old flies were immersed in fixative (1% glutaraldehyde, 1% formaldehyde, and 1 M sodium cacodylate, pH 7.2) for 2 hr, followed by rinsing in water and dehydration via an ethanol series. The samples then were critical point dried and sputter coated (Wolff 2011). The flies were mounted on carbon conductive tabs stuck on aluminum stubs, and images were captured using a Zeiss scanning electron microscope.
Transmission electron microscopy (TEM)
Adult eyes of control animals, angur7 mutant mitotic clones, and rescue genotype animals were prepared for TEM by fixing the eyes for 2 hr at 25° in 2.5% each of paraformaldehyde and glutaraldehyde in 0.1 M cacodylic acid (pH 7.3) containing 7 mM CaCl2. The eyes were postfixed in 2% OsO4 for 2 hr at 4°, as described previously (Meinertzhagen 1996). The samples were visualized at 120 kV using a Zeiss LIBRA 120 transmission electron microscope.
Antibodies and immunocytochemistry
Antibodies against Drosophila α- and β2-adaptin were raised in rabbits. Briefly, the C-terminal region corresponding to 580–940 amino acids of α-adaptin (Gonzalez-Gaitan and Jackle 1997) or that corresponding to 507–914 amino acids of β2-adaptin were expressed in bacteria as His-tag and purified on Ni-NTA beads. The purified protein was injected into rabbits to generate antibodies (Deshpande Laboratories, Bhopal, India). These antibodies were affinity purified and used at 1:100 dilutions. For labeling the NMJs, third instar larvae were dissected in Ca2+-free HL3 and fixed in 4% paraformaldehyde for 30 min. The fillets were washed in PBS containing 0.2% Triton X-100. The following primary antibodies were used for labeling the NMJs: mouse anti-CSP, 1:200 (Zinsmaier et al. 1994); rabbit anti-α-adaptin, 1:100, generated against the same region, as described previously (Gonzalez-Gaitan and Jackle 1997); rabbit anti-β2-adaptin, 1:100 (this study) rabbit anti-Nwk (gift from Barry Ganetzky); mouse anti-Futsch, 1:50 (Roos et al. 2000), rabbit anti-Endophilin 1, 1:200 (Rikhy et al. 2002); rabbit anti-Dynamin 1, 1:200 (Estes et al. 1996); rabbit anti-Dap160, 1:400 (Roos and Kelly 1998); guinea-pig anti-Eps15, 1:200 (Koh et al. 2007); and rabbit anti-pMAD, 1:1000 (Persson et al. 1998), and HRP conjugated to FITC or Rhodamine, secondary antibodies conjugated to Alexa-488/568, and anti-GFP conjugated to Alexa-488 (Molecular Probes, Life Technologies) were used at 1:400. Images were captured with a Zeiss LSM780 confocal microscope and processed using Adobe Photoshop software. The synaptic fluorescence intensity of the various neuronal markers in the control, mutant, and rescue animals was quantified by capturing the images using the same parameters and settings. The average fluorescence intensity around individual boutons was calculated using the ImageJ software (ImageJ, National Institutes of Health). At least 50 boutons from four animals of each genotype were used for fluorescence intensity quantification. An identical area was selected in the background, and the intensity value was subtracted from the individual values. Statistical analysis was carried out using GraphPad Prism software (GraphPad Software, San Diego).
Western blotting
Third instar larval brains of control, mutant, and rescued animals were dissected out and homogenized in buffer containing 2% SDS (50 mM Tris-HCl, pH 6.8, 25 mM KCl, 2 mM EDTA, 0.3 M sucrose, and 2% SDS) at 75° in a water bath (Ruiz-Canada et al. 2004). The samples were further centrifuged at 4000 × g. The protein concentration was calculated using bicinchoninic acid (BCA) assay, and then lysates were boiled in 2× Laemmli buffer. Then 50 μg of protein of each genotype was separated on 10% SDS-PAGE and then transferred to Hybond-LFP PVDF membrane (Amersham, GE Healthcare Life Sciences). The membrane was blocked with 5% fat-free milk for 1 hr at room temperature and then incubated with primary antibody at 4° overnight followed by 1 hr of incubation with HRP-conjugated secondary antibody at 1:20,000 dilution. Visualization of signals was accomplished by using the ECL-Plus detection system (Amersham, GE Healthcare Life Sciences) based on the standard protocol. Primary antibodies against the following proteins and dilutions were used: α-adaptin, 1:4000; β2-adaptin, 1:4000; Dap160, 1:5000; Endophilin 1, 1:10,000; Dynamin 1, 1:10,000; Nwk, 1:10,000; Eps15, 1:3000; and α-actin, 1:5000. The synaptic level of clathrin was assessed using anti-GFP antibody at 1:5000.
FM1-43 dye uptake
FM1-43 dye uptake experiments were performed as described previously (Ramaswami et al. 1994). Briefly, larvae were dissected on Sylgard plates in HL3 medium and then incubated for 3 min in HL3 containing 90 mM KCl and 4 μM FM1-43. For endo-exo cycling pool (ECP) estimation, nerves innervating muscles six and seven were stimulated at 3 Hz for 3 min in HL3 buffer containing 1.5 mM Ca2+ and 4 μM FM1-43. For estimating ECP, 4 min after cessation of high-frequency stimulation, HL3 buffer was replaced with HL3 containing 1.5 mM Ca2+ and 4 μM FM1-43, and the nerve was again stimulated at 3 Hz for 3 min. The fillets were quickly washed five times with Ca2+-free HL3 to remove excess dye. Preparations were imaged under a 63× water immersion objective lens using a QuantEM 512SC EMCCD camera (Photometrics, Tucson, AZ) mounted on an upright Axio Examiner D1 microscope with AxioVision software (Carl Zeiss). The average synaptic FM1-43 fluorescence was quantified using ImageJ.
SynaptopHluorin Imaging
SynaptopHluorin imaging was done using a Zeiss Axio Examiner D1 with a 63× 0.9-NA water immersion objective as described previously (Kumar et al. 2009). Briefly, third instar larvae were dissected in Ca2+-free HL3; the buffer was later exchanged with 2.0 mM Ca2+ containing HL3 for imaging. Time-series imaging was performed by continuous exposure of 200 msec and intervals of 500 msec. Images were acquired using a cooled Evolve 512 Delta 512b EMCCD camera (Roper Scientific). The change in fluorescence (∆F/F) was calculated at each time point by defining a region of interest (ROI) that included at least 30 boutons from five different animals. The fluorescence intensity of the particular ROI at each time point after the stimulus was subtracted from the average fluorescence before stimulus. The tau (τ) value for SpH kinetics was calculated using GraphPad PRISM 5.01 by fitting the fluorescence decay curve after stimulus to one phase exponential.
Electrophysiology
Intracellular recordings were made from muscle 6 of the A2 hemisegment using sharp glass microelectrodes having resistance between 12 and 20 MΩ, and the technique has been described previously (Rikhy et al. 2002; Verstreken et al. 2002). All recordings were performed in HL3 containing 1.5 mM Ca2+. Spontaneous release events were recorded for 60 sec. Nerves were stimulated at 1 Hz to record evoked junction potential (EJP). For synaptic depression experiments, nerves were stimulated at 10 Hz in 1.5 mM Ca2+ for 5 min. For recovery experiments, the larvae were stimulated at 10 Hz for 5 min followed by 90 sec of rest, and then test stimuli were given every 30 sec. Only muscles whose resting membrane potential was between −60 and −75 mV were used for recording. The quantal content was calculated by dividing average EJP amplitude by average miniature EJP (mEJP) amplitude for each NMJ. The signal was amplified using an Axoclamp 900A amplifier, and the data were acquired using a Digidata 1440A data-acquisition system and pClamp10 software (Axon Instruments). For data analysis, an offline software minianalysis (Synaptosoft) was used.
Intracellular recordings at 34° were performed at muscles 6/7 of the A2 hemisegment using sharp microelectrodes with 20- to 30-MΩ resistance, as described previously (Delgado et al. 2000). Briefly, wandering third instar larvae were dissected in 1.5 mM Ca2+ containing HL3 maintained at 30°. During recordings, temperature was maintained at 34° using a QE-1HC quick-exchange heated/cooled platform combined with a CL-100 bipolar temperature controller and a TCM-1 thermal cooling module (Warner Instruments).
Estimation of vesicle pool size
Third instar wandering larvae were dissected in Ca2+-free HL3. Intracellular recordings were made from ventral longitudinal muscles 6/7 of the A2 abdominal hemisegment using sharp microelectrodes with 40–60 MΩ of resistance filled with a 1:1 mixture of 3M KAc and 3M KCl. For calculating ECP, the synapses were incubated in HL3 containing 1.0 mM of Ca2+ and 1.0 μM of Bafilomycin A1 for 15 min at room temperature. mEJPs were recorded for 60 sec, followed by continuous stimulation of synapses at 3 Hz until depletion in EJPs was detected. Quantal content was calculated by dividing the average EJP amplitude of each recording by the average mEJP amplitude. In order to calculate the cycling vesicle pool, the cumulative plot of quanta released over stimulus number was plotted, and linear regression to stimuli number 3800–6000 was exerted. ECP was measured from the Y-intercept of regression, calculated by back-extrapolating from stimuli number 3800–6000 (Kim et al. 2009). To obtain the total vesicle pool, the synapses were continuously stimulated at 10 Hz after recording spontaneous events in the presence of 1 μM Bafilomycin A1. The total vesicle pool was estimated by integrating quantal content over stimuli number in a synaptic depression plot until depletion was achieved (Delgado et al. 2000; Kim et al. 2009). Recordings in which a change in Vm was <20% were used for analysis. Data were acquired using pClamp 10, and analysis was done in Clampfit 10 software.
22C10 loop quantification
Third instar larval fillets were double stained with HRP and 22C10, and images were captured using a Zeiss LSM780 confocal microscope with a 40× objective. Only NMJs of muscles 6/7 of hemisegment A2 were used, and ImageJ was used for quantification. The image was digitally magnified, and the total number of boutons was first determined by manually counting the number of HRP-positive varicosities. This was followed by counting the number of complete looped structures that co-localized with HRP. Incomplete loops and loops with diffused or interrupted staining were not included in the count. It is to be noted that in the case of mutants, the faintly stained terminal loops were included in the count. The total number of loops was divided by the total bouton number to arrive at the percentage of boutons with loops (Roos et al. 2000).
Data availability
Antibodies and Drosophila lines generated and used in this manuscript will be made available upon request. Supplemental data is available as Figure S1, Figure S2, Figure S3, Table S1 and Table S2.
Results
angur is an allele of σ2-adaptin, the smallest subunit of the AP2 complex
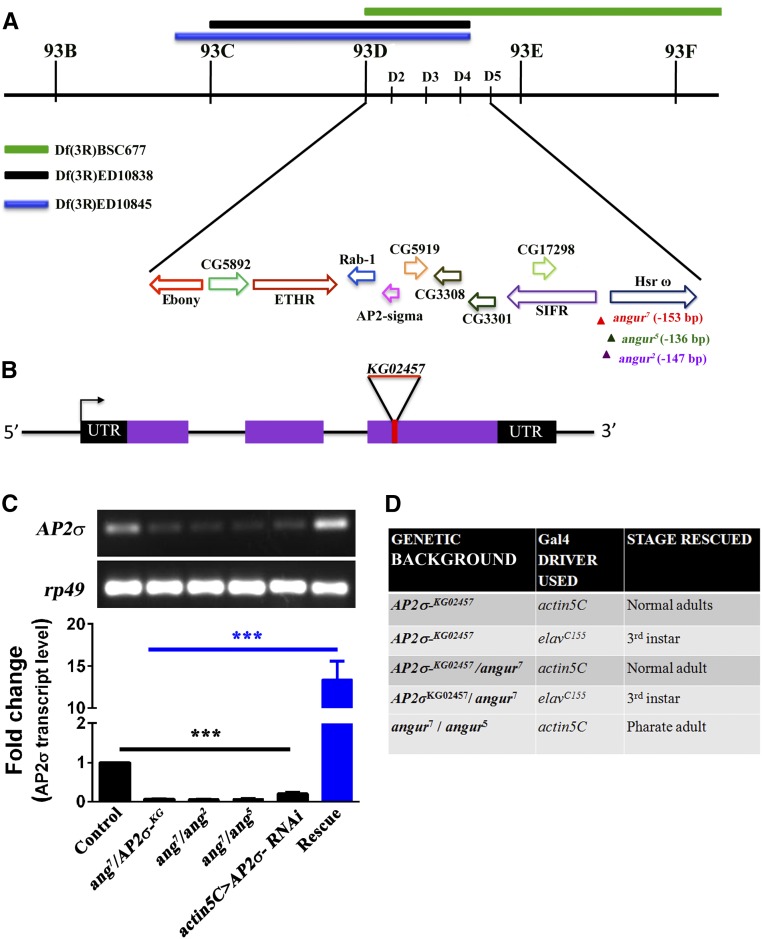
Three alleles of angur were obtained as second-site mutations through Δ2–3-mediated P-element mobilization of BL17200 and BL14472. All the heteroallelic mutant animals died at the third instar larval stage. The NMJ synapse in the mutants showed aberrant morphology resembling a bunch of grapes. Hence we named these mutants angur (a Hindi word meaning “bunch of grapes”). While angur7 and angur2 were obtained through P-element mobilization of SyndEP877/TM6 (BL17200) located 2416 bp upstream of the Syndapin ORF, angur5 was obtained independently through P-element mobilization of KG06118b (BL14472) located 406,920 bp downstream of the Syndapin ORF. Complementation analysis of these mutants showed that they belonged to the same complementation group. To ascertain the affected gene, we performed mapping with various deficiency lines spanning chromosome arm 3R. The deficiency lines BL9485, BL9487, and BL26529 (having chromosomal deletion between 93B6 and 93F14) failed to complement the angur alleles (Figure 1A). We further checked for complementation of angur alleles with smaller deficiencies and mutations in this region. Interestingly, one of these mutant lines, BL13478 (AP2σKG02457), which is a P-element insertion in the third exon of the σ2-adaptin ORF (Figure 1B), did not complement the angur alleles. To find the new genomic position of the P-elements in angur alleles, we performed plasmid rescue followed by sequencing. angur2 and angur7 were found to be present 147 and 153 bp upstream of a non-protein-coding gene, Heat shock RNA omega (Hsrω), respectively. Also, angur5 was found to be present 136 bp upstream of Hsrω. Semiquantitative RT-PCR from these animals, however, showed the Hsrω transcript to be comparable to controls (Figure S1).
Figure 1.
angur is an allele of σ2-adaptin, the smallest subunit of adapter protein 2 (AP2) complex. (A) Deficiency mapping showed the angur locus to be located in the region of 93D1–93D4 on the third chromosome of Drosophila. The three deficiency lines that uncovered the angur locus—Df(3R)BSC677, Df(3R)ED10838, and Df(3R) ED10845—are labeled. Solid bars mark the deleted regions in these deficiency lines. The 93D1–93D4 region of the chromosome contains at least 10 identified genes. Different colored arrows represent the relative positions and orientations of the genes in the 93D1–93D4 region. While angur7 and angur2 were obtained from mobilization of EP0877, angur5 was obtained from mobilization of KG06118b. The P-element in angur alleles was located in the 5′ end of the hsrω gene but did not affect expression of the hsrω gene. (B) Genomic organization of the σ2-adaptin locus showing exons (represented by solid boxes) and introns (represented by thin lines). The transcription start site is represented by an arrow, and the untranslated regions are shown by black solid boxes. The insertion site of P-element KG02457 lies in the third exon of the gene. (C) Semiquantitative (top panel) and quantitative RT-PCR (bottom panel) depicting transcript levels of σ2-adaptin in controls, angur7/AP2σKG02457, angur7/angur2, angur7/angur5, actin5C-Gal4-driven σ2-adaptin RNAi, and rescued heteroallelic mutant (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457), respectively. σ2-adaptin transcript level is dramatically reduced in the heteroallelic mutants or actin5C-Gal4-driven σ2-adaptin RNAi. rp49 transcript level was used as an internal concentration control for messenger RNA (mRNA). The error bars in the bottom panel represent SEM. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. (D) Rescue of AP2σKG02457 mutants and heteroallelic combinations in homozygous or transheterozygous combinations using ubiquitous actin5C-Gal4 or the neuronal elavC155-Gal4 drivers.
Surprisingly, sequencing of the σ2-adaptin genomic region in angur alleles revealed that the ORF was intact. However, RT-qPCR analysis showed a severe reduction in the σ2-adaptin transcript level in all the heteroallelic mutants or when σ2-adaptin was ubiquitously knocked down using an RNAi (Figure 1C). Furthermore, both the lethality and the NMJ morphological defects could be rescued by ubiquitous expression of the σ2-adaptin ORF in heteroallelic as well as AP2σKG02457 homozygous mutant animals (Figure 1D). Pan-neuronal expression of the σ2-adaptin ORF using elavC155-Gal4 in angur heterozygous mutants (angur7/AP2σKG02457 or angur7/angur5) or in AP2σKG02457 homozygous mutants could rescue the synaptic phenotype but not the lethality. Thus, the upstream genomic region of Hsrω appears to contain regulatory sequences that control the expression of σ2-adaptin. To assess whether the P-element insertions upstream of Hsrω affected the expression of other genes in this region, we performed RT-qPCR (data not shown) as well as semiquantitative RT-PCR and analyzed the expression of ETHR, SIFaR, Rab1, and CG5919, the genes flanking σ2-adaptin. We did not detect any change in the expression of these genes, suggesting that the P-element insertions specifically disrupted σ2-adaptin expression (Figure S1). Conversely, we also assessed whether synaptic morphology was altered in two of the Hsrω alleles that have been reported previously to affect Hsrω transcript levels, Hsrω66 (∼1.6 kb deletion in the Hsrω promoter region and the first nine bases of the first exon) and EP93D (an EP insertion upstream of Hsrω) (Sengupta and Lakhotia 2006; Lakhotia et al. 2012). We found that the synaptic morphology in these larvae was comparable to that in controls, and the σ2-adaptin levels were unaltered (Figure S1), as assessed by α-adaptin staining. To avoid potential complications of the P-element insertions near the Hsrω locus, we also analyzed the allelic combinations of AP2σKG02457 with two of the deficiency lines, Df(3R)ED10838 and Df(3R)ED10845. These larvae showed similar synaptic phenotypes and severely reduced synaptic α-adaptin levels as AP2σKG02457 homozygous larvae or AP2σKG02457/angur7 heteroallelic mutants (Figure S1). All these data suggest that angur is an allele of σ2-adaptin and that the lethality and synaptic phenotype are due to the specific loss of σ2-adaptin.
Mitotic clones of angur show a defect in eye morphology
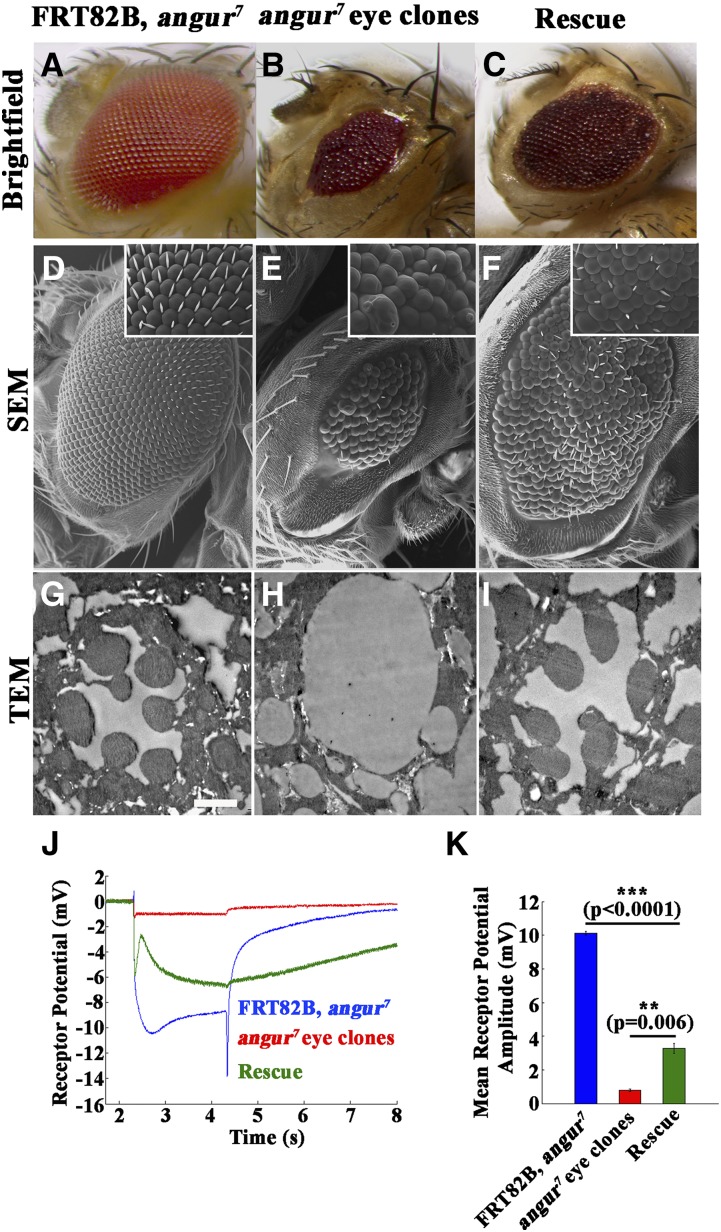
Because angur mutants are homozygous lethal, we made mutant eye clones using the eyFLP system (Stowers and Schwarz 1999; Rikhy et al. 2002). The homozygous angur mutant eyes (EGUF/+; FRT82B, angur7/FRT82B, GMR-hid) showed severe morphological defects with roughening and loss of bristles. The mutant eyes were also smaller than control eyes (Figure 2, A and B). Scanning electron microscopy revealed severely disorganized ommatidia of uneven size. Bristles were sparsely present in the mutant clones compared to the evenly spaced bristles in control eyes (FRT82B, angur7) (Figure 2, D and E). The morphological defects and eye size could be partly restored by expressing the σ2-adaptin transgene in the mutant eyes (EGUF/+; FRT82B, angur7, UAS-AP2σ/FRT82B, GMR-hid) (Figure 2, C and F). To delve into the ultrastructural defects, we performed TEM on these genotypes. The photoreceptor cells R1–R7 could be distinctly visualized in control eyes (Figure 2G). Interestingly, the homozygous mutant eye clones lacked the photoreceptor cells (Figure 2H), and this could be restored in the rescued animals (Figure 2I). We counted the number of photoreceptors in all genotypes from four different cartridges and found that it was always seven in control and rescue animals. In the mutant clones, however, we could not see any photoreceptor cells. Because photoreceptor cells were absent in the mutant clones, we hypothesized that synaptic transmission must be affected in them. To test this, we recorded ERGs and found that angur mutant eye clones showed minimal depolarization, suggesting that they failed to perceive light (Figure 2J). The eye morphology and ERG defects were partially restored by expressing the σ2-adaptin transgene in the eyes. Rescue of the ERG defect was significant compared to the mutant (P = 0.006) (Figure 2K). Taken together, these data suggest that σ2-adaptin-dependent endocytosis plays a crucial role in the biogenesis of rhabdomeres.
Figure 2.
angur mutant eye clones show severe defects in rhabdomere development. (A–C) Brightfield images of (A) control (FRT82B, angur7), (B) mutant angur7 eye clone (EGUF/+; FRT82B, angur7/FRT82B, GMR-hid), and (C) transgene-rescued animal (EGUF/+; FRT82B, angur7, UAS-AP2σ/FRT82B, GMR-hid). (D–F) SEMs of (D) control (FRT82B, angur7), (E) mutant angur7 eye clone (EGUF/+; FRT82B, angur7/FRT82B, GMR-hid), and (F) transgene-rescued animal (EGUF/+; FRT82B, angur7, UAS-AP2σ/FRT82B, GMR-hid). (G–I) TEMs of (G) control (FRT82B, angur7), (H) mutant angur7 eye clone (EGUF/+; FRT82B, angur7/FRT82B, GMR-hid), and (I) transgene-rescued animal (EGUF/+; FRT82B, angur7, UAS-AP2σ/FRT82B, GMR-hid). Scale bar, 2 μm. (J) ERG traces of control animals (FRT82B, angur7) shown in blue, mutant angur7 eye clone animals (EGUF/+; FRT82B, angur7/FRT82B, GMR-hid) shown in red, and transgene-rescued animal (EGUF/+; FRT82B, angur7, UAS-AP2σ/FRT82B, GMR-hid) shown in green. (K) Histogram showing quantification of receptor potential amplitudes of the preceding genotypes. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. Note that compared to the mutant eye clones, the rescued animals showed mild but significant rescue of receptor potential depolarization.
Subunits of AP2 are required for normal synapse morphology
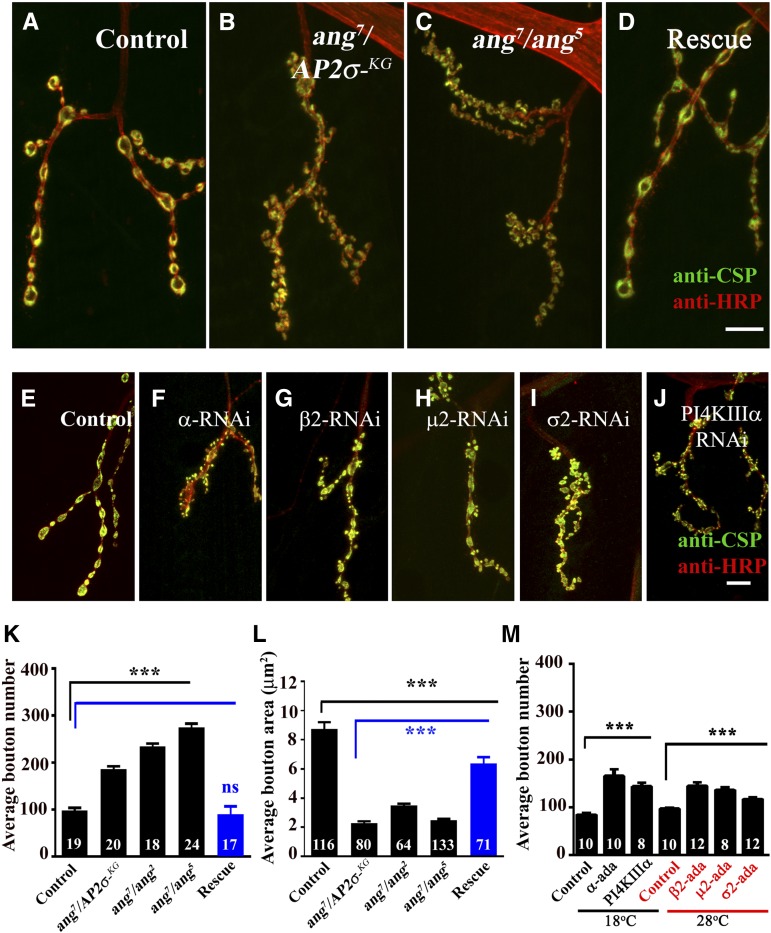
The AP2 complex is believed to be essential for CME, and a block in the process results in defects in synaptic transmission (Gonzalez-Gaitan and Jackle 1997; Gu et al. 2013). Similarly, defects in the CME pathway due to mutations in proteins implicated in the pathway also lead to morphological defects at the Drosophila NMJs (Rikhy et al. 2002; Verstreken et al. 2002; Song and Zinsmaier 2003; Dickman et al. 2006). Our observations revealed severe structural defects (Figure 3, A–C) in σ2-adaptin mutants suggesting that σ2-adaptin-dependent endocytosis plays a crucial role in synaptic development. We observed that boutons in σ2-adaptin mutants were clustered in contrast to controls, where they are uniformly spaced. The σ2-adaptin mutants showed an over twofold increase in the number of boutons (Figure 3K) (control: 98 ± 4.8; angur7/AP2σKG02457: 185 ± 5.9; angur7/angur5: 274 ± 8.6), while there was a significant decrease in the bouton area (Figure 3L) (control: 8.7 ± 0.5 μm2; angur7/AP2σKG02457: 2.3 ± 0.13 μm2; angur7/angur5: 2.5 ± 0.1 μm2). Both lethality and morphological defects were rescued by ubiquitously expressing a σ2-adaptin transgene in homozygous AP2σKG02457 or heteroallelic σ2-adaptin mutants using the actin5C-Gal4 driver (Figure 3D). Synaptic morphology defects but not lethality also could be rescued by expressing a σ2-adaptin transgene using pan-neuronal drivers elavC155-Gal4 or nSyb-Gal4, supporting the idea that morphological defects are due to a selective lack of σ2-adaptin in neurons (Figure 1D). Average number of boutons reverted to wild-type levels in mutant animals rescued with a σ2-adaptin transgene (actin5C/+; angur7/AP2σKG02457, UAS-AP2σ: 89.88 ± 4.06). Similarly, the average size of an individual bouton also was restored to wild-type values in the rescued animals (actin5C/+; angur7/AP2σKG02457, UAS-AP2σ: 6.38 ± 0.42 μm2).
Figure 3.
Mutation in σ2-adaptin causes aberrant neuromuscular junction formation. (A–D) Confocal image of NMJ synapses at muscle 4 of (A) control animals, (B and C) heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457 and angur7/angur5), and (D) transgene-rescued animal (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) double immunolabeled with a presynaptic marker CSP (green) and neuronal membrane marker HRP (red) to reveal the bouton outline. Compared to the control NMJ, the heteroallelic combination of σ2-adaptin shows severely altered NMJ morphology. Scale bar, 15 μm. (E–J) Confocal images of NMJ synapses at muscle 4 co-labeled with CSP (green) and HRP (red) of (E) control and (F–J) neuronally expressing RNAi against AP2 complex subunits or PI4KIIIα: (F) elavC155/+; α-adaptin RNAi/+, (G) elavC155/+; β2-adaptin RNAi/UAS-Dicer, (H) elavC155/+; μ2-adaptin RNAi/UAS-Dicer, (I) elavC155/+; σ2-adaptin RNAi/UAS-Dicer, and (J) elavC155/+; PIK4IIIαRNAi/+. Note that neuronal knockdown of any of the subunits of the AP2 complex or PIK4IIIα phenocopies σ2-adaptin mutations. Scale bar, 15 μm. (K) Histogram showing average number of boutons at muscles 6/7 of the A2 hemisegment in control animals (98.2 ± 4.8), heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457: 185.4 ± 6; angur7/angur2: 233.4 ± 7; angur7/angur5: 274 ± 8.5) and the rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 89.9 ± 4.0). The numbers in columns represent the number of 6/7 NMJs of the A2 segment used for bouton quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (L) Histogram showing average bouton area in control animals (8.7 ± 0.5 μm2), heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457: 2.3 ± 0.13 μm2; angur7/angur2: 3.5 ± 0.12 μm2; angur7/angur5: 2.5 ± 0.1 μm2) and rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 6.4 ± 0.4 μm2). The numbers in columns represent the number of boutons used for quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (M) Histogram showing quantification of average bouton numbers at muscles 6/7 of the A2 hemisegments of third instar larvae in control animals and neuronally expressing RNAi against AP2 subunits (control at 18°, 85 ± 3; control at 28°, 98 ± 2; α-adaptin, 167 ± 13; β2-adaptin, 145 ± 7.0; μ2-adaptin, 137 ± 5; σ2-adaptin, 118 ± 4) or PI4KIIIα (144 ± 7). At least 16 NMJ synapses of muscles 6/7 of the A2 hemisegment of each genotype were used for counting the number of boutons. ***P < 0.0001. The numbers in the columns represent the number of animals used for quantification. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test.
To analyze the consequence of loss of other subunits of the AP2 complex, we next asked whether reducing other components of AP2 would result into similar morphological defects. Predictably, we observed that RNAi-mediated knockdown of any of the subunits of AP2 with various tissue-specific Gal4 drivers yielded a wide range of phenotypes (Table S1). Importantly, knocking down any of the subunits of the AP2 complex in neurons phenocopied the σ2-adaptin mutation (Figure 3, E–I and M, and Figure S2). Consistent with this observation, disrupting AP2 complex assembly in neurons by RNAi-mediated knockdown of phosphatidylinositol-4-kinase IIIα (PI4KIIIα), an enzyme that mediates the synthesis of PI(4)P, a precursor for biosynthesis of PI(4,5)P2, affected synapse morphology similar to other subunits of the AP2 complex (Figure 3J). The synaptic α-adaptin staining and the β2-adaptin staining (not shown) were significantly reduced when the AP2 subunit or PI4KIIIα was neuronally knocked down (Figure S2). Taken together, these data suggest that all the subunits of AP2 along with phospholipid PI(4,5)P2 are obligate partners for regulating morphological plasticity at Drosophila NMJs.
σ2-adaptin facilitates basal synaptic transmission
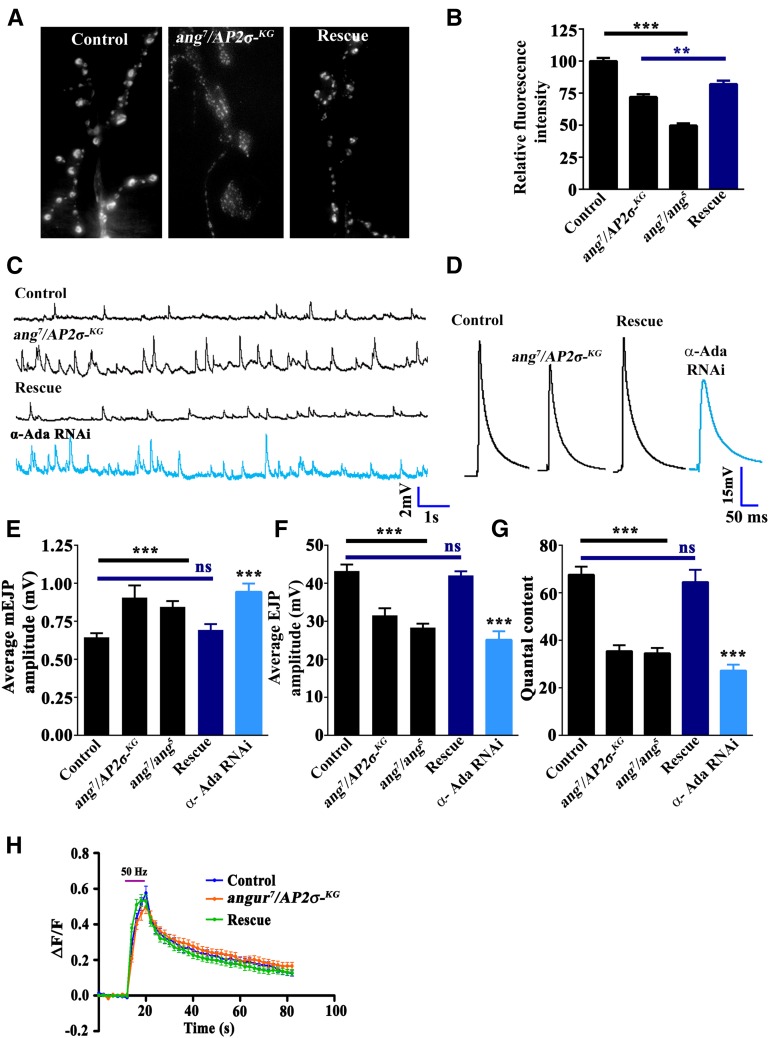
The AP2 complex is believed to be the major adapter for clathrin assembly during CME at the synapses and hence is believed to be essential for SV retrieval and regeneration of SVs for subsequent rounds of neurotransmitter release (Gonzalez-Gaitan and Jackle 1997; Collins et al. 2002; Heerssen et al. 2008; Cheung et al. 2010; Gu et al. 2013; Watanabe et al. 2014). Hence, in order to assess the need for the AP2 complex in endocytosis, we loaded σ2-adaptin mutant and control synaptic terminals with FM1-43. Surprisingly, we found that dye uptake in angur7/AP2σKG02457was reduced only by ∼30% compared to control synapses (Figure 4, A and B) (compared to controls, FM1-43 fluorescence intensity in angur7/AP2σKG02457 was 72.03 ± 2.2%; angur7/angur5 was 49.73 ± 1.6%; and actin5C/+; angur7/AP2σKG02457, UAS-AP2σ was 82.08 ± 2.6%). Next, to ascertain the functional importance of σ2-adaptin in synaptic transmission, we performed intracellular recordings at the NMJ of third instar larvae from σ2-adaptin mutants. We observed that both mEJP amplitude and frequency were significantly higher in σ2-adaptin mutants compared to controls (Figure 4, C and E). However, EJP amplitude in σ2-adaptin mutants was reduced compared to control synapses (control: 43.25 ± 1.7 mV; angur7/AP2σKG02457: 31.60 ± 1.9 mV; angur7/angur5: 28.34 ± 1.1 mV) (Figure 4, D and F). Moreover, the quantal content in σ2-adaptin mutants was significantly reduced (control: 67.63 ± 3.37; angur7/AP2σKG02457: 35.48 ± 2.5; angur7/angur5: 34.54 ± 2.3) (Figure 4G). All the physiological characteristics were restored to normal levels when a σ2-adaptin transgene was expressed in the mutant neurons (EJP amplitude in actin5C/+; angur7/AP2σKG02457, UAS-AP2σ: 42.06 ± 1.1 mV; quantal content in actin5C/+; angur7/AP2σKG02457, UAS-AP2σ: 64.54 ± 5.2). Consistent with this observation, reducing α-adaptin levels in neurons resulted in a similar reduction in EJP amplitude and quantal content (Figure 4, D, F, and G) (EJP amplitude in elavC155/+; α-adaptin RNAi/+: 25.17 ± 2.2 mV; quantal content in elavC155/+; α-adaptin RNAi/+: 27.26 ± 2.53). Taken together, these data suggest that σ2-adaptin facilitates the basal synaptic transmission at Drosophila NMJ synapses.
Figure 4.
σ2-adaptin facilitates basal synaptic transmission as well as synaptic vesicle endocytosis. (A) Representative images of FM1-43 dye uptake by control, heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457), and transgene-rescued (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) synapses. (B) Relative synaptic FM1-43 fluorescence uptake in control, heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457: 72.1 ± 2.3% and angur7/angur5: 49.73 ± 1.7%) and transgene-rescued (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 82.1 ± 2.6%) synapses. ***P < 0.0001; **P < 0.001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (C) Representative traces of mEJP in control animals, heteroallelic σ2-adaptin mutant combinations (angur7/AP2σKG02457 and angur7/angur5), transgenic-rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457), and elavC155/+; α-adaptin RNAi/+ animals. (D) Representative traces of EJPs in control animals, σ2-adaptin heteroallelic mutant combinations (angur7/AP2σKG02457 and angur7/angur5), transgene-rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457), and elavC155/+; α-adaptin RNAi/+ animals. (E) Histogram showing average mEJP amplitude in control animals (0.65 ± 0.03 mV), heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457: 0.91 ± 0.08 mV; angur7/angur5: 0.85 ± 0.04 mV), transgene-rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 0.69 ± 0.04 mV), and elavC155/+; α-adaptin RNAi/+ (0.94 ± 0.06 mV) animals. At least 8 NMJ recordings of each genotype were used for quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (F) Histogram showing average EJP amplitude in control animals (43.25 ± 1.67 mV), heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457: 31.60 ± 1.9 mV; angur7/angur5: 28.34 ± 1.05 mV), transgene-rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 42.06 ± 1.14 mV), and elavC155/+; α-adaptin RNAi/+ (25.17 ± 2.24 mV) animals. At least 8 NMJ recordings of each genotype were used for quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (G) Quantification of quantal content in control animals (67.63 ± 3.37), heteroallelic σ2-adaptin mutants (angur7/AP2σKG02457: 35.48 ± 2.45; angur7/angur5: 34.54 ± 2.25), transgene-rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457: 64.54 ± 5.18), and elavC155/+; α-adaptin RNAi/+ (27.26 ± 2.53) animals. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (H) Graph showing the SpH fluorescence response to a 50-Hz stimulus train for 10 sec in 2.0 mM Ca2+ containing HL3 of control (blue, n = 37 boutons, 5 animals; elav3E1, UAS-SpH/+), mutant (orange, n = 33 boutons, 5 animals; elav3E1, UAS-SpH, AP2σKG02457/ang7), and rescued (green, n = 29 boutons, 5 animals; elav3E1, UAS-SpH, AP2σKG02457/ang7, UAS-AP2σ) synapses. The pink horizontal bar represents the stimulation time. Tau values were calculated after 10 sec of stimulation by fitting the fluorescence decay curve to one-phase exponential. σ2-adaptin mutant (7.60 ± 0.17) shows a mild but significant difference in SpH kinetics compared to control (6.91 ± 0.17) and rescue (6.60 ± 0.23).
The functional requirement of the AP2 complex in SV membrane retrieval at mammalian hippocampal synapses has been debated (Kononenko et al. 2014; Watanabe et al. 2014). Hence, in order to test the functional requirement of σ2-adaptin in SV re-formation from the presynaptic membrane, we directly measured the rate of re-formation of normal-pH synaptic vesicles using synaptopHluorin imaging (Poskanzer et al. 2006; Kumar et al. 2009). Interestingly, we found that the kinetics of membrane retrieval and acidification was mildly but significantly different in σ2-adaptin mutants compared to control animals. The rates of re-formation of synaptic vesicles were τ = 6.9 ± 0.17 sec and τ = 7.6 ± 0.17 sec for control anmals and σ2-adaptin mutants, respectively, when fit to a single exponential function (Figure 4H). Hence, taken together, our data suggest that the AP2 complex has a minimal role in SV retrieval from the presynaptic membrane at Drosophila NMJ synapses.
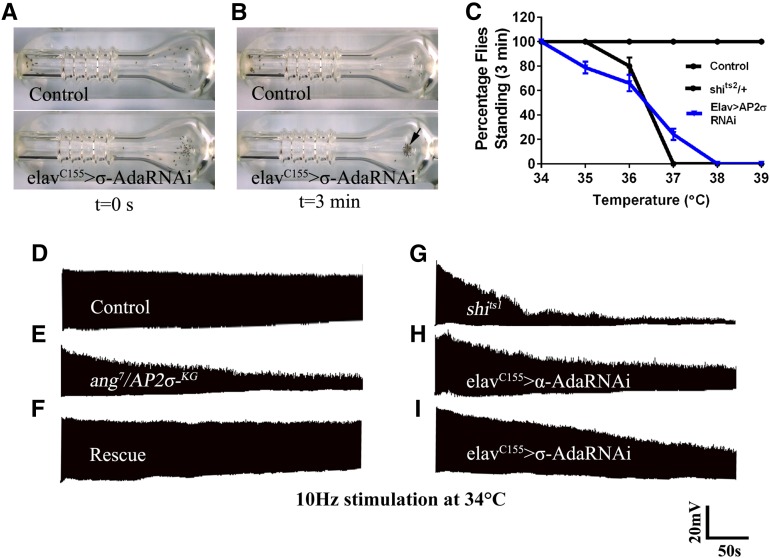
Compromised SV trafficking in Drosophila leads to synaptic fatigue at high-frequency nerve stimulation and also leads to temperature-dependent paralysis (Kosaka and Ikeda 1983; Zinsmaier et al. 1994; Rikhy et al. 2002; Verstreken et al. 2002; Koh et al. 2004). Hence, we tested whether reducing σ2-adaptin levels leads to paralysis and synaptic fatigue at elevated temperature. Interestingly, we found that neuronal knockdown of σ2-adaptin (elavC155/+; σ2-adaptin RNAi/+) results in temperature-dependent paralysis above 35° (Figure 5, A–C). We found that high-frequency nerve stimulation in angur mutants or in animals with neuronally reduced σ2- or α-adaptin at 34° leads to an activity-dependent decline of EJP amplitude; however, unlike with shibirets alleles, the neurotransmitter release was not completely abolished (Figure 5, D–I). These data suggest that unlike essential endocytic proteins such as Endophilin and Shibire, AP2-modulated vesicle trafficking and its activity are also required during elevated temperatures to sustain neurotransmitter release.
Figure 5.
Reducing σ2-adaptin levels in neurons causes temperature-sensitive paralysis at elevated temperature. (A and B) Control (elavC155/+) 2- to 3-day-old adult flies do not suffer paralysis in 3 min at 38ο, whereas similarly aged adult flies expressing σ2-adaptin RNAi (elavC155/+; σ2-adaptin RNAi/+) in neurons showed complete paralysis in 3 min at 38ο. These animals recovered within 2 min when shifted to 25ο (not shown). (C) Paralysis profile of control animals and animals expressing RNAi against σ2-adaptin in neurons (elavC155/+; σ2-adaptin RNAi/+) and shits2/+. While the control animals do not suffer paralysis in 3 min, the RNAi-expressing animals are paralyzed above 35ο. Five trials were performed for each genotype at each temperature in a sushi cooker (Ramaswami et al. 1993). Each trial contained 10–15 animals. Error bars represent standard error of the mean. (D–I) Representative traces of EJPs under high-frequency stimulation of indicated genotypes stimulated at 10 Hz for 5 min at 34° in 1.5 mM Ca2+ containing HL3. Unlike shibrets1 (G), mutant (E) and neuronally reduced α- or σ2- adaptin (H and I) animals do not show complete reduction in EJP amplitude at the end of 5 min of stimulation.
σ2-adaptin regulates synaptic vesicle regeneration under high-frequency nerve firing
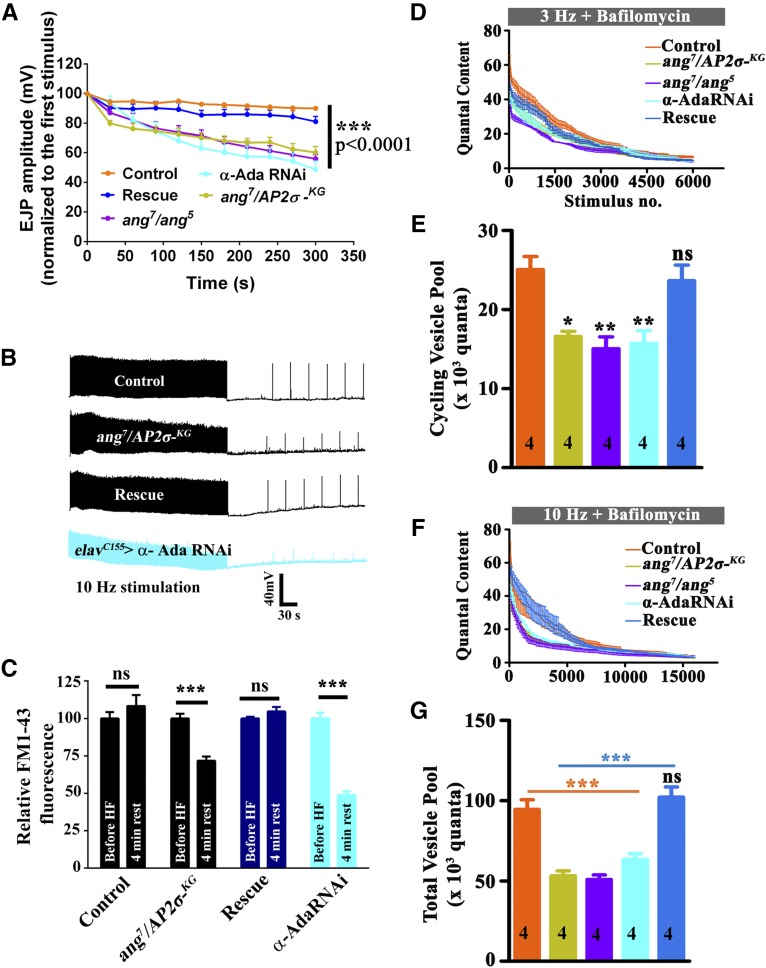
To further assess a requirement for σ2-adaptin in SV recycling, we stimulated the σ2-adaptin mutant synapses in larval body wall muscle for 5 min at 10 Hz and recorded EJPs. While the control and rescued synapses (actin5C/+; angur7/AP2σKG02457, UAS-AP2σ) did not show stimulation-dependent decline in EJP amplitudes over 5 min, mutant synapses (angur7/AP2σKG02457 and angur7/angur5) exhibited a significant decline in EJP amplitude (Figure 6, A and B). The decline in EJP amplitude at the end of 5 min of 10 Hz of nerve stimulation was ∼50% relative to the first stimulus. Similar results were obtained when α-adaptin was neuronally reduced (elavC155/+; α-adaptin RNAi/+) (Figure 6, A and B). Thus, these data suggest that not only is AP2 required for maintaining basal synaptic transmission, but it is also crucial under high-frequency nerve firing to resupply SVs at Drosophila NMJ synapses.
Figure 6.
σ2-adaptin function is essential under high-frequency stimulation to regenerate synaptic vesicles. (A) Normalized EJP amplitudes in control, heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457and angur7/angur5), rescued larvae (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457), and neuronally knocked down α-adaptin (elavC155/+; α-adaptin RNAi/+) animals stimulated at 10 Hz for 5 min in 1.5 mM Ca2+. The mutant larvae showed stimulus-dependent decline in the EJP amplitudes over time. (B) Representative traces of indicated genotypes under high-frequency stimulation at 10 Hz for 5 min in 1.5 mM Ca2+ containing HL3. After 1.5 min of rest, test stimuli were given every 30 sec to assess the recovery of EJP from synaptic depression. Note that the mutant synapses do not recover to the first stimulus levels even after 4 min of rest from high-frequency stimulation. (C) Estimation of endo-exo cycling pool at NMJ synapses of indicated genotypes before high-frequency stimulation and after 4 min of rest from high-frequency stimulation. While the controls and rescue synapses maintained the ECP pool to its initial value after 4 min of rest, the σ2-adaptin mutant synapses and the elavC155/+; α-adaptin RNAi/+ synapses show significant decline in the endo-exo cycling pool and do not recover to the initial value after 4 min of rest. n = 50 boutons from eight different NMJs of muscles 6/7 of the A2 hemisegment were counted. (D) Synaptic depression curve showing the depletion in quantal content over stimulus number in synapses stimulated at 3 Hz in the presence of 1 μM Bafilomycin A1 to deplete ECP. (E) Histogram showing ECP estimates of control (25,050 ± 1511), heteroallelic mutants (angur7/AP2σKG02457: 16,590 ± 974; angur7/angur5: 15,040 ± 870), elavC155/+; α-adaptin RNAi/+ (15,730 ± 1031), and rescued animals (actin5C-GAL4/+; angur7/UAS-AP2σ, AP2σKG02457: 23,635 ± 1358) obtained from Y-intercept of regression, calculated by back-extrapolating from stimulus numbers 3800–6000 on a cumulative plot of Figure 6D. **P ≤ 0.0006; *P ≤ 0.0007. Numbers in the columns represent the number of animals used for experiments and quantification. The error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (F) Synaptic depression curve showing the depletion of the total vesicle pool over stimulus number estimated by stimulating the synapses at 10 Hz in presence of 1 μM Bafilomycin A1. (G) Histogram showing the estimation of total vesicle pool of control animals (94,610 ± 5929), heteroallelic mutants (angur7/AP2σKG02457: 53,220 ± 3156; angur7/angur5: 50,930 ± 2877), elavC155/+; α-adaptin RNAi/+ (63,460 ± 3650), and the rescued animals (actin5C-GAL4/+;angur7/UAS-AP2σ, AP2σKG02457: 102,200 ± 6315) calculated by integrating quantal content over stimulus number in synaptic depression plot of Figure 6F until depletion was achieved. ***P < 0.0001. Numbers in the columns represent the number of animals used for experiments and quantification. The error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test.
Synaptic mutants implicated in CME, such as endophilin and dap160, show complete recovery from synaptic depression within a few minutes of rest after cessation of high-frequency stimulation, suggesting that synaptic membrane retrieval for vesicle regeneration in these mutants is stimulus dependent (Marie et al. 2004; Dickman et al. 2005). In contrast, we found that σ2-adaptin mutants or animals with neuronally reduced α-adaptin do not recover from synaptic depression even after 4 min of rest following cessation of high-frequency stimulation. This observation suggests that in addition to its requirement in synaptic membrane retrieval, AP2 complex function is also required during the much slower process of SV trafficking, possibly at one of the rate-limiting steps in SV regeneration (Figure 6B). Hence, we reasoned that the mutants do not recover from synaptic depression because of compromised SV regeneration. To address this directly, we labeled larval NMJ synapses of control and mutant animals (angur7/AP2σKG02457 and angur7/angur5) with FM1-43 before or after 4 min of rest following cessation of high-frequency stimulation and estimated the ECP. Surprisingly, we found that even after 4 min of rest, the total recycling pool did not recover to its initial value in the mutants or at the synapses where α-adaptin was reduced in the neurons (elavC155/+; α-adaptin RNAi/+) (Figure 6C). Taken together, these data uncover a role for AP2 in the regeneration of synaptic vesicles under high-frequency stimulation in Drosophila.
To further investigate the underlying mechanism of synaptic fatigue in angur mutants, we estimated ECP and the total vesicle pool at the NMJ synapses. In order to measure ECP, synapses were continuously stimulated in the presence of 1 μM Bafilomycin A1 at 3 Hz until synaptic depression was attained. The initial decline in quantal content during the short duration of neuronal activity or at 3 Hz stimulation mostly represents ECP; however, the later depletion reflects the mobilization of vesicles from the reserve pool (Kim et al. 2009). Consistent with our previous observations, the number of vesicles in ECP was found to be significantly lower in angur mutants (angur7/AP2σKG02457: 16,590 ± 974; angur7/angur5: 15,040 ± 870; controls: 25,050 ± 1511). Similar results were obtained when α-adaptin levels were neuronally reduced (elavC155-GAL4 > α-adaptin RNAi: 15,730 ± 1033; controls: 25,050 ± 1511) (Figure 6, D and E). The number of SVs in the ECP of rescued synapses (actin5C-GAL4/+; angur7/UAS-AP2σ, AP2σKG02457: 23,635 ± 1358.75) was comparable to that in control animals (Figure 6, D and E). The total vesicle pool was measured by continuously stimulating the synapses at 10 Hz in the presence of 1 μM Bafilomycin A1. We quantified the total vesicle pool by integrating the quantal content from the depression curve (Figure 6F) until remarkable depletion was attained. Our data suggest significant reduction of the total vesicle pool in angur mutant (angur7/AP2σKG02457: 53,220 ± 3156; angur7/angur5: 50,930 ± 2877) or neuronally reduced α-adaptin (elavC155-GAL4 > α-adaptin RNAi: 63,460 ± 3650) synapses compared to control (94,610 ± 5929) and rescued synapses (actin5C-GAL4/+; angur7/UAS-AP2σ, AP2σKG02457: 102,200 ± 6315) (Figure 6G). Taken together, these data suggest that σ2-adaptin is required to maintain the vesicle pool at Drosophila NMJ synapses. The synaptic depression observed under high-frequency stimulation is possibly due to less availability of SVs at the σ2-adaptin mutant synapses.
σ2-adaptin is an obligate member for stabilizing the AP2 complex and clathrin but not other synaptic proteins
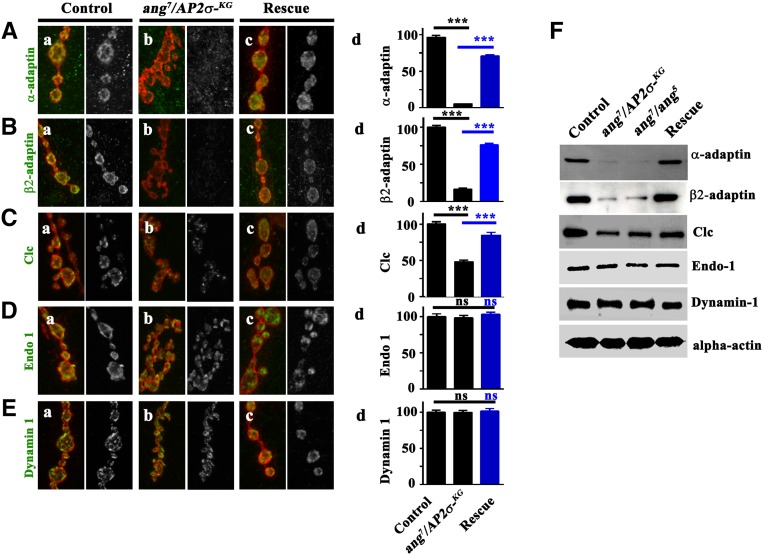
Because the AP2 complex acts as a hub for several molecular interactions during CME, we next asked whether σ2-adaptin affects localization or stability of other synaptic proteins (Schmid and McMahon 2007). To address this, we quantified the synaptic immunofluorescence levels of various synaptic proteins in σ2-adaptin mutants. We observed a severe reduction in synaptic α-adaptin (compared to controls, the α-adaptin fluorescence intensity in angur7/AP2σKG02457 was 5.6 ± 0.2%; in angur7/angur5 was 1.4 ± 0.1%, and in actin5C/+; angur7/AP2σKG02457, UAS-AP2σ was 73.46 ± 1.6%), and β2-adaptin immunoreactivity (compared to controls, the β2-adaptin fluorescence intensity in angur7/AP2σKG02457 was 16.53 ± 1.4%; in angur7/angur5 was 31.97 ± 1.6%; and in actin5C/+; angur7/AP2σKG02457, UAS-AP2σ was 76.07 ± 2.0%) at the σ2-adaptin mutant synapses (Figure 7, A and B). Because the AP2 complex is involved in clathrin recruitment, we speculated that the levels of clathrin at the mutant synapses might be altered. Hence, to assess the levels of clathrin at the σ2-adaptin mutant synapses, we overexpressed an EYFP-tagged Clc transgene in neurons of heteroallelic σ2-adaptin mutants (elavC155/+; UAS-Clc/+; angur7/AP2σKG02457) or in the rescue animals (elavC155/+; UAS-Clc/+; angur7/AP2σKG02457, UAS-AP2σ). Compared to controls (elavC155/+; UAS-Clc/+), the EYFP fluorescence intensity at mutant (48.5 ± 2.3%) synapses was dramatically reduced but was significantly restored in the rescue (84.7 ± 4.1%) synapses (Figure 7C and Figure S3). Other synaptic proteins implicated in CME were not altered in σ2-adaptin mutants (Figure 7, D and E, and Figure S3) Taken together, these data suggest that all the subunits of the AP2 complex are specifically essential for recruitment and/or stability of clathrin at the synapse.
Figure 7.
σ2-adaptin is required specifically for AP2 complex and clathrin stability. (A–E) Representative images of third instar larval boutons from control, heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457), and transgene-rescued (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) NMJ synapses co-labeled with HRP (red) and other synaptic proteins (green): (A) α-adaptin; (B) β2-adaptin; (C); EYFP-Clc (D) Endophilin 1, and (E) Dynamin 1. The histograms on the right show quantification of these synaptic proteins in the boutons, expressed as percentage of control levels. The fluorescence intensity of at least 50 individual boutons was measured and the background subtracted for the quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (F) Western blot analysis of various synaptic proteins in larval brain of control, heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457 and angur7/angur5), and rescued animals (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457). Level of α-actin was used as loading control. The level of clathrin was assessed by probing the blots with anti-GFP antibody. For the EYFP-Clc experiment, the control genotype was elavC155/+; EYFP-Clc/+; the heteroallelic mutant genotype was elavC155/+; EYFP-Clc/+; angur7/AP2σKG02457; and the rescue genotype was elavC155/+; EYFP-Clc/+; angur7/UAS-AP2σ, AP2σKG02457.
The altered synaptic abundance of α-adaptin, β2-adaptin, or clathrin in σ2-adaptin mutants could be the result of either degradation of these proteins or a defect in synaptic targeting. To address this, we performed Western blots on lysates prepared from third instar larval brain. We observed a severe reduction in the α-adaptin, β2-adaptin, and clathrin protein levels in σ2-adaptin mutants (Figure 7F). Consistent with the immunofluorescence studies, the levels of other synaptic proteins, such as Endophilin1, Dynamin1, Dap160, Eps15, and Nwk, were not significantly altered in σ2-adaptin mutants (Figure 7, D and E, and Figure S3). Hence, these data suggest that σ2-adaptin is critically important for stabilizing not only the AP2 complex but also clathrin. Moreover, AP2 appears to be specific adapter for clathrin at the synapses of Drosophila NMJ.
Microtubule-based cytoskeleton is disorganized in σ2-adaptin mutants
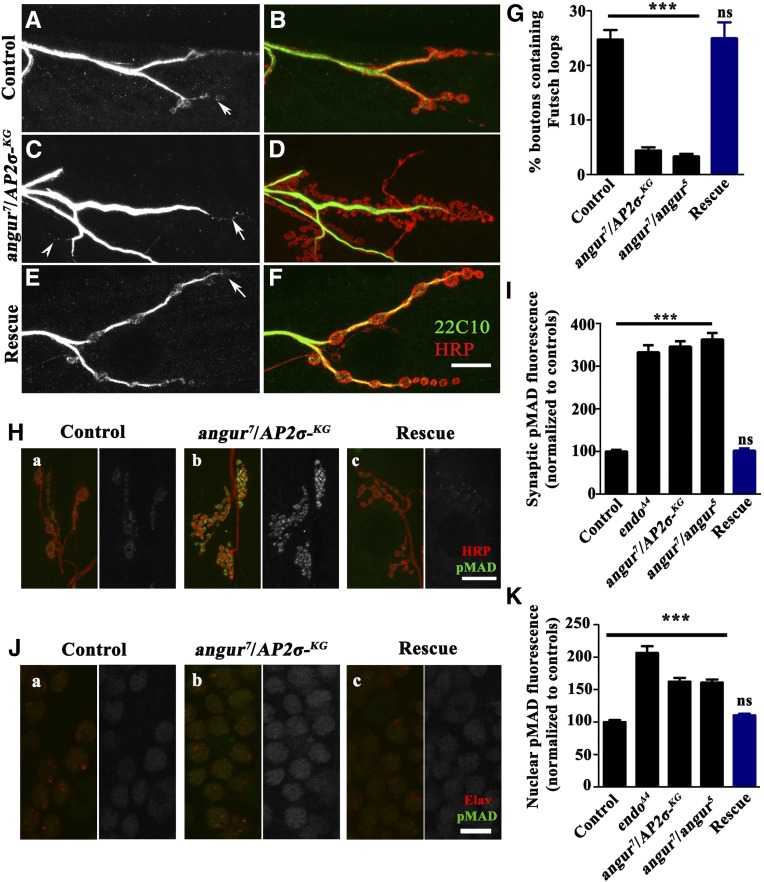
It has been shown that synaptic growth is controlled via regulation of the synaptic microtubule cytoskeleton and that any alteration of this cytoskeleton affects synaptic growth (Roos et al. 2000). To examine whether the microtubule cytoskeleton was disrupted in the mutant NMJ synapses, we labeled the synapses with MAb 22C10. This antibody labels Futsch, which is associated with the dendritic, axonal, and nerve-terminal cytoskeleton (Roos et al. 2000). In control boutons, the microtubules appeared continuous with periodic loop structures (Figure 8, A, B, and G) (24.75 ± 1.7% of type Ib boutons on muscles 6 and 7 of the A2 hemisegment). Contrary to the control boutons, the microtubules in the σ2-adaptin mutant boutons appeared fragmented, with almost total disappearance of the periodic loop structures (Figure 8, C, D, and G) (angur7/AP2σKG02457: 4.42 ± 0.6% and angur7/angur5: 3.33 ± 0.5% of type Ib boutons on muscles 6 and 7 of the A2 hemisegment). These periodic loop structures could be rescued by ubiquitous σ2-adaptin transgene expression (Figure 8, E, F, and G) (actin5C/+; angur7/AP2σKG02457, UAS-AP2σ: 24.98 ± 2.9% of type Ib boutons on muscles 6 and 7 of the A2 hemisegment). RNAi-mediated knockdown of any of the subunits of AP2 or PI4KIIIα in neurons also resulted in a significant decrease in the number of periodic loop structures (Figure S2). These data suggest that σ2-adaptin regulates NMJ morphology by regulating the cytoskeletal architecture.
Figure 8.
Mutations in σ2-adaptin cause disruption of the presynaptic cytoskeleton and upregulated BMP signaling. (A–F) Representative images of third instar larval NMJs from (A and B) control animals, heteroallelic σ2-adaptin mutants (C and D) (angur7/AP2σKG02457), and transgene-rescued (E and F) (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) animals co-labeled with HRP (red) and 22C10 (green). (G) Histogram showing quantification of the percentage of boutons containing Futsch loops of the indicated genotypes. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. n = 6 NMJ synapses of muscles 6/7 of the A2 hemisegment. (H) Representative images of third instar larval boutons from (a) control, (b) heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457), and (c) transgene-rescued (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) NMJ synapses co-labeled with HRP (red) and pMAD (green). (I) Histogram showing quantification of synaptic pMAD fluorescence of the indicated genotypes. The fluorescence intensity of at least 50 individual boutons was measured and the background subtracted for the quantification.***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test. (J) Representative images of motor nuclei of third instar larval VNC from (a) control, (b) heteroallelic σ2-adaptin mutant (angur7/AP2σKG02457), and (c) transgene-rescued (actin5C/+; angur7/UAS-AP2σ, AP2σKG02457) animals co-labeled with Elav (red) and pMAD (green). (K) Histogram showing quantification of the nuclear pMAD fluorescence of the indicated genotypes. The fluorescence intensities of at least 50 motor nuclei were measured both for Elav and for pMAD, and the ratio of pMAD/elav represented in the quantification. ***P < 0.0001. Error bars represent standard error of the mean. Statistical analysis based on one-way ANOVA followed by post-hoc Tukey’s multiple-comparison test.
Because BMP signaling is required for a normal microtubule cytoskeleton (Wang et al. 2007), we assessed whether this signaling pathway was affected in σ2-adaptin mutants. We estimated the synaptic levels of pMAD and found that the mutant synapses had over threefold higher levels of pMAD compared to controls (Figure 8, H and I) (compared to controls, the pMAD fluorescence intensity in endoΔ4 was 332.5 ± 16.7%; angur7/AP2σKG02457 was 345.8 ± 12.6%; angur7/angur5 was 362.8 ± 14.6%, and actin5C/+; angur7/AP2σKG02457, UAS-AP2σ was 102.2 ± 5.1%). Because pMAD has been shown to accumulate in the motor nuclei of synaptic mutants, we analyzed nuclear pMAD levels in σ2-adaptin mutants. Consistent with other endocytic mutants (Vanlandingham et al. 2013), we found significantly elevated pMAD levels in the motor nuclei of σ2-adaptin mutants (angur7/AP2σKG02457: 162.5 ± 5.6% and angur7/angur5: 160.9 ± 4.7%) (Figure 8, J and K). Our data thus suggest that mutation in σ2-adaptin causes a BMP pathway-dependent aberrant NMJ morphology by altering the presynaptic cytoskeleton. We suggest that σ2-adaptin-dependent endocytosis is essential for attenuating BMP-dependent synaptic growth signaling.
Discussion
AP2/CME has been proposed to play an essential role in SV endocytosis. Moreover, mutation in the proteins affecting CME also results in altered NMJ development in Drosophila, suggesting regulation of synaptic signaling by CME. In this study, we analyzed the role of AP2, one of the major adapters for clathrin at synapses, in the context of its physiological relevance and synaptic signaling. Our results demonstrate that AP2 facilitates basal synaptic transmission and SV endocytosis but also is essential during high-frequency nerve firing to regenerate SVs. Moreover, we provide evidence that AP2 regulates morphological plasticity at the Drosophila NMJ by stabilizing the microtubule loops and attenuating the BMP signaling pathway.
Role of σ2-adaptin in regulating rhabdomere biogenesis
The biogenesis of rhabdomeres is regulated by endocytosis and intracellular trafficking (Schuck and Simons 2004). Components of the endocytic machinery, when perturbed, lead to defects in rhabdomere formation. One such example is the disruption of the rhabdomere base when shits flies are grown at restrictive temperature for 4 hr (Pinal and Pichaud 2011). Rh1-Gal4-driven shits1 flies raised at 19° led to an enlargement of photoreceptor cell bodies with the inter-rhabdomeric space being reduced (Gonzalez-Bellido et al. 2009). Similarly, when α-adaptin was knocked down using GMR-Gal4, rhabdomere biogenesis was disrupted, with smaller rhabdomeres (Raghu et al. 2009). In accordance with these observations, the TEM analysis of angur mutant eye clones showed no detectable rhabdomeres. Consistent with the defect in rhabdomere formation, we also observed no membrane depolarization in angur mutant eye clones. The visual cascade in Drosophila begins with rhodopsin being converted to meta-rhodopsin by light, followed by a subsequent phosphorylation by GPRK1. The phosphorylated meta-rhodopsin binds to arrestins, where its activity is quenched. The rhodopsin-arrestin complex is endocytosed, and rhodopsin is degraded in the lysosomes (Wang and Montell 2007). Thus, endocytosis regulates rhodopsin turnover in the photoreceptor cells, and blocking of the endocytic pathway components by mutations in Arr1, Arr2, or AP2 leads to retinal degeneration and photoreceptor cell death. This further establishes a strong link between endocytosis and retinal degeneration. Thus, it is likely that defective endocytosis in angur mutant eye clones affects meta-rhodopsin turnover, resulting into defective rhabdomeres and disorganized ommatidia. Moreover, endocytosis of several signaling receptors also has been shown to regulate morphogenesis during Drosophila eye development. The Notch signaling pathway has been studied extensively, and it has been shown that the Notch extracellular domain is transendocytosed during this process into the Delta-expressing cells (Weinmaster 2000). The localization of Notch and Delta is disrupted when endocytosis is acutely blocked, and this prevents internalization of Notch in the Delta-expressing cells (Parks et al. 2000). We thus speculate that the endocytic defect in angur mutant clones affects turnover and internalization of signaling molecules that may lead to defective rhabdomere development.
σ2-adaptin regulates regeneration of SVs during high-frequency nerve firing
The functional analysis of the σ2-adaptin mutants as well as of other subunits of AP2 reveals its requirement in maintaining basal synaptic transmission and regeneration of synaptic vesicles under high-frequency nerve firing at Drosophila NMJ synapses. Our data are consistent with previous reports showing the requirement for Drosophila α-adaptin in SV recycling at NMJ synapses (Gonzalez-Gaitan and Jackle 1997). However, in contrast to its role at mammalian central synapses, we found that loss of AP2 at Drosophila NMJ synapses affects basal synaptic transmission and SV endocytosis, albeit not very strongly. Moreover, the kinetics of SV re-formation in σ2-adaptin mutants was only mildly affected. Consistent with the recent observation that loss of the AP2 complex at mammalian central synapses or its subunits in C. elegans affects synaptic vesicle biogenesis, we observed compromised synaptic transmission and SV re-formation after high-frequency stimulation, suggesting that at Drosophila NMJ synapses, AP2 function is required not only for SV trafficking but also for SV regeneration under high-frequency nerve stimulation (Gu et al. 2013; Kononenko et al. 2014). Our direct estimation of vesicle pool size in σ2-adaptin mutants further supports its requirement in the regulation of SV pool size and is consistent with a recent report (Gu et al. 2013). Because loss of AP2 in Drosophila does not completely abrogate SV re-formation, it remains possible that other known synaptic clathrin adapters such as AP180, Eps15, and Epsin might partially compensate for loss of the AP2 complex (Zhang et al. 1998; Jakobsson et al. 2008; Cheung and Cousin 2012). This is corroborated by the observation that even a 96% reduction in synaptic α-adaptin level caused only an ∼50% reduction in clathrin, suggesting that other clathrin adapters might contribute to retention and/or stability of synaptic clathrin.
The striking physiological defect in σ2-adaptin mutants or neuronally reduced α-adaptin was their inability to recover from synaptic depression even after 4 min of cessation of high-frequency stimulation. Consistent with these observations, the endo-exo cycling pool did not recover to its initial value even after 4 min of rest following high-frequency nerve stimulation. This suggests that in addition to its requirement in synaptic vesicle endocytosis, AP2 complex functions at a relatively slower step downstream of membrane retrieval, possibly during membrane sorting from endosomes to regenerate fusion-competent synaptic vesicles (Gu et al. 2013; Kononenko et al. 2014).
PI(4,5)P2 and subunits of the AP2 complex are obligate partners for synapse growth in Drosophila
Several studies in cell culture support a model that indicates that the AP2 complex is an obligate heterotetramer for subunit stability and function (Motley et al. 2003; Boucrot et al. 2010). However, studies of AP2 function in C. elegans suggest that α/σ2 subunits and β2/µ2 subunits may constitute two hemicomplexes that can carry out the minimal function of the AP2 complex independent of one another (Gu et al. 2013). Does AP2 in Drosophila form hemicomplexes and contribute to vesicle trafficking? Our results do not support this model for Drosophila. First, unlike C. elegans, in which individual mutants for subunits of the AP2 complex are viable, the Drosophila mutant for α-adaptin is early larval lethal (Gonzalez-Gaitan and Jackle 1997). Consistent with these findings, neuronal knockdown of α-adaptin or β2-adaptin resulted in third instar lethality. Second, if the hemicomplexes were functional in Drosophila, one would observe significant levels of synaptic β2-adaptin in α-adaptin knockdown or σ2-adaptin mutants. However, we observed that removing any of the subunits of the Drosophila AP2 complex in neurons resulted into an unstable AP2 complex and degradation of α− and β2-adaptins. Third, inhibiting AP2 complex assembly by reducing synaptic PI(4,5)P2 levels also resulted into an unstable AP2 complex. These observations strongly suggest that in contrast to C. elegans, all the subunits of AP2 in Drosophila are obligate partners for a stable AP2 complex for clathrin-dependent SV trafficking.
Mutations in genes implicated in regulating CME, BMP signaling, or actin cytoskeleton dynamics all show abnormal NMJ development in Drosophila, characterized by either supernumerary boutons or an increased number but smaller boutons (Dickman et al. 2006; Wang et al. 2007; O’Connor-Giles et al. 2008; Nahm et al. 2013). The NMJ phenotype due to loss of σ2-adaptin is consistent with that of other known endocytic mutants implicated in CME. The contrasting synaptic overgrowth observed in σ2-adaptin mutants suggests a role for AP2 in mediating presynaptic growth signaling. Such synaptic overgrowth also was observed when any of the subunits of AP2 were downregulated or its assembly was interfered with by downregulating neuronal PI(4,5)P2. This suggests a pathway in which PI(4,5)P2 and the AP2 complex interact obligatorily to regulate synapse growth. The NMJ phenotype in angur mutants is strikingly more severe from that of the other synaptic mutants and points toward multiple growth signaling pathways that are possibly affected by an AP2-dependent endocytic deficit.
The abnormal synapse morphology in angur mutants is a consequence of alteration of the neuronal cytoskeleton
The morphology of the synapse is a consequence of the neuronal cytoskeleton network that shapes the growing synapse. Futsch, a protein with MAP1B homology, regulates the synaptic microtubule cytoskeleton, thereby controlling synaptic growth at the Drosophila NMJ (Roos et al. 2000). The hypothesis that the microtubule organization could be altered was strengthened by the dramatic decrease in the number of Futsch-positive loops in the σ2-adaptin mutants. Futsch-positive loops have long been known to be associated with stable synaptic boutons, while the absence or disruption of these loops is indicative of boutons undergoing division or sprouting. The dramatic increase in the number of boutons in these mutants with a corresponding decrease in the number of loops correlates well with the fact that these boutons might be undergoing division. The phenotype associated with futsch mutants is fewer and larger boutons with impaired microtubule organization, which is an expected phenotype when bouton division is impaired. The σ2-adaptin mutants, however, show a larger number but smaller-sized boutons, indicating that bouton division is enhanced. One of the signaling events that has been shown to dictate this process is BMP signaling (Wang et al. 2007). Further, BMP signaling is also required during developmental synaptic growth (Aberle et al. 2002; Marques et al. 2002; McCabe et al. 2003). Consistent with this, we found elevated pMAD levels in σ2-adaptin mutants, indicating that BMP signaling is upregulated in these mutants. It has also been demonstrated that BMP signaling plays a role in maintenance of the presynaptic microtubule network (Wang et al. 2007). Drosophila spichthyin and spartin mutants have upregulated BMP signaling with significantly increased microtubule loops. It has been proposed that Futsch acts downstream of BMP signaling to regulate synaptic growth (Wang et al. 2007; Nahm et al. 2013). The σ2-adaptin mutants also show upregulated BMP signaling, but in contrast to spichthyin and spartin mutants, σ2-adaptin mutants have fewer Futsch loops, which also appeared fragmented. This suggests that deregulated BMP signaling, whether upregulation or downregulation, impairs microtubule stability. dap160 mutants also show supernumerary boutons, with fragmented Futsch staining suggesting that the microtubule dynamics are misregulated in these mutants. Interestingly, Nwk levels are drastically altered in dap160 mutants, suggesting that Nwk levels are important for maintaining microtubule stability (Koh et al. 2004). Because σ2-adaptin mutants have normal Nwk levels, the observed synaptic overgrowth is independent of Nwk and suggests regulation of Futsch through Nwk-independent pathways.
Acknowledgments
We thank Hugo Bellen, Barry Ganetzky, and Peter Dijke for sharing antibodies and the Bloomington Drosophila Stock Center and Hybridoma Bank, University of Iowa, for fly lines and monoclonal antibodies. We thank Abdul Rahim Mohammad and Aseem Shrivastava for their help with initial characterization of the mutant and RNAi lines used in this study. We thank Seema Shirolikar, Lalit C. Borde, and Krishanu Ray (TIFR, Mumbai) for help with TEM. We thank Praveen Verma, NIPGR, New Delhi, for help with SEM. We thank Subhabrata Sanyal, Susy Kim, and Shanker Jha for many useful comments on the manuscript. This work was supported by project grants from the Department of Biotechnology, Government of India, to V.K. We thank IISER Bhopal for generous intramural funds and the Central Instrumentation Facility at IISER Bhopal.
Footnotes
Communicating editor: R. J. Duronio
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.183863/-/DC1.
These authors contributed equally to this work.
Deceased.
Literature Cited
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhaes T. R., et al. , 2002. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558. [DOI] [PubMed] [Google Scholar]
- Boucrot E., Saffarian S., Zhang R., Kirchhausen T., 2010. Roles of AP-2 in clathrin-mediated endocytosis. PLoS One 5: e10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Cheung G., Cousin M. A., 2012. Adaptor protein complexes 1 and 3 are essential for generation of synaptic vesicles from activity-dependent bulk endosomes. J. Neurosci. 32: 6014–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G., Jupp O. J., Cousin M. A., 2010. Activity-dependent bulk endocytosis and clathrin-dependent endocytosis replenish specific synaptic vesicle pools in central nerve terminals. J. Neurosci. 30: 8151–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J., 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109: 523–535. [DOI] [PubMed] [Google Scholar]
- Coyle I. P., Koh Y. H., Lee W. C., Slind J., Fergestad T., et al. , 2004. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron 41: 521–534. [DOI] [PubMed] [Google Scholar]
- Delgado R., Maureira C., Oliva C., Kidokoro Y., Labarca P., 2000. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron 28: 941–953. [DOI] [PubMed] [Google Scholar]
- Dickman D. K., Horne J. A., Meinertzhagen I. A., Schwarz T. L., 2005. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell 123: 521–533. [DOI] [PubMed] [Google Scholar]
- Dickman D. K., Lu Z., Meinertzhagen I. A., Schwarz T. L., 2006. Altered synaptic development and active zone spacing in endocytosis mutants. Curr. Biol. 16: 591–598. [DOI] [PubMed] [Google Scholar]
- Dittman J., Ryan T. A., 2009. Molecular circuitry of endocytosis at nerve terminals. Annu. Rev. Cell Dev. Biol. 25: 133–160. [DOI] [PubMed] [Google Scholar]
- Estes P. S., Roos J., van der Bliek A., Kelly R. B., Krishnan K. S., et al. , 1996. Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. J. Neurosci. 16: 5443–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bellido P. T., Wardill T. J., Kostyleva R., Meinertzhagen I. A., Juusola M., 2009. Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors. J. Neurosci. 29: 14199–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M., Jackle H., 1997. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell 88: 767–776. [DOI] [PubMed] [Google Scholar]
- Granseth B., Odermatt B., Royle S. J., Lagnado L., 2006. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51: 773–786. [DOI] [PubMed] [Google Scholar]
- Granseth B., Odermatt B., Royle S. J., Lagnado L., 2007. Clathrin-mediated endocytosis: the physiological mechanism of vesicle retrieval at hippocampal synapses. J. Physiol. 585: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Liu Q., Watanabe S., Sun L., Hollopeter G., et al. , 2013. AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. eLife 2: e00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen H., Fetter R. D., Davis G. W., 2008. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr. Biol. 18: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., 1973. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57: 315–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Sudhof T. C., 1994. Synaptic vesicles and exocytosis. Annu. Rev. Neurosci. 17: 219–246. [DOI] [PubMed] [Google Scholar]
- Jakobsson J., Gad H., Andersson F., Low P., Shupliakov O., et al. , 2008. Role of epsin 1 in synaptic vesicle endocytosis. Proc. Natl. Acad. Sci. USA 105: 6445–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Ryan T. A., 2009. Synaptic vesicle recycling at CNS snapses without AP-2. J. Neurosci. 29: 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. M., Kumar V., Lin Y. Q., Karunanithi S., Ramaswami M., 2009. Fos and Jun potentiate individual release sites and mobilize the reserve synaptic vesicle pool at the Drosophila larval motor synapse. Proc. Natl. Acad. Sci. USA 106: 4000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh T. W., Verstreken P., Bellen H. J., 2004. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 43: 193–205. [DOI] [PubMed] [Google Scholar]
- Koh T. W., Korolchuk V. I., Wairkar Y. P., Jiao W., Evergren E., et al. , 2007. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J. Cell Biol. 178: 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N. L., Puchkov D., Classen G. A., Walter A. M., Pechstein A., et al. , 2014. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82: 981–988. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K., 1983. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J. Neurobiol. 14: 207–225. [DOI] [PubMed] [Google Scholar]
- Kumar V., Alla S. R., Krishnan K. S., Ramaswami M., 2009. Syndapin is dispensable for synaptic vesicle endocytosis at the Drosophila larval neuromuscular junction. Mol. Cell. Neurosci. 40: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhotia S. C., Mallik M., Singh A. K., Ray M., 2012. The large noncoding hsrω-n transcripts are essential for thermotolerance and remobilization of hnRNPs, HP1 and RNA polymerase II during recovery from heat shock in Drosophila. Chromosoma 121: 49–70. [DOI] [PubMed] [Google Scholar]
- Marie B., Sweeney S. T., Poskanzer K. E., Roos J., Kelly R. B., et al. , 2004. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron 43: 207–219. [DOI] [PubMed] [Google Scholar]
- Marques G., Bao H., Haerry T. E., Shimell M. J., Duchek P., et al. , 2002. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33: 529–543. [DOI] [PubMed] [Google Scholar]
- Matsui W., Kirchhausen T., 1990. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry 29: 10791–10798. [DOI] [PubMed] [Google Scholar]
- McCabe B. D., Marques G., Haghighi A. P., Fetter R. D., Crotty M. L., et al. , 2003. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39: 241–254. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Boucrot E., 2011. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12: 517–533. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen I. A., 1996. Ultrastructure and quantification of synapses in the insect nervous system. J. Neurosci. Methods 69: 59–73. [DOI] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S., 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M., Lee M. J., Parkinson W., Lee M., Kim H., et al. , 2013. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 77: 680–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor-Giles K. M., Ho L. L., Ganetzky B., 2008. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 58: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks A. L., Klueg K. M., Stout J. R., Muskavitch M. A., 2000. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127: 1373–1385. [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., et al. , 1998. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434: 83–87. [DOI] [PubMed] [Google Scholar]
- Pinal N., Pichaud F., 2011. Dynamin- and Rab5-dependent endocytosis is required to prevent Drosophila photoreceptor degeneration. J. Cell Sci. 124: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K. E., Fetter R. D., Davis G. W., 2006. Discrete residues in the c(2)b domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron 50: 49–62. [DOI] [PubMed] [Google Scholar]
- Raghu P., Coessens E., Manifava M., Georgiev P., Pettitt T., et al. , 2009. Rhabdomere biogenesis in Drosophila photoreceptors is acutely sensitive to phosphatidic acid levels. J. Cell Biol. 185: 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M., Rao S., van der Bliek A., Kelly R. B., Krishnan K. S., 1993. Genetic studies on dynamin function in Drosophila. J. Neurogenet. 9: 73–87. [DOI] [PubMed] [Google Scholar]
- Ramaswami M., Krishnan K. S., Kelly R. B., 1994. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron 13: 363–375. [DOI] [PubMed] [Google Scholar]
- Rikhy R., Kumar V., Mittal R., Krishnan K. S., 2002. Endophilin is critically required for synapse formation and function in Drosophila melanogaster. J. Neurosci. 22: 7478–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J., Kelly R. B., 1998. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J. Biol. Chem. 273: 19108–19119. [DOI] [PubMed] [Google Scholar]
- Roos J., Hummel T., Ng N., Klambt C., Davis G. W., 2000. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 26: 371–382. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canada C., Ashley J., Moeckel-Cole S., Drier E., Yin J., et al. , 2004. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron 42: 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y., De Camilli P., 2012. Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 4: a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ernstrom G. G., Watanabe S., Weimer R. M., Chen C. H., et al. , 2009. Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc. Natl. Acad. Sci. USA 106: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E. M., McMahon H. T., 2007. Integrating molecular and network biology to decode endocytosis. Nature 448: 883–888. [DOI] [PubMed] [Google Scholar]
- Schuck S., Simons K., 2004. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 117: 5955–5964. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Lakhotia S. C., 2006. Altered expressions of the noncoding hsrω gene enhances poly-Q-induced neurotoxicity in Drosophila. RNA Biol. 3: 28–35. [DOI] [PubMed] [Google Scholar]
- Song W., Zinsmaier K. E., 2003. Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis. Neuron 40: 665–667. [DOI] [PubMed] [Google Scholar]
- Stimson D. T., Estes P. S., Rao S., Krishnan K. S., Kelly L. E., et al. , 2001. Drosophila stoned proteins regulate the rate and fidelity of synaptic vesicle internalization. J. Neurosci. 21: 3034–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R. S., Schwarz T. L., 1999. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., 2003. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandingham P. A., Fore T. R., Chastain L. R., Royer S. M., Bao H., et al. , 2013. Epsin 1 promotes synaptic growth by enhancing BMP signal levels in motoneuron nuclei. PLoS One 8: e65997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P., Kjaerulff O., Lloyd T. E., Atkinson R., Zhou Y., et al. , 2002. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell 109: 101–112. [DOI] [PubMed] [Google Scholar]
- Wang T., Montell C., 2007. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 454: 821–847. [DOI] [PubMed] [Google Scholar]
- Wang X., Shaw W. R., Tsang H. T., Reid E., O’Kane C. J., 2007. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Trimbuch T., Camacho-Perez M., Rost B. R., Brokowski B., et al. , 2014. Clathrin regenerates synaptic vesicles from endosomes. Nature 515: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., 2000. Notch signal transduction: a real rip and more. Curr. Opin. Genet. Dev. 10: 363–369. [DOI] [PubMed] [Google Scholar]
- Wolff T., 2011. Preparation of Drosophila eye specimens for scanning electron microscopy. Cold Spring Harb. Protoc. 2011: 1383–1385. [DOI] [PubMed] [Google Scholar]
- Zhang B., Koh Y. H., Beckstead R. B., Budnik V., Ganetzky B., et al. , 1998. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron 21: 1465–1475. [DOI] [PubMed] [Google Scholar]
- Zinsmaier K. E., Eberle K. K., Buchner E., Walter N., Benzer S., 1994. Paralysis and early death in cysteine string protein mutants of Drosophila. Science 263: 977–980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Antibodies and Drosophila lines generated and used in this manuscript will be made available upon request. Supplemental data is available as Figure S1, Figure S2, Figure S3, Table S1 and Table S2.