WHEN Meuwissen et al. published this landmark article in 2001, most agricultural genomics research was focused on detecting quantitative trait loci (QTL) using experimental crosses or existing family relationships. Around this time the human genome was nearing its completion, but no livestock or crop genomes were yet available. Genomic markers in livestock were mainly microsatellites, complemented by Amplified Fragment Length Polymorphisms in crops, with Diversity Arrays Technology markers on the horizon. Such genomic information was intended to be used in artificial selection mainly via Marker-Assisted Selection and Marker-Assisted Introgression. In these methods, selection decisions are made based on only a few markers that individually show association with the relevant trait.
So it required considerable foresight by Meuwissen et al. (2001) to develop a new approach that required a sufficiently high marker density such that every QTL affecting a relevant trait would be in linkage disequilibrium with at least one marker. Meuwissen et al. (2001) argued that decisions to select for breeding could be based on the joint merit of all markers across the genome. Before this, breeders used a combination of polygenic effects and genotypes of painstakingly selected and validated QTL. The concept introduced by Meuwissen et al., now known as “genomic selection,” has allowed unprecedented advances in commercial breeding in the past 15 years, including a doubling of dairy cattle improvement per generation compared to traditional selection.
Meuwissen et al. explored these concepts using a simulated data set with a mere 1010 genetic markers and 1000 putative QTL. They applied different statistical modeling approaches: linear regression, Best Linear Unbiased Selection (BLUP), and two Bayesian approaches, dubbed BayesA and BayesB. The Bayesian approaches have been extensively tweaked and revised by other researchers, with many improvements related to the prior distributions of QTL effect [giving rise to the “Bayesian Alphabet” coined by Dan Gianola (Gianola et al. 2009; Gianola 2013)]. It is notable that despite many years of methods development BayesB is still among the gold standards for evaluating new approaches, while a modified implementation of the BLUP approach (GBLUP) is used in many practical applications of genomic selection (reviewed by de los Campos et al. 2013 and Daetwyler et al. 2013).
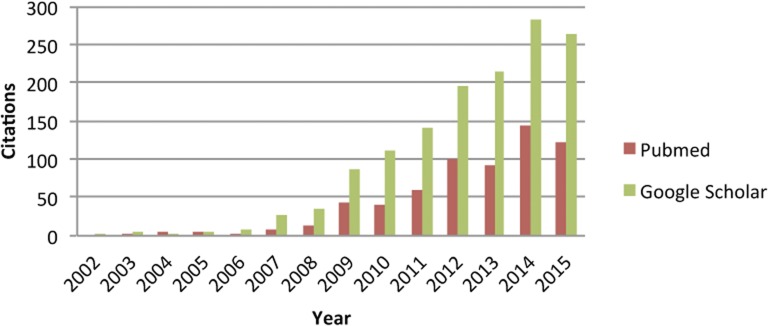
At the time of publication the work in Meuwissen et al. was hailed as a breakthrough by the breeding communities, but the tools needed for implementation were not yet available. So for the next few years genomic selection was just a proposal. However, after 2006, when medium-density SNP chips were becoming routinely available for the main livestock species, genomic selection suddenly became a hot research topic (Figure 1).
Figure 1.
Citations to Meuwissen et al. (2001) according to PubMed and Google Scholar.
Since then, industry uptake of the method, both in terms of speed and magnitude, has been remarkable. In dairy cattle breeding, genomic selection has all but replaced the traditional selection based on progeny testing, with unparalleled results. Other livestock species are following suit. Maize and wheat are at the forefront of genomic selection in crops, not the least because of large international efforts by the International Maize and Wheat Improvement Center (CIMMYT).
So it is fair to say that Meuwissen et al. changed selective breeding for many, if not most, agriculturally important species within 15 years of publication. The principles of genomic selection are now also increasingly being applied in studies of human disease. Researchers in this area, not the least Theo Meuwissen, Ben Hayes, and Mike Goddard, continue to refine the applications of genomic selection including the optimal use of whole-genome sequence information.
The Genetics Society of America journals GENETICS and G3:Genes|Genomes|Genetics have special collections on genomic selection, which currently has >50 papers (http://www.genetics.org/collection/genomic-selection). This collection includes reviews, methods, tools, and applications, including access to valuable data sets for comparison and benchmarking of new approaches.
Footnotes
Communicating editor: C. Gelling
ORIGINAL CITATION
Theodorus H. E. Meuwissen, Benjamin J. Hayes, and Michael E. Goddard
GENETICS April 1, 2001 157: 1819–1829
Literature Cited
- Daetwyler H. D., Calus M. P., Pong-Wong R., de los Campos G., Hickey J. M., 2013. Genomic prediction in animals and plants: simulation of data, validation, reporting, and benchmarking. Genetics 193: 347–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Campos G., J. M. Hickey, R. Pong-Wong, H. D. Daetwyler, and M. P. Calus, 2013. Whole-genome regression and prediction methods applied to plant and animal breeding. Genetics 193: 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianola D., 2013. Priors in whole-genome regression: the Bayesian alphabet returns. Genetics 194: 573–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianola D., de Los Campos G., Hill W. G., Manfredi E., Fernando R., 2009. Additive genetic variability and the Bayesian alphabet. Genetics 183: 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading in GENETICS
- de Koning D.-J., McIntyre L., 2012. Setting the standard: a special focus on genomic selection in GENETICS and G3. Genetics 190: 1151–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. G., 2014. Applications of population genetics to animal breeding, from Wright, Fisher, and Lush to genomic prediction. Genetics 196: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Other GENETICS Articles by Meuwissen et al.
- Calus M. P. L., Meuwissen T. H. E., de Roos A. P. W., Veerkamp R. F., 2008. Accuracy of genomic selection using different methods to define haplotypes. Genetics 178: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain A. J., McPartlan H. C., Goddard M. E., 2007. The number of loci that affect milk production traits in dairy cattle. Genetics 177: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daetwyler H. D., Wiggans G. R., Hayes B. J., Woolliams J. A., Goddard M. E., 2011. Imputation of missing genotypes from sparse to high density using long-range phasing. Genetics 189: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daetwyler H. D., Hayden M. J., Spangenberg G. C., Hayes B. J., 2015. Selection on optimal haploid value increases genetic gain and preserves more genetic diversity relative to genomic selection. Genetics 200: 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos A. P. W., Hayes B. J., Spelman R. J., Goddard M. E., 2008. Linkage disequilibrium and persistence of phase in Holstein-Friesian, Jersey, and Angus cattle. Genetics 179: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos A. P. W., Hayes B. J., Goddard M. E., 2009. Reliability of genomic predictions across multiple populations. Genetics 183: 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier M., Barcelona R. R., Fritz S., Grohs C., Druet T., et al. , 2006. Fine mapping and physical characterization of two linked quantitative trait loci affecting milk fat yield in dairy cattle on BTA26. Genetics 172: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjuvsland A. B., Hayes B. J., Omholt S. W., Carlborg O., 2007. Statistical epistasis is a generic feature of gene regulatory networks. Genetics 175: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall J. M., Goddard M. E., 1999. Multiple-trait mapping of quantitative trait loci after selective genotyping using logistic regression. Genetics 151: 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M. C., Visscher P. M., Goddard M. E., 2011. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics 189: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehammer M., Goddard M. E., Nilsen H., Sehested E., Olsen H. G., et al. , 2008. Quantitative trait locus-by-environment interaction for milk yield traits on Bos taurus autosome 6. Genetics 179: 1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan T., Woolliams J. A., Lien S., Kent M., Svendsen M., et al. , 2009. The accuracy of genomic selection in Norwegian red cattle assessed by cross-validation. Genetics 183: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod I. M., Hayes B. J., Goddard M. E., 2014. The effects of demography and long-term selection on the accuracy of genomic prediction with sequence data. Genetics 198: 1671–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T. H., Goddard M. E., 1997. Estimation of effects of quantitative trait loci in large complex pedigrees. Genetics 146: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T. H., Goddard M. E., 2000. Fine mapping of quantitative trait loci using linkage disequilibria with closely linked marker loci. Genetics 155: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T. H. E., Goddard M. E., 2007. Multipoint identity-by-descent prediction using dense markers to map quantitative trait loci and estimate effective population size. Genetics 176: 2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T., Goddard M., 2010a Accurate prediction of genetic values for complex traits by whole-genome resequencing. Genetics 185: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T., Goddard M., 2010b The use of family relationships and linkage disequilibrium to impute phase and missing genotypes in up to whole-genome sequence density genotypic data. Genetics 185: 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T. H., Hayes B. J., Goddard M. E., 2001. Prediction of total genetic value using genome-wide dense marker maps. Genetics 157: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen T. H. E., Karlsen A., Lien S., Olsaker I., Goddard M. E., 2002. Fine mapping of a quantitative trait locus for twinning rate using combined linkage and linkage disequilibrium mapping. Genetics 161: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard J., Yazdi M. H., Sonesson A. K., Meuwissen T. H. E., 2009. Incorporating desirable genetic characteristics from an inferior into a superior population using genomic selection. Genetics 181: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen H. G., Lien S., Gautier M., Nilsen H., Roseth A., et al. , 2005. Mapping of a milk production quantitative trait locus to a 420-kb region on bovine chromosome 6. Genetics 169: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce J. E., Hayes B. J., Bolormaa S., Goddard M. E., 2011. Polymorphic regions affecting human height also control stature in cattle. Genetics 187: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor C., Farnir F., Hansoul S., Coppieters W., Meuwissen T., et al. , 2006. Linkage disequilibrium on the bovine X chromosome: characterization and use in quantitative trait locus mapping. Genetics 173: 1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. M., Goddard M. E., 2015. A general unified framework to assess the sampling variance of heritability estimates using pedigree or marker-based relationships. Genetics 199: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]