Abstract
The use of probiotics has been widely documented to benefit human health, but their clinical value in surgical patients remains unclear. The present study investigated the effect of perioperative oral administration of probiotic bifidobacteria to patients undergoing colorectal surgery. Sixty patients undergoing colorectal resection were randomized to two groups prior to resection. One group (n=31) received a probiotic supplement, Bifidobacterium longum BB536, preoperatively for 7–14 days and postoperatively for 14 days, while the other group (n=29) received no intervention as a control. The occurrences of postoperative infectious complications were recorded. Blood and fecal samples were collected before and after surgery. No significant difference was found in the incidence of postoperative infectious complications and duration of hospital stay between the two groups. In comparison to the control group, the probiotic group tended to have higher postoperative levels of erythrocytes, hemoglobin, lymphocytes, total protein, and albumin and lower levels of high sensitive C-reactive proteins. Postoperatively, the proportions of fecal bacteria changed significantly; Actinobacteria increased in the probiotic group, Bacteroidetes and Proteobacteria increased in the control group, and Firmicutes decreased in both groups. Significant correlations were found between the proportions of fecal bacteria and blood parameters; Actinobacteria correlated negatively with blood inflammatory parameters, while Bacteroidetes and Proteobacteria correlated positively with blood inflammatory parameters. In the subgroup of patients who received preoperative chemoradiotherapy treatment, the duration of hospital stay was significantly shortened upon probiotic intervention. These results suggest that perioperative oral administration of bifidobacteria may contribute to a balanced intestinal microbiota and attenuated postoperative inflammatory responses, which may subsequently promote a healthy recovery after colorectal resection.
Keywords: probiotics, Bifidobacterium, colorectal surgery, postoperative complications, microbiota, nutrition, inflammation
INTRODUCTION
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [1]. Bifidobacteria and lactic acid bacteria are the main genera of probiotic microorganisms. Probiotics are widely documented for their roles in prevention and treatment of diseases, primarily via improving the intestinal environment and enhancing host defense functions [2, 3]. Clinical trials have illustrated that prophylactic administration of probiotics, and in some cases together with prebiotics (as synbiotics), to patients undergoing abdominal surgery reduced postoperative infections [4,5,6,7,8]. Systematic reviews and meta-analyses showed that the use of pro-/synbiotics has positively affected clinical outcomes after abdominal surgery [9, 10]. However, another study demonstrated that the effect of prophylactic administration of probiotics to patients undergoing different types of gastrointestinal surgeries was insignificant [11]. The effects of pro-/synbiotics on postoperative infections in colorectal cancer patients remain controversial, and studies involving specific strains of probiotics for specific groups of patients are needed to better justify their efficacy.
The present study investigated the effect of a probiotic strain, Bifidobacterium longum BB536, which has been reported to have various physiological effects [12, 13], on immune functions, systemic inflammatory responses, and postoperative infectious complications in patients undergoing colorectal surgery. Fecal microbial profiling was also performed to better understand the possible roles of microbiota on health conditions of patients and to justify the positive outcomes of probiotic intervention.
MATERIALS AND METHODS
Study design and participants
Patients who had colorectal cancer at 20–85 years of age and were scheduled to undergo colorectal cancer resection at Mitoyo General Hospital between October 2008 and December 2012 were enrolled in this study. The exclusion criteria were severe diseases of the liver, kidney, heart, and lung and the presence of a food allergy. Patients with bowel obstruction were also excluded. Written informed consent for participation was obtained from each patient before enrollment. All study protocols were reviewed and approved by the Local Ethics Committee of Mitoyo General Hospital, Takamatsu, Japan, and complied with the Helsinki Declaration as revised in 1983. A flow diagram of participants is shown in Fig. 1.
Fig. 1.
Flow diagram for the study.
This was a randomized, single-center, single-blinded trial. Physicians involved with the diagnosis of infections and researchers performing the statistical analyses were not involved in the randomization process. The subjects were randomized before surgery to either the probiotic group or control group using a computer-generated permuted-block randomization. Subjects in the probiotic group received a sachet containing 2 g of B. longum BB536 powder (approximately 5 × 1010 colony-forming units/2 g) daily for 7–14 days preoperatively and 14 days postoperatively (starting one day after surgery). The subjects were advised to ingest this powder by drinking it with milk or water. Subjects who did not receive the intervention were used as a control group.
Clinical observation and blood analyses
All patients started fasting one day before surgery and underwent preoperative bowel preparation by orally taking an electrolyte-compounding agent (2 L, Niflec, Ajinomoto Pharmaceuticals). All patients received antibiotic prophylaxis (cefmetazole) as a single intravenous drip infusion on the day of surgery and one day after surgery. An additional postoperative antibiotic was prescribed when recommended by the physicians who diagnosed the patients. Detailed daily records of each patient’s postoperative course were kept, and infectious complications were recorded for 14 days after surgery in accordance with the Guideline for the Prevention of Surgical Site Infection (1999) [14].
Blood samples were collected before and after surgery (days: –14, 1, 4, 7, and 14) to assess biomarkers related to the health condition of the patients, with the exception of natural killer (NK) cell activity and interleukin (IL)-6, which were assessed on days –14, 1, and 7. NK cell activity (%) was measured by a 51Cr-release assay [15]. All measurements were performed by SRL, Inc. (Tokyo, Japan).
Analysis of fecal microbiota
Fecal samples were collected from each subject before probiotic ingestion at prior to surgery, and 1 week after surgery using a sampling kit (Techno Suruga Laboratory Co., Ltd., Shizuoka, Japan), which enabled quick stabilization of DNA and processing of samples at normal room temperature. Six patients failed to provide samples before or after surgery; a total of 54 full sets of samples were collected (28 in the probiotic group and 26 in the control group).
DNA was extracted from the fecal samples as previously described [16] with some modifications. Fifty microliters of 10% sodium dodecyl sulfate, 500 µl of phenol-chloroform, and 300 mg of glass beads (diameter, 0.1 mm) were added to 450 µl of fecal suspension. The mixture was vigorously vortexed at 2,700 rpm for 3 minutes at 4°C using a Multi-beads shocker (MBS01(S), Yasui Kikai Corporation, Osaka, Japan). After centrifugation at 14,000×g for 5 min, 400 µl of supernatant was extracted with phenol-chloroform, and 250 µl of the supernatant was precipitated with isopropanol. Inhibitors were removed using a High Pure PCR Template Preparation Kit (Roche). The purified DNA was suspended in 200 µl of Tris-EDTA buffer (pH 8.0).
The V3-V4 region of bacterial 16S rRNA genes was amplified by PCR with a TaKaRa Ex Taq HS kit (TaKaRa Bio, Shiga, Japan) and the primer set of Tru357F (5′-CGCTCTTCCGATCTCTG TACGGRAGGCAGCAG-3′) and Tru806R (5′-CGCTCTTCCGATCTGAC GGACTACHVGGGTWTCTAAT-3′). DNA was amplified according to the following program: preheating at 94°C for 3 min; 25 cycles of denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec, and extension at 72°C for 30 sec; and a final terminal extension at 72°C for 10 min. Upon checking the size and the quantity of the amplified DNA using a QIAxcel system (Qiagen, Valencia, CA, USA), 1 µl of the PCR products were amplified with a 2nd primer set, adapted for the Illumina MiSeq. DNA was amplified using the same program as described above, except that a 15 amplification cycles were used instead of 25. After checking the size and the quantity of the 2nd set of amplified DNA products using the QIAxcel system, the products were purified using a QIAquick 96 PCR Purification Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The purified products were quantified using a Quant-iT PicoGreend dsDNA Assay kit (Life Technologies, Carlsbad, CA, USA). Subsequently, equal amounts of the amplicons from different samples were pooled, and primer-dimers were removed by gel extraction with a QIAquick PCR Purification Kit. The pooled libraries were sequenced using an Illumina Miseq instrument with a MiSeq v2 Reagent kit.
The Illumina paired-end reads that passed the quality filters were combined by the fastq-join script in EA-Utils (ver. 1.1.2-537) [17]. The sequences were then analyzed using the QIIME software package version 1.6.0 [18, 19] (http://quime.org/). Potential chimeric sequences were removed using UCHIME, assigned to operational taxonomic units (OTUs) using open-reference OTU picking [20] with a 97% threshold of pairwise identity, and then classified taxonomically using the Greengenes reference database (http://greengenes.secondgenome.com/downloads/database/12_10) and a confidence threshold of 60% [21]. As a result, 1,363,590 sequences were assigned to 108 samples, in which the average ± standard deviation of the read number per sample was 12,625 ± 3,370. UniFrac distances were calculated from the Illumina V3-4 16S rRNA gene dataset.
Statistical analyses
Postoperative infectious complication was predetermined as the primary efficacy variable. The secondary variables were as follows: additional postoperative antibiotic treatment, hospital stay, and changes in blood parameters and the microbiota. Regarding sample size, a minimum of 60 subjects were required to demonstrate a reduction in postoperative infectious complications from 40% to 15% at a 5% significant level with 80% power, similar to that seen in another study [22].
Results of blood parameters are expressed as the mean ± SD or SE. Statistical analyses were performed using the paired and unpaired Student’s t-test for continuous variables and Fisher’s exact test for categorical variables in comparison between the control and probiotic groups. Variables that did not follow a normal distribution were analyzed after natural log transformation. The data for the microbiota were expressed as the median and interquartile range (IQR) of the proportion of each bacterial category in the microbiota, and the within-group difference and between-group difference at each time point were analyzed using the Wilcoxon signed-rank test and Mann-Whitney U-test, respectively. Statistical significance was determined at p<0.05.
RESULTS
Demographics of patients and clinical observation
A total of 60 patients, 31 in the probiotic group and 29 in the control group, were enrolled. The baseline characteristics of the probiotic and control groups are shown in Table 1. No significant differences in sex, age, BMI, types of surgery, and length of operation were found between the two groups (Table 1). In addition, no significant differences were found in the numbers of subjects with preoperative diabetes and preoperative complications. The probiotic group included 11 colon and 19 rectal cancer patients, whereas the control group included 12 patients with colon cancer, 13 patients with rectal cancer, 1 patients with anal canal cancer, 1 patients with local postoperative recurrence of rectal cancer, 1 patients with rectovaginal fistula, and 1 colostomy patient. The study groups did not differ significantly in preoperative values of blood parameters.
Table 1. Background characteristics for all patients and those patients who received preoperative chemoradiotherapy (CRT).
| All patients†
|
CRT patients†
|
|||||
|---|---|---|---|---|---|---|
| Control (n=29) | Probiotic (n=31) | Control (n=7) | Probiotic (n=8) | |||
| Gender (Male/Female) | 15/14 | 20/11 | 6/1 | 7/1 | ||
| Age (years) | 71.2 ± 9.5 | 68.9 ± 10.4 | 71.3 ± 8.8 | 65.9 ± 8.2 | ||
| Body mass index (BMI) | 24.1 ± 3.4 | 22.4 ± 3.7 | 24.0 ± 2.6 | 21.3 ± 3 | ||
| Preoperative chemoradiotherapy (CRT) | 7 (24.1%) | 8 (25.8%) | - | - | ||
| Preoperative diabetes | 6 (20.7%) | 6 (19.4%) | 0 | 0 | ||
| Preoperative complication | 5 (17.2%) | 7 (22.6%) | 2 (28.6%) | 2 (25.0%) | ||
| Operation time (min) | 230.0 ± 84.4 | 218.9 ± 88.7 | 305.9 ± 110.4 | 281.0 ± 121.3 | ||
| Types of surgery | ||||||
| Colon | 13 | 11 | 0 | 0 | ||
| Rectum | 12 | 19 | 6 | 8 | ||
| Others | 4‡ | 0 | 1§ | 0 | ||
| Blood parameters before surgery (D–14) | ||||||
| White blood cells (/µl) | 5,603 ± 1,910 | 5,446 ± 1,559 | 4,570 ± 968 | 4,950 ± 1,331 | ||
| Erythrocytes (104 /µl) | 433 ± 46 | 417 ± 42 | 438.4 ± 51.8 | 413.9 ± 32.2 | ||
| Hemoglobin (g/dl) | 12.3 ± 1.8 | 12.4 ± 2.1 | 13.2 ± 1.8 | 1 ± 1.4 | ||
| Lymphocytes (%) | 27.8 ± 8.5 | 24.4 ± 10.3 | 18.3 ± 4.2 | 27.3 ± 9.2** | ||
| Platelet (104 /µl) | 22.8 ± 9.5 | 26.6 ± 10.2 | 20.0 ± 6.6 | 19.6 ± 9.1 | ||
| Total protein (g/dl) | 7.0 ± 0.6 | 6.8 ± 0.7 | 6.8 ± 0.7 | 6.4 ± 0.9 | ||
| Albumin (g/dl) | 4.0 ± 0.4 | 3.8 ± 0.6 | 4.0 ± 0.4 | 3.5 ± 0.7* | ||
| hCRP (mg/dl) | 0.5 ± 1.5 | 0.5 ± 0.6 | 0.2 ± 0.1 | 0.4 ± 0.3 | ||
| IL-6 (pg/ml) | 6.0 ± 9.1 | 6.6 ± 10.2 | 3.5 ± 1.7 | 11.1 ± 18.8 | ||
| NK cell activity (%) | 40.3 ± 18.1 | 43.8 ± 13.6 | 40.0 ± 14.4 | 42.6 ± 15.4 | ||
†Groups were compared using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. *p<0.05, **p<0.01. ‡Anal canal cancer (n=1), postoperative local recurrence of rectum cancer (n=1), rectovaginal fistula (n=1), colostomy (n=1). §Anal canal cancer.
Among the patients with rectal and anal canal cancer, 8 in the probiotic group and 7 in the control group received preoperative chemoradiotherapy (CRT). The CRT was performed by oral administration of 5-FU/l-LV for 7 to 9 weeks accompanied by irradiation treatment (3 Gy per day, up to a total of 45 Gy). Surgery was performed 6 to 8 weeks after the CRT. The study groups did not differ significantly in preoperative values of blood parameters with the exception of lymphocytes and albumin. Among the CRT patients, a significantly higher level of lymphocytes (p<0.01) and lower level of albumin (p<0.05) were observed in the probiotic group compared with the control group (Table 1).
The rates of postoperative infections did not differ significantly between the probiotic and control groups (p>0.1); 16.1% and 24.1% for superficial surgical site infections (SSI), 6.5% and 10.3% for deep SSI, and 9.7% and 17.2% for anastomotic leakage, respectively, for the probiotic and control groups. Insignificant differences (p>0.1) were also observed in the frequency of additional postoperative antibiotic treatment and duration of hospital stay between the two groups (Table 2).
Table 2. Clinical observations for all patients and those patients who received preoperative chemoradiotherapy (CRT).
| All patients†
|
CRT patients†
|
|||
|---|---|---|---|---|
| Control (n=29) | Probiotic (n=31) | Control (n=7) | Probiotic (n=8) | |
| Hospital stay (day) | 23.0 ± 13.8 | 21.4 ± 10.1 | 39.6 ± 15.6 | 21.6 ± 11.7 * |
| Surficial infection | 7 (24.1%) | 5 (16.1%) | 4 (57.1%) | 3 (37.5%) |
| Deep infection | 3 (10.3%) | 2 (6.5%) | 3 (42.9%) | 2 (25.0%) |
| Anastomotic leak | 5 (17.2%) | 3 (9.7%) | 4 (57.1%) | 0 |
| Additional antibiotic treatment | 7 (24.1%) | 8 (25.8%) | 5 (71.4%) | 3 (37.5%) |
†Groups were compared using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. *p<0.05.
The occurrences of postoperative infections in patients who received preoperative CRT treatment were higher than those without CRT treatment (Table 2). Four patients in the control group but none in the probiotic group experienced postoperative anastomotic leakage (p=0.10, probiotic vs control). In addition, the duration of hospital stay was significantly shortened in the probiotic group (39.6 ± 15.6 days, p=0.03) compared with the control group (21.6 ± 11.7 days).
Changes in blood parameters
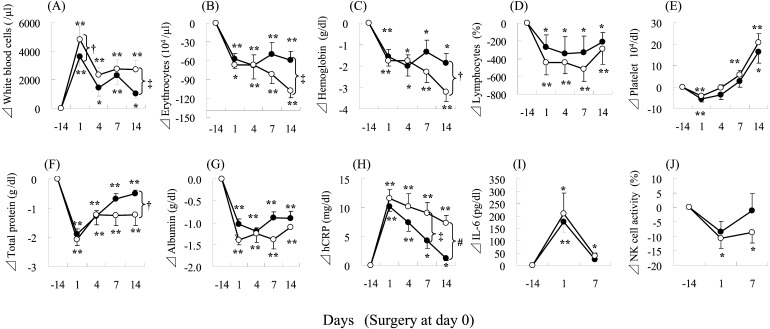
The blood parameters changed significantly after surgery in both the control and the probiotic groups (Fig. 2). There were significant increases in white blood cells and high-sensitive C-reactive protein (hCRP) and significant decreases in erythrocytes, hemoglobin, lymphocytes, total protein, albumin, and NK cell activity after surgery (D1) in the control and probiotic groups (Fig. 2). Significant between-group differences were found in the changes from preoperative levels (D–14) of erythrocytes (D14), total protein (D1), and albumin (D1 and D7). Tendencies for differences (p<0.1) were also found in the changes from baseline values (D–14) of hemoglobin (D14), lymphocytes (D1), and hCRP (D14) between the two groups (Fig. 2).
Fig. 2.
Changes in blood parameters during the trial period for patients who underwent colorectal surgery.
Changes in values, compared with D–14, are shown as means ± SE. ○, control group; ●, probiotic group. *p<0.05, **p<0.01, paired t-test with Bonferroni correction for within-group differences from baseline (D–14). †p<0.1, ‡p<0.05; Student’s t-test for between-group differences at each time point. (A) white blood cell; (B) erythrocytes; (C) hemoglobin; (D) lymphocytes; (E) platelets; (F) total protein; (G)albumin; (H) high sensitive C-reactive protein; (I) interleukin-6; (J) natural killer cell activity.
Among those patients who received CRT treatment, similar within-group changes and between-group differences as found in the whole groups were observed (Fig. 3). In particular, the levels of hCRP remained high at two weeks after surgery in the control group, but declined to near preoperative levels in the probiotic group (Fig. 3). In addition, 7 days after surgery, NK cell activity recovered to near preoperative levels in the probiotic group, but remained low in the control group (Fig. 3). Significant between-group differences were found in the changes from preoperative levels (D–14) of white blood cells (D14), erythrocytes (D14), and hCRP (D7 and D14). Tendencies of differences (p<0.1) were also found in the changes from baseline values (D–14) of white blood cells (D1), hemoglobin (D14), and total protein (D14) between the two groups (Fig. 3).
Fig. 3.
Changes in blood parameters during the trial period among patients who received preoperative chemoradiotherapy.
Changes in values, compared with D–14, are shown as means ± SE. ○, control group; ●, probiotic group. *p<0.05, **p<0.01, paired t-test with Bonferroni correction for within-group differences from baseline (D–14). †p<0.1, ‡p<0.05, #p<0.01, Student’s t-test for between-group differences at each time point. (A) white blood cell; (B) erythrocytes; (C) hemoglobin; (D) lymphocytes; (E) platelets; (F) total protein; (G) albumin; (H) high-sensitive C-reactive protein; (I) interleukin-6; (J) natural killer cell activity.
Changes in fecal microbiota
High-throughput sequencing analysis of the fecal microbiota at the phylum level indicated significant postoperative increases in the proportion of Bacteroidetes and Proteobacteria, but significant decreases in those of Firmicutes and unclassified bacterial groups in the control group (Table 3). On the other hand, the proportion of Actinobacteria increased and proportion of Firmicutes decreased significantly in the probiotic group. The proportions of these bacterial groups did not differ significantly between the two groups at before or after surgery (Table 3).
Table 3. The proportions of bacterial phyla in the fecal microbiota.
| Category at phylum level | Control group |
BB536 group |
||||||
|---|---|---|---|---|---|---|---|---|
| Before surgery | After surgery | Before surgery | After surgery | |||||
| median (%) | IQR (%) | median (%) | IQR (%) | median (%) | IQR (%) | median (%) | IQR (%) | |
| Actinobacteria | 1.95 | 0.32–4.89 | 0.63 | 0.21–2.60 | 0.70 | 0.24–1.90 | 1.71 | 0.36–3.09* |
| Bacteroidetes | 24.52 | 18.32–32.01 | 32.80 | 27.17–40.60* | 24.72 | 18.88–32.89 | 29.56 | 24.76–32.87 |
| Firmicutes | 66.57 | 57.18–75.96 | 56.82 | 46.77–64.24* | 62.31 | 52.34–72.98 | 56.51 | 48.46–64.15* |
| Fusobacteria | 0.23 | 0.08–1.37 | 0.25 | 0.07–3.76 | 0.18 | 0.10–2.09 | 0.69 | 0.09–2.75 |
| Proteobacteria | 1.74 | 1.50–2.16 | 3.54 | 2.90–5.84* | 2.05 | 1.54–5.06 | 3.43 | 2.27–9.75 |
| Unclassified | 0.50 | 0.37–0.55 | 0.37 | 0.26–0.51* | 0.33 | 0.25–0.51 | 0.32 | 0.21–0.46 |
The data for the microbiota were expressed as the median and interquartile range (IQR) of the proportion of each bacterial category. *p<0.05, intra-group difference.
Correlation between compositions of fecal microbiota with blood parameters
Taking into consideration the whole samples collected preoperatively and postoperatively, analysis revealed the following correlations between the proportions of bacterial groups with blood parameters: 1) Actinobacteria significantly correlated positively with erythrocytes, hemoglobin, albumin, and NK cell activity; 2) Firmicutes significantly correlated positively with lymphocytes, total protein, and albumin, but negatively with white blood cells, hCRP, and IL-6; 3) the proportions of Bacteroidetes significantly correlated negatively with total protein and albumin but positively with hCRP and IL-6; and 4) the proportions of Proteobacteria significantly correlated negatively with erythrocytes, hemoglobin, lymphocytes, total protein, and albumin but positively with white blood cells, hCRP and IL-6 (Table 4). The correlations associated with Actinobacteria were observed in samples collected both preoperatively or postoperatively; however, the correlations associated with Proteobacteria were not observed in samples collected preoperatively (Table 4).
Table 4. Correlations between blood biochemical parameters and the composition of fecal microbiota of all patients.
| Blood parameters | Data analysis | Spearman’s correlation |
||||
|---|---|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Firmicutes | Proteobacteria | Unclassified | ||
| White blood cells | Before+after | –0.062 | 0.127 | –0.246* | 0.205* | –0.114 |
| Before | –0.183 | –0.013 | –0.016 | 0.037 | –0.078 | |
| After | 0.017 | 0.048 | –0.287* | 0.099 | 0.02 | |
| Erythrocytes | Before+after | 0.211* | –0.095 | 0.046 | –0.308** | 0.06 |
| Before | 0.204 | 0.061 | –0.206 | –0.132 | 0.105 | |
| After | 0.312* | 0.082 | –0.102 | –0.165 | –0.158 | |
| Hemoglobin | Before+after | 0.361** | –0.052 | 0.032 | –0.241* | 0.138 |
| Before | 0.301* | 0.054 | –0.11 | –0.045 | 0.153 | |
| After | 0.472** | 0.074 | –0.141 | –0.196 | –0.013 | |
| Lymphocytes | Before+after | 0.131 | –0.152 | 0.317** | –0.322** | 0.189† |
| Before | 0.234† | –0.054 | 0.037 | 0.025 | 0.186 | |
| After | 0.035 | –0.016 | 0.366** | –0.282* | 0.034 | |
| Total protein | Before+after | 0.099 | –0.267** | 0.311** | –0.373** | 0.039 |
| Before | 0.031 | –0.131 | 0.134 | –0.094 | –0.13 | |
| After | 0.228 | –0.144 | 0.122 | –0.252† | –0.074 | |
| Albumin | Before+after | 0.242* | –0.240* | 0.311** | –0.391** | 0.171† |
| Before | 0.383* | 0.083 | –0.056 | –0.158 | 0.034 | |
| After | 0.294* | –0.194 | 0.300* | –0.297* | –0.022 | |
| hCRP | Before+after | –0.163† | 0.222* | –0.263** | 0.398** | –0.277** |
| Before | –0.179 | –0.02 | 0.112 | 0.159 | –0.227† | |
| After | –0.247† | 0.043 | –0.203 | 0.154 | –0.173 | |
| IL-6 | Before+after | –0.231* | 0.199** | –0.204** | 0.327*** | –0.236* |
| Before | –0.321* | 0.005 | –0.136 | 0.185 | –0.13 | |
| After | –0.348* | 0.004 | –0.184 | 0.155 | –0.074 | |
| NK cell activity | Before+after | 0.231* | 0.026 | 0.02 | 0.049 | 0.034 |
| Before | 0.249† | 0.052 | 0.012 | 0.139 | 0.122 | |
| After | 0.271† | 0.002 | 0.047 | 0.047 | 0.157 | |
Analysis was based on data of blood parameters and the composition of fecal microbiota obtained at before and/or after surgery of all patients. Blood parameters employed for before and after operation were those obtained at D–14 and D7, respectively. †p<0.1,*p<0.05, **p<0.01, ***p<0.001, Spearman’s correlation analysis.
DISCUSSION
Postoperative infections remain significant risk factors for patients undergoing surgery for cancers, such as colorectal cancer and biliary cancer, and are often indicators of postoperative health and recovery. Zhang, Liu and colleagues [22,23,24] evaluated the effects of administering perioperative probiotic supplements containing L. plantarum, L. acidophilus, and B. longum to patients undergoing colorectal resection. The probiotic group recovered postoperative peristalsis more rapidly, accompanied by lower incidences of diarrhea and infectious-related complications [22].
In the present study, although the incidence of SSI was higher in the control group than in the probiotic group, the difference was not significant. However, among the patients who received preoperative CRT, the duration of postoperative hospital stay differed significantly between the two groups. CRT, which is usually applied to patients with severe case, often disrupts the colonization of indigenous microbiota and subsequently affects gut immunity [25, 26]. We observed a higher incidence of SSI in the CRT patients compared with those without CRT treatment (Table 1). The patients in the control group also had increased levels of hCRP and IL-6, accompanied by decreased levels of NK cell activity two weeks after surgery (Fig. 3). High postoperative levels of CRP are associated with bacterial translocation and prolonged hospitalization and are often critical indicators of post-operative infections [22]. Our results showed significantly earlier postoperative recovery of serum levels of hCRP and NK cell activity in the probiotic group compared with the control group among CRT patients. This is in agreement with previous reports that demonstrated the potential of B. longum BB536 in suppressing inflammatory reactions and maintaining innate immune responses [13, 27].
Surgery led to significant declines in the levels of erythrocytes, hemoglobin, total protein and albumin, however, an improvement in postoperative recovery of hematological and nutritional parameters was observed in the probiotic group. To our knowledge, this is the first report on the possible effects of perioperative probiotic intervention on postoperative hematological and nutritional and parameters of patients undergoing colectomy for cancer. The exact mechanisms of bifidobacteria in improving hematological and nutritional parameters remain unclear. However, several possibilities have been suggested: (1) increased mineral solubility through the production of short-chain fatty acids; (2) production of a phytase that reduces phytate as an antinutrient that leads to mineral unavailability; (3) reducing inflammation, which negatively impacts mineral uptake and nutritional status; and (4) hydrolysis of glycoside bonds of food in the intestines [28]. In addition, some bifidobacteria, including B. longum BB536, have been reported to have a high potential for producing folate [29, 30]. Folate is crucial for its roles in essential functions of cell metabolism, such as DNA replication, repair, and methylation and synthesis of nucleotides, vitamins, and some amino acids, and has been known to promote the generation of erythrocytes [31, 32]. In addition, B. longum BB536 has also been demonstrated to possess anti-inflammatory effects [27]. We postulate that the improved postoperative nutritional status observed in the present study was attributed to the anti-inflammatory effects and the production of folate and phytase in the intestine by B. longum BB536. However, further study is needed to elucidate the details of these mechanisms.
Several studies have demonstrated the effects of perioperative probiotic supplementation on the gastrointestinal microbiota [5, 6, 8, 22]. We observed significant changes at the phylum level in the fecal microbiota 1 to 2 weeks after surgery (Table 3); Firmicutes increased in both groups, Proteobacteria and Bacteroidetes increased in the control group but not in the probiotic group, and Actinobacteria increased in the probiotic group but not in the control group. In addition, we found some feeble but significant correlations between the proportions of the microbiota and blood parameters. The proportions of Proteobacteria and Bacteroidetes positively correlated with the levels of inflammatory parameters (CRP, IL-6, and white blood cells), while the proportions of Actinobacteria and Firmicutes tended to be negatively correlated with these parameters. All patients received antibiotic prophylaxis (cefmetazole) as a single intravenous drip infusion on the days of surgery and one day after surgery, which may be one of the causes of the microbiota changes. Considering the physiological functions of each bacterial group, Proteobacteria are normally present at low levels in a healthy gut environment but often overgrow in inflammatory states [33, 34], leading to opportunistic infections. Biagi et al. [35] showed positive correlations between opportunistic enterobacteria and some pro-inflammatory markers (IL-6 and IL-8). Contrarily, the genus Bifidobacterium is a bacterial group known to downregulate the pro-inflammatory response of the gut epithelium [36, 37]. These observations suggest a possible association between the balance of the microbiota and systemic inflammatory response of the patients.
On the other hand, the current study showed that the proportion of Actinobacteria positively correlated with the levels of erythrocytes, hemoglobin, albumin, and NK cell activity and that the proportion of Firmicutes positively correlated with the levels of lymphocytes, total protein, and albumin; all these parameter tended to be negatively correlated with the proportions of Proteobacteria and Bacteroidetes. As described above, the anti-inflammatory effects and the metabolites generated in the intestine by bifidobacteria, the main components of Actinobacteria, might have contributed to the improvement of the immune function, hematological, and nutritional parameters of these patients. Several studies have demonstrated the effects of administration of B. longum BB536 in maintaining balance and eliminating harmful bacteria [38,39,40]. Therefore, we think that probiotic ingestion contributed to the increased proportion of Actinobacteria in the microbiota, which might have also influenced the proportions of other components of the microbiota. Based on these findings, we conclude that maintaining the balance of the gut microbiota is important for maintaining host defenses and nutritional status, especially during recovery from major surgery.
This study has several limitations. We were unable to perform a placebo-controlled trial due to ethical issues in this hospital. Although the physicians who diagnosed infections were not directly involved in the randomization, we could not exclude the possibility of bias in the clinical observation of the infectious symptoms. However, administration of probiotics had objective effects on blood parameters and the composition of the microbiota. Another limitation is that the number of subject might have been insufficient to detect possible clinical significance, because of the low incidence of postoperative infection. We expected an incidence of 40% of postoperative infectious complications in the control group, but it was much lower (approximately 31%). Therefore, future large-scale randomized controlled trial (RCT) studies are needed to confirm the health benefits of the ingestion of B. longum BB536 for patients with unbalanced health conditions.
In conclusion, this study shows the potential of the B. longum BB536 intervention in balancing the postoperative intestinal microbiota, attenuating systemic postoperative inflammatory responses, and improving recovery of hematological and nutritional conditions in patients undergoing colorectal surgery. These beneficial effects are likely to reduce the risk of postoperative infectious complications and to promote healthy recovery after colorectal resection. Our findings suggest that the administration of B. longum BB536 may represent an effective strategy to maintain a balance in the microbiota and promote healthy recovery in patients undergoing surgery.
Acknowledgments
The authors thank Dr. Liong MT at School of Industrial Technology, Universiti Sains Malaysia, for her critical check of this manuscript.
References
- 1.Food and Agriculture Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid, 2001. Bacteria. Available at: http://www.fao.org/es/ESN/food/food_probio_en.stm.
- 2.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane GT, Cummings JH. 2002. Probiotics, infection and immunity. Curr Opin Infect Dis 15: 501–506. [DOI] [PubMed] [Google Scholar]
- 4.Rayes N, Seehofer D, Hansen S, Boucsein K, Müller AR, Serke S, Bengmark S, Neuhaus P. 2002. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 74: 123–127. [DOI] [PubMed] [Google Scholar]
- 5.Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. 2005. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 390: 104–113. [DOI] [PubMed] [Google Scholar]
- 6.Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. 2006. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 244: 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, Sato N, Kanbayashi C, Tanaka O. 2007. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 54: 661–663. [PubMed] [Google Scholar]
- 8.Okazaki M, Matsukuma S, Suto R, Miyazaki K, Hidaka M, Matsuo M, Noshima S, Zempo N, Asahara T, Nomoto K. 2013. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: a prospective, randomized control trial. Nutrition 29: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 9.Peitsidou K, Karantanos T, Theodoropoulos GE. 2012. Probiotics, prebiotics, synbiotics: is there enough evidence to support their use in colorectal cancer surgery? Dig Surg 29: 426–438. [DOI] [PubMed] [Google Scholar]
- 10.He D, Wang HY, Feng JY, Zhang MM, Zhou Y, Wu XT. 2013. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: a meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 37: 406–415. [DOI] [PubMed] [Google Scholar]
- 11.Jeppsson B, Mangell P, Thorlacius H. 2011. Use of probiotics as prophylaxis for postoperative infections. Nutrients 3: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao JZ. Bifidobacterium longum BB536. In: Lee KL, Salminen S (eds). 2009. Hanbook of Probiotics and Prebiotics, 2nd ed. John Wiley & Sons, New Jersey, pp. 488–491. [Google Scholar]
- 13.Akatsu H, Iwabuchi N, Xiao JZ, Matsuyama Z, Kurihara R, Okuda K, Yamamoto T, Maruyama M. 2013. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. JPEN J Parenter Enteral Nutr 37: 631–640. [DOI] [PubMed] [Google Scholar]
- 14.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Hospital Infection Control Practices Advisory Committee. 1999. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 20: 250–278, quiz 279–280. [DOI] [PubMed] [Google Scholar]
- 15.Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. 2010. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem 74: 939–945. [DOI] [PubMed] [Google Scholar]
- 16.Odamaki T, Xiao JZ, Iwabuchi N, Sakamoto M, Takahashi N, Kondo S, Miyaji K, Iwatsuki K, Togashi H, Enomoto T, Benno Y. 2007. Influence of Bifidobacterium longum BB536 intake on faecal microbiota in individuals with Japanese cedar pollinosis during the pollen season. J Med Microbiol 56: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 17.Aronesty E. 2013. Comparison of sequencing utility programs. Open Bioinforma J 7: 1–8. [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. 2011. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Current Protocols in Bioinformatics Chapter 10: Unit 10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Yang Z, Wang Y, Zheng Q. 2011. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery —a double-blind study. Aliment Pharmacol Ther 33: 50–63. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. 2012. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 343: 199–205. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, Lan P, Peng JS, Yang Z, Xia Y, Liu WJ, Yang J, Qin HL, Wang JP. 2013. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 97: 117–126. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Teng F, Huang S, Lin Z, Yuan X, Zeng X, Yang F. 2014. Changes of saliva microbiota in nasopharyngeal carcinoma patients under chemoradiation therapy. Arch Oral Biol 59: 176–186. [DOI] [PubMed] [Google Scholar]
- 26.Fraunholz IB, Haberl A, Klauke S, Gute P, Rödel CM. 2014. Long-term effects of chemoradiotherapy for anal cancer in patients with HIV infection: oncological outcomes, immunological status, and the clinical course of the HIV disease. Dis Colon Rectum 57: 423–431. [DOI] [PubMed] [Google Scholar]
- 27.Tomosada Y, Villena J, Murata K, Chiba E, Shimazu T, Aso H, Iwabuchi N, Xiao JZ, Saito T, Kitazawa H. 2013. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 8: e59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parvaneh K, Jamaluddin R, Karimi G, Erfani R. 2014. Effect of probiotics supplementation on bone mineral content and bone mass density. Scientific World Journal 2014: 595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deguchi Y, Morishita T, Mutai M. 1985. Comparative studies on synthesis of water-soluble vitamins among human species of bifidobacteria. Agric Biol Chern 49: 13–19. [Google Scholar]
- 30.Sugahara H, Odamaki T, Hashikura N, Abe F, Xiao JZ. 2015. Differences in folate production by bifidobacteria of different origins. Biosci Microbiota Food Health 34: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer R, Tomar SK. 2009. Folate: a functional food constituent. J Food Sci 74: R114–R122. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc JG, Laiño JE, del Valle MJ, Vannini V, van Sinderen D, Taranto MP, de Valdez GF, de Giori GS, Sesma F. 2011. B-group vitamin production by lactic acid bacteria—current knowledge and potential applications. J Appl Microbiol 111: 1297–1309. [DOI] [PubMed] [Google Scholar]
- 33.Pédron T, Sansonetti P. 2008. Commensals, bacterial pathogens and intestinal inflammation: an intriguing ménage à trois. Cell Host Microbe 3: 344–347. [DOI] [PubMed] [Google Scholar]
- 34.Sansonetti PJ. 2011. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol 4: 8–14. [DOI] [PubMed] [Google Scholar]
- 35.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. 2010. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 5: e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EM. 2013. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4: 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagar S, Morgan ME, Chen S, Vos AP, Garssen J, van Bergenhenegouwen J, Boon L, Georgiou NA, Kraneveld AD, Folkerts G. 2014. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir Res 15: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogata T, Takahashi S, Fukuwatari Y, Ishibashi N, Fujisawa T, Iino H. 1997. Effect of Bifidobacterium longum BB536 administration on the intestinal environment, defecation frequency and fecal characteristics of human volunteers. Bioscience Microflora 16: 53–58. [Google Scholar]
- 39.Yaeshima T, Takahashi S, Matsumoto N, Ishibashi N, Hayasawa H, Iino H. 1997. Effect of yogurt containing Bifidobacterium longum BB536 on the intestinal environment, fecal characteristics and defecation frequency: a comparison with standard yogurt. Bioscience Microflora 16: 73–77. [Google Scholar]
- 40.Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao JZ. 2012. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe 18: 14–18. [DOI] [PubMed] [Google Scholar]