Abstract
Fermented milk supplemented with two probiotic strains (Bifidobacterium lactis Bi-07 and Lactobacillus acidophilus NCFM) and a prebiotic (isomaltooligosaccharide) was orally administered to Wistar rats for 30 days using three dosages. A commercial yogurt was used as a placebo. After treatment, the total protein, hemoglobin, and albumin levels in serum were significantly increased in female rats compared with those in the control group (p<0.05), whereas no significant change occurred in the male rats. A significant decrease in serum glucose levels was observed in male rats administered a low dosage of the tested fermented milk (p<0.05). The serum triglyceride level was significantly decreased in both male and female rats (p<0.05). No significant differences were found between rats groups in body weight, food intake, food utilization rate, red blood cell counts, white blood cell counts, alanine aminotransferase, aspartate aminotransferase, urea nitrogen, creatinine, and total cholesterol. These results suggest that the fermented milk supplemented with synbiotics altered the nutritive status of the host animal and contributed to their health. However, such potent health-promoting effects could be deeply associated with the dose and sex specific. Therefore, different physiological targets and population characteristics should be managed with different combinations of probiotics and prebiotics.
Keywords: fermented milk, probiotic, prebiotic, nutritional status, Wistar rat
INTRODUCTION
The normal intestinal microbiota is important for maintaining host health because it provides energy in the form of short-chain fatty acids [1, 2] and nutrients such as vitamins K and B12 [3] and provides protection against invading organisms by exerting colonization resistance [4, 5]. Changes in the gastrointestinal tract, as well as modifications to the diet and host immune system, inevitably affect the colonic microbiota, thereby allowing bacterial population changes to occur. Therapeutic strategies to counteract these changes have been suggested for aging people. These include dietary supplements containing probiotics or prebiotics, with combinations of the two being called synbiotics [6].
Fuller [7] defined probiotics as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.” The following is a definition published by an Expert Consultation Committee at a meeting convened by the Food and Agriculture Organization/World Health Organization in 2001: “Probiotics are live microorganisms which when administered in adequate amounts confer health benefits on the host.” Currently, the major probiotics that have the ability to colonize the human intestine are Lactobacillus spp. and Bifidobacterium spp [5]. Emerging evidence from recent clinical and animal studies suggests that probiotic lactobacilli, particularly certain selected strains, can modify host innate and acquired immune responses and thus protect against respiratory infections [8,9,10].
Prebiotics can be defined as nondigestible food ingredients that beneficially affect the host by stimulating the growth and/or activity of one or a limited number of desired bacteria in the colon, such as bifidobacteria, which are regarded as beneficial to human health [11]. Some of the effects attributed to prebiotics include modulation of key physiological functions, such as the absorption of calcium, lipid metabolism, and glycemia; modulation of intestinal microbiota composition, which plays a primary role in gastrointestinal physiology; and reduced risk of colon cancer [12]. Synbiotics, combinations of probiotics and prebiotics, might improve the survival of bacteria crossing the upper part of the gastrointestinal tract, thereby enhancing their effects in the large bowel. In addition, their effects might be additive or even synergistic [12]. However, more studies are necessary to obtain additional information needed to understand the combined action of probiotics and prebiotics.
In the present study, fermented milk supplemented with two probiotic strains, Bifidobacterium lactis Bi-07 and Lactobacillus acidophilus NCFM, and a prebiotic isomaltooligosaccharide (IMO), was orally administered to 100 healthy Wistar rats. Blood and biochemical indexes and body parameters were measured, and the influences of sex and dosage were investigated.
MATERIALS AND METHODS
Preparation of fermented milk and placebo yogurt
The tested synbiotic-supplemented fermented milk (SSFM) was prepared using fresh milk, sugar, and a stabilizer, which was fermented with a starter culture containing Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus (Danisco, Copenhagen, Denmark). The SSFM was supplemented with the probiotic strains L. acidophilus NCFM and B. lactis Bi-07 (Danisco) and the prebiotic IMO (Shandong Tianmei Biotechnology Co., Ltd., Heze, Shandong, China).
To prepare the SSFM, the sugar, stabilizer, and IMO were first dissolved in fresh milk (2.8% protein, 3.0% milk fat), and the milk mixture was heated to 41°C, followed by homogenization (60°C, 20 MPa) and sterilization (95°C, 5 min) before cooling to 42°C. The SSFM was inoculated with the starter culture and probiotic bacteria and was fermented to completion at 42°C for approximately 5 h. The final preparation contained B. lactis 8.0×107colony forming units (cfu)/g, S. thermophilus (1.5×108cfu/g), Lactobacillus (6.6×107cfu/g), IMO (0.6%), protein (2.5%), fat (2.7%), and soluble solids (13%).
For the animal tests, the SSFM was modified using the recommended amount (480 g/day) and by adding 20-fold more functional ingredients (probiotic bacteria and IMO) to produce a high dosage treatment. A medium dosage treatment (10-fold) was prepared by diluting the high dosage treatment with a common commercial yogurt, which has been fermented with L. delbrueckii ssp. bulgaricus and S. thermophilus. This commercial yogurt fermented with L. delbrueckii ssp. bulgaricus and S. thermophilus was also used as the placebo.
Test animals
One hundred (50 females and 50 males) four-week-old SPF-grade Wistar rats (weighing 60–82 g) were purchased from the Experimental Animal Center at the Military Medical Science Academy of the People’s Liberation Army (Beijing, China). The rats were housed and fed in single cages under constant conditions (temperature, 20.0–20.9°C; humidity, 40.1–49.9%) with a 12-hr light dark cycle. The rats were allowed free access to sterile water and a general powder feed purchased from the Experimental Animal Center at the Military Medical Science Academy of the People’s Liberation Army (Beijing, China).
After 1 week, all rats were divided into a male group and female group. Each group was randomly divided into five subgroups (n=10/subgroup) based on body weight to ensure there were no significant differences in average body weights between groups. Then the rats were fed deionized water (control), the common commercial yogurt (placebo), or the test sample SSFM at doses of 1-fold (low dosage), 10-fold (medium dosage), or 20-fold (high dosage) based on grouping. The treatments were administered through a gastric tube for 30 days with 1 ml/100 g body weight. Nontreatment food and water were supplied freely during the test. All experiments were in accordance with the guidelines of the ethics committee of Shijiazhuang Junlebao Dairy Co., Ltd. for the care and use of the laboratory animals.
Analysis of nutritional status
General performance, behavior, poisoning symptoms, and death of rats were monitored and recorded daily. Body weight and food intake were recorded, and the food utilization rate was calculated weekly.
The rats were fasted for 16 hr after the final administration, anesthetized with diethyl ether, and euthanized by exsanguination from the carotid artery. Their blood was collected to determine hemoglobin levels, red blood cell counts, white blood cell (WBC) counts, and WBC classes. The serum was analyzed for alanine aminotransferase, aspartate aminotransferase, urea nitrogen, creatinine, glucose, triglyceride, total cholesterol, total protein, and albumin using a HITACHI 7020 automatic biochemical analyzer.
The rats were dissected, and the heart, liver, spleen, stomach, kidneys, testes, and ovaries were collected, observed, and weighed; the organ/body ratios were then calculated.
Statistical analyses
Data were expressed as the mean ± SEM, and all analyses were performed using SPSS 17.0 (SPSS Inc, Chicago. IL, USA). Differences between groups were analyzed by analysis of variance after the homogeneity of variance of the data was confirmed using the F test. Calculating the F-value revealed no significant differences between groups. An F≥0.05 and p≤0.05 comparison of group means and pairwise comparison were performed. Data with heterogeneity of variance or abnormal distributions were analyzed after variable transition. The rank-sum test was used if the variable transition was unsuitable. Differences were considered significant at the 5.0% and 1.0% probability levels.
RESULTS
After 30 days of treatment, no death or poisoning symptoms were observed, and no aberrant behavior or performance was observed. Body weight and nontreatment food intake were normal, and the weekly food utilization rate was not significantly different between the treatment subgroups.
Significant differences were observed in serum total protein, hemoglobin, albumin, serum glucose, and serum triglyceride levels between the groups. The other parameters were not significantly different.
Serum total protein, hemoglobin, and albumin
After 30 days of treatment, no significant difference was observed in protein, hemoglobin, and albumin levels in male rats at any dosage compared with the control and yogurt groups. However, these three parameters were significantly higher in the female rats in the treatment groups (Figs. 1, 2, 3).
Fig. 1.
Effect of SSFM on total protein in the hematology of Wistar rats.
Effect of SSFM on total protein in the hematology of Wistar rats after 30 days of intervention. Results are expressed as the mean ± SE (g/l). Compared with the control group, total protein was significantly higher in female rats in the low dosage group (**p<0.01) and medium dosage group (**p<0.01). However, there was no significant difference in male rats at any dosage compared with the control group.
*Compared with the control group (p<0.05); **Compared with the control group (p<0.01); #Compare with the yogurt group (p<0.05); ##Compared with the yogurt group (p<0.01).
Fig. 2.
Effect of SSFM on hemoglobin in the hematology of Wistar rats.
Effect of SSFM on hemoglobin in the hematology of Wistar rats after 30 days of intervention. Results are expressed as the mean ± SE (g/l). Compared with the control group, hemoglobin was significantly higher in female rats in the low dosage group (**p<0.01) and medium dosage group (**p<0.01). Hemoglobin was also significantly higher in the low dosage group (#p<0.05) compared with the yogurt group. However, there was no significant difference in male rats at any dosage compared with the control group.
*Compared with the control group (p<0.05); **Compared with the control group (p<0.01); #Compare with the yogurt group (p<0.05); ##Compared with the yogurt group (p<0.01).
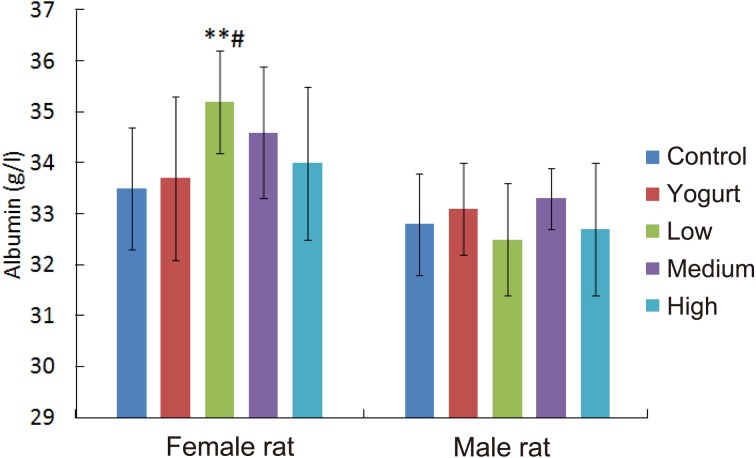
Fig. 3.
Effect of SSFM on albumin in the hematology of Wistar rats.
Effect of SSFM on albumin in the hematology of Wistar rats after 30 days of intervention. Results are expressed as the mean ± SE (g/l). Compared with the control and yogurt group, albumin was significantly higher in female rats in the low dosage group (**p<0.01; #p<0.05). However, there was no significant difference in male rats at any dosage compared with the control group.
*Compared with the control group (p<0.05); **Compared with the control group (p<0.01); #Compare with the yogurt group (p<0.05); ##Compared with the yogurt group (p<0.01).
Compared with the levels of the control group, both the serum total protein and hemoglobin levels were significantly higher in the female rats in the low dosage group (**p<0.01; **p<0.01) and medium dosage group (**p<0.01; **p<0.01). The female rats administered the low dosage treatment had significantly higher hemoglobin (#p<0.05) and albumin (#p<0.05) levels than the female rats in the yogurt group.
Serum glucose
The serum glucose levels of female rats administered the SSFM at different dosages did not significantly differ between the control and yogurt groups. The serum glucose levels of male rats were significantly lower at the low dosages (**p<0.01, ##p<0.01) compared with both the control group and yogurt group (Fig. 4).
Fig. 4.
Effect of SSFM on glucose in the hematology of Wistar rats.
Effect of SSFM on serum glucose in the hematology of Wistar rats after 30 days of intervention. Results are expressed as the mean ± SE (g/l). Compared with the control and yogurt group, glucose was significantly lower in male rats in the low dosage group (**p<0.01; ##p<0.05). However, there was no significant difference in female rats with at any dosage compared with the control group.
*Compared with the control group (p<0.05); **Compared with the control group (p<0.01); #Compare with the yogurt group (p<0.05); ##Compared with the yogurt group (p<0.01).
Serum triglyceride
Significant differences in serum triglyceride levels were observed in both female and male rats. The high dosage group of female rats had significantly lower (*p<0.05) serum triglyceride levels compared with the control group. On the other hand, compared with the levels of the control group, the serum triglyceride levels of male rats in the low dosage group (*p<0.05) and medium dosage group (*p<0.05) were significantly lower (Fig. 5).
Fig. 5.
Effect of SSFM on triglyceride in the hematology of Wistar rats.
Effect of SSFM on triglyceride in the hematology of Wistar rats after 30 days of intervention. Results are expressed as the mean ± SE (mmol/l). A significant difference was observed in both female and male rats. The female rats of the high dosage group had a significantly lower (*p<0.05) triglyceride level compared with the control group. On the other hand, the triglyceride of male rats in low dosage group and medium dosage group were significantly lower compared with the control group (*p<0.05; *p<0.05).
*Compared with the control group (p<0.05); **Compared with the control group (p<0.01); #Compare with the yogurt group (p<0.05); ##Compared with the yogurt group (p<0.01).
DISCUSSION
L. acidophilus NCFM is one of the most frequently studied probiotic strains, and it is widely used in the dairy and nutrition industry [13]. This bacterium adheres well to human cells in vitro, and successful passing of this bacterium into the human intestine has been indicated in several studies [13]. B. lactis Bi-07 is also a common probiotic strain [14, 15]. Although there have been few studies on the health-promoting effects of B. lactis Bi-07, this bacterium was found to effectively bind to intestinal mucus and improve the intestinal environment of infants and the elderly [16]. In a previous study, oral administration of a synbiotic fermented milk prepared with L. acidophilus NCFM, B. lactis Bi-07, and IMO (or SSFM) significantly increased the populations of fecal lactobacilli and bifidobacteria and decreased the populations of enterobacilli in humans as well as in mice, indicating that this synbiotic fermented milk might alter the gastrointestinal microbiota of the host animal. This previous study also found that the synbiotic fermented milk prepared with L. acidophilus NCFM, B. lactis Bi-07, and IMO (or SSFM) could significantly enhance the immunity of the tested mice too [17]. However, it still remains unclear whether an orally administrated synbiotic fermented milk could influence the nutritional status of the host animal and thereby be beneficial for the health of the animal. The currently emerging scientific evidence indicates that the gastrointestinal microbiota might be closely associated with the nutrition status of the host animals [18]. Therefore, we hypothesize that the present synbiotic fermented milk might implicate more physiological responses of the host animal besides its impact on immunity such as that observed in a previous study [17].
Plasma proteins, also termed serum proteins or blood proteins, are proteins present in blood plasma [19]. Serum albumin, which accounts for 55% of total blood proteins, is one of the plasma proteins, whereas globulins and fibrinogens comprise 38% and 7%, respectively. The remaining of the plasma proteins (1%) consist of regulatory proteins, such as enzymes, proenzymes, and hormones. These proteins serve several different functions, including transport of lipids, hormones, vitamins, and metals in the circulatory system and regulation of acellular activity and functioning in the immune system. Other blood proteins act as enzymes, complement components, protease inhibitors, or kinin precursors. Therefore, the serum proteins could be considered one of the most important parameters in monitoring of the nutritional and physiological state of the host animal.
In the present study, oral administration of the fermented milk prepared with two probiotic strains (B. lactis Bi-07 and L. acidophilus NCFM) and a prebiotic IMO (SSFM) significantly increased total protein, hemoglobin, and albumin levels in the female rats. These results indicate that the tested fermented milk (SSFM) could improve the serum protein profile of the tested females, which could contribute to the health of the host animal. There have been numerous studies in which probiotic and prebiotics have been demonstrated to possess an ability to maintain or improve the function of the intestines by promoting the recovery of intestinal cells and function of tight junctions [20, 21]. Therefore, the positive effects of SSFM on the serum protein profile could result from their ability to enhance nutrient absorption in the intestine, at least partly.
An unbalanced intestinal microbiota can modify gut permeability, and metabolic endotoxemia can be caused by bacteria and/or bacterial fragments such as lipopolysaccharides that pass through into the blood [22, 23]. These molecules can stimulate macrophage infiltration and activate the synthesis of inflammatory cytokines [22, 24], and the increased cytokine signaling can inhibit protein synthesis and enhance catabolism [24, 25]. For example, 1–20% of circulating plasma lysine and threonine are derived from the intestinal microbiota [26, 27]. Urea generated by host tissues passes into the gut and is hydrolyzed to ammonia by the intestinal microflora [28]. Our previous study suggested that SSFM increases the beneficial intestinal microbiota [17]. By decreasing the cytokine signaling and increasing the sources of nitrogen and some amino acids for protein synthesis, this modulation may be responsible for the increased serum protein levels in the present study [17].
On the other hand, a significant decrease in serum glucose levels was observed in the male rats exposed to low dosages of the tested fermented milk in the present study. Oral administration of probiotics and/or prebiotics has frequently been found to decrease serum glucose levels. However, a specific animal model such as obese mice or diabetic mice and high-calorie and high-glucose loadings have been used in the majority of studies [29]. In the present study, normal animals without excessive food loading were used to test the potential effects of the tested synbiotic fermented milk to examine the serum sugar level with a relatively longer time of exposure to the tested fermented milk. The results obtained in the present study indicate that the tested fermented milk prepared with the selected strains can modify blood glucose metabolism even under normal conditions. However, it is still unclear why only a low dosage can show such an ability to modify serum glucose metabolism.
Daily administration of viable Lactobacillus rhamnosus GG (LGG) cells was found to decrease the fasting blood glucose level in KK-Ay mice before the development of diabetes and in KK-Ay mice that developed severe diabetes [30]. These results are similar to that reported by [30]. It is suggested that LGG decreases postprandial blood glucose through suppression of glucose absorption by decreasing the glucose available from digestion of sucrose and starch in ICR mice. In other studies, the tested bacteria, L. acidophilus NCFM and B. lactis Bi-07, were demonstrated to have a potential ability to successfully pass into and colonize the human intestine and hence to change the intestinal microbiota [13]. Therefore, suppression of glucose absorption by decreasing the glucose available from digestion caused by colonized L. acidophilus NCFM and B. lactis Bi-07 might be a possible underlying mechanism of the change in serum glucose caused by the tested fermented milk.
Insulin responses are positively correlated with plasma leucine, phenylalanine, and tyrosine concentrations [31, 32]. When fermented dairy products are consumed, the amino acid level increases in vivo. In other words, as the insulin levels increase, the amino acid levels increase, thus promoting transport of blood sugars into tissue or muscle; as a result, blood glucose values decrease as well [31]. In addition, the increase in protein results in enhanced function of skeletal muscles, which can regulate insulin resistance and insulin-sensitizing effects, under the regulation of the Wnt signaling pathway, thereby reducing the blood sugar levels [32]. However, in another study, groups of mice treated with Lactobacillus gained significantly less body weight than control mice; in addition, the serum insulin levels were lower in those treated with Lactobacillusreuteri ATCC PTA 4659, but no significant effects were observed in glucose or insulin tolerance tests [33].
Oral administration of the fermented milk prepared with the two probiotic strains (B. lactis Bi-07 and L. acidophilus NCFM) and the prebiotic (IMO) also significantly decreased the serum triglyceride level in the male rats. Some lactobacilli were reported to decrease the serum triglyceride level in the host animal. For example, decreases in triglyceride levels of 33% were observed in a group administered with milk with L. reuteri CRL1098 (104 cfu/day) in comparison with a group given milk alone [34]. A 30% decrease in triglyceride plasma levels was observed in hamsters fed an enriched diet containing a fermented milk product supplemented with Lactobacillus casei strain Shirota compared with those fed a control diet (unfermented milk); this may have been due to an alteration in the regulation of lipogenic enzyme activities [35]. Triglyceride levels decreased when rats were administered milk and non-fermented milk supplemented with Lactobacillus gasseri SBT0270# (109 cfu/ml) in relation to a control group (water); the authors of the paper attributed the effect of L. gasseri SBT0270 to its ability to suppress the reabsorption of bile acids into the enterohepatic circulation (by deconjugation) and enhance the excretion of acidic steroids in feces [36]. Some prebiotics also possess a property that decreases the triglyceride level of the host animal. After being fed oligofructose in their diet for 30 days (20 g/100 g), a large decrease in the concentration of serum triglycerides was reported in the tested rats compared with the levels measured in isocalorically fed control rats [37]. In addition, the effects of oligofructose on lipid metabolism in obese Zucker rats were examined. After 10 weeks, a 57% decrease in the hepatic concentration of triglycerides, relative to a control group, was reported [38]. It is commonly accepted that the principal mechanism for the hypotriacylglycerolemic effect of oligofructose and inulin is a reduction in hepatic de novo fatty acid and triacylglycerol synthesis through a coordinated reduction in the activity of all lipogenic enzymes [39, 40].
In this study, oral administration of a synbiotic fermented milk prepared with L. acidophilus NCFM, B. lactis Bi-07, and IMO (or SSFM) had different impacts on the tested female and male rats. These results suggest that the influence of the tested synbiotic fermented milk on the nutritional status of the tested animal might be sex dependent or sex specific. These results are good agreement with those of previous studies in which males and females responded differently to treatment with pathologic and probiotic microorganisms, suggesting that a “one-size-fits-all” approach to treatment of gastrointestinal tract inflammation may be inadequate [41]. In this study, males generally displayed increased expression of Th2 and B-cell mediators, and females showed repressed cytokine expression after MAP infection (IL-6, TNF-α, and IL-1 among others). Additionally, females responded positively to use lactobacilli when compared to males. The differences observed suggest that male and female gut tissues and microbiota respond to newly introduced microorganisms differently and that gut-associated microorganisms with host immune system responses and metabolic activity are supported by biology distinct to the host sex [42]. However, further study should be conducted to obtain additional information about the underlying mechanism behind the sex-dependent response of the host animal to treatment with probiotics.
In conclusion, our results suggest that the fermented milk supplemented with synbiotics can alter some nutritive indexes of the host animal and that the health-promoting properties of this fermented milk are sex specific and dependent on the dosage of probiotic and prebiotic. However, significant differences were observed in serum total protein, hemoglobin, albumin, serum glucose, and serum triglyceride levels in female rats when compared with control group but not when compared with the yogurt group. Therefore, the changes observed in this study are not induced by the probiotics and prebiotics alone, and the synbiotic effects of probiotic strains and IMO need further study.
Acknowledgments
Junjie Miao and Chunhui Lang contributed equally to this manuscript. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.Cummings JH, Macfarlane GT. 1991. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 70: 443–459. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JH, Macfarlane GT. 1997. Role of intestinal bacteria in nutrient metabolism. J Parenter Enteral Nutr 21: 357–365. [DOI] [PubMed] [Google Scholar]
- 3.Deguchi Y, Morishita T, Mutai M. 1985. Comparative studies on synthesis of water-soluble vitamins among human species of bifidobacteria. Agric Biol Chem 49: 13–19. [Google Scholar]
- 4.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 69: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolfe RD. 1997. Colonization resistance. In Gastrointestinal Microbiology Vol. 2 ed. Mackie RI and White BA. Chapman and Hall, New York, pp. 501–536. [Google Scholar]
- 6.Woodmansey EJ. 2007. Intestinal bacteria and ageing. J Appl Microbiol 102: 1178–1186. [DOI] [PubMed] [Google Scholar]
- 7.Fuller R. 1989. Probiotics in man and animals. J Appl Bacteriol 66: 365–378. [PubMed] [Google Scholar]
- 8.Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, Yausi H. 2010. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol 50: 597–602. [DOI] [PubMed] [Google Scholar]
- 9.Kawase M, He F, Kubota A, Harata G, Hiramatsu M. 2010. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett Appl Microbiol 51: 6–10. [DOI] [PubMed] [Google Scholar]
- 10.Kawase M, He F, Kubota A, Yoda K, Miyazawa K, Hiramatsu M. 2012. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol Med Microbiol 64: 280–288. [DOI] [PubMed] [Google Scholar]
- 11.Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401–1412. [DOI] [PubMed] [Google Scholar]
- 12.Roberfroid MB. 2000. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 71Suppl: 1682S–1687S, discussion 1688S–1690S. [DOI] [PubMed] [Google Scholar]
- 13.Sanders ME, Klaenhammer TR. 2001. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci 84: 319–331. [DOI] [PubMed] [Google Scholar]
- 14.Quigley EM. 2011. Probiotics in functional bowel disorders getting it right. J Clin Gastroenterol 45: 481–482. [DOI] [PubMed] [Google Scholar]
- 15.Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, Ringel Y. 2011. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacteriumlactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol 45: 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candela M, Bergmann S, Vici M, Vitali B, Turroni S, Eikmanns BJ, Hammerschmidt S, Brigidi P. 2007. Binding of human plasminogen to Bifidobacterium. J Bacteriol 189: 5929–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Zhu H, Lu C, Kang Z, Luo Y, Feng L, Lu X. 2012. Fermented milk supplemented with probiotics and prebiotics can effectively alter the intestinal microbiota and immunity of host animals. J Dairy Sci 95: 4813–4822. [DOI] [PubMed] [Google Scholar]
- 18.Bindels LB, Delzenne NM. 2013. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol 45: 2186–2190. [DOI] [PubMed] [Google Scholar]
- 19.Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. 2002. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics 1: 947–955. [DOI] [PubMed] [Google Scholar]
- 20.Yoda K, Miyazawa K, Hosoda M, Hiramatsu M, Yan F, He F. 2014. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur J Nutr 53: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan F, Polk DB. 2010. Probiotics: progress toward novel therapies for intestinal diseases. Curr Opin Gastroenterol 26: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manco M, Putignani L, Bottazzo GF. 2010. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 31: 817–844. [DOI] [PubMed] [Google Scholar]
- 23.Pendyala S, Walker JM, Holt PR. 2012. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142: 1100–1101.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guigoz Y, Doré J, Schiffrin EJ. 2008. The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care 11: 13–20. [DOI] [PubMed] [Google Scholar]
- 25.Jensen GL. 2006. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr 30: 453–463. [DOI] [PubMed] [Google Scholar]
- 26.Metges CC, El-Khoury AE, Henneman L, Petzke KJ, Grant I, Bedri S, Pereira PP, Ajami AM, Fuller MF, Young VR. 1999. Availability of intestinal microbial lysine for whole body lysine homeostasis in human subjects. Am J Physiol 277: E597–E607. [DOI] [PubMed] [Google Scholar]
- 27.Metges CC, Petzke KJ, El-Khoury AE, Henneman L, Grant I, Bedri S, Regan MM, Fuller MF, Young VR. 1999. Incorporation of urea and ammonia nitrogen into ileal and fecal microbial proteins and plasma free amino acids in normal men and ileostomates. Am J Clin Nutr 70: 1046–1058. [DOI] [PubMed] [Google Scholar]
- 28.Forsythe SJ, Parker DS. 1985. Nitrogen metabolism by the microbial flora of the rabbit caecum. J Appl Bacteriol 58: 363–369. [DOI] [PubMed] [Google Scholar]
- 29.Honda K, Saneyasu T, Hasegawa S, Tominaga Y, Yokota S, Kamisoyama H. 2013. Effect of licorice flavonoid oil on cholesterol metabolism in high fat diet rats. Biosci Biotechnol Biochem 77: 1326–1328. [DOI] [PubMed] [Google Scholar]
- 30.Tabuchi M, Morita H, He F, Hosoda M, Yamada N, Ishida T. 2005. Effect of administration of Lactobacillus rhamnosus GG on postprandial blood glucose levels in rats. Milk Science 54: 17–21. [Google Scholar]
- 31.van Loon LJ, Saris WH, Kruijshoop M, Wagenmakers AJ. 2000. Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr 72: 106–111. [DOI] [PubMed] [Google Scholar]
- 32.Zhou D, Strakovsky RS, Zhang X, Pan YX. 2012. The skeletal muscle Wnt pathway may modulate insulin resistance and muscle development in a diet-induced obese rat model. Obesity (Silver Spring) 20: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 33.Fåk F, Bäckhed F. 2012. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS ONE 7: e46837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. 2000. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J Dairy Sci 83: 401–403. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi-Hayakawa H, Shibahara-Sone H, Osada K, Onodera-Masuoka N, Ishikawa F, Watanuki M. 2000. Lower plasma triglyceride level in Syrian hamsters fed on skim milk fermented with Lactobacillus casei strain Shirota. Biosci Biotechnol Biochem 64: 466–475. [DOI] [PubMed] [Google Scholar]
- 36.Usman HA, Hosono A. 2000. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J Dairy Sci 83: 1705–1711. [DOI] [PubMed] [Google Scholar]
- 37.Delzenne NM, Kok N, Fiordaliso MF, Deboyser D, Goethals FM, Roberfroid MB. 1993. Dietary fructooligosaccharides modify lipid metabolism in rats. Am J Clin Nutr 57Suppl: 820S. [Google Scholar]
- 38.Daubioul CA, Taper HS, De Wispelaere LD, Delzenne NM. 2000. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J Nutr 130: 1314–1319. [DOI] [PubMed] [Google Scholar]
- 39.Fiordaliso M, Kok N, Desager JP, Goethals F, Deboyser D, Roberfroid M, Delzenne N. 1995. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats. Lipids 30: 163–167. [DOI] [PubMed] [Google Scholar]
- 40.Kok N, Roberfroid M, Robert A, Delzenne N. 1996. Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats. Br J Nutr 76: 881–890. [DOI] [PubMed] [Google Scholar]
- 41.Karunasena E, McMahon KW, Chang D, Brashears MM. 2014. Host responses to the pathogen Mycobacterium avium subsp. paratuberculosis and beneficial microbes exhibit host sex specificity. Appl Environ Microbiol 80: 4481–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karunasena E, McMahon KW, Kurkure PC, Brashears MM. 2014. A comparison of cell mediators and serum cytokines transcript expression between male and female mice infected with Mycobacterium avium subspecies paratuberculosis and/or consuming probiotics. Pathog Dis 72: 104–110. [DOI] [PubMed] [Google Scholar]