Abstract
Methylthioadenosine phosphorylase (MTAP), a key enzyme in the adenine and methionine salvage pathways, catalyzes the hydrolysis of methylthioadenosine (MTA), a compound suggested to affect pivotal cellular processes in part through the regulation of protein methylation. MTAP is expressed in a wide range of cell types and tissues, and its deletion is common to cancer cells and in liver injury. The aim of this study was to investigate the proteome and methyl proteome alterations triggered by MTAP deficiency in liver cells to define novel regulatory mechanisms that may explain the pathogenic processes of liver diseases. iTRAQ analysis resulted in the identification of 216 differential proteins (p < 0.05) that suggest deregulation of cellular pathways as those mediated by ERK or NFκB. R-methyl proteome analysis led to the identification of 74 differentially methylated proteins between SK-Hep1 and SK-Hep1+ cells, including 116 new methylation sites. Restoring normal MTA levels in SK-Hep1+ cells parallels the specific methylation of 56 proteins, including KRT8, TGF, and CTF8A, which provides a novel regulatory mechanism of their activity with potential implications in carcinogenesis. Inhibition of RNA-binding proteins methylation is especially relevant upon accumulation of MTA. As an example, methylation of quaking protein in Arg242 and Arg256 in SK-Hep1+ cells may play a pivotal role in the regulation of its activity as indicated by the up-regulation of its target protein p27kip1. The phenotype associated with a MTAP deficiency was further verified in the liver of MTAP± mice. Our data support that MTAP deficiency leads to MTA accumulation and deregulation of central cellular pathways, increasing proliferation and decreasing the susceptibility to chemotherapeutic drugs, which involves differential protein methylation. Data are available via ProteomeXchange with identifier PXD002957 (http://www.ebi.ac.uk/pride/archive/projects/PXD002957).
Liver disorders are the fifth leading cause of death, with steadily increasing incidence in Western countries (1). Most of the predominant risk factors are well known and include hepatitis B and C viral infections, abusive alcohol consumption, autoimmune hepatitis, metabolic dysfunction, and genetic determinants as for hemochromatosis and Alpha 1-antitrypsin deficiency (1, 2). Regardless of the etiology, chronic tissue injury and inflammation are considered drivers of liver disease progression from fatty liver disease and fibrosis to hepatocellular carcinoma (HCC)1 (3). The molecular principles underlying the inflammation–fibrosis–cancer axis in the liver are extensively studied leading to the identification of intermediate molecules (1, 3) whose targeting have demonstrated an efficient interference with the progression and onset of liver injury in animal models (4). However, despite this indubitable progress, there is still an urgent need for new procedures to control the progression of fibrosis and liver ailments in humans, and therefore a systematic analysis of the molecular pathogenesis is required to define new intermediate proteins, allowing earlier diagnostics and more efficient therapies.
Metabolic remodeling is a recognized feature that is common to many liver disorders from steatosis to HCC, where cancerous hepatocytes adapt their metabolism to the inherent proliferative requirements of the transformed phenotype, a condition first described by Warburg (5). The maintenance of the differentiated and quiescent state of hepatocytes is highly dependent of one carbon metabolism, pathway that must be finely tuned to preserve central cellular processes as lipid homeostasis (6), epigenetic regulation (7), cell growth and apoptosis (8), stem cell programming, (9) and axonal myelinization (10). One carbon metabolism integrates a complex network of enzymatic reactions leading to the synthesis of pivotal biomolecules, including, among others, proteins, DNA, polyamines, folates, glutathione, and S-adenosylmethionine (SAMe), the main alkylating agent in living cells (11) and is recognized as the nexus of intermediary metabolism and epigenetic regulation (12). SAMe participates in many reactions as ATP, transferring its methyl moiety to a wide array of acceptors, and tight regulation of its intracellular levels arises as a remarkable issue for hepatocytes as both accumulation and depletion associate with the progression of fatty liver disease and HCC (13). In addition to the enzyme catalyzing SAMe synthesis, methionine adenosyltransferase (MAT) (4), methylthioadenosine phosphorylase (MTAP) might also play a central role in maintaining the cellular methylation balance.
MTAP was first identified in rat prostate (14), and since then, it has been detected and purified from various tissues of many different organisms (15–18). In the human being, MTAP is a ubiquitous enzyme, but the liver exhibits the highest expression level (19). Abrogation of MTAP has been associated with the progression of human diseases, including cancer, as its expression is compromised in many cancer cell lines and tumors (20), leading to activation of ornithine decarboxylase (21). Interestingly, activation of ornithine decarboxylase correlates with the malignancy of hepatocellular carcinoma (22). Intracellular accumulation of 5′-methylthioadenosine (MTA), the MTAP substrate, likely mediates these pathogenic effects.
MTA is a hydrophobic nucleoside that is synthesized as a by-product of polyamine synthesis in all mammalian tissues (23). The aminopropyl group of decarboxylated S-adenosylmethionine is transferred to putrescine and to spermidine in two consecutive reactions to yield two molecules of MTA (24). MTA is then catabolized by the enzyme (MTAP), leading to the formation of adenine and methylthioribose-1-phosphate (25). Adenine is used to replenish adenine nucleotides reservoirs, and methylthioribose-1-phosphate is isomerized to methylthioribulose-1-phosphate that is transformed into 4-methylthio-2-oxobutanoic acid through several complex oxidation reactions. 4-methylthio-2-oxobutanoic acid is finally converted into methionine, completing the methionine salvage pathway that recycles back MTA into the one carbon metabolism to promote SAMe synthesis and therefore contribute to the maintenance of the methylation capacity of the cell. Modification of MTA levels has been associated with regulation of cell proliferation (26), apoptosis (27), and inflammatory reactions (19). However, the underlying mechanisms are still only partially understood and besides other regulatory systems, it may involve the control of protein methylation reactions.
In this study, we have investigated the mechanisms by which a deficiency of MTAP, leading to MTA accumulation, might condition liver cell fate by assessing changes in the cellular proteome and methyl proteome.
EXPERIMENTAL PROCEDURES
Materials
MTAP-deficient mice were from the Mutant Mouse Resource and Research Center (CA). Cell lines HepG2, SK-Hep1 and AML12 were from the American type culture collection. Culture media were from Life Technology (Carlsbad, CA) and the rest of cell culture reagents were from Gibco/Invitrogen (Paisley, UK). MTA, HGF, AdOx, DOXO, and MTT were from Sigma-Aldrich (St. Louis, MO) and additional reagents and chemicals used in this study were from different sources, always with analytical grade quality. All electrophoresis material and reagents were from BioRad (Hercules, CA). Antibodies were from: b-actin, cyclin A, CYP2E1, and HuR, from Abcam (Cambridge, UK); GAPDH, MAP-K 1/2, p-Erk (Ser117/221), p-NFκB (Ser536), and mono-methyl-R were from Cell Signaling (Boston, MA); proliferating cell nuclear antigen was from Santa Cruz Biotechnology (Santa Cruz, CA); and MTAP antibody was kindly provided by Dr. D. A. Carson University of California (San Diego, CA). Secondary antibodies were goat anti-mouse IgG HRP, Santa Cruz Biotechnology, and goat anti-rabbit IgG HRP, Sigma Aldrich.
Isolation of Primary Hepatocytes, Cell Culture, and Treatments
Hepatocytes were isolated from male 3-month-old C57/BL6 WT and MTAP± mice by liberase perfusion as described previously (28). After isolation, hepatocytes were cultured according to García-Trevijano et al. (29). Cell viability was measured by trypan blue exclusion, and no significant differences were observed at any time between controls and any of the various treatments performed in this study.
HepG2 and Sk-Hep1 cells were incubated in DMEM, AML-12 cells in DMEM-F12, and primary hepatocytes in Williams E medium with glutamine (2 mm). In all cases, culture media were supplemented with 10% FBS and antibiotics, 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. In addition, DMEM-F12 was also supplemented with 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 5 ng/ml selenium, and 40 ng/ml dexamethasone. Sk-Hep1+ cells expressing MTAP were obtained in our laboratory in a previous project (30). Cells were always grown at 37 °C in 5% CO2.
Doxorubicin was added when required at the indicated concentrations, and cellular viability was assessed after 24 h by addition of the tetrazolium salt MTT and measuring the absorbance at 570 nm. For estimation of MTAP activity in living primary hepatocytes, culture medium was supplemented with 10% horse serum, which lacks MTAP activity, instead of FBS and 200 μm MTA. Medium was collected at the indicated time intervals and the remaining MTA was measured as described below.
CCl4 Mouse Model of Liver Injury
MTAP± and WT mice were housed in a temperature-controlled room with a 12 h light cycle and given fresh water and food ad libitum. Studies were approved by the University of Navarra Committee on Animal Care and satisfied National Institutes of Health guidelines for human treatment of animals.
Three-month-old male WT and MTAP± mice were challenged by intraperitoneal injection of CCl4 (1 μl/gr) in olive oil (1:1). For animal operations, male MTAP± and WT littermates were anesthetized with isoflurane (IsoFlo®). At the indicated periods of time, serum samples, obtained after centrifugation at 1500 × g from 500 μl of blood collected by retro-orbital puncture were analyzed in a Cobas Itachi C311 analyzer (Roche). Mice were sacrificed for histological examination of the liver upon hematoxylin/eosin staining. Liver and specimens were snap frozen in liquid nitrogen and stored at −80 °C.
Western Blotting
Protein extracts (10–30 μg) were resolved in 12.5% SDS-PAGE gels. Protein electrophoresis and blotting were done as described previously (31). Primary antibodies were used at dilutions as indicated by the manufacturer. After incubation with the appropriate horseradish peroxidase-conjugated secondary antibody (1:5000), the immunoreactivity was visualized by enhanced chemiluminescence (Perkin Elmer). Equal loading of the gels was assessed by Ponceau staining and by β-actin detection with a specific antibody.
MTA, SAMe, and SAH Determinations
MTA, SAMe, and SAH concentrations were determined by HPLC as previously described (32). Samples were homogenized in methanol:1 M acetic acid (80:20 v/v) (MeOH:GAA) and after sonication, extracts were spun down at 24,100 × g for 5 min at 4 °C. Pellets were re-extracted twice with 200 ml of the same buffer. After solvent evaporation, residues were resuspended in 50 μl buffer A (110 mm ammonium formate, 4 mm heptanosulphonic acid, pH 4) and analyzed on an Spherisorb ODS-2 column (2 × 250 mm, 5 μ) equilibrated in the same buffer. LC separation was carried out using a linear acetonitrile gradient (0–40% in 23 min) at a flow rate of 0.2 μl/min. Elution was monitored by recording the absorbance at 254, nm and data were processed with Unicorn 5.0 (Amersham Biosciences).
Methyl Proteome Analysis and Immunoprecipitation of Methyl-R Peptides
Differential analysis of overall protein methylation was performed by two-dimensional electrophoresis Western blot. Cell extracts were obtained in radioimmune precipitation assay buffer (150 mm NaCl; 50 mm Tris, pH 7.4; 0.1% SDS; 1% Triton X-100; 0.5% sodium deoxycholate), containing 1 mm sodium ortovanadate, 10 mm sodium fluoride, 100 mm β-glycerophosphate, and protease inhibitors (Roche). Samples were then sonicated and centrifuged for 30 min at 25,000 g and 4 °C and stored at -80 °C until use. Protein concentration was determined in the supernatants with the Bradford assay kit (Bio-Rad) using bovine serum albumin as standard protein. For 2DE analysis, supernatant aliquots containing 50 μg protein were precipitated with the ReadyPrep 2-D Cleanup kit (BioRad) and resuspended in 7 m urea, 2 m thiourea, 4% CHAPS, 1% DTT, and 0.5% Bio-Lyte 3–10 ampholytes. First-dimensional isoelectrofocusing was performed on a Protean IEF cell (Bio-Rad) using 7 cm, pH 3–10 NL ReadyStrips IPG strips (Bio-Rad). Sample (25 μg protein) was loaded and active rehydration was performed for 12 h at 50 V and 20 °C, and gels were run following the manufacturer recommendations. IPG strips were equilibrated in 50 mm Tris/HCl, pH 7.5; 6 m urea; 30% glycerol; 2% SDS; and 2% DTT and then incubated in the same buffer containing 2.5% iodoacetamide and bromphenol blue in the absence of DTT. IPG strips were directly loaded onto 12.5% polyacrylamide gels (18 cm × 20 cm × 1 mm) and sealed with low-melting-point agarose. Second-dimensional SDS-PAGE gels were run for 4 h at 50 V. Then WB was performed as previously indicated.
For methyl-R peptides immunoprecipitation, cell extracts (10 mg protein) were processed as described by Shevchenko et al. (33), and the resulting peptides were treated according to Guo et al. (34). Briefly, after reduction and alkylation, proteins were trypsin digested (1:100) for 16 h at 37 °C. The resulting peptides were acidified with 0.1% TFA and desalted and purified in a C18 Sep-Pack cartridge (Waters), eluted with acetonitrile, evaporated in a Speedvac, and resuspended in IP buffer (radioimmune precipitation assay buffer with 1 mm EDTA). Upon a preclearing step with G protein Dynabeads (Invitrogen) 150 μg mono-methyl-R specific antibody were added, and the mixture was incubated overnight at 4 °C in an orbital shaker. Dynabeads were then added (8 μg IgG/mg Dynabeads) and incubated for 4 h at 4 °C. After four washing steps with cold PBS, bound peptides were eluted in 100 μl 0.15% TFA, desalted, and concentrated in a C18 ZipTip column (Millipore). For methyl protein enrichment from liver tissue, a similar procedure was followed starting from a liver extract (10 mg protein) in radioimmune precipitation assay buffer containing protease inhibitors as described above, and the resulting enriched mixture was interrogated for QKI-5 by Western blotting.
Sample Preparation and iTRAQ Labeling
Quantitative analysis was performed using iTRAQ 8-PLEX labeling kit (AB SCIEX) according to the protocol previously described (35) with the modifications recommended by Bonzon-Kulichenko (36). The proteome of Sk-Hep1 and SK-Hep1+ cells was concentrated using 10% SDS-PAGE at 50 V. The process was stopped when the bromphenol blue entered 3 mm into the resolving gel. The resulting band containing the whole proteome (Coomassie staining) was excised and subjected to trypsin digestion at 10:1 protein:trypsin (w/w) ratio in 50 mm triethylammonium bicarbonate buffer, pH 8.8. Peptides were then extracted by 1 h incubation in 50% acetonitrile solution with 0.1% formic acid, vacuum dried, iTRAQ labeled according with the AB SCIEX standard protocol (Supplemental Fig. 1A), pooled, dried again, and stored at −20 °C until analysis.
LC-MS/MS Data-Dependent Acquisition
iTRAQ-labeled peptides were reconstituted in 45 μl buffer A (5 mm ammonium bicarbonate, pH 9.8) and resolved on a reversed phase XTerra RP18 3.5 μm, 1.0 × 150 mm column (Waters) equilibrated in the same buffer. Elution was carried out with a 70 min 0–65% linear gradient of acetonitrile in buffer A, at 50 μl/min. Six 150 μl fractions were collected and vacuum dried. Samples from both iTRAQ and methyl-R-IP analysis were resuspended in 6 μl 0.1% TFA and 2% acetonitrile and chromatographed on a nano LC 1D+ Eksigent system using a nano LC precolumn (Eksigent C18-CL 3 μm) connected to a C18-CL column (Eksigent, 3 μm, 75 μm × 25 cm), equilibrated in 0.1% formic acid, 5% acetonitrile. Elution was performed with a 90 min (120 min in the case of methyl-R-IP peptides) 5–35% linear gradient of acetonitrile in the same in 0.1% formic acid at a flow rate of 300 nl/min.
Eluted peptides were analyzed on a nanoESI QTOF 5600 Triple TOF mass spectrometer (AB SCIEX). The instrument was operated in data dependent acquisition mode with m/z scan range a 350–1250 in 25 s. The 25 (iTRAQ) and 50 (methyl-R-IP) most intense parent ions with 2–5 charges were selected for fragmentation (100–1500 m/z, 0.075 s).
MS Data and Statistical Analysis
For iTRAQ, the peaklist-generating and MS data processing were performed with ProteinPilot (Version 4.5_2012, AB SCIEX), while for methylation analyses the files generated with ProteinPilot were converted to mascot generic file for sequence assignments and protein identifications with MASCOT (Version 2.3, Matrix Science). The database used was UniprotKB (Release 2014_03; 79,824,243 entries). Mass tolerance was 10 ppm and 0.2 Da for precursor and fragment ions, respectively. Only assignments below 1% false discovery rate were accepted. One trypsin-missed cleavage was allowed (four in the case of methyl-R peptides) as well as cysteine carbamidomethylation (+57,021) as fixed modification and methionine oxidation (+15.995) and R mono-methylation (+14.015) as variable modifications (for analyses of methyl-R peptides only). For methylated peptides, trypsin missed-cleavage as well as the presence of GAR consensus sequences was assessed and de novo sequencing was performed to verify the expected Δ-mass in the parent and in the corresponding fragment ions. For iTRAQ experiments, quantitative analysis was performed with R/Bioconductor (37). Quantile normalization and filtering of reporter ions was performed to discard proteins with peptides displaying low quantitative correlation values. Significant differential expression between SK-Hep1 and SK-Hep1+ cells (p < 0.05) was estimated with linear models for microarray data (38). Differentially expressed proteins and their corresponding fold-change were based on the median quantification value of all their peptides. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (39) via the PRIDE partner repository with the dataset identifier PXD002957.
RESULTS
MTAP Deficiency Leads to Accumulation of MTA and Deep Proteome Changes in SK-Hep1 Cells
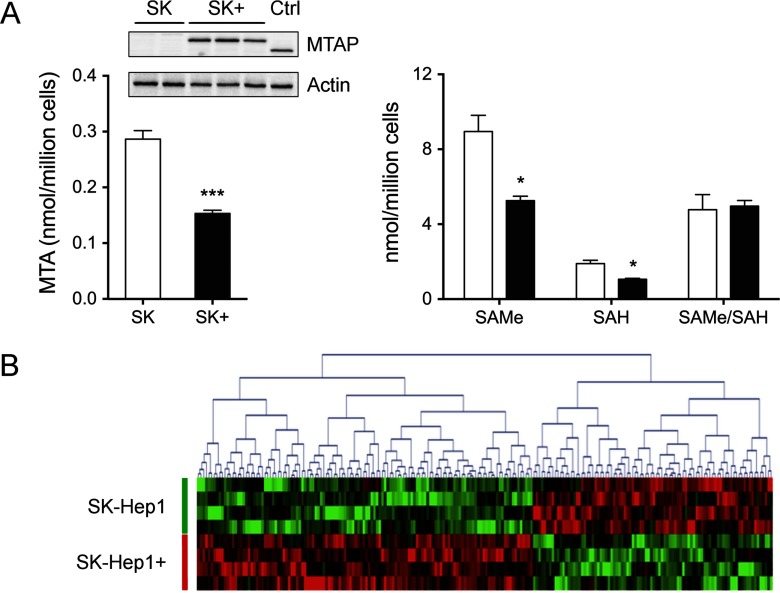
A deficiency of MTAP has been reported in many cancer types. To elucidate if this alteration can be also related with liver cancer as well as the mechanisms involved, we have compared the proteome of SK-Hep1 cells lacking MTAP (40) and SK-Hep1+, a variant of this cell line that was engineered in our lab to express MTAP (30). MTAP expression in SK-Hep1+ cells was similar to basal levels detected in HepG2 liver hepatoma cells and allowed a twofold decrease of intracellular MTA levels (Fig. 1A). SAMe (8.95 ± 0.5 and 5.26 ± 0.5 nmol/106 cells) and SAH (1.89 ± 0.11 and 1.06 ± 0.11 nmol/106 cells) levels were also decreased in SK-Hep1+ cells (Fig. 1A), which might result from the inhibitory effect of MTA that is prevented in SK-Hep1+ cells. Further supporting this hypothesis, the clearance of accumulated SAH after cell exposure to 5 μm AdOx is significantly retarded in cells lacking MTAP (Supplemental Fig. 2). It is, however, remarkable that the SAMe/SAH ratio is the same in both cell lines (4.77 ± 0.5 versus 4.97 ± 0.5) (Fig. 1A). Since this is one of the critical factors involved in the regulation of methylation reactions, no changes should be expected between Sk-Hep1 and SK-Hep1+ cells unless other factors were involved.
Fig. 1.
One-carbon metabolites and proteome analysis in SK-Hep1+ cells. (A) MTAP, MTA, SAMe, and SAH levels. HepG2 extracts were used as positive control. MTA was down-regulated in MTAP expressing cells while SAMe/SAH ratio remained unchanged. (B) Heat map representing the differential proteins resulting from SK-Hep1 and SK-Hep1+ iTRAQ comparison.
To characterize the phenotypic changes of SK-Hep1 cells upon expression of MTAP, a differential proteome analysis was performed through isobaric labeling and LC-MS/MS analysis. Four biological replicates were compared in an 8-PLEX iTRAQ experiment. Overall, 2006 proteins were successfully identified (false discovery rate <1%) based on two or more unique peptides (Supplemental Table I). Using p < 0.05 as a threshold value (LIMMA), 146 differential proteins were assessed (Supplemental Table II); 57 and 89 decreased and increased their steady-state levels in SK-Hep1+ cells, respectively (Fig. 1B). Analysis with ingenuity pathway analysis indicated that differential proteins significantly enrich and therefore are mainly involved in cell growth and proliferation, cell death and survival, cellular assembly and organization, cell cycle, and different metabolic pathways of principal biomolecules, including nucleic acids (Supplemental Fig. 3).
MTA Prevents Apoptosis and Provides Resistance to Chemotherapy in SK-Hep1 Cells
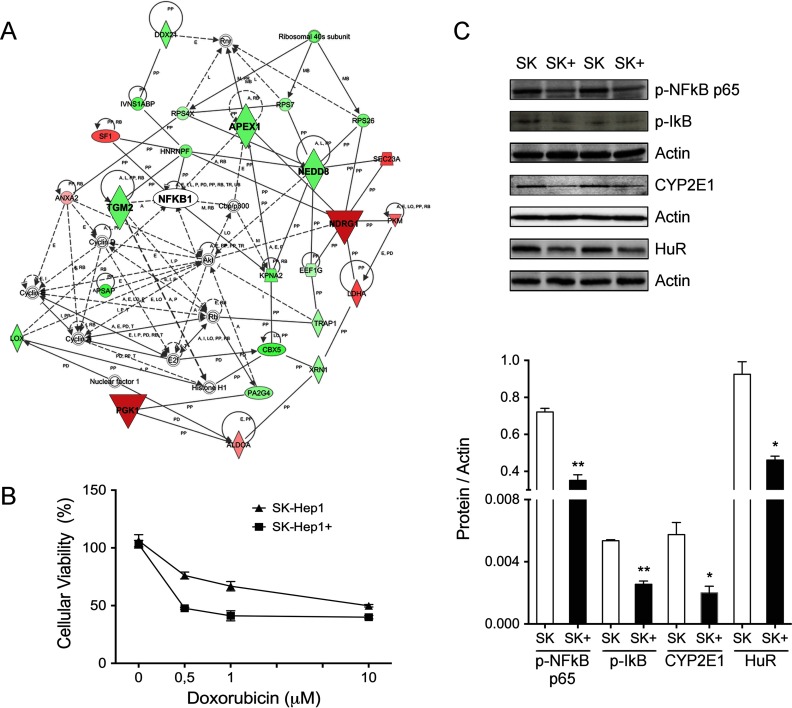
To get further insight on the functional implications of proteins regulated by MTA, network analysis was performed with IPA. Network 1 (score 47, threshold 30) highlights deregulation of ribosomal proteins and glycolytic enzymes like PGK1 that have been associated with many cancer types (41) and angiogenesis (42), respectively (Fig. 2A). Decreased expression of NDRG1 in SK-Hep1 cells might also be associated with their differentiation state (43). APEX1 and TGM2 are increased when MTA accumulates and may modulate apoptosis and provide drug resistance (44). Supporting this idea, SK-Hep1 cells were less sensitive to exposure to the cytostatic agent doxorubicin than the SK-Hep1+ counterparts (Fig. 2B). Interestingly, although no alteration was observed on NFκB levels, this is one of the hubs proposed by IPA since it might be regulated by TGM2 and some of its targets are altered upon MTAP expression. Therefore, we decided to study NFκB activation using specific antibodies. Expression of MTAP and the concomitant down-regulation of MTA reduced p-NFκB p65 levels and induced IκB phosphorylation (Fig. 2C) in SK-Hep1+ cells, suggesting the implication of this pivotal factor in the prevention of apoptosis in response to accumulation of MTA in SK-Hep1 liver cancer cells. We were also interested in NEDD8, a protein from the ubiquitin family that has been associated with HCC and SAMe signaling (45) and that regulates detoxification processes by controlling enzymes including cytochromes P450 via aryl hydrocarbon receptor (46). To study if NEDD8 activity correlated the observed down-regulation in SK-Hep1+ cells, expression of CYP2E1 and HuR levels were measured. We found a threefold and twofold decrease of CYP2E1 and HuR levels, respectively (Fig. 2C), providing functional support to our proteomic observations.
Fig. 2.
MTA prevents apoptosis and provides drug resistance in SK-Hep1 cells. (A) IPA network representing functional connections between up- and down-regulated proteins in SK-Hep1+ cells. Nodes in green and red correspond to down- and up-regulated proteins in SK-Hep1+, respectively. Noncolored nodes are proposed by IPA and suggest potential targets functionally coordinated with the differential proteins. APEX1 and TGM2 levels deregulation suggested a drug resistance condition in SK-Hep1 cells. (B) Cellular sensitivity to doxorubicin exposure. MTAP deletion provides increased resistance to doxorubicin-induced apoptosis. (C) Functional validation of hypothesis based on IPA network. NFκB activation, CYP2E1, and HuR overexpression in liver hepatoma cells lacking MTAP was demonstrated by Western blotting.
MTA Increases the Proliferation Rate of SK-Hep1 Cells
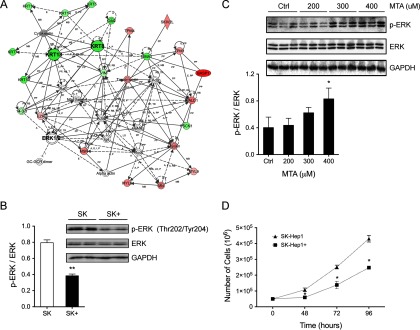
A second IPA network (score 34, threshold 30) connects 24 differential proteins (Fig. 3A) mainly involved in cell proliferation and organization such as KRT8 and 18 that have been associated with chronic liver damage and HCC (47). Similarly to NFκB, a change of ERK 1/2 activity is suggested, although its cellular levels remain constant upon expression of MTAP. Considering the pivotal role of this intermediate protein of the MAP kinases pathway in the regulation of cell survival and proliferation, we studied its phosphorylation-mediated activation. In agreement with previous studies indicating MTA-induced ERK hyperactivation in melanoma cells (48), we found a decrease of ERK Thr202/Thr204 phosphorylation in SK-Hep1+ cells (Fig. 3B). The stimulatory effect of MTA inducing ERK phosphorylation in liver cell types was further assessed in mouse AML12 cells, which is considered a nontransformed hepatocyte cell line (49). Exposure to increasing MTA concentrations resulted in a dose-dependent ERK phosphorylation (Fig. 3C), supporting the notion that expression of MTAP reduces ERK activation in SK-Hep1+ cells that might decrease their proliferative capacity with respect to the SK-Hep1 cells. In agreement with these observations, cell counting revealed a significant reduction of cell growth in SK-Hep1+ cells (Fig. 3D), leading to the conclusion that MTAP deficiency provides a proliferative advantage to liver cancer cells according to a mechanism dependent on activation of the MAPK pathway.
Fig. 3.
MTA increases the proliferation rate according to a mechanism dependent on activation of the MAPK pathway. (A) IPA network MTAP from differentially regulated proteins mainly involved in cell proliferation and organization such as KRT8 and 18 in SK-Hep1 cells. (B) ERK1/2 activation was assessed by Western blotting, showing phosphorylation on Thr202/Tyr204 residues. (C) A dose-dependent MTA activation of ERK by phosphorylation was further confirmed in AML12 cells. (D) Cell proliferation was significantly reduced in SK-Hep1+ cells expressing MTAP as evidenced by cell counting.
Increased MTA Inhibits Protein Methylation in SK-Hep1 Cells, Remarkably RNA-Binding Proteins
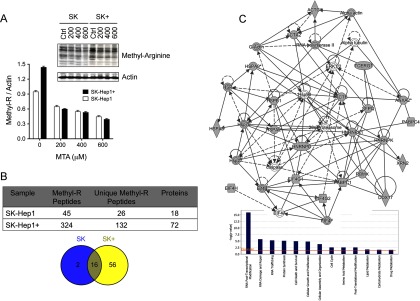
MTA arises as a potential regulator of protein methylation, as has been demonstrated for rapidly accelerated fibrosarcoma in melanoma cells (48). Therefore, we wondered if the phenotypic differences between SK-Hep1 and SK-Hep1+ cells may involve changes in the overall protein methylation pattern in addition to the modifications in protein abundance already described. To investigate the effect of MTA on protein methylation, SK-Hep1 and SK-Hep1+ cells were incubated with increasing MTA concentrations, and overall protein R-methylation was measured. SK-Hep1+ cells showed higher protein methylation levels than SK-Hep1 cells, and in both cell types, MTA treatment reduced protein methylation in a dose-dependent manner (Fig. 4A). The inhibitory effect of MTA was further demonstrated in AML12 cells upon incubation with 200 mm MTA (Supplemental Fig. 4A). To dig deeper into the MTA effects on the methyl-proteome, methyl peptide enrichment coupled to high-resolution LC-MS/MS analysis were performed. We used a mono-methyl-R antibody based on the reproducibility of the preliminary results, assuming the limited coverage of the methyl proteome as dimethylation events would not be, in principle, detected (Supplemental Fig. 4B). Methyl-peptide enrichment was done by incubation of 10 mg protein with 150 mg mono-methyl-R antibody, according to the workflow summarized in the supplemental material (Supplemental Fig. 1B), where chromatograms indicate similar peptide recovery throughout the process. LC-MS/MS analysis allowed the assignment (false discovery rate <1%, Mascot score >25) of 26 and 132 unique methyl-peptides and 116 novel mono-methyl-R sites, corresponding to 18 and 72 proteins in SK-Hep1 and SK-Hep1+ cells, respectively (Supplemental Table III), from which 16 were common to both cell lines, two were specifically detected in Sk-Hep1 cells, and 56 were only found upon expression of MTAP (Fig. 4B), likely resulting from the reduction of MTA and its associated inhibitory capacity of methylation reactions. It is worth mentioning that most of the detected methylation events occur in common methyltransferases target sites such as GlyAlaArg motifs (50) and ProGlyMet sites (51). Moreover, 52.5% of the detected methylation sites were assigned as missed cleavages, as should be expected from the inhibitory effect that R-methylation have on trypsin processing. These observations support our assignments that, however, were further confirmed by de novo sequencing (Supplemental Figs. 5 and 6, available from PRIDE).
Fig. 4.
Cellular methylation profile was significantly reduced upon incubation with MTA. (A) Overall protein R methylation analysis in untreated and MTA-treated SK-Hep1 and Sk-Hep1+ cells that confirm a dose-dependent methylation reduction. (B) Table and Venn diagram representing the number of R-peptides identified in SK-Hep1 and SK-Hep1+ cells. (C) IPA network from differential R-methylated proteins. Nodes in gray correspond to mono-R proteins specifically methylated in SK-Hep1+. The functional analysis of the 56 proteins revealed that they are mainly involved in RNA processing, protein synthesis and several processes regulating cell fate.
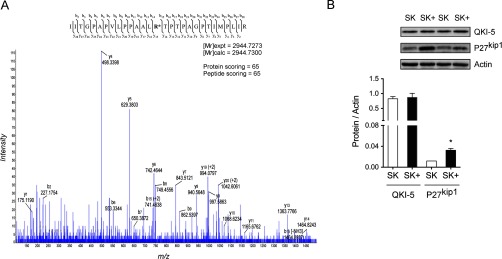
Functional analysis revealed that most of the methylated proteins were involved in the cancer-associated processes. Methylation of laminin B1 and ribosomal protein S10 (RS10) was detected only in SK-Hep1 cells, proteins that have been associated with the regulation of gene expression and protein synthesis (52). Moreover, RS10 is involved in cell proliferation and cancer, according to a mechanism dependent on its PRMT5-mediated methylation (52). Up to 56 proteins were found to be specifically methylated in SK-Hep1+ cells that are mainly involved in RNA processing, protein synthesis, and several associated processes regulating cell fate (Fig. 4C). Nineteen of the 56 methylated proteins in SK-Hep1+ cells were successfully connected on an IPA network (score 47, threshold 30) and can be classified in three major groups: heat shock proteins (HSP), translation initiation factors, and ribonucleoproteins (hnRNP) (Fig. 4C). Moreover, as previous studies in our laboratory demonstrated that QKI-5 mediates the response of liver tumoral cells to a therapeutic oncolytic vector (31), we wonder whether its methylation could be a mechanism to regulate its activity. Two QKI-5 methyl peptides were identified, containing R242, in agreement with previous reports (53), and R242 and 256 (Fig. 5A). To investigate the functional implications of these methylation events, the abundance of p27kip1 was monitored in Sk-Hep1 and SK-Hep1+ cells. It is known that QKI-5 binds to and stabilizes p27kip1 RNA, leading to the accumulation of the corresponding protein, which induces cell cycle arrest (54). We found increased levels of p27kip1 in SK-Hep1+ cells, in parallel to methylation of QKI-5, suggesting that methylation might increase QKI-5 activity as no change on its steady-state levels were detected (Fig. 5B).
Fig. 5.
Profiling arginine methylation of quaking protein. (A) MS/MS spectrum of mono-methylated R242 residue identified from SK-Hep1+ cells using the Me-R4-100 antibody. Y15 ion corresponding to the methylated-R residue is highlighted. (B) The activation of p27kip1, a target of QKI, was assessed by Western blotting suggesting that methylation might increase QKI-5 activity as no change on its steady-state levels were detected.
Partial Deletion of MTAP Increases the Sensitivity to Liver Injury in Mice
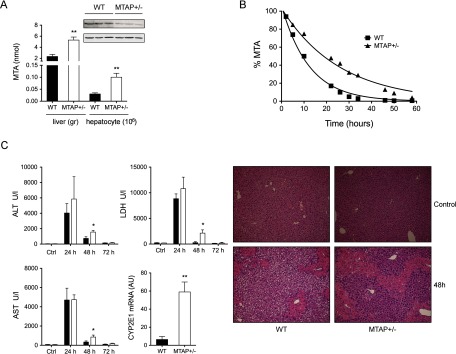
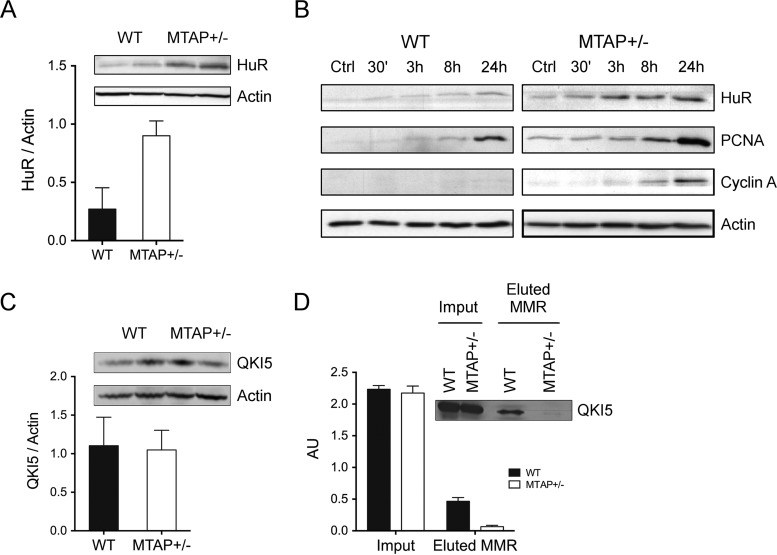
In order to find further support to the functional alterations mediated by MTA metabolism impairment in liver cells, we characterized the effects of a partial MTAP deletion in murine liver. In this study, heterozygous MTAP± mice were used since total deletion of this enzyme leads to early death during embryogenesis (55), suggesting its physiological importance. As expected, MTAP± mice expressed roughly 40% less MTAP as that measured in WT littermates and, concomitantly, a twofold increase of MTA in the liver (Fig. 6A). Moreover, primary hepatocytes isolated from WT and MTAP-deficient livers showed differential MTA metabolic capacity. In agreement with our measurements in the liver, hepatocytes from MTAP± mice accumulated MTA (0.1 ± 0.01 versus 0.03 ± 0.003 nmol/106 hepatocytes in WT cells, not shown), and their consumption of exogenous MTA was twofold slower than in WT hepatocytes (V50MTAP+/- = 22 h versus V50WT = 10 h) (Fig. 6B). Although no apparent phenotype was associated with the partial deletion of MTAP, the recovery capacity upon an acute liver challenge with CCl4 was significantly reduced in MTAP± mice. Acute treatment by intraperitoneal administration of 1 μl/gr CCl4 resulted in a significant aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase increase and liver parenchymal cells necrosis in both groups, but while biochemical and histological parameters returned to control values in WT mice, they remain significantly higher in MTAP deficient mice (Fig. 6C), suggesting that MTAP deficiency and accumulation of MTA might be a factor increasing the risk for chronic liver disease progression. The initial step in CCl4 metabolism in the liver is its reductive dehalogenation by P-450 cytochromes, mainly CYP2E1. This reaction induces high levels of oxidative stress and greatly contributes to CCl4-associated damage, as has been demonstrated by preventing the deleterious effect of CCl4 by inhibition of CYP2E1 (56). We then investigated if the differential response of MTAP± mice could be associated to a CYP2E1 overexpression. CYP2E1 gene expression was ninefold increased in MTAP-deficient livers with respect to WT (Fig. 6C), similarly to what we observed in SK-Hep1 cells, highlighting the regulatory role of MTA on CYP2E1 expression. Finally, we wanted to address if the significant deregulation of RNA-binding proteins found in SK-Hep1 cells was also paralleled in vivo upon accumulation of MTA in MTAP± mice livers. A threefold HuR accumulation was found in MTAP-deficient livers (Fig. 7A) and in isolated MTAP± hepatocytes (Fig. 7B). Moreover, stimulation with HGF, a hepatocyte mitogen whose receptor c-Met is overexpressed by many cancer types (57), leads to a time-dependent HuR accumulation significantly higher in MTAP-deficient hepatocytes than in their WT counterpart. The differential stimulatory effect was paralleled by HuR-targets, including PCNA and cyclin A (Fig. 7B). Regarding QKI-5, no change of steady-state levels was measured when WT and MTAP± livers were compared (Fig. 7C). However, R-methyl-protein-IP combined with WB analysis revealed QKI-5 hypermethylation (Fig. 7D), as was also found in SK-Hep1+ cells containing normal MTA levels. Collectively, this evidence suggests that MTA accumulation prevents QKI-5 methylation impairing its activity, which contributes to hepatocytes dedifferentiation and proliferation.
Fig. 6.
Liver sensitivity induced by CCl4 injury in MTAP deficient mice. (A) Characterization of MTAP and MTA level was performed by Western blotting and HPLC analysis, respectively, and showed that a partial deletion of MTAP+-/(40% decrease) in liver mice lead to a twofold increase of MTA hepatic level. (B) In agreement, consumption of exogenous MTA was twofold slower in MTAP± hepatocytes than in WT counterparts. (C) Exposure to a sublethal dose of CCl4 (1 μl/g) induced a more severe damage in MTAP-deficient livers as evidenced by the more extent necrotic area and the larger increase on serum transaminases. Impaired recovery capacity upon 48 h in WT littermates was also observed. Up-regulation of CYP2E1 RNA level may explain the increased sensitivity to CCl4.
Fig. 7.
HuR and QKI-5 proteins are deregulated in MTAP± mice as in SK-Hep1 cells. (A) Western blotting analysis revealed a threefold increase of HuR in MTAP± liver mice. (B) HuR activation by accumulation of MTA was further confirmed in WT and MTAP-deficient hepatocytes upon HGF stimulation. Furthermore, PCNA and cyclin A, HuR targets, were up-regulated. (C) QKI steady-state levels were similar in WT and MTAP± livers. (D) R-methyl-protein-IP combined with WB analysis revealed QKI-5 hypermethylation, providing support to our findings in SK-Hep1+ cells containing normal MTA levels.
DISCUSSION
MTAP deficiency leading to intracellular accumulation of MTA has been increasingly recognized as one of the factors involved in the pathogenesis of several pathogenic syndromes, including cancer, in particular those afflicting the liver (20, 58). Although the regulatory activity of MTA is viewed as one of the key players regulating the balance between cell death and survival and proliferation in hepatocytes (19), the molecular mechanisms behind it are largely unknown. To investigate this, we studied the changes in protein abundance and methylation associated to fluctuations of MTA concentration in SK-Hep1 hepatoma cells and in MTAP± mouse liver.
Expression of MTAP in SK-Hep1 cells led to normalization of MTA levels with clear implications in the proliferative capacity of these cells as evidenced by overexpression of PGK1 and NDRG1 and down-regulation of PRDX3. PGK1 participates in angiostatin synthesis and release, a potent inhibitor of angiogenesis and tumoral growth (42). NDRG1 is an N-myc down-regulated protein that is considered a tumor suppressor with the capacity to revert metastatic processes (43). In addition to its capacity to regulate cell growth, PRDX3 might be also providing drug resistance mechanisms in cancer cells (59) as its activity scavenges reactive oxygen species mitigating the apoptotic effects of chemotherapy (60). Down-regulation of KRT 8 and 18 was of special interest as they have been associated with the maintenance of the quiescent state of adult hepatocytes (61). Moreover, decreased KRT 8 and 18 promotes the inactivation of ERK1/2 and apoptosis upon activation of the FAS receptor (62). Interestingly, accumulation of MTA in SK-Hep1 cells led to ERK hyperphosphorylation while expression of MTAP and recovery of control MTA concentration induced ERK dephosphorylation, in agreement with previous observations in melanoma cells, suggesting the regulation of the rat sarcoma–rapidly accelerated fibrosarcoma–ERK pathway by MTA (63). The RAF protein has a PRMT5-specific GlyArgGly motif where R563 is dimethylated symmetrically, favoring its degradation (64). Accumulation of intracellular MTA inhibits RAF Arg563 methylation increasing its stability, which might lead to ERK overactivation (48, 63).
CYP2E1 overexpression increases liver susceptibility to hepatotoxic substances, including haloalkanes (65), as a result of the generation of reactive species as by-products of its activity. CYP2E1 regulation by MTA has not been so far described, but since it is down-regulated in rodents fed with a methyl groups reach diet (66), MTA-induced inhibition of PMRTs activity might provide a plausible explanation for CYP2E1 stimulation both in SK-Hep1 cells and in MTAP± liver. Moreover, increased NEDD8 in MTAP-deficient cells might stimulate the AhR pathway (46), leading to the transcriptional activation of genes encoding enzymes such as CYPs (67). NEDD8 binds covalently to Lys residues in proteins according to an ubiquitin-like mechanism known as NEDDylation that has been involved in the regulation of an increasing number of cellular processes (68). Interestingly, HuR is stabilized by NEDDylation on it Lys313 and Lys326 residues in an ubiquitin ligase E3-like reaction catalyzed by the oncogenic protein Mdm2 (69). Therefore, NEDD8 accumulation provides a suggestive mechanism to explain HuR up-regulation as well as the maintenance of the transformed phenotype associated to the increased MTA levels in MTAP deficient liver cells.
MTA provides mechanisms to evade drug-induced apoptosis in tumoral cells. The reduced sensitivity of SK-Hep1 cells to doxorubicin might be partially explained by alterations in APEX and TGM2 expression patterns. Similarly to what we found in SK-Hep1 cells, APEX1 is up-regulated in many cancer cell types and MTA prevents its repression (70) and the concomitant response to drugs (44). Regarding TGM2, its elevation upon MTA accumulation correlates with the restricted doxorubicin-induced apoptotic response, as also reported in other studies (71) and with NFκB activation likely mediated by its kinase activity (72). In contrast with our results, there are studies reporting the proapoptotic and antiproliferative effects induced in hepatoma cells upon exposure to pharmacological concentrations of MTA (27). This apparent discrepancy might be reconciled assuming that the MTA concentration used in other investigations is much higher than the physiological/pathological levels reported in this study. Moreover, the therapeutic effect attributed to MTA appears to be cell type, microenvironment, and injury specific (58). It might be speculated that MTA has a dual role in the regulation of cellular homeostasis, as is the case for other cell cycle regulators, oncogenes, and growth factors (73).
The inhibitory effect of MTA on PRMT enzymes induced changes on the methylation pattern of HSP, hnRNP, and translation initiation factors, proteins that explain some of the mechanisms underlying the proliferative and antiapoptotic phenotype of MTAP-deficient liver cells. HSPs are a highly HSPs are a highly phylogenetically conserved protein family whose activity in mammals is frequently regulated by posttranslational modifications. In particular, Lys methylation modulates substrate and cofactors specificity of HSP70 (74), and variations on its methylation state may participate in cancer progression (75). Methylation of many RNA-binding proteins (RBP), including hnRNP, can be explained by the high frequency of GAR and PGM motifs in their sequences. It has been indeed estimated that asymmetric Arg dimethylation of RBPs represent about 65% of this modification type in the nucleus (76) and that 12% Arg residues of RBP might be methylated (77, 78). The phylogenetic conservation of consensus methylation sites in RBPs suggests a relevant functional role of this modification (77). Methylation might change the RNA binding protein–RNA interaction kinetics, suggesting an interesting mechanism to regulate RNA processing, shuttling, and translation, as it was evidenced upon PRMT1 inhibition (79), with remarkable effects in cell proliferation and apoptosis. For instance, hnRNP-K inhibition increases apoptosis in response to cytotoxic agents (80), while hnRNP-AB promotes proliferation and metastasis of pancreatic cancer cells (81). In addition to RBPs, other proteins whose methylation has been demonstrated in this study for the first time (CTF8A and TFG) might also explain the enhanced survival capacity of liver tumor cells (82, 83) and how it might be regulated by MTA.
QK1–5 mono-methylation in Arg242 and Arg256 residues arises as a regulatory mechanism of its activity that provides evidence to support the implication of MTAP deficiency and MTA accumulation in the progression of liver cancer. QKI-5 is a member of signal transduction activator of RNA metabolism proteins that induces cell cycle arrest by increasing p27kip1 levels (84) and has a relevant participation in cell differentiation in the CNS (85) while its function in the liver is largely unknown. We have recently demonstrated that modulation of QKI-5 levels, as well as its nucleus–cytoplasm shuttling capacity constitutes an essential intermediate mechanism orchestrating the response of liver cells to an oncolytic viral vector (31). Enhanced p27kip1 in SK-Hep1+ cells upon MTAP expression points out to methylation mediated by PRMT4/CARM4 (53) as the regulatory mechanism responsible of QKI-5 activity regulation, considering that no changes at the protein level were observed. Supporting this hypothesis are observations that indicate modulation of other STAR proteins shuttling, including Sam68, by methylation events (86).
Collectively, our data demonstrate that MTAP deficiency and accumulation of MTA induce deregulation of central cellular pathways that control cell fate and contributes to the onset of a proliferative and antiapoptotic phenotype in the liver. Changes in the proteome and methyl-proteome suggest protein intermediates leading to impairment of MAPK and NFκB pathways and a severe alteration of RNA BP activity. In light of our data, it is tempting to suggest that the lack of MTAP is a bad prognostic hallmark in cancer.
Supplementary Material
Acknowledgments
The technical assistance of Carmen Miqueo, Manuela Molina, María I Mora, Leticia Odriozola, and Rocío Martínez de Grado is greatly acknowledged.
Footnotes
Author contributions: E.B. and F.J.C. performed the research and F.J.C. wrote the paper.
* This work was supported by the Carlos III Health Institute of Spain (ISCIII, FIS PI11/02114 and FIS PI14/01538)-Fondos FEDER (EU); grants SAF2014–5478-R from Ministerio de Economía y Competitividad. The Proteomics Unit belongs to ProteoRed, PRB2-ISCIII, supported by grant PT13/0001L.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- DOXO
- doxorubicin
- HCC
- hepatocellular carcinoma
- methyl-R
- methyl-arginine
- methyl-R-IP
- methyl-arginine immunoprecipitation
- MMR
- mono-methyl-arginine
- MTAP
- methylthioadenosine phosphorylase
- MTA
- methylthioadenosine
- PRMT
- protein arginine methyltransferase
- QKI
- quaking protein
- R
- arginine
- SAH
- S-adenosylhomocisteine
- SAMe
- S-adenosylmethionine
- SK
- SK-Hep1 cells
- SK+
- SK-Hep1+ cells.
REFERENCES
- 1. Siegel A. B., and Zhu A. X. (2009) Metabolic syndrome and hepatocellular carcinoma: Two growing epidemics with a potential link. Cancer 115, 5651–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang H., Lafdil F., Kong X., and Gao B. (2011) Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 7, 536–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams R. (2006) Global challenges in liver disease. Hepatology 44, 521–526 [DOI] [PubMed] [Google Scholar]
- 4. Berasain C., Sampedro A., Mauleon I., Goni S., Latasa M. U., Matscheko N., Garcia-Bravo M., Unzu C., Corrales F. J., Enriquez de Salamanca R., Prieto J., Avila M. A., and Fontanellas A. (2009) Epidermal growth factor receptor ligands in murine models for erythropoietic protoporphyria: Potential novel players in the progression of liver injury. Cell. Mol. Biol. 55, 29–37 [PubMed] [Google Scholar]
- 5. Vander Heiden M. G., Cantley L. C., and Thompson C. B. (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Uña M., Varela-Rey M., Cano A., Fernández-Ares L., Beraza N., Aurrekoetxea I., Martínez-Arranz I., Garcia-Rodríguez J. L., Buqué X., Mestre D., Luka Z., Wagner C., Alonso C., Finnell R. H., Lu S. C., Martínez-Chantar M. L., Aspichueta P., and Mato J. M. (2013) Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology 58, 1296–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ulanovskaya O. A., Zuhl A. M., and Cravatt B. F. (2013) NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 9, 300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu S. C., and Mato J. M. (2008) S-Adenosylmethionine in cell growth, apoptosis and liver cancer. J. Gastroenterol. Hepatol. 23, S73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shyh-Chang N., Locasale J. W., Lyssiotis C. A., Zheng Y., Teo R. Y., Ratanasirintrawoot S., Zhang J., Onder T., Unternaehrer J. J., Zhu H., Asara J. M., Daley G. Q., and Cantley L. C. (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varela-Rey M., Iruarrizaga-Lejarreta M., Lozano J. J., Aransay A. M., Fernandez A. F., Lavin J. L., Mósen-Ansorena D., Berdasco M., Turmaine M., Luka Z., Wagner C., Lu S. C., Esteller M., Mirsky R., Jessen K. R., Fraga M. F., Martínez-Chantar M. L., Mato J. M., and Woodhoo A. (2014) S-adenosylmethionine levels regulate the Schwann cell DNA methylome. Neuron 81, 1024–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mato J. M., and Lu S. C. (2007) Role of S-adenosyl-L-methionine in liver health and injury. Hepatology 45, 1306–1312 [DOI] [PubMed] [Google Scholar]
- 12. Locasale J. W. (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mato J. M., Martínez-Chantar M. L., and Lu S. C. (2013) S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 12, 183–189 [PMC free article] [PubMed] [Google Scholar]
- 14. Pegg A. E., and Williams-Ashman H. G. (1969) On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J. Biol. Chem. 244, 682–693 [PubMed] [Google Scholar]
- 15. Toorchen D., and Miller R. L. (1991) Purification and characterization of 5′-deoxy-5′-methylthioadenosine (MTA) phosphorylase from human liver. Biochem. Pharmacol. 41, 2023–2030 [DOI] [PubMed] [Google Scholar]
- 16. Della Ragione F., Oliva A., Gragnaniello V., Russo G. L., Palumbo R., and Zappia V. (1990) Physicochemical and immunological studies on mammalian 5′-deoxy-5′-methylthioadenosine phosphorylase. J. Biol. Chem. 265, 6241–6246 [PubMed] [Google Scholar]
- 17. Shugart L., Tancer M., and Moore J. (1979) Methylthioadenosine nucleoside phosphorylase activity in Drosophila melangoaster. Int. J. Biochem. 10, 901–904 [DOI] [PubMed] [Google Scholar]
- 18. Carteni'-Farina M., Oliva A., Romeo G., Napolitano G., De Rosa M., Gambacorta A., and Zappia V. (1979) 5′-Methylthioadenosine phosphorylase from Caldariella acidophila. Purification and properties. Eur. J. Biochem. 101, 317–324 [DOI] [PubMed] [Google Scholar]
- 19. Avila M. A., García-Trevijano E. R., Lu S. C., Corrales F. J., and Mato J. M. (2004) Methylthioadenosine. Int. J. Biochem. Cell Biol. 36, 2125–2130 [DOI] [PubMed] [Google Scholar]
- 20. Bertino J. R., Waud W. R., Parker W. B., and Lubin M. (2011) Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: Current strategies. Cancer Biol. Ther. 11, 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subhi A. L., Diegelman P., Porter C. W., Tang B., Lu Z. J., Markham G. D., and Kruger W. D. (2003) Methylthioadenosine phosphorylase regulates ornithine decarboxylase by production of downstream metabolites. J. Biol. Chem. 278, 49868–49873 [DOI] [PubMed] [Google Scholar]
- 22. Kubo S., Tamori A., Nishiguchi S., Omura T., Kinoshita H., Hirohashi K., Kuroki T., and Otani S. (1998) Relationship of polyamine metabolism to degree of malignancy of human hepatocellular carcinoma. Oncol. Rep. 5, 1385–1388 [DOI] [PubMed] [Google Scholar]
- 23. Pegg A. E. (1988) Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 48, 759–774 [PubMed] [Google Scholar]
- 24. Williams-Ashman H. G., Seidenfeld J., and Galletti P. (1982) Trends in the biochemical pharmacology of 5′-deoxy-5′-methylthioadenosine. Biochem. Pharmacol. 31, 277–288 [DOI] [PubMed] [Google Scholar]
- 25. Savarese T. M., Crabtree G. W., and Parks R. E. Jr. (1981) 5′-Methylthioadenosine phosphorylase-L. Substrate activity of 5′-deoxyadenosine with the enzyme from sarcoma 180 cells. Biochem. Pharmacol. 30, 189–199 [DOI] [PubMed] [Google Scholar]
- 26. Pascale R. M., Simile M. M., De Miglio M. R., and Feo F. (2002) Chemoprevention of hepatocarcinogenesis: S-adenosyl-L-methionine. Alcohol 27, 193–198 [DOI] [PubMed] [Google Scholar]
- 27. Ansorena E., García-Trevijano E. R., Martínez-Chantar M. L., Huang Z. Z., Chen L., Mato J. M., Iraburu M., Lu S. C., and Avila M. A. (2002) S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology 35, 274–280 [DOI] [PubMed] [Google Scholar]
- 28. Avila M. A., Carretero M. V., Rodriguez E. N., and Mato J. M. (1998) Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology 114, 364–371 [DOI] [PubMed] [Google Scholar]
- 29. García-Trevijano E. R., Latasa M. U., Carretero M. V., Berasain C., Mato J. M., and Avila M. A. (2000) S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: A new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 14, 2511–2518 [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Irigoyen J., Santamaría M., Sánchez-Quiles V., Latasa M. U., Santamaría E., Muñoz J., Sánchez Del Pino M. M., Valero M. L., Prieto J., Avila M. A., and Corrales F. J. (2008) Redox regulation of methylthioadenosine phosphorylase in liver cells: Molecular mechanism and functional implications. Biochem. J. 411, 457–465 [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Quiles V., Mora M. I., Segura V., Greco A., Epstein A. L., Foschini M. G., Dayon L., Sanchez J. C., Prieto J., Corrales F. J., and Santamaria E. (2011) HSV-1 Cgal+ infection promotes quaking RNA binding protein production and induces nuclear-cytoplasmic shuttling of quaking I-5 isoform in human hepatoma cells. Mol. Cell. Proteomics 10, M111.009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirovski G., Stevens A. P., Czech B., Dettmer K., Weiss T. S., Wild P., Hartmann A., Bosserhoff A. K., Oefner P. J., and Hellerbrand C. (2011) Down-regulation of methylthioadenosine phosphorylase (MTAP) induces progression of hepatocellular carcinoma via accumulation of 5′-deoxy-5′-methylthioadenosine (MTA). Am. J. Pathol. 178, 1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shevchenko A., Wilm M., Vorm O., and Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 34. Guo A., Gu H., Zhou J., Mulhern D., Wang Y., Lee K. A., Yang V., Aguiar M., Kornhauser J., Jia X., Ren J., Beausoleil S. A., Silva J. C., Vemulapalli V., Bedford M. T., and Comb M. J. (2014) Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell. Proteomics 13, 372–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glibert P., Van Steendam K., Dhaenens M., and Deforce D. (2014) iTRAQ as a method for optimization: Enhancing peptide recovery after gel fractionation. Proteomics 14, 680–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonzon-Kulichenko E., Perez-Hernandez D., Nunez E., Martinez-Acedo P., Navarro P., Trevisan-Herraz M., Ramos Mdel C., Sierra S., Martinez-Martinez S., Ruiz-Meana M., Miro-Casas E., Garcia-Dorado D., Redondo J. M., Burgos J. S., and Vazquez J. (2011) A robust method for quantitative high-throughput analysis of proteomes by 18O labeling. Mol. Cell. Proteomics 10, M110.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gentleman R., Carey V., Dudoit S., Irizzary R., and Huber W. (2005) Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, New York [Google Scholar]
- 38. Smyth G. K. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 [DOI] [PubMed] [Google Scholar]
- 39. Vizcaíno J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Ríos D., Dianes J. A., Sun Z., Farrah T., Bandeira N., Binz P. A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R. J., Kraus H. J., Albar J. P., Martinez-Bartolomé S., Apweiler R., Omenn G. S., Martens L., Jones A. R., and Hermjakob H. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berasain C., Hevia H., Fernández-Irigoyen J., Larrea E., Caballería J., Mato J. M., Prieto J., Corrales F. J., Garcia-Trevijano E. R., and Avila M. A. (2004) Methylthioadenosine phosphorylase gene expression is impaired in human liver cirrhosis and hepatocarcinoma. Biochim. Biophys. Acta 1690, 276–284 [DOI] [PubMed] [Google Scholar]
- 41. van Riggelen J., Yetil A., and Felsher D. W. (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 10, 301–309 [DOI] [PubMed] [Google Scholar]
- 42. Lay A. J., Jiang X. M., Daly E., Sun L., and Hogg P. J. (2002) Plasmin reduction by phosphoglycerate kinase is a thiol-independent process. J. Biol. Chem. 277, 9062–9068 [DOI] [PubMed] [Google Scholar]
- 43. Melotte V., Qu X., Ongenaert M., van Criekinge W., de Bruïne A. P., Baldwin H. S., and van Engeland M. (2010) The N-myc downstream regulated gene (NDRG) family: Diverse functions, multiple applications. FASEB J. 24, 4153–4166 [DOI] [PubMed] [Google Scholar]
- 44. Chattopadhyay R., Das S., Maiti A. K., Boldogh I., Xie J., Hazra T. K., Kohno K., Mitra S., and Bhakat K. K. (2008) Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol. Cell. Biol. 28, 7066–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernández-Ramos D., and Martínez-Chantar M. L. (2015) NEDDylation in liver cancer: The regulation of the RNA binding protein Hu antigen R. Pancreatology 15, S49–54 [DOI] [PubMed] [Google Scholar]
- 46. Antenos M., Casper R. F., and Brown T. J. (2002) Interaction with Nedd8, a ubiquitin-like protein, enhances the transcriptional activity of the aryl hydrocarbon receptor. J. Biol. Chem. 277, 44028–44034 [DOI] [PubMed] [Google Scholar]
- 47. Strnad P., Paschke S., Jang K. H., and Ku N. O. (2012) Keratins: Markers and modulators of liver disease. Curr. Opin. Gastroenterol. 28, 209–216 [DOI] [PubMed] [Google Scholar]
- 48. Limm K., Ott C., Wallner S., Mueller D. W., Oefner P., Hellerbrand C., and Bosserhoff A. K. (2013) Deregulation of protein methylation in melanoma. Eur. J. Cancer 49, 1305–1313 [DOI] [PubMed] [Google Scholar]
- 49. Wu J. C., Merlino G., and Fausto N. (1994) Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc. Natl. Acad. Sci. U.S.A. 91, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gary J. D., and Clarke S. (1998) RNA and protein interactions modulated by protein arginine methylation. Progress Nucleic Acids Res. Mol. Biol. 61, 65–131 [DOI] [PubMed] [Google Scholar]
- 51. Cheng D., Côté J., Shaaban S., and Bedford M. T. (2007) The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell 25, 71–83 [DOI] [PubMed] [Google Scholar]
- 52. Ren J., Wang Y., Liang Y., Zhang Y., Bao S., and Xu Z. (2010) Methylation of ribosomal protein S10 by protein-arginine methyltransferase 5 regulates ribosome biogenesis. J. Biol. Chem. 285, 12695–12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weimann M., Grossmann A., Woodsmith J., Özkan Z., Birth P., Meierhofer D., Benlasfer N., Valovka T., Timmermann B., Wanker E. E., Sauer S., and Stelzl U. (2013) A Y2H-seq approach defines the human protein methyltransferase interactome. Nat. Methods 10, 339–342 [DOI] [PubMed] [Google Scholar]
- 54. Yang G., Fu H., Zhang J., Lu X., Yu F., Jin L., Bai L., Huang B., Shen L., Feng Y., Yao L., and Lu Z. (2010) RNA-binding protein quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology 138, 231–240.el-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kadariya Y., Yin B., Tang B., Shinton S. A., Quinlivan E. P., Hua X., Klein-Szanto A., Al-Saleem T. I., Bassing C. H., Hardy R. R., and Kruger W. D. (2009) Mice heterozygous for germ-line mutations in methylthioadenosine phosphorylase (MTAP) die prematurely of T-cell lymphoma. Cancer Res. 69, 5961–5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manibusan M. K., Odin M., and Eastmond D. A. (2007) Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 25, 185–209 [DOI] [PubMed] [Google Scholar]
- 57. Ma P. C., Tretiakova M. S., Nallasura V., Jagadeeswaran R., Husain A. N., and Salgia R. (2007) Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: Implications for tumour invasion. Br. J. Cancer 97, 368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Czech B., Dettmer K., Valletta D., Saugspier M., Koch A., Stevens A. P., Thasler W. E., Müller M., Oefner P. J., Bosserhoff A. K., and Hellerbrand C. (2013) Expression and function of methylthioadenosine phosphorylase in chronic liver disease. PLoS ONE 8, e80703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kalinina E. V., Berezov T. T., Shtil' A. A., Chernov N. N., Glazunova V. A., Novichkova M. D., and Nurmuradov N. K. (2012) Expression of peroxiredoxin 1, 2, 3, and 6 genes in cancer cells during drug resistance formation. Bull. Exp. Biol. Med. 153, 878–881 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y. G., Li L., Liu C. H., Hong S., and Zhang M. J. (2014) Peroxiredoxin 3 is resistant to oxidation-induced apoptosis of Hep-3b cells. Clin. Transl. Oncol. 16, 561–566 [DOI] [PubMed] [Google Scholar]
- 61. Kakehashi A., Kato A., Inoue M., Ishii N., Okazaki E., Wei M., Tachibana T., and Wanibuchi H. (2010) Cytokeratin 8/18 as a new marker of mouse liver preneoplastic lesions. Toxicol. Appl. Pharmacol. 242, 47–55 [DOI] [PubMed] [Google Scholar]
- 62. Gilbert S., Loranger A., and Marceau N. (2004) Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol. Cell. Biol. 24, 7072–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Andreu-Pérez P., Esteve-Puig R., de Torre-Minguela C., López-Fauqued M., Bech-Serra J. J., Tenbaum S., Garcia-Trevijano E. R., Canals F., Merlino G., Avila M. A., and Recio J. A. (2011) Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci. Signal. 4, ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wrighton K. H. (2011) Cell signalling: PRMT5 restricts ERK activity. Nat. Rev. Mol. Cell Biol. 12, 689. [DOI] [PubMed] [Google Scholar]
- 65. Weber L. W., Boll M., and Stampfl A. (2003) Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 33, 105–136 [DOI] [PubMed] [Google Scholar]
- 66. Powell C. L., Bradford B. U., Craig C. P., Tsuchiya M., Uehara T., O'Connell T. M., Pogribny I. P., Melnyk S., Koop D. R., Bleyle L., Threadgill D. W., and Rusyn I. (2010) Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicol. Sci. 115, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bock K. W. (1994) Aryl hydrocarbon or dioxin receptor: Biologic and toxic responses. Rev. Physiol. Biochem. Pharmacol. 125, 1–42 [DOI] [PubMed] [Google Scholar]
- 68. Brown J. S., and Jackson S. P. (2015) Ubiquitylation, neddylation and the DNA damage response. Open Biol. 5, 150018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Embade N., Fernández-Ramos D., Varela-Rey M., Beraza N., Sini M., Gutiérrez de Juan V., Woodhoo A., Martínez-López N., Rodriguez-Iruretagoyena B., Bustamante F. J., de la Hoz A. B., Carracedo A., Xirodimas D. P., Rodríguez M. S., Lu S. C., Mato J. M., and Martínez-Chantar M. L. (2012) Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology 55, 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomasi M. L., Iglesias-Ara A., Yang H., Ramani K., Feo F., Pascale M. R., Martínez-Chantar M. L., Mato J. M., and Lu S. C. (2009) S-adenosylmethionine regulates apurinic/apyrimidinic endonuclease 1 stability: Implication in hepatocarcinogenesis. Gastroenterology 136, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herman J. F., Mangala L. S., and Mehta K. (2006) Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 25, 3049–3058 [DOI] [PubMed] [Google Scholar]
- 72. Mann A. P., Verma A., Sethi G., Manavathi B., Wang H., Fok J. Y., Kunnumakkara A. B., Kumar R., Aggarwal B. B., and Mehta K. (2006) Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 66, 8788–8795 [DOI] [PubMed] [Google Scholar]
- 73. Blagosklonny M. V. (1999) A node between proliferation, apoptosis, and growth arrest. Bioessays 21, 704–709 [DOI] [PubMed] [Google Scholar]
- 74. Jakobsson M. E., Moen A., Bousset L., Egge-Jacobsen W., Kernstock S., Melki R., and Falnes P. Ø. (2013) Identification and characterization of a novel human methyltransferase modulating Hsp70 protein function through lysine methylation. J. Biol. Chem. 288, 27752–27763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cho H. S., Shimazu T., Toyokawa G., Daigo Y., Maehara Y., Hayami S., Ito A., Masuda K., Ikawa N., Field H. I., Tsuchiya E., Ohnuma S., Ponder B. A., Yoshida M., Nakamura Y., and Hamamoto R. (2012) Enhanced HSP70 lysine methylation promotes proliferation of cancer cells through activation of Aurora kinase B. Nat. Commun. 3, 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bedford M. T., and Richard S. (2005) Arginine methylation an emerging regulator of protein function. Mol. Cell 18, 263–272 [DOI] [PubMed] [Google Scholar]
- 77. Liu Q., and Dreyfuss G. (1995) In vivo and in vitro arginine methylation of RNA-binding proteins. Mol. Cell. Biol. 15, 2800–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sprung R., Chen Y., Zhang K., Cheng D., Zhang T., Peng J., and Zhao Y. (2008) Identification and validation of eukaryotic aspartate and glutamate methylation in proteins. J. Proteome Res. 7, 1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Herrmann F., Pably P., Eckerich C., Bedford M. T., and Fackelmayer F. O. (2009) Human protein arginine methyltransferases in vivo–distinct properties of eight canonical members of the PRMT family. J. Cell Sci. 122, 667–677 [DOI] [PubMed] [Google Scholar]
- 80. van Domselaar R., Quadir R., van der Made A. M., Broekhuizen R., and Bovenschen N. (2012) All human granzymes target hnRNP K that is essential for tumor cell viability. J. Biol. Chem. 287, 22854–22864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen Z. Y., Cai L., Zhu J., Chen M., Chen J., Li Z. H., Liu X. D., Wang S. G., Bie P., Jiang P., Dong J. H., and Li X. W. (2011) Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis 32, 1419–1426 [DOI] [PubMed] [Google Scholar]
- 82. Sun M., Ma L., Xu L., Li J., Zhang W., Petrovics G., Makarem M., Sesterhenn I., Zhang M., Blanchette-Mackie E. J., Moul J., Srivastava S., and Zou Z. (2002) A human novel gene DERPC on 16q22.1 inhibits prostate tumor cell growth and its expression is decreased in prostate and renal tumors. Mol. Med. 8, 655–663 [PMC free article] [PubMed] [Google Scholar]
- 83. Miranda C., Roccato E., Raho G., Pagliardini S., Pierotti M. A., and Greco A. (2006) The TFG protein, involved in oncogenic rearrangements, interacts with TANK and NEMO, two proteins involved in the NF-kappaB pathway. J. Cell. Physiol. 208, 154–160 [DOI] [PubMed] [Google Scholar]
- 84. Larocque D., Galarneau A., Liu H. N., Scott M., Almazan G., and Richard S. (2005) Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat. Neurosci. 8, 27–33 [DOI] [PubMed] [Google Scholar]
- 85. Chen Y., Tian D., Ku L., Osterhout D. J., and Feng Y. (2007) The selective RNA-binding protein quaking I (QKI) is necessary and sufficient for promoting oligodendroglia differentiation. J. Biol. Chem. 282, 23553–23560 [DOI] [PubMed] [Google Scholar]
- 86. Côté J., Boisvert F. M., Boulanger M. C., Bedford M. T., and Richard S. (2003) Sam68 RNA binding protein is an in vivo substrate for protein arginine N-methyltransferase 1. Mol. Biol. Cell 14, 274–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.