Abstract
Lipopolysaccharide dispersed in the blood by Gram-negative bacteria can be a potent inducer of septic shock. One research focus has been based on antibody sequestration of lipid A (the endotoxic principle of LPS); however, none have been successfully developed into a clinical treatment. Comparison of a panel of anti-lipid A antibodies reveals highly specific antibodies produced through distinct germ line precursors. The structures of antigen-binding fragments for two homologous mAbs specific for lipid A, S55-3 and S55-5, have been determined both in complex with lipid A disaccharide backbone and unliganded. These high resolution structures reveal a conserved positively charged pocket formed within the complementarity determining region H2 loops that binds the terminal phosphates of lipid A. Significantly, this motif occurs in unrelated antibodies where it mediates binding to negatively charged moieties through a range of epitopes, including phosphorylated peptides used in diagnostics and therapeutics. S55-3 and S55-5 have combining sites distinct from anti-lipid A antibodies previously described (as a result of their separate germ line origin), which are nevertheless complementary both in shape and charge to the antigen. S55-3 and S55-5 display similar avidity toward lipid A despite possessing a number of different amino acid residues in their combining sites. Binding of lipid A occurs independent of the acyl chains, although the GlcN-O6 attachment point for the core oligosaccharide is buried in the combining site, which explains their inability to recognize LPS. Despite their lack of therapeutic potential, the observed motif may have significant immunological implications as a tool for engineering recombinant antibodies.
Keywords: lipid A, lipopolysaccharide (LPS), monoclonal antibody, protein structure, X-ray crystallography, recognition pocket
Introduction
Bacterial Gram-positive and Gram-negative infections can lead to septic shock, with estimates as high as one million annual cases in the United States with a mortality rate as high as 50% (1–3). The amphipathic lipopolysaccharide (LPS) responsible for Gram-negative-induced septic shock is normally shed from the bacterial outer membrane but can be released in great amounts upon cell death (4). Lipid A, the endotoxic principle of LPS, is an acylated glucosamine phosphate disaccharide that anchors the LPS molecule to the bacterial outer membrane (5, 6). The presence of intact LPS (or lipid A) in blood can induce a potentially fatal inflammatory cascade in humans (7), initiated by the formation of a signaling complex of the lipid A with Toll-like receptor 4 (TLR4) and co-receptor myeloid differentiation factor 2 (MD-2) (8–11).
Efforts to develop therapeutic antibodies to inhibit the formation of the LPS·TLR4·MD-2 complex by sequestering LPS have proved challenging (12–14). Although antibodies specific for the various LPS components have been reported (15–24), the structural variation in the core and O-polysaccharide regions together with the rapid onset of septic shock have hindered their introduction into clinical use (4, 12, 25–27). To date, only the inner core binding mAb WN1 222-5 has been reported successful in neutralizing a wide range of Gram-negative bacteria, including Escherichia, Salmonella, Shigella, and Citrobacter (15, 28, 29).
There have been considerable efforts to sequester lipid A with a view to treatment of sepsis (17–19, 30, 31). Antibodies believed to be specific for lipid A were first observed during immunization with acid-treated bacterial LPS, where the liberated lipid A fragment can act as a neoantigen when embedded into erythrocytes or liposomes (30, 32). Despite numerous reports of antibodies shown to be specific for lipid A, none have led to successful clinical implementation (17–19, 30).
Recently, the structure of antigen-binding fragments (Fabs)4 from monoclonal antibodies (mAbs) A6 (IgG2b) (33) and S1-15 (IgG2b), also referred to as S1 (30), were determined both in complex with lipid A and in the unliganded form to high resolution (34). The structures provided a structural basis for the observed failure of anti-lipid A antibodies to bind intact LPS, as the free hydroxyl on the β-glucosamine C-6 attachment point for LPS inner core residues (35) was observed to be buried in the antibody-combining site.
Although the search for monoclonal antibodies specific for lipid A did not produce any that would recognize the free LPS, it did produce a number of antibodies of different germ line origin. Generally, carbohydrate antigens produce antibodies from a limited number of germ line genes (a phenomenon called V-region restriction (36–38)). The different germ line origins of the published antibodies specific for lipid A is especially interesting given that none bind intact LPS.
Antibodies S55-3 and S55-5 stem from different germ lines than S1-15 and A6 and show significantly different reactivities toward differentially phosphorylated variants of lipid A. We now report binding data and crystal structures of unliganded and liganded antigen-binding fragments (Fabs) for lipid A-binding mAbs, S55-3 and S55-5, as a step toward the elucidation of the basis for specificity toward lipid A.
Experimental Procedures
Generation of Lipid A Immunogen
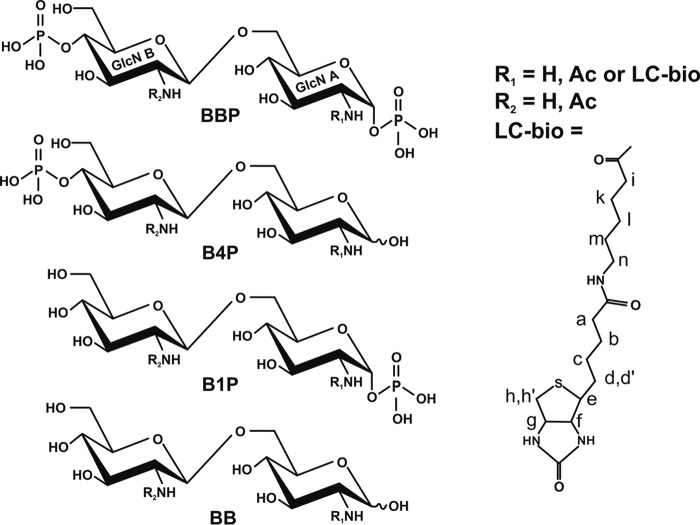
The lipid A backbone β-d-GlcN4P(1→6)α-d-GlcN1P (BBP) of Escherichia coli was prepared as described (40). 10.9 mg (20 μmol) of BBP were transferred into 2 ml of NaHCO3 saturated, 1 ml of CHCl3, and 1 mmol of chloroacetic anhydride (dissolved in 1 m dioxane). After reaction for 30 min at 0 °C, the sample was kept at room temperature for 1 h followed by a second addition of the same amount of chloroacetic anhydride. Saturated NaHCO3 was then added to adjust the pH between 8 and 9. The reaction was continued for 18 h at room temperature under stirring. The aqueous phase was collected, and the organic phase re-extracted twice with water. The water phases were combined, and the sample was dried in a vacuum by rotary evaporation. Gel filtration using Sephadex G-10 (1.5 × 68 cm) gave 11.9 mg of product (BBP-CA) after lyophilization (84% yield). For ammonolysis, BBP-CA (11.9 mg) was then dissolved in 1 ml of (NH4)2CO3 saturated, and a small amount of solid (NH4)2CO3 was additionally added, and the sample was kept at 85 °C for 18 h, then desalted on Sephadex G-10 in 10 mm NH4HCO3, and lyophilized (BBP-bis-glycine, yield 9.8 mg, 15.6 μmol, 78%).
The BBP-bis-glycine was conjugated to bovine serum albumin with divinyl sulfone (DVS) as described previously (39). One hundred fifty μl of DVS-BSA (75 nmol) were mixed with 200 μl of BBP-bis-glycine (containing 3.75 μmol, ratio 1:50) and 100 μl of Na2CO3 saturated in water. After 48 h at room temperature, the reaction was stopped by addition of 50 μl of 1 m glycine and incubation for 2 h at room temperature. The sample was purified by gel filtration using Sephadex G-50 in NH4HCO3 and lyophilized (BBP-bis-Gly-DVS-BSA). After determination of the protein concentration, the sample was dissolved in phosphate-buffered saline to make a 1 mg/ml solution and sterile-filtered. The ligand concentration was determined by measuring the GlcN content photometrically (Morgan-Elson) after hydrolysis in 4 m HCl for 16 h at 100 °C (92 nmol ligand/mg of BSA).
Generation of Monoclonal Antibodies
Monoclonal antibodies S55-3 (IgG2b, κ) and S55-5 (IgG1, κ) were obtained by immunization with BBP-bis-Gly-DVS-BSA of four BALB/c mice as described (41) with minor modifications. Mice received their second immunization on day 33 and booster injections on 3 consecutive days starting on day 74 after the first injection. Hybridomas were obtained after fusion of spleen cells from one mouse and screened by EIA with immobilized acylated lipid A (100 ng/cup) as the solid-phase antigen as described (30). The lipid A was prepared by hydrolysis of E. coli F515 LPS in acetate buffer, pH 4.5, for 90 min at 100 °C. mAbs S55-3 and S55-5 were isolated from ascites by affinity chromatography on BBP conjugated to AH-Sepharose (80 mg of ligand/2.5 ml of packed beads) followed by elution with 0.1 m glycine-HCl, pH 3.2, and addition of NaHCO3 to pH 4. Production and purification of mAb A6 were described previously (33, 34).
Biotinylation of Ligands
For biotinylation of BBP, 2 mg (4 μmol, Mr 500.29) were dissolved in 1 ml of 0.1 m borate buffer, pH 9.0, and 44.6 mg of sulfo-NHS-LC-biotin (Pierce, Mr 556.59, 80 μmol, 20-fold excess) were added and allowed to react for 2 h at room temperature. The sample was desalted on Sephadex G-10 (100 × 1.5 cm) using NH4HCO3 as eluent at a flow rate of 1 ml/min and dried under reduced pressure, which was repeated two times after addition of water (∼1 ml each). The dried sample was redissolved in 0.2 ml of water, and excess biotin was removed by semi-preparative high performance anion-exchange chromatography (HPAEC) under alkaline conditions on CarboPac PA100 (Dionex Corp., Germany) using a Dionex HPLC system. The sample was eluted by a gradient run over 45 min from 0 to 100% sodium acetate in 0.1 m NaOH. After neutralization with HCl, the product BBPbio was desalted by gel filtration as above, and after 3-fold evaporation, it was lyophilized (yield 2.6 mg, Mr 839.74, 3.1 μmol, 77%).
Hydrolysis of 1 mg of BBPbio (1.2 μmol) in 0.5 ml of 0.1 m HCl for 30 min at 100 °C, neutralization with NaOH, and gel filtration on Sephadex G-10 as above gave B4Pbio in a yield of 91% (dried and lyophilized (0.75 mg, 1 μmol, Mr 759.76)). B1P was obtained by hydrolysis of BBP (122 mg, 0.1 m HCl, 100 °C, 60 min), separation by HPAEC, and desalting by gel filtration (yield 15.5 mg from 80 mg of BBP). B1Pbio (Mr 759.76) was then obtained by biotinylating B1P (Mr 420.31, 1 mg, 2.4 μmol) as described for BBP. After preparative HPAEC, gel filtration, and lyophilization, 1.0 mg of B1Pbio was obtained (yield 1.4 μmol, 58%). Alternatively, B1Pbio was obtained by treatment of BBPbio (1 mg, 1.2 mmol) with alkaline phosphatase at 56 °C for 45 min as described (42) in a yield of 92% (0.83 mg, 1.1 μmol) after gel filtration. The biotinylated dephosphorylated backbone BBbio (Mr 679.78) was generated by acid hydrolysis of B1Pbio (0.8 mg, 1 μmol) and gel filtration as above in a yield of 90% (0.6 mg, 0.9 μmol).
NMR Spectroscopy
For NMR spectroscopy, 0.5 mg of the purified and lyophilized biotinylated oligosaccharides were exchanged with D2O three times by evaporation and finally dissolved in 400 μl of D2O (99.98%, Deutero GmbH, Germany). NMR spectra were recorded at 300 K on a Bruker Avance III 700 MHz ultrashielded plus spectrometer equipped with a 5-mm CPQCI 1H-31P/13C/15N/D Z-GRD probehead. One-dimensional 1H, 13C, and 31P NMR spectra, two-dimensional 1H,1H-DQF-COSY (cosydfphpr), 1H,13C HMQC and 1H31P HMQC (hmqcphpr and hsqcgpph) were recorded using the indicated Bruker standard pulse programs. The spectra were referenced to the methyl signals of acetone (1H, 2.225 ppm), (13C, 31.5 ppm), and external phosphoric acid (85% in water, 31P, 0 ppm) and analyzed using Bruker TopSpin version 3.0 software.
Determination of Avidities
Relative avidities of mAbs S55-3, S55-5, and A6 (33) were determined using EIA with biotinylated lipid A analogues BBPbio, B4Pbio, B1Pbio, and BBbio. First, Neutravidin (Pierce, Thermo Fisher) was dissolved in carbonate buffer (5 μg/ml), 50 μl/well transferred to 96-well ELISA plates (MaxiSorp, Nunc), and kept for 24 h at 4 °C. Then ligand in 50 μl of carbonate buffer, pH 9.2, was added filling rows with either 2 or 20 pmol per well, and the plates were incubated for 1 h at 37 °C. Wells were then blocked with casein (2.5% w/v) in phosphate-buffered saline (PBS), pH 7.2, containing 0.05% Tween 20 (PBST-C) for 1 h at 37 °C. The mAbs S55-3 and S55-5 and A6 were titrated in duplicates with 1 + 1 dilution steps over 12 wells and a starting concentration of 0.5 μg/ml. Bound primary mAb was determined after addition of secondary horseradish peroxidase-conjugated goat anti-mouse IgG(H+L) antibody (Dianova) diluted 1:500 in 5% BSA in PBST-C, incubation for 1 h at 37 °C, and addition of 2,2′-azino-di(3-ethylbenzthiazoline)sulfonic acid with hydrogen peroxide, as substrate. Color development was read after 30 min at 37 °C at 405 nm (reference wavelength 490 nm) using a plate reader. Binding curves were generated by non-linear regression fitting of the duplicate data points to the logistic function y = A2 + (A1 − A2)/(1 + (x/x0)p) using Origin software version 7.0 SR4 (OriginLab Corp., Northampton, MA). For data not reaching saturation, A2 was kept constant and assumed to adopt a value of A405 = 4.2.
Germ Line Gene Usage Analysis
Amino acid sequences of Vh and Vl of both clones were determined from cDNA after T/A cloning as described (43). The IMGT/V-quest and junctional analysis web applications (44, 45) were used to analyze the variable genes of S55-3 and S55-5 and to determine the murine germ line gene segments from which the lipid A-specific antibodies were derived.
Fab Preparation and Crystallization
Fab fragments of each antibody were prepared by digestion of the intact immunoglobulin with papain (Sigma). IgG was dialyzed into 25 mm HEPES (Sigma), pH 7.5, diluted to a concentration of 1.0–0.8 mg/ml, 2 mm EDTA (Sigma), and 5–6 mm DTT (Sigma). The digestion reaction was carried out at room temperature using a papain-to-IgG ratio of 1:200 (in mg) for 2–3 h. The reaction was quenched by the addition of 10 mm iodoacetamide (Sigma) and dialyzed overnight into 25 mm HEPES buffer, pH 7.4. Fab fragment was purified by cation-exchange chromatography on a Shodex CM-825 column (Phenomenex, Torrance, CA) using a linear gradient of 0.0 to 0.5 m NaCl in 20 mm HEPES, pH 7.4. Purified Fab was concentrated to 10–12 mg/ml stock and stored at 4.0 °C, except for S55-5 Fab, which was concentrated to 7 mg/ml, since precipitate was observed at higher concentrations.
The Fabs of S55-3 and S55-5 mAbs were mixed with 4 mm lipid A bisphosphate backbone carrying an acetamido group at the C-2 position as follows: βGlcNAc4P(1→6)αGlcNAc1P (Fig. 1) designated BBP-NAc (16) for liganded crystallization screening. Sitting drops were set up in 16 °C room with 96-well plates using Gryphon Xtallization Robot (Art Robbins Instruments, San Jose, CA). Both antibodies formed crystals of variable sizes under a JCSG+ crystal screen (Qiagen, Toronto, Canada) condition 26 (1.0 m LiCl, 0.1 m citric acid, pH 4.0, and 20% (w/v) PEG 6000). Larger plate crystals (0.5 × 0.2 × 0.35 mm3) of S55-5 were grown using a pH of 5.0 instead of the initial 4.0, using the hanging drop method.
FIGURE 1.
Chemical structure of lipid A analogues used for ELISA and crystallization trials. BBP, B4P, B1P, and dephosphorylated lipid A backbone (BB) are shown. GlcN, glucosamine; in the deacylated lipid A backbone R1 and R2 = H; after N-acetylation R1 and R2 = Ac, and after spacered biotinylation R1 = LC-Bio and R2 = H. Shown is the structure designated LC-bio, which is attached to the free amine by reaction with sulfo-NHS-LC-biotin. The labels a–n indicate the location of protons assigned by NMR (Figs. 2 and 3).
Fresh papain digests of intact mAbs S55-3 and S55-5 using the same protocol as above was used for crystallization screening of unliganded Fabs. Conditions were set using the Gryphon Xtallization Robot, and each 96-well sitting drop plate was incubated in a 16 °C room. Unliganded crystals for S55-3 Fab initially were grown under various conditions using the low ionic strength screen (Hampton Research, Aliso Viejo, CA) using lower protein concentration (diluted to 8 mg/ml from 12 mg/ml with 20 mm HEPES, pH 7.4). Optimized crystals (0.5 × 0.5 × 0.6 mm3) were grown in hanging drop setup using 0.05 m glycine, pH 9.5, 4% (w/v) PEG 3350 in the drop with ratios of 2:1:2.5 for protein/buffer/precipitant, respectively, and 24% (w/v) PEG 3350 as the reservoir. Unliganded crystals for S55-5 Fab (7 mg/ml) were obtained under condition 78 (15% (w/v) PEG 6000 and 5% (w/v) glycerol) of the PEG II suite crystal screen.
Data Collection, Molecular Replacement, and Structure Refinement
Liganded S55-3, S55-5, and unliganded S55-3 crystals were removed from the mother liquor and carefully dehydrated by successive trials in a 16 °C room until concentration of cryoprotectant (PEG 3350 and PEG 6000) reached appropriate levels. The crystals obtained for unliganded S55-5 were transferred to drops containing 15% (w/v) PEG 6000 and 10% (w/v) glycerol following a short (30 s) dehydration step prior to being flash-frozen to −160 °C using an Oxford Cryostream 700 crystal cooler (Oxford Cryosystems, Oxford, UK). X-ray diffraction data sets were collected at the Canadian Macromolecular Crystallography Facility on beamline 08ID-1 (CMCF-ID) of the Canadian Light Source (Saskatoon, Saskatchewan, Canada) at 0.979 Å wavelength, with a MarMosaic CCD300 detector, and processed using HKL2000 (HKL Research Inc. Charlottesville, VA).
The unliganded S55-3 structure was solved by molecular replacement using Phaser (46) with the variable fragment (Fv) from mouse mAb 1121B (47) (PDB code 3S35) and constant domains from mAb BV04–01 IgG2b (48) (PDB code 1NBV) as search models. Liganded structure of S55-3 was solved using the unliganded Fab structure as a search model. The liganded S55-5 Fab structure was solved using the constant domains of mAb S25-26 (IgG1) (49) (PDB 4M93) and unliganded Fv structure of S55-3 as the search models, and subsequently the unliganded S55-5 Fab structure was solved using the liganded structure as a search model.
Manual fitting of σA-weighted Fo − Fc and 2Fo − Fc electron density maps was carried out with Coot (50). Restrained refinement and translation, libration, and screw (TLS) refinement was carried out using REFMAC5 (51, 52). All stereo figures and r.m.s.d. calculations presented in this paper were made using SetoRibbon (available upon request from author S. V. E.). Electrostatic surface potential figures were made using Chimera molecular visualization software (53). Marvin version 5.7.0 from ChemAxon was used for drawing chemical structures.
Results
Mouse Immunization and mAb Isolation
BALB/c mice were successfully immunized with BBP-bis-Gly-DVS-BSA as shown by the appearance of serum antibodies against the immunizing antigen. The animal with the highest titer in enzyme-linked immunosorbent assay (ELISA) against the immunizing antigen was used for fusion. Of 2.1 × 108 spleen cells fused and seeded into 750 wells, 409 primary hybridomas were obtained (54%), 19 of which produced specific antibody. Monoclonal antibodies S55-3 (IgG2b) and S55-5 (IgG1) were selected based on their reactivity in ELISA and isotype.
Biotinylation of Lipid A Disaccharides
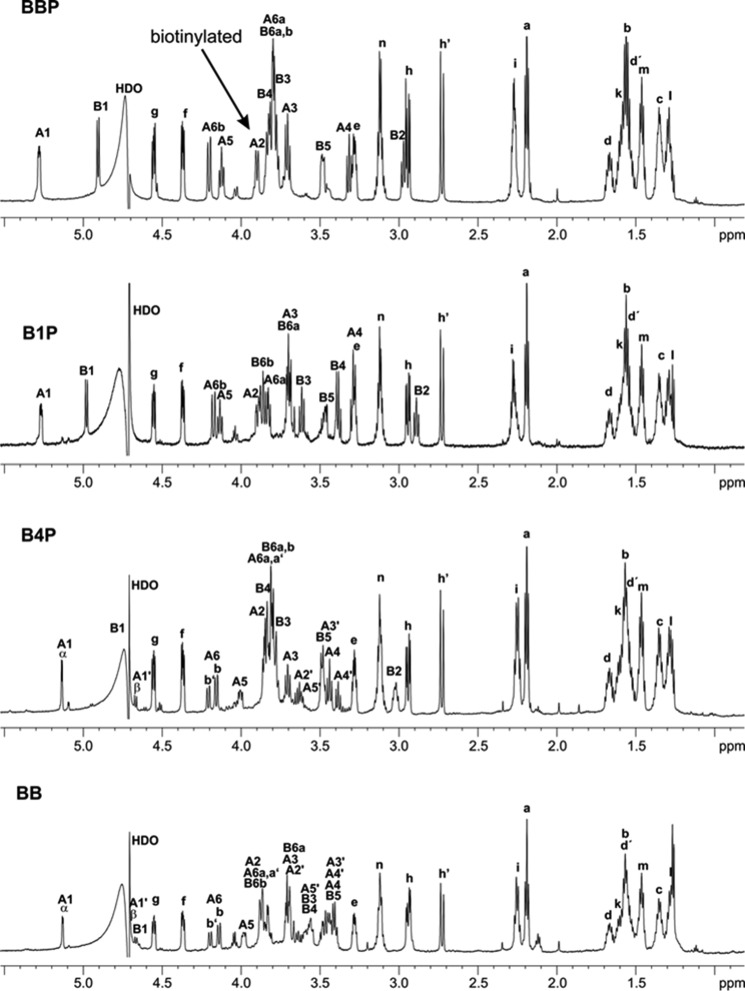
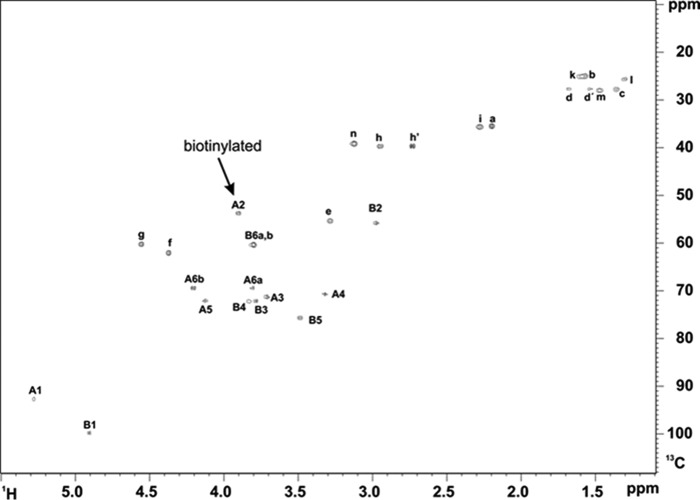
The deacylated bisphosphorylated lipid A backbone BBP was successfully biotinylated at pH 9.0 in borate buffer using a 20-fold molar excess of sulfo-NHS-LC-biotin. From biotinylated BBP other compounds could be obtained either by chemical hydrolysis or enzymatic alkaline phosphatase treatment. The one- and two-dimensional NMR analysis (Figs. 2 and 3) revealed that only a single biotin was incorporated at the 2-position of αGlcN1P, whereas the amine of βGlcN4P remained underivatized. B1Pbio was obtained from BBPbio by acid hydrolysis of the 1-phosphate in moderate yields. Alkaline phosphatase treatment removed only the 4′-phosphate from BBPbio and the 1-phosphate remained. Thus, B4Pbio could be obtained in very high yields (>90%) only by gel filtration without the need of further purification. Complete removal of the 4′-phosphate was proven by 1H,13C HSQC, 1H,31P HMQC, and 31P NMR. Mutarotation of the dephosphorylated GlcN (residue A) resulted in two separate signals of protons A6b (Fig. 2). The integration of these signals showed that 40% were present in β-configuration in B4Pbio and BBbio.
FIGURE 2.
1H NMR spectra and proton assignments of BBP, B4P, B1P, and BB after reaction with sulfo-NHS-LC-biotin and purification by HPAEC. For structures see Fig. 1.
FIGURE 3.
1H,13C NMR spectrum and assignments of BBP after reaction with sulfo-NHS-LC-biotin and purification by HPAEC showing that under the conditions chosen only the proton of A2 (GlcN A) is shifted to higher frequency due to biotinylation. For structure see Fig. 1.
Characterization of Lipid A-specific Antibodies through ELISA
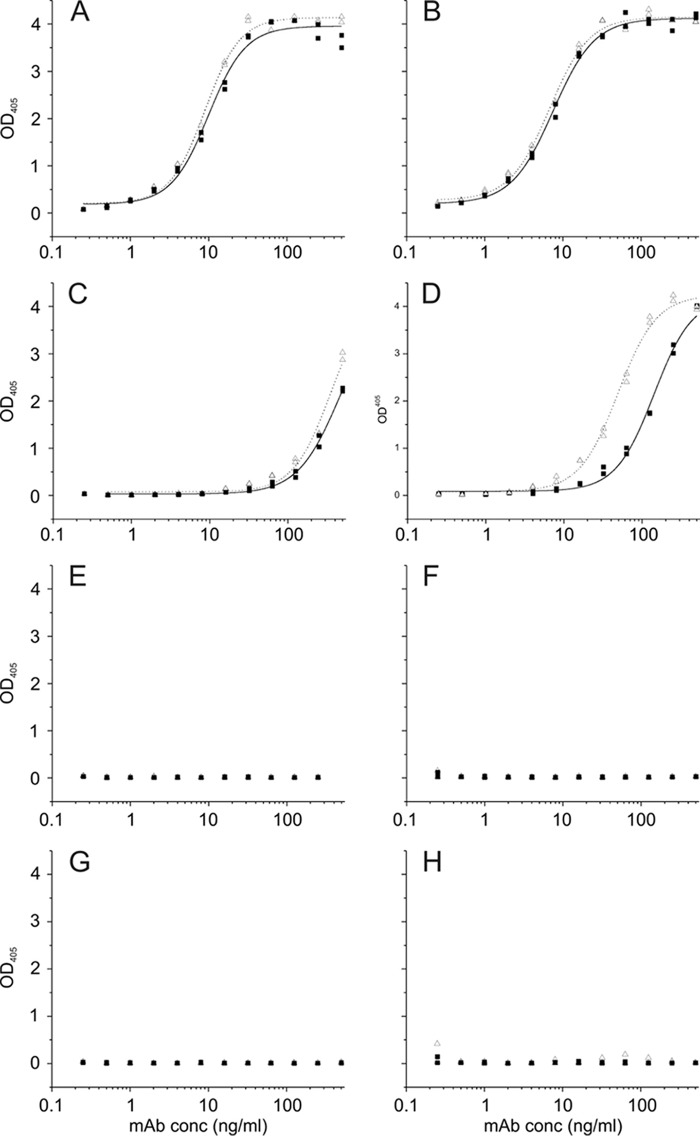
ELISA against the immobilized biotinylated ligands are presented in Fig. 4. BBPbio displaying the natural epitope with two phosphates was bound by both antibodies with highest affinity (Fig. 4, A and B). When ligand was immobilized at 20 pmol (Fig. 4B), half-maximum binding was achieved at 7 ng/ml for S55-3 and S55-5, and an OD of >0.2 was achieved at 0.5 ng/ml for both antibodies. Lowering the amount of ligand to 2 pmol/cup had no effect (Fig. 4A). Reactivity with B4Pbio immobilized at 20 pmol (Fig. 4D) was reduced 20-fold for S55-3 and 7-fold for S55-5 in comparison with BBPbio. Half-maximum binding was observed for S55-3 at 142 ng/ml (OD of >0.2 at 16 ng/ml) and for S55-5 at 49 ng/ml (OD of >0.2 at 5 ng/ml). Lowering the amount of immobilized ligand to 2 pmol (Fig. 4C) reduced binding a further 3-fold (50% binding at 465 ng/ml, OD of >0.2 at 64 ng/ml) for S55-3 and 7-fold for S55-5 (50% at 346 ng/ml, OD of >0.2 at 32 ng/ml). Both antibodies neither bound B1P (Fig. 4, E and F) nor BB (Fig. 4, G and H) at the concentrations tested.
FIGURE 4.
Quantitative ELISA coated with graded concentrations of biotinylated neoglycoconjugates corresponding to 2 pmol (left panel) and 20 pmol (right panel) of ligand per well and reacted with mAbs S55-3 (black squares) and S55-5 (open triangles) at concentrations indicated on the x axis. Measurements were performed as duplicates, and binding curves were generated by fitting the data to a logistic function. Ligands used were BBP (A and B), B4P (C and D), B1P (E and F), and BB (G and H). For structures see Fig. 1.
X-ray Diffraction Data, Solution, and Refinement
Data collection and refinement statistics for liganded and unliganded structures of the Fabs from lipid A-specific mAbs S55-3 (IgG2b) and S55-5 (IgG1) are given in Table 1. The Fabs of both anti-lipid A antibodies were also crystallized in the unliganded form.
TABLE 1.
Data collection and refinement statistics for liganded and unliganded Fabs of lipid A specific mAbs S55–3 and S55–5
| Crystal | S55–3 BBP-NAc | S55–3 Unliganded | S55–5 BBP-NAc | S55–5 Unliganded |
|---|---|---|---|---|
| PDB codes | 5DQ9 | 4ODS | 5DQD | 5DQJ |
| Resolution (Å) | 25.0–1.95 (2.02–1.95)a | 25.0–1.94 (2.01–1.94) | 25.0–1.94 (2.01–1.94) | 25.0–2.60 (2.69–2.60) |
| Space group | C2 | C2 | P21212 | P21 |
| a (Å) | 338.1 | 135.6 | 56.4 | 53.6 |
| b (Å) | 52.9 | 44.3 | 64.8 | 139.9 |
| c (Å) | 75.4 | 85.4 | 129.8 | 72.1 |
| α, β, γ (°) | 90,101.0,90 | 90,111.6,90 | 90,90,90 | 90,110.7,90 |
| Volume Å3 | 1.32 × 106 | 4.77 × 105 | 4.74 × 105 | 5.05 × 105 |
| Wavelength (Å) | 0.979 | 0.979 | 0.979 | 0.979 |
| Mean B-factor (Å2) | 39.3 | 27.1 | 23.1 | 67.6 |
| Z | 3 | 1 | 1 | 2 |
| Unique reflections | 89318 | 33590 | 36013 | 30002 |
| Redundancy | 4.1 (4.2) | 3.7 (3.7) | 5.7 (5.7) | 3.7 (3.8) |
| 〈I/σ(I)〉 | 14.4 (3.04) | 18.4 (2.93) | 15.8 (3.93) | 19.7 (2.78) |
| Rsym (%) | 9.20 (57.7)b | 6.10 (42.7) | 11.1 (47.8) | 6.00 (53.7) |
| Completeness (%) | 98.7 (98.7) | 95.3 (92.2) | 100.0 (99.9) | 99.2 (100.0) |
| Protein atoms | 9802 | 3312 | 3359 | 6718 |
| Solvent atoms | 484 | 240 | 395 | 54 |
| Ligand atoms | 111 | 0 | 37 | 0 |
| Ramachandran outliers | 7 | 4 | 2 | 2 |
| Solvent content (%) | 47.0 | 51.0 | 50.3 | 53.4 |
| Refinement | ||||
| Rwork (%) | 21.4 | 18.7 | 17.5 | 19.3 |
| Rfree (%)c | 24.6 | 23.3 | 22.2 | 22.8 |
| rmsd bond lengths (Å) | 0.010 | 0.010 | 0.010 | 0.010 |
| rms bond angles (°) | 1.47 | 1.42 | 1.46 | 1.35 |
a Values in the parentheses refer to the highest resolution shell.
b Rsym = ΣhklΣi|Ihkl, i − (Ihkl)|/Σhkl Σi Ihkl, i, where (Ihkl) is the average of Friedel-related observations (i) of a unique reflection (hkl).
c 5% of reflections were omitted for Rfree calculations.
d r.m.s. is root mean square.
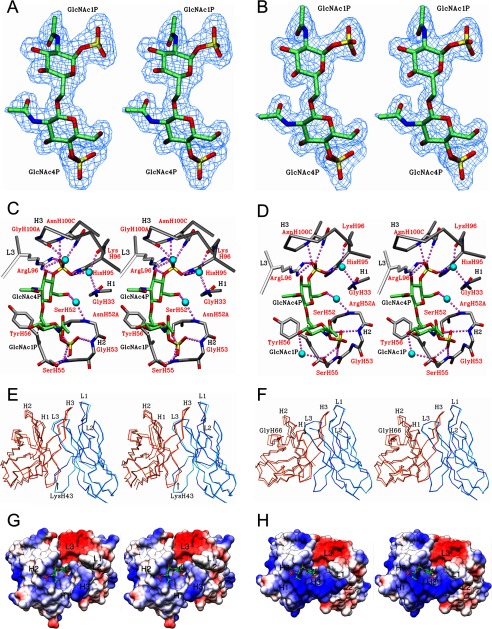
Data were collected to 1.95 Å resolution for S55-3 Fab crystals in the presence of BBP-NAc and with an Rsym = 0.092 in space group C2. The structure contained three molecules in the asymmetric unit. Excellent electron density was observed for the antigen (Fig. 5A) in all three combining sites. Residues 199–202 on the light chain and residues 157–161 on the heavy chain constant domain were disordered, and few of these residues were excluded from the final model. Data were collected on an unliganded crystal of S55-3 Fab to a resolution of 1.95 Å with an Rsym of 0.061 in the monoclinic space group C2. The S55-3 unliganded structure contained one molecule in the asymmetric unit with excellent electron density for the entire Fab, except residues 158–160 on the heavy chain, which were excluded from the final model.
FIGURE 5.
Stereo diagram of 2Fo − Fc electron density map (blue) contoured at 1.0 σ for BBP-NAc lipid A analogue observed in the combining site of S55-3 (A) and S55-5 (B) mAbs post-refinement is shown. Stereo view of S55-3 (C) and S55-5 (D) in complex with BBP-NAc shows hydrogen bonds (purple dashed spheres) and water (blue spheres) bridges between the antigen and mAbs. CDR loops of the light and heavy chain are colored white and gray, respectively. Strong hydrophobic contact are shown as dashed lines (black). C-6 hydroxyl group of second glucosamine is the attachment point to inner core residues normally found on LPS. Stereo view of Fv structure alignments between liganded and unliganded structures of S55-3 (E) and S55-5 (F). Alignments were carried out using the α-carbon trace of the liganded variable light as the reference structure for each antibody. Displacement of CDR L1 and H3 is highlighted. Dark blue, liganded light chain. Cyan, unliganded light chains. Orange, unliganded heavy chains. Red, liganded heavy chain. The stereo image of the electrostatic surface potentials for Fv structures of S55-3 (G) and S55-5 (H) bound to BBP-NAc.
Data were collected to 1.94 Å from a crystal of S55-5 Fab grown in the presence of the BBP-NAc lipid A analogue. The data set was solved in space group P21212 with an Rsym of 0.061. The structure contained one complex in the asymmetric unit with excellent electron density observed for the antigen (Fig. 5B). An unliganded data set for S55-5 Fab was solved to 2.35 Å resolution, with an Rsym = 0.060 in the monoclinic space group P21, containing two molecules in the asymmetric unit. Both liganded and unliganded S55-5 Fabs showed excellent electron density along the polypeptide chains, except for residues 127 to 133 on the heavy chain.
Germ Line Gene Usage and Sequence Comparison
The primary sequence comparisons for the variable region of mAbs S1-15, A6, S55-3, and S55-5 are given in Table 2, along with their germ line sequences. Sequences for S1-15 and A6 antibodies were reported previously (34). As expected, IMGT germ line database analysis revealed that the nucleotide sequences of mAbs S55-3 and S55-5 arise from identical variable genes on both Vl and Vh chain. The S55-3 Vl chain was found to share 288/291 and 32/36 nucleotide identity with the V-gene IGKV3–5*015 and J-gene IgKJ1*01, respectively. S55-5 showed identical nucleotide identity to the V-gene as S55-3 and shared 31/36 nucleotide identity with the respective J-gene on the Vl chain. The V-, D-, and J-genes of S55-3 and S55-5 belong to IGHV5–6*01, IGHD2–2*01, and IGHJ4*01, respectively. Mutations from the S55-3 germ line resulted in 15 amino acid mutations of which 8 are in the CDRs, whereas S55-5 contains 13 mutations, with 7 residue changes in the CDRs.
TABLE 2.
Amino acid sequences of the CDR regions for mAbs S1–15, A6, S55–3, and S55–5
Numbering is based on the Kabat scheme. The sequence of S1–15 originates from germ line 1 (GL-1) sequences. A6 H-chain belongs to the GL-2 sequence and the L-chain to GL-1. MAbs S55–3 and S55–5 both belong to GL-3 sequences. Underlined amino acids are mutated from the germ line and residues in bold are contacting the lipid A antigen directly or through water bridges.
S55-3 and S55-5 Contacts with BBP-NAc
Both S55-3 and S55-3 structures show a total of nine hydrogen bonds to the bisphosphorylated lipid A (Table 3) and four bridging interactions involving three water molecules (Fig. 5, C and D). Both antibodies form two charged interactions to the 4P on the second GlcNAc (N-acetylglucosamine); a bidentate salt bridge formed with Arg(L)-96 (residues are identified as L or H chain to donate the light and heavy chains, respectively) and a salt bridge with His(H)-95 residue. Both antibodies form two hydrogen bonds with the 4P via backbone amide and primary amine group of AsnH100C. Four hydrogen bonds are formed between 1P of the first GlcNAc and CDR H2 residues of S55-3 and S55-5. These include backbone amide and hydroxyl of Ser(H)-55, backbone amide of Gly(H)-53, and a final hydrogen bond from Ser(H)-52 side chain. Both S55-3 and S55-5 form a hydrophobic contact (3.63 and 3.57 Å, respectively) between Cβ of Tyr(H)-56 and the C-6 of the GlcNAc1P residue (Fig. 5, C and D). In one of the Fab molecules in the asymmetric unit of S55-3 structure, a Gly(H)-100A forms a weak hydrogen bond (3.29 Å) through its carbonyl backbone to the C3 hydroxyl of GlcNAc4P; however, this interaction is too distant in the S55-5 structure (3.53 Å).
TABLE 3.
H-bond interactions between mAbs S55-3 and S55-5 to lipid A analogue BBP-NAc
Numbering scheme given in Table 2. The distance cutoff for hydrogen bond assignment was 3.3 Å, except for charged residue interactions where the distance cutoff was 3.9 Å. Hydrogen bond distances shown for S55-3 is averaged over the three molecules in the asymmetric unit.
| Antigen |
Residue |
CDR | Distance (Å) |
|||
|---|---|---|---|---|---|---|
| Residue | Atom(s) | Residue | Atom | S55-3 | S55-5 | |
| GlcN4P | 4PO4 | Arg(L)-96 | NH1 | L3 | 2.94a | 2.96a |
| Arg(L)-96 | NH2 | L3 | 3.08a | 3.00a | ||
| His(H)-95 | ND1 | H3 | 3.00a | 2.88a | ||
| AsnH100C | N | H3 | 2.83 | 2.75 | ||
| AsnH100C | ND2 | H3 | 2.97 | 2.92 | ||
| GlcN1P | 1PO4 | Ser(H)-52 | OG | H2 | 2.69 | 3.06 |
| Gly(H)-53 | N | H2 | 3.17 | 2.80 | ||
| Ser(H)-55 | N | H2 | 3.09 | 2.93 | ||
| Ser(H)-55 | OG | H2 | 2.69 | 2.49 | ||
a Charged residue interaction is shown. Protonation of the His residues is assumed as both proteins were crystallized below pH 5.0.
Least Square Superposition of Liganded and Unliganded Fv Structures
Least square alignments of the α-carbon backbones of the Vl chains of the corresponding liganded and unliganded Fv structures of S55-3 and S55-5 are presented in Fig. 5, E and F. In case of the S55-3-liganded structure, only one of the molecules in the asymmetric unit was used because there were no significant conformational shifts between them. The alignment of S55-3 Fv structures resulted in a mean r.m.s.d. of 0.17 and 0.67 Å, a maximum of 1.41 and 9.70 Å, and a minimum of 0.03 and 0.01 Å for the Vl and Vh, respectively. The large r.m.s.d. observed for the Vh chain belongs to the terminal Glu(H)-1 residue. A modest r.m.s.d. of 4.42 Å was also observed for the Lys(H)-43 residue on Framework Region 2 (FR2) (Fig. 5E). The alignment of S55-5 Fv structures resulted in a mean r.m.s.d. of 0.12 and 0.42 Å, a maximum of 1.02 and 1.90 Å, and a minimum of 0.01 and 0.05 Å for the Vl and Vh respectively. The maximum r.m.s.d. of 1.90 Å corresponds to Gly(H)-66 residue on FR3 (Fig. 5F).
Discussion
Immune Response to Lipid A and Comparison with Other Anti-lipid A Antibodies
The S55-3 and S55-5 combining sites present a complementary pocket to lipid A that displays considerable differences from the CDR composition and recognition strategies of S1-15 and A6 (Table 2) and is an excellent example of how the humoral immune system has evolved redundant responses to a single carbohydrate epitope. This is the first reported structurally characterized example of unrelated germ line gene combinations forming light and heavy chains specific for the identical carbohydrate antigen. Although A6 and S1-15 could be considered to utilize different recognition strategies, their Vl genes are related (34).
S1-15 antibody binds lipid A exclusively through the heavy chain, but A6 uses both the Vl and Vh chain residues. Both S55-3 and S55-5 form a pocket with contacts dominated from the heavy chain (Fig. 5, C, D, G, and H), with only one light chain residue, Arg(L)-96, forming contacts to the BBP-NAc lipid A analogue. Like A6, mAbs S55-3 and S55-5 require both terminal phosphate groups for efficient binding (Fig. 4), whereas binding to the 4-monophosphoryl lipid A is largely retained in the ELISA study for S1-15 (34). One feature common to all four antibodies is a hydrophobic contact between the C-6 carbon of the GlcN1P (Fig. 5, C and D, dashed lines) and tyrosine residues (H)-32 (S1-15), (L)-50 (A6), and (H)-56 (S55-3 and S55-5).
Five-residue Motif in CDR H2 Recognizes Negatively Charged Moieties in Many Antibodies
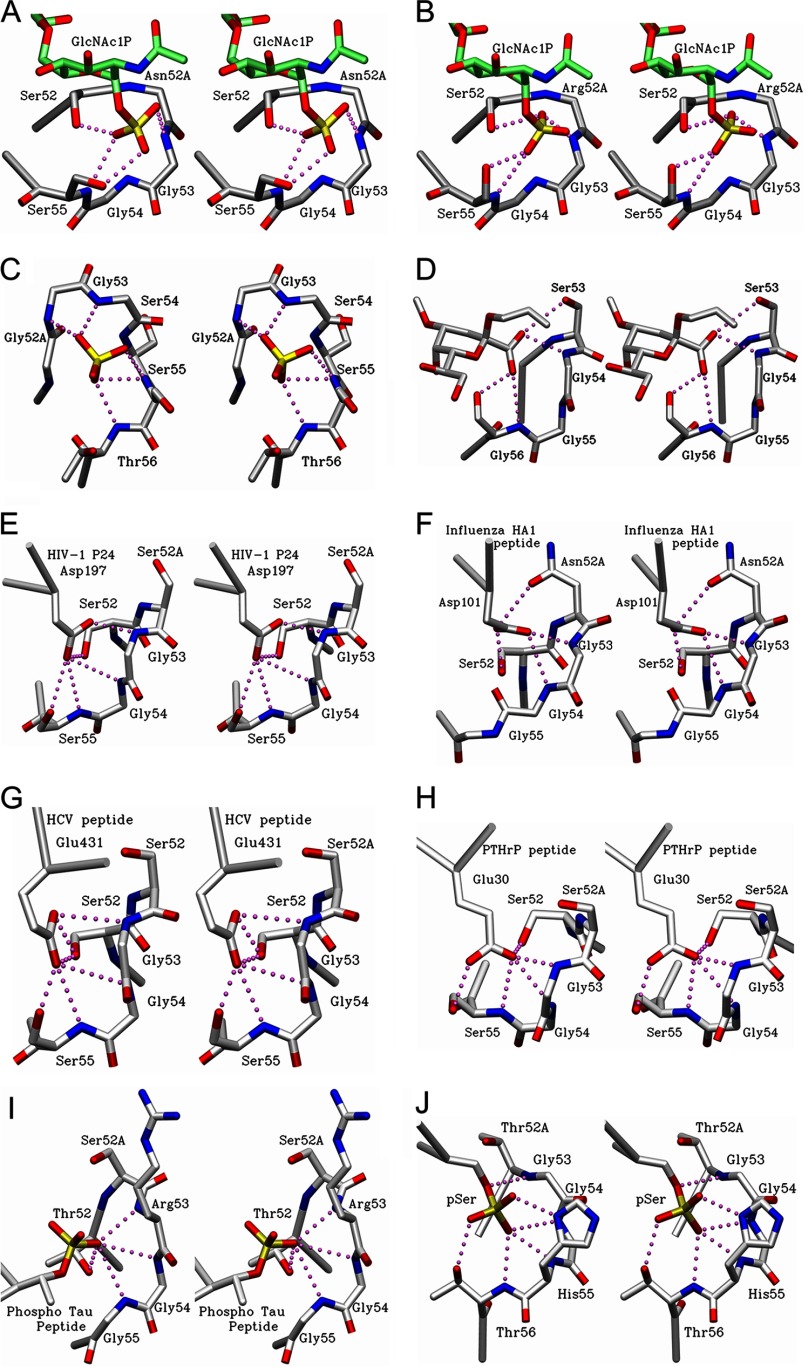
The recognition by S55-3 and S55-5 of the 1P of the first glucosamine is mediated entirely via a short loop in CDR H2 of sequence Ser(H-)52-Asn(H)-52A/Arg(H)-52A-Gly-Gly-Ser(H)-55, respectively (Fig. 6, A and B). A survey of the Protein Data Bank (54) shows that the sequence motif Xaa-Xaa-Gly-Gly-(Ser/Thr/Gly), where one of the Xaa residues is usually a serine or threonine, is involved in binding negatively charged groups in antibodies of otherwise unrelated sequence and specificity. For example, the sequence Gly(H)-52-Gly-Gly-Ser-Ser-Thr(H)-56 (Fig. 6C) in antibody LPT-3 specific for the inner core of LPS from Neisseria meningitidis (55) forms a similar pocket that binds a sulfate group in the complexed structure. Furthermore, the sequence Trp(H)-52-Ser-Gly-Gly-Ser(H)-56 (Fig. 6D) in antibody S25–26 specific for the Chlamydiaceae LPS (49) forms a similar pocket to the carboxyl group of 3-deoxy-d-manno-oct-2-ulosonic acid. Interestingly, these antibodies stem from unrelated heavy chain V-genes. Although CDR H2 sequences in S25–26, S55-3, and S55-5 are germ line-encoded, the CDR H2 sequence of LPT-3 antibody contains mutations GlyH52 and GlyH53 presumably accrued during affinity maturation.
FIGURE 6.
Stereo diagram of CDR H2 loop of antibodies possessing the Xaa-Xaa-Gly-Gly-(Ser/Thr/Gly) motif. Hydrogen bonds are formed via CDR H2 loop of S55-3 (A) and S55-5 Fab (B) (this study) to GlcNAc1P residue of lipid A (this study). C, N. meningitides LPT-3 Fab showing a sulfate group near the binding site and hydrogen bonds formed via the CDR H2 residues. D, CDR H2 loop of anti-chlamydial S25-26 Fab (PDB code 4M7J, Haji-Ghassemi et al. (49) showing hydrogen bonds formed via the CDR H2 residues to 3-deoxy-d-manno-oct-2-ulosonic acid carboxyl group as part of the antigen. E, hydrogen bonds between Asp-197 residue of p24 viral capsid protein and CDR H2 residues of A10F9 Fab (PDB code 3VRL (see Footnote 6)). F, residue contacts between Asp-101 residue of influenza virus hemagglutinin (HA-1) peptide and CDR H2 loop of 17/9 Fab (PDB code 1HIM, Rini et al. (70)). G, contacts between Glu-431 residue of hepatitis C virus envelope peptide and CDR H2 residues of mAb8 Fab (PDB code 4HZL, Deng et al. (57)). H, contacts between Glu-30 residue of a peptide from parathyroid hormone-related protein and CDR H2 residues of anti-PTHrP Fab (PDB code 3FFD, McKinstry et al. (59)). I, contacts between phosphorylated Thr belonging to the Tau peptide and CDR H2 residues of anti-Tau antibody Thr(P)-231/Ser(P)-235 (PDB code 4GLR, Shih et al. (60)). J, contacts between phosphorylated Ser peptide and CDR H2 residues of pSAb (PDB code 4JFZ, Koerber et al. (61)).
The sequence of a glycine residue adjacent to at least one serine residue allows for a more flexible backbone conformation (Table 4) that permits the backbone amides to point toward the anion groups (such as phosphate), with the serine hydroxyl group positioned to form a hydrogen bond to the charged group. The mutual proximity of a number of amide groups results in a formation of a net positively charged pocket, which contributes favorably to binding through enthalpic gains. Adjacent residues with their amide groups pointed inward are ideal anion-binding sites, with three or more so grouped referred to as a “nest” (56). These nests are observed in a variety of different enzymes, like GTPases, iron-sulfur proteins, dehydrogenases, and proteases (56).
TABLE 4.
φ and ψ torsion angles for CDR H2 residues as part of the motif Xaa-Xaa-Gly-Gly-(Ser/Thr/Gly)
Angles are averaged in cases with multiple molecules in the asymmetric unit. Numbers 52–56 refer to the Kabat numbering scheme of CDR H2 residues, and italicized numerals refer to the position of the residue in a β-turn.
| CDR H2 residue dihedral angles (°) |
Antibody | Primary sequence | Resolution | Turn type | PDB code | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 or 52A, 0 | 52A or 53, +1 | 53 or 54, +2 | 54 or 55, +3 | 55 or 56 | ||||||||||
| Å | ||||||||||||||
| φ | ψ | φ | ψ | φ | ψ | φ | ψ | φ | ψ | |||||
| −71.9 | 163.0 | −32.5 | −63.4 | −93.4 | 2.31 | 86.5 | 10.5 | −67.8 | −31.0 | S55-3 | SNGGS | 1.95 | IVa | 4DQ9 |
| −71.0 | 167.2 | −54.2 | −36.4 | −100.4 | −7.86 | 91.7 | 1.64 | −80.6 | −12.2 | S55-5 | SRGGS | 1.94 | I | 5DQD |
| −72.5 | 168.6 | −60.7 | −36.3 | −88.2 | −10.0 | 78.6 | −1.78 | −68.0 | −37.7 | LPT-3 | GGGSS | 2.69 | I | 4C83 |
| −79.7 | 172.0 | −57.6 | −39.4 | −79.1 | −4.48 | 95.5 | −8.60 | −65.9 | 155.7 | S25-26 | WSGGS | 1.95 | I | 4M7J |
| −62.4 | 155.8 | −62.4 | −41.1 | −60.6 | −47.1 | 111.7 | −19.0 | −73.6 | −10.3 | A10F9 Fab | SSGGS | 3.20 | III | 3VRL |
| −64.5 | 155.3 | −61.3 | −28.0 | −98.4 | −5.47 | 90.7 | −26.7 | −62.2 | −41.6 | 17/9 Fab | SNGGG | 2.90 | I | 1HIM |
| −71.5 | 151.3 | −51.5 | −32.3 | −90.2 | −8.04 | 82.2 | 8.61 | −96.8 | −5.02 | mAb8 Fab | SSGGS | 2.85 | I | 4HZL |
| −67.8 | 169.3 | −55.9 | −42.3 | −91.2 | 0.99 | 83.2 | −0.04 | −67.9 | −28.6 | Anti-parathyroid hormone-related protein | SSGGS | 2.00 | I | 3FFD |
| −72.1 | 168.1 | −53.2 | −32.9 | −118.0 | 16.7 | 71.8 | 15.6 | −65.8 | −33.3 | Thr(P)-231/Ser(P)-235 | TSRGGb | 1.90 | I | 4GLR |
| −76.6 | 168.0 | −53.5 | −35.3 | −80.6 | −13.0 | 80.5 | 12.9 | −103.3 | −40.5 | pSAb | ATGGH(T)c | 1.75 | I | 4JFZ |
| −79.7 | 151.3 | −62.4 | −63.4 | −118.0 | −47.1 | 71.8 | −26.7 | −100.3 | −41.6 | Minimum | ||||
| −62.4 | 172.0 | −32.5 | −28.0 | −60.6 | 16.7 | 111.7 | 15.6 | −62.2 | 155.7 | Maximum | ||||
| 17.3 | 20.7 | 29.9 | 35.4 | 57.4 | 63.8 | 39.9 | 42.3 | 38.1 | 65.9 | Difference | ||||
a Turns that do not fall into any known classes are grouped under the miscellaneous type IV class.
b Outlier in terms of the motif sequence involved in binding to negative charge.
c The β-turn starts at the alanine residue, but this residue is not involved in recognition of the anion.
Interestingly, the CDR H2 nest described here to recognize carbohydrate antigens is far more ubiquitous in the recognition of carboxyl groups on glutamate and aspartate residues in protein antigens such as viral proteins (PDB code 3VRL),6 viral peptides (57, 58), and an α-helical peptide from thyroid receptor (59), and phosphopeptides (Table 4 and Fig. 6, E–J) (60–62).
Using phage display, Koerber et al. (61) further refined the CDR H2 residues required in the recognition of phosphorylated peptides corresponding to post-translationally modified proteins, with the observation of two distinct modes. Although one mode involves arginine or lysine side chains, the other is remarkably similar to the more general motif described here with an optimal consensus sequence of Ser(H)-52-Thr(H)-52A-Gly-Gly-Ser(H)-55.
One notable exception is the anti-Tau antibody, which forms a similar pocket against a phosphate group using Thr(H)-52-Ser(H)-52A-Arg-Gly-Gly(H)-55, which deviates slightly from the consensus sequence Xaa-Xaa-Gly-Gly-(Ser/Thr/Gly) (Table 4 and Fig. 6I) (60). Nevertheless, the structure of this CDR H2 requires the backbone amides of glycine residues to point toward the phosphate, and this knowledge was crucial in humanization efforts of this antibody (62).
An extensive search through the Protein Data Bank did not reveal other proteins that utilized this motif in the recognition of negatively charged species, and it appears to be restricted to the CDR H2 loops of antibodies. Given that this is a conserved sequence in unrelated germ lines, it is quite likely that this sequence motif has evolved to recognize the negatively charged moieties on antigens present on a variety different pathogens, and the motif would therefore serve as a prediction tool for other antibodies with specificity toward charged antigens. For example, several DNA-binding antibodies (accession numbers AAB49122 and AAB49121, and AAB53403 EMBL nucleotide database) possess the following CDR H2 sequence, Ser(H)-52-Ser-Gly-Gly-Ser(H)-55 (63), which may form a pocket specific for a DNA backbone phosphate. Other examples that possess this motif include anti-meningococcal polysaccharide C antibody, 1922.2 (64), and a group of antibodies cross-reactive toward a variety of antigens (DNA, carbohydrate, and protein) (65), all of which contain negatively charged groups.
Interestingly, the germ line sequence of mAb A6 possesses the motif Ser(H)-52A-Asn-Gly-Gly-Ser(H)-56, but the last serine was likely mutated to an asparagine during affinity maturation, and the resulting CDR H2 does not form a pocket for the lipid A phosphates (nor do the lipid A phosphates bind the antibody near H2). However, the presence of this motif in the germ line may explain the polyspecific behavior of some anti-lipid A antibodies toward oligonucleotides via binding to their phosphate groups.
S55-3 CDR H2 Possesses a Different β-Turn Type
Turns in proteins are categorized by their main chain dihedral angles. Venkatachalam (66) originally described three types (designated I, II, and III, along with their mirror images I′, II′, and III′), which have since been expanded into 10 distinct types, including the “miscellaneous” type IV (67, 68). Although most of the turns in the H2 loops involved in the recognition of negatively charged groups fall into the type I turn (most common), S55-3 does not match any known criteria and therefore is classed under type IV. Antibody A10F9 Fab displays main chain dihedral angles close to but not precisely within the range of a type III turn (Table 4).
Steric Hindrance Prevents S55-3 and S55-5 from Binding LPS
The structures for mAbs S55-3 and S55-5 in complex with lipid A backbone yield an unequivocal explanation as to why these antibodies cannot recognize lipid A when attached to the inner core LPS structures as neither S55-3 nor S55-5 can accommodate the inner core residues due to steric hindrance. Although there are significant differences between S55-3 and S55-5 and both S1-15 and A6, the latter mAbs fail to bind intact LPS for a similar reason (34, 35, 69). This indicates that the widely reported inability to raise antibodies specific for LPS from free lipid A may be for same general reason.
Recognition Strategy and Binding Surface of Lipid A-specific Antibodies
Antibody binding mechanisms are often be described as “lock and key” or “induced fit,” based on the degree of conformational shift when comparing the CDRs of bound and unbound states of the antibody (20, 37, 58, 70–74). Despite their similarity in sequence, S55-3 and S55-5 displayed variable degrees of induced fit between their unliganded and liganded structures (Fig. 5, E and F). There is a displacement in the Framework Region 2 for the Vh chain of S55-3 (r.m.s.d. of 4.42 Å) between the liganded and unliganded structure; however, no noticeable degree of induced fit is observed for S55-5 in the same region, which is surprising considering their high sequence and structural similarity. This is also unusual because mutations accrued during affinity maturation often generate a more rigid lock-and-key type receptor (75, 76); however, in this case the antibody with the higher number of mutations, namely S55-3, shows a higher degree of induced fit than S55-5, although they show comparable avidities toward the lipid A antigen (Fig. 4).
Furthermore, an antibody-combining site can form a pocket or groove as originally predicted by Kabat (77) or a combination of both depending on the nature of the antigen (24). B-cell receptors often select groove combining site architecture for larger antigens such as proteins, although pocket-type antibodies usually select for oligosaccharides and smaller haptens. As with S1-15 and A6, mAbs S55-3 and S55-5 antibodies form positively charged pockets with high surface complementarity to lipid A phosphate groups (Fig. 5, G and H).
From the ELISAs reported in Haji-Ghassemi et al. (34) and in this study, the relative strength of binding to the bisphosphorylated lipid A backbone can be determined as A6 > S55-3 ≈ S55-5 > S1-15 (Fig. 4). The trend is consistent with the number of contacts and salt bridges formed between these antibodies and lipid A. A6 forms 10 direct contacts, including three charged interactions, whereas S55-3 and S55-5 only form nine hydrogen bonds with two charged interactions to the lipid A. S1-15 forms only eight hydrogen bonds, including three charged interactions. The minor difference in avidity between A6 and S55-3/5 can be explained due to the third (weak) salt bridge formed between Lys(H)-53 of A6 and 4P of lipid A. Most of the hydrogen bonds between S55-3/S55-5 and lipid A are directed toward the 4P group of lipid A, explaining the loss of binding upon its removal as observed from ELISA (Fig. 4). The interactions to the 1P is formed via a net positively charged surface (Fig. 5, G and H) as several backbone amide groups are pointed toward the 1P, accounting for the higher avidity of S55-3 and S55-5 for lipid A compared with S1-15. Although they have comparable binding, there is a noticeable difference in recognition of the 4P between S55-3 and S55-5, where S55-5 shows higher avidity (Fig. 4, C and D) toward the 4-monophosphorylated ligand. The increased number of mutations in or adjacent to the combining site of S55-5 results in subtle changes in the position of residues contacting the antigen. Consequently, S55-5 forms stronger interactions to lipid A (particularly to the 4P) in comparison with S55-3, as evident from hydrogen bond distances as shown in Table 3.
Neither the sequence analysis nor the structures of S55-3 and S55-5 suggest the possibility of recognition of nucleic acids or oligonucleotides as observed for many phospholipid-binding antibodies (18, 78–80). This was not surprising as S55-3 and S55-5 form a small cavity that is unlikely to accommodate an oligonucleotide, and furthermore, the binding sites lack the stacking interactions with nucleotide bases via Tyr and Trp residues observed in anti-nucleotide antibodies (48, 81–83).
Conclusion
The structures of mAbs S55-3 and S55-5 confirm the structural basis for the inability of anti-lipid A antibodies to bind intact LPS and provide an explanation for their binding avidities. All lipid A antibodies structurally characterized thus far bind lipid A such that the GlcN-O6 attachment point for the core oligosaccharide is buried in the combining site, which may explain the general inability of this entire class of antibodies to recognize intact LPS.
The binding mechanism of S55-3 and S55-5 stems from germ line origin distinct from antibodies A6 and S1-15, and it generates unique combining-site pockets that are complementary both in shape and charge to the antigen with a minimum polyspecific potential.
Finally, the sequence motif Xaa-Xaa-Gly-Gly-(Ser/Thr/Gly) commonly present in the CDR H2 loop of unrelated antibodies appears to play an evolutionarily conserved role in recognizing negatively charged groups on carbohydrates, DNA, and proteins. Thus, this motif may help in antibody engineering for recognition of negatively charged ions (such as phosphopeptides) and serve as a platform for mutagenesis and modeling studies where a complexed structure is not available.
Author Contributions
O. H. G. performed many of the experiments, prepared Figs. 5 and 6, analyzed the data, and wrote a major part of the paper. S. M. L. conceived the experiments, provided reagents, sequenced the antibodies, performed NMR and ELISA experiments, analyzed the data, and contributed to the paper. T. R. performed some of the experiments. L. B. generated and initially characterized the antibodies, analyzed the data, and contributed to the paper. H.-D. G. generated the neoglycoconjugate for the immunization of mice. H. B. provided reagents, conceived the generation of the antibodies, and initiated the project. S. V. E. conceived many of the experiments and wrote a major part of the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We gratefully acknowledge the technical assistance of Ute Agge, Veronika Susott, Christine Schneider, and Nadine Harmel at the Research Center, Borstel, Germany. Research described in this paper was performed at the Canadian Light Source, which is supported by the Canada Foundation for Innovation, Natural Sciences and Engineering Research Council of Canada, the University of Saskatchewan, the Government of Saskatchewan, Western Economic Diversification Canada, the National Research Council Canada, and the Canadian Institutes of Health Research.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (to S. V. E.). The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 5DQ9, 5DQD, and 5DQJ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
The gene designations shown with parentheses are based on the IMGT/V-quest database (Brochet et al. (44) and Monod et al. (45)) and are used throughout this paper. The asterisk denotes the allele group.
Y. Gu, F. Cao, S. Li, Y. A. Yuan, and N. Xia, unpublished data.
- Fab
- fragment antigen binding
- BBP
- bisphosphorylated lipid A backbone
- BBP-NAc
- 2,2′-N-acetylated bisphosphorylated lipid A backbone
- B4P
- 4′-monophosphorylated lipid A backbone
- B1P
- 1′-monophosphorylated lipid A backbone
- CDR
- complementarity determining region
- Fv
- fragment variable
- r.m.s.d.
- root mean square deviation
- HPAEC
- high performance anion-exchange chromatography
- PDB
- Protein Data Bank
- Vh
- variable heavy
- Vl
- variable light
- BB
- dephosphorylated lipid A backbone
- EIA
- enzyme immunoassay
- DVS
- divinyl sulfone.
References
- 1. Engel C., Brunkhorst F. M., Bone H. G., Brunkhorst R., Gerlach H., Grond S., Gruendling M., Huhle G., Jaschinski U., John S., Mayer K., Oppert M., Olthoff D., Quintel M., Ragaller M., et al. (2007) Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 33, 606–618 [DOI] [PubMed] [Google Scholar]
- 2. Martin G. S. (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev. Anti Infect. Ther. 10, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., and Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 4. Buttenschoen K., Radermacher P., and Bracht H. (2010) Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbeck Arch. Surg. 395, 597–605 [DOI] [PubMed] [Google Scholar]
- 5. Rietschel E. T., Brade H., Brade L., Brandenburg K., Schade U., Seydel U., Zähringer U., Galanos C., Lüderitz O., and Westphal O. (1987) Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog. Clin. Biol. Res. 231, 25–53 [PubMed] [Google Scholar]
- 6. Kawasaki K. (2009) Endotoxin modifications in the bacterial outer membrane: lipopolysaccharide lipid A remodeling in Salmonella typhimurium. Immun. Endoc. Metab. Agents Med. Chem. 9, 224–233 [Google Scholar]
- 7. Zweigner J., Gramm H. J., Singer O. C., Wegscheider K., and Schumann R. R. (2001) High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98, 3800–3808 [DOI] [PubMed] [Google Scholar]
- 8. Miller S. I., Ernst R. K., and Bader M. W. (2005) LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3, 36–46 [DOI] [PubMed] [Google Scholar]
- 9. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., and Lee J. O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist eritoran. Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 10. Miyake K. (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 19, 3–10 [DOI] [PubMed] [Google Scholar]
- 11. Hold G., and Bryant C. (2011) in Bacterial Lipopolysaccharides (Knirel Y., and Valvano M., eds) pp. 371–387, Springer, Vienna [Google Scholar]
- 12. Müller-Loennies S., Brade L., and Brade H. (2007) Neutralizing and cross-reactive antibodies against enterobacterial lipopolysaccharide. Int. J. Med. Microbiol. 297, 321–340 [DOI] [PubMed] [Google Scholar]
- 13. Wittebole X., Castanares-Zapatero D., and Laterre P. F. (2010) Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010:568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alejandria M. M., Lansang M. A., Dans L. F., and Mantaring J. B. 3rd (2013) Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 9, CD001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomery K., Müller-Loennies S., Brooks C. L., Brade L., Kosma P., Di Padova F., Brade H., and Evans S. V. (2012) Antibody WN1 222-5 mimics Toll-like receptor 4 binding in the recognition of LPS. Proc. Natl. Acad. Sci. U.S.A. 109, 20877–20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brade L., and Brade H. (1985) Characterization of two different antibody specificities recognizing distinct antigenic determinants in free lipid A of Escherichia coli. Infect. Immun. 48, 776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujihara Y., Bogard W. C., Lei M. G., Daddona P. E., and Morrison D. C. (1993) Monoclonal anti-lipid A IgM antibodies HA-1A and E5 recognize distinct epitopes on lipopolysaccharide and lipid A. J. Infect. Dis. 168, 1429–1435 [DOI] [PubMed] [Google Scholar]
- 18. Helmerhorst E. J., Maaskant J. J., and Appelmelk B. J. (1998) Anti-lipid A monoclonal antibody centoxin (HA-1A) binds to a wide variety of hydrophobic ligands. Infect. Immun. 66, 870–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuhn H. M. (1993) Cross-reactivity of monoclonal antibodies and sera directed against lipid A and lipopolysaccharides. Infection 21, 179–186 [DOI] [PubMed] [Google Scholar]
- 20. Blackler R. J., Müller-Loennies S., Brade L., Kosma P., Brade H., and Evans S. V. (2012) in Anticarbohydrate Antibodies: From Molecular Basis to Clinical Application (Kosma P., and Müller-Loennies S., eds) pp. 75–120, Springer, Vienna [Google Scholar]
- 21. Rynkiewicz M. J., Lu Z., Hui J. H., Sharon J., and Seaton B. A. (2012) Structural analysis of a protective epitope of the Francisella tularensis O-polysaccharide. Biochemistry 51, 5684–5694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vulliez-Le Normand B., Saul F. A., Phalipon A., Bélot F., Guerreiro C., Mulard L. A., and Bentley G. A. (2008) Structures of synthetic O-antigen fragments from serotype 2a Shigella flexneri in complex with a protective monoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 105, 9976–9981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cygler M., Rose D. R., and Bundle D. R. (1991) Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science 253, 442–445 [DOI] [PubMed] [Google Scholar]
- 24. Haji-Ghassemi O., Blackler R. J., Martin Young N., and Evans S. V. (2015) Antibody recognition of carbohydrate epitopes. Glycobiology 25, 920–952 [DOI] [PubMed] [Google Scholar]
- 25. Ianaro A., Tersigni M., and D'Acquisto F. (2009) New insight in LPS antagonist. Mini-Rev. Med. Chem. 9, 306–317 [DOI] [PubMed] [Google Scholar]
- 26. Li J., Carr B., Goyal M., and Gaieski D. F. (2011) Sepsis: the inflammatory foundation of pathophysiology and therapy. Hosp. Pract. (1995) 10.3810/hp.2011.08.585 [DOI] [PubMed] [Google Scholar]
- 27. Cross A. S. (2014) Anti-endotoxin vaccines: Back to the future. Virulence 5, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Padova F. E., Brade H., Barclay G. R., Poxton I. R., Liehl E., Schuetze E., Kocher H. P., Ramsay G., Schreier M. H., and McClelland D. B. (1993) A broadly cross-protective monoclonal-antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect. Immun. 61, 3863–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollack M., Ohl C. A., Golenbock D. T., Di Padova F., Wahl L. M., Koles N. L., Guelde G., and Monks B. G. (1997) Dual effects of LPS antibodies on cellular uptake of LPS and LPS-induced proinflammatory functions. J. Immunol. 159, 3519–3530 [PubMed] [Google Scholar]
- 30. Kuhn H. M., Brade L., Appelmelk B. J., Kusumoto S., Rietschel E. T., and Brade H. (1992) Characterization of the epitope specificity of murine monoclonal antibodies directed against lipid A. Infect. Immun. 60, 2201–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yokota S., Ohtsuka H., Kohzuki T., and Noguchi H. (1996) A polyreactive human anti-lipid A monoclonal antibody having cross reactivity to polysaccharide portions of Pseudomonas aeruginosa lipopolysaccharides. FEMS Immunol. Med. Microbiol. 14, 31–38 [DOI] [PubMed] [Google Scholar]
- 32. Galanos C., Lüderitz O., and Westphal O. (1971) Preparation and properties of antisera against the lipid A component of bacterial lipopolysaccharides. Eur. J. Biochem. 24, 116–122 [DOI] [PubMed] [Google Scholar]
- 33. Appelmelk B. J., Verweij-van Vught A. M., Maaskant J. J., Schouten W. F., De Jonge A. J., Thijs L. G., and Maclaren D. M. (1988) Production and characterisation of mouse monoclonal antibodies reacting with the lipopolysaccharide core region of Gram-negative bacilli. J. Med. Microbiol. 26, 107–114 [DOI] [PubMed] [Google Scholar]
- 34. Haji-Ghassemi O., Müller-Loennies S., Rodriguez T., Brade L., Kosma P., Brade H., and Evans S. V. (2015) Structural basis for antibody recognition of lipid A: insights to polyspecificity toward single stranded DNA. J. Biol. Chem. 290, 19629–19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brade L., Engel R., Christ W. J., and Rietschel E. T. (1997) A nonsubstituted primary hydroxyl group in position 6′ of free lipid A is required for binding of lipid A monoclonal antibodies. Infect. Immun. 65, 3961–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pascual V., Victor K., Spellerberg M., Hamblin T. J., Stevenson F. K., and Capra J. D. (1992) Vh restriction among human cold agglutinins. The Vh4-21 gene segment is required to encode anti-I and anti-I specificities. J. Immunol. 149, 2337–2344 [PubMed] [Google Scholar]
- 37. Nguyen H. P., Seto N. O., MacKenzie C. R., Brade L., Kosma P., Brade H., and Evans S. V. (2003) Germline antibody recognition of distinct carbohydrate epitopes. Nat. Struct. Biol. 10, 1019–1025 [DOI] [PubMed] [Google Scholar]
- 38. Caton A. J., Brownlee G. G., Staudt L. M., and Gerhard W. (1986) Structural and functional implications of a restricted antibody-response to a defined antigenic region on the influenza-virus hemagglutinin. EMBO J. 5, 1577–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houen G., and Jensen O. M. (1995) Conjugation to preactivated proteins using divinylsulfone and oodoacetic acid. J. Immunol. Methods 181, 187–200 [DOI] [PubMed] [Google Scholar]
- 40. Holst O., Müller-Loennies S., Lindner B., and Brade H. (1993) Chemical structure of the lipid A of Escherichia coli J-5. Eur. J. Biochem. 214, 695–701 [DOI] [PubMed] [Google Scholar]
- 41. Fu Y., Baumann M., Kosma P., Brade L., and Brade H. (1992) A synthetic glycoconjugate representing the genus-specific epitope of chlamydial lipopolysaccharide exhibits the same specificity as its natural counterpart. Infect. Immun. 60, 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holst O., Thomas-Oates J. E., and Brade H. (1994) Preparation and structural analysis of oligosaccharide monophosphates obtained from the lipopolysaccharide of recombinant strains of Salmonella minnesota and Escherichia coli expressing the genus-specific epitope of Chlamydia lipopolysaccharide. Eur. J. Biochem. 222, 183–194 [DOI] [PubMed] [Google Scholar]
- 43. Müller-Loennies S., Gronow S., Brade L., MacKenzie R., Kosma P., and Brade H. (2006) A monoclonal antibody against a carbohydrate epitope in lipopolysaccharide differentiates Chlamydophila psittaci from Chlamydophila pecorum, Chlamydophila pneumoniae, and Chlamydia trachomatis. Glycobiology 16, 184–196 [DOI] [PubMed] [Google Scholar]
- 44. Brochet X., Lefranc M. P., and Giudicelli V. (2008) IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36, W503–W508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Monod M. Y., Giudicelli V., Chaume D., and Lefranc M. P. (2004) IMGT/Junction Analysis: the first tool for the analysis of the immunoglobulin and T cell receptor complex V-J and V-D-J JUNCTIONs. Bioinformatics 20, i379–385 [DOI] [PubMed] [Google Scholar]
- 46. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Franklin M. C., Navarro E. C., Wang Y., Patel S., Singh P., Zhang Y., Persaud K., Bari A., Griffith H., Shen L., Balderes P., and Kussie P. (2011) The structural basis for the function of two anti-VEGF receptor 2 antibodies. Structure 19, 1097–1107 [DOI] [PubMed] [Google Scholar]
- 48. Herron J. N., He X. M., Ballard D. W., Blier P. R., Pace P. E., Bothwell A. L., Voss E. W. Jr., and Edmundson A. B. (1991) An autoantibody to single-stranded-DNA: comparison of the 3-dimensional structures of the unliganded Fab and a deoxynucleotide Fab complex. Proteins 11, 159–175 [DOI] [PubMed] [Google Scholar]
- 49. Haji-Ghassemi O., Müller-Loennies S., Saldova R., Muniyappa M., Brade L., Rudd P. M., Harvey D. J., Kosma P., Brade H., and Evans S. V. (2014) Groove-type recognition of chlamydiaceae-specific lipopolysaccharide antigen by a family of antibodies possessing an unusual variable heavy chain N-linked glycan. J. Biol. Chem. 289, 16644–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 51. Murshudov G. N., Vagin A. A., and Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 52. Winn M. D., Murshudov G. N., and Papiz M. Z. (2003) Macromolecular TLS refinement in REFMAC at moderate resolutions. Method Enzymol 374, 300–321 [DOI] [PubMed] [Google Scholar]
- 53. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., and Ferrin T. E. (2004) UCSF chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 54. Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., and Bourne P. E. (2000) The Protein Data Bank. Nucleic Acids Res. 28, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parker M. J., Gomery K., Richard G., MacKenzie C. R., Cox A. D., Richards J. C., and Evans S. V. (2014) Structural basis for selective cross-reactivity in a bactericidal antibody against inner core lipooligosaccharide from Neisseria meningitidis. Glycobiology 24, 442–449 [DOI] [PubMed] [Google Scholar]
- 56. Watson J. D., and Milner-White E. J. (2002) A novel main-chain anion-binding site in proteins: The nest. A particular combination of φ, ψ values in successive residues gives rise to anion-binding sites that occur commonly and are found often at functionally important regions. J. Mol. Biol. 315, 171–182 [DOI] [PubMed] [Google Scholar]
- 57. Deng L., Zhong L., Struble E., Duan H., Ma L., Harman C., Yan H., Virata-Theimer M. L., Zhao Z., Feinstone S., Alter H., and Zhang P. (2013) Structural evidence for a bifurcated mode of action in the antibody-mediated neutralization of hepatitis C virus. Proc. Natl. Acad. Sci. U.S.A. 110, 7418–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schulze-Gahmen U., Rini J. M., and Wilson I. A. (1993) Detailed analysis of the free and bound conformations of an antibody. X-ray structures of Fab 17/9 and 3 Different Fab-peptide complexes. J. Mol. Biol. 234, 1098–1118 [DOI] [PubMed] [Google Scholar]
- 59. McKinstry W. J., Polekhina G., Diefenbach-Jagger H., Ho P. W., Sato K., Onuma E., Gillespie M. T., Martin T. J., and Parker M. W. (2009) Structural basis for antibody discrimination between two hormones that recognize the parathyroid hormone receptor. J. Biol. Chem. 284, 15557–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shih H. H., Tu C., Cao W., Klein A., Ramsey R., Fennell B. J., Lambert M., Ní Shúilleabháin D., Autin B., Kouranova E., Laxmanan S., Braithwaite S., Wu L., Ait-Zahra M., Milici A. J., et al. (2012) An ultra-specific avian antibody to phosphorylated Tau protein reveals a unique mechanism for phosphoepitope recognition. J. Biol. Chem. 287, 44425–44434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koerber J. T., Thomsen N. D., Hannigan B. T., Degrado W. F., and Wells J. A. (2013) Nature-inspired design of motif-specific antibody scaffolds. Nat. Biotechnol. 31, 916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baek D. S., and Kim Y. S. (2015) Humanization of a phosphothreonine peptide-specific chicken antibody by combinatorial library optimization of the phosphoepitope-binding motif. Biochem Biophys Res. Commun. 463, 414–420 [DOI] [PubMed] [Google Scholar]
- 63. Krishnan M. R., Jou N. T., and Marion T. N. (1996) Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J. Immunol. 157, 2430–2439 [PubMed] [Google Scholar]
- 64. García-Ojeda P. A., Monser M. E., Rubinstein L. J., Jennings H. J., and Stein K. E. (2000) Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68, 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mertens N. M., Galvin J. E., Adderson E. E., and Cunningham M. W. (2000) Molecular analysis of cross-reactive anti-myosin/anti-streptococcal mouse monoclonal antibodies. Mol. Immunol. 37, 901–913 [DOI] [PubMed] [Google Scholar]
- 66. Venkatachalam C. M. (1968) Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of 3 linked peptide units. Biopolymers 6, 1425–1436 [DOI] [PubMed] [Google Scholar]
- 67. Lewis P. N., Momany F. A., and Scheraga H. A. (1973) Chain reversals in proteins. Biochim. Biophys. Acta 303, 211–229 [DOI] [PubMed] [Google Scholar]
- 68. Hutchinson E. G., and Thornton J. M. (1994) A revised set of potentials for β-turn formation in proteins. Protein Sci. 3, 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. El-Samalouti V. T., Schletter J., Brade H., Brade L., Kusumoto S., Rietschel E. T., Flad H. D., and Ulmer A. J. (1997) Detection of lipopolysaccharide(LPS)-binding membrane proteins by immuno-coprecipitation with LPS and anti-LPS antibodies. Eur. J. Biochem. 250, 418–424 [DOI] [PubMed] [Google Scholar]
- 70. Rini J. M., Schulze-Gahmen U., and Wilson I. A. (1992) Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science 255, 959–965 [DOI] [PubMed] [Google Scholar]
- 71. Wilson I. A., and Stanfield R. L. (1994) Antibody-antigen interactions: new structures and new conformational-changes. Curr. Opin. Struct. Biol. 4, 857–867 [DOI] [PubMed] [Google Scholar]
- 72. Wang W., Ye W., Yu Q., Jiang C., Zhang J., Luo R., and Chen H. F. (2013) Conformational selection and induced fit in specific antibody and antigen recognition: SPE7 as a case study. J. Phys. Chem. B 117, 4912–4923 [DOI] [PubMed] [Google Scholar]
- 73. Sonkaria S., Boucher G., Flórez-Olvarez J., Said B., Hussain S., Ostler E. L., Gul S., Thomas E. W., Resmini M., Gallacher G., and Brocklehurst K. (2004) Evidence for ‘lock and key’ character in an anti-phosphonate hydrolytic antibody catalytic site augmented by non-reaction centre recognition: variation in substrate selectivity between an anti-phosphonate antibody, an anti-phosphate antibody and two hydrolytic enzymes. Biochem. J. 381, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Adhikary R., Yu W., Oda M., Walker R. C., Chen T., Stanfield R. L., Wilson I. A., Zimmermann J., and Romesberg F. E. (2015) Adaptive mutations alter antibody structure and dynamics during affinity maturation. Biochemistry 54, 2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Manivel V., Sahoo N. C., Salunke D. M., and Rao K. V. (2000) Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity 13, 611–620 [DOI] [PubMed] [Google Scholar]
- 76. James L. C., Roversi P., and Tawfik D. S. (2003) Antibody multispecificity mediated by conformational diversity. Science 299, 1362–1367 [DOI] [PubMed] [Google Scholar]
- 77. Kabat E. A. (1957) Size and heterogeneity of the combining sites on an antibody molecule. J. Cell Comp. Physiol. (Suppl.) 50, 79–102 [DOI] [PubMed] [Google Scholar]
- 78. Kaburaki J., Kuwana M., Ogasawara T., Takano M., Funatsu Y., and Tojo T. (1992) Specificity of antibodies to single-stranded (ss)DNA in SLE patients with anti-phospholipid syndrome. Keio J. Med. 41, 10–15 [DOI] [PubMed] [Google Scholar]
- 79. Spellerberg M., Chapman C., Hamblin T., and Stevenson F. (1995) Dual recognition of lipid A and DNA by human antibodies encoded by the VH4–21 gene: a possible link between infection and lupus. Ann. N.Y. Acad. Sci. 764, 427–432 [DOI] [PubMed] [Google Scholar]
- 80. Lafer E. M., Rauch J., Andrzejewski C. Jr., Mudd D., Furie B., Furie B., Schwartz R. S., and Stollar B. D. (1981) Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J. Exp. Med. 153, 897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pokkuluri P. R., Bouthillier F., Li Y., Kuderova A., Lee J., and Cygler M. (1994) Preparation, characterization and crystallization of an antibody Fab fragment that recognizes RNA: crystal structures of native Fab and 3 Fab-mononucleotide complexes. J. Mol. Biol. 243, 283–297 [DOI] [PubMed] [Google Scholar]
- 82. Ou Z., Bottoms C. A., Henzl M. T., and Tanner J. J. (2007) Impact of DNA hairpin folding energetics on antibody-ssDNA association. J. Mol. Biol. 374, 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sanguineti S., Centeno Crowley J. M., Lodeiro Merlo M. F., Cerutti M. L., Wilson I. A., Goldbaum F. A., Stanfield R. L., and de Prat-Gay G. (2007) Specific recognition of a DNA immunogen by its elicited antibody. J. Mol. Biol. 370, 183–195 [DOI] [PubMed] [Google Scholar]